Abstract
Cymbopogon giganteus is a medicinal plant from Burkina Faso whose leaves are used in many traditional recipes to treat several diseases. However, no scientific studies have been reported on the analysis of bioactive molecules of the plant. It is therefore for the first time that flavonoids are isolated from the leaves of the Burkina Faso species. The aim was to quantify, isolate and characterize the major flavonoids in methanol extracts of the plant leaves by spectrophotometry, chromatography and NMR respectively. Flavonoid content analysis showed values ranging from 134 to 270 μg QE/mg extract. HPTLC-MS identified six peaks corresponding to phenolic compounds. By a succession of chromatography on column and by chemical, physicochemical and physical methods, we could isolate and characterize three flavonoids: epicatechin, luteolin 8-C-glucosid and luteolin 6-C-glucosid which structures were characterized by NMR. This study has provided relevant results to contribute to the knowledge of bio-active molecules of the local flora of Burkina Faso for their consideration as an alternative to synthetic products in various fields.
Keywords: Cymbopogon giganteus, Flavonoids, HPLC, HTLC, LC-MS, NMR
Cymbopogon giganteus, Flavonoids, HPLC, HTLC, LC-MS, NMR.
1. Introduction
Emerging diseases apparition and micro-organisms’s resistance development have shown the insufficiency of synthetic molecules in pathologies treatment. In Burkina Faso, the resort to many plant recipes become systematical by population for the treatment of numerous pathologies. Cymbopogon giganteus, is one of the plants widely used by the local population. The different organs of this plant are often used alone or in combination with other plants or plant organs in the management of malaria, hypertension, hepatitis, insomnia, yellow fever, etc. (Moussa et al., 2012 and Ouattara-Sourabie et al., 2017). Although the plant is one of most cited species last decades in many traditional anti-malarial recipes in some regions of the country, the first scientific work on non-volatile extracts of C. giganteus from Burkina Faso was our thesis work (Bationo, 2019). During this work, the antiplasmodial and antifungal efficacy of different plant organs extracts was evaluated (Bationo et al., 2017a,Bationo et al., 2017b). The results showed that methanol extracts of leaves and flowers have, on chloroquine-resistant (K1) and chloroquine-sensitive (3D7) strain, an antiplasmodial activity very similar to quinine [IC50 = 0.17 μg/mL]. The evaluation of antifungal activity showed that the same extracts, at low dose, inhibited to about 23% the radial growth of some foodstuff fungi (Bationo et al., 2017a,Bationo et al., 2017b). However, the chemical structure of the major compounds was still unknown. This work is a continuation of what has been undertaken and its aims is to isolate and characterize the structure of the main molecules in the extracts of C. giganteus. The aim is to contribute to a better knowledge of Burkina Faso flora through an analysis of bioactive molecules contained in the different organs.
2. Material and methods
2.1. Plant collection
Plant material composed of C. giganteus leaves were collected in experimental field (12°25′28.2″N; 1°29′15.06″ W) at the Institut de Recherche en Sciences Appliquées et Technologie (IRSAT) in Ouagadougou. Plant material was shade dried at room temperature for 15 days and then ground. The plant was identified by herbarium of University of Ouagadougou were a specimen was deposited under number 6895.
2.2. Chemicals
The reagents purchased from Sigma-Aldrich (St. Louis, MO) and used were analytical grade: Quercetin, rutin, Sodium Nitrite (NaNO2), Aluminium chlorid (AlCl3) and Sodium hydroxyde (NaOH).
2.3. Extracts preparation
50 g of powder of ground leaves were macerated successively with 300 mL of hexane, dichloromethane, ethyl acetate and methanol under magnetic stirring for 24 h. After filtration on Whatman papers N°3, extractions were repeated twice with the same volume of each solvent for 24 h. Extracts obtained were concentrated under vacuum until almost dried. Dried extracts were stored in the refrigerator for the various tests.
2.4. Determination of total flavonoids content (TFC)
Total flavonoids content was measured using aluminum chloride colorimetric assay as described by Zhishen et al. (1999) with slight modifications (adapted for microplates) (Bationo et al., 2017a,Bationo et al., 2017b). A calibration curve was established using different concentrations (0, 25 μg/L, 50 μg/L, 75 μg/L and 100 μg/L) of Quercetin solutions. The procedure consists of introducing 25 μL of each different concentration solution into microplate wells. Subsequently, 150 μL of distilled water and 10 μL of 5% NaNO2 were added to it. After 5 min and 6 min, 10 μL of 10% AlCl3 and 50 μL of NaOH 1 M were added respectively. The mixture was incubated at 37 °C during 30 min and absorbance were measured at 415 nm using SAFAS spectrometer. For the extracts, the same procedure was used. The only difference is that we replaced quercetin solutions with suitably diluted extracts. Tests were carried out in triplicates and results were expressed as μg of Quercetin equivalent/mg of extract (μg QE/mg).
2.5. HPTLC-mass spectrometry analysis
HPTLC analysis are made on 60 F254 (Merck) silica gel plates in normal phase. TLC plate dimensions for the development were 20 × 10 cm. The system elution was composed of: EtOAc-AcOH–HCOOH– H2O (100:11:11:26). 20 μL (10 mg/mL) were deposited using LINOMAT 5 (CAMAG®). After development using the mobile phase on the TLC plate (3/4 of the length of the plate is traversed by the solvent system), the spot was scraped off the HP-TLC plate, eluted in a tube and analyzed in the Bruker Daltonics micrOTOF-Q™ mass spectrometer in positive and negative mode using a CAMAG TLC-MS interface system.
2.6. HPLC- mass spectrometry (LC-MS) analysis
The analysis was made using a Merck HITACHI Channel chromatograph with L-7100 pump, a L-7200 automatic injector and a L-7400 UV detector, controlled by the D-7000 HSM (Merck) software. 20 μL of crude extract with a concentration of 1 mg/mL were injected. Mobile phase was composed of two solvents A and B. Solvent A is water (H2O) and solvent B is acetonitrile (AcN) which are acidified with 2% of formic acid (AcN-HCOOH 2%). Linear elution gradient from 5% to 100% of B was applied during 40 min. Then an isocratic elution of 100% of B was applied for 5 min. ODS HYPERSIL column (column specifications 5 μm; 250 × 21 mm) was used. Coupled detector is the microtof-QTM (Bruker Daltonics) mass spectrometry described in paragraph 2.5.
2.7. Isolation of pure compounds in methanolic extract
By medium pressure preparative chromatography, 2 g of the methanol crude extract from the leaves were fractionated on normal phase silica gel into seven fractions (F1–F7). The F3 fraction (1252.83 mg) was again fractionated on the same column with the same solvent system. Six subfractions (F3.1–F3.6) were collected. Subfractions F3.2, F3.3, F3.4, and F3.5 have been grouped according to their TLC profile to give an FG fraction. This fraction was then fractionated on a reverse phase silica column with a MeOH–H2O binary system by elution gradient. The sub-fractions from FG had been grouped into four sub-fractions FG.1, FG.2, FG.3 and FG.4. A binary system composed of methanol and water, each with 2% of formic acid, was used in gradient on RP C18 ODS HYPERSIL column (5 μm 250 × 21.2 mm) to isolate compounds A (3.2 mg), E (4.2 mg) and F (3.3 mg) (Table 4).
Table 4.
Isolation efficiencies.
| <!--Col Count:3-->Compound | Isolation ratio |
|
|---|---|---|
| Compound Mass (mg) | Ratio (%) | |
| A | 3.2 | 0.16 |
| E | 4.2 | 0.21 |
| F | 3.3 | 0.165 |
2.8. Pure compounds purification and structural characterization
Isolated compounds were purified using high performance liquid chromatography (HPLC). The binary gradient as described in paragraph 2.7 was applied. The structures of these compounds were determined by chromatographic techniques and physical methods (MS, IR, UV-vis and NMR). Infrared spectra are recorded on a Shimadzu IR Affinity-1-8400S Fourier Transform IR Spectrophotometer (FT-IR). The spectra were collected between 400-4000 cm−1 on each compound’s powder. The UV-vis spectra of the compounds were recorded between 200 and 800 using a monochromatic 3E carry UV spectrophotometer in methanol. The 1H, 13C and homonuclear and heteronuclear COSY NMR spectra were recorded on a Bruker Avance III 400 MHz spectrometer. Approximately 1.5–2 mg of each isolated molecule was dissolved in 0.5 ml of deuterated methanol (D3COD) in 5 mm diameter NMR tubes.
3. Results and discussion
3.1. Total flavonoid contents
The results of flavonoids content (TFC) evaluation was expressed in μg quercetin equivalent/mg extract. All data was processed and statistical analysis was performed using Genstat version 14. The values presented in Table 1 are expressed in mean ± standard deviation. The letters reflect the significant difference between the values.
Table 1.
Total flavonoids content in the leaves extracts of C. giganteus.
| Total flavonoids content | |||
|---|---|---|---|
| Extracts | DCM | EtOcA | MeOH |
| TFC | 132.23 ± 4.05a | 147.55 ± 3.24b | 270.41 ± 5.20c |
DCM: dichloromethane; EtOcA: Ethyl Acetate; MeOH: methanol.
TFC data of different extracts in the table are 134.23; 147.55 and 270.4 μg EQ/mg extract in DCM, ethyl acetate and methanolic extracts respectively. From the analysis of these values, we can say that methanol is a good solvent for extraction of large amount of flavonoids better than ethyl acetate and DCM. These results was confirmed by other researches (Bationo et al., 2017a,Bationo et al., 2017b; Eruygur et al., 2018). The evaluation of flavonoid content is in agreement with previous data (Bationo et al., 2017a,Bationo et al., 2017b). Repetition of this work confirmed that methanolic extracts of C. giganteus leaves contained higher TFC. These data motivated us once again to continue the investigation to isolate and characterize the major flavonoids in these extracts.
3.2. Structural determination of compound in methanolic extract
The determination of the structures of the compounds in methanol extracts was carried out in two steps. The first was to identify the compounds in the methanol extract using thin layer chromatography and chromatography-physical methods coupling. After identification, the majority compounds were isolated and characterized using analytical and preparatory chromatographic methods (CCM, HPLC, HPLC-UV and CC) and spectral methods (MS, IR, UV and NMR).
3.2.1. Structural identification by HPTLC-MS and LC-MS
Crude extract TLC profile (Figure 1) gave five (05) spots, three (03) of which were majority spots. Among three main spots, two have a yellow fluorescence (spots 3 and 4) and one, yellow-orange (spot 1) under UV 365 nm after spraying the Neu reagent. These spots, according to the literature, correspond to glycosylated flavonol derivatives (Wagner and Bladt, 1996). TLC profile of crude extracts is compared with standards namely orientin and luteolin (Table 2). Spots 3 (Rf = 0.68) and 4 (Rf = 0.7) could be oriental derivatives. Tasks 1 and 2 could be a diglucoside or triglucoside derivative of quercetin or myricetin (Wagner and Bladt, 1996). Indeed, in the literature, glycosylated derivatives of quercetin and myricetin have an orange-yellow fluorescence under UV 365 nm after spraying the Neu reagent (Wagner and Bladt, 1996).
Figure 1.
TLC of MeOH Extract (a) and pure compounds (b).
Table 2.
TLC data (Front reference: Rf) of the crude extract and standards.
| Spots | Different Spots TLC of MeOH Extract |
Standards |
|||||
|---|---|---|---|---|---|---|---|
| 5 | 4 | 3 | 2 | 1 | orientin | luteolin | |
| Front reference | 0.86 | 0.70 | 0.68 | 0.4 | 0.37 | 0.7 | 0.96 |
| Fluorescence under UV-365 nm after spraying | ND | yellow | yellow | yellow-orange | yellow-orange | yellow | yellow-orange |
After TLC analyse’s of methanol extracts, majority stains (1, 3 and 4) were analyzed with mass spectrometer using the CAMAG interface. Figure 2 shows the majority spots mass spectrum. This concerns the spot 1 (Rf = 0.37), spot 3 (Rf = 0.67) and spot 4 (Rf = 0.7). On this mass spectra (Figure 2) positive mode ionization electrospray (ESI+) we observe characteristic molecular ion peaks at m/z 441 ; m/z 471 et m/z 473. These molecular peaks correspond respectively to the molecular weight characteristic masses of certain known flavonoids listed in Table 3. Thus, methanol extracts from the leaves would close monoglycosylated derivatives of quercetin and luteolin.
Figure 2.
HPTLC-MS mass spectra of Spot 4 Rf = 0.7 (a) Spot 3 Rf = 0.67 (b) Spot 1 Rf = 0.37 (c).
Table 3.
Different peaks in TLC-MS of flavonoids.
| Spots N° (Rf) |
Molecular ions | Fragment ions | Suggested names of molecular |
|---|---|---|---|
| 4 (0,70) | 469 | 243; 353; 355; 385; 393; 411 | Biochanin A 8-C-glucoside |
| 493 | 180; 179; 185; 471 | Glycyrrhetinic Acid | |
| 271 | 241; 185 | 6-(2-Hydroxyethyl)-2-hydroxymethyl-2,5,7-trimethyl-1-indanone | |
| 3 (0,68) | 186 | ND | ND |
| 271 | ND | ND | |
| 471 | 254; 327; 349; 365; 413; 431 | Luteolin-C-glucoside | |
| 1 (0.37) | 209 | ND | ND |
| 355 | 313; 271; 247 | Laricitrin | |
| 441 | 209; 307; 313 | Aloin | |
| 469 | ND | Baicalin |
ND: Not Determined.
3.2.2. Isolation and characterization of pure compounds in methanolic extract
3.2.2.1. Isolation of pure compounds
Three (03) pure compound’s could be isolated A (TLC spot 5), E (TLC spot 4) and F (TLC spot 4); Figure 1) by chromatography succession (preparative chromatography and semi-preparative HPLC). The masses of pure compounds have been calculated. Isolation efficiencies were calculated and expressed in percentage (%). The values are grouped in Table 4.
Result’s analysis shows that the values are very low. The yield of isolation of molecules in a raw extract is the ratio between the mass of the isolated molecule and the quantity of extract used (Table 4). Several factors such as the succession of methods, influence this yield. However, the yield is an important indicator of efficiency in industrial applications. This yield allows to evaluate or to appreciate the economic profitability in case of large scale application. The molecular structure was then characterized.
3.2.2.2. The thin-layer chromatographic profile of pure compounds
Thin-Layer Chromatographic (TLC) profile of the isolated compounds shows that compounds E and F, like orientin, appear yellow under UV light at 365 nm (Figure 1B). Also, these compounds have the same frontal reference as orientin (Table 5). These compounds are believed to be orientin derivatives (C-glucoside luteolin). Compound A does not fluoresce under UV at 365 nm after spraying with Neu’s reagent (Figure 1B).
Table 5.
TLC front references of pure compounds.
| Pure compounds |
Orientin | Isorhamnetin | Lutéolin | |||
|---|---|---|---|---|---|---|
| A | E | F | ||||
| Rf | 0,87 | 0,7 | 0,7 | 0,7 | 0,75 | 0,96 |
3.2.2.3. Structural determination of isolated compounds by spectral methods
3.2.2.3.1Structural characterization of the compound A
Compound A is a reddish-brown powder. The Infra Red (IR) spectrum (Figure 3) of this powder shows no vibration band at 1600 cm−1 characteristic of carbonyl (C=O). There is also a band around 2900 cm−1 characteristic of methylenes (–CH2–) (Anthoni et al., 2010; Uzan et al., 2011) and a band around 1180 cm−1 is characteristic of secondary alcohols (–CHOH–). Through this data, we can suggest that compound A is a flavanol.
Figure 3.
Compound A IR spectra.
UV-Visible spectrum of compound A (Figure 4) shows two (02) characteristic bands of flavonoids. One in UV around 210 nm and the other between 270 and 300 nm. The band between 280 and 300 nm is a characteristic band of aromatic nuclei of flavonoids. The band around 210 nm is a characteristic of flavanol nuclei (Lhuillier et al., 2007; Michel et al., 2011). Indeed, secondary alcohols absorb towards 180–210 nm (Lhuillier et al., 2007 and T. Michel et al., 2011). Compound A is therefore a flavanol.
Figure 4.
Compound A UV spectra in methanol.
Analytical chromatogram (HPLC) of compound A, shows a retention time (Rt= 10.8 min) comparable to epicatechin taken as standard.
In mass spectrometry (Figure 5), the molecular ion at 289.0714 is coherent with the mass calculated using formula C15H14O6. Indeed, basic structure of flavonoids is a sequence of 15 carbon atoms (Cuyckens and Claeys, 2004 and Michel et al., 2010). With regard to this proposed raw formula and the general formula of flavonoids, compound A is not glycosylated.
Figure 5.
MS/MS spectra in positive mode of compound A.
Analysis of the LC-MS and LC-MS/MS mass spectra (Figure 5) show molecular ions and fragments similar to those of epicatechin. From the analysis of the fragment ions grouped in Table 6, it appears that the compound is of the flavanol family. The characteristic fragment ions observed at m/z 139.039 and 123.044 (Figure 5, Table 6) result respectively from the loss of a C9H10O2 and C9H10O3 group from an epicatechin aglycone. Compound A would probably be epicatechin of the flavanol family. Indeed, in the negative mode fragmentation, epicatechin differs from catechin by the presence of fragment ions at m/z 139.0392 [M-150] and 123.0443 [M-166]. These specific fragment ions of epicatechin are present in our data. So, compound A is epicatechin.
Table 6.
LC-MS/MS data of isolated compounds.
| Compounds | Mol. weight | MS fragment ions | Retention time (min) | Molecular formula | % |
|---|---|---|---|---|---|
| A | 290 | 123; 126; 139; 143; 147; 161; 162; 163; 165; 179; 181; 189; 207; 291 | 10.31 | 100 | |
| E | 448 | 149; 173; 195; 255; 283; 299; 300; 311; 313; 329; 339; 355; 360; 383; 384; 413; 416; 419; 421; 431; 449 | 11.98 | 70.19 | |
| F | 448 | 299; 313; 329; 339; 355; 360; 383; 384; 413; 416; 419; 421; 431; 449 | 12.39 | 80.51 |
The data of NMR proton spectrum (Figure 6) was confirmed the chemical structure of compound A. Indeed, chemical shifts between 6 and 7 ppm are characteristic of the aromatic proton signals of A and B rings of flavonoids [Silverstein et al., 1998]. The aromatic cycle A has 3 protons: H-2′ (6.75 (s)), H-6′ (6.94 (d; 1.86)), H-5′ (6.70 (d; 2.93)), and the aromatic cycle A has 2 protons: H-6 (5.94 (s)), and H-8 (5.90 (s)). The two large singlet lets at 4.14 ppm and 4.56 ppm are characteristic of the CH proton’s at positions 2 (H-2) and 3 (H-3) close to the oxygen atoms. The coupling constant obtained between H2 and H3 shows that these protons are in the Cis position relative to each other [Silverstein et al., 1998 and Markham and Ternai, 2005]. The difference between catechin and epicatechin lies in this configuration at the C2 and C3 carbons. Indeed, in catechin chemical structure we observes (2R, 3S) or (2S, 3R) configurations while in epicatechin it is the (2S, 3S) or (2R, 3R) configurations that we find. The weak coupling constant observed between the protons H-2 in position 2 and H-3 in position 3 (vicinal protons) shows that these protons are in the Cis position relative to each other. Compound A is epicatechin.
Figure 6.
1H NMR spectra of compounds A.
Furthermore, the signals of the two protons in position 4 (2.84 and 2.72 ppm) forming split doubles with large coupling constants J = 16.75 Hz are characteristic of Trans coupling (Silverstein et al., 1998 and Michel et al., 2011). So, the protons being carried by the same carbon, this is a geminal coupling. They are thus two non-equivalent protons of a methylene (–CH2–). These two protons couple with the proton H-3 at 4.14 ppm forming a doublet with coupling constants J = 4.5 Hz and J = 3.0 Hz.
The data of carbon, HSQC and HMBC spectra confirmed that A has fifteen (15) carbon atoms. The signal at 4.56 ppm in the HSQC spectrum shows that the proton is bound to a carbon close to the heterocycle (C–O–). This is the C2 carbon at 78.5 ppm. The protons at 4.14 and 2.84 ppm are respectively bound to the carbons at 66.1 and 27.6 ppm. These chemical shifts are respectively those of the carbons C–OH (C3) and CH2 (C4). In addition, the HMBC and HSQC spectra confirmed the assignment of the A and B rings protons. Indeed, the HMBC spectrum reveals quaternary carbons C1′ (130 ppm); C4′ (141.5 ppm); C5′ (159.1); C7 (156 ppm) and C9 (157 ppm). This information (Table 1) coupled with data from the literature [8–9] confirmed that epicatechin compound A (Figure 7).
Figure 7.
Structure of compound A.
3.2.2.3.2Structural characterization of the compound E
Compound E was isolated from C. giganteus leaves by semi-preparative HPLC and characterized by chemical, chromatographic and spectral (IR, UV-visible, MS, NMR) methods.
Infra-red (IR) spectrum (Figure 8) shows, trough the band at 1670 cm−1, the presence of aromatic ketone in compound E structure. The band around 800 cm−1 characterizes the adjacent protons of the aromatic nucleus. The absence of a band around 2800–3000 cm−1 indicates the absence of sp3-type carbon from saturated hydrocarbons (Anthoni et al., 2010; Uzan et al., 2011). The hydroxyl groups (OH) of phenolic compounds are revealed by the vibration band around 1200 cm−1. Compound E is therefore not a flavane or flavanol derivative but would be a derivative of the flavone or flavonol family.
Figure 8.
Compound E IR spectra.
The UV-visible spectrum of this compound (Figure 9) shows a band around 360 nm. This band is a characteristic band of flavones and flavonols (Lhuillier et al., 2007; Michel et al., 2011). Indeed aromatic hydrocarbons have very characteristic spectra whose richness must be attributed to the great symmetry of these molecules (Silverstein et al., 1998; Lhuillier et al., 2007; Michel et al., 2011). Branching shifts this absorption slightly towards long wavelengths. Indeed, flavones are generally detected at 360 or 370 nm. This result suggests that compound E is a flavone or a flavonol.
Figure 9.
Compound E UV spectra in methanol.
The LC/MS mass spectrum in ESI negative mode gives a peak at m/z of 447.09314. This mass is consistent with that calculated from the gross formula C21H20O11. This formula corresponds to several isomers of aminoglycoside flavonoid. Indeed, flavonoids having 15 carbon atoms, the 6 additional carbon atoms would correspond to those of a hexose as a substituent (Fabre et al., 2001; Lhuillier et al., 2007; Michel et al., 2011).
On other hand, LC-MS/MS in positive (Figure 10) and negative mode from E to 20 eV shows no loss corresponding to a sugar. This data suggests the sugar would be bound to the aglycone by a carbon-carbon bond (C-glycosylation). In the literature, C-glycosylated flavonoids are generally in position 6 or 8. Data (Table 5) analysis and their comparison with the literature shows that E is 8 or 6 C-glucoside luteolin (Wolfender et al., 2000; Cuyckens et al., 2000, 2001; Cuyckens and Claeys, 2005). Indeed, the peak at m/z 299.055 is commonly found among fragment ions of C-glycosylated flavonoids (Wolfender et al., 2000; Cuyckens et al., 2000, 2001; Cuyckens and Claeys, 2005). In general, the MS/MS spectrum can distinguish 8-C isomers from 6-C glycosides (Wolfender et al., 2000; Cuyckens et al., 2000, 2001; Cuyckens and Claeys, 2005). The MS/MS fragmentation of 6-C glycosides differs from that of 8-C glycosides trough the peak at m/z 329.066 [0.2X+]. This peat is specific to 6-C flavonoid glycosides (Wolfender et al., 2000; Waridel et al., 2001). This is observed in mass spectrum of E (Figure 10 and Table 6). During fragmentation the latter may in turn fragment and give the ions at m/z 300.0594 [0.2X+-CHO] and 311.05568 [M + H-120-18; 0.2X+-H2O] observed. The presence of ions at m/z 283 [0.2X+-CH2O2], 300 [0.2X+-CHO] and 311 [0.2X+-H2O] observed with E indicates that E is a 6-C isomer. In addition, there is fragmentation of the C ring in the 6 C-glycosides, giving fragment ions at m/z 195 (1.3A+) and 137 (0.2B+) (Wolfender et al., 2000; Cuyckens et al., 2000, 2001; Cuyckens and Claeys, 2005). These ions could also be obtained in the MS/MS spectrum of the compound E. Compound E would likely be luteolin-6-C-glucoside.
Figure 10.
Mass MS/MS spectra in positive mode of compound E.
The data of 1H-NMR spectra (Figure 11; Table 7) of E and its aglycone in agreement with data in the literature show that it is a glycosylated luteolin derivative (Davis and Brodbelt, 2005; Markham and Ternai, 2005). Protons were unambiguously detected at δ (ppm) 7.51 (H-2′); 7.55 (H-6′); 6.90 (H-5′); 6.27 (H-8) and 6.53 (H-3) (Table 7). In general, the A-ring protons of a flavonoid appear between 6 and 6.5 ppm (Silverstein et al., 1998; Davis and Brodbelt, 2005; Markham and Ternai, 2005). In E compound spectrum (Figure 11, Table 7), the aromatic proton in this range (6.27 ppm) characterizes a flavonoid with a single proton in the A-ring. This proton is either the H-6 or H-8 proton (Silverstein et al., 1998; Davis and Brodbelt, 2005; Markham and Ternai, 2005). The B-ring protons are observed on a 1H-NMR spectrum around 6.7 and 7.9 ppm (Table 7). The 1H-NMR spectrum of E shows three aromatic protons at δH 7.55 ppm (d, J = 8.9 Hz); 7.51 ppm (d, J = 7.65); 6.9 ppm (d, J = 8.3 Hz) whose coupling constants indicate that they form an ABX system on the B-ring. These protons are the protons H-2′, H-6′ and H-5′ respectively.
Figure 11.
1H NMR spectra of compound E.
Table 7.
NMR data of isolated compounds.
| N° | Compound A |
Compound E |
Compound F |
|||
|---|---|---|---|---|---|---|
| 1H ppm (mult; J Hz) | 13C ppm | 1H ppm (mult; J Hz) | 13C ppm | 1H ppm (mult; J Hz) | 13C ppm | |
| 2 | 4.56 (d) | 78.5 | – | 165.8 | – | 164.4 |
| 3 | 4.14 (m) | 66.1 | 6.53 (s) | 103.5 | 6.55 (s) | 103.0 |
| 4 | 2.8 (dd 16.75; 2.67) | 27,6 | – | 183.7 | – | 182.8 |
| 5 | – OH | 159.1 | –OH | 157.1 | –OH | 157.2 |
| 6 | 5.94 (s) | 94.9 | –gluc | 102.1 | 6.49 (s) | 93.9 |
| 7 | – OH | 156 | –OH | 161 | –OH | 163.8 |
| 8 | 5.90 (s) | 94.9 | 6.27 (s) | 99.1 | –gluc | 107.3 |
| 9 | – | 157.3 | – | 165.5 | – | 160.8 |
| 10 | – | – | 104.5 | – | 103.7 | |
| 1′ | – | 130 | – | 122.5 | – | 119.0 |
| 2′ | 6.75 (s) | 113.7 | 7.51 (d; 7.65) | 120.5 | 7.36 (br s) | 120.5 |
| 3′ | – OH | 117.2 | –OH | 149.5 | –OH | 145.9 |
| 4′ | – OH | 141.5 | –OH | 146.1 | –OH | 148.9 |
| 5′ | 6.70 | 144.2 | 6.90 (d; 8.3) | 115.8 | 6.91 (d; 8.4) | 115.4 |
| 6′ |
6.94 (dd; 1.86; 6,7) (s) |
118.4 |
7.55 (d; 8.90) |
114.9 |
7.40 (br d; 7.23) |
112.8 |
| Sugar |
Sugar |
|||||
| 1″ | 4.98 (d; 7.8) | 73.8 | 4.91 (d; 10.2) | 74.02 | ||
| 2″ | 4.18 (t) | 72.4 | 4.15 (t) | 71.1 | ||
| 3″ | 3.49 | 80.12 | 3.47 | 79.12 | ||
| 4″ | 3.47 (br; s) | 71.6 | 3.45 (br s) | 70.4 | ||
| 5″ | 3.44 | 82.4 | 3.42 | 81.0 | ||
| 6″ | 3.74 et 3.88 | 62.6 | 3.73 et 3.86 | 61.4 | ||
The anomeric sugar proton (proton of the carbon having established the bond with the aglycone) is observed at 4.91 ppm. The region between 4.5 and 3 ppm corresponds to the sugar protons that appear in a massif. The massif at δ = 3.66 ppm corresponds to a methylenic signal. The chemical shift of the anomeric proton (δ = 4.96 ppm) is characteristic of a C-glycoside flavonoid (Silverstein et al., 1998; Davis and Brodbelt, 2005; Markham and Ternai, 2005).
In addition, the high value of the coupling constant observed for the anomeric proton signal (J = 7.8 Hz) suggests that it is in the form β-D-glucopyranose (Silverstein et al., 1998; Davis and Brodbelt, 2005; Markham and Ternai, 2005).
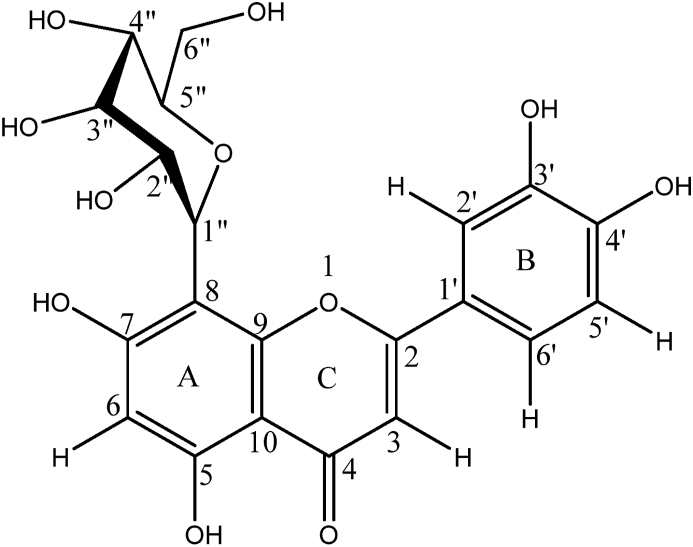
The COSY spectrum which consists of a homonuclear 1H–1H scalar coupling shows that the anomeric proton is coupled to a proton whose chemical shift is δ = 4.18 ppm (H-2″). The proximity of anomeric proton to aglycone proton is not revealed in the COSY spectrum. This confirms that the glycosylated substituent on the aglycone is in position 8 or 6; the C5 and C7 carbons being occupied by hydroxyl. Indeed, the structure indicates that the C5, C7, C9, and C10 carbons do not possess protons. Thus, compound E is identified as luteolin-8-C-glucoside or luteolin-6-C-glucoside. The HSQC, HMBC, and 13C carbon spectra were essential for proton allocation and molecular structure determination.
The HSQC (Heteronuclear Single Quantum Correlation) correlates the protons to nuclei carbon where they are directly attached (the JC-H direct correlation). Indeed, the HSQC spectrum shows that the proton at 7.55 ppm (H-6′) is related to a carbon at 114.9 ppm (C6′). The HSQC, HMBC and 13C-NMR technique’s was used to assign the signals 165.8; 103.5; 183.7; 157.1; 102.1; 161; 99.1; 165.5; 104.5; 122.5; 120.5; 149.5; 146.1; 115.8 and 114.9 respectively to the carbons C2, C3; C4; C5; C6; C7; C8; C9; C10; C1′; C2′; C3′; C4′; C5′ and C6′ (Silverstein et al., 1998; Davis and Brodbelt, 2005; Markham and Ternai, 2005). Sugar carbons occur between 50 and 100 ppm (Silverstein et al., 1998; Davis and Brodbelt, 2005 and Markham and Ternai, 2005). HMBC (Heteronuclear Multiple Bond Correlation) spectral data show a proximity of the anomeric proton to the carbons at 102.1, 157.1 and 161 ppm. These carbons are the C6, C5, and C7 carbons, respectively. Indeed, the HMBC technique detects the long-distance couplings (2JC-H; 3JC-H) between the proton and the two to three bond carbon. The sugar is therefore in position 6. Compound E was therefore luteolin-6-C-glucoside (Figure 12).
Figure 12.
Structure of compound E.
3.2.2.3.3Structural characterization of the compound F
IR spectrum of the compound reveals a vibration band around 1600 cm−1. This band is characteristic of aromatic ketones (νC=O) (Silverstein et al., 1998; Anthoni et al., 2010; Uzan et al., 2011). Compound F is of the same family as compound E and has a C=O group. The 1050 cm−1 band is characteristic of alcohols (νO-H) and phenols (Silverstein et al., 1998; Anthoni et al., 2010; Uzan et al., 2011). Compound F is believed to be a compound of the flavone or flavonol family.
UV-Visible spectrum appears to be identical to that of compound E. There are two characteristic bands of flavonols and their glycosides that are generally detected at 270, 365 and 370 (Lhuillier et al., 2007; Michel et al., 2011). Compound F would be of the same family as compound E. It is therefore a flavonol.
The mass spectrum in ESI negative mode gives a peak m/z 447.0931. This mass is consistent with that calculated using the gross formula C21H20O11. The raw formula shows 6 additional carbons compared to flavonoids. Compound F could therefore be a hexosyl monoglycosylated derivative. However, LC-MS/MS mass spectrum of this majority compound (Figure 6) does not give any secondary ion attributed to the loss of a hexosyl residue (glucosyl or galactosyl, etc.). This suggests that F is a C-glucoside whose C–C bond between aglycone and glycosyl is difficult to break at a low collision energy of the order of 20 eV (Wolfender et al., 2000; Cuyckens et al., 2000, 2001; Waridel et al., 2001; Cuyckens and Claeys, 2005). Assuming the loss of a glycosyl [M-162] by the molecular ion at m/z 447.0931, a secondary ion at m/z 285.093 would be observed, corresponding to that of luteolin.
LC-MS/MS fragments entered into the Global Natural Product Social Molecular Networking (GNPS) database confirm that compound F has a 90% chance of being vitexin-like with excess hydroxyl. The mass spectrum gives m/z peaks grouped in Table 3. The F spectrum obtained from the molecular ion at m/z 449 shows the same characteristic fragmentation of flavonoid C-glycosides observed previously. The masses of all F fragments (Table 3) differ from those of E (Table 2), by the absence of ions at m/z 283 [0.2X+ -CH2O2], 300 [0.2X+-CHO] 311 [0.2X+-H2O], 195 (1.3A+) and 137 (0.2B+) observed with E. This indicates that E is not a 6-C isomer. Indeed, according to Waridel et al. in 2001 a fragmentation of the C cycle in the 8 C-glycosides is rarely observed (Waridel et al., 2001). Compound F is believed to be 8 C-glycoside luteolin. These results are confirmed by those of proton NMR.
The anomeric proton at 4.91 ppm subdivides the 1H-NMR spectrum into two main zones: the 9 to 6 ppm zone corresponds to aglycone protons, and the 4 to 3 ppm zone corresponds to sugar protons.
The analysis of the 1H-NMR spectrum in the 9 to 6 ppm range shows similarities with that of compound E. Like E, F is a monoglycosylated derivative of luteolin. The spectrum shows, respectively, the aromatic proton signals of the aglycone of a flavone substituted at positions 5; 6 or 8; 7; 3′ and 4′: δH-3 (6.55 ppm, s), δH-6 (6.49 ppm, s), (δH-2′ = 7.36 ppm br), δH-5′ (6.91 ppm, d, J = 8.4 Hz) and δH-6′ (7.40 ppm, d, J = 7.23 Hz) [167, 173, 174]. Also, on the 1H-NMR spectra, the range for aglycone is in perfect agreement with data from the literature of other C-monoglycosylated luteolin derivatives (Wolfender et al., 2000; Cuyckens et al., 2000, 2001; Waridel et al., 2001; Cuyckens and Claeys, 2005). The chemical shift of the anomeric proton (δH = 4.91 ppm) is characteristic of a flavonoid C-glycoside (Wolfender et al., 2000; Cuyckens et al., 2000, 2001; Waridel et al., 2001; Cuyckens and Claeys, 2005). In addition, the large value of the coupling constant observed for the anomeric proton signal (J = 10.02 Hz) suggests that it is in the form β-D-glucopyranose.
The COSY spectrum shows no coupling between the anomeric proton (δH1″ = 4.91 ppm) and one of the aglycone protons. This suggests that the glycosyl on the aglycone is either in the 6-C position or in the 8-C position. The presence of the proton at δH 6.55 ppm (H-3) leads to rule out the hypothesis of a flavonol-type structure. Compound F is therefore a flavone. The HSQC, HMBC and carbon 13C spectra were essential for proton allocation and molecular structure determination.
Analysis of the HSQC spectrum, which is a correlation of protons to the carbon nuclei to which they are directly attached (the JC-H direct correlation), shows that the 7.36 ppm proton is related to a 120.5 ppm carbon. This carbon would be a carbon of the aromatic rings that appear between 100 and 140 ppm (Wolfender et al., 2000; Cuyckens et al., 2000, 2001; Waridel et al., 2001; Cuyckens and Claeys, 2005). In an analysis similar to that conducted for the structural determination of E, compound F was identified as luteolin 8-C-β-galactoside (Figure 13).
Figure 13.
Structure of compound F.
4. Conclusion
It is the first time that organic solvent extracts of C. giganteus were studied. It is also the first time that flavonoids could be isolated from this plant. The study, focused on methanol extracts from the leaves of C. giganteus, allowed to evaluate total flavonoids contents of the extracts by spectrophotometric methods and isolated trees flavonoids by chromatographic and spectral methods combination. The evaluation of the flavonoid content showed that the methanol extracts were rich in flavonoids (270 μg EQ/mg). HPTLC analysis showed that the extracts contained a majority of flavonoids. The chromatographic techniques succession allowed to isolate epicatechin, 8-C-glucoside luteolin and 6-C-glucoside luteolin whose structures have been confirmed by NMR. The results show that the extracts of C. giganteus could be a natural source of flavonoids. However, a study on the effectiveness of the isolated products is needed to orient their application.
Declarations
Author contribution statement
Rémy K. Bationo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Constantin M. Dabiré: Analyzed and interpreted the data; Wrote the paper.
Adama Hema: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Roger H. Ch. Nébié, Eloi Palé, Mouhoussine Nacro: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are gratefull to Pascal Lemière for HPLC analysis in UMR 7053 - L2CM and Mass Spectrometry team of Université de Lorraine. We would like to thank the team of the Service de Coopération et d’Action Culturelle (SCAC) and the team of pharmacognosy laboratory of University of Lorraine in Nancy.
References
- Anthoni J., Humeau C., Maia E., Chebil L., Engasser J.-M., Ghoul M. Enzymatic synthesis of oligoesculin: structure and biological activities characterizations. Eur. Food Res. Technol. 2010;231:571–579. [Google Scholar]
- Bationo Rémy K., Ouattara Lamoussa P., Dabire Constantin M., Hema Adama, Sanon Souleymane, Sirima Sodiomon B., Nebie Roger Ch.H., Pale Eloi, Nacro Mouhoussine. In vitro evaluation of the antiplasmodial activity of cymbopogon giganteus extracts from Burkina Faso. J. Soc. Ouest-Afr. Chim. 2017;44:7–14. [Google Scholar]
- Bationo Rémy K., Dabire Constantin M., Hema Adama, Bonzi Shemaeza, Nebie Roger H Ch, Pale Eloi, Somda Irénée, Dhanabal S.P., Nacro Mouhoussine. Phytochemical screening, total phenolics, flavonoids contents, total antioxidant capacity and antifungal activity of cymbopogon giganteus extracts from Burkina Faso. Nat. Prod. Ind. J. 2017;13(2):1141–11412. [Google Scholar]
- Bationo Rémy K. Joseph KI-ZERBO University of Ouagadougou Burkina Faso; 2019. Phytochemical Study and Evaluation of the Biological Properties of Extracts from Different Organs of Cymbopogon Giganteus; p. 192. PhD Thesis. [Google Scholar]
- Cuyckens F., Claeys M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004;39(1):1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- Cuyckens F., Claeys M. Determination of the glycosylation site in flavonoid mono-O'glycosides by collision-induced dissociation of electrospray-generated deprotonated and sodiated molecules. J. Mass Spectrom. 2005;40(3):364–372. doi: 10.1002/jms.794. [DOI] [PubMed] [Google Scholar]
- Cuyckens F., Ma Y.L., Pocsfalvi G., Claeys M. Tandem mass spectral strategies for the structural characterization of flavonoid glycosides. Analusis. 2000;28(10):888–895A. [Google Scholar]
- Cuyckens F., Rozenberg R., de Hoffmann E., Claeys M. Structure characterization of flavonoid O-diglycosides by positive and negative nano-electrospray ionization ion trap mass spectrometry. J. Mass Spectrom. 2001;36(11):1203–1210. doi: 10.1002/jms.224. [DOI] [PubMed] [Google Scholar]
- Davis B.D., Brodbelt J.S. LC-MSn methods for saccharide characterization of monoglycosyl flavonoids using postcolumn manganese complexation. Anal. Chem. 2005;77(6):1883–1890. doi: 10.1021/ac048374o. [DOI] [PubMed] [Google Scholar]
- Eruygur N., Koçyiğit U.M., Taslimi P., Ataş M., Tekin M., Gülçin İ. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. South Afr. J. Bot. 2018;120:141–145. [Google Scholar]
- Fabre N., Rustan I., de Hoffmann E., Quetin-Leclercq J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001;12(6):707–715. doi: 10.1016/S1044-0305(01)00226-4. [DOI] [PubMed] [Google Scholar]
- Lhuillier A., Fabre N., Moyano F., Martins N., Claparols C., Fourasté I., Moulis C. Comparison of flavonoid profiles of Agauria salicifolia (Ericaceae) by liquid chromatography-UV diode array detection–electrospray ionisation mass spectrometry. J. Chromatogr. A. 2007 doi: 10.1016/j.chroma.2007.03.038. In Press. [DOI] [PubMed] [Google Scholar]
- Markham K.R., Ternai B. Carbon-13 NMR of flavonoids-II: flavonoids other then flavone and flavonol aglycones. Tetrahedron. 2005;32:2607–2612. [Google Scholar]
- Michel T., Destandau E., Elfakir C. 2010. Development of an on-line centrifugal partition chromatography (CPC) coupled with high performance liquid chromatography (CLHP) for the fractionation of flavonoids from hippophaë rhamnoides berries. (VIth International Symposium on Counter Current Chromatography (CCC) Lyon, France). [Google Scholar]
- Michel T., Destandau E., Elfakir C. On-line hyphenation of centrifugal partition chromatography (CPC) and high pressure liquid chromatography (CLHP) for the fractionation of flavonoids from hippophaë rhamnoides L. Berries. J. Chromatogr. A. 2011;1218:6173–6178. doi: 10.1016/j.chroma.2011.01.070. [DOI] [PubMed] [Google Scholar]
- Moussa Idrissa, Djibo Alfa K., Ikhiri Khalid, Poupat Christiane, Ahond Alain. Activité cytotoxique et Antivirale de Cymbopogon giganteus (poaceae) J. Soc. Ouest-Afr. Chim. 2012;34:35–37. [Google Scholar]
- Ouattara-Sourabie P.B., Nikiema P.A., Traore A. Antifungal activity of Hyptis spicigera (Lamiaceae) extracts and essential oils of Cymbopogon citratus (Poaceae) and Cymbopogon giganteus against the growth of Aspergillus strains isolated in Bur ina Faso. J. Pharm. Pharmacol. 2017;7:17–27. [Google Scholar]
- Silverstein R.M., Basler G.C., Morill T.C. 5ème éd. De Boeck & Larcier; Paris: 1998. Identification spectrometrique de composés organique; p. 420. [Google Scholar]
- Uzan E., Portet B., Lubrano C., Milesi S., Favel A., Lesage-Meessen L. Pycnoporus laccase-mediated bioconversion of rutin to oligomers suitable for biotechnology applications. Appl. Microbiol. Biotechnol. 2011;90:97–105. doi: 10.1007/s00253-010-3075-4. [DOI] [PubMed] [Google Scholar]
- Wagner H., Bladt S. second ed. 1996. Plant Drug Analysis. Munich. [Google Scholar]
- Waridel Patrice, Wolfender Jean-Luc, Ndjoko Karine, Hobby Kirsten R., Major Hilary J., Hostettmann Kurt. Evaluation of quadrupole time-of-flight tandem mass spectrometry and ion-trap multiple-stage mass spectrometry for the differentiation of C-glycosidic flavonoid isomers. J. Chromatogr. A. 2001;926:29–41. doi: 10.1016/s0021-9673(01)00806-8. [DOI] [PubMed] [Google Scholar]
- Wolfender J.L., Waridel P., Ndjoko K., Hobby K.R., Major H.J., Hostettmann K. Evaluation of Q-TOFMS/MS and multiple stage IT-MSn for the dereplication of flavonoids and related compounds in crude plant extracts. Analusis. 2000;28(10):895–906. [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.