Abstract
VanK is the fourth member of the ubiquitous major facilitator superfamily of transport proteins to be identified that, together with PcaK, BenK, and MucK, contributes to aromatic catabolism in Acinetobacter sp. strain ADP1. VanK and PcaK have overlapping specificity for p-hydroxybenzoate and, most clearly, for protocatechuate: inactivation of both proteins severely impairs growth with protocatechuate, and the activity of either protein alone can mask the phenotype associated with inactivation of its homolog. Furthermore, vanK pcaK double-knockout mutants appear completely unable to grow in liquid culture with the hydroaromatic compound quinate, although such cells on plates convert quinate to protocatechuate, which then accumulates extracellularly and is readily visible as purple staining. This provides genetic evidence that quinate is converted to protocatechuate in the periplasm and is in line with the early argument that quinate catabolism should be physically separated from aromatic amino acid biosynthesis in the cytoplasm so as to avoid potential competition for intermediates common to both pathways. Previous studies of aromatic catabolism in Acinetobacter have taken advantage of the ability to select directly strains that contain a spontaneous mutation blocking the β-ketoadipate pathway and preventing the toxic accumulation of carboxymuconate. By using this procedure, strains with a mutation in structural or regulatory genes blocking degradation of vanillate, p-hydroxybenzoate, or protocatechuate were selected. In this study, the overlapping specificity of the VanK and PcaK permeases was exploited to directly select strains with a mutation in either vanK or pcaK. Spontaneous mutations identified in vanK include a hot spot for frameshift mutation due to contraction of a G6 mononucleotide repeat as well as point mutations producing amino acid substitutions useful for analysis of VanK structure and function. Preliminary second-site suppression analysis using transformation-facilitated PCR mutagenesis in one VanK mutant gave results similar to those using LacY, the prototypic member of the major facilitator superfamily, consistent with the two proteins having a similar mechanism of action. The selection for transport mutants described here for Acinetobacter may also be applicable to Pseudomonas putida, where the PcaK permease has an additional role in chemotaxis.
One of the defining characteristics of a microorganism is the spectrum of compounds that are transported across the cell membrane, and it is therefore of interest to examine the diversity of proteins that have evolved to contribute to an organism’s transport capacity (7). Genome sequencing has revealed that a significant fraction of transport proteins (64) can be grouped either into the ATP-binding cassette (ABC) superfamily or the major facilitator superfamily. In the soil bacterium Acinetobacter sp. strain ADP1, permeases associated with the β-ketoadipate pathway for catabolism of aromatic compounds appear exclusively to be members of the major facilitator superfamily (61): BenK (9) and MucK (77) are involved in uptake of compounds in the benzoate branch of the pathway, and PcaK (8, 45) is involved in the p-hydroxybenzoate branch. This study concerns VanK, a fourth member of the superfamily associated with aromatic catabolism in Acinetobacter.
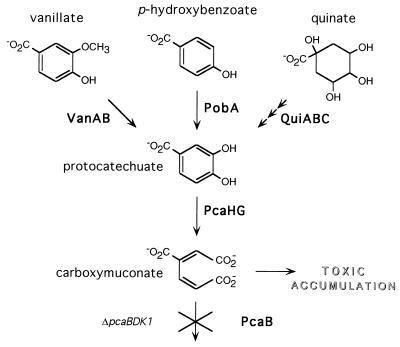
Studies of the β-ketoadipate pathway in Acinetobacter have taken advantage of the ability to use a strain with the engineered ΔpcaBDK1 deletion to directly select derivatives in which a spontaneous mutation prevents the toxic accumulation of carboxymuconate (Fig. 1). By using this procedure, strains that contained a structural gene mutation inactivating the VanAB vanillate demethylase (69), the PobA p-hydroxybenzoate hydroxylase (13, 33), or the PcaHG protocatechuate dioxygenase (12, 28) were selected. Also selected were strains with a regulatory gene mutation inactivating either of two transcriptional activators (14, 26): PobR (14, 27, 43, 44), governing expression of PobA, or PcaU (11), governing expression of PcaH and PcaG (31). As described in this study, the gene for the PcaK transport protein is already inactivated by the ΔpcaBDK1 deletion in the parental strain ADP500, which consequently allowed the selection of derivatives with a mutation in VanK, a protein closely related to and overlapping in specificity with PcaK.
FIG. 1.
Positive selection of mutants blocked in catabolism of aromatic or hydroaromatic compounds. Strain ADP500 contains the engineered ΔpcaBDK1 deletion and cannot grow with succinate in the presence of protocatechuate (or compounds upstream in the β-ketoadipate pathway) due to the toxic accumulation of β-carboxy-cis,cis-muconate. ADP500 derivatives in which a spontaneous mutation blocks the β-ketoadipate pathway upstream of carboxymuconate can therefore be selected. After correction of the ΔpcaBDK1 deletion to wild type, the phenotype of the remaining spontaneous mutation can be revealed by growth tests with single carbon sources.
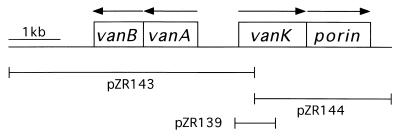
The genetic analysis presented here was made possible by characterization of a chromosomal segment containing vanA and vanB, structural genes for vanillate demethylase (69). As shown in Fig. 2, these genes, essential for growth with vanillate, neighbor an open reading frame designated vanK on the basis of its genetic location and its sequence similarity to other transporters associated with aromatic catabolism (69). The other investigation (69) did not associate a phenotype with vanK. That knowledge emerged from the present study, initiated by discovery that in strains defective in pcaK, mutations in vanK protect cells against toxicity exerted by protocatechuate. The vanK mutations occur with remarkable frequency, and so double mutants unable to express both protocatechuate oxygenase and VanK are likely to emerge from exposure of cells to protocatechuate in a single round of mutant selection (Fig. 1). The genetic bases for the genetic instability of vanK are reported here and elsewhere (69). Mutations blocking both pcaK and vanK impede growth with either protocatechuate or quinate (Fig. 1) and cause extracellular accumulation of protocatechuate from the latter compound. vanK is the first observed chromosomal locus that can specifically influence protocatechuate catabolism and is not within the pca-qui-pob supraoperonic cluster (17, 30, 35).
FIG. 2.
Chromosomal region containing vanA and vanB, genes for vanillate demethylase. Arrows denote direction of transcription. DNA fragments cloned in plasmids pZR139, pZR143, and pZR144 (69) were used to map vanK mutations by marker rescue.
MATERIALS AND METHODS
Strains and culture conditions.
Acinetobacter sp. strain ADP1, originally called Acinetobacter calcoaceticus BD413 (39), was routinely grown with 10 mM succinate in a mineral medium. Unless otherwise indicated, cells were grown at 37°C and the mineral medium was supplemented with 5 mM p-hydroxybenzoate, 3 mM quinate, 3 mM vanillate, 3 mM protocatechuate, or 2.5 mM benzoate. Because of the instability of protocatechuate, only fresh plates or liquid cultures with this carbon source were used, made with stock solutions (pH 7.0) stored frozen until use. For characterization of mutant phenotypes, strains were tested after overnight growth on plates with 10 mM succinate.
DNA manipulations.
Crude cell lysates of Acinetobacter strains for use in transformation reactions were prepared by incubation of pelleted cells (from a 5-ml culture) in 0.5 ml of saline-citrate buffer with 0.05% sodium dodecyl sulfate at 60°C for 1 h. Plasmids were isolated from 5-ml Escherichia coli cultures by using the Wizard miniprep kit (Promega) and assumed to contain 2 μg of DNA. To isolate template DNA for PCR, pelleted Acinetobacter cells from a 5-ml culture were washed and treated with InstaGene Matrix as described by the supplier (Bio-Rad); 5 μl of the resulting solution was then used in 50-μl PCRs including 0.5 U of Taq polymerase (Boehringer Mannheim), 10 pmol of each primer, and 10 pmol of each deoxynucleoside triphosphate. Standard PCR conditions were used: 30 cycles of denaturing at 94°C for 45 s, annealing at 56°C for 45 s, and elongation at 72°C for 1 min 30 s. PCR primers were synthesized by the Keck Biotechnology Resource Laboratory (Yale University).
For efficient transformation of Acinetobacter strains, 200 μl of a 5-ml culture of the recipient strain grown overnight with 10 mM succinate was transferred to a fresh 5-ml culture. After growth in a gyratory shaker for 2 h (to increase competence), 500 μl of the culture was transferred to Falcon 15-ml polypropylene tubes together with 1 μg of plasmid DNA or 20 μl of a 50-μl PCR mixture. After overnight shaking incubation, transformation reactions were transferred to appropriate selective plates.
Selection and characterization of strains deficient in vanK.
Strain ADP500 (32) contains the engineered ΔcatD101::Kmr and ΔpcaBDK1 mutations. To isolate mutants blocked in catabolism of protocatechuate, single colonies of succinate-grown ADP500 were transferred to patches on freshly prepared plates with 10 mM succinate and 3 mM protocatechuate. Cells with spontaneous secondary mutations blocking protocatechuate catabolism do not accumulate toxic levels of β-carboxy cis,cis-muconate and are able to grow (Fig. 1). To prevent analysis of siblings, only one mutant derivative was picked per single colony of ADP500.
To demonstrate coselection of independent mutations in pcaH and pcaG and in vanK, mutant cells of ADP500 were selected for resistance to 3 mM protocatechuate at 37°C and then screened for heat-sensitive mutations in pcaH and -G. Correction of the ΔpcaBDK1 deletion in 16 such strains generated ADP1102 to ADP1117, each with a spontaneous heat-sensitive mutation in either pcaH or -G (12). To identify and to map potential spontaneous mutations in vanK in addition to the conditional mutation in pcaH or -G, the ΔpcaBDK1 deletion in the 16 strains was replaced on the chromosome with ΔpcaK859 (8) instead of wild-type DNA by the following procedures. Recipient strains were patched onto an LB plate together with 1 μl of crude cell lysate of ADP859 (ΔpcaK859). After overnight growth, cells from each patch were transferred to and purified on plates with 5 mM p-hydroxybenzoate incubated at 37°C, thereby demanding correction of the heat-sensitive mutation in pcaH or -G as well as activity of PcaBD. This generated strains ADP7576 to ADP7591 (Table 1; equivalent to strains ADP1102 to ADP1117 with the addition of ΔpcaK859), which were then tested for growth with quinate. Strains severely impaired in growth with quinate (or protocatechuate) due to inactivation of both vanK and pcaK were patched onto an LB plate together with about 50 ng of plasmid pZR139, pZR143, or pZR144 (69) (Fig. 2). After overnight growth, cells from each patch were transferred to a plate with 3 mM quinate: wild-type growth with quinate indicated marker rescue and allowed rough localization of the spontaneous vanK mutation.
TABLE 1.
Strains mutated in vanK
| Straina | Genotype
|
vanK mutation | VanK mutation | |
|---|---|---|---|---|
| pca | van | |||
| ADP7576 | ΔpcaK859 | Δvan1102 | ||
| ADP7577b | ΔpcaK859 | ΔvanK1103 | ΔG (366–371)d | |
| ADP7579 | ΔpcaK859 | Δvan1105 | ||
| ADP7580 | ΔpcaK859 | Δvan1106 | ||
| ADP7582 | ΔpcaK859 | ΔvanK1108 | ΔA (697–704)d | |
| ADP7584 | ΔpcaK859 | Δvan1110 | ||
| ADP7587 | ΔpcaK859 | ΔvanK1113 | G212A | Gly71Asp |
| ADP7592 | ΔpcaBDK1 | ΔvanK1103 | ΔG (366–371)d | |
| ADP7593 | ΔpcaBD1 | ΔvanK1103 | ΔG (366–371)d | |
| ADP7618 | ΔvanK1103 | ΔG (366–371)d | ||
| ADP7602c | ΔpcaK859 | ΔvanK7602 | Δ(A427–T522) | Δ(Ser143–Arg174) |
| ADP7603 | ΔpcaK859 | ΔvanK7603 | vanK::IS1236 | |
| ADP7604 | ΔpcaK859 | ΔvanK7604 | G460T | Gly154Trp |
| ADP7607 | ΔpcaK859 | ΔvanK7607 | Δ(T769–A918) | Δ(Cys257–Ala306) |
| ADP7608 | ΔpcaK859 | ΔvanK7608 | C655T | Gln219Stop |
| ADP7609 | ΔpcaK859 | ΔvanK7609 | G1133C | Arg378Pro |
| ADP7610 | ΔpcaK859 | ΔvanK1113 | G212A | Gly71Asp |
| ΔvanK7610 | T806C | Phe269Ser | ||
| ΔvanK7611 | T812C | Phe271Ser | ||
| ADP7612 | ΔpcaK859 | ΔvanK1113 | G212A | Gly71Asp |
| ΔvanK7612 | G880C | Gly294Arg | ||
ADP7576 to ADP7591 are derived from strains selected for a spontaneous mutation in pcaH or pcaG; ADP7602 to ADP7609 are derived from strains selected for a spontaneous mutation solely in vanK; ADP7610 and ADP7612 are derived from ADP7587 and contain PCR-generated suppressor mutations of vanK1113.
Same as ADP7578, ADP7581, ADP7583, ADP7585, ADP7586, ADP7589, and ADP7590.
Same as ADP7605, and ADP7606.
Frameshift mutation caused by deletion of one residue in a mononucleotide repeat.
An independent collection of mutants was obtained by selecting derivatives of ADP500 mutated so that they resisted 3 mM protocatechuate at 22°C. Strains from this selection (still containing the ΔpcaBDK1 deletion) with a cold-enhanced resistance to p-hydroxybenzoate were found to have a spontaneous mutation affecting transcriptional regulation by PcaU and are described elsewhere. Strains that resisted protocatechuate but not p-hydroxybenzoate at both 37 and 22°C were purified on an LB plate and then tested for the ability to grow on a plate with 10 mM succinate in the presence of 3 mM vanillate (which does not require a functional vanK for its metabolism). Strains from this selection (still containing the ΔpcaBDK1 deletion) with a cold-enhanced resistance to p-hydroxybenzoate were found to have a spontaneous mutation affecting transcriptional regulation by PcaU and were described elsewhere (11). Strains not resistant to p-hydroxybenzoate at either 37 or 22°C were purified on an LB plate and then tested for the ability to grow on a plate with 10 mM succinate in the presence of 3 mM vanillate. For those strains also not resistant to vanillate and therefore assumed to have a spontaneous mutation confined to vanK, crude cell lysates were made. To identify complementation groups, these lysates were tested for the ability to transform strains carrying ΔpcaK859 combined with various sequenced mutations in vanK to wild-type growth with quinate: 5-ml cultures of the double-mutant recipient strains were grown overnight with 10 mM succinate and, after addition of 10 μl of 1 M succinate, incubated for an additional 30 min in a gyratory shaker (to increase competence) before being spread onto selective plates with 3 mM quinate. Lysate (4 μl) of each mutant was spotted onto the cell lawn. Because the ΔpcaBDK1 deletion in the donor strains overlapped the ΔpcaK859 mutation in the recipients, wild-type growth with quinate was possible only when the lysate DNA corrected the vanK mutation; no growth was readily observed when the donor and recipient contained the same or overlapping mutations in vanK. Strains which appeared to have a unique vanK mutation were treated as described above: ΔpcaBDK1 was replaced on the chromosome with ΔpcaK859, generating ADP7602 to ADP7609 (Table 1), and the vanK mutation was mapped by marker rescue.
To reconstruct and to analyze the selection pressure for vanK inactivation, the ΔpcaBDK1 and ΔpcaBD1 deletions each were introduced into the chromosome of an ADP500 derivative carrying only the spontaneous vanK1103 mutation, generating ADP7592 and ADP7593, respectively (Table 1). To construct such strains, liquid cultures were transformed (as described above) with one of two plasmids: pZR301 (32), in which removal of two internal EcoRV fragments from the pZR3 (28, 32) insert generated ΔpcaBDK1; and pZR35, in which removal of one of the internal EcoRV fragments generated ΔpcaBD1. Transformation reactions were streaked onto plates with 10 mM succinate, and single colonies were tested for loss of the ability to grow with p-hydroxybenzoate, indicating chromosomal replacement of wild-type pca genes with the engineered deletion. Generally at least 1 in 500 colonies tested had the desired phenotype.
Transformation-facilitated PCR mutagenesis.
For mutagenesis (12, 43, 44), standard PCR was performed as described above except that the number of cycles was increased to 35. For suppression of vanK1113, a 1.2-kb PCR fragment was amplified from ADP7587 by using primers VK3 (5′-GTTTAGCTATTGCAGGCATCAGC-3′) and VK6 (5′-GCAATAAATTCGGCATGGACTAC-3′). For mutagenesis of pcaK, a 1.7-kb fragment was amplified from ADP7593 by using primers PK1 (5′-CATTGATTATCGCGGGTAGTGC-3′) and PK2 (5′-CTCAAGTGAACGGTTAACATGC-3′). Twenty microliters of each PCR mixture was directly used to transform a liquid culture of the recipient strain (as described above).
DNA sequence analysis.
To isolate template DNA for sequencing, products of PCRs performed under standard conditions were purified with 8 μl of GeneClean Glassmilk according to the supplier (Bio 101, Inc.) and resuspended in 25 μl of water; 8 μl was used for ABI PRISM dye terminator cycle sequencing with AmpliTaq DNA polymerase, FS (Perkin-Elmer). Cycle sequence reactions were processed as previously described (43). PCR primers were synthesized by the Keck Biotechnology Resource Laboratory (Yale University), which also performed some of the DNA sequencing. The nucleotide sequence of a 14-kb EcoRI fragment containing Acinetobacter wild-type vanK has been reported previously (69) and appears in the GenBank nucleotide sequence database under accession no. AF009672.
RESULTS
Additional mutations blocking conversion of vanillate to protocatechuate in strains selected directly for a block in protocatechuate utilization.
Selection for resistance to the toxic accumulation of carboxymuconate in the engineered Acinetobacter mutant ADP500 yields strains with secondary mutations blocking the β-ketoadipate pathway upstream of PcaB (Fig. 1). By using this procedure, a collection of strains each with a spontaneous mutation in pcaH or pcaG, structural genes for protocatechuate 3,4-dioxygenase (12, 28, 31), was generated. One such mutant, ADP6311, contained a 302-bp deletion in pcaG (28). As part of a study of catabolic pathways in Acinetobacter potentially convergent upon protocatechuate, it was shown that unlike the wild-type strain ADP1, ADP6311 and other mutants blocked in pcaH and -G could not grow with vanillate as sole carbon and energy source. This was expected since the first step in vanillate catabolism is its conversion to protocatechuate by vanillate demethylase (69) (Fig. 1). However, when the supernatant of an ADP6311 culture grown in the presence of vanillate was analyzed by high-pressure liquid chromatography (data not shown), no accumulation of protocatechuate was detected, indicating that contrary to expectation, the vanillate remained unmetabolized by this strain. Furthermore, ADP6338 (28), a spontaneous mutant containing a leaky four amino acid deletion in PcaH causing slow growth with protocatechuate, was unable to grow with vanillate.
Since ADP6311 and ADP6338 came from the same collection of spontaneous mutants, the anomalous defect in vanillate catabolism could have been the result of a mutation in the particular isolate of the parental strain ADP500. Therefore, we tested a separate collection of 16 strains (ADP1102 to ADP1117) derived from ADP500 and each with a heat-sensitive mutation in pcaH or -G (12) for growth with vanillate at the permissive temperature (22°C). Although all 16 strains grew with p-hydroxybenzoate at 22°C, four (ADP1102, ADP1105, ADP1106, and ADP1110) could not grow at 22°C with vanillate (Fig. 1). This finding confirmed the original observation with ADP6311 and ADP6338 and indicated that strains selected for a block in conversion of protocatechuate to carboxymuconate could contain a significant fraction with an additional independently selected block in conversion of vanillate to protocatechuate. Suggesting that this additional spontaneous mutation in ADP6338 was a large deletion, transformation of ADP6338 with chromosomal DNA contained in crude cell lysate of wild-type strain ADP1 yielded only rare recombinants able to grow with vanillate.
Identification of vanK.
The apparent presence of a deletion mutation preventing vanillate degradation in ADP6338 raised the possibility that a selected mutation blocked vanK (which proved not to be required for vanillate degradation) and that the deletion mutation extended into vanA and vanB (which are required for vanillate degradation [69] [Fig. 2]). Therefore, other ADP500-derived strains with no apparent block in vanillate degradation may also harbor an undetected mutation, and ADP1103 (12) was used to test this possibility. By demanding growth with p-hydroxybenzoate at 37°C, a strain in which the heat-sensitive pcaH1103 mutation had spontaneously reverted was selected, and a subsequent test showed that growth with vanillate was not detectably impaired. To reveal any mutations other than pcaH1103 that might have been originally selected in this strain, the ΔpcaBDK1 mutation was reintroduced into the chromosome (see Materials and Methods), generating ADP7592 (Table 1). Reconstructing the selection for resistance to carboxymuconate accumulation, it was found that ADP7592, unlike the parental strain ADP500, appeared completely resistant to 1 mM protocatechuate but the 3 mM concentration used in the original selection remained toxic.
The phenotype of ADP7592, sensitive to the difference between 1 and 3 mM protocatechuate, suggested the presence of a hitherto undetected spontaneous vanK mutation affecting protocatechuate catabolism at the level of transport. Fortuitously coincident with this study, a genetic region including genes for conversion of vanillate to protocatechuate was cloned from Acinetobacter (69). DNA sequencing of this region revealed vanA and vanB, encoding the two subunits of vanillate demethylase, and, transcribed divergently from these structural genes, a gene encoding a protein closely related to the Acinetobacter (8, 45) and Pseudomonas (34, 56) PcaK permease (Fig. 2). This gene was designated vanK to reflect its chromosomal location but the role of vanK in vanillate catabolism remains uncertain (see Discussion). Preliminary studies in Acinetobacter (8) indicate that PcaK is involved in uptake of p-hydroxybenzoate and protocatechuate as is the case in Pseudomonas (34, 56). Given the sequence similarity of VanK and PcaK, the vanK gene seemed an ideal candidate for a target of selection that could affect resistance of ADP500 to protocatechuate.
Nucleotide sequence of vanK.
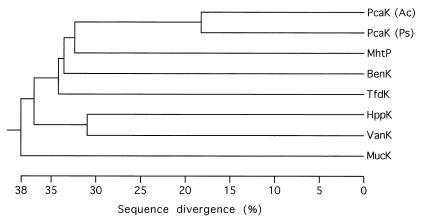
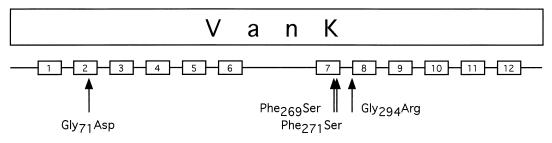
The vanK gene (Fig. 3) encodes a protein of 448 amino acids (Mr = 47,927) whose sequence indicates that it is a member of the major facilitator superfamily of transport proteins (52, 61). In a phylogenetic analysis (Fig. 4), VanK clusters with six other proteins: PcaK (45) and BenK (9) in Acinetobacter strain ADP1, PcaK (34, 56) in Pseudomonas putida, MhpT (20) in E. coli, TfdK (48) in Ralstonia eutropha, and HppK (2) in Rhodococcus globerulus. These six permeases, known or predicted to mediate the uptake of aromatic acids, form a new protein family within the superfamily (2, 61). VanK is most closely related (37% identity over 442 amino acid aligned residues [1]) to HppK, predicted to transport 3-hydroxyphenylpropionate, also the predicted substrate of MhpT. As with LacY, the prototypic member of the major facilitator superfamily, VanK and the other members of the aromatic acid permease family are predicted to contain 12 membrane-spanning α-helical segments (Fig. 3), topologically oriented so that a large cytoplasmic loop is formed by residues in the middle of the linear amino acid sequence. An amino acid signature sequence for this family (61), including most of the residues in transmembrane segment 2, is matched in 11 of 13 positions in the VanK sequence (Fig. 3, VanK residues 62 to 81). This signature sequence in turn just overlaps with amino acids between transmembrane segments 2 and 3 that had been previously identified as characteristic of the superfamily (51, 61).
FIG. 3.
Nucleotide sequence of vanK and deduced amino acid sequence of the encoded protein. Underlined with a dashed line are 12 segments with amino acids potentially forming transmembrane α-helices (identified by the TMpred program of the ISREC-Bioinformatics Group, Geneva, Switzerland). The orientation in the membrane of these segments is predicted to alternate with the first helix oriented inside to outside. Sequenced spontaneous mutations are indicated above vanK nucleotides and above VanK amino acids; all were selected based on loss of VanK function except for the intragenic suppressors of vanK1113: the underlined vanK7610 and vanK7611 (together in ADP7610) and vanK7612 (in ADP7612). The TAG sequence duplicated as a result of IS1236 insertion in vanK7603 is underlined below a vertical arrow. Underlined below an open triangle are the G6 and A7 mononucleotide repeats in which 1 bp is deleted, in ΔvanK1103 and ΔvanK1108, respectively. Horizontal arrows are shown above the first and last nucleotides deleted in ΔvanK7607 and ΔvanK7602, the latter deletion including one of two repeats of the 8-bp sequence GCTGGCGT (underlined). Downstream from vanK is a gene for a putative porin (69) (Fig. 2).
FIG. 4.
Relative sequence divergence in the aromatic acid permease family. The organisms in which these proteins were identified and the substrates, inferred or demonstrated, for the transporters are as follows: PcaK (Ac) in Acinetobacter sp. strain ADP1, protocatechuate and p-hydroxybenzoate (reference 45 and this study); PcaK (Ps) in P. putida, protocatechuate and p-hydroxybenzoate (34, 56); MhtP in E. coli, 3-(3-hydroxyphenyl)propionate (20); BenK in Acinetobacter sp. strain ADP1, benzoate (9); TfdK in Ralstonia eutropha: 2,4-dichlorophenoxyacetate (48); HppK in Rhodococcus globerulus: 3-(3-hydroxyphenyl)propionate (2); VanK in Acinetobacter sp. strain ADP1, protocatechuate and p-hydroxybenzoate (this study); MucK in Acinetobacter sp. strain ADP1, cis,cis-muconate (77). The degree to which each of these proteins may facilitate transport of multiple related compounds remains unknown. A new family of carboxylic acid permeases including MucK, distinct from the aromatic acid permease family (61), may become apparent with additional sequencing (2). The sequence of Acinetobacter PcaK in GenBank has been updated (accession no. AF009672, June 1998).
Spontaneous mutations inactivating vanK can be masked by PcaK activity.
The ΔpcaBDK1 mutation in ADP500, besides blocking the β-ketoadipate pathway at the level of carboxymuconate, also inactivates pcaK. This loss of pcaK function, however, does not protect ADP500 from the toxic accumulation of carboxymuconate during growth in the presence of even micromolar quantities of protocatechuate. Furthermore, growth on plates with protocatechuate is not dramatically impeded in ADP859 (8), in which the ΔpcaK859-engineered 4-bp deletion (of one of the tandem TTAA repeats between nucleotides [nt] 4945 and 4952 [45]) inactivates the encoded permease in an otherwise wild-type genetic background. This apparent redundancy of pcaK function suggested that spontaneous mutations in vanK might contribute to protocatechuate resistance in ADP500 but then go undetected after PcaK activity was restored by correction of pcaBDK to wild type.
To reveal vanK mutations potentially masked by PcaK activity in the 16 spontaneous mutant strains, ADP1102 to ADP1117 (12), the heat-sensitive mutation in pcaH or pcaG in each strain was corrected to wild type, and simultaneously the ΔpcaK859 mutation was introduced into the chromosome, inactivating pcaK (see Materials and Methods). Tests of the resulting strains, ADP7576 to ADP7591 (Table 1), revealed that 14 of 16, including the four strains with apparent deletions overlapping vanK, were now severely impaired in growth with protocatechuate or quinate. Excluding the four deletion mutants, the spontaneous mutation in the remaining 10 strains was localized to vanK by marker rescue with cloned DNA in plasmids pZR139, pZR143, and pZR144 (69) (Fig. 2). Subsequently, DNA sequencing of the appropriate region, in 8 of the 10 strains, revealed ΔvanK1103 (Fig. 3), a single-base deletion in a string of six G residues (nt 366 to 371). The remaining two strains had different mutations (Fig. 3): ΔvanK1108, a single-base deletion in a string of seven A residues (nt 697 to 704) or vanK1113, a point mutation causing substitution of Gly71 with Asp71 in the VanK protein. These results confirm the prediction that vanK can be a target of spontaneous mutation during selection with strain ADP500 and that these mutations can be masked by the activity of PcaK.
Role of vanK during selection of ADP500 derivatives resistant to protocatechuate.
The reconstruction experiment with ADP7592 showed that the ΔvanK1103 spontaneous mutation alone could not provide resistance to protocatechuate at the concentration (3 mM) used in the original selection. This explains the necessity for the pcaH1103 mutation in addition to ΔvanK1103 in the original ADP500 derivative but does not explain the order in which these two mutations arose. To determine when vanK mutations may have arisen, the selection for mutant derivatives of ADP500 was repeated. Mutant colonies growing in the presence of 3 mM protocatechuate were purified by two successive transfers on selective plates as in the original selections (12, 28). Mutant cells generally grew poorly during these transfers, and frequently there appeared a mix of colony sizes. In a particularly clear case after the second round of transfer, a crude cell lysate was made from one relatively large colony. In contrast to lysates of other samples of this mutant strain from the first and second transfers, the DNA in the lysate of the large colony could not transform ADP7576 (Table 1), a strain deleted in vanK and containing the ΔpcaK859 mutation, to wild-type growth with protocatechuate. This negative transformation result indicates that the large colony had acquired an additional mutation preventing correction of the ADP7576 vanK defect and implies that a mutation inactivating vanK can arise by unintended selection during purification on selective media of a mutant strain already impaired in protocatechuate degradation.
Direct selection and characterization of strains with a spontaneous mutation inactivating vanK.
The resistance of ADP7592 (ΔvanK1103 ΔpcaBDK1) to growth at 37°C in the presence of 1 mM or less protocatechuate suggested that these conditions could be used to directly select ADP500 derivatives with spontaneous mutations only in vanK. Indeed, such strains had almost certainly already been isolated: it had been previously noted (65) that by using these conditions to select ADP500 derivatives with spontaneous mutations in pcaH or pcaG, strains with no change in phenotype apparent after replacement of the ΔpcaBDK1 deletion on the chromosome with wild-type DNA were also isolated, consistent with the presence of a vanK mutation. Also consistent with the use of 1 mM protocatechuate expanding the potential mutational targets compared to selection with 3 mM protocatechuate, the former conditions consistently yielded significantly more spontaneous mutant derivatives per ADP500 CFU.
In another study, ADP500 derivatives were selected for resistance to 3 mM protocatechuate during growth with succinate at 22°C rather than 37°C (11). During these experiments, it was noticed that consistently more than half of the protocatechuate-resistant colonies first appearing on the selective plate, when subsequently tested, could not grow in the presence of 5 mM p-hydroxybenzoate at either 22 or 37°C. Because this anomalous resistance to protocatechuate but not the upstream metabolite p-hydroxybenzoate was suggestive of the phenotype resulting from vanK inactivation, 46 such strains had been saved. Of these 46 strains, 25 were resistant to 3 mM vanillate, implying that structural van genes as well as vanK had been inactivated by a deletion mutation (Fig. 2). The remaining 21 strains, in which vanK alone appeared to be mutant, were purified, and cell lysates of these strains were used in marker rescue experiments with three recipients: ADP7577, ADP7582, and ADP7587 (Table 1). Lysates of 13 strains could correct all but the ΔvanK1103 mutation in ADP7582; because these strains probably also carried ΔvanK1103, they were not analyzed further.
Each of the eight remaining strains, lysates of which could correct the vanK mutation in all three recipients, appeared to have a new mutation in vanK. As before, the ΔpcaBDK1 deletion in these mutants was replaced on the chromosome with ΔpcaK859, generating ADP7602 to ADP7609 (Table 1), and the vanK mutation was localized by marker rescue with plasmids pZR139, pZR143, and pZR144. DNA sequencing of the relevant vanK regions revealed three cases of point mutations producing amino acid substitutions in VanK: Gly154Trp in VanK7604, Gln219Stop in VanK7608, and Arg378Pro in VanK7609 (Fig. 3). One strain had a 150-bp deletion (ΔvanK7607) flanked by the 4-bp sequence ATTA, but the deletion left both repeats present (Fig. 3). Three strains had ΔvanK7602 in which 96 bp is deleted, including one flanking 8-bp repeat of the sequence GCTGGCGT (Fig. 3). Intriguingly, a sequence identical in seven of eight positions is involved in a spontaneous deletion mutation, mucK51 (77), in an Acinetobacter gene encoding a permease closely related to VanK (Fig. 4), and the 3′ endpoint of the deletion is in the equivalent position of both genes.
PCR amplification of DNA containing the remaining mutation, vanK7603, indicated an insertion of 1.2 kb, and sequencing confirmed the presence of the Acinetobacter insertion sequence IS1236 (27). Insertion of IS1236 in the direction of vanK transcription generated a 3-bp target site duplication (Fig. 3). This insertion behavior, characteristic of the IS3 family (24), was also seen in previous studies in the two regulator genes pobR (27) and pcaU (11), in contrast to three of four insertions in the pcaH structural gene (28). Tn5613, a newly discovered compound transposon with identical copies of IS1236 at either end, contributes to spontaneous mutation in Acinetobacter vanA and vanB and also generates a 3-bp target site duplication (69).
Overlapping specificities of VanK and PcaK revealed during catabolism of protocatechuate, p-hydroxybenzoate, and quinate.
To systematically identify compounds in the β-ketoadipate pathway (Fig. 1) for which VanK and PcaK had overlapping specificities, ADP7592 (ΔvanK1103 ΔpcaBDK1) was tested for growth with 10 mM succinate at two temperatures (37 and 22°C) in the presence of various concentrations (0.1 to 5 mM) of protocatechuate, p-hydroxybenzoate, quinate, or vanillate. ADP500 (ΔpcaBDK1) was sensitive to these compounds under all conditions and concentrations tested, indicating that pcaK inactivation alone was not sufficient to provide resistance. At 37°C, besides being resistant to 1 mM protocatechuate, ADP7592 grew in the presence of 5 mM quinate or 0.1 mM p-hydroxybenzoate. Higher concentrations of p-hydroxybenzoate of vanillate at even 0.1 mM were toxic, consistent with the phenotype observed during isolation of other mutants, including those later shown to have larger deletions within vanK (Fig. 3). Emphasizing the importance of growth temperature, ADP7592 was resistant to 3 mM protocatechuate during growth at 22°C but not 37°C. To test if inactivation of vanK alone was sufficient for any resistance, strain ADP7593 (ΔvanK1103 ΔpcaBD1) was constructed (see Materials and Methods). Growth tests with ADP7593 showed that no protection against toxicity was provided only by vanK inactivation; therefore, the observed resistance phenotypes required inactivation of both vanK and pcaK.
In a genetic background in which pcaB was blocked, the enhanced resistance described above in strains in which both vanK and pcaK were inactivated suggested that these two genes could have overlapping roles in growth with protocatechuate, p-hydroxybenzoate, and quinate. More direct evidence for these roles came from growth tests with strains in which the only mutations were in vanK and pcaK. In strains with the ΔpcaK859 mutation in combination with ΔvanK7602 or any of the other sequenced vanK mutations (Fig. 3), growth on plates with 5 mM quinate or 3 mM protocatechuate as sole carbon and energy source was barely detectable after overnight incubation. Inactivation of both vanK and pcaK was required for this phenotype: transformation of the double-mutant strains with cloned DNA containing either vanK or pcaK yielded recombinants able to grow with quinate or protocatechuate at a level disguising the remaining mutation. In contrast, overnight growth with p-hydroxybenzoate or vanillate, either on plates or in liquid culture, was not clearly impaired for strains in which both vanK and pcaK were dysfunctional. This finding is consistent with the observation that inactivation of the two permease genes in ADP7592 did not prevent the toxic accumulation of carboxymuconate during growth in the presence of vanillate and conferred resistance to p-hydroxybenzoate only when supplied at a low concentration (0.1 mM). Furthermore, these same phenotypes were also observed in an engineered strain (ADP7614) in which ΔpcaK859 was combined with an insertion into the SphI site in vanK (69) of the chloramphenicol resistance gene from pCAT19 (22).
VanK PcaK double mutants exhibited an even stronger phenotype with protocatechuate and quinate in liquid culture. Strain ADP7602 (ΔvanK7602 ΔpcaK859) was tested since phenotypic revertants were readily obtained from other strains, especially those with ΔvanK1103 (Fig. 3). Indeed, the first attempt to sequence this latter mutation in ADP7585 and ADP7589 (Table 1) gave results consistent with a mixed population of cells, including those in which ΔvanK1103 had directly reverted. CFU from a 5-ml culture inoculated with approximately 107 stationary-phase cells of succinate-grown ADP7602 and provided either with 3 mM protocatechuate or 3 mM quinate consistently had decreased 10- or 100-fold, respectively, after 24 h of shaking incubation. Cultures with protocatechuate incubated for an additional 24 h would generally become turbid, consistent with another transport system in the cell having been activated by mutation. In contrast, cultures with quinate continued to decrease in CFU, and no growth was ever observed even after prolonged incubation (14 days).
Extracellular accumulation of protocatechuate during growth in the presence of quinate of strains blocked in vanK and pcaK.
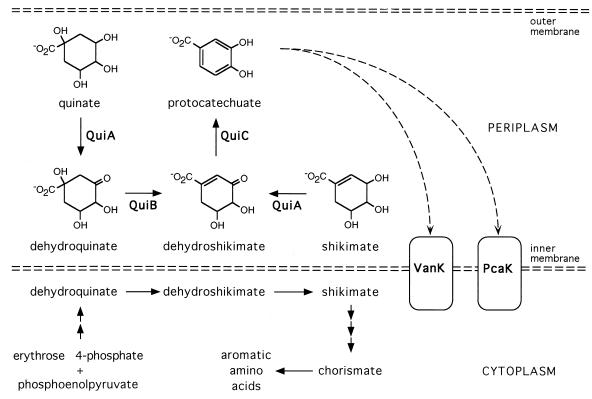
That the substrate specificity of VanK and PcaK might overlap to include the structurally similar aromatic compounds protocatechuate and p-hydroxybenzoate was unsurprising, but the strong phenotype associated with growth with the hydroaromatic compound quinate was unexpected (Fig. 1). A clue that the phenotype with quinate was indirect came from the observation that strains without a functional vanK and pcaK, growing on succinate in the presence of quinate, stained the medium a dramatic purple color characteristic of protocatechuate accumulation. This extracellular accumulation could be readily explained if conversion of quinate to protocatechuate in Acinetobacter takes place in the periplasm, outside the inner cell membrane (Fig. 5). Such compartmentation may cause a further obstacle to growth of vanK pcaK double mutants with quinate since the final step in conversion of quinate to protocatechuate, catalyzed by QuiC (Fig. 1), is susceptible to feedback inhibition by protocatechuate in at least one member of the genus Acinetobacter (74).
FIG. 5.
Predicted periplasmic localization of quinate and shikimate catabolism to protocatechuate. Genetic evidence from this study suggests that the sequential action of the QuiA membrane-bound quinate/shikimate dehydrogenase (17), the QuiB dehydroquinate dehydratase (18), and the QuiC dehydroshikimate dehydratase (18) produces protocatechuate in the periplasm. Protocatechuate thus formed could then be transported across the inner membrane (dashed horizontal lines) into the cytoplasm by the PcaK and VanK permeases. Such compartmentation would reduce competition for intermediates that are common to both the catabolic pathway and the biosynthetic pathway for generation of aromatic amino acids. The putative porin QuiX (18) may mediate entry of quinate and shikimate into the periplasm from outside the cell.
Extracellular accumulation of protocatechuate can reach concentrations high enough to significantly affect neighboring cells and was detected by monitoring growth of ADP603 (Δqui1 ΔpcaBDK1) on a plate with 10 mM succinate and 5 mM quinate (Fig. 6). Deletion in ADP603 of qui genes (17), required for conversion of quinate to protocatechuate, prevents the toxic accumulation of carboxymuconate during growth of this strain in the presence of quinate (Fig. 1). Protocatechuate itself, however, remains toxic to ADP603. Growth of ADP603, when adjacent to cells of ADP7577 (ΔvanK1103 ΔpcaK859), was drastically inhibited as expected if protocatechuate was accumulating extracellularly during growth of ADP7577 in the presence of quinate (Fig. 6). This effect was also produced, although less intensely, by protocatechuate diffusing from cells in which either vanK or pcaK was dysfunctional (Fig. 6).
FIG. 6.
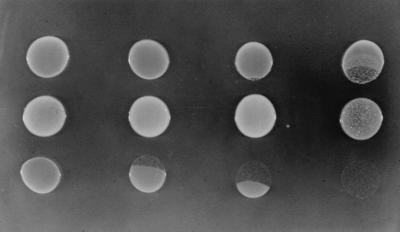
Detection of extracellular protocatechuate generated by mutant strains growing in the presence of quinate. A 20-μl aliquot of an LB culture of each strain was spotted onto a plate with 10 mM succinate and 5 mM quinate and incubated overnight at 37°C. In the top and bottom rows are cells of ADP603 (Δqui1 ΔpcaBDK1) added simultaneously (top row) or 5 h after (bottom row) strains in the middle row (from left to right): ADP1 (wild type), ADP859 (ΔpcaK859), ADP7618 (ΔvanK1103), and ADP7577 (ΔpcaK859 ΔvanK1103). Deletion of quinate genes in ADP603 prevents the toxic accumulation of carboxymuconate during growth of this strain in the presence of quinate but not protocatechuate (Fig. 1). Growth of ADP603 can therefore serve as a detector of protocatechuate diffusing from adjacent cells of mutant strains impaired to various degrees in transport of protocatechuate across the inner cell membrane after its generation from quinate in the periplasm (Fig. 5). The speckling observed in the spot of ADP7577 cells is due to reversion of ΔvanK1103.
Positive selection of strains with a mutation in pcaK.
In strains without PcaB function (Fig. 1), resistance to the toxic accumulation of carboxymuconate produced during growth in the presence of protocatechuate requires inactivation of both vanK and pcaK. This requirement was exploited to select strains derived from ADP500 (ΔpcaBDK1), each with a spontaneous mutation in vanK (Table 1; Fig. 3). The success of this selection in turn suggested that in the appropriate genetic background, pcaK mutants could be isolated by positive selection as well. Previously, PCR-generated mutations had been introduced into the Acinetobacter chromosome by natural transformation facilitating genetic analysis of the structural genes pcaH and pcaG (12, 43) and the regulatory genes, pobR and pcaU (43, 44). Applying this technique to pcaK, transformation of ADP7593 (ΔvanK1103 ΔpcaBD1) with PCR-amplified DNA containing the pcaK gene was followed by selection for growth with succinate at 22°C in the presence of 1 mM protocatechuate. Consistent with the introduction of PCR-generated mutations into pcaK on the chromosome, transformants of ADP7593 that were resistant to protocatechuate appeared at a frequency over 200-fold above the spontaneous mutation frequency of approximately 1 in 106 CFU.
Intragenic suppression of a vanK point mutation.
The Gly71Asp substitution in VanK1113 (Fig. 3) changes an amino acid residue conserved in all eight transport proteins in Fig. 4. This conservation suggests that Gly71 is particularly important for VanK function. Therefore, to gain insight into how structure influences function in VanK, PCR mutagenesis was again used, this time to obtain intragenic suppressors of vanK1113. PCR amplification across vanK but excluding the vanK1113 locus near the start of the gene produced DNA that yielded recombinants of ADP7587 (vanK1113 ΔpcaK859) that could grow at 30°C like wild-type cells with quinate. DNA sequencing of two such recombinants revealed in one of the strains, in addition to vanK1113, two new mutations, vanK7610 and vanK7611, causing Phe269Ser and Phe271Ser substitutions, respectively (Fig. 3). In the other recombinant, one new mutation, vanK7612, produced a Gly294Arg substitution (Fig. 3).
DISCUSSION
Multiple spontaneous mutations in strains derived from a single genetic selection.
The first evidence for the existence of vanK came from the detection of two spontaneous mutations in ADP500-derived strains selected for resistance to the toxic accumulation of carboxymuconate produced during growth in the presence of protocatechuate. One of these mutations was in pcaH or pcaG, structural genes for protocatechuate 3,4-dioxygenase, and the other inactivated vanK, encoding a putative transport system able to act on protocatechuate. A remarkably similar result was described in two recent Acinetobacter studies involving a parallel branch of the β-ketoadipate pathway. Selection for resistance to the toxic internal accumulation of muconate also generated strains with two spontaneous mutations, one in a structural gene for benzoate or anthranilate degradation and one inactivating the muconate transporter MucK (5, 77). The transporter mutation in this case, although not sufficient to allow growth (77), could have been beneficial by blocking the uptake of muconate accumulating extracellularly at the onset of selection from the lawn of cells of the parental strain (5, 23, 77). In the case of vanK, multiple mutations may have been an artifact of the selection procedure: in at least one ADP500 derivative already resistant to protocatechuate, inactivation of vanK appeared to confer additional resistance and was identified by its forming a relatively large colony during purification on a plate with selective media. The exact nature of the additional resistance provided by the block in protocatechuate uptake is unclear but may reflect the relative toxicity of this aromatic compound (62).
The unexpected spontaneous transport protein mutations in Acinetobacter described above illustrate the potential complexity of genetic selections. This complexity is consistent with the suggestion that sequential mutations can confer incremental benefits during nonlethal genetic selection, although only one mutation may ultimately be detected (25, 47). Furthermore, the results of this study highlight the influence of selection conditions and the genetic background of the starting strain on the range of genetic loci revealed by mutation, a conclusion that is probably particularly relevant to analyses of transport proteins (3).
A hot spot for frameshift mutation in a poly(G) tract in vanK.
In the 10 Acinetobacter strains analyzed by DNA sequencing that had a spontaneous mutation in both vanK and pcaH or pcaG, 8 contained ΔvanK1103, a single-nucleotide deletion in a string of 6 G residues (Fig. 3). When strains in which only vanK had acquired a mutation were selected, a greater variety of mutations emerged, but still the majority (13 of 21) contained ΔvanK1103. The frequent selection of ΔvanK1103 identifies the mononucleotide repeat as a hot spot for frameshift mutation. Furthermore, this poly(G) tract is not conserved in the genes for the five permeases most closely related to VanK, raising the possibility that the genetic instability reflected by ΔvanK1103 may be of adaptive significance to Acinetobacter in the soil environment. High-frequency frameshifting in a poly(A) tract in a gene encoding an ABC transporter component (72) has been suggested to generate alternative transport capabilities in mycoplasmas organisms with apparently limited transport systems (72), and phase variation due to reversible frameshifting in a poly(G) tract has been described for several bacterial genes involved in pathogenesis (6, 10, 38). The mutability of these contingency genes (55) can facilitate adaptation to rapidly changing and unpredictable environmental challenges (15, 55, 67), and this may apply to Acinetobacter vanK as well.
Insight into VanK structure and function from second-site suppressor mutations.
By demanding growth of ADP500 (ΔpcaBDK1) on succinate at 22°C in the presence of 3 mM protocatechuate, strains with secondary spontaneous mutations in vanK were isolated. Subsequent tests of lysates of these spontaneous mutants in marker rescue experiments rapidly identified strains with mutations not caused by the ΔvanK1103 frameshift hot spot. By using this simple procedure, a VanK missense mutation was found in three strains (Fig. 3): two mutations altered a glycine, Gly71Asp (VanK1113) and Gly154Trp (VanK7604), the former introducing a charged residue into a putative membrane-spanning helix, and the third mutation was a substitution with a proline residue, Arg378Pro (VanK7609). A combination of selection with transformation-facilitated PCR mutagenesis (12, 43, 44), successfully tested on chromosomal pcaK in this study, will allow the isolation of a large collection of strains with point mutations useful for identifying amino acid residues critical for structure and function of the two homologous Acinetobacter transport proteins, VanK and PcaK.
A signature sequence for the aromatic acid transporter family within the major facilitator superfamily includes most of the residues forming predicted transmembrane segment 2 (61). A completely conserved glycine residue in this sequence is changed by the Gly71Asp mutation in VanK1113 (Fig. 3). PCR mutagenesis of vanK DNA encoding putative transmembrane helices 4 to 12 in this mutant led to the isolation of two phenotypic revertants, one with a double mutation, Phe269Ser Phe271Ser, in helix 7 and one with a Gly294Arg substitution adjacent to helix 8 (Fig. 7). Substitution of a glycine residue in transmembrane segment 2 that inactivates LacY, the prototypic member of the major facilitator superfamily, is suppressed by second-site mutations, including ones in the second half of the protein in transmembrane segments 7 and 8 (37). In combination with biochemical data, this finding led to a model of LacY function whereby substrate binding generates a global conformation change propagated from helix 8 and mediated by helix 2 at the interface of the two halves of the protein (37, 40). The congruence of the LacY suppression analysis with the preliminary VanK results is consistent with the two proteins having a similar mechanism of action. This shared mechanism may be underlaid by the fact that all the members of the major facilitator superfamily are believed to be derived from a six-transmembrane-helix protein, the gene for which underwent an ancient tandem duplication (51, 61).
FIG. 7.
Intragenic suppression of VanK1113 (Gly71Asp). Transformation-facilitated PCR mutagenesis in ADP7587 (vanK1113 ΔpcaK859) generated two strains with second-site suppressors of VanK1113, a Gly71Asp substitution in VanK transmembrane helix 2. ADP7610 had two suppressors in transmembrane helix 7, Phe269Ser and Phe271Ser, and ADP7612 had one suppressor adjacent to transmembrane helix 8, Gly294Arg (Fig. 3).
Evidence that quinate is converted to protocatechuate in the periplasm.
In Acinetobacter strain ADP1, the hydroaromatic compound quinate (Fig. 1) is converted to protocatechuate by the sequential action of three enzymes (QuiA, QuiB, and QuiC) encoded by genes in the pca-qui-pob supraoperonic cluster (17, 18, 30, 35). The reactions catalyzed by the catabolic enzymes QuiB and QuiC can interfere with the same reactions in the opposite physiological direction, performed by enzymes in the shikimate pathway for aromatic amino acid biosynthesis (18) (Fig. 5). In fungi, channeling of intermediates in an aggregate of the shikimate pathway biosynthetic enzymes prevents access by catabolic enzymes during growth with quinate (29, 46). Evidence for physical segregation of the two classes of enzymes has also been described in Acinetobacter (18, 74). Further evidence presented here supports catabolism of quinate to protocatechuate taking place in the periplasm: pcaK vanK double mutants severely impaired in growth with protocatechuate because of their inability to transport this compound across the inner membrane into the cytoplasm also are severely impaired in growth with quinate, although quinate appears to be efficiently converted to extracellular protocatechuate (Fig. 6). Periplasmic localization of QuiABC is consistent with the amino acid sequence of QuiA (17), which identifies it as a member of a family of membrane-associated, PQQ-dependent dehydrogenases (16), as well as a putative leader peptide in the N terminus of QuiB (18) which could mediate its export out of the cytoplasm.
Spontaneous mutant derivatives of ADP500 resistant to the toxic accumulation of carboxymuconate during growth in the presence of quinate never contained mutations confined to qui genes and were always blocked at the level of protocatechuate degradation (19). This observation can now be explained by the ease with which protocatechuate accumulates extracellularly after its generation in the periplasm from quinate. This is particularly true in strains blocked in PcaK such as ADP500 (Fig. 6) but is probably also relevant to the wild-type strain growing in the more natural condition of multiple carbon sources: significant amounts of protocatechuate accumulated when Acinetobacter was provided with shikimate, another substrate of QuiA (Fig. 5), simultaneously with three other carbon sources (23). This accumulation may reach local extracellular concentrations that are toxic to some competing soil microorganisms (Fig. 6). On the other hand, this “overflow metabolism” may mediate mutually beneficial cross-feeding (42). Similarly, bacterial growth on aromatic compounds can lead to extracellular accumulation of β-ketoadipate (23, 41), a chemoattractant that has been suggested to mediate the formation of “catabolic consortia” (63) of soil bacteria. In this light, reversible inactivation of Acinetobacter vanK by mutation at the frameshift hot spot may underlie a switch in membrane permeability important for cell-cell interactions.
Membership of Acinetobacter QuiA in a family of PQQ-dependent membrane-bound dehydrogenases suggests a further link between oxidation of quinate, a compound ubiquitous in the soil environment (36), and active transport. The single step of oxidation of glucose to gluconate by a dehydrogenase closely related to QuiA has been shown in several organisms to generate a proton motive force which could efficiently power secondary transport systems (75). Conversion of quinate to dehydroquinate may likewise power transport by PcaK and VanK, facilitating scavenging of aromatic compounds from the environment even in the absence of growth. Such a role may explain why transcription of Acinetobacter quiA appears, at least in part, to be independently regulated (18). Also, as has been suggested for gluconate production from glucose in Pseudomonas (68), periplasmic oxidation of quinate by Acinetobacter cells may serve to rapidly remove this compound from access by competing soil microorganisms.
Overlapping specificity of the VanK and PcaK transport proteins.
The pca operon encodes the PcaK transport protein together with all the enzymes for conversion of protocatechuate to citric acid cycle intermediates (45). The role of PcaK in protocatechuate transport can be masked by the overlapping specificity of VanK, encoded by a gene distant on the chromosome from the pca-qui-pob supraoperonic cluster and part of a separate cluster of genes for conversion of vanillate to protocatechuate (69) (Fig. 2). Despite the location of vanK, no evidence indicating that the encoded protein transports vanillate has yet been found, although the activities of yet to be identified transporters may well mask such a role. The full spectrum of compounds that can be transported by PcaK and VanK remains to be elucidated.
The results of this study, consistent with studies of P. putida PcaK (34, 56), indicate that both VanK and PcaK can mediate uptake of protocatechuate and p-hydroxybenzoate: inactivation of both proteins severely impairs growth with protocatechuate and, in cells lacking PcaB function, prevents toxic accumulation of carboxymuconate during growth in the presence of low levels of p-hydroxybenzoate. This latter phenotype should allow the genetic dissection of transport of these two compounds by Acinetobacter PcaK. After transformation-facilitated PCR mutagenesis of chromosomal pcaK in ADP7593 (ΔvanK1103 ΔpcaBD1), recombinant cells selected for resistance to protocatechuate can be screened for lack of resistance to an appropriate concentration of p-hydroxybenzoate. This procedure should identify strains with a PcaK amino acid substitution in which only protocatechuate uptake is significantly impaired.
The requirement for either VanK or PcaK for efficient growth with protocatechuate may support the idea that transport systems play a fundamental role in regulating cytoplasmic levels of metabolites that are also inducers of gene expression (58, 59). This concept has been recently emphasized (2) following studies of aromatic catabolism which revealed linkage on the chromosome of a gene for a PcaK family member and a gene for a regulatory protein predicted to respond to the aromatic substrate of the transporter. In this light, the apparent redundancy of VanK and PcaK is consistent with the need to concentrate the same inducer-metabolite in two spatially segregated cellular compartments. The most likely candidate for this inducer is protocatechuate, given that it activates pca operon expression (26, 44) and, although it awaits determination in Acinetobacter, it also appears to induce expression of vanA and vanB in Pseudomonas (76).
Facilitating the channeling of protocatechuate after it enters the cytoplasm, the gene clusters containing vanK and pcaK may become associated with the cytoplasmic membrane by coupling of transcription of these genes with translation and membrane insertion of the encoded proteins (49, 50). The substrate specificity of the transport protein encoded in each gene cluster would thereby become pivotal: capable of either preventing cross talk between biochemical pathways or conversely, of efficiently mediating such interactions (66). A more general role in the β-ketoadipate pathway of metabolic channeling by enzyme complexes (21, 53, 60, 71) has been suggested to underly the supraoperonic clustering (23) of genes in Acinetobacter as well as an explanation of the different effects caused by an inducer added extracellularly versus made endogenously as described for both Acinetobacter (23) and Pseudomonas (54).
A previous study of Acinetobacter examined the genetic flexibility of two closely related transcriptional activators associated with protocatechuate catabolism and found that no more than two amino acid substitutions were required for each protein to functionally replace its homolog (44). Similarly, only one amino acid substitution was required for protocatechuate dioxygenase to functionally replace catechol dioxygenase (12). This study outlines a genetic system for studying the contribution to protocatechuate catabolism of two homologous transport proteins, VanK and PcaK, already overlapping in specificity. Recognition of this overlap is particularly important given that the activity of the LacY permease can generate nongenetic heterogeneity of lac operon expression in E. coli cells exposed to suboptimal levels of inducer (57, 73). While this phenomenon provides yet another source of variability for bacteria to deal with environmental change (73), it is likely to complicate laboratory measurements of transport activity, gene expression, and growth rates in Acinetobacter if the activities of both VanK and PcaK are not taken into account. Genetic analysis of these two permeases in Acinetobacter also may complement characterization of P. putida PcaK, which has an additional role in chemotaxis (34, 56). Such a comparative approach may reveal new properties of aromatic acid transport systems, such as a spatial organization in the membrane (4, 70), and should be particularly useful in elucidating the molecular biology of self-identity (35).
ACKNOWLEDGMENTS
This research was supported by grants DAAG55-98-1-0232 from the Army Research Office and MCB-9603980 from the National Science Foundation. A.S. was supported by a postdoctoral fellowship from the Spanish Ministerio de Educación y Ciencia.
The overlapping specificity of VanK and PcaK was recognized thanks to the faithful recording of anomalous phenotypes of ADP500 derivatives by D. Elsemore, D. Eulberg, T. Plaggemeier, and P. West. Discussion with E. P. Greenberg prompted consideration of possible additional roles played by the membrane-associated QuiA dehydrogenase. The plate photograph was taken by W. Sacco.
Footnotes
Publication 20 from the Biological Transformation Center in the Yale Biospherics Institute.
REFERENCES
- 1.Altschul S F, Madden T L, Schäfer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes M R, Duetz W A, Williams P A. A 3-(3-hydroxyphenyl)propionic acid catabolic pathway in Rhodococcus globerulus PWD1: cloning and characterization of the hpp operon. J Bacteriol. 1997;179:6145–6153. doi: 10.1128/jb.179.19.6145-6153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson S A, DeCloux A M, Munro J. Mutant bias in nonlethal selections results from selective recovery of mutants. Genetics. 1991;129:647–658. doi: 10.1093/genetics/129.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray D, Levin M D, Morton-Firth C J. Receptor clustering as a cellular mechanism to control sensitivity. Nature. 1998;393:85–88. doi: 10.1038/30018. [DOI] [PubMed] [Google Scholar]
- 5.Bundy B M, Campbell A L, Neidle E L. Similarities between the antABC-encoded anthranilate dioxygenase and the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADP1. J Bacteriol. 1998;180:4466–4474. doi: 10.1128/jb.180.17.4466-4474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll P A, Tashima K T, Rogers M B, DiRita V J, Calderwood S B. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol Microbiol. 1997;25:1099–1111. doi: 10.1046/j.1365-2958.1997.5371901.x. [DOI] [PubMed] [Google Scholar]
- 7.Clayton R A, White O, Ketchum K A, Venter J C. The first genome from the third domain of life. Nature. 1997;387:459–462. doi: 10.1038/387459a0. [DOI] [PubMed] [Google Scholar]
- 8.Coco W M, Ornston L N. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Acinetobacter PcaK is a multispecificity active facilitator of aromatic acid transport, abstr. K-81; p. 355. [Google Scholar]
- 9.Collier L S, Nichols N N, Neidle E L. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J Bacteriol. 1997;179:5943–5946. doi: 10.1128/jb.179.18.5943-5946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danaher R J, Levin J C, Arking D, Burch C L, Sandlin R, Stein D C. Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation. J Bacteriol. 1995;177:7275–7279. doi: 10.1128/jb.177.24.7275-7279.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Argenio, D. A., A. Segura, P. V. Bünz, and L. N. Ornston. Unpublished data.
- 12.D’Argenio, D. A., M. W. Vetting, D. H. Ohlendorf, and L. N. Ornston. Unpublished data.
- 13.DiMarco A A, Averhoff B A, Kim E E, Ornston L N. Evolutionary divergence of pobA, the structural gene for p-hydroxybenzoate hydroxylase in an Acinetobacter calcoaceticus strain well-suited for genetic analysis. Gene. 1993;125:25–33. doi: 10.1016/0378-1119(93)90741-k. [DOI] [PubMed] [Google Scholar]
- 14.DiMarco A A, Averhoff B, Ornston L N. Identification of the transcriptional activator pobR and characterization of its role in the expression of pobA, the structural gene for p-hydroxybenzoate hydroxylase in Acinetobacter calcoaceticus. J Bacteriol. 1993;175:4499–4506. doi: 10.1128/jb.175.14.4499-4506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiRita V J, Mekalanos J J. Genetic regulation of bacterial virulence. Annu Rev Genet. 1989;23:455–482. doi: 10.1146/annurev.ge.23.120189.002323. [DOI] [PubMed] [Google Scholar]
- 16.Duine J A. Quinoproteins: enzymes containing the quinoid cofactor pyrroloquinoline quinone, topaquinone or tryptophan-tryptophan quinone. Eur J Biochem. 1991;200:271–284. doi: 10.1111/j.1432-1033.1991.tb16183.x. [DOI] [PubMed] [Google Scholar]
- 17.Elsemore D A, Ornston L N. The pca-pob supraoperonic cluster of Acinetobacter calcoaceticus contains quiA, the structural gene for quinate-shikimate dehydrogenase. J Bacteriol. 1994;176:7659–7666. doi: 10.1128/jb.176.24.7659-7666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsemore D A, Ornston L N. Unusual ancestry of dehydratases associated with quinate catabolism in Acinetobacter calcoaceticus. J Bacteriol. 1995;177:5971–5978. doi: 10.1128/jb.177.20.5971-5978.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsemore, D. A., P. West, and L. N. Ornston. Personal communication.
- 20.Ferrández A, García J L, Díaz E. Genetic characterization and expression in heterologous hosts of the 3-(3-hydroxyphenyl)propionate catabolic pathway of Escherichia coli K-12. J Bacteriol. 1997;179:2573–2581. doi: 10.1128/jb.179.8.2573-2581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraenkel D G. Genetics and intermediary metabolism. Annu Rev Genet. 1992;26:159–177. doi: 10.1146/annurev.ge.26.120192.001111. [DOI] [PubMed] [Google Scholar]
- 22.Fuqua W C. An improved chloramphenicol resistance gene cassette for site-directed marker replacement mutagenesis. BioTechniques. 1992;12:223–225. [PubMed] [Google Scholar]
- 23.Gaines G L, III, Smith L, Neidle E L. Novel nuclear magnetic resonance spectroscopy methods demonstrate preferential carbon source utilization by Acinetobacter calcoaceticus. J Bacteriol. 1996;178:6833–6841. doi: 10.1128/jb.178.23.6833-6841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galas J G, Chandler M. Edited by D. E. Berg and M. M. Howe (ed.), Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. Bacterial insertion sequences; pp. 109–162. [Google Scholar]
- 25.Galitski T, Roth J R. A search for a general phenomenon of adaptive mutability. Genetics. 1996;143:645–659. doi: 10.1093/genetics/143.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerischer U, Segura A, Ornston L N. PcaU, transcriptional activator of genes for protocatechuate utilization in Acinetobacter. J Bacteriol. 1997;180:1512–1524. doi: 10.1128/jb.180.6.1512-1524.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerischer U, D’Argenio D A, Ornston L N. IS1236, a newly discovered member of the IS3 family, exhibits varied patterns of insertion into the Acinetobacter calcoaceticus chromosome. Microbiology. 1996;142:1825–1831. doi: 10.1099/13500872-142-7-1825. [DOI] [PubMed] [Google Scholar]
- 28.Gerischer U, Ornston L N. Spontaneous mutations in pcaH and -G, structural genes for protocatechuate 3,4-dioxygenase in Acinetobacter calcoaceticus. J Bacteriol. 1995;177:1336–1347. doi: 10.1128/jb.177.5.1336-1347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giles N H, Partridge C W H, Ahmed S I, Case M E. The occurrence of two dehydroquinases in Neurospora crassa, one constitutive and one inducible. Proc Natl Acad Sci USA. 1967;58:1930–1937. doi: 10.1073/pnas.58.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gralton E M, Campbell A L, Neidle E L. Directed introduction of DNA cleavage sites to produce a high-resolution genetic and physical map of the Acinetobacter sp. strain ADP1 (BD413) chromosome. Microbiology. 1997;143:1345–1357. doi: 10.1099/00221287-143-4-1345. [DOI] [PubMed] [Google Scholar]
- 31.Hartnett C, Neidle E L, Ngai K-L, Ornston L N. DNA sequence of genes encoding Acinetobacter calcoaceticus protocatechuate 3,4-dioxygenase: evidence indicating shuffling of genes and of DNA sequences within genes during their evolutionary divergence. J Bacteriol. 1990;172:956–966. doi: 10.1128/jb.172.2.956-966.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartnett G B. Ph.D. thesis. New Haven, Conn: Yale University; 1993. [Google Scholar]
- 33.Hartnett G B, Averhoff B, Ornston L N. Selection of Acinetobacter calcoaceticus mutants deficient in the p-hydroxybenzoate hydroxylase gene (pobA), a member of a supraoperonic cluster. J Bacteriol. 1990;172:6160–6161. doi: 10.1128/jb.172.10.6160-6161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harwood C S, Nichols N N, Kim M-K, Ditty J L, Parales R E. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176:6479–6488. doi: 10.1128/jb.176.21.6479-6488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harwood C S, Parales R E. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins A R, Lamb H K, Moore J D, Charles I G, Roberts C F. The pre-chorismate (shikimate) and quinate pathways in filamentous fungi: theoretical and practical aspects. J Gen Microbiol. 1993;139:2891–2899. doi: 10.1099/00221287-139-12-2891. [DOI] [PubMed] [Google Scholar]
- 37.Jessen-Marshall A E, Parker N J, Brooker R J. Suppressor analysis of mutations in the loop 2-3 motif of lactose permease: evidence that glycine-64 is an important residue for conformational changes. J Bacteriol. 1997;179:2616–2622. doi: 10.1128/jb.179.8.2616-2622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonsson A-B, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juni E, Janick A. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaback H R. A molecular mechanism for energy coupling in a membrane transport protein, the lactose permease of Escherichia coli. Proc Natl Acad Sci USA. 1997;94:5539–5543. doi: 10.1073/pnas.94.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilby B A. The formation of β-ketoadipic acid by bacterial fission of aromatic rings. Biochem J. 1951;49:671–674. doi: 10.1042/bj0490671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch A L. Microbial physiology and ecology of slow growth. Microbiol Mol Biol Rev. 1997;61:305–318. doi: 10.1128/mmbr.61.3.305-318.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kok R G, D’Argenio D A, Ornston L N. Combining localized PCR mutagenesis and natural transformation in direct genetic analysis of a transcriptional regulator gene, pobR. J Bacteriol. 1997;179:4270–4276. doi: 10.1128/jb.179.13.4270-4276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kok R G, D’Argenio D A, Ornston L N. Mutation analysis of PobR and PcaU, closely related transcriptional activators in Acinetobacter. J Bacteriol. 1998;180:5058–5069. doi: 10.1128/jb.180.19.5058-5069.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowalchuk G A, Hartnett G B, Benson A, Houghton J E, Ngai K-L, Ornston L N. Contrasting patterns of evolutionary divergence within the Acinetobacter calcoaceticus pca operon. Gene. 1994;146:23–30. doi: 10.1016/0378-1119(94)90829-x. [DOI] [PubMed] [Google Scholar]
- 46.Lamb H K, van den Hombergh J P T W, Newton G H, Moore J D, Roberts C F, Hawkins A R. Differential flux through the quinate and shikimate pathways. Implications for the channeling hypothesis. Biochem J. 1992;284:181–187. doi: 10.1042/bj2840181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenski R E, Slatkin M, Ayala F J. Mutation and selection in bacterial populations: alternatives to the hypothesis of directed mutation. Proc Natl Acad Sci USA. 1989;86:2775–2778. doi: 10.1073/pnas.86.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leveau J H, Zehnder A J B, van der Meer J R. The tfdK gene product facilitates uptake of 2,4-dichlorophenoxyacetate by Ralstonia eutropha JMP134(pJP4) J Bacteriol. 1998;180:2237–2243. doi: 10.1128/jb.180.8.2237-2243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lodge J K, Kazic T, Berg D E. Formation of supercoiling domains in plasmid pBR322. J Bacteriol. 1989;171:2181–2187. doi: 10.1128/jb.171.4.2181-2187.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch A S, Wang J C. Anchoring of DNA to the bacterial cytoplasmic membrane through cotranscriptional synthesis of polypeptides encoding membrane proteins or proteins for export: a mechanism of plasmid hypernegative supercoiling in mutants deficient in DNA topoisomerase I. J Bacteriol. 1993;175:1645–1655. doi: 10.1128/jb.175.6.1645-1655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maiden M C J, Davis E O, Baldwin S A, Moore D C M, Henderson P J F. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987;325:641–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- 52.Marger M D, Saier M H., Jr A major superfamily of transmembrane facilitators that catalyze uniport, symport, and antiport. Trends Biochem Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 53.Mathews C K. The cell-bag of enzymes or network of channels? J Bacteriol. 1993;175:6377–6381. doi: 10.1128/jb.175.20.6377-6381.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meagher R B, McCorkle G M, Ornston M K, Ornston L N. Inducible uptake system for β-carboxy-cis,cis-muconate in a permeability mutant of Pseudomonas putida. J Bacteriol. 1972;111:465–473. doi: 10.1128/jb.111.2.465-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moxon E R, Rainey P B, Nowak M A, Lenski R E. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr Biol. 1994;4:24–33. doi: 10.1016/s0960-9822(00)00005-1. [DOI] [PubMed] [Google Scholar]
- 56.Nichols N N, Harwood C S. PcaK, a high-affinity permease for the aromatic compounds 4-hydroxybenzoate and protocatechuate from Pseudomonas putida. J Bacteriol. 1997;179:5056–5061. doi: 10.1128/jb.179.16.5056-5061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novick A, Weiner M. Enzyme induction as an all-or-none phenomenon. Proc Natl Acad Sci USA. 1957;43:553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ondrako J M, Ornston L N. Biological distribution and physiological role of the β-ketoadipate transport system. J Gen Microbiol. 1980;120:199–209. doi: 10.1099/00221287-120-1-199. [DOI] [PubMed] [Google Scholar]
- 59.Ornston L N, Parke D. The evolution of induction mechanisms in bacteria: insights derived from the study of the β-ketoadipate pathway. Curr Top Cell Regul. 1977;12:209–262. doi: 10.1016/b978-0-12-152812-6.50011-1. [DOI] [PubMed] [Google Scholar]
- 60.Ovádi J. Physiological significance of metabolic channeling. J Theor Biol. 1991;152:1–22. [PubMed] [Google Scholar]
- 61.Pao S S, Paulsen I T, Saier M H., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parke D, Ornston L N. Nutritional diversity of Rhizobiaceae revealed by auxanography. J Gen Microbiol. 1984;130:1743–1750. [Google Scholar]
- 63.Parke D, Rivelli M, Ornston L N. Chemotaxis to aromatic and hydroaromatic acids: comparison of Bradyrhizobium japonicum and Rhizobium trifoli. J Bacteriol. 1985;163:417–422. doi: 10.1128/jb.163.2.417-422.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paulsen I T, Sliwinski M K, Saier M H., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 65.Plaggemeier, T., and D. Eulberg. Unpublished data.
- 66.Reid C J, Poole P S. Roles of DctA and DctB in signal detection by the dicarboxylic acid transport system of Rhizobium leguminosarum. J Bacteriol. 1998;180:2660–2669. doi: 10.1128/jb.180.10.2660-2669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robertson B D, Meyer T F. Genetic variation in pathogenic bacteria. Trends Genet. 1992;8:422–427. doi: 10.1016/0168-9525(92)90325-x. [DOI] [PubMed] [Google Scholar]
- 68.Schleissner C, Reglero A, Luengo J M. Catabolism of d-glucose by Pseudomonas putida U occurs via extracellular transformation into d-gluconic acid and induction of a specific gluconate transport system. Microbiology. 1997;143:1595–1603. doi: 10.1099/00221287-143-5-1595. [DOI] [PubMed] [Google Scholar]
- 69.Segura A, Bünz P V, D’Argenio D A, Ornston L N. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J Bacteriol. 1999;181:3494–3504. doi: 10.1128/jb.181.11.3494-3504.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shapiro L. Protein localization and asymmetry in the bacterial cell. Cell. 1993;73:841–855. doi: 10.1016/0092-8674(93)90266-s. [DOI] [PubMed] [Google Scholar]
- 71.Srere P A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- 72.Theiss P, Wise K S. Localized frameshift mutation generates selective, high-frequency phase variation of a surface lipoprotein encoded by a Mycoplasma ABC transporter operon. J Bacteriol. 1997;179:4013–4022. doi: 10.1128/jb.179.12.4013-4022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tolker-Nielsen T, HolmstrØm K, Boe L, Molin S. Non-genetic population heterogeneity studied by in situ polymerase chain reaction. Mol Microbiol. 1998;27:1099–1105. doi: 10.1046/j.1365-2958.1998.00760.x. [DOI] [PubMed] [Google Scholar]
- 74.Tresguerres M E F, Ingledew W M, Cánovas J L. Potential competition for 5-dehydroshikimate between the aromatic biosynthetic route and the catabolic hydroaromatic pathway. Arch Mikrobiol. 1972;82:111–119. [Google Scholar]
- 75.van Schie B J, Hellingwerf K J, van Dijken J P, Elferink M G L, van Dijl J M, Kuenen J G, Konings W N. Energy transduction by electron transfer via a pyrrolo-quinoline quinone-dependent glucose dehydrogenase in Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter calcoaceticus (var. lwoffi) J Bacteriol. 1985;163:493–499. doi: 10.1128/jb.163.2.493-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Venturi V, Zennaro F, Degrassi G, Okeke B C, Bruschi C V. Genetics of ferulic acid bioconversion to protocatechuic acid in plant-growth-promoting Pseudomonas putida WCS358. Microbiology. 1998;144:965–973. doi: 10.1099/00221287-144-4-965. [DOI] [PubMed] [Google Scholar]
- 77.Williams P A, Shaw L E. mucK, a gene in Acinetobacter calcoaceticus ADP1 (BD413), encodes the ability to grow on exogenous cis,cis-muconate as the sole carbon source. J Bacteriol. 1997;179:5935–5942. doi: 10.1128/jb.179.18.5935-5942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]