Abstract
Coronary computed tomography angiography (CCTA) derived machine learning fractional flow reserve (ML-FFRCT) can assess the hemodynamic significance of coronary artery stenoses. We aimed to assess sex differences in the association of ML-FFRCT and incident cardiovascular outcomes. We studied a retrospective cohort of consecutive patients who underwent clinically indicated CCTA and single photon emission computed tomography (SPECT). Obstructive stenosis was defined as ≥ 70% stenosis severity in non-left main vessels or ≥ 50% in the left main coronary. ML-FFRCT was computed using a machine learning algorithm with significant stenosis defined as ML-FFRCT < 0.8. The primary outcome was a composite of death or non-fatal myocardial infarction (D/MI). Our study population consisted of 471 patients with mean (SD) age 65 (13) years, 53% men, and multiple comorbidities (78% hypertension, 66% diabetes, 81% dyslipidemia). Compared to men, women were less likely to have obstructive stenosis by CCTA (9% vs. 18%; p = 0.006), less multivessel CAD (4% vs. 6%; p = 0.25), lower prevalence of ML-FFRCT < 0.8 (39% vs. 44%; p = 0.23) and higher median (IQR) ML-FFRCT (0.76 (0.53–0.86) vs. 0.71 (0.47–0.84); p = 0.047). In multivariable adjusted models, there was no significant association between ML-FFRCT < 0.8 and D/MI [Hazard Ratio 0.82, 95% confidence interval (0.30, 2.20); p = 0.25 for interaction with sex.]. In a high-risk cohort of symptomatic patients who underwent CCTA and SPECT testing, ML-FFRCT was higher in women than men. There was no significant association between ML-FFRCT and incident mortality or MI and no evidence that the prognostic value of ML-FFRCT differs by sex.
Subject terms: Cardiovascular diseases, Ischaemia
Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of morbidity and mortality among women1. Women typically present later in life and have atypical symptoms. Furthermore, women are less likely to be referred for diagnostic procedures and receive guideline-directed medical treatment and lifestyle interventions2–5. Despite having less obstructive coronary artery disease (CAD), women often have worse outcomes than men6. Non-invasive assessment of CAD in women may be limited by smaller body habitus, reduced peak exercise achievement, and breast attenuation.
Coronary computed tomography angiography (CCTA) offers the advantage of direct visualization of the coronary tree without the inherent risks of invasive angiography and with acceptable accuracy7. However, similar to invasive coronary angiography (ICA), CCTA does not provide information of the hemodynamic significance of coronary stenoses. Recent advances in computational fluid dynamics have enabled the non-invasive assessment of coronary blood flow using CCTA derived fractional flow reserve (FFRCT). FFRCT has been shown to improve the diagnostic accuracy of ischemia compared to other noninvasive tests8. FFRCT has also been suggested to play a role as both a gatekeeper to ICA by decreasing the likelihood of nonobstructive CAD at ICA9, and as guide to revascularization as it has been shown to predict standard of care guided coronary revascularization10. Importantly, FFRCT has similar discriminatory power for detection of ischemia by ICA regardless of sex11.
Machine learning based FFRCT (ML-FFRCT) is the latest tool to non-invasively determine coronary blood flow without the need for high computation demand or off-site data transfer. Although not approved for clinical use, a recent meta-analysis has shown how sensitivity and specificity are comparable to both computational flow and gold standard invasive angiography-based determination of FFR12.
A prior study from the Assessing Diagnostic Value of Non-invasive FFRCT in Coronary Care (ADVANCE) registry found that women have higher FFRCT compared to men for the same degree of coronary stenosis13. Among patients with positive FFRCT (< 0.80) women were found to have less obstructive CAD and less revascularization. However, the study did not assess the association of FFRCT with incident cardiovascular outcomes. In the present analysis, we compare machine learning FFRCT among men and women to determine whether there are sex differences in the distribution of ML-FFRCT by degree of coronary stenosis and specific coronary vessel involvement. We also evaluate sex differences in the prognostic significance of ML-FFRCT in relation to incident cardiovascular outcomes.
Methods
Study population
Our study population consisted of 471 consecutive patients who underwent a clinically indicated CCTA and single positron emission computed tomography (SPECT) for suspected CAD between January 1, 2016 to June 22, 2020 at a large referral center.
This cohort of patients was initially established to assess the comparative prognostic role of functional assessment with SPECT vs ML-FFRCT when added to CCTA anatomic assessment, which we have published before14. Dual SPECT-CCTA testing was done at the discretion of the treating physician. In the current sub-analysis, we present sex-specific differences. A flow diagram is provided (Supplementary Fig. 1).
Approval from the Institutional Review Board at the Houston Methodist Academic Institute was obtained prior to the start of the study. Informed consent was waived by Institutional Review Board at the Houston Methodist Academic Institute due to the retrospective nature of the study. All methods were carried out in accordance with relevant guidelines and regulations.
Assessment of covariates
Information on sociodemographic variables, medical history, comorbidities and medication use was obtained using chart review of electronic health records within 30 days of imaging.
Follow-up and outcome
The primary outcome was death or non-fatal myocardial infarction (D/MI). Secondary outcome included major adverse cardiovascular events (MACE), a composite of death, MI, and unplanned revascularization—PCI or CABG occurring more than 90 days after index imaging). Myocardial infarction was defined as the 4th universal definition of Myocardial Infarction15. All outcomes were obtained from chart review and adjudicated by expert physicians in a blinded manner. All patients were followed from the date of CCTA imaging to either the occurrence of outcomes or the last known date of contact noted on the patient records.
CCTA
CCTA scans were performed using 3rd generation SOMATOM FORCE Scanner (Siemens, Forchheim, Germany). Image acquisition was performed in accordance with the Society of Cardiovascular Computed Tomography (SCCT) guidelines and has been described before14,16. Briefly, intravenous metoprolol was administered for patients with a heart rate ≥ 65 beats/min and sublingual nitroglycerin 0.4 mg was administered immediately before image acquisition. During image acquisition, 60–100 cc of contrast was injected, followed by saline flush. Axial scans were obtained with prospective electrocardiographic gating. Image acquisition was performed to include the coronary arteries, left ventricle and proximal ascending aorta.
Images were analyzed with a 3-dimensional workstation using one of several post-processing methods including axial, multiplanar reformat, maximum intensity projection and cross-sectional analysis. Type and location of lesion were visually evaluated using an 18-segment model according to SCCT guidelines16. In each segment, atherosclerosis was defined as tissue structures > 1 mm2 within the coronary artery lumen or adjacent to the lumen that could be discriminated from pericardial tissue, epicardial fat or vessel lumen itself. Coronary stenosis was classified as none (0%), mild (1–49%), moderate (50–69%), or severe (≥ 70%) based on degree of narrowing of the luminal diameter. Anatomically obstructive CAD by CCTA was defined at 70% stenosis severity in non-left main vessels and 50% in the left main artery. Findings were reported using SCCT Coronary Artery Disease Reporting & Data System (CAD-RADS). All interpretations were done by experienced imaging cardiologists (at least 10 years of experience).
ML-FFRCT
ML-FFRCT was determined using a machine learning based computation of fractional flow reserve (ML-FFRCT, cFFR 3.2, Siemens Healthcare GmbH, Forchheim, Germany, not available for commercial use). Methods for ML-FFRCT determination have been described before14. The coronary tree was isolated semi-automatically to generate a 3-dimensional coronary model. All vessels and branches with a diameter of at least 2 mm were included. ML-FFRCT was determined at the midpoint of a segment for normal segments and 1 cm distal to stenosis for segments with lesions based on prior work showing higher prognostic role of measurements distal to stenosis17. Details on the derivation of ML-FFRCT values, diagnostic accuracy and concordance of results with invasive ML-FFRCT have been reported previously12,18. A quantitative 3-dimensional model of the coronary tree was reconstructed and a 17-segment model was used. Image processing was done by two investigators blinded to results from other tests. Both investigators underwent extensive training prior to image processing. The reproducibility of findings was assessed on a random subset of patients and was found to be high (ICC 0.981 per patient and 0.970 per segment, absolute mean difference 0.019 per and 0.027 per segment) and across image quality and degrees of stenosis by CAD-RADS. The minimum ML-FFRCT values were recorded in each segment per patient and summarized using median (interquartile range) and categorized. Hemodynamically significant ischemia was defined as ML-FFRCT < 0.8 consistent with prior literature19–21.
Statistical analysis
Continuous variables were presented as mean (standard deviation)/median (inter-quartile range) and categorical variables were presented as count (percentage) stratified by sex. Results were compared using Student’s t test for continuous normally distributed variables and median testing for continuous non-normally distributed variables or chi-square test for categorical variables.
Median (IQR) and per-patient minimum ML-FFRCT were calculated for each CAD-RAD category (0, 1–2, 3, 4A and 4B) per patient and for each coronary vessel and stratified by sex to unmask potential differences by severity of stenosis. The prevalence of ML-FFRCT < 0.8 was similarly calculated for each CAD-RAD category and coronary vessel stratified by sex. The results were graphically depicted using bar charts and box plots as appropriate.
Multivariable-adjusted Cox proportional hazards models were used to study the association between ML-FFRCT < 0.80 and incident outcomes stratified by sex after confirming the proportionality assumption using log–log plots. Models were adjusted for age, hypertension, diabetes mellitus, dyslipidemia, ever cigarette smoking, indication for CCTA testing, early revascularization (PCI or CABG within 90 days of testing), and degree of coronary stenosis by CCTA. Results were further stratified by CAD-RAD score (≤ 2 vs. > 2). In sensitivity analyses, we utilized median per-patient ML-FFRCT and lower thresholds (significant ML-FFRCT defined as < 0.75 and < 0.70).
To determine whether there should be separate cutoffs for ML-FFRCT for men versus women, mean ML-FFRCT for each coronary vessel proximal, mid, and distal segment were summarized for the overall study cohort and stratified by sex. Difference across segments was assessed using the Kruskal–Wallis equality of proportions rank test.
All analyses were conducted using Stata 17.0 (StataCorp, College Station, Texas) and a p-value of < 0.05 was considered statistically significant.
Results
The study population consisted of 471 patients with mean (SD) age 64 (13) years, 47% women, and multiple comorbidities (78% hypertension, 66% diabetes, 81% dyslipidemia). The median number of days between SPECT and CCTA was 24 days (IQR 3–118 days) and nearly two-thirds of the cohort had CCTA prior to or on the same day as SPECT (337 CT then SPECT vs 134 SPECT then CT). Women had a similar distribution of cardiovascular risk factors except they were less likely to report history of smoking (19% vs. 31%). Compared to men, women were less likely to have obstructive stenosis (9% vs. 18%; p = 0.006), less often had advanced (higher CAD-RADS) stenosis and less likely to have multivessel CAD (4% vs. 6%; p = 0.25) (Tables 1, 2).
Table 1.
Baseline characteristics of the study population by sex.
| Sociodemographic | Total | Sex | ||
|---|---|---|---|---|
| Male | Female | p | ||
| N = 471 | N = 249 | N = 222 | ||
| Age, N (%) | 63.83 (12.60) | 63.30 (12.70) | 64.42 (12.48) | 0.34 |
| Comorbidities | ||||
| Hypertension, N (%) | 369 (78.3%) | 199 (79.9%) | 170 (76.6%) | 0.38 |
| Diabetes, N (%) | 309 (65.6%) | 160 (64.3%) | 149 (67.1%) | 0.51 |
| Dyslipidemia, N (%) | 382 (81.1%) | 202 (81.1%) | 180 (81.1%) | 0.99 |
| Ever smoker, N (%) | 118 (25.1%) | 77 (30.9%) | 41 (18.5%) | 0.002 |
| Symptoms | ||||
| Chest pain or shortness of breath, N (%) | 279 (59.2%) | 140 (56.2%) | 139 (62.6%) | 0.16 |
| Medication | ||||
| Aspirin/clopidogrel, N (%) | 372 (79.0%) | 198 (79.5%) | 174 (78.4%) | 0.76 |
| Statin, N (%) | 349 (74.1%) | 184 (73.9%) | 165 (74.3%) | 0.92 |
| ACE/ARB, N (%) | 295 (62.6%) | 152 (61.0%) | 143 (64.4%) | 0.45 |
| Beta blockers, N (%) | 357 (75.8%) | 190 (76.3%) | 167 (75.2%) | 0.78 |
| Calcium channel blockers, N (%) | 199 (42.3%) | 105 (42.2%) | 94 (42.3%) | 0.97 |
p-value comparing women and men was calculated using Student’s t test for continuous normally-distributed variables and median testing for continuous non-normally distributed variables or chi-square test for categorical variables.
ACE Angiotensin converting enzyme, ARB angiotensin receptor blocker.
Table 2.
Imaging characteristics of the study population by sex.
| Total | Sex | |||
|---|---|---|---|---|
| Male | Female | p | ||
| CCTA stenosis | ||||
| CCTA CAD-RAD, N (%) | < 0.001 | |||
| CAD-RAD 0 | 115 (24.4%) | 44 (17.7%) | 71 (32.0%) | |
| CAD-RAD 1/2 | 208 (44.2%) | 108 (43.4%) | 100 (45.0%) | |
| CAD-RAD 3 | 76 (16.1%) | 49 (19.7%) | 27 (12.2%) | |
| CAD-RAD 4A | 50 (10.6%) | 33 (13.3%) | 17 (7.7%) | |
| CAD-RAD 4B | 22 (4.7%) | 15 (6.0%) | 7 (3.2%) | |
| CCTA obstructive stenosis, N (%) | 64 (13.6%) | 44 (17.7%) | 20 (9.0%) | 0.006 |
| CCTA multi-vessel disease, N (%) | 25 (5.3%) | 16 (6.4%) | 9 (4.1%) | 0.25 |
| FFRct | ||||
| Minimum FFRct | ||||
| Minimum FFRct per patient, median (IQR) | 0.74 (0.51–0.85) | 0.71 (0.47–0.84) | 0.76 (0.53–0.86) | 0.047 |
| Ischemic stenosis | ||||
| ML-FFRct < 0.70 on any proximal/mid segment, N (%) | 120 (25.5%) | 67 (26.9%) | 53 (23.9%) | 0.45 |
| ML-FFRct < 0.75 on any proximal/mid segment, N (%) | 147 (31.2%) | 85 (34.1%) | 62 (27.9%) | 0.15 |
| ML-FFRct < 0.80 on any proximal/mid segment, N (%) | 196 (41.6%) | 110 (44.2%) | 86 (38.7%) | 0.23 |
p-value comparing women and men was calculated using Student’s t test for continuous normally-distributed variables and median testing for continuous non-normally distributed variables or chi-square test for categorical variables.
CCTA Coronary computed tomography angiography, FFRCT fractional flow reserve derived using computed tomography.
Women had higher median (IQR) ML-FFRCT compared to men: 0.76 (0.53–0.86) vs. 0.71 (0.47–0.84; p = 0.047). In stratified results, median ML-FFRCT was generally higher among women compared to men regardless of CAD-RADS score or specific vessel involvement (Fig. 1; Supplementary Fig. 2). Results were similar comparing per-patient minimum FFRCT stratified by CAD-RADS (Supplementary Fig. 3). The prevalence of ML-FFRCT < 0.8 was lower among women compared to men (39% vs. 44% respectively; p = 0.23). No major differences were seen when stratifying by CAD-RADS. (Fig. 2; Supplementary Fig. 4). Sensitivity analysis using lower cut-offs showed similar results (Supplementary Fig. 5). Similarly, no major differences were seen in per-patient minimum FFRCT values when stratified by CAD-RADS (Supplementary Table 1). While non-significant, FFRCT was lower among women with significant coronary stenosis (CAD-RAD 4A/4B).
Figure 1.
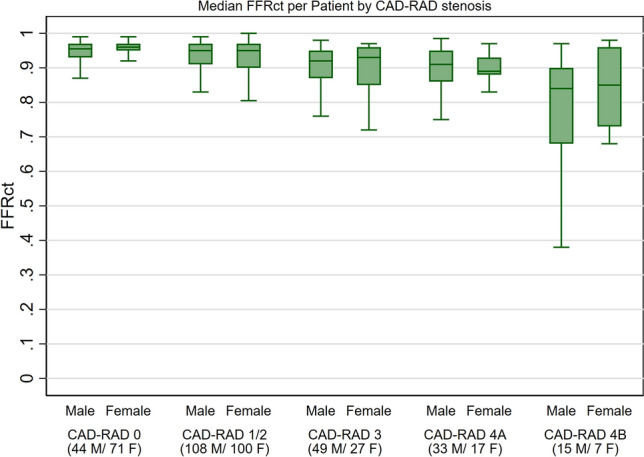
Median FFRCT per patient and for each coronary vessel stratified by sex and CAD-RAD score. FFRCT Fractional flow reserve derived using computed tomography, M male, F female.
Figure 2.

Prevalence of FFRCT < 0.8 in any segment per patient and for each coronary vessel stratified by sex and CAD-RAD score. FFRCT Fractional flow reserve derived using computed tomography, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery, M male, F female.
Over a median follow-up time of 18 months, there were 33 incident D/MI (38 D/20 MI) and 38 MACE (38 D/20 MI/42 PCI/5 CABG) events which corresponded to 3.48 and 4.05 events per 1000 person-years respectively (Table 3). There was no significant difference in event rates between men and women. In multivariable adjusted models, there was no significant association between ML-FFRCT < 0.8 and D/MI: Hazard Ratio 0.82, 95% confidence interval (0.30, 2.20); p = 0.254 for interaction with sex (Table 4). There was no significant association in analyses stratified by CAD-RADS score (≤ 2 vs. > 2). Similar results were observed for the secondary outcome of MACE (Table 4) and when significant ML-FFRCT was defined as < 0.70 or < 0.75 (results not shown).
Table 3.
Outcomes of the study population by sex.
| Total | Sex | |||
|---|---|---|---|---|
| Male | Female | p | ||
| Outcomes | ||||
| Death/MI | ||||
| Incidence Rate (per 1000 person-year) | 2.89 | 3.55 | 2.25 | 0.204 |
| N (%) | 33 (7.0%) | 20 (8.0%) | 13 (5.9%) | 0.36 |
| MACE | ||||
| Incidence rate (per 1000 person-year) | 3.36 | 4.32 | 2.43 | 0.086 |
| N (%) | 38 (8.1%) | 24 (9.6%) | 14 (6.3%) | 0.18 |
| All-cause death, N (%) | 20 (4.2%) | 12 (4.8%) | 8 (3.6%) | 0.51 |
| Myocardial infarction, N (%) | 13 (2.8%) | 8 (3.2%) | 5 (2.3%) | 0.53 |
| PCI 90-days post imaging, N (%) | 42 (8.9%) | 22 (8.8%) | 20 (9.0%) | 0.95 |
| CABG 90-days post imaging, N (%) | 5 (1.1%) | 4 (1.6%) | 1 (0.5%) | 0.22 |
p-value comparing women and men was calculated using Student’s t test for continuous normally-distributed variables and median testing for continuous non-normally distributed variables or chi-square test for categorical variables.
MACE major adverse cardiovascular events, MI myocardial infarction, PCI percutaneous coronary intervention, CABG coronary artery bypass graft.
Table 4.
Hazard ratios for the association of ML-FFRCT < 0.8 and incident outcomes.
| Death or all-cause mortality | Major adverse cardiovascular outcomes | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | p for interaction | Unadjusted | Adjusted | p for interaction | |
| Overall | 1.87 | 0.82 | 0.690 | 1.69 | 0.95 | 0.921 |
| CAD-RAD ≤ 2 | 2.44 | 1.22 | 0.781 | 2.44 | 1.23 | 0.770 |
| CAD-RAD > 2 | 0.82 | 0.30 | 0.112 | 0.67 | 0.51 | 0.283 |
ML-FFRCT refers to fractional flow reserve derived using computed tomography and was categorized as < 0.8
Models were adjusted for age, hypertension, diabetes mellitus, dyslipidemia, ever cigarette smoking, indication for CCTA testing, early revascularization (PCI or CABG within 90 days of testing), and degree of coronary stenosis by CCTA.
p-for interaction refers to multiplicative interaction between ML-FFRCT and sex (women vs. men).
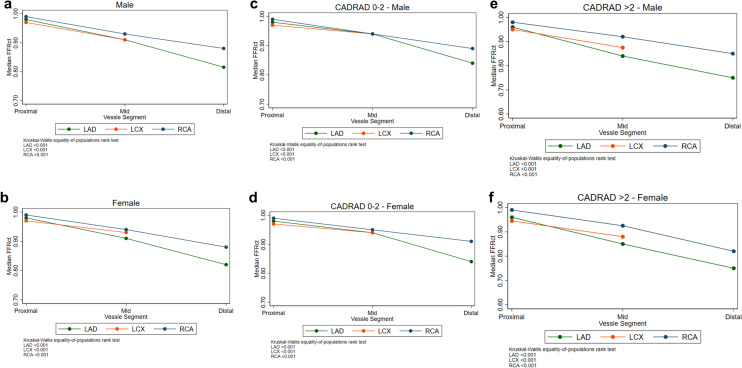
Median ML-FFRCT decreased moving from proximal to distal segments in each coronary vessel for both men and women (Fig. 3). These results were similar stratifying by CAD-RADS score (≤ 2 vs. > 2). In multivariable adjusted models, there was no significant association between median ML-FFRCT with the primary and secondary outcome (Supplementary Table 2).
Figure 3.
Proximal-to-distal median FFRCT stratified for each coronary vessel stratified by sex (a,b) and CAD-RAD score (c–f). FFRCT Fractional flow reserve derived using computed tomography, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery, M male, F female.
Discussion
In our study of high-risk stable CAD patients who underwent concomitant CCTA and SPECT testing, we found that women had higher ML-FFRCT when compared to men but this was likely due to a lower burden of obstructive coronary atherosclerotic disease. However, hemodynamically significant stenosis as defined by ML-FFRCT < 0.8 was higher among women than men for advanced degrees of stenosis (i.e. CAD-RAD 4A-4B). ML-FFRCT was not associated with incident adverse outcomes and there was no significant interaction with sex.
Women constituted half of our study population and had a similar cardiovascular risk factor profile compared to men except for lower prevalence of smoking. Despite having higher ML-FFRCT, less obstructive CAD, and less advanced stenosis in our study, women experienced similar rates of death and mortality compared to men in our study. While the prevalence of ML-FFRCT < 0.8 was higher among women, analyses stratified by degree of coronary stenosis revealed that women more likely to have hemodynamically significant coronary stenosis among those with more advanced stenosis burden (CAD-RAD 4A/B), which might have contributed to excess event rates in this group. This discordance points to the importance of coronary CT perfusion data that is afforded by ML-FFRCT. Lastly, we did not find that the prognostic value of ML-FFRCT differs by sex and there does not appear to be a sex-specific cut-off for ML-FFRCT. Taken together, our results suggest that ML-FFRCT has similar prognostic value among women and men.
Women are less likely to achieve maximum exercise capacity on exercise stress testing and more likely to have common attenuation artifacts that can reduce the diagnostic performance of SPECT for diagnosing CAD22. Therefore, a complementary anatomical (CCTA) and functional approach (FFRCT) may improve the sensitivity of coronary CT for diagnosing obstructive CAD among women. Improving the diagnostic accuracy of CAD among women can help decrease the excess burden of CAD among women and poorer outcomes compared to men6.
Few randomized controlled studies have evaluated the use of CCTA vs. standard of care (including ICA and SPECT) in patients with stable chest pain. The SCOT-HEART trial found that CCTA did improve clinical outcomes23, whereas use of CCTA in the PROMISE trial did not24. In the latter study there was no evidence of a significant interaction of CCTA with sex suggesting a similar prognostic value of CCTA in both men and women. A functional evaluation of coronary stenosis using FFRCT may help further improve diagnostic accuracy of CCTA. In the FAME 2 trial of patients with stable chest pain, invasive FFR-guided PCI decreased the rate of urgent revascularization among those with functionally significant stenosis. These results were similar among men and women without significant interaction by sex25.
Knegt et al. found that CT perfusion resulted in improved discrimination compared with CCTA alone for the diagnosis of hemodynamically significant CAD defined by FFR and quantitative ICA26. In the CREDENCE trial, CCTA was superior to SPECT for predicting invasive FFR though addition of FFRCT to atherosclerotic plaque predictors did not significantly improve discrimination27. The AUC for CCTA was similar for men and women (0.83 vs. 0.88) but sex-stratified results for FFRCT were not presented in that study. In the ReASSESS Study, FFRCT and SPECT were similar in identifying hemodynamically significant stenosis21. However, FFRCT was more sensitive than SPECT in the overall study population and in both men and women suggesting similar prognostic value by sex.
In a subgroup analysis from the ADVANCE registry, FFRCT was higher among women compared to men regardless of the degree of coronary stenosis suggesting that women have less hemodynamically significant lesions13. Similarly we found that ML-FFRCT was higher among women though functionally significant FFRCT was higher among women with a higher burden of stenosis. This was a surprise finding as while women tend to have less obstructive CAD compared to men, a higher degree of stenosis may be more hemodynamically significant in women as our study indicates. This could be related to the very high prevalence of comorbidities in our study population (for example 66% vs. 22% diabetes) and subsequent higher event rates compared to the ADVANCE registry (4% death, 3% MI vs. event rates of 0.6–1.16%)26,27 However, there was no significant association between FFRCT and incident outcomes in our study either in the overall population or among those with higher degree of coronary stenosis.
An interesting finding is the high rates of hemodynamically significant stenosis in those with non-obstructive stenosis (CAD-RADS 0–2). Our results are similar to those from prior studies using the only FFRCT tool approved for clinical use where ischemia was detected in 33% of vessels with normal CCTA and 42% with mild (1–49%) stenosis28.
Limitations
Our study is not without limitations. Our patients likely constitute a particularly high-risk population as they were referred to concomitant CCTA and SPECT testing and therefore results may not be generalizable to otherwise lower risk patients. We used a machine learning prototype to measure FFRCT which is not currently validated or widely adopted in clinical practice. We did not evaluate for plaque characteristics such as plaque volume which could have affected analysis of FFRCT by sex. Our study was likely underpowered to evaluate incident outcomes given the small number of events our patients experienced as well as the relatively short duration of follow-up. There was no information on cause of death so we could not determine the association of FFRCT with cardiac-specific mortality outcomes. Similarly, we could not identify if the culprit vessel had the lowest ML-FFRCT in patients with an MI because such data was not available for our cohort. Patients with non-obstructive stenosis (CAD-RADS 0–2) were included primarily for comparability with prior studies that also included patients with similar range of stenosis28,29. ML-FFRCT could not be processed in 12% of the cohort. This rate is similar to those from prior studies using computational flow-based determination9,30,31. We used chart review to determine baseline characteristics and outcomes, potentially resulting in under-detection. However, obituaries and linked electronic records from other institutions were checked for patients who had not been seen at our institution for more than a year. Lastly, we cannot exclude the possibility of residual confounding.
Conclusion
In conclusion, among a high-risk cohort of symptomatic patients who underwent CCTA and SPECT testing, the degree of coronary atherosclerotic disease was lower and ML-FFRCT was higher in women than men. There is no significant association between FFRCT and incident mortality or MI and no evidence that the prognostic value of ML-FFRCT differs by sex.
Supplementary Information
Author contributions
The idea for this study was conceived by M.H.A. and M.A. Data collection and statistical analysis was done by M.A., A.I.A. and Y.H. Literature search and drafting of the manuscript was by M.A. and A.I.A. All authors commented on previous versions of the manuscript. All authors reviewed and approved the manuscript.
Data availability
Deidentified data for this study will be made available by the corresponding author upon reasonable request.
Competing interests
Dr Al-Mallah receives research support from Siemens, unrelated to this work. Drs Cocker, Schwemmer and Ramirez-Giraldo are employed by Siemens. All other authors have nothing to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mahmoud Al Rifai and Ahmed Ibrahim Ahmed.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17875-9.
References
- 1.Cho L, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:2602–2618. doi: 10.1016/j.jacc.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehran R, Vogel B, Ortega R, Cooney R, Horton R. The Lancet Commission on women and cardiovascular disease: Time for a shift in women's health. Lancet. 2019;393:967–968. doi: 10.1016/S0140-6736(19)30315-0. [DOI] [PubMed] [Google Scholar]
- 3.Pagidipati NJ, Peterson ED. Acute coronary syndromes in women and men. Nat. Rev. Cardiol. 2016;13:471–480. doi: 10.1038/nrcardio.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters SAE, Woodward M, Jha V, Kennedy S, Norton R. Women's health: A new global agenda. BMJ Glob. Health. 2016;1:e000080. doi: 10.1136/bmjgh-2016-000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldassarre LA, et al. Noninvasive imaging to evaluate women with stable ischemic heart disease. JACC Cardiovasc. Imaging. 2016;9:421–435. doi: 10.1016/j.jcmg.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crea F, Battipaglia I, Andreotti F. Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis. 2015;241:157–168. doi: 10.1016/j.atherosclerosis.2015.04.802. [DOI] [PubMed] [Google Scholar]
- 7.Arbab-Zadeh A, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J. Am. Coll. Cardiol. 2012;59:379–387. doi: 10.1016/j.jacc.2011.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danad I, et al. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: A meta-analysis. Eur. Heart J. 2017;38:991–998. doi: 10.1093/eurheartj/ehw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas PS, et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: The prospective longitudinal trial of FFR(CT): Outcome and resource impacts study. Eur. Heart J. 2015;36:3359–3367. doi: 10.1093/eurheartj/ehv444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packard RRS, Li D, Budoff MJ, Karlsberg RP. Fractional flow reserve by computerized tomography and subsequent coronary revascularization. Eur. Heart J. Cardiovasc. Imaging. 2017;18:145–152. doi: 10.1093/ehjci/jew148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson AG, et al. Diagnostic accuracy and discrimination of ischemia by fractional flow reserve CT using a clinical use rule: Results from the determination of fractional flow reserve by anatomic computed tomographic angiography study. J. Cardiovasc. Comput. Tomogr. 2015;9:120–128. doi: 10.1016/j.jcct.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Agasthi P, et al. Comparison of computed tomography derived fractional flow reserve to invasive fractional flow reserve in diagnosis of functional coronary stenosis: A meta-analysis. Sci. Rep. 2018;8:11535. doi: 10.1038/s41598-018-29910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairbairn TA, et al. Sex differences in coronary computed tomography angiography-derived fractional flow reserve: Lessons from ADVANCE. JACC Cardiovasc. Imaging. 2020;13:2576–2587. doi: 10.1016/j.jcmg.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed AI, et al. Prognostic value of computed tomography derived fractional flow reserve comparison with myocardial perfusion imaging. J. Am. Coll. Cardiol. 2021;77:1267. doi: 10.1016/S0735-1097(21)02625-5. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, et al. Fourth Universal Definition of Myocardial Infarction (2018) J. Am. Coll. Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 16.Abbara S, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI) J. Cardiovasc. Comput. Tomogr. 2016;10:435–449. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Kueh SH, et al. Fractional flow reserve derived from coronary computed tomography angiography reclassification rate using value distal to lesion compared to lowest value. J. Cardiovasc. Comput. Tomogr. 2017;11:462–467. doi: 10.1016/j.jcct.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Coenen A, et al. Diagnostic accuracy of a machine-learning approach to coronary computed tomographic angiography-based fractional flow reserve: Result from the MACHINE consortium. Circ. Cardiovasc. Imaging. 2018;11:e007217. doi: 10.1161/CIRCIMAGING.117.007217. [DOI] [PubMed] [Google Scholar]
- 19.Min JK, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308:1237–1245. doi: 10.1001/2012.jama.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo B, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J. Am. Coll. Cardiol. 2011;58:1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 21.Sand NPR, et al. Prospective comparison of FFR derived from coronary CT angiography with SPECT perfusion imaging in stable coronary artery disease: The ReASSESS study. JACC Cardiovasc. Imaging. 2018;11:1640–1650. doi: 10.1016/j.jcmg.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LJ, et al. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: Results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation. 2011;124:1239–1249. doi: 10.1161/CIRCULATIONAHA.111.029660. [DOI] [PubMed] [Google Scholar]
- 23.Newby DE, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N. Engl. J. Med. 2018;379:924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 24.Douglas PS, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N. Engl. J. Med. 2015;372:1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Bruyne B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 26.de Knegt MC, et al. Stress myocardial perfusion with qualitative magnetic resonance and quantitative dynamic computed tomography: Comparison of diagnostic performance and incremental value over coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging. 2020;22:jeaa270. doi: 10.1093/ehjci/jeaa270. [DOI] [PubMed] [Google Scholar]
- 27.Stuijfzand WJ, et al. Stress myocardial perfusion imaging vs coronary computed tomographic angiography for diagnosis of invasive vessel-specific coronary physiology: Predictive modeling results from the computed tomographic evaluation of atherosclerotic determinants of myocardial ischemia (CREDENCE) trial. JAMA Cardiol. 2020;5:1338–1348. doi: 10.1001/jamacardio.2020.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel MR, et al. 1-Year impact on medical practice and clinical outcomes of FFRCT: The ADVANCE registry. JACC Cardiovasc. Imaging. 2020;13:97–105. doi: 10.1016/j.jcmg.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Fairbairn TA, et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: Lessons from the ADVANCE Registry. Eur. Heart J. 2018;39:3701–3711. doi: 10.1093/eurheartj/ehy530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nørgaard BL, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) J. Am. Coll. Cardiol. 2014;63:1145–1155. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 31.Lu MT, et al. Noninvasive FFR derived from coronary CT angiography: Management and outcomes in the PROMISE trial. JACC Cardiovasc. Imaging. 2017;10:1350–1358. doi: 10.1016/j.jcmg.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data for this study will be made available by the corresponding author upon reasonable request.