Abstract
Chinese baijiu brewing is an open, complex, and synergetic functional microbiota fermentation process. Microbial interaction is pivotal for the regulation of microbial structure and function in the brewing microecosystem, consequently affecting the flavor and quality of baijiu. This article mainly summarizes the effect of microbial interactions among functional microbiota on the growth performance, flavor formation, and safe quality of baijiu fermentation process. In addition, the review specifically emphasizes on the microbial interactions for the regulation of “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing” in Chinese strong-flavor baijiu. Furthermore, the construction of synthetic microbiota by metabolic characteristics of the functional microbes and their interactions for regulating and controlling flavor quality of Chinese baijiu is also reviewed and prospected.
Keywords: Chinese baijiu, flavor quality, microbial interaction, synergistic fermentation, synthetic microbiota
Introduction
Chinese baijiu, one of the well-known traditional fermented foods, possesses strong ethnic characteristics in Chinese culture and industrial advantages in the national economy. For example, owing to its unique flavor and aroma, the output and revenue of Chinese baijiu achieved 7.1 billion liters and 583.6 billion RMB in 2020, respectively, reaching a total profit of 131.2 billion RMB. The distinctive flavor and taste of Chinese baijiu is attributed to the composition and proportion of multifarious flavor compounds. Typically, four organic acids (acetic acid, lactic acid, butanoic acid, and hexanoic acid) and their corresponding ethyl esters, especially, caproic acid and ethyl caproate, have been confirmed to be dominant compounds and important contributors to the characteristic flavor of strong-flavor baijiu (1, 2). In fact, 12 flavor types of Chinese baijiu contain more than 1,870 flavor compounds, namely, acids, alcohols, esters, ketones, aldehydes, aromatics, nitrogenous compounds, terpenes, and sulfur compounds (3). In addition, the potential functional component in Chinese baijiu was also uncovered in recent years. For instance, a tetrapeptide (Ala-Lys-Arg-Ala) had been successfully identified in sesame-flavor baijiu and exhibited preventive effects against 2,2-Azobis (2-methyl-propanimidamidine) dihydrochloride-induced oxidative stress in HepG2 cells (4).
The formation of these flavor and functional compounds is extremely complicated and can be fluctuated mainly by dynamic succession of functional microbiota during the fermentation process. These functional microbes are supposed responsible for the production of flavor compounds by their extensive interactions. For example, the flavor compounds, namely, fatty acids, esters, terpenes, and aromatic compounds produced by Saccharomyces cerevisiae were correlated with the mixing ratio of Bacillus licheniformis in Chinese maotai−flavor baijiu fermentation (5). Moreover, the microbial interaction is a crucial factor for maintaining the co-occurring in microbiota structure, which will influence the microbial metabolism and flavor formation during the wine fermentation (6). Therefore, revealing the mechanism of the microbial interactions on flavor metabolism is important for regulation of Chinese baijiu fermentation. Based on this, some related studies have already been focused on the microbial interactions and how to achieve the targeted regulation by these interactions in baijiu fermentation (7, 8).
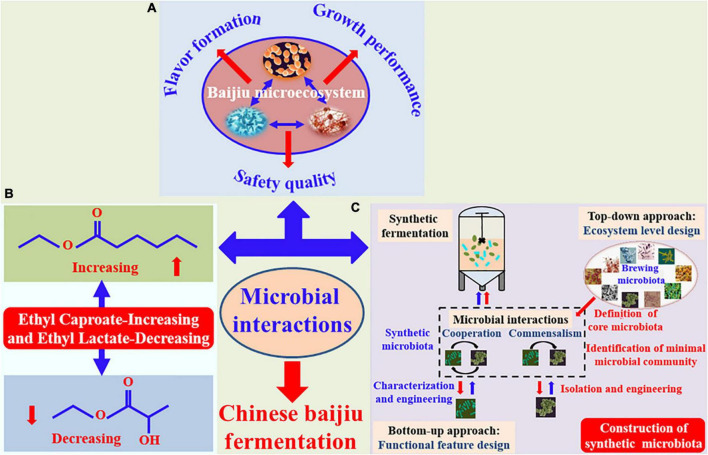
In this review, recent researches relating to the effect of microbial interactions on growth metabolism, flavor formation, and safe quality in Chinese baijiu fermentation (Figure 1A), especially for regulation of “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing” in strong-flavor baijiu (Figure 1B) are summarized and discussed. Furthermore, the construction of synthetic microbiota by considering the metabolic features of functional microbes and their interactions (Figure 1C), is also described and prospected in the regulation of flavor quality for Chinese baijiu fermentation.
FIGURE 1.
Effect of microbial interactions on regulation of growth and flavor metabolism (A) and “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing” (B), as well as construction of synthetic microbiota (“top-down” approach was red arrows, and “bottom-up” approach was blue arrows) (C) in Chinese baijiu fermentation process.
Interactions among functional microbes
Chinese baijiu is produced by the traditional spontaneous solid-state fermentation process containing various functional microbes and their complex interactions (9). In general, microbial interactions are mainly classified through ecological typing into competition, mutualism (cooperation), commensalism, amensalism, or parasitism, and these interactions can be regulated by modifying the metabolic pathway, intercellular communication, and spatial structures, thereby accomplishing the specific functions (10). In fact, there are mainly synergistic (cooperation) and antagonistic (competition) effects involved in the microbial interactions in the baijiu brewing microecosystem. Here, we describe the effect of interactions among functional microbes on the growth performance, flavor formation, and safety characteristic (Figure 1A) in the baijiu fermentation process.
Effect of microbial interactions on growth performance
In fact, microbial interactions are usually deemed as cooperative networks with functional microbiota working together toward an ultimate goal during the baijiu-brewing process. This implicates that the cooperation and interaction can influence growth performance and even metabolic activity of the microbial consortia. For example, the biomass of S. cerevisiae increased when it was co-cultured with Aspergillus oryzae at the ratios of 1:0.1, 1:0.5, and 1:1, and this was attributed to providing more glucose for S. cerevisiae growth by inducing enzyme system of starch hydrolyzation in A. oryzae (11). This means that the metabolites produced by microbe have synergistic or antagonistic effect on other microbes, also known as metabolite regulation mechanism of the microbial interactions. But, on the contrary, the antagonistic interaction between both species was also uncovered. The growth and biomass of S. cerevisiae and A. oryzae were inhibited in the mixed culture system, but the protein synthesis for the cell wall of S. cerevisiae was significantly promoted (12). In addition, the occurrence and effectiveness of synergistic interactions within functional microbiota are affected easily and restricted by the environmental factors in the natural succession process (13). For instance, the growth of Zygosaccharomyces bailii was normal at 30°C, but was inhibited at 37°C in the co-culture system with B. licheniformis for Chinese maotai-flavor baijiu fermentation (14). This is mainly related to the stress mechanism of fermentation environment, that is, the growth and survival of brewing microbiota are declined under multiple environmental stresses, namely, alcoholic, acidic, thermal, and oxidative during baijiu production.
Most notably, the antagonistic effect between non-Saccharomyces yeasts and S. cerevisiae is essential for Chinese baijiu brewing. Many researches have demonstrated that S. cerevisiae could inhibit the growth of non-Saccharomyces such as Z. bailii (15), Wickerhamomyces anomalus (16), and Issatchenkia orientalis (17) when they were co-cultured. These interactions might be resulted from the non-specific competition for nutrients among yeasts (18) and the inhibition of metabolites (such as ethanol) produced by S. cerevisiae (19). So, microbial interactions inevitably influence the growth performance of brewing microbiome during the baijiu fermentation process, consequently altering the metabolic activity and even the flavor formation of the final products.
Effect of microbial interactions on flavor formation
There are multifarious strategies for improving the flavor quality of traditional fermented foods (20, 21), and the core point of strategy is regulation of the microbial community and their interactions in the fermentation process. For example, the contents of caproic acid and ethyl caproate were improved in strong-flavor baijiu microecosystem by increasing the abundances of caproic acid bacteria and methanogens and also the hydrogen transfer interaction among them with fortified Daqu fermentation (22). Interestingly, although the growth performances of functional strains are repressed, their activities of flavor metabolism are not weakened in the co-culture fermentation system. For instance, when Z. bailii and B. licheniformis were coexisted, the growth of B. licheniformis was significantly inhibited, but the genes, namely, GAPDH, PGM1, ENO1, PDC1, COX1, and MEP2 involved in glycolysis, Ehrlich, and oxidative phosphorylation pathways in Z. bailii were upregulated, thereby producing more alcohols, acids, esters, and aldehydes in co-culture (15). Actually, the inhibition of growth but promotion of flavor metabolism activity in the co-culture system with non-Saccharomyces yeasts and S. cerevisiae is ubiquitous in the baijiu fermentation process (15–17). For instance, compared with the single culture of W. anomalus, higher yield of ethyl acetate was observed when S. cerevisiae and W. anomalus were co-cultured (16). This result indicated that the higher content of ethyl acetate could be attributed to synergy between non-Saccharomyces yeast and S. cerevisiae in co-culture (23). S. cerevisiae could produce acetic acid and ethanol, which were critical for W. anomalus in generating ethyl acetate in co-culture synergistic fermentation.
Besides aforementioned, some microbes have really poor ability to produce flavor compounds in the fermentation process, but they can coordinate the metabolic activity with those flavor producers. For example, Bacillus amyloliquefaciens and Pichia membranaefaciens were not effective flavor producers, but they could improve the flavor compounds with S. cerevisiae, I. orientalis, and B. licheniformis co-cultured in the sesame-flavor baijiu fermentation (17). In examples like this, the synergetic interactions between yeasts and lactic acid bacteria are the most widespread in the fermented alcoholic beverages (24). For instance, the content of ethyl lactate was significantly increased when co-cultured with P. membranifaciens and Lactobacillus acetotolerans in the strong-flavor baijiu fermentation compared with the mono-culture of P. membranifaciens (25). In addition, He et al. (26) reported that improvement of esters production and fruity flavor of strong-flavor baijiu was observed when fermented with the fortified Daqu in the brewing process, in which the synergistic interaction between Lactobacillus and Candida was considered to be an important driving factor. Another example of synergistic effect on higher production of 3-(methylthio)-1-propanol and dimethyl disulfide was also obtained by co-culturing S. cerevisiae with Lactobacillus buchneri in baijiu fermentation (27). This synergy mechanism between S. cerevisiae and L. buchneri was revealed by transcriptome analysis. S. cerevisiae upregulated expression of genes for generation of 3-(methylthio)-1-propanol and dimethyl disulfide in the presence of L. buchneri, which can regenerate the precursors methionine and S-adenosylmethionine by methyl recycle (27). On the contrary, yeast and lactic acid bacteria could provide nutrients to each other, and promote the growth metabolism of them (28).
Effect of microbial interactions on safe quality
Although the traditional baijiu brewing has been produced for thousands of years, traditional hand-making in an open and complex brewing environment without strict control leads to low production, inconsistent quality, and even security risk (29). Based on the security of baijiu, current researches mainly pay attentions to the regulation and reduction of ethyl carbamate (EC), a class 2A carcinogen (30). For example, there are reports already that the concentrations of EC and its precursor cyanide effectively decreased in raw baijiu by pot still second distillation process (31, 32). Unfortunately, the flavor and quality of baijiu would be affected in part by altering the production process to eliminate the EC content. So, some researchers have focused on the microbial intervention methods for removing the EC, especially for microbial interactions (33, 34). For instance, low amounts of urea (the precursor of EC) was produced by Lactobacillus species with non-conventional yeasts, namely, Pichia, Schizosaccharomyces, and Zygosaccharomyces species co-fermentation in Chinese maotai-flavor baijiu (35). Moreover, Fang et al. (36) reported that EC generation by S. cerevisiae was significantly inhibited in the co-culture with Lactobacillus brevis and provided valuable insights into the molecular mechanism of EC formation by transcriptomic analysis.
Besides EC, some odor compounds were also detected in the baijiu fermentation process, thereby affecting the flavor and safety quality. For example, the compound p-cresol is the major off-odor and toxic component of strong-flavor baijiu (37). Another research indicated that Clostridium was the primary microbial source for p-cresol production and the formation of p-cresol could be inhibited by increasing the proportions of Lactobacillus (38). In particular, the concentration of p-cresol in sesame-flavored baijiu is decreased by hydrogen bond interactions with the non-volatile tetrapeptide Ala-Lys-Arg-Ala (39). Taken together, these studies inspire that intensification of interspecies interactions between Clostridium with relevant Lactobacillus or Ala-Lys-Arg-Ala-producing strains is a possible strategy for eliminating the p-cresol in baijiu fermentation.
Regulation of “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing” by microbial interactions
At present, one of the tricky problems for many Chinese strong-flavor baijiu production enterprises is how to decrease lactic acid and increase caproic acid accumulation during the fermentation process (40). This technical challenge inevitably results in weakness of the body aroma by ethyl caproate, thinness of flavor and texture, and shortness of aftertaste, which consequently destroys the typical style of strong-flavor baijiu. To address this problem, many researches have been devoted to regulate and control the process parameters during the fermentation, such as scientific construction of fermentation cellar, optimization of pit mud, improvement the quality of fermentation starter, and regulation of the conditions for pit entry (41). However, these works focused mainly on uncovering the correlations between the lactic acid and caproic acid contents with the technical parameters in the baijiu fermentation process. The vital information related to the alteration of functional microbiota caused by these process parameters for regulation of “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing” is still fragmented.
According to this, some researchers have paid attentions on microbial interactions for regulation of “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing” (Figure 1B) in these years. For instance, the co-culture fermentation broth with Clostridium kluyveri and Methanogen was subjected to strong-flavor baijiu pit-entry fermentation, the concentration of caproic acid and ethyl caproate in the raw baijiu, respectively, increased by 114.7 and 142.8%, while that of ethyl lactate decreased by 64.1% (42). Interestingly, recent study indicated the relative abundance of caproic acid bacteria significantly increased after 15-day fed-batch fermentation with lactate as carbon sources, which meaned that the brewing microbiota exhibited a regular and directional evolutionary pattern for effectively achieving “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing” (43). However, in general, it is difficult to control the natural evolution of microbiota and easily fluctuated by environmental factors and process parameters.
Thus, it is of urgent need to develop feasible strategy for directionally regulating fermentation and accelerate the enrichment of functional microbiota. Most notably, an effective regulation approach is the interspecies hydrogen transfer interactions between the hexanoic acid producers and methanogenic archaea (44). The synergistic interaction between caproic acid bacteria and methanogens is extensively existed in baijiu-brewing microecosystem, which is conducive to maintain the stability of the microbial succession and also produce more caproic acid and ethyl caproate (42, 45, 46). In addition, considering the negative correlations between Lactobacillus and Bacillus reported by many researches, He et al. (22) performed a novel strategy for regulating strong-flavor baijiu fermentation by directional bioturbation with fortified Daqu (inoculation of Bacillus subtilis and Bacillus velezensis). The results demonstrated that the bioturbation by fortified Daqu was feasible for “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing” by interspecies interactions of functional microbiota, namely, Bacillus, Lactobacillus, Caproiciproducens, Clostridium, Candida, Aspergillus, Methanobacterium, and Methanosarcina.
Construction of synthetic microbiota by the microbial interactions
Synthetic microbiota, constructed artificially by co-culturing of multiple species with well-defined genetic background and specific functions, is provided with low complexity, high controllability, and stability (47, 48). In recent years, some researchers have focused on how to transform to the synthetic fermentation from spontaneous fermentation for regulation of microbial metabolism and production of high-quality foods. For example, Acetobacter pasteurianus, Lactobacillus brevis, and Lactobacillus fermentum were co-cultured and constructed an acetoin-producing synthetic community, when it was applied in the traditional vinegar fermentation, the content of acetoin in vinegar increased significantly (49). In addition, it was reported that a tractable microbiota system was constructed by 24 widely distributed and culturable genera and conducted to the cheese rind fermentation (50). These studies provide references for synthetic microbiota in the different fermented foods. It means that the manipulation and repeatability of microbial succession and dynamic can be obtained in vitro. This also affords an opportunity to construct a synthetic microbiota system for food fermentation with prospective flavor and quality.
However, Chinese baijiu brewing is an open, complex, and synergetic of the functional microbiota fermentation process. It seems arduous to construct the synthetic microbiota for regulation and control of the flavor compounds formation in such a microecosystem. Fortunately, the methods of synthetic biology and microbiome make it possible by identification and isolation of the core microbiota with the development of modern biotechnology (51). For the construction of synthetic microbiota, revealing the phylogeny, metabolic functions, especially interspecies interaction of the selected strains are of paramount importance (52). Synthetic microbiota is generally constructed according to the top–down or bottom–up approach (Figure 1C), and the microbial interactions play an important role in the construction process by both the approaches (53).
For example, although the growth performances of S. cerevisiae, P. membranaefaciens, I. orientalis, B. licheniformis, and B. amyloliquefaciens were inhibited by each other, the co-culture of these five species could coordinate the metabolic activities by their interactions and produce the largest amount of flavor compounds in the Chinese sesame-flavor baijiu (17). For another example, the microbial interaction was analyzed by co-occurring network and the synthetic microbiota composed by L. acetotolerans, Pichia kudriavzevii, Geotrichum candidum, Candida vini, and S. cerevisiae was constructed, 77.27% of the flavor compounds produced by the synthetic microbiota exhibited a similar dynamic profile with that in situ system, and the flavor profile presented a similar composition (54). In recent, the core microbiota, namely, Lactobacillus, Thermoactinomyces, Aquabacterium, Aspergillus, and Kazachstania was identified in the fermented grains of Chinese strong-flavor baijiu by finding ubiquitous, dominant, flavor associated, and co-occurring microbiota together (55). And, the results lay the foundation of construction the synthetic microbiota for regulating the baijiu fermentation process and achieving the homogenization of product quality. Therefore, once we can understand the interaction mechanism of functional microbiota for constructing the synthetic microbiota, we will be able to regulate and control the fermentation process for production of high-quality baijiu.
Conclusion
Chinese baijiu is produced by the spontaneous solid-state fermentation process involved in the multifarious microbes and their extensive and complex interactions. These interactions are propitious to flavor and safety improvement, such as “Ethyl Caproate-Increasing and Ethyl Lactate-Decreasing,” and to stability enhancement of baijiu-brewing microecosystem. Moreover, revealing the mechanism of microbial interactions is beneficial for rational construction of synthetic microbiota, and achieving the directional regulation for flavor quality in Chinese baijiu fermentation process.
Author contributions
LG performed the literature search and wrote the manuscript. JZ performed the literature search and contributed to the manuscript revision. GH contributed to the literature summary, manuscript revision, and overall support of this work. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Southwest University of Science and Technology for supporting this work.
Funding
This work was funded by the Sichuan Province Key Research and Development Project (2021YFS0340), Doctoral Scientific Fund Project of Southwest University of Science and Technology (20zx7130), and Open Fund of Key Laboratory in Luzhou (NJ202201).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Fan W, Qian M. Characterization of aroma compounds of Chinese “Wuliangye” and “jiannanchun” liquors by aroma extract dilution analysis. J Agric Food Chem. (2006) 54:2695–704. 10.1021/jf052635t [DOI] [PubMed] [Google Scholar]
- 2.Fan W, Qian M. Identification of aroma compounds in Chinese “Yanghe Daqu” liquor by normal phase chromatography fractionation followed by gas chromatography/olfactometry. Flavour Frag J. (2010) 21:333–42. 10.1002/ffj.1621 [DOI] [Google Scholar]
- 3.Liu H, Sun B. Effect of fermentation processing on the flavor of baijiu. J Agric Food Chem. (2018) 66:5425–32. 10.1021/acs.jafc.8b00692 [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Huo J, Huang M, Zhao M, Luo X, Sun B. Structural characterization of a tetrapeptide from sesame flavor-type baijiu and its preventive effects against AAPH-induced oxidative stress in HepG2 cells. J Agric Food Chem. (2017) 65:10495–504. 10.1021/acs.jafc.7b04815 [DOI] [PubMed] [Google Scholar]
- 5.Meng X, Wu Q, Wang L, Wang D, Chen L, Xu Y. Improving flavor metabolism of Saccharomyces cerevisiae by mixed culture with Bacillus licheniformis for Chinese maotai-flavor liquor making. J Ind Microbiol Biot. (2015) 42:1601–8. 10.1007/s10295-015-1647-0 [DOI] [PubMed] [Google Scholar]
- 6.Ciani M, Comitini F. Yeast interactions in multi-starter wine fermentation. Curr Opin Food Sci. (2015) 1:1–6. 10.1016/j.cofs.2014.07.001 [DOI] [Google Scholar]
- 7.Wang B, Wu Q, Xu Y, Sun B. Synergistic effect of multi-saccharifying enzymes on alcoholic fermentation for Chinese baijiu production. Appl Environ Microb. (2020) 86:e00013–20. 10.1128/AEM.00013-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Wu Q, Xu Y, Sun B. Multiple sugars promote microbial interactions in Chinese baijiu fermentation. LWT Food Sci Technol. (2021) 138:110631. 10.1016/j.lwt.2020.110631 [DOI] [Google Scholar]
- 9.Zou W, Zhao C, Luo H. Diversity and function of microbial community in Chinese strong-flavor baijiu ecosystem: a review. Front Microbiol. (2018) 9:671. 10.3389/fmicb.2018.00671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song H, Ding M, Jia X, Ma Q, Yuan Y. Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev. (2014) 43:6954–81. 10.1039/c4cs00114a [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Chen B, Xu Y. Regulating yeast flavor metabolism by controlling saccharification reaction rate in simultaneous saccharification and fermentation of Chinese maotai-flavor liquor. Int J Food Microbiol. (2015) 200C:39–46. 10.1016/j.ijfoodmicro.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 12.Ge X, Qian H, Zhang W. Influence of mixed culture system on the growth performance of Aspergillus oryzae and Saccharomyces cerevisiae. Afr J Biotechnol. (2013) 12:3272–7. 10.5897/AJB09.025 [DOI] [Google Scholar]
- 13.Nuno MO, Rene N, Kevin R. Evolutionary limits to cooperation in microbial communities. Proc Natl Acad Sci USA. (2014) 111:17941–6. 10.1073/pnas.1412673111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuang X, Wu Q, Xu Y. Physiological characteristics of Zygosaccharomyces bailii and its interaction with Bacillus licheniformis in Chinese maotai-flavor liquor making. Microbiol China. (2017) 44:251–62. 10.13344/j.microbiol.china.160174 [DOI] [Google Scholar]
- 15.Xu Y, Wu Q, Xu Y. Effects of main functional strains on Zygosaccharomyces bailii in Chinese maotai-flavor liquor fermentation. Microbiol China. (2018) 45:42–53. 10.13344/j.microbiol.china.170190 [DOI] [Google Scholar]
- 16.Zha M, Sun B, Wu Y, Yin S, Wang C. Improving flavor metabolism of Saccharomyces cerevisiae by mixed culture with Wickerhamomyces anomalus for Chinese baijiu making. J Biosci Bioeng. (2018) 126:189–95. 10.1016/j.jbiosc.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 17.Wu Q, Ling J, Xu Y. Starter culture selection for making Chinese sesame-flavored liquor based on microbial metabolic activity in mixed-culture fermentation. Appl Environ Microb. (2014) 80:4450–9. 10.1128/AEM.00905-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang J, Wang H, Xu Y. Effect of mixed culture of Saccharomyces cerevisiae and Pichia anomala on fermentation efficiency and flavor compounds in Chinese liquor. Microbiol China. (2012) 39:921–30. [Google Scholar]
- 19.Wu Q, Kong Y, Xu Y. Flavor profile of Chinese liquor is altered by interactions of intrinsic and extrinsic microbes. Appl Environ Microb. (2016) 82:422–30. 10.1128/AEM.02518-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Zheng Y, Zhou C, Pan D, Geng F, Cao J, et al. Kinetic response of conformational variation of duck liver globular protein to ultrasonic stimulation and its impact on the binding behavior of n-alkenals. LWT Food Sci Technol. (2021) 150:111890. 10.1016/j.lwt.2021.111890 [DOI] [Google Scholar]
- 21.Lin X, Tang Y, Hu Y, Lu Y, Sun Q, Lv Y, et al. Sodium reduction in traditional fermented foods: challenges, strategies, and perspectives. J Agric Food Chem. (2021) 69:8065–80. 10.1021/acs.jafc.1c01687 [DOI] [PubMed] [Google Scholar]
- 22.He G, Huang J, Wu C, Jin Y, Zhou R. Bioturbation effect of fortified Daqu on microbial community and flavor metabolite in Chinese strong-flavor liquor brewing microecosystem. Food Res Int. (2019) 129:108851. 10.1016/j.foodres.2019.108851 [DOI] [PubMed] [Google Scholar]
- 23.Sadoudi M, Tourdot R, Rousseaux S, Steyer D, Gallardo J, Ballester J, et al. Yeast-yeast interactions revealed by aromatic profile analysis of sauvignon Blanc wine fermented by single or co-culture of non-saccharomyces and Saccharomyces yeasts. Food Microbiol. (2012) 32:243–53. 10.1016/j.fm.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 24.Gammacurta M, Marchand S, Moine V, Revel G. Influence of different yeast/lactic acid bacteria combinations on the aromatic profile of red bordeaux wine. J Sci Food Agr. (2017) 97:4046–57. 10.1002/jsfa.8272 [DOI] [PubMed] [Google Scholar]
- 25.Luo Q, Zheng J, Zhao D, Qiao Z, An M, Zhang X, et al. Interaction between dominant lactic acid bacteria and yeasts strains in strong aroma baijiu. China J Appl Environ Biol. (2019) 25:1192–9. 10.19675/j.cnki.1006-687x.2018.12031 [DOI] [Google Scholar]
- 26.He G, Huang J, Zhou R, Wu C, Jin Y. Effect of fortified Daqu on the microbial community and flavor in Chinese strong-flavor liquor brewing process. Front Microbiol. (2019) 10:56. 10.3389/fmicb.2019.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Wu Q, Wang P, Lin J, Huang L, Xu Y. Synergistic effect in core microbiota associated with sulfur metabolism in spontaneous Chinese liquor fermentation. Appl Environ Microb. (2017) 83:e01475–17. 10.1128/stocktickerAEM.01475-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendes F, Sieuwerts S, de Hulster E, Almering MJ, Luttik MA, Pronk JT, et al. Transcriptome-based characterization of interactions between Saccharomyces cerevisiae and Lactobacillus delbrueckii subsp. bulgaricus in lactose-grown chemostat cocultures. Appl Environ Microb. (2013) 79:5949–61. 10.1128/AEM.01115-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin G, Zhu Y, Xu Y. Mystery behind Chinese liquor fermentation. Trends Food Sci Tech. (2017) 63:18–28. 10.1016/j.tifs.2017.02.016 [DOI] [Google Scholar]
- 30.Wang C, Wang M, Zhang M. Ethyl carbamate in Chinese liquor (baijiu): presence, analysis, formation, and control. Appl Microbiol Biot. (2021) 105:4383–95. 10.1007/s00253-021-11348-1 [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Fan W, Xu Y. Influence of different types of double distillation on removal efficiency of ethyl carbamate in Chinese strong aroma type liquor. Food Ferment Ind. (2015) 41:1–7. 10.13995/j.cnki.11-1802/ts.201506001 [DOI] [Google Scholar]
- 32.Wang J, Fan W, Xu Y, Yang J, Xie G, Sun L, et al. Influence of pilot scale pot still second distillation on ethyl carbamate and cyanide in baijiu and the quality of raw liquor. Food Ferment Ind. (2022) 48:53–7. 10.13995/j.cnki.11-1802/ts.030800 [DOI] [Google Scholar]
- 33.Cui K, Wu Q, Xu Y. Biodegradation of ethyl carbamate and urea with Lysinibacillus sphaericus MT33 in Chinese liquor fermentation. J Agric Food Chem. (2018) 66:1583–90. 10.1021/acs.jafc.7b05190 [DOI] [PubMed] [Google Scholar]
- 34.Wei T, Jiao Z, Hu J, Lou H, Chen Q. Chinese yellow rice wine processing with reduced ethyl carbamate formation by deleting transcriptional regulator Dal80p in Saccharomyces cerevisiae. Molecules. (2020) 25:3580. 10.3390/molecules25163580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du H, Song Z, Xu Y. Ethyl carbamate formation regulated by lactic acid bacteria and nonconventional yeasts in solid-state fermentation of Chinese moutai-flavor liquor. J Agric Food Chem. (2017) 66:387–92. 10.1021/acs.jafc.7b05034 [DOI] [PubMed] [Google Scholar]
- 36.Fang L, Zhou W, Chen Q. Ethyl carbamate regulation and genomic expression of Saccharomyces cerevisiae during mixed-culture yellow rice wine fermentation with Lactobacillus sp. Food Chem. (2019) 292:90–7. 10.1016/j.foodchem.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 37.Dong W, Guo R, Liu M, Shen C, Sun X, Zhao M, et al. Characterization of key odorants causing the roasted and mud-like aromas in strong-aroma types of base baijiu. Food Res Int. (2019) 125:108546. 10.1016/j.foodres.2019.108546 [DOI] [PubMed] [Google Scholar]
- 38.Du H, Liu B, Wang X, Xu Y. Exploring the microbial origins of p-cresol and its co-occurrence pattern in the Chinese liquor-making process. Int J Food Microbiol. (2017) 260:27–35. 10.1016/j.ijfoodmicro.2017.08.016 [DOI] [PubMed] [Google Scholar]
- 39.Huang M, Huo J, Wu J, Zhao M, Zheng F, Sun J, et al. Interactions between p-cresol and Ala-Lys-Arg-Ala (AKRA) from sesame-flavor-type baijiu. Langmuir. (2018) 34:12549–59. 10.1021/acs.langmuir.8b02662 [DOI] [PubMed] [Google Scholar]
- 40.Tao Y, Hu X, Zhu X, Jin H, Xu Z, Tang Q, et al. Production of butyrate from lactate by a newly isolated Clostridium sp. BPY5. Appl Biochem Biotech. (2016) 179:361–74. 10.1007/s12010-016-1999-6 [DOI] [PubMed] [Google Scholar]
- 41.He P, Hu X, Zheng Y, Shen X, Li S, Li X, et al. Research and application progress of “Ethyl Caproate-increasing and Ethyl Lactate-decreasing” in brewing of Chinese luzhou-flavor liquor. J Light Ind. (2018) 33:1–12. 10.3969/j.issn.2096-1553.2018.04.001 [DOI] [Google Scholar]
- 42.Yan S, Dong D. Improvement of caproic acid production in a Clostridium kluyveri H068 and Methanogen 166 co-culture fermentation system. AMB Expr. (2018) 8:175. 10.1186/s13568-018-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chai L, Qian W, Zhong X, Zhang X, Lu Z, Zhang S, et al. Mining the factors driving the evolution of the pit mud microbiome under the impact of long-term production of strong-flavor baijiu. Appl Environ Microb. (2021) 87:e0088521. 10.1128/AEM.00885-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thauer R, Kaster A, Seedorf H, Buckel W, Hedderich R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol. (2008) 6:579–91. 10.1038/nrmicro1931 [DOI] [PubMed] [Google Scholar]
- 45.Hu X, Du H, Ren C, Xu Y. Illuminating anaerobic microbial community and cooccurrence patterns across a quality gradient in Chinese liquor fermentation pit muds. Appl Environ Microb. (2016) 82:2506–15. 10.1128/AEM.03409-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao Y, Wang X, Li X, Wei N, Jin H, Xu Z, et al. The functional potential and active populations of the pit mud microbiome for the production of Chinese strong-flavour liquor. Microb Biotechnol. (2017) 10:1603–15. 10.1111/1751-7915.12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy K, Marzorati M, Abbeele P, Wiele T, Boon N. Synthetic microbial ecosystems: an exciting tool to understand and apply microbial communities. Environ Microbiol. (2014) 16:1472–81. 10.1111/1462-2920.12343 [DOI] [PubMed] [Google Scholar]
- 48.Shong J, Diaz M, Collins C. Towards synthetic microbial consortia for bioprocessing. Curr Opin Biotech. (2012) 23:798–802. 10.1016/j.copbio.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 49.Lu Z, Liu N, Wang L, Wu L, Gong J, Yu Y, et al. Elucidating and regulating the acetoin production role of microbial functional groups in multispecies acetic acid fermentation. Appl Environ Microb. (2016) 82:5860–8. 10.1128/AEM.01331-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfe B, Button J, Santarelli M, Dutton R. Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell. (2014) 158:422–33. 10.1016/j.cell.2014.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Q, Zhu Y, Fang C, Wijffels R, Xu Y. Can we control microbiota in spontaneous food fermentation? – Chinese liquor as a case example. Trends Food Sci Tech. (2021) 110:321–31. 10.1016/j.tifs.2021.02.011 [DOI] [Google Scholar]
- 52.Vorholt J, Vogel C, Carlstrm C, Müller D. Establishincg ausality: opportunities of synthetic communities for plant microbiome research. Cell Host Microbe. (2017) 22:142–55. 10.1016/j.chom.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 53.Lawson C, Harcombe W, Hatzenpichler R, Lindemann S, Mcmahon K. Common principles and best practices for engineering microbiomes. Nat Rev Microbiol. (2019) 17:725–41. 10.1038/s41579-019-0255-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, Wu Q, Nie Y, Wu J, Xu Y. Construction of synthetic microbiota for reproducible flavor metabolism in Chinese light aroma type liquor produced by solid-state fermentation. Appl Environ Microb. (2019) 85:e03090–18. 10.1101/510610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiao W, Xie F, Gao L, Du L, Wei Y, Zhou J, et al. Identification of core microbiota in the fermented grains of a Chinese strong-flavor liquor from Sichuan. LWT Food Sci Technol. (2022) 158:113140. 10.1016/j.lwt.2022.113140 [DOI] [Google Scholar]