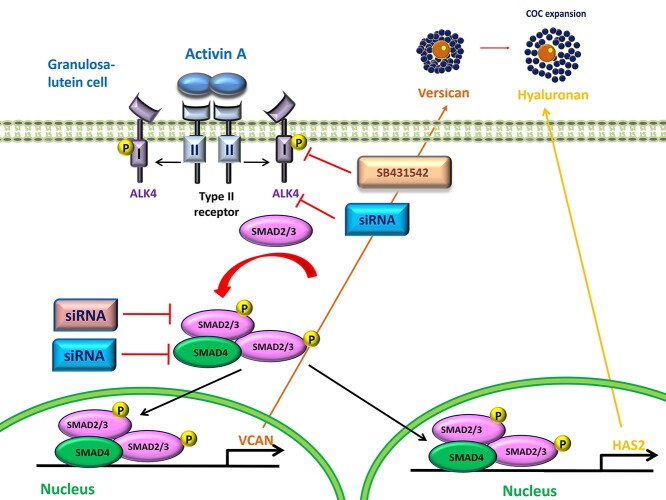
Abstract
Hyaluronan is a structural component of the expanded cumulus matrix, and hyaluronan synthase 2 is the major enzyme for the synthesis of hyaluronan in humans. Versican cross-links the hyaluronan-rich matrix to cumulus cells and is critical for successful ovulation. Activin A is a critical intrafollicular regulator of ovarian function. Although activin A has been shown to promote cumulus matrix expansion in mice, the functional role of activin A in the regulation of cumulus expansion in the human ovary remains to be elucidated. Using primary and immortalized human granulosa-lutein cells as study models, we provide the first data showing that activin A increased the production of hyaluronan by upregulating the expression of hyaluronan synthase 2 in these cells. Additionally, activin A also promoted the expression of the hyaluronan-binding protein versican. Moreover, using inhibitor- and small interfering RNA-mediated inhibition approaches, we found that these stimulatory effects of activin A are most likely mediated through the type I receptor activin receptor-like kinase (ALK4)-mediated Sma- and Mad-related protein (SMAD2)/SMAD3-SMAD4 signaling pathway. Notably, the chromatin immunoprecipitation analyses demonstrated that SMAD4 could bind to human hyaluronan synthase 2 and VERSICAN promoters. The results obtained from this in vitro study suggest that locally produced activin A plays a functional role in the regulation of hyaluronan production and stabilization in human granulosa-lutein cells.
Keywords: activin A, hyaluronan, HAS2, versican, human granulosa cells, ovulation
The results obtained from this in vitro study suggest that a locally produced intraovarian growth factor (activin A) may play a functional role in the regulation of hyaluronan production and stabilization in the human ovary.
Graphical abstract
Introduction
Mammalian ovulation is a complex event that requires synchronization of the oocyte and its surrounding somatic cells [1, 2]. In response to the luteinizing hormone (LH) surge, granulosa cells and theca cells secrete locally produced factors, which promote oocyte maturation, cumulus matrix expansion, rapid angiogenesis, and differentiation of luteal cells [1]. Specifically, the LH surge induces cumulus cells that display a unique pattern of gene expression a few hours prior to ovulation, leading to the production of a hyaluronan-rich extracellular matrix (ECM) that surrounds the cumulus-oocyte complex (COC) [1]. In this regard, appropriate formation and stabilization of the expanded COC is essential for successful ovulation [1].
In the developing COC, the major component and backbone of the expanded cumulus matrix is hyaluronan, a polysaccharide that provides strong osmotic force and viscoelastic properties for the expanded ECM [3]. Mammalian hyaluronan is synthesized by three hyaluronan synthases (HAS1, hyaluronan synthase 2 [HAS2], and HAS3), and HAS2 is rapidly induced by LH in cumulus cells [4]. In addition to the mass production of hyaluronan, the stabilization of the expanded cumulus matrix depends on several cross-linkers that bind hyaluronan through its link module domain [5]. Among those hyaluronan-binding factors, versican is a large proteoglycan that cross-links hyaluronan and interacts with cell surface proteins (e.g., integrins) to anchor the hyaluronan matrix to cumulus cells [1]. In mice, versican is mainly produced by mural granulosa cells. This critical hyaluronan-binding protein is rapidly incorporated into the cumulus matrix, which is subsequently cleaved by the metalloprotease ADAMTS-1 [6]. Targeted depletion of ADAMTS-1 in mice leads to undetectable cleaved versican, showing decreased ovulation rates with strikingly disrupted COC expansion [7].
Belonging to the transforming growth factor-β superfamily, activins are homodimers or heterodimers of inhibin subunits (βA, βB, and βC), which are predominantly expressed in the granulosa cells of developing follicles [8]. The results obtained from animal studies and clinical samples indicate that activins are important intrafollicular regulators of various ovarian functions, including follicular development, ovarian steroidogenesis, oocyte maturation, ECM remodeling, and luteal function [9–13]. Indeed, granulosa cell-specific activin βA subunit knockout mice exhibited subfertility with enhanced corpus luteum accumulation [14]. Systemic deletion of the βA subunit in mice results in death within 24 h [15], while activin βB-deficient mice remain alive but with lower numbers of preovulatory follicles and reduced estrogen levels [16], indicating that activin A is functionally dominant over activin B. Notably, activin A is also a critical regulator in follicular ECM remodeling, as our previous study has shown that activin A promotes lysyl oxidase (a key enzyme essential for ECM formation) activity in human granulosa-lutein (hGL) cells [12]. Furthermore, animal studies using a mouse model showed that activin A enables cumulus matrix expansion in the oocytectomized complex, and this effect is neutralized by the activin inhibitor follistatin [17]. Taken together, these findings suggest that activin A plays an important role in the regulation of cumulus expansion.
Given that the hyaluronan-rich COC matrix is essential for female reproductive function, studies investigating the regulation of hyaluronan synthase and its binding proteins in the ovarian follicle have been a focus of research in this area. Previous studies have shown that activin A promotes ECM formation by increasing versican production in leiomyomas [18]. In mouse periovulatory follicles, activin A induces cumulus expansion by promoting Has2 gene expression [19]. To date, the functional role of activin A in the regulation of HAS2 and versican expression in the human ovary remains to be elucidated. We thus aimed to investigate the effects of activin A on the expression of HAS2 and versican and the underlying molecular mechanisms in hGL cells.
Materials and methods
Cell culture
In this study, we used a nontumorigenic immortalized human granulosa SVOG cell line. This cell line was produced by transfecting hGL cells obtained from women undergoing in vitro fertilization (IVF) with simian virus 40 large T antigen [20]. The SVOG cells were seeded (2–4 × 105 cells/mL) in six-well plates. Cells were cultured in DMEM/F12 medium (Sigma-Aldrich Corp., Oakville, ON) supplemented with 10% charcoal-/dextran-treated fetal bovine serum (HyClone, Logan, UT). The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2.
Primary hGL cells were obtained from patients undergoing IVF procedures with informed consent, following approval from the University of British Columbia Research Ethics Board. The hGL cells were purified by density centrifugation as previously described [21, 22]. Each individual primary culture comprised cells from one individual patient. Purified hGL cells were seeded (2 × 105 cells/mL in six-well plates) and were cultured with DMEM/F12 medium (Sigma-Aldrich Corp.) supplemented with 10% fetal bovine serum (HyClone), 100 U/mL penicillin, and 100 μg/mL streptomycin sulfate (Life Technologies, Inc/BRL, Grand Island, NY). The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. After 24 h, the culture medium was changed to low serum medium (containing 0.5% FBS), and the cells were treated with medium containing a vehicle control (Tris-buffered saline) or exogenous human recombinant activin A (R&D, Minneapolis, MN) for specified time points.
Antibodies and reagents
Monoclonal anti-α-tubulin and anti-HAS2 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, sc-8035 and sc-34068). The anti-versican antibody was obtained from Abcam (Cambridge, MA, ab19345). Polyclonal anti-SMAD4 (9515) and anti-phospho-SMAD2 (138D4) antibodies and monoclonal anti-SMAD2 (L16D3), anti-phospho-SMAD3 (C25A9), and anti-SMAD3 (C67H9) antibodies were obtained from Cell Signaling Technology (Beverly, MA). Peroxidase-conjugated goat anti-mouse (1706520) and goat anti-rabbit (1706515) IgG were obtained from Bio-Rad Laboratories (Hercules, CA). SB431542 (301836-41-9) was obtained from Sigma-Aldrich Corp.
Reverse transcription-quantitative PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Life Technologies, Burlington, ON) according to the manufacturer’s instructions. Reverse transcription was performed using 3 μg RNA, random primers, and M-MLV reverse transcriptase (Promega, Madison, WI). The primers used for SYBR Green reverse transcription-quantitative PCR (RT-qPCR) are presented in Table 1. For each 20 μL TaqMan reaction, 100 ng of cDNA was mixed with 10 mL of 2× TaqMan Gene Expression Master Mix (Applied Biosystems) and 1 mL of 20× TaqMan Gene Expression Probe. The specificity of each assay was validated using dissociation curve analysis and agarose gel electrophoresis of the PCR products. Assay performance was validated by evaluating amplification efficiencies using calibration curves to ensure that the plot of the log input amount versus ΔCt had a slope < |0.1|. The relative quantification of the mRNA levels was performed using the comparative cycle threshold (Ct) method with the formula 2−ΔΔCt, and GAPDH was used as the reference gene.
Table 1.
Primer used in this study
| Sense | Antisense | |
|---|---|---|
| HAS2 | 5′-GACCAAGAGCTGAACAAGATGC-3′ | 5′- GGTGTGATGCCAAAAAGGCA-3′ |
| VCAN | 5´-TCA GCC TAC CTT GTC ATT TTT C-3′ | 5´-CAT TTG ATG CGG AGA AAT TCA C-3′ |
| SMAD2 | 5´-GCC TTT ACA GCT TCT CTG AAC AA-3′ | 5′-ATG TGG CAA TCC TTT TCG AT-3′ |
| SMAD3 | 5´-CCC CAG CAC ATA ATA ACT TGG-3´ | 5′- AGG AGA TGG AGC ACC AGA AG-3´ |
| SMAD4 | 5′-TGG CCC AGG ATC AGT AGG T-3′ | 5´-CAT CAA CAC CAA TTC CAG CA-3′ |
| GAPDH | 5′-GAG TCA ACG GAT TTG GTC GT-3´ | 5´-GAC AAG CTT CCC GTT CTC AG-3´ |
Western blotting
After treatment, cells were lysed in lysis buffer (Cell Signaling Technology) with a protease inhibitor cocktail (Sigma-Aldrich Corp.). Extracts were centrifuged at 20 000 g for 15 min at 4°C, and protein concentrations were measured using a DC Protein Assay (Bio-Rad Laboratories). Equal amounts (40 ng) of protein were separated by 10% SDS-PAGE and were transferred to polyvinylidene fluoride membranes. The membranes were blocked in Tris-buffered solution containing 0.05% Tween 20% and 5% nonfat dried milk for 1 h and were then incubated overnight at 4°C with primary antibodies. The membranes were washed and incubated with a peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG secondary antibody (Bio-Rad) for 1 h. Immunoreactive bands were detected using enhanced chemiluminescence reagents or a SuperSignal West Femto chemiluminescence substrate (Pierce, Rockford, IL), followed by exposure to CL-XPosure film (Thermo Fisher, Waltham, MA). The membranes were stripped with stripping buffer (25 mN glycine-HCl, 10% SDS, 10 mM sodium phosphate, 0.9% (v/v) NaCl) at 50°C for 30 min and were reprobed with the relevant antibodies when necessary. The band intensities were quantified by densitometry (Scion Image software, Scion Corporation, Frederick, MD), and target protein levels were normalized to respective loading control. The results were expressed as fold changes relative to the respective control.
Small interfering RNA transfection
To knock down endogenous ALK4, ALK5, ALK7, SMAD2, SMAD3, and SMAD4, the cells were transfected with 50 nM ON-TARGETplus SMARTpool small interfering RNA (siRNA) (Dharmacon, Lafayette, CO) using Lipofectamine RNAiMAX (Invitrogen, Life Technologies). The siControl non-targeting pool siRNA (Dharmacon) was used as the transfection control. The knockdown efficiency was determined by RT-qPCR and western blotting.
Measurement of hyaluronan
After treatment, the accumulated levels of hyaluronan in culture medium were measured using a hyaluronan Quantikine solid phase sandwich enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, DHYAL0). A human hyaluronan ELISA kit was used according to the manufacturer’s instructions. The inter- and intra-assay coefficients of variation for these assays were 7.7% and 8.9%, respectively. The detection limit of hyaluronan was 0.068 ng/mL. Hyaluronan levels were normalized to the protein concentrations from the cell lysates. Normalized hyaluronan values from the treatments are presented relative to the control.
Chromatin immunoprecipitation assay
The ChIP-IT Express Enzymatic Magnetic Chromatin Immunoprecipitation kit and Enzymatic Shearing kit (Catalog Nos. 53009 and 53035, Active Motif, Carlsbad, CA, USA) were used to conduct the chromatin immunoprecipitation (ChIP) assay according to the manufacturer’s protocol as previously described [23, 24]. The anti-SMAD4 antibody (#46535) and control IgG antibody (#3900) were obtained from Cell Signaling Technology (Beverly, MA). The purified DNA was subjected to RT-qPCR amplification for the SMAD4 binding site located at the human HAS2 and VERSICAN promoters using specific primers: HAS2, forward (5′-GTAGAAGCGAACAGCCCAG -3′) and reverse (5′- CGCGTCTGTCATCCCTGTAG-3′) primers (Figure 9C); VERSICAN, forward (5′-CTGCCAAACTGCTTTTCGCT-3′) and reverse (5′-CCAACACCTTTCCTCAACTGGC-3′) (Figure 9E). The promoter sequences were scanned with transcription factor binding sites matrix obtained from the publicly available JASPAR database (http://jaspar.genereg.net/). We confirmed the selected primers using a silico PCR program (GENOME) and ensured that the generation of an amplicon is from the human genomic DNA.
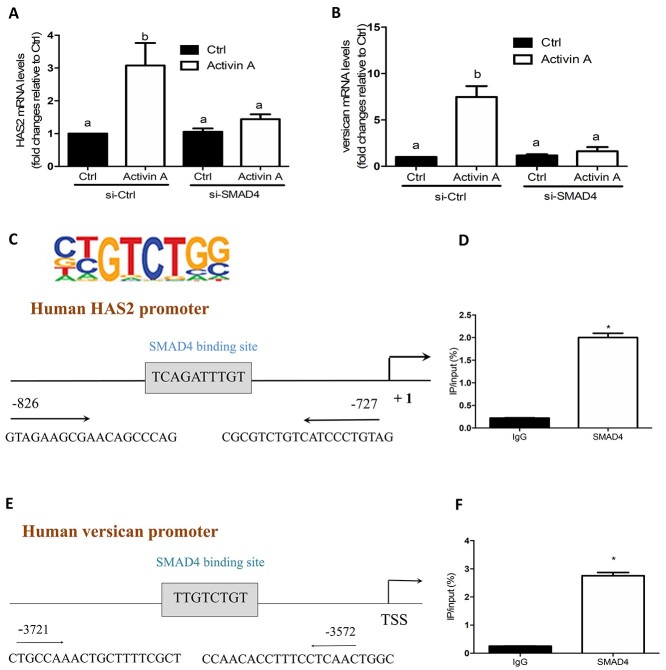
Figure 9.
SMAD4 is required for activin A-induced upregulation of HAS2 and versican expression, and SMAD4 binds to human HAS2 and VERSICAN promoters in primary hGL cells. (A and B) Primary hGL cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siSMAD4 for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA levels of HAS2 (A) and versican (B) were examined using RT-qPCR. (C and E) The SMAD4 binding sites in the human HAS2 and VERSICAN promoter are highlighted by a box. The primers (forward and reverse) for ChIP assay are listed underlined. (D and F) Primary hGL cells were treated with 25 ng/ml of activin A for 6 h before being subjected to a ChIP assay. Cell extracts were precipitated with anti-SMAD4 or control IgG antibodies. Subsequent RT-qPCR was performed using specific primers complementary to the SMAD4 binding site at HAS2 and VERSICAN promoter regions. Percent input method was used to analyze the ChIP-qPCR data. The results are expressed as the mean ± SEM from at least three independent experiments (* indicates P < 0.05). Ctrl, control.
Statistical analysis
The results are presented as the mean ± SEM of at least three independent experiments. The data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests using Prism software (GraphPad Software, Inc., San Diego, CA). Data were considered significantly different if P < 0.05.
Results
Activin A upregulates the expression of HAS2 and versican in SVOG cells
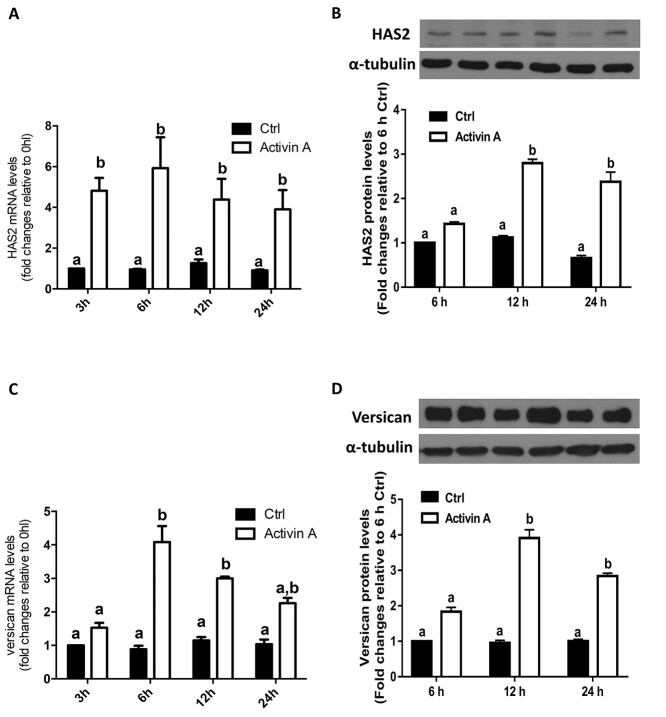
During the periovulatory stage, HAS2 and versican are expressed in mammalian granulosa cells and have been shown to play critical roles in COC expansion [1, 4]. To test the effects of activin A on the expression of HAS2 and versican, we first performed a time-course study in immortalized hGL (SVOG) cells. The SOVG cells were treated with a vehicle control (Ctrl) or 25 ng/mL activin A for 24 h. As shown in Figure 1A, activin A started to increase the mRNA levels of HAS2 at 3 h after treatment, and the effects persisted until 24 h. The western blotting results showed that activin A started to increase the protein levels of HAS2 at 12 h, and the effects persisted until 24 h (Figure 1B). Similarly, treatment with 25 ng/mL activin A significantly increased the mRNA and protein levels of versican starting at 3 h and 12 h, respectively (Figure 1C and D).
Figure 1.
Activin A upregulates the expression of HAS2 and versican in SVOG cells. (A and C) SVOG cells (n = 3) were treated with a vehicle control (Ctrl) or 25 ng/mL activin A for 3, 6, 12, and 24 h, and the mRNA levels of HAS2 (A) and versican (C) were examined using RT-qPCR. (B and D) SVOG cells (n = 3) were treated with Ctrl or 25 ng/mL activin A for 6, 12, and 24 h, and the protein levels of HAS2 (B) and versican (D) were examined using western blotting. The results are expressed as the means ± SEM of at least three independent experiments. Values without common letters are significantly different (P < 0.05).
The TGF-β type I receptor inhibitor SB431532 reverses the activin A-induced upregulation of HAS2 and versican in SVOG cells
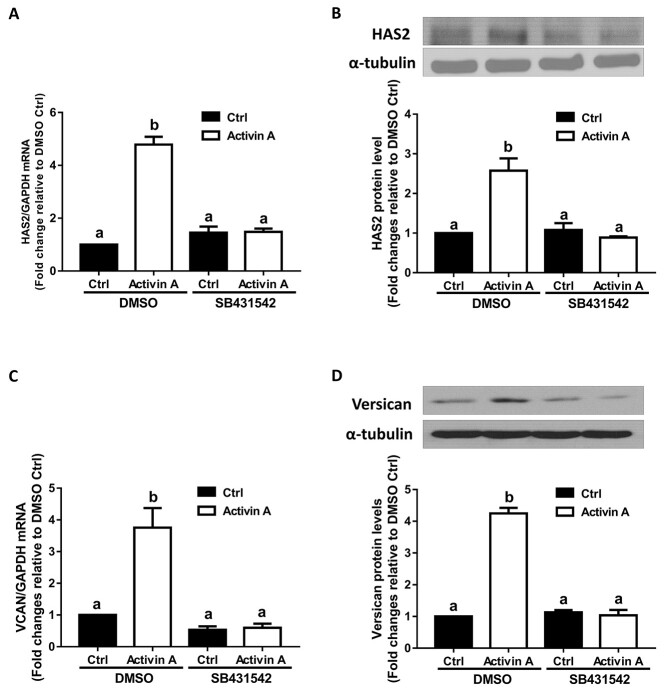
Next, we sought to investigate the molecular mechanisms by which activin A upregulated the expression of HAS2 and versican in hGL cells. Similar to other TGF-β superfamily members, activin A initiates cellular activities by binding to two transmembrane serine/threonine kinase receptors (type I and type II receptors). In most cell types, type II receptor kinases are constitutively active, and upon ligand binding, type I receptors are the principle determinants of signal specificity and transmit intracellular signals by phosphorylating different downstream proteins [25]. To investigate whether the TGF-β type I receptor is involved in the activin A-mediated upregulation of HAS2 and versican expression, we pretreated SVOG cells with DMSO (as a vehicle control) or 10 μM SB431542 (a selective inhibitor of activin receptor-like kinase 4/5/7 (ALK4/5/7)) [26] for 30 min and then treated the cells with 25 ng/mL activin A for an additional 12 h. The results showed that the increased mRNA and protein levels of HAS2 induced by activin A were completely reversed by pretreatment with SB431542 (Figure 2A and B). Similarly, the addition of SB431542 completely reversed the increased mRNA and protein levels of versican in response to activin A (Figure 2C and D). These results indicate that the TGF-β type I receptor is required for the activin A-induced upregulation of HAS2 and versican in SVOG cells.
Figure 2.
The effects of the type I receptor inhibitor SB431542 on the activin A-induced upregulation of HAS2 and versican in SVOG cells. (A and B) SVOG cells (n = 3) were pretreated with DMSO or SB431542 (10 μM) for 30 min and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (A) and protein (B) levels of HAS2 were examined using RT-qPCR and western blotting, respectively. (C and D) SVOG cells (n = 3) were pretreated with DMSO or SB431542 (10 μM) for 30 min and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (C) and protein (D) levels of versican were examined using RT-qPCR and western blotting, respectively. The results are expressed as the means ± SEM of at least three independent experiments. Values without common letters are significantly different (P < 0.05).
SMAD2- and SMAD3-mediated signaling pathways are required for the activin A-induced upregulation of HAS2 and versican in SVOG cells
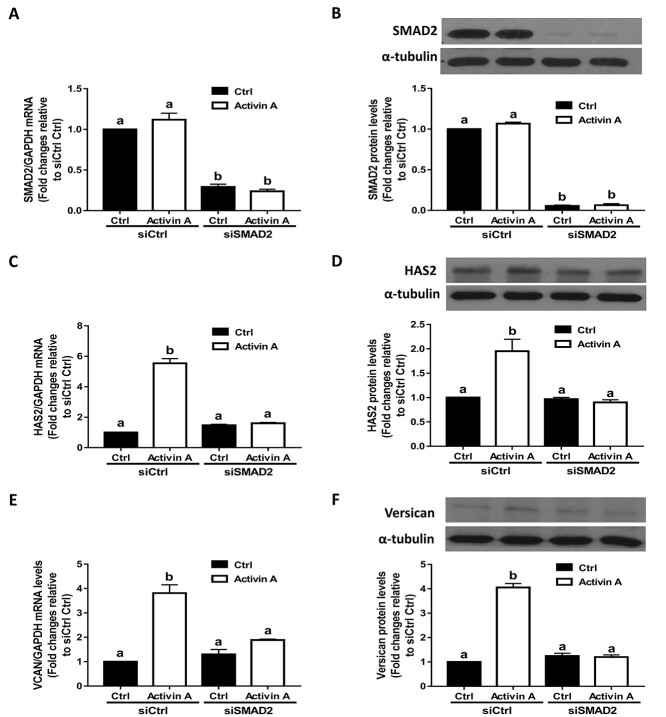
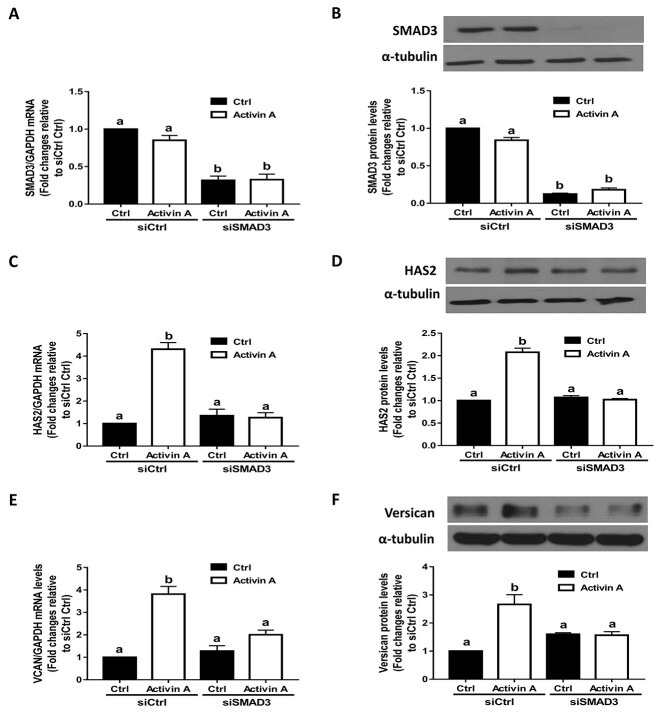
To confirm that activin A can induce the phosphorylation of SMAD2 and SMAD3 in hGL cells, we performed additional experiments using SVOG cells. As shown in Supplementary Figure 1, treatment with 25 ng/mL activin A for 30 min significantly increased the protein levels of phosphorylated SMAD and SMAD3, and these effects were abolished by the addition of kinase inhibitor SB431542 in SVOG cells. To investigate whether the canonical SMAD signaling pathway is involved in the upregulation of HAS2 and versican induced by activin A, we used an siRNA-mediated knockdown approach. As shown in Figure 3A and B, transfection of SVOG cells with siRNAs targeting SMAD2 for 48 h significantly decreased the mRNA and protein levels of SMAD2. Notably, knocking down SMAD2 using siRNAs completely reversed the stimulatory effects of activin A on the expression of HAS2 at both the mRNA and protein levels (Figure 3C and D). Similarly, knockdown of SMAD2 completely reversed the stimulatory effects of activin A on the expression of versican at both the mRNA and protein levels (Figure 3E and F). Using the same inhibition approach, we found that transfection of SVOG cells with siRNAs targeting SMAD3 for 48 h significantly decreased the mRNA and protein levels of SMAD3 (Figure 4A and B). Most importantly, knocking down SMAD3 completely reversed the stimulatory effects of activin A on the expression of HAS2 (Figure 4C and D) and versican (Figure 4E and F) at the mRNA and protein levels in SVOG cells. These results indicate that both SMAD2 and SMAD3 are involved in the activin A-induced upregulation of HAS2 and versican in SVOG cells.
Figure 3.
The effects of SMAD2 knockdown on the activin A-induced upregulation of HAS2 and versican in SVOG cells. (A and B) SVOG cells (n = 3) were transfected with 50 nM control siRNA (siCtrl) or 50 nM siRNA targeting SMAD2 (siSMAD2) for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (A) and protein (B) levels of SMAD2 were examined using RT-qPCR and western blotting, respectively. (C and D) SVOG cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siSMAD2 for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (C) and protein (D) levels of HAS2 were examined using RT-qPCR and western blotting, respectively. (E and F) SVOG cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siSMAD2 for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (E) and protein (F) levels of versican were examined using RT-qPCR and western blotting, respectively. The results are expressed as the means ± SEM. Values without a common letter were significantly different (P < 0.05).
Figure 4.
The effects of SMAD3 knockdown on the activin A-induced upregulation of HAS2 and versican in SVOG cells. (A and B) SVOG cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siRNA targeting SMAD3 (siSMAD3) for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (A) and protein (B) levels of SMAD3 were examined using RT-qPCR and western blotting, respectively. (C and D) SVOG cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siSMAD3 for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (C) and protein (D) levels of HAS2 were examined using RT-qPCR and western blotting, respectively. (E and F) SVOG cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siSMAD3 for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (E) and protein (F) levels of versican were examined using RT-qPCR and western blotting, respectively. The results are expressed as the means ± SEM. Values without a common letter were significantly different (P < 0.05).
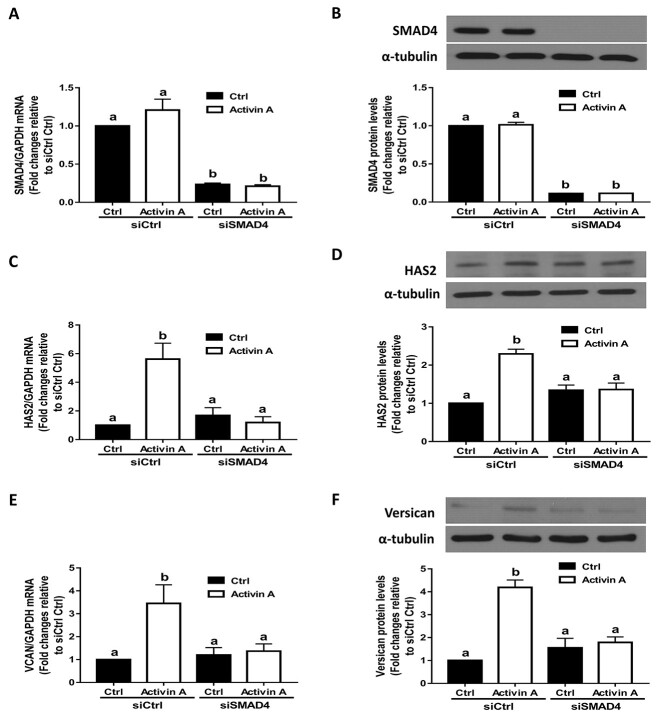
SMAD4 is required for the activin A-induced upregulation of HAS2 and versican expression in SVOG cells
In the SMAD-dependent signaling pathway, SMAD4 is the downstream transcription factor that is associated with R-SMADs. To confirm the functional role of SMAD4 in activin A-induced cellular activity in SVOG cells, we used an siRNA-mediated knockdown approach. The knockdown efficiency was confirmed using RT-qPCR and western blotting. The results showed that transfection with siRNAs targeting SMAD4 for 48 h significantly decreased endogenous mRNA and protein levels of SMAD4 (Figure 5A and B). Notably, activin A-induced increases in the mRNA and protein levels of HAS2 were completely abolished by knocking down SMAD4 (Figure 5C and D). Furthermore, knocking down SMAD4 completely abolished increases in the mRNA and protein levels of versican in response to activin A in SVOG cells (Figure 5E and F).
Figure 5.
The effects of SMAD4 knockdown on the activin A-induced upregulation of HAS2 and versican in SVOG cells. (A and B) SVOG cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siRNA targeting SMAD4 (siSMAD4) for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (A) and protein (B) levels of SMAD4 were examined using RT-qPCR and western blotting, respectively. (C and D) SVOG cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siSMAD4 for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (C) and protein (D) levels of HAS2 were examined using RT-qPCR and western blotting, respectively. (E and F) SVOG cells (n = 3) were transfected with 50 nM siCtrl or 50 nM siSMAD4 for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (E) and protein (F) levels of versican were examined using RT-qPCR and western blotting, respectively. The results are expressed as the means ± SEM. Values without a common letter were significantly different (P < 0.05).
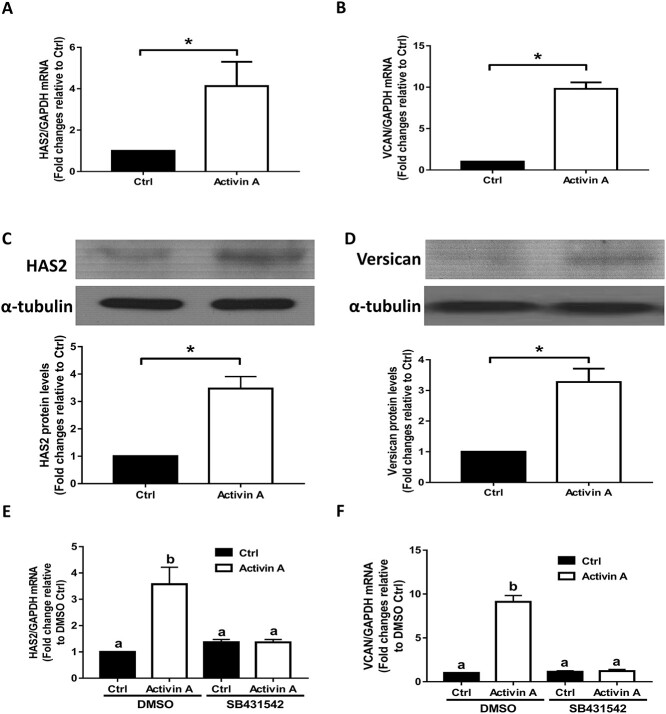
SB431532 reverses the activin A-induced upregulation of HAS2 and versican in primary hGL cells
Using nonimmortalized primary hGL cells obtained from patients undergoing IVF treatment, we further confirmed that treatment with activin A for 12 h significantly increased both the mRNA and protein levels of HAS2 (Figure 6A and C) and versican (Figure 6B and D). In addition, using the TGF-β type I receptor inhibitor SB431542, we found that the addition of SB431542 completely abolished the activin A-induced increase in the mRNA levels of HAS2 and versican in primary hGL cells (Figure 6E and F).
Figure 6.
The effects of the type I receptor inhibitor SB431542 on the activin A-induced upregulation of HAS2 and versican in primary hGL cells. (A and B) Primary hGL cells were treated with Ctrl or 50 ng/mL activin A for 12 h, and the mRNA levels of HAS2 (A) and versican (B) were examined using RT-qPCR. (C and D) Primary hGL cells were treated with Ctrl or 50 ng/mL activin A for 12 h, and the protein levels of HAS2 (C) and versican (D) were examined using western blotting. (E and F) Primary hGL cells were pretreated with DMSO or SB431542 (10 μM) for 30 min and then the cells were treated with Ctrl or 50 ng/mL activin A for an additional 12 h. The mRNA levels of HAS2 (E) and versican (F) were examined using RT-qPCR. The results are expressed as the means ± SEM of at least three independent experiments. Student’s t-test was used to analyze two groups (*P < 0.05). One-way ANOVA was used to analyze multiple groups (values without a common letter were significantly different, P < 0.05).
ALK4 type I receptor is required for activin A-induced hyaluronan production in SVOG and primary hGL cells
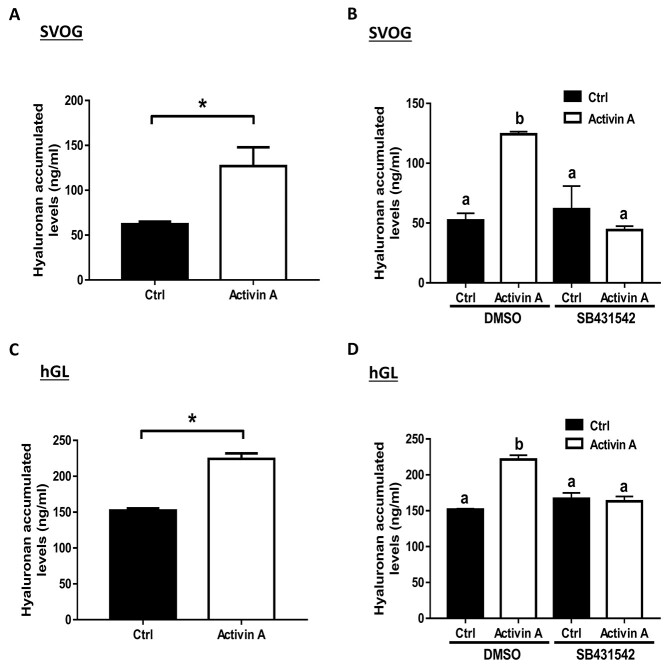
Finally, we sought to investigate whether the activin A-induced upregulation of HAS2 expression contributes to the increased levels of accumulated hyaluronan in SVOG cells. An ELISA was used to examine the accumulated levels of hyaluronan in conditioned medium obtained from cultured SVOG cells, and the results showed that treatment with activin A (25 ng/mL) significantly increased the concentrations of hyaluronan produced by SVOG cells (Figure 7A). Additionally, the addition of SB431542 completely abolished the increased levels of accumulated hyaluronan induced by activin A in SVOG cells (Figure 7B). Similarly, we further confirmed that treatment with activin A (50 ng/mL) significantly increased the concentrations of hyaluronan produced by primary hGL cells, and this stimulatory effect was completely abolished by the addition of SB431542 (Figure 7C and D). These results indicate that either ALK4, ALK5, or ALK7 is involved in activin A-mediated cellular activities in SVOG cells.
Figure 7.
The effects of the type I receptor inhibitor SB431542 on the activin A-induced increase in hyaluronan accumulation in SVOG and primary hGL cells. (A and C) SVOG (A) and primary hGL (C) cells were treated with 25 ng/mL and 50 ng/mL activin A for 12 h, respectively. The accumulated levels of hyaluronan in conditioned medium were measured using ELISA. (B and D) SVOG (B) and primary hGL (D) cells were pretreated with DMSO or SB431542 (10 μM) for 30 min, and the cells were treated with Ctrl or 25 ng/mL (SOVG hGL) and 50 ng/mL (primary hGL) activin A for an additional 12 h. The accumulated levels of hyaluronan in conditioned media were examined using ELISA. The results are expressed as the means ± SEM of at least three independent experiments. Student’s t-test was used to analyze two groups (*P < 0.05). One-way ANOVA was used to analyze multiple groups (values without a common letter were significantly different, P < 0.05).
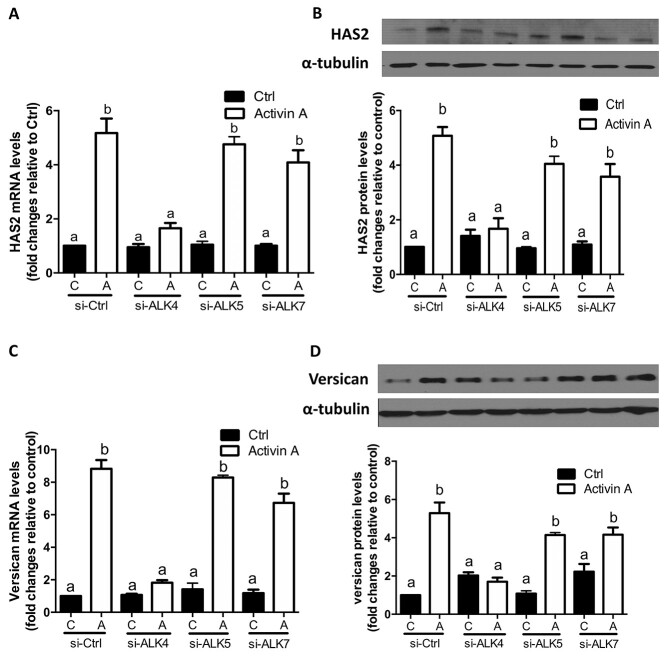
To further determine which ALK type I receptor-mediated activin A-induced cellular activities in primary hGL cells, we used siRNA-based knockdown approach. As shown in Figure 8A and B, transfection of primary hGL cells with siRNA targeting ALK4 completely reversed the stimulatory effects of activin A on the mRNA and protein levels of HAS2. However, siRNA targeting ALK5 or ALK7 did not have this effect. Similarly, targeted knockdown of ALK4 completely reversed activin A-induced increases in the mRNA and protein levels of versican in primary hGL cells, whereas knockdown of ALK5 or ALK7 did not have this effect (Figure 8C and D). Taken together, these results indicate that the activin A-induced upregulation of HAS2 and versican is mediated by ALK4 type I receptor in primary hGL cells.
Figure 8.
The effects of ALK4, ALK5, or ALK7 knockdown on the activin A-induced upregulation of HAS2 and versican in primary hGL cells. (A and B) SVOG cells (n = 3) were transfected with 50 nM siCtrl, 50 nM siRNA targeting ALK4 (siALK4), 50 nM siRNA targeting ALK5 (siALK5), or 50 nM siRNA targeting ALK7 (siALK7) for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (A) and protein (B) levels of HAS2 were examined using RT-qPCR and western blotting, respectively. (C and D) SVOG cells (n = 3) were transfected with 50 nM siCtrl, 50 nM siALK4, 50 nM siALK5, or 50 nM siALK7 for 48 h and then the cells were treated with Ctrl or 25 ng/mL activin A for an additional 12 h. The mRNA (C) and protein (D) levels of versican were examined using RT-qPCR and western blotting, respectively. The results are expressed as the means ± SEM. Values without a common letter were significantly different (P < 0.05).
SMAD4 is required for activin A-induced upregulation of HAS2 and versican expression, and SMAD4 binds to human HAS2 and VERSICAN promoters in primary hGL cells
To confirm the functional role of SMAD4 in activin A-induced cellular activity in primary hGL cells, we used an siRNA-mediated knockdown approach. The results showed that activin A-induced increases in the mRNA levels of HAS2 and versican were completely abolished by knocking down SMAD4 (Figure 9A and B).
At present, there are no data to show that SMAD4 molecules may bind to human HAS2 or VERSICAN promoter in human granulosa cells. We thus investigated the interaction of SMAD4 protein with the human HAS2 and VERSICAN promoters by using a ChIP analysis and the anti-SMAD4 antibody. Putative SMAD binding element was identified in the HAS2 and VERSICAN promoters using the Regulatory Sequence Analysis Tools. Using specific primers targeting human HAS2 and VERSICAN promoters, the purified DNA was subjected to examine by RT-qPCR (Figure 9C and E). The ChIP assay showed that treatment of primary hGL cells with activin A increased the binding of endogenous SMAD4 to the human HAS2 and VERSICAN promoters compared to nonspecific IgG (Figure 9D and F).
Discussion
In the current study, using primary and immortalized hGL cells as study models, we sought to investigate the effects of activin A on the expression of two key proteins contributing to ovarian cumulus expansion, HAS2 and versican. Our results showed that activin A significantly upregulated the expression of HAS2 and increased the production of hyaluronan in hGL cells. Our previous studies demonstrated that rather than HAS1 or HAS3, HAS2 was the principal enzyme responsible for hyaluronan accumulation in hGL cells [27, 28]. In addition to the endocrine system, the expression of HAS2 and production of hyaluronan can be regulated by granulosa cell-derived factors in an autocrine/paracrine manner in mammalian ovarian follicles. We and other research groups have previously reported that locally produced TGF-β1 (granulosa cell-derived), BMP4 (theca cell-derived), and GDF9 (oocyte-derived) can increase hyaluronan production by upregulating the expression of HAS2 in hGL cells [27–29]. Consistent with these results, animal studies also revealed that activin A promotes ovarian cumulus expansion in mice [17]. Activin A has been shown to promote follicular growth and development by increasing the granulosa cell response to FSH stimulation and oocyte maturation [9, 30]. Additionally, our previous studies showed that activin A increases prostaglandin E2 production by upregulating the expression of cyclooxygenase-2 (COX-2) in hGL cells [31]. Indeed, the serum level of activin in humans reaches a highest level at the mid-menstrual cycle [32]. Taken together, these findings suggest that activin A may play a functional role in the regulation of human ovulation.
Additionally, activin A promoted the expression of the hyaluronan-binding protein versican at both the mRNA and protein levels. Interestingly, HAS2 knockout mice and versican-deficient mice share an identical phenotype of defects in the developing heart [33]. Additionally, HAS2 and versican exhibit an overlapping expression pattern in embryonic stem cell differentiation [34], indicating a close functional link between HAS2 and versican. Indeed, a recent study revealed a strongly stabilized interaction between versican fragments and a hyaluronan-rich expanding matrix before ovulation [35]. Moreover, clinical studies have shown that versican levels in cumulus cells are associated with human oocyte development competence [36] and better IVF outcomes [37]. However, the absence of versican during in vitro maturation (IVM) of COC may account for the poor outcome of the IVM procedure [7]. Previous studies and our results clearly indicate that HAS2 and versican tightly cooperate in activin-mediated cumulus expansion. Given the importance of versican in mammalian ovulation, these findings provide a better understanding of how versican is regulated in human granulosa cells.
In the human fetal ovary, the activin-induced SMAD2/3 signaling pathway is involved in granulosa cell growth and follicular development [38]. Additionally, a conditional knockout of Smad2/3 in mice results in minimal ovarian cumulus expansion [39]. In human embryonic stem cells, activin/SMAD facilitates cell differentiation by upregulating the expression of HAS2 [40]. Using an inhibitor-mediated inhibition approach, we showed that the activin A-induced upregulation of HAS2 and versican was completely reversed by the specific TGF-β type I receptor inhibitor SB-431542 in SVOG cells. This result is in agreement with a previous animal study showing that SB-431542 inhibits COC expansion in mice [17]. These results indicate that the type I receptor is required for the activin A-induced regulation of hyaluronan and versican. Similarly, a recent study showed that type I and II receptors are equally functional in the activin-regulated muscle growth [41]. Considering the off-target effect of kinase inhibitor, we used the siRNA-mediate knockdown approach to demonstrate that activin A-induced cellular activities (increases in mRNA levels of HAS2 and versican) in primary hGL cells are mediated by the TGF-β type I receptor ALK4 (ACVR1B) but not by ALK5 (TGFBR1) or ALK7 (ACVR1C). Indeed, recent studies have demonstrated the therapeutic perspective of several ALK inhibitors, including LY-2157299 and SB431542, in tumor progression and stem cell differentiation [42]. Consistent with these results, our previous studies also showed that activin A-induced upregulation of plasminogen activator inhibitor I (PAI-1) is mediated by ALK4 but not by ALK5 nor ALK7 in hGL cells [13].
Using an siRNA-mediated depletion approach, our results showed that knockdown of SMAD2 or SMAD3 completely abolished the activin A-induced upregulation of HAS2 and versican. These results indicate that the canonical SMAD2/3-SMAD4 signaling pathway is required for activin A-induced cellular activities in hGL cells. These results are in agreement with our previous studies showing that SMAD2 and SMAD3 are equally involved in the activin A-induced regulation of the expression of another ovulation-related gene (COX-2) in hGL cells [31]. However, inconsistent with these results, only SMAD3 but not SMAD2 mediates the activin A-induced upregulation of PAI-1 [13], whereas only SMAD2 but not SMAD3 mediates the activin A-induced regulation of StAR expression [11]. Therefore, the specific SMAD (SMAD2 or SMAD3) that mediates activin A-induced gene regulation in hGL cells is target gene-dependent. In the canonical SMAD-dependent signaling pathway, SMAD4 is the downstream transcription factor that is associated with SMAD2 and SMAD3. In this study, we also found that knockdown of SMAD4 completely abolished the stimulatory effects of activin A on the regulation of HAS2 and versican in both SVOG and primary hGL cells. Indeed, our ChIP assay results confirmed that activin A stimulated the binding of endogenous SMAD4 to the human HAS2 and VERSICAN promoters in primary hGL cells. These results are consistent with our previous results showing that SMAD4 is required for the activin A-induced regulation of downstream target genes in hGL cells, indicating that SMAD4 is essential for cumulus expansion in human ovaries [11, 13, 31]. Indeed, the selective knockout of SMAD4 in mouse ovarian follicles results in multiple defects in ovulation [43].
Animal studies using mouse granulosa cells have shown that human BMP15 homodimer and mouse GDF9 enhance COC expansion by upregulating the expression of COC expansion-related genes (including Has2, Ptgs2, and Ptx3) [29]. In this study, our results demonstrated that activin A increases hyaluronan production by upregulating the expression of HAS2 in hGL cells. Our previous studies also showed that BMP4 and BMP15 upregulated the expression of HAS2 and promoted hyaluronan production via a noncanonical SMAD2/SMAD3 signaling pathway [28]. Traditionally BMPs activate SMAD1/5/8 via ALK2/3; however, BMP4 and BMP15 may upregulate the expression of HAS2 via the activation of SMAD2 and SMAD3 in hGL cells [28]. Additionally, studies performed by our research group have shown that TGF-β1 and activin A can promote the production of prostaglandin E2 by upregulating the expression of PTGS2 in hGL cells [31, 44]. All these results provide a new insight into the regulation of COC expansion by members of the TGFβ superfamily in human granulosa cells.
In summary, this study provided the first data showing that activin A increased the production of hyaluronan by upregulating the expression of HAS2 in immortalized and primary hGL cells. Additionally, the important hyaluronan-binding protein versican was also positively regulated by activin A, which may enhance the stabilization of the hyaluronan network. Moreover, these activin A-induced cellular activities were mediated by the TGF-β type I receptor and its downstream SMAD2/SMAD3-SMAD4 signaling pathway. The results obtained from this in vitro study suggest that locally produced activin A plays a functional role in the regulation of hyaluronan production and stabilization in hGL cells, which may provide new insight into the potential functional role of activin A in the modulation of cumulus expansion and ovulation in the human ovary.
Supplementary Material
Acknowledgment
We thank the patients for providing the follicular aspirates used for the purification of primary human granulosa cells.
Contributor Information
Shen Tian, Department of Reproductive Medicine, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China; Department of Obstetrics and Gynaecology, British Columbia Children’s Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada.
Han Zhang, Department of Obstetrics and Gynaecology, British Columbia Children’s Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Center for Reproductive Medicine, Center for Prenatal Diagnosis, Department of Obstetrics and Gynecology, First Hospital, Jilin University, Changchun, Jilin, China.
Hsun-Ming Chang, Department of Obstetrics and Gynaecology, British Columbia Children’s Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Reproductive Medicine Center, Department of Obstetrics and Gynecology, China Medical University Hospital, Taichung, Taiwan.
Christian Klausen, Department of Obstetrics and Gynaecology, British Columbia Children’s Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada.
He-Feng Huang, The Key Laboratory of Reproductive Genetics, Department of Obstetrics and Gynecology, Ministry of Education (Zhejiang University), Hangzhou, Zhejiang, China; Shanghai Key Laboratory of Embryo Original Diseases, Shanghai JiaoTong University, School of Medicine, Shanghai, China.
Min Jin, Department of Reproductive Medicine, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Peter C K Leung, Department of Obstetrics and Gynaecology, British Columbia Children’s Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada.
Data availability
The data used to generate the results for this this article will be shared upon request to the corresponding author.
Authors’ roles
S.T. and H.Z. contributed to the study design, execution, analysis and interpretation of data, article drafting, and critical discussion. C.K. contributed to the study design and analysis of data. H.-M.C. contributed to revise the manuscript critically. H.-F.H. contributed to the interpretation of data and critical discussion. M.J. and P.C.K.L. contributed to the supervision of the study and final approval of the version to be published.
Conflict of Interest The authors have declared that no conflict of interest exists.
References
- 1. Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update 2007; 13:289–312. [DOI] [PubMed] [Google Scholar]
- 2. Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update 2016; 23:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Motte CA, Drazba JA. Viewing hyaluronan: imaging contributes to imagining new roles for this amazing matrix polymer. J Histochem Cytochem 2011; 59:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer APet al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 1999; 274:25085–25092. [DOI] [PubMed] [Google Scholar]
- 5. Nagyova E. The biological role of Hyaluronan-rich oocyte-cumulus extracellular matrix in female reproduction. Int J Mol Sci 2018; 19:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem 2003; 278:42330–42339. [DOI] [PubMed] [Google Scholar]
- 7. Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, Russell DL. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod 2010; 83:549–557. [DOI] [PubMed] [Google Scholar]
- 8. Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P. Chemical and biological characterization of the inhibin family of protein hormones. Recent Prog Horm Res 1988; 44:1–34. [DOI] [PubMed] [Google Scholar]
- 9. Knight PG, Satchell L, Glister C. Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol 2012; 359:53–65. [DOI] [PubMed] [Google Scholar]
- 10. Chang HM, Cheng JC, Klausen C, Taylor EL, Leung PC. Effects of recombinant activins on steroidogenesis in human granulosa-lutein cells. J Clin Endocrinol Metab 2014; 99:E1922–E1932. [DOI] [PubMed] [Google Scholar]
- 11. Chang HM, Cheng JC, Huang HF, Shi FT, Leung PC. Activin A, B and AB decrease progesterone production by down-regulating StAR in human granulosa cells. Mol Cell Endocrinol 2015; 412:290–301. [DOI] [PubMed] [Google Scholar]
- 12. Chang HM, Cheng JC, Liu Y, Klausen C, Xu C, Leung PC. Activin A-induced increase in LOX activity in human granulosa-lutein cells is mediated by CTGF. Reproduction 2016; 152:293–301. [DOI] [PubMed] [Google Scholar]
- 13. Chen B, Chang HM, Zhang Z, Cao Y, Leung PCK. ALK4-SMAD3/4 mediates the effects of activin A on the upregulation of PAI-1 in human granulosa lutein cells. Mol Cell Endocrinol 2020; 505:110731. [DOI] [PubMed] [Google Scholar]
- 14. Pangas SA. Growth factors in ovarian development. Semin Reprod Med 2007; 25:225–234. [DOI] [PubMed] [Google Scholar]
- 15. Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 1995; 374:356–360. [DOI] [PubMed] [Google Scholar]
- 16. Vassalli A, Matzuk MM, Gardner HA, Lee KF, Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev 1994; 8:414–427. [DOI] [PubMed] [Google Scholar]
- 17. Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod 2007; 76:848–857. [DOI] [PubMed] [Google Scholar]
- 18. Islam MS, Protic O, Janjusevic M, Gray PCSRG, Ciavattini A, Lamanna PALT, Petraglia F, Castellucci M, Ciarmela AP. Role of activin-A and myostatin and their signaling pathway in human myometrial and leiomyoma cell function. J Clin Endocrinol Metab 2014; 99:E775–E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilchrist RB, Ritter LJ. Differences in the participation of TGFB superfamily signalling pathways mediating porcine and murine cumulus cell expansion. Reproduction 2011; 142:647–657. [DOI] [PubMed] [Google Scholar]
- 20. Lie BL, Leung E, Leung PC, Auersperg N. Long-term growth and steroidogenic potential of human granulosa-lutein cells immortalized with SV40 large T antigen. Mol Cell Endocrinol 1996; 120:169–176. [DOI] [PubMed] [Google Scholar]
- 21. Chang HM, Klausen C, Leung PC. Antimullerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril 2013; 100:585, e581–592. [DOI] [PubMed] [Google Scholar]
- 22. Chang HM, Fang L, Cheng JC, Taylor EL, Sun YP, Leung PC. Effects of growth differentiation factor 8 on steroidogenesis in human granulosa-lutein cells. Fertil Steril 2016; 105:520–528. [DOI] [PubMed] [Google Scholar]
- 23. Chang HM, Fang Y, Liu PP, Cheng JC, Yang X, Leung PC. Connective tissue growth factor mediates growth differentiation factor 8-induced increase of lysyl oxidase activity in human granulosa-lutein cells. Mol Cell Endocrinol 2016; 434:186–198. [DOI] [PubMed] [Google Scholar]
- 24. Liu C, Chang HM, Yi Y, Fang Y, Zhao F, Leung PCK, Yang X. ALK4-SMAD2/3-SMAD4 signaling mediates the activin A-induced suppression of PTX3 in human granulosa-lutein cells. Mol Cell Endocrinol 2019; 493:110485. [DOI] [PubMed] [Google Scholar]
- 25. Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol 2001; 187:265–276. [DOI] [PubMed] [Google Scholar]
- 26. Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 2002; 62:65–74. [DOI] [PubMed] [Google Scholar]
- 27. Wang F, Chang HM, Yi Y, Li H, Leung PCK. TGF-β1 promotes hyaluronan synthesis by upregulating hyaluronan synthase 2 expression in human granulosa-lutein cells. Cell Signal 2019; 63:109392. [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Tian S, Klausen C, Zhu H, Liu R, Leung PC. Differential activation of noncanonical SMAD2/SMAD3 signaling by bone morphogenetic proteins causes disproportionate induction of hyaluronan production in immortalized human granulosa cells. Mol Cell Endocrinol 2016; 428:17–27. [DOI] [PubMed] [Google Scholar]
- 29. Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A 2013; 110:E776–E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wijayarathna R, Kretser DM. Activins in reproductive biology and beyond. Hum Reprod Update 2016; 22:342–357. [DOI] [PubMed] [Google Scholar]
- 31. Liu PP, Chang HM, Cheng JC, Leung PC. Activin A upregulates PTGS2 expression and increases PGE2 production in human granulosa-lutein cells. Reproduction 2016; 152:655–664. [DOI] [PubMed] [Google Scholar]
- 32. Muttukrishna S, Fowler PA, Groome NP, Mitchell GG, Robertson WR, Knight PG. Serum concentrations of dimeric inhibin during the spontaneous human menstrual cycle and after treatment with exogenous gonadotrophin. Hum Reprod 1994; 9:1634–1642. [DOI] [PubMed] [Google Scholar]
- 33. Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest 2000; 106:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shukla S, Nair R, Rolle MW, Braun KR, Chan CK, Johnson PY, Wight TN, McDevitt TC. Synthesis and organization of hyaluronan and versican by embryonic stem cells undergoing embryoid body differentiation. J Histochem Cytochem 2010; 58:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagyova E, Salustri A, Nemcova L, Scsukova S, Kalous J, Camaioni A. Versican G1 fragment establishes a strongly stabilized interaction with hyaluronan-rich expanding matrix during oocyte maturation. Int J Mol Sci 2020; 21:2267–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen Q, Chen M, Zhao X, Liu Y, Ren X, Zhang L. Versican expression level in cumulus cells is associated with human oocyte developmental competence. Syst Biol Reprod Med 2020; 66:176–184. [DOI] [PubMed] [Google Scholar]
- 37. Ocampo A, Pedraza J, Ortiz G, Hernández-Pérez E, Porchia L, López-Bayghen E. Assessment of prostaglandin-endoperoxide synthase 2 and versican gene expression profile from the cumulus cells: association with better in vitro fertilization outcomes. J Ovarian Res 2018; 11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coutts SM, Childs AJ, Fulton N, Collins C, Bayne RA, McNeilly AS, Anderson RA. Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev Biol 2008; 314:189–199. [DOI] [PubMed] [Google Scholar]
- 39. Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol 2008; 28:7001–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu X, Wang L, Liu B, Xie W, Chen YG. Activin/Smad2 and Wnt/β-catenin up-regulate HAS2 and ALDH3A2 to facilitate mesendoderm differentiation of human embryonic stem cells. J Biol Chem 2018; 293:18444–18453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee SJ, Lehar A, Liu Y, Ly CH, Pham QM, Michaud M, Rydzik R, Youngstrom DW, Shen MM, Kaartinen V, Germain-Lee EL, Rando TA. Functional redundancy of type I and type II receptors in the regulation of skeletal muscle growth by myostatin and activin A. Proc Natl Acad Sci U S A 2020; 117:30907–30917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cui X, Shang S, Lv X, Zhao J, Qi Y, Liu Z. Perspectives of small molecule inhibitors of activin receptor-like kinase in anti-tumor treatment and stem cell differentiation (Review). Mol Med Rep 2019; 19:5053–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu C, Zhang YL, Fan HY. Selective Smad4 knockout in ovarian preovulatory follicles results in multiple defects in ovulation. Mol Endocrinol 2013; 27:966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fang L, Chang HM, Cheng JC, Leung PC, Sun YP. TGF-beta1 induces COX-2 expression and PGE2 production in human granulosa cells through Smad signaling pathways. J Clin Endocrinol Metab 2014; 99:E1217–E1226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to generate the results for this this article will be shared upon request to the corresponding author.