Abstract
Background:
Acetabular aseptic loosening due to bone defect in total hip arthroplasty revisions is a great challenge and several solutions have been proposed, but a broadly accepted consensus in the literature has not been reached yet. The aim of this study is to compare the clinical and radiographic results of acetabular bone defects treatment with biological-only graft or with a mixture of bone graft substitute and biological graft.
Methods:
33 patients had revision hip arthroplasty using impaction grafting with biological-only graft (21 patients, Group A) or a 1/3 mixture of allograft and tricalcium phosphate bone graft substitute (12 patients, Group B). Patients were reassessed at a minimum of one year after surgery with new x-rays and the Harris Hip Score (HHS).
Results:
Survivorship of bone graft was 86% in Group A and 100% in Group B at a mean follow-up of 35 months. No statistical difference between the two groups was found in terms of implants survivorship (P=0.28), clinical (P=0.08) or radiographic (P=0.27) outcomes.
Conclusion:
In our experience the use of tricalcium phosphate bone graft substitutes in combination with allo and autograft provides good outcomes, low risk of failure and great clinical and radiographic results. Further investigations on larger samples are needed to impact clinical practice.
Key Words: abbr
Introduction
Total hip arthroplasty (THA) is one of the most successful procedures in orthopedic surgery, with excellent clinical outcomes in terms of pain relief, restoration of joint function and patient satisfaction; the number of primary hip arthroplasties performed is consistently growing worldwide and the numbers of younger patients are also increasing (1). As a result, an increase in revision surgery is expected in the future (2,3).
Massive bone defects and prosthetic components-induced osteolysis are known indications for revision THA (4,5). When a primary THA requires revision, the acetabular component alone has been reported to be involved 40% of the time (6). Acetabular defects can be managed with bone grafting, either autografts or allografts, as well as metal augments or cemented rings (7-9). Cancellous bone autograft, harvested from the iliac crest, has been shown to have good incorporation in the remaining bone (10). However, its use has limitations, including: the amount of available bone can be inadequate, harvesting is time consuming and sometimes the donor site is complicated by residual pain. On the other hand, the use of allografts is expensive (during an impaction grafting procedure the equivalent of five femoral heads may be used) and can be associated with risk of transmitting infections and of immunological rejection (11-14). Although autologous bone grafting is still considered the ‘‘gold standard’’ in bony defect repair, significant recent advances in the development of alternatives to natural bone have been made (15). Bone graft substitutes can be used alone or in combination with auto or allografts. Their use is convenient due to inadequate amount of graft material and may exploit potential advantages such as better mechanical properties and reduced risk of infection (16). Possible disadvantages of bone graft substitutes are poor integration with the remaining bone and foreign body reaction (17).
The aim of this study is to compare the clinical and radiographic results of patients with bone defects from aseptic acetabular loosening treated with biological-only graft or with a mixture of bone graft substitute and biological graft in total hip arthroplasty revisions.
Materials and Methods
Each author certifies that all investigations were conducted in conformity with ethical principles of research. In our hospital between January 2012 and December 2016 a total of 40 patients underwent acetabular-only revision THA with bone impaction grafting. Informed consent was signed, charts and imaging were reviewed retrospectively. Preoperative planning was based on plain anteroposterior and Judet oblique views of the pelvis. Preoperative computed tomography (CT) scans were not routinely used, but only for the most complex cases. The acetabular defect was classified according to Paprosky and only patients affected by Paprosky 2 or greater were included in the study (18). Thirty-three patients met inclusion criteria. Age at surgery, gender, complications and comorbidities were recorded.
All the patients were treated with surgical posterolateral approach. Acetabular bone defect classification was intraoperatively confirmed. When biological derived bone grafts were chosen, acetabular gaps were filled with a mix of morsellized femoral head allografts (which were stored at −70°, thawed, and decorticated) and little autografts (periacetabular and trocanteric ossifications, residual bone reamed from the acetabulum) [Figure 1]. As bone graft substitute, tricalcium phosphate (Vitoss®, Stryker) was used and mixed with a 3:1 ratio with biological graft [Figure 2]. Graft choice was based on graft availability and surgeon preference. In cases of large segmental acetabular defects, cemented Burch-Schneider® rings (Zimmer) or cementless metal augments were used, regardless of the type of graft that was used.
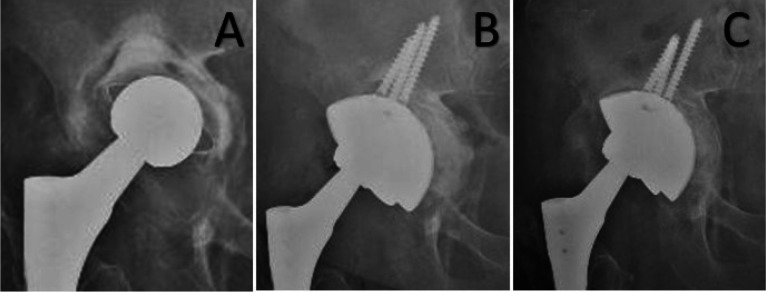
Figure 1.
A 73 years old patient affected by Paprosky 3b acetabular defect treated with a cementless implant (Delta One TT®, Lima) associated with bone allograft. A (preoperative x-ray); B (immediate postoperative x-ray), C (36 months follow-up)
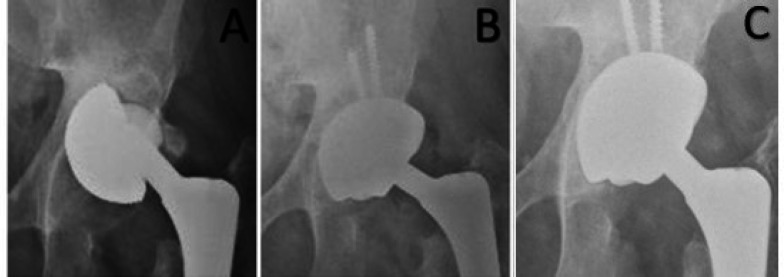
Figure 2.
A 56 years old patient affected by Paprosky 2a acetabular defect and cup migration treated with a cementless implant (Delta One TT®, Lima) associated with Vitoss® bone graft substitute: A (preoperative x-ray); B (immediate postoperative x-ray), C (12 months follow-up)
Patients were divided into Group A, with biological-only graft used, and Group B, cases with combination of biological and bone graft substitutes.
Patients were reassessed at least one year after surgery with new x-rays and clinical evaluation with the Harris Hip Score (HHS) (19,20). X-rays allowed the evaluation of incorporation of the graft in the three zones described by DeLee and Charnley, any radiolucent lines, localized resorption, and migration of the prosthetic components according to Hodgkinson (21,22).
Statistical analysis was performed using the Fisher’s test and the Student t-test to compare nominal and continuous variables respectively. P values of <0.05 were considered to be significant. Values are reported as mean ± standard deviation [range].
Results
Among the 33 patients studied, 17 (52%) were male and 16 (48%) were female, with a mean age of 68.0±12.8 [31-89] years. Twelve patients (36%) were affected by Paprosky type 2a defects, 2 (6%) by type 2b, 4 (12%) by type 2c, 4 (12%) by type 3a, and 11 (33%) by type 3b. Acetabular revision was performed using cementless cups (Pinnacle Multihole®, DePuy Synthes; Omnia®, AdlerOrtho; Delta One TT®, Lima) in 26 cases (79%) (associated with metal augments in five cases of large segmental defects), while in 7 cases (21%) the Burch-Schneider® ring (Zimmer) with a cemented PolarCup® (Smith & Nephew) was used [Figure 3; Table 1].
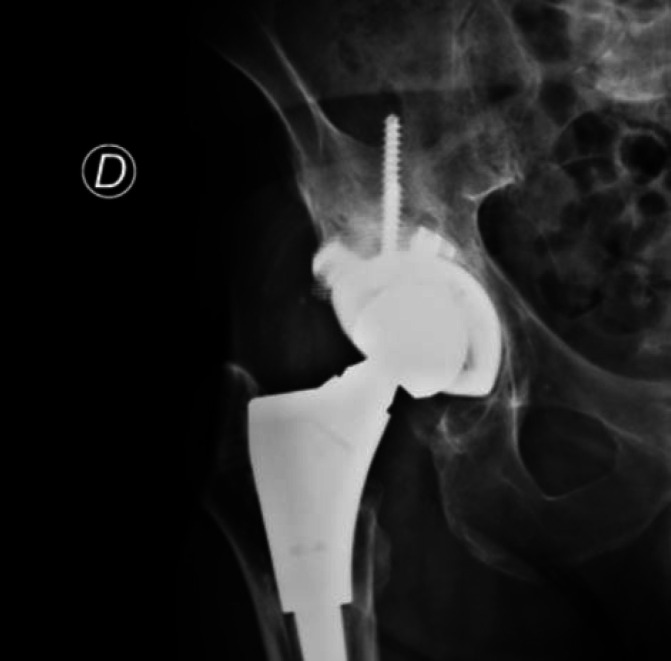
Figure 3.
A 65 years old patient treated with a cementless implant (Delta One TT®, Lima) associated with metal augment
Table 1.
Patients’ Demographics
| GROUP A | GROUP B | TOTAL | |
|---|---|---|---|
| Number of patients | 21 | 12 | 33 |
| Gender (male:female) | 10:11 | 7:5 | 17:16 |
| Mean age, y (range) | 67 (31-84) | 69 (50-89) | 68 (31-89) |
| Mean clinical Follow up, months (range) | 36 (13-60) | 33 (12-60) | 35 (12-60) |
| Mean radiographic Follow up, months (range) | 29 (12-60) | 26 (12-60) | 28 (12-60) |
| Preop. Paprosky 2a (patients) Preop. Paprosky 2b Preop. Paprosky 2c Preop. Paprosky 3a Preop. Paprosky 3b Uncemented/cemented implant |
7 2 1 4 7 18/3 |
5 / 3 / 4 8/4 |
12 2 4 4 11 26/7 |
The mean clinical follow-up time was 34.6±15.0 [12-60] months and the mean radiographic follow-up time was 27.6±14.2 [12-60] months. At the latest follow-up, the mean HHS was 77.9±14.1 [41.8-97.7].
In 29 patients (88%) there was no radiographic evidence of graft resorption or cup migration. In 3 patients (9%) affected by Paprosky 3a (1 patient) and 3b (2 patients) acetabular defect graft resorption with cup migration was observed and required a second THA revision [Figure 4]. In 1 case (3%) affected by Paprosky 2a acetabular defect, signs of lysis and resorption of the graft were detected without mobilization of the cup and without symptoms and the patient did not require any further intervention. One patient, underwent biological grafting, presented with clinical signs (pain, local heat, redness and little purulence from the wound associated with alteration of blood tests) of early infection (< 2 months) without any radiographic evidence of graft resorption at follow-up, and was treated with targeted antibiotic therapy. IV antibiotics were used, while DAIR (debridement, antibiotics and implant retention) was not performed due to the patient’s poor clinical condition and because the patient refused any additional surgery. Up to now (four years from surgery) no further surgery has been needed [Table 2].
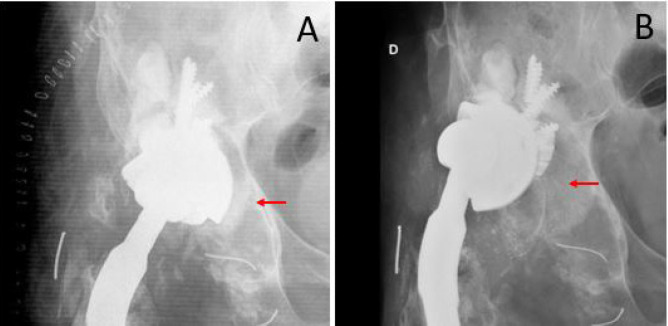
Figure 4.
A 62 years old patient with a cementless implant (Delta One TT®, Lima) associated with metal augment and bone allograft. A (immediate postoperative x-ray), B (implant failure at 12 months follow-up). Bone graft (red arrow)
Table 2.
Outcomes
| Group A (n.21) | Group B (n.12) | TOT (n.33) | |
|---|---|---|---|
|
COMPLICATIONS - N° (%) Early infection* |
1 (5) | None | 1 (3.03) |
|
GRAFT OUTCOME - N°(%) No lysis/resorption signs Graft resorption which required new revision Lysis/resorption of the graft, but no further intervention Implant survivorship at latest follow-up - N° (%) |
17 (81) 3 (14) 1 (5) 18 (86) |
12 (100) None None 12 (100) |
29 (87.9) 3 (9.1) 1 (3.03) 30 (91) |
| Mean postoperative HHS (range) | 75 (42-96) | 83 (55-98) | 78 (42-98) |
* (< 2 month after surgery)
In 21 patients (64%) biological-only grafts were used (Group A), while in 12 (36%) Vitoss®bone graft substitute mix was used (Group B).
The mean age was 67±13 [31-84] years in Group A and 69±13[50-89] years in Group B (P=0.67). There were no significant differences between the two groups in terms of the Paprosky type distribution (P=0.13), gender distribution (P=0.72), clinical and radiographic follow-up time (P=0.56 and P=0.58 respectively) and percentage of use of cemented/uncemented implants (P=0.37) or metal augments (P=0.13). Three patients reported rheumatoid arthritis in their past medical history (one in Group A, two in Group B) with none having bone resorption and implant loosening.
In Group A the mean HHS was 74.7±14.6 [41.8-95.5]. In 17 hips (81%) there were no radiographic signs of graft resorption or cup migration, 3 patients (14%) needed further surgical revision due to radiographic evidence of graft resorption associated with symptoms, 1 patient (5%) had signs of resorption of the graft without symptoms and did not need any further surgery. In these three failed cases, nearly an entire femoral head thawed and morselized plus residual bone reamed from the remaining acetabulum was used. In Group B the mean HHS was 83.3±11.9 [55.0-97.7] and there were no radiographic signs of graft resorption or cup migration at the latest follow-up.
Survivorship (with revision as the end point) of bone graft was 86% in Group A and 100% in Group B at a mean follow-up of 35 months. The no statistical difference between the two groups was found in terms of implants survivorship (P=0.28), clinical (P=0.08) or radiographic (P=0.27) outcomes [Table 2].
Discussion
THA revision surgery is becoming more frequent and acetabular component loosening due to osteolysis is a known mechanism of failure (4). In this scenario, the use of bone grafts, bone substitutes or a combination are options (23). The aim of our study was to compare the results of patients who underwent revision THA either with only biological graft or with a mixture of bone graft substitute and biological graft. Our results showed the value of bone graft substitutes in the management of acetabular defects in THA revisions, without significant differences between the groups studied.
Several studies have evaluated the role of bone graft substitutes in the context of spine and head and neck surgery, but there is still little evidence of its use in hip surgery (17, 24). The use of bone graft in acetabular bone stock deficiency has been discussed in some studies, but a widely accepted consensus has not been reached yet. Therefore surgeon’s choice is often guided by personal preference, with a large variety of techniques proposed (25-28).
Some authors have reported on the use of pure biological bone grafts. Oommen, et alshowed good outcomes in 26 out of 30 hips treated with auto or allograft to restore bone deficiencies at a mean follow-up of 23.4 months in both cemented and uncemented THA (27). Joong-Myung Lee, et al. analyzed the clinical and radiographic results of acetabular revision arthroplasty using an impacted morselized allograft and a cementless cup inserted via a press-fit technique performed on 71 hips, reporting 95,8% survival rate at a mean follow-up of 12 years (29).
Other authors have described the use of synthetic bone substitutes. Huang, et al. identified 89 patients with acetabular or femoral bone defects that underwent revision THA with the use of bone graft substitute made of hydroxyapatite and type I collagen to fill all these defects (30). None of the components needed re-revision at a mean follow-up of 33.6 months. Schwartz and Bordei used biphasic phospho-calcium ceramics in 32 hips to fill cavities or to reconstruct segmental deficiencies in revision THA with severe acetabular bone loss (31). After a mean follow-up of 5.5 years, the authors showed radiographic evidence of good integration of bone and ceramic. Rates of common complications as dislocations and infections were considered similar to those in their series with allograft. Schwartz, et al. confirmed these results at a long-term follow up (32).
Most of the time surgeons prefer to mix bone substitutes with bone grafts. This is due to the fact that hydroxyapatite and tricalcium phosphate have osteoconductive potential with poor osteoinductive properties (33,34). Recent studies reported on the incorporation of mesenchymal stem cells into bone graft substitutes to improve osteoinductive and osteogenic properties with encouraging results (35,36). Mixing bone graft substitutes with the residual reamed acetabular bone could be a good option in order to compensate for the lack of osteoinduction. Abdullah, et al. reported on the effectiveness of bone impaction grafting mixed with hydroxyapatite (with a 1:1 ratio) for 47 acetabular defects in primary and revision hip surgeries; survivorship, with revision as an end point, of bone impaction grafting was 100% at 10-year follow-up (37). In contrast with good clinical results, x-ray showed lysis in 8 cases and cup migration in 4 cases, suggesting that close monitoring is mandatory. Whitehouse, et al. conducted a retrospective review of 43 consecutive patients that underwent impaction grafting of acetabular defects with a biphasic porous ceramic bone graft substitute (80% sintered tricalcium phosphate and 20% hydroxyapatite)(38). This was used in a 1:1 mix with femoral head allograft with a reported 94% survivorship of the grafted acetabulum and of the acetabular component at 7 years after surgery, confirming the short-term results previously reported in this cohort by Blom, et al (39).
The current literature does not define what graft to bone substitute ratio is the best, with several studies reporting a 1:1 ratio (37-40). In an animal study with an ovine hemiarthroplasty model, Blom et Al. tested different ratios of bone autograft with biphasic ceramic bone substitutes (1:1 and 1:9) and showed that a higher proportion of ceramic bone substitute can be used without compromising implant outcomes (41). In our study in Group B we used a 3:1 ratio, with 1 being the biologic graft. This was chosen in order to avoid the use of too much acetabular bone, which is often mixed with fibrous and scar tissue.
Few studies have compared the use of bone graft with the use of a bone substitute mix. In a cadaveric study, Jacofsky, et al. compared the initial as well as long-term stability of acetabular components during simulated walking when a standardized acetabular defect was filled either with an impacted, reverse-reamed, cancellous allograft or with a injectable, bioresorbable, calcium phosphate bone substitute (42). The calcium phosphate provided markedly superior initial stability and cup stability lasted longer during cyclic loading. In a retrospective clinical study, Kumar, et al. reported after either acetabular or femoral revision using a 1:1 mixture of allograft and synthetic bone graft or allograft alone (43). There were no significant clinical or radiographic differences between the two groups. Aulakh, et al. described a cohort of 65 patients who underwent THA revision with the impaction grafting technique (44). The authors compared the outcomes of pure morsellized allograft (42 patients) with a 1:1 mixture of allograft and solid particulate hydroxyapatite (23 patients). After 13 years of follow up, the authors concluded that prosthesis survival (82% in patients treated with bone substitutes + allograft versus 84% in patients treated with pure allograft), graft incorporation and hip function were similar in both groups. The cohort Aulakh, et al. reported was heterogeneous, the impacted component was femoral in 27 patients, acetabular in 9 patients and both in 29 patients, making a difficult to compare with our results. However, both studies support the use of bone substitutes mixed with bone graft as a valid option.
In our series the graft resorption rate was 12% and revision rate 9%, which is in line with what reported in the literature regarding the impaction grafting technique (45,46).
Although no significant differences were detected, it has to be noted that in group B the rate of radiographic failure was 0% and this does not seem to be related to the age of patients, type of defect, length of follow-up and use of cement.
In our series one patient presented clinical signs of infection in the “biological-only” group and none in the “bone graft substitutes” but, although an increased risk of infection appears to be usually considered attributable to the use of biological grafts, this patient underwent multiple previous hip surgeries which is confounding (39).
Economic aspects are worth being taken into consideration. Leung, et al. compared the costs of fresh frozen femoral head banking with the costs of bone graft substitutes including the costs of screening tests, power consumption of freezer storage, and manpower costs (47). They estimated the cost of cancellous bone allograft material as US$ 78–86 per gram compared to US$ 9 per gram for calcium phosphate ceramic bone graft. Although in our study we did not focus on economic aspects, since we showed no significant differences between the use of biological-only grafts and bone graft substitutes, costs should be taken into consideration by the surgeon during his decision process in THA revision.
Our study has limitations and therefore it must be interpreted with caution. First, this is a retrospective study. Second, we do not have preoperative HHS values, although the pre-operative functional state of a patient has significant effect on the final outcome (44). Third, we have used cementless as well as cemented implants; cemented implants are currently used in association with bone grafting, but could make radiographic evaluation more difficult (48,49). Fourth, the number of patients in this study is low. Fifth, CT scan was not used for preoperative or postoperative assessment in all patients, but only in the most complex cases. Finally, although radiographic healing was established, further follow-up to assess long term survivorship is necessary.
Both biological-only grafts and bone graft substitutes seem to be valid option in the management of acetabular defects in THA revisions. In our experience tricalcium phosphate bone graft substitutes used in combination with allo and autograft was associated with lower risk of failure and better clinical and radiographic results, without statistically significant differences compared to those obtained using biological-only grafts. Although our clinical results appear promising, longer follow-up are required to establish whether this technique is as effective as using biological grafts alone.
References
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508–19. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Jbjs. 2007;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Kurcz B, Lyons J, Sayeed Z, Anoushiravani AA, Iorio R. Osteolysis as it pertains to total hip arthroplasty. Orthopedic Clinics. 2018;49(4):419–35. doi: 10.1016/j.ocl.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Haynes JA, Stambough JB, Sassoon AA, Johnson SR, Clohisy JC, Nunley RM. Contemporary surgical indications and referral trends in revision total hip arthroplasty: a 10-year review. The journal of arthroplasty. 2016;31(3):622–5. doi: 10.1016/j.arth.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. JBJS. 2009;91(1):128–33. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Durbhakula S, Berry DJ, Naessens JM, Rappaport K, Cisternas M, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. The Journal of arthroplasty. 2005;20:17–25. doi: 10.1016/j.arth.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Borland WS, Bhattacharya R, Holland JP, Brewster NT. Use of porous trabecular metal augments with impaction bone of porous trabecular metal augments with impaction bone. Acta Orthop. 2012;83:347–52. doi: 10.3109/17453674.2012.718518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendrich C, Mehling I, Sauer U, Kirschner S, Martell JM. Cementless acetabular reconstruction and structural bone-grafting in dysplastic hips. J Bone Joint Surg. 2006;88:387–94. doi: 10.2106/JBJS.D.02373. [DOI] [PubMed] [Google Scholar]
- 9.Joong-Myung Lee, Tae-ho Kim. Acetabular Cup Revision Arthroplasty Using Morselized Impaction Allograft. Hip Pelvis. 2018;30(2):65–77. doi: 10.5371/hp.2018.30.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brydone AS, Meek D, Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng. 2010;224:1329–43. doi: 10.1243/09544119JEIM770. [DOI] [PubMed] [Google Scholar]
- 11.Holt G, Arthur A, Frame D, Muirhead A. Human skeletal allograft collection – room for improvement? Scott Med J. 2004;49:146–8. doi: 10.1177/003693300404900410. [DOI] [PubMed] [Google Scholar]
- 12.Friedlaender GE, Strong DM, Tomford WW, Mankin HJ. Long-term follow-up of patients with osteochondral allografts A correlation between immunologic responses and clinical outcome. Orthop Clin North. 1999;30:583–8. doi: 10.1016/s0030-5898(05)70111-5. [DOI] [PubMed] [Google Scholar]
- 13.Stepanović ŽLj, Ristić BM. Bacterial infections associated with allogenic bone transplantation. Vojnosanit Pregl. 2015;72(5):427–30. doi: 10.2298/vsp1505427s. [DOI] [PubMed] [Google Scholar]
- 14.Graham SM, Leonidou A, Aslam-Pervez N, Hamza A, Panteliadis P, Heliotis M, et al. Biological therapy of bone defects: the immunology of bone allo-transplantation. Expert Opinion on Biological Therapy. 2010;10(6):885–901. doi: 10.1517/14712598.2010.481669. [DOI] [PubMed] [Google Scholar]
- 15.Campana V, Milano GI, Pagano E, Barba M, Cicione C, Salonna G, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. Journal of Materials Science: Materials in Medicine. 2014;25(10):2445–61. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNamara IR. Impaction bone grafting in revision hip surgery: past, present and future. Cell Tissue Bank. 2010;11:57–73. doi: 10.1007/s10561-009-9147-y. [DOI] [PubMed] [Google Scholar]
- 17.Beswick A, Blom AW. Bone graft substitutes in hip revision surgery: A comprehensive overview. Injury Int. J Care Injured. 2011;42: S40–S46. doi: 10.1016/j.injury.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. J Arthroplasty. 1994;9:33–44. doi: 10.1016/0883-5403(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 19.Nilsdotter A, Bremander A. Measures of hip function and symptoms: Harris hip score (HHS), hip disability and osteoarthritis outcome score (HOOS), Oxford hip score (OHS), Lequesne index of severity for osteoarthritis of the hip (LISOH), and American Academy of orthopedic surgeons (AAOS) hip and knee questionnaire. Arthritis care & research. 2011;63(S11):S200–7. doi: 10.1002/acr.20549. [DOI] [PubMed] [Google Scholar]
- 20.WH , Harris Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty An end-resultstudy using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 21.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop. 1976;21:20–32. [PubMed] [Google Scholar]
- 22.Hodgkinson J P, Shelley P, Wroblewski B M. The correlation between the roentgenographic appearance and operative findings at the bone-cement junction of the socket in Chamley low friction arthroplasties. Clin Orthop. 1988;228:105–9. [PubMed] [Google Scholar]
- 23.Calori GM, Mazza E, Colombo M, Ripamonti C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury, Int. J. Care Injured. 2011;42: S56–S63. doi: 10.1016/j.injury.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Coetzee AS. Regeneration of bone in the presence of calcium sulfate. Arch Otolaryngol. 1980;106:405–9. doi: 10.1001/archotol.1980.00790310029007. [DOI] [PubMed] [Google Scholar]
- 25.Board TN, Brunskill S, Doree C, Hyde C, Kay PR, Meek RD, et al. Processed versus fresh frozen bone for impaction bone grafting in revision hip arthroplasty. Cochrane Database of Systematic Reviews. 2009:4. doi: 10.1002/14651858.CD006351.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris WH, Crothers O, Oh I. Total hip replacement and femoral-head bone-grafting for severe acetabular deficiency in adults. J Bone Joint Surg Am. 1977;59:752–9. [PubMed] [Google Scholar]
- 27.Oommen AT, Krishnamoorthy VP, Poonnoose PM, Korula RJ. Fate of bone grafting for acetabular defects in total hip replacement. Indian J Orthop. 2015;49:181–6. doi: 10.4103/0019-5413.152462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haddad FS. The role of impaction grafting: the when and how. Orthopedics (Online) 2009;32(9):675. doi: 10.3928/01477447-20090728-19. [DOI] [PubMed] [Google Scholar]
- 29.Lee JM, Nam HT. Acetabular Revision Total Hip Arthroplasty Using an Impacted Morselized Allograftand a Cementless Cup: Minimum 10-Year Follow-Up. The Journal of Arthroplasty. 2011;26(7):1057–60. doi: 10.1016/j.arth.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Qin L, Yan W, Weng X, Huang X. Clinical evaluation following the use of mineralized collagen graft for bone defects in revision total hip arthroplasty. Regenerative Biomaterials. 2015:245–249. doi: 10.1093/rb/rbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz C, Bordei R. Biphasic phospho-calcium ceramics used as bone substitutes are efficient in the management of severe acetabular bone loss in revision total hip arthroplasties. Eur J Orthop Surg Traumatol. 2005;15:191–6. [Google Scholar]
- 32.C Schwartz, M. Vautrin. Phosphocalcium ceramics are efficient in the management of severe acetabular loss in revision hip arthroplasties. A 22 cases long-term follow-up study. Eur J Orthop Surg Traumatol. 2015;25:227–232. doi: 10.1007/s00590-014-1476-2. [DOI] [PubMed] [Google Scholar]
- 33.Bohner M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury. 2000;31: S37–47. doi: 10.1016/s0020-1383(00)80022-4. [DOI] [PubMed] [Google Scholar]
- 34.Hollinger JO, Brekke J. Role of bone substitutes. Clin Orthop Relat Res. 1996;324:55–65. doi: 10.1097/00003086-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein P, Bornhauser M, Gunther K, Stiehler M. Bone tissue engineering in clinical application Assessment of the current situation. Orthopade. 2009;38:1029–37. doi: 10.1007/s00132-009-1493-8. [DOI] [PubMed] [Google Scholar]
- 36.Šponer P, Kučera T, Brtková J, Urban K, Kočí Z, Měřička P, et al. Comparative study on the application of mesenchymal stromal cells combined with tricalcium phosphate scaffold into femoral bone defects. Cell transplantation. 2018;27(10):1459–68. doi: 10.1177/0963689718794918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdullah KM, Hussain N, Parsons SJ, Porteous MJ, Atrey A. 11-Year Mean Follow-Up of Acetabular Impaction Grafting With a Mixture of Bone Graft and Hydroxyapatite Porous Synthetic Bone Substitute. The Journal of Arthroplasty. 2018 ;33(5):1481–6. doi: 10.1016/j.arth.2017.11.065. [DOI] [PubMed] [Google Scholar]
- 38.Whitehouse MR, Dacombe PJ, Webb JCJ, Blom AW. Impaction grafting of the acetabulum with ceramic bone graft substitute mixed with femoral head allograft: High survivorship in 43 patients with a median follow-up of 7 years. Acta Orthopaedica. 2013;84(4):365–370. doi: 10.3109/17453674.2013.792031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blom A W, Wylde V, Livesey C, Whitehouse M R, Eastaugh-Waring S, Bannister G C, et al. Impaction bone grafting of the acetabulum at hip revision using a mix of bone chips and a biphasic porous ceramic bone graft substitute. Acta Orthop. 2009;80(2):150–4. doi: 10.3109/17453670902884767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara I, Deshpande S, Porteous MJ. Impaction grafting of the acetabulum with a mixture of frozen, ground irradiated bone graft and porous synthetic bone substitute (Apapore 60) J Bone Joint Surg [Br] 2010;92: 617– 23. doi: 10.1302/0301-620X.92B5.23044. [DOI] [PubMed] [Google Scholar]
- 41.Blom AW, Cunningham JL, Hughes G, Lawes TJ, Smith N, Blunn G, et al. The compatibility of ceramic bone graft substitutes as allograft extenders for use in impaction grafting of the femur. J Bone Joint Surg Br. 2005;87(3):421–5. doi: 10.1302/0301-620x.87b3.14337. [DOI] [PubMed] [Google Scholar]
- 42.Jacofsky DJ, McCamley JD, Jaczynski AM, Shrader MW, Jacofsky MC. Improving initial acetabular component stability in revision total hip arthroplasty: Calcium phosphate cement vs reverse reamed cancellous allograft. The Journal of Arthroplasty. 2012;27(2):305–9. doi: 10.1016/j.arth.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Kumar V, Ricks M, Sherif Abouel-Enin, Dunlop DG. Long term results of impaction Bone grafting using a synthetic graft (Apapore) in revision hip surgery. Journal of Orthopaedics. 2017;14: 290–293. doi: 10.1016/j.jor.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aulakh TS, Jayasekera N, Kuiper JH, Richardson JB. Long-term clinical outcomes following the use of synthetic hydroxyapatite and bone graft in impaction in revision hip arthroplasty. Biomaterials. 2009;30: 1732–1738. doi: 10.1016/j.biomaterials.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 45.Van Egmond N, De Kam DC, Gardeniers JW, Schreurs BW. Revisions of extensive acetabular defects with impaction grafting and a cement cup. Clin Orthop Relat Res. 2011;469:562–73. doi: 10.1007/s11999-010-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Haaren EH, Heyligers IC, Alexander FG, Wuisman PI. High rate of failure of impaction grafting in large acetabular defects. J Bone Joint Surg. 2007;89:296–300. doi: 10.1302/0301-620X.89B3.18080. [DOI] [PubMed] [Google Scholar]
- 47.Leung HB, Fok MW, Chow LC, Yen CH. Cost comparison of femoral head banking versus bone substitutes. J Orthopaedic Surg. 2010;18:50–4. doi: 10.1177/230949901001800111. [DOI] [PubMed] [Google Scholar]
- 48.Welten ML, Schreurs BW, Buma P, Verdonschot N, Slooff TJ. Acetabular Reconstruction With Impacted Morcellized Cancellous Bone Autograft and Cemented Primary Total Hip Arthroplasty. The Journal of Arthroplasty. 2000:15–7. doi: 10.1054/arth.2000.7110. [DOI] [PubMed] [Google Scholar]
- 49.Ibrahim MS, Raja S, Haddad FS. Acetabular impaction bone grafting in total hip replacement. The Bone & Joint Journal. 2013;95(11_Supple_A):98–102. doi: 10.1302/0301-620X.95B11.32834. [DOI] [PubMed] [Google Scholar]