Abstract
Background
Evidence from epidemiological studies on the role of tea drinking in gastric cancer risk remains inconsistent. We aimed to investigate and quantify the relationship between tea consumption and gastric cancer in the Stomach cancer Pooling (StoP) Project consortium.
Methods
A total of 9438 cases and 20,451 controls from 22 studies worldwide were included. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) of gastric cancer for regular versus non-regular tea drinkers were estimated by one and two-stage modelling analyses, including terms for sex, age and the main recognised risk factors for gastric cancer.
Results
Compared to non-regular drinkers, the estimated adjusted pooled OR for regular tea drinkers was 0.91 (95% CI: 0.85–0.97). When the amount of tea consumed was considered, the OR for consumption of 1–2 cups/day was 1.01 (95% CI: 0.94–1.09) and for >3 cups/day was 0.91 (95% CI: 0.80–1.03). Stronger inverse associations emerged among regular drinkers in China and Japan (OR: 0.67, 95% CI: 0.49–0.91) where green tea is consumed, in subjects with H. pylori infection (OR: 0.68, 95% CI: 0.58–0.80), and for gastric cardia cancer (OR: 0.64, 95% CI: 0.49–0.84).
Conclusion
Our results indicate a weak inverse association between tea consumption and gastric cancer.
Subject terms: Gastric cancer, Risk factors, Cancer epidemiology
Introduction
Tea is the most consumed beverage in the world after water, with an estimated global consumption of about 6.3 billion kilograms in 2020 and an estimated increase of 17.4% by 2025 [1]. Tea is made from the dried leaves of the Camellia sinensis plant and its different types, mainly green tea, black tea, oolong tea and white tea, depending on the level of fermentation of the leaves.
In vitro and in vivo studies suggest that tea polyphenols have beneficial effects on inflammation and the prevention of chronic diseases, including certain cancers, diabetes and cardiovascular diseases [2]. However, studies on the association between tea consumption and gastric cancer showed conflicting results [3–15]. Recent meta-analyses, based on data from both cohort and case-control studies, including between 5430 and 23,764 cases of gastric cancer, found weak evidence to support an association between tea drinking and gastric cancer risk [16–18]. A few studies of tea and gastric cancer by anatomic location or histological type have been performed in the last decade [19, 20]. The most recent report from the World Cancer Research Fund and the American Institute for Cancer Research (WCRF/AICR) on non-alcoholic drinks and the risk of cancer concluded that there is too limited evidence to conclude the potential protective effect of tea consumption on gastric cancer [21].
Therefore, to evaluate whether tea consumption is associated with gastric cancer risk, we carried out an individual participant pooled analysis of studies included in a global consortium, the Stomach cancer Pooling (StoP) Project [22].
Methods
Study population
This study was based on the third release (v 3.2) of the StoP Project dataset, which includes 34 case-control (CC) studies or nested within cohort case-control (NCC) studies, for a total of 13,121 cases of gastric cancer and 31,420 controls. The StoP Project is a consortium of epidemiological studies on gastric cancer that was initiated in July 2012. Its main objective is to analyse the role of lifestyle and genetic factors in gastric cancer risk through pooled analyses of individual data following the collection and validation of the original study datasets [22]. Principal investigators of potentially relevant studies were invited to participate in the StoP Project and share original data, such as demographic, lifestyle and clinical variables, including information on known and suspected risk factors for gastric cancer. All datasets were harmonised at the pooling centre (University of Milan) according to a pre-specified format. The StoP Project received ethical approval from the Review Board of the University of Milan (reference 19/15 on 01/04/2015).
For the present analyses, data from 22 studies with information on tea intake (any type of tea) were used, including 9438 gastric cancer cases and 20,451 controls from Greece (two studies [23, 24]), Italy (three studies [25–27]), Canada [28], Russia [29], Portugal [30], USA (three studies [31–33]), Spain [34], Japan [35], Mexico [36], Brazil (three studies [37–39]), Iran [40] and China (four studies [5, 41–43]). The study Italy 3 [44] was excluded from the present analysis due to a high proportion (i.e. >60%) of missing values in tea consumption.
Tea consumption
We searched the original datasets and questionnaires of each study for any information on tea intake, including total tea, green tea, black tea and/or other types of tea. Dietary habits were assessed by food frequency questionnaires (FFQs) (interview-administered or self-administered) focusing on food and beverage consumption before gastric cancer diagnosis (for cases), the onset of disease, hospital admission (for hospital-based controls) or recruitment (for population-based controls).
Participants were asked about their tea-drinking habits (drinkers or non-drinkers), the frequency, the amount of tea consumed, the temperature of tea drinking (in six studies), how strong the tea was (in four studies) and the specific types of tea consumed (in eight studies). When information on different types of tea consumption was collected separately, we considered the combined intake. Tea consumption was expressed in the standard unit of cups per day for all studies by considering the number of cups or times tea was consumed or the frequency of consumption specified in each study. When tea intake was indicated in categories of consumption, the amount of tea consumed was converted into cups per day by considering the average number of teacups or times tea was consumed and dividing it by the number of days considered. Total tea consumption was categorised as non-regular tea drinkers (no tea at all for the studies: Italy 1 [26], China 1 [5], Italy 4 [25], Canada [28], China 2 [41], Russia [29], China 3 [42], China 4 [43], Portugal [30], Mexico 1 [36], USA 3 [33], USA 4 [31], Brazil 3 [39] or less than one cup/day for the studies: Italy 2 [27], Greece 1 [24], USA 1 [32], Spain 2 [34], Brazil 1 [38], Brazil 2 [37] and Greece 2 [23]) and tea drinkers as those who consumed at least one cup/day. For studies Iran 1 [40] and Japan 3 [35], since tea consumption was reported more frequently compared to the other studies, non-regular drinkers were considered those with consumption of fewer than three cups/day and less than two cups/day, respectively, based on the distribution of tea consumption in these studies. In studies where information on the amount and frequency of tea consumed was available, we further grouped regular drinkers as follows: ≥1 to <2 cups/day, ≥2 to <3 cups/day and ≥3 cups/day. Additionally, we considered the study-specific levels of consumption, which were computed based on the distribution of tea consumption for each study, namely non-regular tea drinkers (including non-tea drinkers), low, moderate and high tea consumption. Tea-drinking temperature was assessed as cold or warm, hot or very hot and the strength of tea as strong or very strong and regular or light.
Statistical analysis
We used both a two-stage and a one-stage modelling analysis to estimate the association between tea consumption (regular versus non-regular) and gastric cancer.
We used a two-stage analysis to include the studies that provided original individual data and locally computed estimates, namely the study Greece 2 [23]. For each study, we estimated the odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) between regular tea drinkers compared to non-regular tea drinkers and gastric cancer using multivariable conditional or unconditional logistic regression models, as appropriate. In the second stage, the summary (pooled) effect estimates were obtained through a random-effect model [45]. The heterogeneity between studies in the two-stage analysis was quantified through the I2 statistic [46].
In the one-stage analysis, we estimated the pooled ORs and the corresponding 95% CIs of gastric cancer across the categories of tea consumption using generalised linear mixed-effects models with a logistic link function and a random intercept for each study. We estimated the p-values for trends to test for the significance of linear trends across levels of tea consumption, i.e. considering the variable as ordinal in the models. Further, we assessed the dose-response relationship between cups of tea (continuously) and gastric cancer using a one-stage linear random-effects model with natural cubic splines and three knots at fixed percentiles of tea consumption (50, 75 and 90th) [47].
We also used the one-stage analysis to evaluate the association between tea consumption and gastric cancer across strata of selected covariates. We estimated the effect of tea consumption (regular versus non-regular tea drinkers) across strata of geographic area, sex, age group (<65 years, ≥65 years), socioeconomic status (low, intermediate, high) based on education level, income or occupation in each original study, smoking (never smokers, former smokers, current smokers), alcohol drinking (<1 drink/day, 1–3 drinks/day, ≥4 drinks/day, where 12 g of alcohol is 1 drink), vegetable and fruit intake (low, intermediate, high), salt intake (low, intermediate, high), family history of gastric cancer (no, yes), Helicobacter pylori (Hp) infection (no, yes as defined by serology), type of controls (hospital-based, population-based), study design (CC studies, NCC studies), cancer anatomical site (cardia, non-cardia) and histological type (intestinal, diffuse, mixed/unspecified). The studies from China and Japan were also analysed separately since green tea is the most consumed type of tea in those countries. For the strata of Hp infection, we did not include the Spain 2 study [34] since the information was only collected for cases. For cancer anatomical site (cardia and non-cardia) and histological type (intestinal, diffuse and mixed/unspecified by Lauren classification), we used multinomial mixed-effects models to estimate the ORs for each type of gastric cancer and histological type separately. The heterogeneity across strata was assessed through the Q test statistics.
As a sensitivity analysis, to avoid misclassification bias, we performed a one-stage analysis for the association between tea consumption and gastric cancer, excluding the studies Iran 1 [40] and Japan 3 [35] where non-regular drinkers were defined as those who consumed at least two cups per day. All models (two- and one-stage analyses) were adjusted for sex, 5-year age groups (<40, 40–44 to 70–74, ≥75 years), socioeconomic status (study-specific low, intermediate, high), tobacco smoking (never smoker, former smoker: stopped smoking for more than 1 year, current smoker low: ≤10 cigarettes/day, current smoker intermediate: >10–20 cigarettes/day, current smoker high: >20 cigarettes/day), alcohol drinking (never, low: ≤12 g/day, intermediate: >12 and ≤47 g/day, high: >47 g/day), family history of gastric cancer (no, yes), salt intake (study-specific low, intermediate, high) and vegetable and fruit intake (study-specific tertiles: low, intermediate, high). Subjects with missing values in the study-specific confounders were included in the models either as a separate category or by including them in the lower categories of the variables when there was a low proportion of missing (i.e. <1%).
Results
Table 1 shows the distribution of sociodemographic characteristics and the main lifestyle risk factors among gastric cancer cases and controls. Cases were more frequently men (66.1% versus 59.6%), older (52.8% versus 45.4%, individuals ≥65 years old) and had a lower socioeconomic status (50.6% versus 37.3%) than controls. They were also more frequently high current smokers (8.2% versus 6.7%), heavy drinkers (15.0% versus 11.2%) and had a more frequent family history of gastric cancer (18.4% versus 8.3%).
Table 1.
Main characteristics of gastric cancer cases and controlsa in the Stomach cancer Pooling (StoP) Project consortium.
| Cases | Controls | |||
|---|---|---|---|---|
| N | % | N | % | |
| Total | 9438 | 100.0 | 20,451 | 100.0 |
| Study center | ||||
| Europe | 3750 | 39.7 | 7030 | 34.4 |
| Greece 1 [24] | 110 | 1.2 | 100 | 0.5 |
| Greece 2 [23] | 82 | 0.9 | 410 | 2 |
| Italy 1 [26] | 769 | 8.1 | 2081 | 10.2 |
| Italy 2 [27] | 230 | 2.4 | 547 | 2.7 |
| Italy 4 [25] | 1016 | 10.8 | 1159 | 5.7 |
| Portugal [30] | 692 | 7.3 | 1667 | 8.2 |
| Russia [29] | 450 | 4.8 | 611 | 3 |
| Spain 2 [34] | 401 | 4.2 | 455 | 2.2 |
| Asia | 1686 | 17.9 | 2789 | 13.6 |
| China 1 [5] | 266 | 2.8 | 533 | 2.6 |
| China 2 [41] | 206 | 2.2 | 415 | 2 |
| China 3 [42] | 711 | 7.5 | 711 | 3.5 |
| China 4 [43] | 133 | 1.4 | 433 | 2.1 |
| Iran 1 [40] | 217 | 2.3 | 394 | 1.9 |
| Japan 3 [35] | 153 | 1.6 | 303 | 1.5 |
| Americas | 4002 | 42.4 | 10,632 | 52.0 |
| Brazil 1 [38] | 226 | 2.4 | 226 | 1.1 |
| Brazil 2 [37] | 93 | 1 | 186 | 0.9 |
| Brazil 3 [39] | 368 | 3.9 | 738 | 3.6 |
| Canada [28] | 1182 | 12.5 | 5039 | 24.6 |
| Mexico 1 [36] | 248 | 2.6 | 478 | 2.3 |
| USA 1 [32] | 132 | 1.4 | 132 | 0.6 |
| USA 3 [33] | 170 | 1.8 | 502 | 2.5 |
| USA 4 [31] | 1583 | 16.8 | 3331 | 16.3 |
| Sex | ||||
| Men | 6243 | 66.1 | 12,185 | 59.6 |
| Women | 3195 | 33.9 | 8266 | 40.4 |
| Age | ||||
| <40 | 312 | 3.3 | 1600 | 7.8 |
| 40–44 | 314 | 3.3 | 1212 | 5.9 |
| 45–49 | 542 | 5.7 | 1521 | 7.4 |
| 50–54 | 754 | 8.0 | 1781 | 8.7 |
| 55–59 | 1083 | 11.5 | 2214 | 10.8 |
| 60–64 | 1450 | 15.4 | 2828 | 13.8 |
| 65–69 | 1846 | 19.6 | 3532 | 17.3 |
| 70–74 | 1902 | 20.1 | 3408 | 16.7 |
| ≥75 | 1235 | 13.1 | 2342 | 11.5 |
| Socioeconomic status (study-specific) | ||||
| Low | 4704 | 50.6 | 7529 | 37.3 |
| Intermediate | 3107 | 33.4 | 7451 | 36.9 |
| High | 1478 | 15.9 | 5215 | 25.8 |
| Tobacco smoking | ||||
| Never | 3736 | 41.2 | 9033 | 45.3 |
| Former | 3100 | 34.2 | 6372 | 32.0 |
| Current | ||||
| Low | 547 | 6.0 | 1364 | 6.8 |
| Intermediate | 929 | 10.3 | 1812 | 9.1 |
| High | 744 | 8.2 | 1344 | 6.7 |
| Alcohol drinking (g/day)b | ||||
| Never | 2602 | 31.3 | 5997 | 32.5 |
| <1 drink/day | 2075 | 25.0 | 5964 | 32.4 |
| 1–3 drinks/day | 2387 | 28.7 | 4388 | 23.8 |
| ≥4 drinks/day | 1249 | 15.0 | 2069 | 11.2 |
| Vegetable and fruit intake (study-specific tertiles)c | ||||
| Low | 2862 | 31.1 | 5829 | 29.3 |
| Intermediate | 3087 | 33.6 | 6784 | 34.2 |
| High | 3250 | 35.3 | 7248 | 36.5 |
| Salt intake (study-specific tertiles)d | ||||
| Low | 2957 | 40.4 | 7202 | 41.7 |
| Intermediate | 2529 | 34.6 | 5675 | 32.8 |
| High | 1830 | 25.0 | 4413 | 25.5 |
| Family history of gastric cancere | ||||
| No | 3541 | 81.6 | 7220 | 91.6 |
| Yes | 800 | 18.4 | 658 | 8.3 |
| H. pylori infectionf | ||||
| No | 754 | 33.3 | 1361 | 31.9 |
| Yes | 1511 | 66.7 | 2900 | 68.1 |
| Type of controlsg | ||||
| Hospital-based | 3198 | 33.9 | 5912 | 28.9 |
| Population-based | 6240 | 66.1 | 14,539 | 71.1 |
| Study designh | ||||
| Case-control | 7773 | 82.4 | 16,710 | 81.7 |
| Nested case-control | 1665 | 17.6 | 3741 | 18.3 |
| Subsitei | ||||
| Cardia | 1607 | 28.4 | 20,451 | 100.0 |
| Non-cardia | 4057 | 71.6 | 20,451 | 100.0 |
| Histological typej | ||||
| Intestinal | 1897 | 29.3 | 20,451 | 100.0 |
| Diffuse | 1218 | 18.8 | 20,451 | 100.0 |
| Mixed/unspecified | 3368 | 51.9 | 20,451 | 100.0 |
aFor some variables, the sum does not add to the total because of missing values in age (13 controls), social class (149 cases, 256 controls), tobacco smoking (382 cases, 526 controls), alcohol drinking (281 cases, 889 controls), family history of gastric cancer (1025 controls, 2071 cases), vegetable and fruit intake (106 cases, 157 controls) and salt intake (203 cases, 781 controls), or because the variables were not available for some studies.
bThe studies China 3 [42] and China 4 [43] did not collect data on alcohol drinking.
cThe study China 4 [43] did not collect data on vegetable and fruit intake.
dThe studies Greece 1 [24], Greece 2 [23], China 3 [42] and Italy 4 [25] did not collect data on salt intake.
eThe studies China 1 [5], Canada [28], China 3 [42], Mexico 1 [36], Greece 2 [23] and USA 4 [31] did not collect data on family history of gastric cancer.
fThe studies China 2 [41], Russia [29], Iran 1 [40], China 4 [43], Portugal [30], Mexico 1 [36], Brazil 1 [38], Brazil 2 [37], Japan 3 [35] and Brazil 3 [39] collected data on H. pylori infection. The study Spain 2 [34] was not included because no information on H. pylori infection was available for controls.
gThe studies Italy 4 [25], Canada [28], China 2 [41], Iran 1 [40], China 3 [42], China 4 [43], Portugal [30], Mexico 1 [36] and USA 3 [33] include population-based controls.
hThe studies Greece 2 [23] and USA 4 [31] are nested case-control studies (NCC).
iThe studies China 1 [5], China 2 [41], China 3 [42] and China 4 [43] did not collect data on cancer subsite.
jThe studies Italy 1 [26], China 1 [5], Greece 1 [24], China 2 [41], China 3 [42], China 4 [43], Japan 3 [35] and Greece 2 [23] did not collect data on histological type.
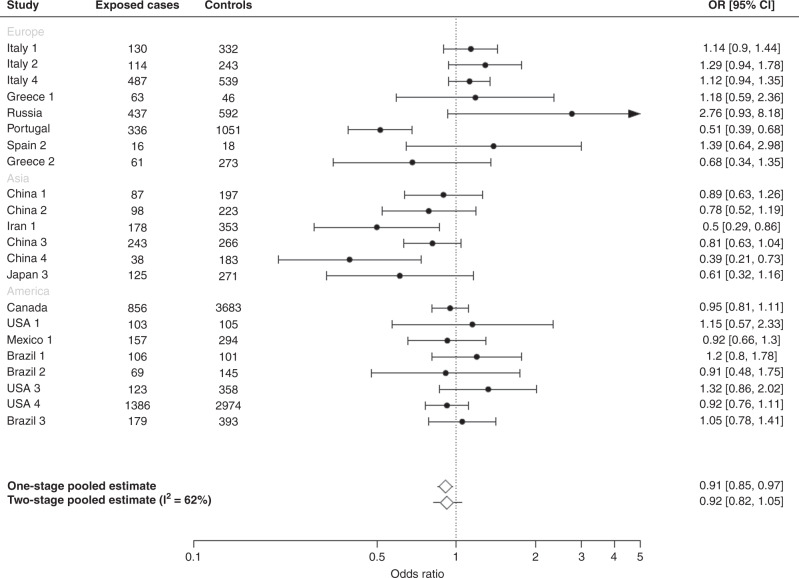
Figure 1 shows the study-specific and adjusted pooled OR of gastric cancer and the corresponding 95% CIs for regular tea drinkers compared with non-regular drinkers. The one-stage pooled OR was 0.91 (95% CI: 0.85–0.97) for regular tea drinkers compared to non-regular tea drinkers. The summary (pooled) OR for gastric cancer obtained from the two-stage analysis was similar to that obtained from the one-stage approach (OR: 0.92, 95% CI: 0.82–1.05), and with an estimated heterogeneity of I2 = 62%. The summary estimates from the two-stage analysis did not change when study Greece 2 [23] was excluded.
Fig. 1. Overall regular tea consumption and gastric cancer.
Study-specific, adjusted pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) of gastric cancer for regular tea drinkers compared with non-regular tea drinkers in the Stomach cancer Pooling (StoP) Project consortium.
The distribution of cases and controls according to the levels of tea consumption and the adjusted pooled ORs of gastric cancer from the one-stage analysis is given in Table 2. About 57.6% of cases and 63.0% of controls reported ever consuming tea, with a pooled OR of 0.91 (95% CI: 0.85–0.97). Tea-drinking intensity was collected in 18 studies, and 26.2% of cases and 30.6% of controls reported consumption of ≥1 cup per day of tea. Compared with non-regular tea drinkers, the one-stage adjusted pooled ORs were 1.03 (95% CI: 0.94–1.12) for ≥1 to <2 cups per day, 0.98 (95% CI: 0.88–1.10) for ≥2 to <3 cups per day and 0.91 (95% CI: 0.80–1.03) for ≥3 cups of tea per day, with a non-significant trend in risk (p = 0.27). Similar results were observed when data were analysed through study-specific categories of tea consumption. A significant inverse association with gastric cancer was found for subjects consuming cold or warm tea compared to non-tea drinkers (OR: 0.65, 95% CI: 0.53–0.79) as well as those consuming regular or light tea (OR: 0.77, 95% CI: 0.63–0.93), while a null association was found for subjects consuming hot or very hot tea (OR: 1.04, 95% CI: 0.88–1.23) and strong or very strong tea (OR: 1.11, 95% CI: 0.89–1.39).
Table 2.
Distribution of gastric cancer cases and controlsa according to tea-drinking habits, adjusted pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for gastric cancer in the Stomach cancer Pooling (StoP) Project consortium.
| Cases | Controls | OR (95% CI)b | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Tea-drinking statusc | |||||
| Non-regular drinkers | 3921 | 42.4 | 7271 | 37.0 | 1 |
| Regular drinkers | 5331 | 57.6 | 12,362 | 63.0 | 0.91 (0.85, 0.97) |
| Tea-drinking intensityd | |||||
| Non-regular drinkers | 5804 | 73.8 | 12,303 | 69.4 | 1 |
| 1 cup/day | 920 | 11.7 | 2198 | 12.4 | 1.03 (0.94, 1.12) |
| 2 cups/day | 557 | 7.1 | 1725 | 9.7 | 0.98 (0.88, 1.10) |
| ≥3 cups/day | 586 | 7.4 | 1507 | 8.5 | 0.91 (0.80, 1.03) |
| p-trend | 0.27 | ||||
| Study-specific tea-drinking intensityd | |||||
| Non-regular drinkers | 3529 | 45.8 | 7850 | 45.1 | 1 |
| Low | 2417 | 31.4 | 5246 | 30.1 | 0.92 (0.85, 0.99) |
| Moderate | 979 | 12.7 | 2432 | 14.0 | 0.98 (0.89, 1.07) |
| High | 776 | 10.1 | 1879 | 10.8 | 0.90 (0.80, 1.01) |
| p-trend | 0.10 | ||||
| Temperature of tea drinkinge | |||||
| Non-tea drinkers | 797 | 40.8 | 1132 | 36.5 | 1 |
| Cold/warm | 372 | 19.0 | 929 | 30.0 | 0.65 (0.53, 0.79) |
| Hot/very hot | 786 | 40.2 | 1041 | 33.6 | 1.04 (0.88, 1.23) |
aFor some variables, the sum does not add to the total because of missing values in tea-drinking status (104 cases, 408 controls), tea-drinking intensity (218 cases, 886 controls), study-specific tea-drinking intensity (94 cases, 211 controls) and tea-drinking temperature (85 cases, 195 controls).
bOne-stage pooled ORs were estimated using a mixed-effects model adjusted for sex, age category, social class, smoking status, salt intake, vegetable and fruit intake, alcohol intake and family history of gastric cancer.
cThe study Greece 2 [23] only provided locally computed estimates and thus was not included in one-stage analyses.
dInformation on tea-drinking intensity was not available for the studies Greece 1 [24], Russia [29], China 3 [42] and Greece 2 [23].
eInformation on the temperature of tea drinking was available for the studies China 1 [5], China 2 [41], Russia [29], Iran 1 [40], USA 3 [31] and China 3 [42].
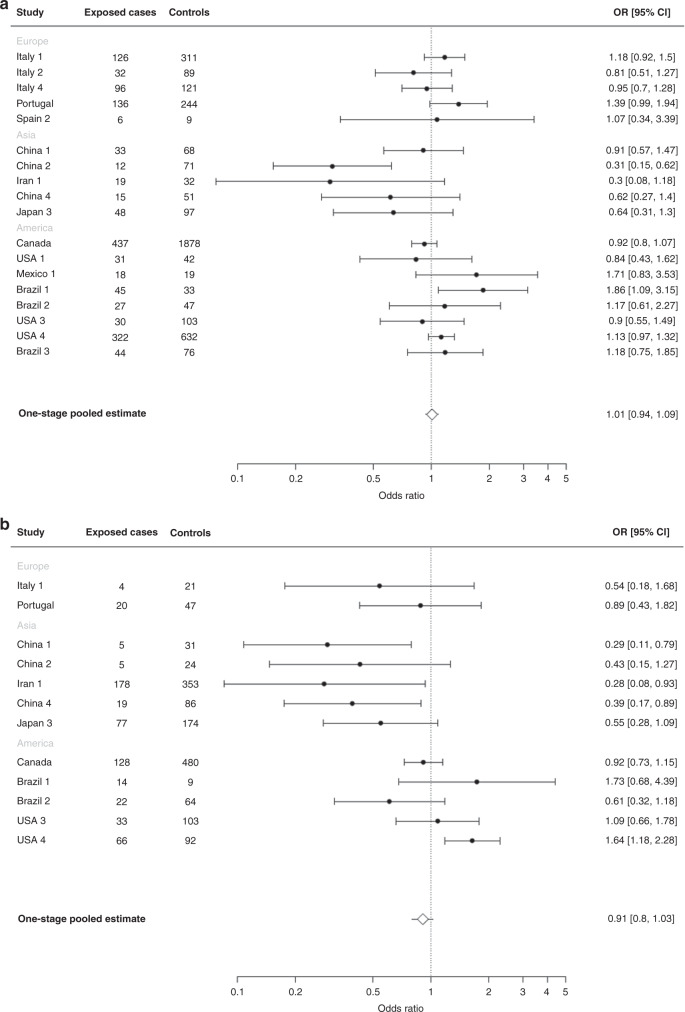
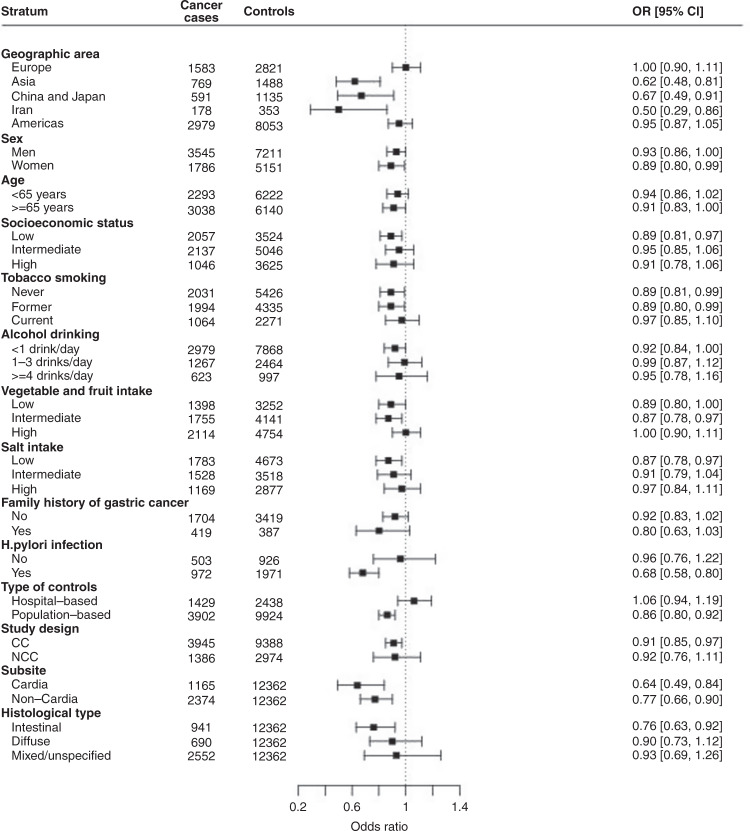
Figure 2 shows the study-specific and one-stage adjusted pooled OR of gastric cancer and the corresponding 95% CIs for consumption of 1–2 cups of tea per day (a) and ≥3 cups of tea per day (b) as compared with non-regular drinkers. The pooled ORs were 1.01 (95% CI: 0.94–1.09) for 1–2 cups/day (panel a) and 0.91 (95% CI: 0.80–1.03) for ≥3 cups of tea per day (panel b). When Iran 1 [40] and Japan 3 [35] studies were excluded from the pooled analysis, the ORs for 1–2 cups/day were 1.06 (95% CI: 0.92–1.22) and 0.81 (95% CI: 0.62–1.07) for ≥3 cups/day. The results from the stratified analysis of tea drinkers are presented in Fig. 3. Heterogeneity was evident across categories of the geographic area of the studies (Q = 11.5, p < 0.001), categories of Hp infection (Q = 5.7, p = 0.017) and type of controls (Q = 8.5, p = 0.003). A reduced risk was found in studies from Asia (OR: 0.62, 95% CI: 0.48–0.81 for studies in China, Japan and Iran, OR: 0.67, 95% CI: 0.49–0.91 for studies in China and Japan only), while null associations emerged from the European and American studies. An inverse association was found among subjects with Hp infection while no association emerged among non-infected subjects (OR: 0.68, 95% CI: 0.58–0.80 versus OR: 0.96, 95% CI: 0.76–1.22). An inverse relation for tea consumers—in the absence of significant heterogeneity across strata—was evident for cardia cancer (OR: 0.64, 95% CI: 0.49–0.84), for non-cardia cancer (OR: 0.77, 95% CI: 0.66–0.90), and intestinal histological type (OR: 0.76, 95% CI: 0.63–0.92), while no associations were detected for diffuse and mixed/unspecified histological types. The reduced risk was restricted to studies using population-based controls (OR: 0.86, 95% CI: 0.80–0.92). Results were similar in the CC (OR: 0.91, 95% CI: 0.85–0.97) and NCC (OR: 0.92, 95% CI: 0.76–1.11) studies, but not significant in the NCC studies.
Fig. 2. Categories of tea consumption and gastric cancer.
Study-specific, adjusted pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) of gastric cancer for tea drinkers of 1–2 cups per day (a) and ≥3 cups per day (b) compared with non-regular tea drinkers in the Stomach cancer Pooling (StoP) Project consortium. Studies with more than five subjects in exposed cases or controls are shown in figures (a) and (b).
Fig. 3. Overall tea consumption and gastric cancer in strata of selected variables.
Adjusted pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for regular tea consumption compared to non-regular tea consumption, according to strata of selected variables in the Stomach cancer Pooling (StoP) Project consortium.
Figure 4 shows the dose-response relationship between tea-drinking intensity (as a continuous variable) and gastric cancer, fitted by natural cubic splines. There was a progressive risk reduction from three cups per day to higher levels of consumption.
Fig. 4. Tea-drinking intensity (as a continuous variable) and gastric cancer.
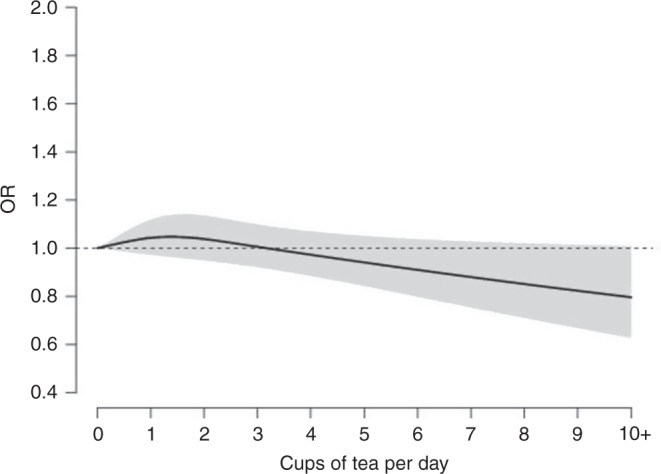
Dose-response relationship between tea consumption and gastric cancer (adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs)) fitted by natural cubic splines in a one-stage linear random-effects model in the Stomach cancer Pooling (StoP) Project consortium.
Discussion
This study, based on data from a global consortium of studies on gastric cancer, found a weak inverse association between tea consumption and gastric cancer, with a 9% lower risk among regular tea drinkers compared to non-regular tea drinkers. A significantly reduced risk of 9% remained in tea drinkers when only CC studies were included, but not for NCC studies, possibly due to the limited numbers. However, the dose-response analysis suggested an increased level of protection at very high intakes. Stronger inverse associations were observed in studies from Asia, in subjects with Hp infection, and those with gastric cardia cancer. The consumption of warm or cold tea was associated with reduced risk, whereas drinking hot or very hot tea was not associated with gastric cancer.
We found a 38% significantly reduced risk of gastric cancer in studies from Asia, driven by an OR of 0.67 obtained by restricting the analysis to studies from China and Japan only and an OR of 0.50 obtained in the Iranian study. These findings could be related to higher amounts of tea consumed, along with the type of tea, mainly green tea in China and Japan, and black tea in Iran [11, 48–50].
Only a few studies have investigated the relationship between tea drinking and gastric cancer by anatomic location or histological type. Our results for cancer subsites are in line with those of the EPIC (European Prospective Investigation into Cancer and Nutrition) study [20] that found a higher risk reduction for cardia cancers than non-cardia cancers, although non-significant. Similarly, the EPIC study reported decreased, although not significant, risks for intestinal- and diffuse-type cancers for higher tea consumption. A cohort study in the United States, including 231 gastric cardia and 224 gastric non-cardia cancer patients, found no association between hot tea consumption and gastric cardia and non-cardia cancers [19].
When we analysed the effect of tea drinking across categories of Hp infection, we found an inverse association only among infected subjects. This finding can be attributed to the potential protective effect of tea consumption against Hp infection. In fact, studies in animal models showed that green tea compounds may be an effective strategy for inhibiting bacterial growth, including Hp, as well as related Hp atrophic gastritis conditions and gastric tumorigenesis [51, 52]. In addition, a study of 150 dyspeptic patients found that the consumption of green/black tea was associated with a reduced prevalence of Hp infection (OR: 0.45, 95% CI: 0.21–0.95) [53].
Concerning tea-drinking temperature, our results are in line with those reported in a case-control study in a Chinese population (266 gastric cancer cases and 533 controls) and found a reduced gastric cancer risk among drinkers of green tea when consumed at cold to warm temperatures (OR: 0.61, 95% CI: 0.45–0.82), but not at hot temperatures [5]. In contrast to what has been observed in other studies, the consumption of hot tea was not associated with a significantly increased risk of gastric cancer. In previous analyses, hot tea consumption was associated with a high risk of gastric cancer with ORs ranging from 1.82 to 2.85 [54, 55], and higher risks were observed for very hot temperatures of consumption (55–60°) with ORs ranging from 3.07 to 7.60 [54, 55]. In this regard, the time elapsed between tea being poured and drunk, which was not considered in our analysis, could have at least partly masked the association [56]. Moreover, four out of six studies reporting information on drinking temperature were conducted in Asia. Thus, it is difficult to evaluate if the observed association is attributable to drinking temperature per se or is rather related to the geographic area.
Among the limitations of the study, most of our results are based on retrospective studies, so information on tea consumption and other dietary habits may suffer from recall bias. However, when we examined CC and NCC studies separately, the findings on the association between tea drinking and gastric cancer were similar, although no significant results were obtained for the NCC studies. Additionally, some studies enrolled hospital-based controls, which could have affected the reported prevalence of dietary factors, but the inverse association between tea drinking and gastric cancer emerged mainly in population-based studies. Differences in the types of questions on tea consumption across studies may represent a source of heterogeneity and may partially explain some of the inconsistent results found in different studies for high levels of consumption. The small effect found for regular versus non-regular tea drinkers could be in part caused by bias or lack of confounding control in some of the included studies. Reverse causation is conceivable, assuming that (strong) tea drinking may cause heartburn in patients, though this remains undefined. We had information on how strong a cup of tea was only in four studies, indicating no heterogeneity. This may also be due to a greater prevalence of Hp in selected Asian countries, and in our analysis, the inverse association was stronger in Hp positive subjects. We had information on Hp positivity in cases from 12 studies conducted in 8 countries [57]. This was 80.3% overall, 82.1% in Japan, and around 88% on average in the three Iranian studies. The apparently greater inverse relationship with tea in Asia cannot, therefore, be largely or totally explained by higher Hp positivity in Asian studies.
The large dataset from a global consortium is the main strength of the study. We included detailed individual-level data on tea drinking and important covariates on over 9000 cases and 19,000 controls. The patient-level approach allowed us to perform in-depth multivariate analyses. Thus, models were adjusted for several potential sociodemographic and lifestyle confounders, such as 5-year age groups, smoking habits, alcohol drinking, salt intake, vegetable and fruit intake and family history of gastric cancer. The associations for gastric cancer were also examined by anatomical site and histological type.
In conclusion, our results, based on a unique pool of studies globally, provide evidence of a weak inverse association between tea consumption and gastric cancer.
Supplementary information
Acknowledgements
We thank the European Cancer Prevention (ECP) Organization for providing support for the StoP meetings.
Author contributions
Study concept and design: GM, CP, CL and EN. Acquisition, processing and reporting of samples: RB, JH, KCJ, CSR, LML, RS, ZFZ, MD, NL, SM, DP, MF, GPY, ST, AH, MPC, EDN, DZ, DM, JV, MGH, LLC, RUHR, GSH, MHW, LM, RM, FP, AT, AK, RCK, AL, PL, SB, PB and MCC. Analysis and interpretation of the data: GM, CP, GA, MR and CL. Drafting of the manuscript: GM, CP, GA and CL. Critical revision of the manuscript: all authors.
Funding
This study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Project no. 21378 (Investigator Grant) and by the Italian League for the Fight Against Cancer (LILT). Brazilian data collection received support from Fundação de Amparo à Pesquisa do Estado de São Paulo (14/26897-0). NL and SM are funded under the Unidade de Investigação em Epidemiologia—Instituto de Saúde Pública da Universidade do Porto (EPIUnit; UIDB/04750/2020) financed by national funds from the Foundation for Science and Technology—FCT (Portuguese Ministry of Science, Technology and Higher Education). SM also received funding under the scope of the project ‘NEON-PC—Neuro-oncological complications of prostate cancer: longitudinal study of cognitive decline’ (POCI-01-0145-FEDER-032358; Ref. PTDC/SAU-EPI/32358/2017) funded by FEDER through the Operational Program Competitiveness and Internationalization, and national funding from FCT, and the EPIUnit—Junior Research—Prog Financing (UIDP/04750/2020).
Data availability
Data supporting the results reported in the paper cannot be found on publicly available databases. Data can be provided on reasonable request from the authors.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The participating studies were performed in accordance with laws, regulations and guidelines for the protection of human subjects (including consent from the participants) applicable at the time of study conduction, and in accordance with the Declaration of Helsinki. All identifying information was removed before data were pooled at the study coordinating centre located at the University of Milan. The StoP Project received ethical approval from the University of Milan Review Board (reference 19/15 on 01/04/2015).
Consent for publication
Not applicable
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01856-w.
References
- 1.Statista. Volume of tea consumption worldwide from 2012 to 2025 (in million kilograms). 2021. https://www.statista.com/statistics/940102/global-tea-consumption/. Accessed 2 September 2021.
- 2.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–21. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setiawan VW, Zhang ZF, Yu GP, Lu QY, Li YL, Lu ML, et al. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. Int J Cancer. 2001;92:600–4. doi: 10.1002/ijc.1231. [DOI] [PubMed] [Google Scholar]
- 4.Yu GP, Hsieh CC, Wang LY, Yu SZ, Li XL, Jin TH. Green-tea consumption and risk of stomach cancer: a population-based case-control study in Shanghai, China. Cancer Causes Control. 1995;6:532–8. doi: 10.1007/BF00054162. [DOI] [PubMed] [Google Scholar]
- 5.Deandrea S, Foschi R, Galeone C, La Vecchia C, Negri E, Hu J. Is temperature an effect modifier of the association between green tea intake and gastric cancer risk? Eur J Cancer Prev. 2010;19:18–22. doi: 10.1097/CEJ.0b013e328330eb1a. [DOI] [PubMed] [Google Scholar]
- 6.Kono S, Ikeda M, Tokudome S, Kuratsune M. A case-control study of gastric cancer and diet in northern Kyushu, Japan. Jpn J Cancer Res. 1988;79:1067–74. doi: 10.1111/j.1349-7006.1988.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, Yatsuya H, et al. A nested case-control study of stomach cancer in relation to green tea consumption in Japan. Br J Cancer. 2004;90:135–8. doi: 10.1038/sj.bjc.6601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshiyama Y, Sasaba T. A case-control study of single and multiple stomach cancers in Saitama Prefecture, Japan. Jpn J Cancer Res. 1992;83:937–43. doi: 10.1111/j.1349-7006.1992.tb02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol. 1998;27:173–80. doi: 10.1093/ije/27.2.173. [DOI] [PubMed] [Google Scholar]
- 10.Nagano J, Kono S, Preston DL, Mabuchi K. A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan) Cancer Causes Control. 2001;12:501–8. doi: 10.1023/A:1011297326696. [DOI] [PubMed] [Google Scholar]
- 11.Tsubono Y, Nishino Y, Komatsu S, Hsieh CC, Kanemura S, Tsuji I, et al. Green tea and the risk of gastric cancer in Japan. N Engl J Med. 2001;344:632–6. doi: 10.1056/NEJM200103013440903. [DOI] [PubMed] [Google Scholar]
- 12.Fujino Y, Tamakoshi A, Ohno Y, Mizoue T, Tokui N, Yoshimura T. Prospective study of educational background and stomach cancer in Japan. Prev Med. 2002;35:121–7. doi: 10.1006/pmed.2002.1066. [DOI] [PubMed] [Google Scholar]
- 13.Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, Yatsuya H, et al. A prospective study of stomach cancer death in relation to green tea consumption in Japan. Br J Cancer. 2002;87:309–13. doi: 10.1038/sj.bjc.6600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasazuki S, Inoue M, Hanaoka T, Yamamoto S, Sobue T, Tsugane S. Green tea consumption and subsequent risk of gastric cancer by subsite: the JPHC Study. Cancer Causes Control. 2004;15:483–91. doi: 10.1023/B:CACO.0000036449.68454.42. [DOI] [PubMed] [Google Scholar]
- 15.Khan MM, Goto R, Kobayashi K, Suzumura S, Nagata Y, Sonoda T, et al. Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex. Asian Pac J Cancer Prev. 2004;5:58–65. [PubMed] [Google Scholar]
- 16.Kim TL, Jeong GH, Yang JW, Lee KH, Kronbichler A, van der Vliet HJ, et al. Tea consumption and risk of cancer: an umbrella review and meta-analysis of observational studies. Adv Nutr. 2020;11:1437–52. doi: 10.1093/advances/nmaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Jin Z, Jiang H, Xiang C, Tang J, Li T, et al. Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer. 2014;14:197. doi: 10.1186/1471-2407-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao LG, Li ZY, Feng GS, Ji XW, Tan YT, Li HL, et al. Tea drinking and risk of cancer incidence: a meta-analysis of prospective cohort studies and evidence evaluation. Adv Nutr. 2021;12:402–12. [DOI] [PMC free article] [PubMed]
- 19.Ren JS, Freedman ND, Kamangar F, Dawsey SM, Hollenbeck AR, Schatzkin A, et al. Tea, coffee, carbonated soft drinks and upper gastrointestinal tract cancer risk in a large United States prospective cohort study. Eur J Cancer. 2010;46:1873–81. [DOI] [PMC free article] [PubMed]
- 20.Sanikini H, Dik VK, Siersema PD, Bhoo-Pathy N, Uiterwaal CS, Peeters PH, et al. Total, caffeinated and decaffeinated coffee and tea intake and gastric cancer risk: results from the EPIC cohort study. Int J Cancer. 2015;136:E720–30. doi: 10.1002/ijc.29223. [DOI] [PubMed] [Google Scholar]
- 21.World Cancer Research Fund/American Institute for Research. Continuous Update Project Expert Report 2018. Non-alcoholic drinks and the risk of cancer. 2018. https://www.wcrf.org/wp-content/uploads/2021/02/Non-alcoholic-drinks.pdf. Accessed 2 September 2021.
- 22.Pelucchi C, Lunet N, Boccia S, Zhang ZF, Praud D, Boffetta P, et al. The stomach cancer pooling (StoP) project: study design and presentation. Eur J Cancer Prev. 2015;24:16–23. doi: 10.1097/CEJ.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 23.Benetou V, Trichopoulou A, Orfanos P, Naska A, Lagiou P, Boffetta P, et al. Conformity to traditional Mediterranean diet and cancer incidence: the Greek EPIC cohort. Br J Cancer. 2008;99:191–5. doi: 10.1038/sj.bjc.6604418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagiou P, Samoli E, Lagiou A, Peterson J, Tzonou A, Dwyer J, et al. Flavonoids, vitamin C and adenocarcinoma of the stomach. Cancer Causes Control. 2004;15:67–72. doi: 10.1023/B:CACO.0000016619.18041.b0. [DOI] [PubMed] [Google Scholar]
- 25.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, et al. A case-control study of gastric cancer and diet in Italy. Int J Cancer. 1989;44:611–6. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 26.La Vecchia C, D’Avanzo B, Negri E, Decarli A, Benichou J. Attributable risks for stomach cancer in northern Italy. Int J Cancer. 1995;60:748–52. doi: 10.1002/ijc.2910600603. [DOI] [PubMed] [Google Scholar]
- 27.Lucenteforte E, Scita V, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Food groups and alcoholic beverages and the risk of stomach cancer: a case-control study in Italy. Nutr Cancer. 2008;60:577–84. doi: 10.1080/01635580802054512. [DOI] [PubMed] [Google Scholar]
- 28.Mao Y, Hu J, Semenciw R, White K. Active and passive smoking and the risk of stomach cancer, by subsite, in Canada. Eur J Cancer Prev. 2002;11:27–38. doi: 10.1097/00008469-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V. Alcohol consumption, smoking and risk of gastric cancer: case-control study from Moscow, Russia. Cancer Causes Control. 2000;11:363–71. doi: 10.1023/A:1008907924938. [DOI] [PubMed] [Google Scholar]
- 30.Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev. 2007;16:312–27. doi: 10.1097/01.cej.0000236255.95769.22. [DOI] [PubMed] [Google Scholar]
- 31.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZF, Kurtz RC, Klimstra DS, Yu GP, Sun M, Harlap S, et al. Helicobacter pylori infection on the risk of stomach cancer and chronic atrophic gastritis. Cancer Detection Prev. 1999;23:357–67. doi: 10.1046/j.1525-1500.1999.99041.x. [DOI] [PubMed] [Google Scholar]
- 33.Ward MH, Sinha R, Heineman EF, Rothman N, Markin R, Weisenburger DD, et al. Risk of adenocarcinoma of the stomach and esophagus with meat cooking method and doneness preference. Int J Cancer. 1997;71:14–9. doi: 10.1002/(SICI)1097-0215(19970328)71:1<14::AID-IJC4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Santibañez M, Alguacil J, de la Hera MG, Navarrete-Muñoz EM, Llorca J, Aragonés N, et al. Occupational exposures and risk of stomach cancer by histological type. Occup Environ Med. 2012;69:268–75. doi: 10.1136/oemed-2011-100071. [DOI] [PubMed] [Google Scholar]
- 35.Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, et al. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer. 2004;7:46–53. doi: 10.1007/s10120-004-0268-5. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Ramírez RU, Galván-Portillo MV, Ward MH, Agudo A, González CA, Oñate-Ocaña LF, et al. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int J Cancer. 2009;125:1424–30. doi: 10.1002/ijc.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamada GS, Kowalski LP, Nishimoto IN, Rodrigues JJ, Iriya K, Sasazuki S, et al. Risk factors for stomach cancer in Brazil (II): a case-control study among Japanese Brazilians in São Paulo. Jpn J Clin Oncol. 2002;32:284–90. doi: 10.1093/jjco/hyf061. [DOI] [PubMed] [Google Scholar]
- 38.Nishimoto IN, Hamada GS, Kowalski LP, Rodrigues JG, Iriya K, Sasazuki S, et al. Risk factors for stomach cancer in Brazil (I): a case-control study among non-Japanese Brazilians in São Paulo. Jpn J Clin Oncol. 2002;32:277–83. doi: 10.1093/jjco/hyf060. [DOI] [PubMed] [Google Scholar]
- 39.Bartelli TF, Senda de Abrantes LL, Freitas HC, Thomas AM, Silva JM, Albuquerque GE, et al. Genomics and epidemiology for gastric adenocarcinomas (GE4GAC): a Brazilian initiative to study gastric cancer. Appl Cancer Res. 2019;39:12. doi: 10.1186/s41241-019-0081-4. [DOI] [Google Scholar]
- 40.Pourfarzi F, Whelan A, Kaldor J, Malekzadeh R. The role of diet and other environmental factors in the causation of gastric cancer in Iran-a population based study. Int J Cancer. 2009;125:1953–60. doi: 10.1002/ijc.24499. [DOI] [PubMed] [Google Scholar]
- 41.Mu LN, Lu QY, Yu SZ, Jiang QW, Cao W, You NC, et al. Green tea drinking and multigenetic index on the risk of stomach cancer in a Chinese population. Int J Cancer. 2005;116:972–83. doi: 10.1002/ijc.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setiawan VW, Yu GP, Lu QY, Lu ML, Yu SZ, Mu L, et al. Allium vegetables and stomach cancer risk in China. Asian Pac J Cancer Prev. 2005;6:387–95. [PMC free article] [PubMed] [Google Scholar]
- 43.Setiawan VW, Zhang ZF, Yu GP, Li YL, Lu ML, Tsai CJ, et al. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2000;9:73–80. [PubMed] [Google Scholar]
- 44.Boccia S, Sayed-Tabatabaei FA, Persiani R, Gianfagna F, Rausei S, Arzani D, et al. Polymorphisms in metabolic genes, their combination and interaction with tobacco smoke and alcohol consumption and risk of gastric cancer: a case-control study in an Italian population. BMC Cancer. 2007;7:206-. doi: 10.1186/1471-2407-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 46.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 47.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–57. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 48.Tong GX, Liang H, Chai J, Cheng J, Feng R, Chen PL, et al. Association of risk of gastric cancer and consumption of tobacco, alcohol and tea in the Chinese population. Asian Pac J Cancer Prev. 2014;15:8765–74. doi: 10.7314/APJCP.2014.15.20.8765. [DOI] [PubMed] [Google Scholar]
- 49.Lin Y, Wu C, Yan W, Guo S, Lin S, Liu B. Sociodemographic and lifestyle factors in relation to gastric cancer in a high-risk region of China: a matched case-control study. Nutr Cancer. 2020;72:421–30. doi: 10.1080/01635581.2019.1638425. [DOI] [PubMed] [Google Scholar]
- 50.Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. 2020;42:e2020004. doi: 10.4178/epih.e2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong M, Park JM, Han YM, Kangwan N, Kwon SO, Kim BN, et al. Dietary intervention of artemisia and green tea extracts to rejuvenate helicobacter pylori-associated chronic atrophic gastritis and to prevent tumorigenesis. Helicobacter. 2016;21:40–59. doi: 10.1111/hel.12229. [DOI] [PubMed] [Google Scholar]
- 52.Stoicov C, Saffari R, Houghton J. Green tea inhibits Helicobacter growth in vivo and in vitro. Int J Antimicrob Agents. 2009;33:473–8. doi: 10.1016/j.ijantimicag.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyanova L, Ilieva J, Gergova G, Vladimirov B, Nikolov R, Mitov I. Honey and green/black tea consumption may reduce the risk of Helicobacter pylori infection. Diagn Microbiol Infect Dis. 2015;82:85–6. doi: 10.1016/j.diagmicrobio.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Mao XQ, Jia XF, Zhou G, Li L, Niu H, Li FL, et al. Green tea drinking habits and gastric cancer in southwest China. Asian Pac J Cancer Prev. 2011;12:2179–82. [PubMed] [Google Scholar]
- 55.Huang Y, Chen H, Zhou L, Li G, Yi D, Zhang Y, et al. Association between green tea intake and risk of gastric cancer: a systematic review and dose-response meta-analysis of observational studies. Public Health Nutr. 2017;20:3183–92. doi: 10.1017/S1368980017002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Duan H, Yang H. A case-control study of stomach cancer in relation to Camellia sinensis in China. Surg Oncol. 2015;24:67–70. doi: 10.1016/j.suronc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Morais S, Peleteiro B, Araújo N, Malekzadeh R, Ye W, Plymoth A, et al. Identifying the profile of Helicobacter pylori-negative gastric cancers: a case-only analysis within the Stomach Cancer Pooling (StoP) Project. Cancer Epidemiol Biomark Prev. 2022;31:200–9. doi: 10.1158/1055-9965.EPI-21-0402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the results reported in the paper cannot be found on publicly available databases. Data can be provided on reasonable request from the authors.