Abstract
Expression of heat shock genes is controlled in Escherichia coli by the antagonistic action of the ς32 subunit of RNA polymerase and the DnaK chaperone system, which inactivates ς32 by stress-dependent association and mediates ς32 degradation by the FtsH protease. A stretch of 23 residues (R122 to Q144) conserved among ς32 homologs, termed region C, was proposed to play a role in ς32 degradation, and peptide analysis identified two potential DnaK binding sites central and peripheral to region C. Region C is thus a prime candidate for mediating stress control of ς32, a hypothesis that we tested in the present study. A peptide comprising the central DnaK binding site was an excellent substrate for FtsH, while a peptide comprising the peripheral DnaK binding site was a poor substrate. Replacement of a single hydrophobic residue in each DnaK binding site by negatively charged residues (I123D and F137E) strongly decreased the binding of the peptides to DnaK and the degradation by FtsH. However, introduction of these and additional region C alterations into the ς32 protein did not affect ς32 degradation in vivo and in vitro or DnaK binding in vitro. These findings do not support a role for region C in ς32 control by DnaK and FtsH. Instead, the ς32 mutants had reduced affinities for RNA polymerase and decreased transcriptional activities in vitro and in vivo. Furthermore, cysteines inserted into region C allowed cysteine-specific cross-linking of ς32 to RNA polymerase. Region C thus confers on ς32 a competitive advantage over other ς factors to bind RNA polymerase and thereby contributes to the rapidity of the heat shock response.
The major heat shock proteins (HSPs) of Escherichia coli are molecular chaperones and proteases that constitute a cytosolic system for folding, repair and degradation of proteins (5, 6, 11). Their synthesis is induced as part of the cellular heat shock response after exposure to a large variety of stress conditions which appear to have in common the ability to cause protein misfolding (4, 7, 10, 16, 40). When induced by upshift of the cells to a nonlethal temperature (e.g., 42°C), the heat shock response is transient and consists of a rapid induction phase followed by a shutoff phase starting approximately 5 to 10 min. after upshift.
Expression of HSPs is positively controlled at the transcriptional level by the heat shock promoter-specific ς32 subunit of RNA polymerase, encoded by rpoH (4, 11, 42). Stress-dependent changes in heat shock gene expression are mediated by the antagonistic action of ς32 and negative modulators which act upon ς32 (34–36). These modulators are the DnaK chaperone and its DnaJ and GrpE cochaperones, which inactivate ς32 by direct association and mediate its degradation by proteases (8, 9, 20, 21, 34, 35, 38). Degradation of ς32 is mediated mainly by FtsH (HflB), an ATP-dependent metalloprotease associated with the inner membrane (14, 37, 39, 40). FtsH degrades free ς32 but not RNA polymerase-bound ς32, indicating that protease and RNA polymerase compete for binding to ς32 (40). The role of the chaperones in ς32 degradation is poorly understood. Inactivation of ς32 occurs by association of DnaK and DnaJ with the free form of ς32, thereby preventing its binding to RNA polymerase (8, 9, 20, 22). There is increasing evidence that the sequestration of the DnaK chaperone system through binding to misfolded proteins is a direct determinant of the induction of the heat shock response (4, 7, 37, 40). Conversely, the shutoff of the heat shock response is assumed to result from HSP-mediated repair and degradation of misfolded proteins, which frees the DnaK chaperone system to inactivate ς32 and to promote its degradation. Furthermore, a competition may exist in vivo between ς32 and other sigma factors including ς70 for association with RNA polymerase. This competition is subject to stress-dependent changes and, consequently, leads to alterations in transcriptional activity of ς32 (2).
A central open question is the identity of the binding sites within ς32 for DnaK, DnaJ, FtsH, and the core of RNA polymerase and the functional interplay between these sites. Previous work showed that the in vivo half-life of fusions between N-terminal fragments of ς32 and β-galactosidase increased when a stretch of 23 residues (R122 to Q144), located between conserved regions 2 and 3 of ς32 and termed region C (Fig. 1), is deleted or replaced by other residues (27). Within region C, a segment of 9 amino acids between residues 132 and 140 of ς32 (QRKLFFNLR) is almost entirely conserved within ς32 homologs but not other sigma factors; it was therefore termed the RpoH box (28). This specific conservation strongly suggests a regulatory role for the RpoH box. Consistent with this assumption were the results of a study in which a ς32-derived peptide library was screened for DnaK binding sites. A high-affinity DnaK binding site exists within the RpoH box in the center of region C, and a second binding site was found close to the RpoH box at the periphery of region C (between residues L118 and K125) (25). Based on this peptide analysis, the RpoH box, and possibly the peripheral DnaK binding motif, is a prime candidate for a regulatory site within the ς32 protein which allows binding of DnaK and possibly also degradation by FtsH (25).
FIG. 1.
Mutational alterations of ς32. ς32 is shown schematically, with the locations of conserved regions 1 to 4 and details of region C including the RpoH box indicated. Sequences of two potential DnaK binding sites identified by peptide scanning are boxed, and mutational alterations of amino acid residues introduced into peptides and/or ς32 proteins are indicated.
The aim of the present study is to experimentally investigate the regulatory role of region C, in particular of the two DnaK binding motifs. In contrast to our expectations, we did not find evidence for a role of region C in chaperone binding and degradation by FtsH. Instead, we found that region C was involved in high-affinity binding of ς32 to RNA polymerase, thereby providing to ς32 a competitive advantage over other sigma factors in association with RNA polymerase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Cells of strains BB2019 [GW1000 recA441 sulA11 Δ(argF-lac)U169 supC(Ts) rpoH165(Am) pDMI,1] (8) and BB7089 (C600 thr-1 leuB6 thi-1 lacY supE44 rfbD1 fhuA21 lacIq PA1/lacO-1dnaKJ) (40) were grown aerobically at 30 or 42°C in Luria broth or M9 minimal medium supplemented with 0.2% glucose (M9-Glu) or 0.2% maltose (M9-Mal) as the carbon source, thiamine (20 μg/ml), and appropriate amino acids (50 μg/ml). The growth media were also supplemented with 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG) for BB7089 and kanamycin (40 μg/ml) and ampicillin (100 μg/ml) when required. The wild-type rpoH gene cloned into plasmid pUHE21-2fdΔ12 (9) was used as template for mutagenesis by the method of Kunkel et al. (17). pBAD30 (rpoH) (wild type or mutant) was obtained by cloning the EcoRI-HindIII fragment from pUHE21-2fdΔ12 (rpoH) into pBAD30 (12). For production of hexahistidine-tagged ς32 variants, wild-type and mutant alleles of rpoH were subcloned by inserting the EcoRI-PstI fragment into pUHE211-1 (rpoH) (carboxy-terminal histidine fusion, used for all mutant ς32) or the MluI-HindIII fragment into pUHE212-1 (amino-terminal histidine fusion, used for ς32-F137E) (8, 9). For C-terminal fusion of ς32 to the intein-chitin binding domain, we amplified by PCR a 0.3-kb fragment corresponding to the coding sequence of the C-terminal 80 residues of ς32, using primers P1 (5′-GGAATTCTGCAGGATAAATCATCTAAC) and P2 (5′-GGGGTACCCTTGGCAAAGCACGCTTCAATGGCAGCACGC) and pUHE21-2fdΔ12 (rpoH) as the template. The P1 sequence comprises internal coding sequences of rpoH containing an additional EcoRI site at the 5′ end. The 3′ end of P2 is complementary to the 3′-end coding sequence of rpoH (from the codon for amino acid 276 to the stop codon). The 5′ end of P2 is complementary to the sequence coding for intein (the N-terminal 6 amino acids) and contains a KpnI site. The sequence complementary to the stop codon of rpoH (CAT) was changed to CAC, allowing us to genetically fuse ς32 with the first cysteine of intein. The EcoRI-PstI fragment from pCYB1 (New England Biolabs) was cloned into pUHE21-2fdΔ12 by using as the linker a small DNA fragment containing a multiple-cloning site (XhoI, BglII, and HindIII). The 0.3-kb fragment obtained by PCR was cloned in this pUHE21-2fdΔ12 derivative (EcoRI-KpnI). Finally, the 5′ end of the rpoH gene (EcoRI-PstI) (wild-type or mutant alleles) was cloned into the resulting plasmid.
Half-life determination.
For determination of ς32 stability in strain BB2019, the cells were grown at 30°C in M9-Glu without methionine or IPTG to an optical density at 600 nm of 0.4. A 1-ml volume of culture was labeled for 2 min with 70 μCi of [35S]methionine, and this was followed by chase with unlabeled methionine (final concentration, 200 μg/ml). Aliquots of 100 μl were collected at the indicated times, mixed with 10% (vol/vol) trichloroacetic acid (TCA), and incubated for 15 min on ice. After centrifugation for 15 min at 16.000 × g, the pellets were washed with acetone and resuspended in 50 mM Tris-HCl (pH 8.0)–1% sodium dodecyl sulfate (SDS)–1 mM EDTA. Half of each sample was subjected to SDS-polyacrylamide gel electrophoresis (PAGE), and the other half was used for immunoprecipitation with ς32-specific rabbit antiserum as described previously (41). The immunoprecipitated material was separated by SDS-PAGE and quantified by phosphorimaging with MacBas software (Fuji Film Co.). For half-life determination of ς32 in strain BB7089, the cells were grown in M9-Mal without methionine but with 100 μM IPTG to an optical density at 600 nm of 0.4. Expression of rpoH was induced by addition of arabinose (100 μM) for 5 min. Then [35S]methionine to label the cells and glucose (0.4%) to stop further expression of rpoH were added, and the chase and immunoprecipitation steps were performed as described above.
Protein and peptide purification.
Histidine-tagged ς32 proteins were purified essentially as described previously (8), except that cell lysis was performed with the French press (500 lb/in2) and buffer containing 100 mM NaCl, 20 mM Tris-HCl (pH 7.9), 0.05% sodium deoxycholate, and 1 mM phenylmethylsulfonyl fluoride. After elution from Ni2+-nitrilotriacetic acid columns, the pooled fractions containing ς32 were subjected to gel filtration on a Superose 12 column (Pharmacia) with a mobile buffer containing 40 mM HEPES-KOH (pH 8.0), 100 mM KCl, 0.1 mM EDTA, 10% glycerol, and 1 mM β-mercaptoethanol. A final purification step was performed by using a MonoQ column (HR5/5; Pharmacia) and elution of the bound protein by a linear 100 to 1,000 mM KCl gradient. Untagged ς32 and ς32-F137E were purified as fusion proteins with self-cleavable intein-chitin binding domains on chitin affinity columns as specified by the supplier (New England Biolabs), except that a different running buffer was used (20 mM Hepes-KOH, 500 mM NaCl, 0.5% Triton X-100) and an additional washing step of the cell extract-loaded chitin column with 20 mM HEPES-KOH–500 mM NaCl–5 mM MgCl2–5 mM ATP was performed to elute DnaK bound to ς32. The chitin bound intein-ς32 was eluted from the intein moiety with running buffer containing 50 mM dithiothreitol (DTT), which induces self-splicing, and further purified using a MonoQ column. RNA polymerase (holoenzyme and core), ς70, DnaK, DnaJ, and FtsH were purified (purity of approximately 70% for ς70 and >90% for the other proteins) as described previously (3, 23, 32, 39). Protein concentrations were routinely determined by a Bradford assay (Bio-Rad) with bovine serum albumin (BSA) as the standard and, for ς32, calibrated by the bicinchoninic acid protein assay (Pierce). The peptides used were synthesized by R. Franck (ZMBH, University of Heidelberg, Heidelberg, Germany) and (for ς32-E115-A131-C/I123D) by Jerini Bio Tools (Berlin, Germany). Peptide concentrations were determined by measurement of the absorption at 280 nm.
3H labeling of proteins and in vitro degradation assays.
To assay the degradation of ς32, the proteins were labeled with N-succinimidyl-[2,3-3H]propionate (Amersham) as described previously (9), except that free N-succinimidyl-[2,3-3H]propionate was removed by dialysis against transcription buffer (20 mM Tris-HCl [pH 8.0], 200 mM KCl, 5 mM MgCl2, 1 mM DTT, 5% glycerol). Degradation of labeled ς32 by FtsH was assayed in a purified system adapted from that of Tomoyasu et al. (39). Briefly, FtsH (1.2 μg) preincubated on ice for 30 min with 50 μM Zn2+–25 mM Tris-acetate (pH 8.0)–2.5 mM magnesium acetate (final concentration) was mixed with ς32 (1 μM, final concentration) in a final volume of 20 μl of reaction buffer (50 mM Tris-acetate [pH 8.0], 5 mM magnesium acetate, 2 mM β-mercaptoethanol, 50 mM KCl, 5 mM ATP) and incubated at 42°C. Aliquots of 2 μl were withdrawn at the indicated times, mixed with BSA (0.5 mg/ml) and EDTA (20 mM), and precipitated with TCA (10%, vol/vol). Radioactivity in the supernatant was determined in a scintillation cocktail (Roth) with a Perkin-Elmer counter. To assay the degradation of peptides, the final volume of the reaction mixture was 60 μl and the concentration of peptide was 50 μM. At the indicated time points, aliquots of 18 μl were mixed with 92 μl of 0.5% trifluoroacetic acid to stop the reaction. Products were analyzed by reverse-phase chromatography with a 5 to 80% acetonitrile gradient in 0.1% trifluoroacetic acid.
Analysis of protein interactions.
Association of ς32 with DnaK, DnaJ, and RNA polymerase core enzyme (RNAP core) was determined by gel filtration with a Superdex 200 column essentially as described previously (9). To determine the association of ς32 with RNAP core, 0.5 μM (in competition experiments) or 1 μM ς32 was incubated with 1.5 μM RNAP core for 10 min at 30°C in transcription buffer (20 μl, final volume). To determine the association of ς32 with DnaK, 5 μM DnaK was incubated for 2 h at 30°C in transcription buffer (to discourage oligomerization), mixed with 1 μM ς32 in a final volume of 20 μl, and further incubated for 30 min at 30°C. These mixtures were shifted to ice, adjusted to 100 μl by addition of transcription buffer which for competition experiments contained a 10- or 30-fold excess of unlabeled ς70 or ς32, respectively, and loaded on a Superdex 200 column at 4°C. Labeled ς32 was detected in the elution fractions by liquid scintillation counting.
Cross-linking experiments.
To couple the cysteine-specific cross-linker N-(4-azido-2,3,5,6-tetrafluorobenzyl)-3-maleimidylpropionamide (TFPAM-3) to cysteine-containing ς32 mutant proteins, 50 μl of a 20 μM protein solution was dialyzed against buffer A (20 mM HEPES-KCl [pH 8.0], 200 mM KCl, 5 mM EDTA), incubated for 1 h at 30°C in the dark with a 10-fold molar excess of TFPAM-3, and then dialyzed against buffer B (20 mM HEPES-KOH [pH 8.0], 200 mM KCl, 5 mM MgCl2) to remove free TFPAM-3. All other proteins were dialyzed against buffer B before use. For cross-linking, the proteins were mixed as indicated (150 pmol each) in 20 μl of buffer B and incubated at 30°C for 2 h in the dark. DnaJ (150 pmol) and ATP (5 mM) were added when indicated, and the mixtures were incubated for a further 10 min on ice. Cross-linking was induced on ice by illumination under UV light (360 nm) for 5 min. After addition of sample buffer (18) and boiling for 5 min, the samples were subjected to electrophoresis on SDS–10% polyacrylamide gels. The gels were silver stained or electroblotted onto polyvinylidene difluoride membranes (Amersham) for immunodetection. Immunoblots were developed with a Vistra ECF fluorescence Western blotting kit (Amersham, Inc.), using Fluoroimager and MacBas software (Fuji Film Co.).
RESULTS
In vitro analysis of region C-derived peptides.
To investigate whether region C comprises binding sites for DnaK and FtsH that are responsible for the control of ς32 activity and stability, we designed amino acid alterations predicted to perturb these sites (Fig. 1). For DnaK, this approach is straightforward since two potential binding sites central and peripheral to region C had been identified (25). Furthermore, the consensus sequence motif recognized by DnaK and the sequence features which prevent DnaK binding have been elucidated. The binding motif consists of a hydrophobic core of up to five consecutive hydrophobic residues flanked by segments enriched in basic residues (30), features which are compatible with the architecture of the substrate binding cavity of DnaK (29, 44). Introduction of negatively charged residues into the hydrophobic core prevent DnaK binding (30). These findings provide a rational basis for introducing alterations into the two potential DnaK binding sites central and peripheral to region C. Recent results concerning the substrate specificity of FtsH (40a) led us to speculate that this protease also recognizes hydrophobic stretches within protein sequences. We therefore changed hydrophobic residues within the hydrophobic stretches located in region C to negatively charged residues in order to perturb DnaK binding and degradation by FtsH.
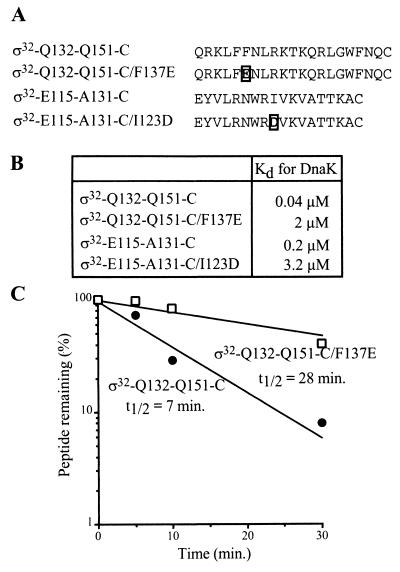
As an experimental starting point, we used peptides comprising either the RpoH box (including the central DnaK binding site) or the peripheral DnaK binding site to test the effects of sequence alterations in vitro. This approach is suitable since peptides can bind DnaK with high affinity in an ATP-dependent fashion (25, 31) and can be degraded efficiently by FtsH in the presence of ATP (40a). A 21-mer peptide comprising the wild-type sequence of the RpoH box (ς32-Q132-Q151-C) has very high affinity for DnaK (Kd, 40 nM) and is rapidly degraded by FtsH in the presence of ATP (t1/2, 7 min) (Fig. 2). To perturb the single DnaK binding motif within the RpoH box, we replaced F137, located in the center of the hydrophobic core, by E (ς32-Q132-Q151-C/F137E). This replacement increased the Kd of the ς32-DnaK complex by 50-fold (Kd, 2 μM) and strongly reduced the efficiency of degradation by FtsH (t1/2, of 28 min) (Fig. 2). A 18-mer peptide comprising the DnaK binding site located peripheral to region C (ς32-E115-A131-C) has high affinity for DnaK (Kd, 0.2 μM) (Fig. 2) but is only slowly degraded by FtsH in the presence of ATP (t1/2, 60 min) (data not shown). A replacement I123 by D, predicted to prevent DnaK binding to its binding site within this peptide (ς32-E115-A131-C/I123D), caused a strong decrease in affinity for DnaK (Kd, 3.4 μM) (Fig. 2) and no observable degradation by FtsH (data not shown).
FIG. 2.
DnaK binding and FtsH-mediated degradation of peptides derived from region C. (A) Amino acid sequences of the peptides used. Mutated residues are boxed. (B) Dissociation constants (Kd) of the peptide-DnaK complexes. The Kd values were determined by peptide titration with fluorescently labeled peptide ς32-Q132-Q144-C-IAANS as competitor as described previously (25). (C) In vitro degradation of peptides by FtsH. Degradation is shown as a percentage of the amount of peptide remaining.
These results show that at the peptide level, the RpoH box contains overlapping or identical recognition sites for DnaK and FtsH which are efficiently perturbed by the F137E exchange and that the N-terminal end of region C contains a binding site for DnaK which is perturbed by the I123D exchange.
In vivo activity of ς32 mutant proteins with altered region C.
The above results formed the basis for a rational design of mutational alterations of region C within the ς32 protein (Fig. 1). rpoH was mutated to introduce the F137E (rpoH-F137E) or I123D mutation (rpoH-I123D), or the rpoH-WRI121,122,123ART mutation. This mutation generates a mutant protein with increased similarity to ς70. The mutant rpoH genes were cloned into plasmid pUHE21-2fdΔ12 (9) such that their expression is controlled by the IPTG-regulatable PA1/lacO-1 promoter. When produced to the levels used in the experiments described below, the three mutant ς32 proteins were recovered in the soluble fractions of cells growing at 30 and 42°C (data not shown). The mutational alterations therefore did not cause structural changes in ς32 leading to aggregation, allowing further analysis of the in vivo activities of the mutant proteins.
We first tested the ability of plasmids containing the rpoH mutant alleles to complement the temperature-sensitive growth of rpoH165(Am) mutant cells (BB2019) in liquid culture and on agar plates. The rpoH-WRI121,122,123ART and rpoH-I123D alleles allowed IPTG-dependent complementation of growth at 42°C, but the colonies were smaller than those formed by cells expressing wild-type rpoH. The rpoH-F137E mutant allele allowed only partial complementation of growth at 42°C, leading to formation of a reduced number of slow-growing colonies (data not shown).
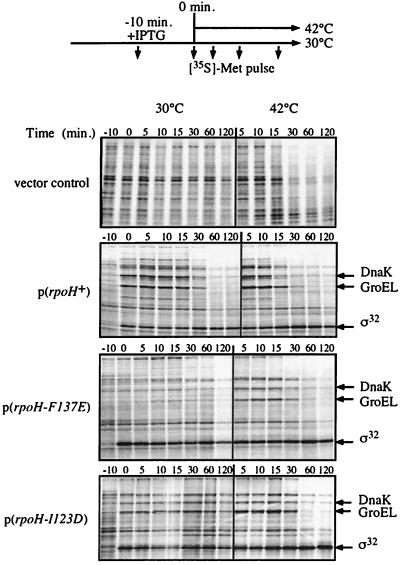
We then determined in pulse experiments the ability of the plasmid-borne mutant alleles to restore the heat shock response in rpoH165(Am) cells after a shift from 30 to 42°C. The induction of expression of the rpoH-WRI121,122,123ART (data not shown) and rpoH-I123D (Fig. 3) alleles by IPTG allowed the induction of expression of heat shock genes at 30 and 42°C. However, the amplitude of the response was two- and fourfold lower for cells expressing rpoH-WRI121,122,123ART and rpoH-I123D, respectively, than for cells expressing wild-type rpoH. Furthermore, the induction of the heat shock response was delayed by 5 to 10 min in cells expressing rpoH-I123D. The induction of expression of the rpoH-F137E allele allowed an increase in heat shock gene expression only after the temperature upshift to 42°C, and the induction of the heat shock response was delayed (10 min) compared to the response in cells expressing wild-type rpoH. However, in cells producing either one of the mutant proteins, a shutoff phase of the heat shock response was observed, suggesting that the DnaK-mediated inactivation of ς32 is operative in vivo. These results correlate well with the growth complementation profile of the mutant allele. Together, these data indicate that the mutational alterations I123D and WRI121,122,123ART of ς32 cause only partial regulatory defects of ς32 whereas the F137E mutation causes stronger regulatory defects in vivo.
FIG. 3.
Ability of rpoH mutant alleles to restore the heat shock response in rpoH165(Am) cells. Cells of strain BB2019 [rpoH165(Am)] which carry plasmids (pUHE21-2fdΔ12) expressing wild-type or mutant rpoH were grown in M9-Glu without methionine. At midexponential growth phase, expression of plasmid-borne rpoH alleles was induced by IPTG (500 μM) for 10 min. The cultures were split and further grown at 30 and 42°C. At the indicated times, aliquots (160 μl) were pulse-labeled with [35S]methionine (7.5 μCi) for 1 min and 40 μl of fivefold-concentrated sample buffer was added. Aliquots were subjected to SDS-PAGE (12% polyacrylamide) followed by development with a phosphorimager. The positions of GroEL, DnaK, and ς32 are indicated.
Proteolysis of ς32 mutant proteins in vivo.
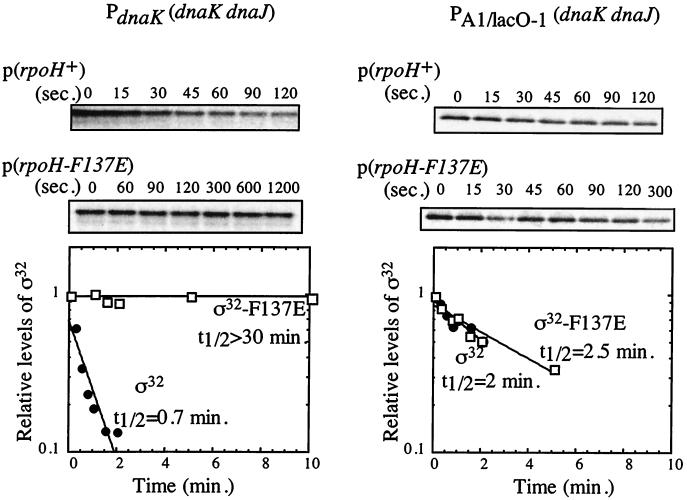
We investigated the effects of the mutational alterations in region C on ς32 stability by performing pulse-chase experiments followed by immunoprecipitation of ς32. The ς32-F137E mutant protein produced from a plasmid was stabilized in rpoH165(Am) mutant cells at 30°C (t1/2, 30 min) (Fig. 4) and at 42°C (t1/2, 17 min [data not shown]). In comparison, wild-type ς32 produced from plasmids was rapidly degraded after prolonged growth at both temperatures (t1/2, ca. 1 min) (Fig. 4).
FIG. 4.
In vivo stability of the ς32-F137E mutant protein at 30°C. Wild-type ς32 and the ς32-F137E mutant protein were produced from plasmids containing rpoH and tested for their stabilities in both BB2019 cells expressing the dnaK and dnaJ genes from authentic ς32-dependent heat shock promoters (left panel) and BB7089 cells expressing the dnaK and dnaJ genes from the IPTG-regulated PA1/lacO-1 promoter. After pulse-labeling with [35S]methionine and a chase step, aliquots were taken at the indicated time points followed by immunoprecipitation of ς32 (top). The bottom panels show quantification of the precipitated proteins relative to time zero. Mean values of the results of at least two experiments are given.
The above experiments were complicated by our finding that the in vivo activity of ς32-F137E was low at 30°C (Fig. 3). Consequently, in the rpoH165(Am) mutant cells producing ς32-F137E, the synthesis of HSPs was reduced compared to that in cells producing wild-type ς32. This reduction includes the synthesis of DnaK and DnaJ, which are limiting for activity and stability control of ς32 (37, 40), and this may profoundly perturb our stability determinations. We therefore tested whether the observed stabilization of ς32-F137E in rpoH165(Am) mutant cells is due to the low levels of DnaK and DnaJ. For this purpose, ς32 stability experiments were performed with a C600 strain derivative (BB7089) in which DnaK and DnaJ levels can be kept constant by replacement of the chromosomal dnaK dnaJ operon by an IPTG-regulatable artificial operon (40). The concentration of IPTG was adjusted such that dnaK and dnaJ were not overexpressed compared to their expression in the parental C600 wild-type strain. Under our experimental conditions for immunoprecipitation, the chromosomally encoded wild-type ς32 was not detectable due to its low abundance. Wild-type and mutant rpoH alleles were expressed from plasmids under the control of an arabinose-inducible promoter (pBAD30) (12), which allows for tight repression in the absence of inducer. This promoter was induced for only a short time (5 min) with 100 μM arabinose, so that the synthesis of ς32 produced from plasmids increased only slightly, just enough to detect labeled ς32 by immunoprecipitation. At this short time after induction of ς32 synthesis, the levels of HSPs were not increased detectably (data not shown). In these cells, only minor differences existed between the half-lives of wild-type ς32 (1.3 ± 1 min) and the three ς32 mutant proteins (1.1 ± 0.1 min for ς32-WRI-ART, 2.8 ± 2 min for ς32-I123D, and 1.6 ± 1 min for ς32-F137E). Figure 4 shows the data for ς32-F137E. These results indicate that the mutations introduced into region C did not affect the half-life of ς32 in vivo provided that the levels of DnaK and DnaJ were kept constant.
Proteolysis of ς32 mutant proteins in vitro.
To further substantiate these in vivo results, we investigated the half-life of each mutated ς32 proteins in vitro by using purified histidine-tagged ς32 and FtsH. The three ς32 mutant proteins had wild-type-like elution profiles during gel filtration and ion-exchange chromatography. Furthermore, they were indistinguishable from the wild type with respect to the proteolysis pattern obtained by partial proteinase K and trypsin digestion (data not shown). We thus have no indication for changes in their overall tertiary structures.
All three mutant proteins (ς32-F137E, ς32-I123D, and ς32-WRI121,122,123ART) were degraded by FtsH in presence of ATP, with similar kinetics to those of wild-type ς32 (Fig. 5 and data not shown). The histidine tags fused to the ς32 proteins were not responsible for the degradation, since the authentic ς32-F137E mutant protein, purified after cleavage from an intein-chitine fusion, was degraded with the same efficiency as the tagged derivative (data not shown). Thus, consistent with the in vivo data, the alterations introduced within region C did not affect the efficiency of ς32 degradation by FtsH in vitro.
FIG. 5.
In vitro degradation of ς32 mutant proteins by FtsH. 3H-labeled ς32 proteins and 3H-labeled ς70 (as control) were incubated with FtsH in the absence or presence of 5 mM ATP, followed by TCA precipitation at the indicated time points. The curves represent the percentage of the radioactivity in the supernatants which contain the proteolytic fragments. ς32 and ς32-I123D have C-terminal histidine tags, and ς32-F137E has an N-terminal histidine tag. N-terminally and C-terminally histidine tagged ς32 did not differ in the kinetics of degradation (data not shown). Open squares, ς32-F137E; solid circles, wild-type ς32; solid triangles, ς32-I123D; open circles, ς70; open triangles, wild-type ς32 without ATP.
DnaK and DnaJ binding to ς32 mutant proteins in vitro.
Several in vitro approaches were used to test whether DnaK and DnaJ binding to ς32 is impaired by the mutational alterations in region C. In one approach, we used gel filtration to detect complexes between ς32 and the chaperones. 3H-labeled wild-type and mutant ς32 proteins, all histidine tag fusions, were incubated with DnaK and subjected to gel filtration to separate DnaK-ς32 complexes from free ς32 (Fig. 6). Under the conditions used, approximately 75% of wild-type 3H-labeled ς32 was recovered in complex with DnaK (eluting in fractions 12 to 17). The 3H-labeled ς32 mutant proteins (ς32-F137E, ς32-I123D, ς32-WRI121,122,123ART) showed similar efficiencies of complex formation, even under chase conditions in which the complexes were separated after the addition of a 30-fold molar excess of unlabeled wild-type ς32. Thus, the mutations introduced into region C of ς32 had no defect in binding of DnaK. For the ς32-F137E mutant protein and wild-type ς32, we verified that these results are also valid for authentic proteins lacking histidine tags (data not shown). Furthermore, no defect in chaperone binding was observed when the ς32 mutant proteins were incubated with DnaK together with DnaJ in the presence of ATP (data not shown).
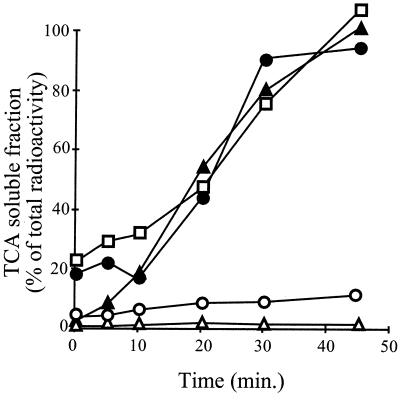
FIG. 6.
Binding of ς32 mutant proteins to DnaK. 3H-labeled ς32, ς32-F137E, and ς32-I123D proteins were incubated with DnaK and the reaction mixtures were subjected to gel filtration either immediately (− competitor) or after further incubation with a 30-fold excess of unlabeled wild-type ς32 (+ competitor). Labeled protein was quantified in the elution fractions and is expressed as a percentage of total radioactivity. The peaks corresponding to the DnaK-ς32 complex and free ς32 are indicated. Solid circles, wild-type ς32; open circles, ς32-I123D; solid triangles, ς32-F137E.
In a second approach, we tested the interaction of DnaK with ς32 proteins by a functional assay which relies on the ability of substrates to stimulate the ATPase activity of DnaK (25, 31). In single-turnover ATPase assays in the presence of DnaJ, wild-type ς32 and the ς32-F137E mutant protein stimulated ATP hydrolysis to similar extents (approximately 10-fold) (data not shown). The ς32-I123D mutant protein stimulated ATP hydrolysis by DnaK efficiently, although to a slightly lower level compared to the two other proteins. We do not consider this difference to be significant with respect to the DnaK-ς32 interaction.
In a third approach, we used plasmon surface resonance spectroscopy to analyze the ability of the ς32 mutant proteins to interact with DnaJ. This method was used previously to detect the interaction of DnaJ with wild-type ς32 (9). We did not observe any difference between the wild type and the three ς32 mutant proteins in affinity for DnaJ (data not shown). Taken together, these data indicate that none of the mutational alterations within region C affects the affinity of DnaK and DnaJ for ς32.
RNAP binding of ς32 mutant proteins in vitro.
Our findings that the mutational alterations introduced into region C of ς32 failed to show defects in the interaction with FtsH and DnaK-DnaJ led us to search for other roles for this region. Since several ς32 mutant proteins analyzed in this study had defects in activity, we investigated whether the mutated segments of region C are involved in the interaction of ς32 with the RNAP core enzyme. We determined the efficiency of association of 3H-labeled ς32 with RNAP by using gel filtration. We focused on the ς32-F137E and ς32-I123D mutant proteins, since they showed functional defects in vivo. The relative amounts of the ς32-I123D mutant protein recovered in association with RNAP (eluting in fractions 8 to 15) were similar to those of wild-type ς32. In contrast, the amounts of ς32-F137E recovered in association with RNAP were reduced two- to threefold compared to those of wild-type ς32 (data not shown). We then determined the half-lives of the RNAP holoenzymes in chase experiments as outlined in Fig. 7A. The amounts of holoenzymes containing 3H-labeled wild-type or mutant ς32 were determined by gel filtration at various time points after the addition of excess unlabeled wild-type ς32. Differences in the stability of the mutant ς32-core complexes were evident immediately after addition of the competitor, and 5 min after addition almost no holoenzymes containing the ς32-F137E and ς32-I123D mutant proteins were recovered (Fig. 7B). We therefore chose shorter chase times to determine the half-lives of the holoenzymes (Fig. 7C, left panel). The half-lives of the holoenzymes containing 3H-labeled ς32-F137E (0.7 ± 0.07 min) and 3H-labeled ς32-I123D (0.8 ± 0.2 min) were five- and fourfold reduced, respectively, compared to the half-life of the holoenzyme containing the wild-type 3H-labeled ς32 (3.5 ± 0.2 min). For the 3H-labeled ς32-F137E mutant protein, we performed additional experiments with a 10-fold excess of ς70 as competitor and found a 6-fold decrease in the half-life (6.4 ± 0.6 min for the holoenzyme containing 3H-labeled ς32 and 1 ± 0.1 min for the holoenzyme containing 3H-labeled ς32-F137E) (Fig. 7C, right panel). These results show that the mutational alterations in region C decrease the affinity of ς32 for RNAP by four- to sixfold.
FIG. 7.
Binding of ς32 mutant proteins to RNAP. 3H-labeled ς32 proteins (wild type [WT], ς32-F137E, and ς32-I123D) (A) were incubated with RNAP, and a 30-fold excess of wild-type unlabeled ς32 (B and C, left) or a 10-fold excess of unlabeled ς70 (C, right) was added. At the indicated times, samples were subjected to gel filtration, and the amount of labeled protein was quantified and is expressed as the percentage of the total labeled protein (B) or as a relative amount of RNAP-bound ς32 recovered at time zero after the addition of competitor (C). (B) Open circles, 0-min chase with competitor; solid squares, 5-min chase; solid triangles, 60-min chase. (C) Open squares, ς32-F137E; solid circles, wild-type ς32; solid triangles, ς32-I123D.
Transcriptional activity of ς32 mutant proteins in vitro.
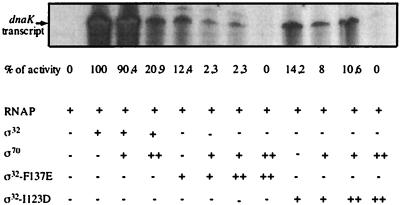
The reduced affinity of the ς32 mutant proteins for RNAP may have consequences for their activity in the transcription of heat shock genes, in particular in a situation of competition with other sigma factors. This possibility was tested by runoff transcription assays with the ς32-dependent P2 heat shock promoter of the dnaK dnaJ operon as a template. The reactions were performed in the presence or absence of ς70 as competitor, with the ς32-F137E and ς32-I123D mutant proteins, since they had reduced activities in vivo and reduced ability to compete with ς70 for RNAP binding in vitro. In the absence of competitor, both ς32 mutant proteins had strongly reduced activities compared to wild-type ς32 (Fig. 8). Moreover, the addition of equimolar concentrations of ς70 was sufficient to strongly reduce the activities of both ς32 mutant proteins, whereas the activity of wild-type ς32 was affected only in the presence of a fivefold excess of ς70 (Fig. 8). The ς32-F137E and ς32-I123D mutant proteins thus had reduced activities in heat shock gene transcription, which were further reduced in the presence of ς70 as competitor.
FIG. 8.
In vitro transcriptional activity of ς32 mutant proteins. Runoff transcription assays were performed in transcription buffer (20 mM Tris-HCl [pH 8.0], 200 mM KCl, 5 mM MgCl2, 1 mM DTT, 5% glycerol) as described previously (9), with a template consisting of a linear 360-bp DNA fragment (blunt ends) containing the P2 promoter of dnaK. The transcription assay mixtures contained 120 nM RNAP, ς70, wild-type ς32, ς32-F137E, or ς32-I123D as indicated. ++, fivefold molar excess (600 nM) of the corresponding protein over the other proteins in the assay. Transcripts were analyzed by polyacrylamide-urea gel electrophoresis (9) followed by autoradiography. The relative amounts of transcripts were quantified and are expressed as a percentage of the transcript obtained with wild-type ς32 in the absence of ς70 (defined as 100%).
Cross-linking of region C of ς32 to RNAP.
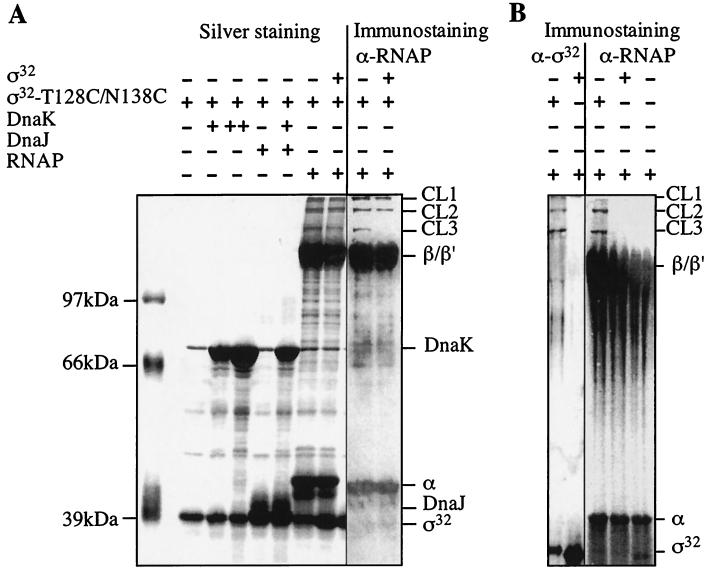
To obtain physical evidence for a role of region C in the association of ς32 with RNAP, we determined by cysteine-specific cross-linking whether region C is surface exposed within ς32 and in proximity to the RNAP in the holoenzyme. This approach was facilitated by the fact that ς32 lacks cysteine, which allowed us to specifically engineer cysteines into region C. Plasmid-borne rpoH was mutagenized to encode a ς32 mutant protein (ς32-TN128,138CC) which has two residues within region C replaced by cysteines (Fig. 1). The ς32-TN128,138CC protein retained full activity in vivo, was a soluble monomer when purified as His tag fusion, and showed similar efficiencies in complex formation with DnaK and RNAP to those of wild-type ς32 (data not shown).
After coupling of the cysteine-specific, heterobifunctional cross-linker TFPAM-3 with purified ς32-TN128,138CC, we tested whether DnaK, DnaJ, and RNAP can be specifically cross-linked. To control for nonspecific cross-linking of proteins to ς32-TN128,138CC we tested several unrelated proteins (BSA, lysozyme, immunoglobulin G [data not shown]) and wild-type ς32 treated with TFPAM-3 (Fig. 9B). In the presence of a high concentration of DnaK, DnaJ, and DnaK plus DnaJ and ATP, no specific cross-linking product was observed in silver-stained gels (Fig. 9A) or after immunostaining (data not shown). In contrast, three low-abundance cross-linking products of more than 100 kDa (CL1, CL2, and CL3) formed in the presence of RNAP. These products were not generated when wild-type ς32 lacking cysteines was used (Fig. 9B). Immunostaining revealed that the cross-linking products contain RNAP and ς32-TN128,138CC (Fig. 9). The amount of the lower-molecular-weight band (CL3) strongly decreased in the presence of an equimolar amount of wild-type ς32 as competitor and thus shows specificity (Fig. 9A). The amounts of the other two cross-linking products (CL1 and CL2) showed only a slight but detectable reduction upon addition of ς32. These results are consistent with a direct role of region C in the association of ς32 with RNAP but not with DnaK and DnaJ.
FIG. 9.
Cysteine-specific cross-linking of ς32-TN128,138CC with RNAP. RNAP, DnaK, wild-type ς32, or ς32-TN128,138CC proteins were mixed as indicated (+, 150 pmol; ++, 450 pmol). After exposure to UV light, the samples were separated by SDS-PAGE followed by silver staining or immunostaining with ς32- or RNAP-specific antiserum. Cross-linking products (CL1, CL2, and CL3), DnaK, DnaJ, ς32, and subunits of RNAP (α, β, β′) are indicated.
DISCUSSION
The aim of this study was to establish whether region C plays a role in the regulation of ς32. Such a role had been suggested by (i) the high conservation of region C, in particular of the nonameric RpoH box, specifically among ς32 homologs (28); (ii) demonstration that the in vivo stability of protein fusions between ς32 segments and β-galactosidase strongly increases when region C is deleted or replaced by other residues (27); and (iii) identification at the peptide level of two high-affinity binding sites for DnaK within the RpoH box and peripheral to region C (25).
In view of the above evidence, it seemed plausible to postulate that region C provides recognition sites for DnaK and FtsH which allow the regulation of ς32 activity and stability. To analyze this possibility, we mutationally altered region C by rational design. Using peptides, McCarty et al. found that a 31-mer peptide comprising the wild-type sequence of region C is a high-affinity substrate for DnaK (Kd, ca. 80 nM) (25). This qualifies region C as recognition site for DnaK. We found that the 21-mer peptide comprising only the RpoH box is an excellent substrate for DnaK (Kd, 40 nM), in accordance with our earlier findings (25), but also for FtsH (t1/2, 7 min). Replacement of a single hydrophobic residue in this 21-mer peptide, positioned in the hydrophobic core segment of the DnaK binding motif, by a negatively charged residue (F137E) strongly decreased the affinity for DnaK (Kd, 2 μM) and the efficiency of degradation by FtsH (t1/2, 28 min). To our knowledge, this is the first evidence that a protease and a chaperone recognize the same sequence stretch within a substrate, possibly establishing a competitive relationship allowing kinetic partitioning of the substrate between the chaperone and the protease.
The identification of amino acid substitutions within region C peptides that affect the recognition of DnaK and FtsH provided a rational basis for specific mutagenesis of ς32. It was surprising that ς32 mutant proteins carrying the I123D and F137E substitutions, as well as a third mutant protein carrying the WRI121,122,123ART substitution which renders the ς32 sequence more similar to ς70, showed no defects in affinity for DnaK or in degradation by FtsH. With respect to DnaK, wild-type-like interactions with the ς32 mutant proteins were found in vitro by gel filtration, surface plasmon resonance spectroscopy, and a functional assay for substrate binding to DnaK. Furthermore, in cells producing ς32-F137E, ς32-I123D, or ς32-WRI121,122,123ART, a DnaK-mediated shutoff of the heat shock response was still observed. However, in these cells the amplitude of the heat shock response was reduced, and in cells producing the ς32-F137E and ς32-I123D mutant proteins the induction kinetics were slower than in cells expressing wild-type ς32. These differences are assumed to result from reduced affinities of the ς32 mutant proteins for RNAP (see below). With respect to proteolytic susceptibility, the half-life of each ς32 mutant protein was almost normal in vivo at 30°C, provided that the DnaK and DnaJ levels were adjusted, and in vitro in FtsH- and ATP-dependent degradation assays. It is important to note that the amino acid substitutions in the three ς32 mutant proteins do not appear to cause overall structural changes, as evidenced by the unaltered partial proteolysis pattern and solubility of the proteins. It is therefore unlikely that the ability of DnaK and FtsH to recognize the ς32 mutant proteins is caused by exposure of novel recognition sites induced by unfolding. Together, our data indicate that region C is not a regulatory site in ς32 that is essential for binding of DnaK and degradation by FtsH. Recently, it was proposed that the C termini of protein substrates including λcI and ς32 are determinants for degradation by FtsH (1, 13), although recent experiments from our laboratory question the importance of the C terminus of ς32 for degradation (unpublished results).
In retrospect, the identification of DnaK and FtsH recognition sites at the peptide level did not lead to elucidation of essential DnaK and FtsH recognition sites within the ς32 protein. Several reasons may account for this failure. It is possible that region C plays a nonessential role in the DnaK- and FtsH-dependent regulation. Alternatively, and perhaps more probably, the segments within region C that act as recognition sites for DnaK and FtsH at the peptide level may not be accessible to interactions with these ligands in the context of the folded ς32. For DnaK, this may be caused by helix formation by the respective segments in the folded state of ς32, a conformation that is incompatible with the architecture of the substrate binding cavity (29, 44). It should be emphasized that the approach of using peptides as first indicators for potential chaperone and protease binding sites in protein substrates is not devalued by our findings. However, as expected for folded protein substrates, peptides do not provide information on the accessibility of such sites in the context of a folded protein. Validation of peptide data by transfer of corresponding mutations into the protein substrate is therefore an essential step of this approach. Here we investigated two of approximately seven major DnaK binding regions within the ς32 sequence. Further experiments will be performed to investigate the regulatory potential of the other regions.
Our experimental results provide evidence for a role of region C in the interaction of ς32 with the core enzyme of RNAP. This is consistent with a similar conclusion obtained from an independent study of mutational alterations within the entire ς32 protein (15). By glycerol gradient sedimentation analysis, Joo et al. showed that a ς32 mutant protein altered in region C (F136L) has decreased affinity for RNAP and only partial transcriptional activity in vitro. However, this study did not provide quantitative data on the dissociation rate of the RNAP holoenzyme and did not investigate the in vivo activity of the ς32 mutant protein and the interaction with FtsH and DnaK. Our present work thus considerably extends the findings of Joo et al. We quantified the RNAP binding defects of the ς32-F137E or ς32-I123D mutant proteins by measuring the half-lives of the RNAP holoenzymes in the presence of competitor. For both proteins, a sixfold-decreased half-life was obtained compared to that of the holoenzyme containing wild-type ς32. These defects in core binding are sufficient to account for the reduced activities of the mutant proteins in vivo at 30 and 42°C and in runoff transcription in vitro at 30°C. However, the in vivo activity of the ς32-F137E mutant protein is more dramatically affected than that of the ς32-I123D mutant protein. The reason for this difference is unclear. We speculate that the ς32-F137E mutant protein has additional defects in promoter recognition.
The ability of region C to increase the affinity of ς32 for RNAP has physiological consequences. Region C may provide competitive advantages of both ς32 over other sigma factors for RNAP binding and RNAP over DnaK, DnaJ, and FtsH for ς32 binding. Region C thereby may increase the efficiency and speed by which the heat shock response is induced upon temperature upshift and the efficiency of heat shock gene transcription under steady-state conditions. In fact, only 10 to 30 molecules of ς32 that exist in a cell growing at 30°C (35) are sufficient to produce HSPs that account for at least 5% of the total cytosolic protein (11). The observed delay in the induction of the heat shock response in cells producing the ς32-F137E and ς32-I123D mutant proteins is consistent with this proposed role of region C.
Our cross-linking experiments provide the first indication that region C is in close proximity to the RNAP. It is possible that region C provides directly the physical contacts which increase the affinity of ς32 for RNAP core. However, since the sequence of region C is absent in other sigma factors not belonging to the ς32 branch, it is clear that this region cannot be involved in essential contacts to RNAP but that it seems to be required to enhance affinity. Several regions within the polypeptide chains of ς32 and ς70, including the conserved regions 2.1., 2.2., 3, and 4, have been implicated in core interaction (15, 19, 24, 26, 33, 43). This multitude of potential interacting sites suggests a high structural complexity of the sigma factor-RNAP interaction which may be fully understood only upon elucidation of the atomic structures of the involved proteins.
ACKNOWLEDGMENTS
We thank T. Laufen for assistance with the ATPase assays, S. Rüdiger for determination of the Kd of DnaK-peptide complexes, K. Paal for analysis by plasmon surface resonance spectroscopy, and M. P. Mayer and T. Ogura for fruitful discussions and comments on the manuscript.
F.A. is a recipient of a Marie Curie training grant of the EEC. This work was support by a grant of the Deutsche Forschungsgemeinschaft to B.B. and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Blaszczak A, Georgopoulos C, Liberek K. On the mechanism of FtsH-dependent degradation of the ς32 transcriptional regulator of Escherichia coli and the role of the DnaK chaperone machine. Mol Microbiol. 1999;31:157–166. doi: 10.1046/j.1365-2958.1999.01155.x. [DOI] [PubMed] [Google Scholar]
- 2.Blaszczak A, Zylicz M, Georgopoulos C, Liberek K. Both ambient temperature and the DnaK chaperone machine modulate the heat shock response in Escherichia coli by regulating the switch between ς70 and ς32 factors assembled with RNA polymerase. EMBO J. 1995;14:5085–5093. doi: 10.1002/j.1460-2075.1995.tb00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchberger A, Valencia A, McMacken R, Sander C, Bukau B. The chaperone function of DnaK requires the coupling of ATPase activity with substrate binding through residue E171. EMBO J. 1994;13:1687–1695. doi: 10.1002/j.1460-2075.1994.tb06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukau B. Regulation of the E. coli heat shock response. Mol Microbiol. 1993;9:671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 5.Bukau B, editor. Molecular chaperones and folding catalysts—regulation, cellular function and mechanisms. Amsterdam, The Netherlands: Harwood Academic Publishers; 1999. [Google Scholar]
- 6.Burkholder W F, Gottesman M E. Genetic evidence for the roles of molecular chaperones in Escherichia coli metabolism. In: Bukau B, editor. Molecular chaperones and folding catalysts—regulation, cellular function and mechanisms. Amsterdam, The Netherlands: Harwood Academic Publishers; 1999. pp. 105–138. [Google Scholar]
- 7.Craig E A, Gross C A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991;16:135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- 8.Gamer J, Bujard H, Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, GrpE and the bacterial heat shock transcriptional factor ς32. Cell. 1992;69:833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- 9.Gamer J, Multhaup G, Tomoyasu T, McCarty J S, Rüdiger S, Schönfeld H-J, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the E. coli heat shock transcription factor ς32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 10.Goff S A, Goldberg A L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985;4:587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- 11.Gross C A. Function and regulation of the heat shock proteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1382–1399. [Google Scholar]
- 12.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBad promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman C, Thévenet D, Boulox P, Walker G C, D’Ari R. Degradation of carboxy-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB (FtsH) Genes Dev. 1998;12:1348–1355. doi: 10.1101/gad.12.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman C, Thévenet D, D’Ari R, Bouloc P. Degradation of sigma 32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci USA. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joo D M, Nolte A, Calendar R, Zhou Y N, Jin D J. Multiple regions on the Escherichia coli heat shock transcription factor ς32 determine core RNA polymerase binding specificity. J Bacteriol. 1998;180:1095–1102. doi: 10.1128/jb.180.5.1095-1102.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanemori M, Mori H, Yura T. Induction of heat shock proteins by abnormal proteins results from stabilization and not increased synthesis of sigma 32 in Escherichia coli. J Bacteriol. 1994;176:5648–5653. doi: 10.1128/jb.176.18.5648-5653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel T A, Bebenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lesley S A, Burgess R R. Characterization of the Escherichia coli transcription factor sigma 70: localization of a region involved in the interaction with core RNA polymerase. Biochemistry. 1989;28:7728–7734. doi: 10.1021/bi00445a031. [DOI] [PubMed] [Google Scholar]
- 20.Liberek K, Galitski T P, Zylicz M, Georgopoulos C. The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the ς32 transcription factor. Proc Natl Acad Sci USA. 1992;89:3516–3520. doi: 10.1073/pnas.89.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberek K, Georgopoulos C. Autoregulation of the Escherichia coli heat shock response by the DnaK and DnaJ heat shock proteins. Proc Natl Acad Sci USA. 1993;90:11019–11023. doi: 10.1073/pnas.90.23.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberek K, Wall D, Georgopoulos C. The DnaJ chaperone catalytically activates the DnaK chaperone to preferentially bind the sigma 32 heat shock transcriptional regulator. Proc Natl Acad Sci USA. 1995;92:6224–6228. doi: 10.1073/pnas.92.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe P A, Hager D A, Burgess R R. Purification and properties of the ς subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1978;18:1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra A, Severinova E, Darst S A. Crystal structure of a ς70 subunit fragment from E. coli RNA polymerase. Cell. 1996;87:127–136. doi: 10.1016/s0092-8674(00)81329-x. [DOI] [PubMed] [Google Scholar]
- 25.McCarty J S, Rüdiger S, Schönfeld H-J, Schneider-Mergener J, Nakahigashi K, Yura T, Bukau B. Regulatory region C of the E. coli heat shock transcription factor, ς32, constitutes a DnaK binding site and is conserved among eubacteria. J Mol Biol. 1996;256:829–837. doi: 10.1006/jmbi.1996.0129. [DOI] [PubMed] [Google Scholar]
- 26.Nagai H, Shimamoto N. Regions of the Escherichia coli primary sigma factor ς70 that are involved in interaction with RNA polymerase core enzyme. Genes Cells. 1998;2:725–734. doi: 10.1046/j.1365-2443.1997.1600357.x. [DOI] [PubMed] [Google Scholar]
- 27.Nagai H, Yuzawa H, Kanemori M, Yura T. A distinct segment of the ς32 polypeptide is involved in DnaK-mediated negative control of the heat shock response in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:10280–10284. doi: 10.1073/pnas.91.22.10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakahigashi K, Yanagi H, Yura T. Isolation and sequence analysis of rpoH genes encoding sigma 32 homologs from gram negative bacteria: conserved mRNA and protein segments for heat shock regulation. Nucleic Acids Res. 1995;23:4383–4390. [PMC free article] [PubMed] [Google Scholar]
- 29.Rüdiger S, Buchberger A, Bukau B. Interaction of Hsp70 chaperones with substrates. Nat Struct Biol. 1997;4:342–349. doi: 10.1038/nsb0597-342. [DOI] [PubMed] [Google Scholar]
- 30.Rüdiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell R, Jordan R, McMacken R. Kinetic characterization of the ATPase cycle of the DnaK molecular chaperone. Biochemistry. 1998;37:596–607. doi: 10.1021/bi972025p. [DOI] [PubMed] [Google Scholar]
- 32.Schönfeld H-J, Schmidt D, Schröder H, Bukau B. The DnaK chaperone system of Escherichia coli: quaternary structures and interactions of the DnaK and GrpE components. J Biol Chem. 1995;270:2183–2189. doi: 10.1074/jbc.270.5.2183. [DOI] [PubMed] [Google Scholar]
- 33.Severinova E, Severinov K, Fenyö D, Marr M, Brody E N, Roberts J W, Chait B T, Darst S A. Domain organization of the Escherichia coli RNA polymerase ς70 subunit. J Mol Biol. 1996;263:637–647. doi: 10.1006/jmbi.1996.0604. [DOI] [PubMed] [Google Scholar]
- 34.Straus D, Walter W, Gross C. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of ς32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 35.Straus D B, Walter W A, Gross C A. The heat shock response of E. coli is regulated by changes in the concentration of ς32. Nature (London) 1987;329:348–350. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- 36.Straus D B, Walter W A, Gross C A. The activity of ς32 is reduced under conditions of excess heat shock protein production in Escherichia coli. Genes Dev. 1989;3:2003–2010. doi: 10.1101/gad.3.12a.2003. [DOI] [PubMed] [Google Scholar]
- 37.Tatsuta T, Tomoyasu T, Bukau B, Kitagawa M, Mori H, Karata K, Ogura T. Heat shock regulation in the ftsH null mutant of Escherichia coli: dissection of stability and activity control mechanisms of sigma32 in vivo. Mol Microbiol. 1998;30:583–593. doi: 10.1046/j.1365-2958.1998.01091.x. [DOI] [PubMed] [Google Scholar]
- 38.Tilly K, McKittrick N, Zylicz M, Georgopoulos C. The DnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983;34:641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- 39.Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rutman A J, Oppenheim A B, Yura T, Yamanaka K, Niki H, Hiraga S, Ogura T. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor sigma 32. EMBO J. 1995;14:2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomoyasu T, Ogura T, Tatsuta T, Bukau B. Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in E. coli. Mol Microbiol. 1998;30:567–581. doi: 10.1046/j.1365-2958.1998.01090.x. [DOI] [PubMed] [Google Scholar]
- 40a.Tomoyasu, T., and B. Bukau. Unpublished data.
- 41.Yano R, Nagai H, Shiba K, Yura T. A mutation that enhances synthesis of sigma 32 and suppresses temperature-sensitive growth of the rpoH15 mutant of Escherichia coli. J Bacteriol. 1990;172:2124–2130. doi: 10.1128/jb.172.4.2124-2130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yura T, Nagai H, Mori H. Regulation of the heat-shock response in bacteria. Annu Rev Microbiol. 1993;47:321–350. doi: 10.1146/annurev.mi.47.100193.001541. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y N, Walter W A, Gross C A. A mutant sigma 32 with a small deletion in conserved region 3 of sigma has reduced affinity for core RNA polymerase. J Bacteriol. 1992;174:5005–5012. doi: 10.1128/jb.174.15.5005-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu X, Zhao X, Burkholder W F, Gragerov A, Ogata C M, Gottesman M, Hendrickson W A. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]