Abstract
Pyruvate dehydrogenase kinases (PDKs)-pyruvate dehydrogenase E1α subunit (PDHE1α) axis plays an important role in regulating glucose metabolism in mammals. However, the regulatory function of PDKs-PDHE1α axis in the glucose metabolism of fish is not well known. This study determined whether PDKs inhibition could enhance PDHE1α activity, and improve glucose catabolism in fish. Nile tilapia fingerlings (1.90 ± 0.11 g) were randomly divided into 4 treatments in triplicate (30 fish each) and fed control diet without dichloroacetate (DCA) (38% protein, 7% lipid and 45% corn starch) and the control diet supplemented with DCA, which inhibits PDKs through binding the allosteric sites, at 3.75 (DCA3.75), 7.50 (DCA7.50) and 11.25 g/kg (DCA11.25), for 6 wk. The results showed that DCA3.75, DCA7.50 and DCA11.25 significantly increased weight gain, carcass ratio and protein efficiency ratio (P < 0.05) and reduced feed efficiency (P < 0.05) of Nile tilapia. To investigate the effects of DCA on growth performance of Nile tilapia, we selected the lowest dose DCA3.75 for subsequent analysis. Nile tilapia fed on DCA3.75 significantly reduced the mesenteric fat index, serum and liver triglyceride concentration and total lipid content in whole fish, and down-regulated the expressions of genes related to lipogenesis (P < 0.05) compared to the control. The DCA3.75 treatment significantly improved glucose oxidative catabolism and glycogen synthesis in the liver, but significantly reduced the conversion of glucose to lipid (P < 0.05). Furthermore, the DCA3.75 treatment significantly decreased the PDK2/4 gene and protein expressions (P < 0.05), accordingly stimulated PDHE1α activity by decreasing the phosphorylated PDHE1α protein level. In addition, DCA3.75 treatment significantly increased the phosphorylated levels of key proteins involved in insulin signaling pathway and glycogen synthase kinase 3β (P < 0.05). Taken together, the present study demonstrates that PDK2/4 inhibition by using DCA promotes glucose utilization in Nile tilapia by activating PDHE1α and improving insulin sensitivity. Our study helps to understand the regulatory mechanism of glucose metabolism for improving dietary carbohydrate utilization in farmed fish.
Keywords: Dichloroacetate, Glucose utilization, Insulin sensitivity, Nile tilapia, PDK2/4-PDHE1α axis
1. Introduction
Carbohydrates are abundant plant ingredients, and are generally considered as the most economical energy source in omnivorous and herbivorous fish nutrition, because of their relatively low cost and protein-sparing effect (Kamalam et al., 2017; Shrestha et al., 2011). Thus, increasing carbohydrate percentages in aquafeeds is one of the most economical nutritional strategies. Currently, high carbohydrate diets are widely used in aquaculture, especially in Nile tilapia (Oreochromis niloticus), gibel carp (Carassius auratus var. Gibelio), blunt snout bream (Megalobrama amblycephala) and grass carp (Ctenopharyngodon idellus) (Boonanuntanasarn et al., 2018a; Li et al., 2021; Limbu et al., 2020; Tan et al., 2009; Su et al., 2021; Shi et al., 2018). Fish species possess the key metabolic pathways and complete enzymatic systems for glucose catabolism (Kamalam et al., 2017). However, fish are poor users of dietary carbohydrates, and often display prolonged hyperglycemia, excessive fat accumulation, growth retardation, reduced feed utilization, decreased antioxidant ability and immune functions after feeding them with high carbohydrate diets for a long period (Jin et al., 2014; Li et al., 2021; Xu et al., 2018). Although the precise reasons for poor utilization of carbohydrates by fish are not fully understood, it has been suggested that the low capacity of fish to use efficiently carbohydrate for energy is caused partly by the imbalance between the glucose breakdown (glycolysis and tricarboxylic acids [TCA] cycle) and synthesis (gluconeogenesis) (Hemer et al., 2002; Kamalam et al., 2017). Therefore, promoting complete glucose catabolism in fish, especially those fed on high carbohydrate diets, is an important research topic for fish nutritionists.
Activities for living animals depend on the availability of energy in the form of adenosine triphosphate (ATP), delivered from substrate fuels through the oxidative phosphorylation process (Stacpoole, 2017). Glucose is initially converted into pyruvate through several glycolytic intermediates in cytoplasm. On one hand, pyruvate is converted to acetyl-coenzyme A (acetyl-CoA) in mitochondria, which then enters the TCA cycle and oxidative phosphorylation to produce ATP, CO2 and H2O (Pithukpakorn, 2005). Moreover, pyruvate is also converted to other 3-carbon molecules for the synthesis of fatty acids and steroids (Wahren and Ekberg, 2007). Therefore, the final metabolic fate of pyruvate determines the efficient use of glucose directly as an energy supply substance by animals. The rate of glucose-derived pyruvate oxidation is dictated, in large part, by the multisubunit enzyme-pyruvate dehydrogenase (PDH) (Gopal et al., 2017; Takubo et al., 2013; Zhang et al., 2014), which is the key enzyme system connecting glycolysis to the TCA cycle and the subsequent oxidative phosphorylation (Schafer et al., 2018; Wu et al., 2000, 2018a). PDH activity is regulated by reversible covalent modification via PDHE1α subunit (PDHE1α) phosphorylation, which is mediated by PDH kinases (PDKs) (Jeoung and Harris, 2008; Harris et al., 2002). Four PDK (PDK1-4) isoforms are expressed in a tissue-specific manner, with unique expression profiles in response to different physiological conditions (Wu et al., 2000). In mammals, PDK1 and PDK3 are mainly expressed in heart, kidney and testes. PDK2 and PDK4 are widely expressed in many tissues, including heart, brain, liver, islets, skeletal muscle and the adipose tissue (Klyuyeva et al., 2019; Wang et al., 2005). PDK1-4 are all serine-specific kinases, which can phosphorylate and inactivate PDHE1α, and among them PDK2 and PDK4 are the major PDKs, which are responsible for the regulation of PDHE1α activity in the liver (Harris et al., 2002; Jeoung and Harris, 2008). Similar to mammals, different PDK isoenzymes have been identified in common killifish (Fundulus heteroclitus) and zebrafish (Danio rerio) (Fukuda et al., 2020; Richards et al., 2008; Kuang et al., 2016). However, studies on the regulatory function of the PDKs-PDHE1α axis in glucose metabolism in Nile tilapia are currently lacking. PDKs-PDHE1α axis might be a potential regulatory target for improving the oxidative catabolism of glucose in fish.
Dichloroacetate (DCA) is a widely studied pyruvate mimetic, which inhibits PDKs through binding to the allosteric sites of PDK1, 2, 3 and 4 in mammals (Kato et al., 2007). By inhibiting the PDKs, the flux of pyruvate into the mitochondria is increased, thus promoting glucose oxidation over glycolysis. Previous studies have indicated that DCA inhibited PDK4 expression, and activated PDHE1α expression by dephosphorylation at the serine 293 and 300 residues in mouse C2C12 cells (Thoudam et al., 2019) and H9C2 cardiac myocytes (Gopal et al., 2017). In normal and obese mice, DCA improved markedly glucose tolerance and insulin sensitivity, and enhanced total carbohydrate oxidation (Younghoon et al., 2016; Wu et al., 2018). In addition, DCA also increased glucose translocation and consumption in porcine intestinal epithelial (IPEC-J2) cells (An et al., 2018). In zebrafish, some studies showed that the DCA-mediated PDK2 inhibition reduced lactate production; DCA also inhibited PDK4, and induced a shift in energy supply from fatty acids to glucose in cardiomyocytes (Fukuda et al., 2020; Kuang et al., 2016). Therefore, we hypothesized that DCA may act as an effector to improve the glucose utilization in fish by regulating the PDKs-PDHE1α axis.
Nile tilapia is one of the most important economic omnivorous species in global aquaculture (FAO, 2020). Generally, diets containing 300-350 g/kg carbohydrate do not affect Nile tilapia growth performance (Boonanuntanasarn et al., 2018b; Wang et al., 2005). Our previous studies showed that diets containing 450 g/kg carbohydrate led to excessive fat accumulation and reduced growth performance in Nile tilapia (Li et al., 2020b; Liu et al., 2018; Luo et al., 2020; Xu et al., 2021; Limbu et al., 2020). Normally, the adverse effects of high carbohydrate diets on Nile tilapia are ascribed to the imbalance between glucose catabolism for energy provision and conversion to fats (Liu et al., 2018; Prisingkorn et al., 2017). In the present study, Nile tilapia were fed with a control diet containing 450 g/kg corn starch (DCA0), and the control diet supplemented with different concentrations of DCA at 3.75 (DCA3.75), 7.50 (DCA7.50) and 11.25 g/kg (DCA11.25) levels for 6 wk. The aim was to evaluate the regulatory roles of the PDKs-PDHE1α axis on the glucose utilization efficiency and fat accumulation in fish. Our study demonstrates that PDK2/4 inhibition by using DCA promoted glucose utilization in Nile tilapia by activating PDHE1α and improving the insulin sensitivity. These results help to understand the regulatory mechanism of glucose metabolism for improving energy supply from dietary carbohydrates for farmed fish. This is also the first study elucidating the potential regulatory function of the PDKs-PDHE1α axis in improving the carbohydrate utilization in fish.
2. Materials and methods
2.1. Ethical statement
All experiments were conducted strictly under the Guidance Suggestions for the Care and Use of Laboratory Animals formulated by the Ministry of Science and Technology of China. In the present study, all the animal experimental procedures were conducted in compliance with the Ethics of Animal Experiments of East China Normal University (approval number F20210101).
2.2. Experimental fish, diets and study design
Nile tilapia (all-male) fingerlings were purchased from Bairong Fish Breeding Farm in Guangzhou, China. Before the formal trial, the fish were acclimated in a recirculating aquaculture system for 2 wk. During the acclimation process, fish were fed a purified diet containing 380 g/kg protein, 50 g/kg lipid and 300 g/kg corn starch. Four isonitrogenous and isolipidic purified experimental diets were formulated, including a control diet (DCA0, 450 g/kg corn starch), and the control diet supplemented with 3 doses of DCA at 3.75 (DCA3.75), 7.50 (DCA7.50) and 11.25 g/kg (DCA11.25). Protein was supplied by casein and gelatin. Dietary lipid was supplied by soybean oil. Corn starch was used to provide the required dietary carbohydrate level. In our previous studies, 450 g/kg corn starch was used to formulate the high carbohydrate diets for Nile tilapia (Luo et al., 2020; Limbu et al., 2020). The diets contained approximately 380 g/kg protein and 70 g/kg lipid, which met the nutritional requirements of Nile tilapia according to our previous studies (Li et al., 2021; Limbu et al., 2020; Luo et al., 2020). Feed formulation and proximate composition of the diets used in the present study are presented in Table 1. The ingredients were finely ground, mixed homogeneously, and pelletized by using a double helix plodder (F-26, SCUT industrial factory, Guangdong, China). The experimental diets were dried at 40 °C for 24 h, and stored at −20 °C until use. After the acclimation period, 360 Nile tilapia fingerlings with no exterior injuries and deformities (initial mean weight: 1.90 ± 0.11 g) were divided randomly into 12 tanks (each tank held 30 fish, in 3 replicates) in a recirculating aquaculture system. The size of each tank was 0.8 m × 0.6 m × 0.6 m (containing 250 L water), and the water flow rate was 200 L/h. The fish were hand-fed by using the four experimental diets twice a day (08:30 and 17:30) at a daily feeding rate of 4% body weight for 6 wk. The daily intake of DCA for fish was 0, 150, 300 and 450 mg/kg body weight, respectively. The dietary DCA doses were determined based effective dosages already used in previous studies in rat and zebrafish (Wu et al., 2018; Hassoun et al., 2005). Body weight of all fish in each tank was bulk measured once every week by using an electronic weighing scale to adjust their daily feed rations. The remaining feeds after 1 h of feeding were siphoned, dried and weighed for the determination of feed intake. During the feeding trial period, the water temperature, pH, dissolved oxygen and ammonia nitrogen were 27.00 ± 2.00 °C, 7.80 ± 0.20, >7.00 mg/L and <0.05 mg/L, respectively.
Table 1.
The ingredients and proximate composition of the experimental diets.
| Item | DCA0 | DCA3.75 | DCA7.50 | DCA11.25 |
|---|---|---|---|---|
| Ingredients, g/kg | ||||
| Casein1 | 336.0 | 336.0 | 336.0 | 336.0 |
| Gelatin2 | 84.0 | 84.0 | 84.0 | 84.0 |
| Soybean oil3 | 70.0 | 70.0 | 70.0 | 70.0 |
| Corn starch4 | 450.0 | 450.0 | 450.0 | 450.0 |
| Vitamin premix5 | 10.0 | 10.0 | 10.0 | 10.0 |
| Mineral premix6 | 10.0 | 10.0 | 10.0 | 10.0 |
| Ca(H2PO4)27 | 7.75 | 7.75 | 7.75 | 7.75 |
| Carboxy methyl cellulose8 | 26.0 | 26.0 | 26.0 | 26.0 |
| Choline chloride9 | 5.0 | 5.0 | 5.0 | 5.0 |
| Dimethyl-β-propiothetin10 | 1.0 | 1.0 | 1.0 | 1.0 |
| Butylated hydroxytoluene11 | 0.25 | 0.25 | 0.25 | 0.25 |
| Dichloroacetate (DCA)12 | 0.00 | 3.75 | 7.50 | 11.25 |
| Total | 1,000 | 1,000 | 1,000 | 1,000 |
| Proximate composition, % dry matter basis | ||||
| Dry matter | 92.37 | 92.44 | 92.71 | 92.33 |
| Protein | 37.95 | 37.89 | 37.96 | 38.03 |
| Lipid | 6.99 | 6.85 | 6.98 | 6.83 |
| Ash | 3.31 | 3.21 | 3.22 | 3.30 |
| Nitrogen-free extract13 | 44.12 | 44.49 | 44.55 | 44.17 |
| Available energy14, MJ/kg | 16.43 | 16.40 | 16.40 | 16.46 |
| DCA12, g/kg | – | 3.02 | 6.88 | 10.88 |
Casein: Wan Ling, Changzhou Linghao Biotechnology Co., Ltd., Jiangsu, China.
Gelatin: Sangon Biotech (Shanghai) Co., Ltd., China.
Soybean oil: Arawana Brand, Yihai Kerry Investments Co., Ltd., Hubei, China.
Corn starch: Shijiazhuang Tangtian starch Co., Ltd., Hebei, China.
Vitamin premix provided the following per kilogram of diet: 500,000 IU vitamin A, 50,000 IU vitamin D3, 2,500 mg vitamin E, 1,000 mg vitamin K3, 5,000 mg vitamin B1, 5,000 mg vitamin B2, 5,000 mg vitamin B6, 5,000 mg vitamin B12, 25,000 mg inositol, 10,000 mg pantothenic acid, 100,000 mg cholin, 25,000 mg niacin, 1,000 mg folic acid, 250 mg biotin, 10,000 mg vitamin C.
Mineral premix provided the following per kilogram of diet: 147.4 g MgSO4·7H2O, 49.8 g NaCl, 10.9 g Fe (II) gluconate, 3.12 g MnSO4·H2O, 4.67 g ZnSO4·7H2O, 0.62 g CuSO4·5H2O, 0.16 g KI, 0.08 g CoCl2·6H2O, 0.06 g NH4 molybdate, 0.02 g NaSeO3.
Ca(H2PO4)2: Sangon Biotech (Shanghai) Co., Ltd., China.
Carboxy methyl cellulose: Shandong Dongda Commerce Co., Ltd., China.
Choline chloride: Sangon Biotech (Shanghai) Co., Ltd., China.
Dimethyl-β-propiothetin: Sangon Biotech (Shanghai) Co., Ltd., China.
Butylated hydroxytoluene: Sangon Biotech (Shanghai) Co., Ltd., China.
Dichloroacetate (DCA): Aladdin Biotech (Shanghai) Co., Ltd., China. The actual DCA concentrations were determined by high-performance liquid chromatography.
Calculated as 100 - (moisture + protein + lipid + ash).
Based on 16.7 MJ/kg protein, 37.6 MJ/kg lipid and 16.7 MJ/kg nitrogen-free extract (NFE).
2.3. Samples collection for analyses
At the end of the 6 wk feeding trial, all fish were fasted overnight and anaesthetized by using MS222 (20 mg/L). The survived fish in each tank were counted to determine their number and bulk weighed. Three fish per tank were sampled randomly and individually measured for their body weight and length. After weight and length measurements, blood was drawn from the caudal vein of the 9 fish by using 2-mL syringes (Klmedical, China). Blood samples were immediately centrifuged at 1,000 × g for 10 min at 4 °C, the serum was placed into polypropylene tubes for biochemical analysis. The remaining fish after blood collection were dissected individually for collecting liver, muscle and mesenteric samples. The mesenteric fat and liver samples were weighed for organ indices analysis. The liver and muscle samples were instantly frozen in liquid nitrogen, and then stored at −80 °C for the analyses of glycogen and triglyceride (TG) contents and mRNA expression of genes. The fish head, fin and visceral were removed from all the 4 fish per tank (12 fish per treatment), and were weighed for the determination of carcass ratio. Another 6 fish from each treatment were randomly collected and stored at −20 °C for body proximate composition analysis.
2.4. Estimation of growth performance, survival rate, feed efficiency, and organ indices
The data obtained on weight, number of fish, length, amount of feed and organs weight were used to compute growth performance, survival rate, feed efficiency, and organ indices by using the following formulae:
Weight gain (WG, %) = 100 × [(final weight, g) – (initial weight, g)]/(initial weight, g);
Survival rate (SR, %) = 100 × final fish number/initial fish number;
Feed conversion ratio (FCR) = (feed fed during the entire study, g)/(biomass gained during the study, g);
Hepatosomatic index (HSI, %) = 100 × (liver weight, g)/(individual fish weight, g);
Mesenteric fat index (%) = 100 × (mesenteric fat weight, g)/(individual fish weight, g);
Feed intake (FI, %) = 100 × (dry feed consumed, g)/{[(final weight, g) + (initial weight, g)]/2}/d;
Protein efficiency ratio (PER) = (biomass gained during the study, g)/(total protein intake, g DM);
Condition factor (CF, %) = 100 × (final weight, g)/(final body length, cm3);
Carcass ratio (CR, %) = 100 × [(body weight, g) – (head weight, g) – (fin weight, g) – (visceral weight, g)]/(body weight, g).
2.5. Biochemical parameters analyses
Specific commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to measure the contents of TG (Kit F001-1) and glycogen (Kit A043-1) in the liver and muscle. Similarly, the concentrations of TG, glucose (Kit F006-1) and insulin (Kit H203-1) were analyzed in the serum by using specific commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All measurement steps were performed according to the relevant kit protocols. Furthermore, the total lipid in the whole fish was extracted and determined by using the chloroform/methanol (2:1, vol:vol) method (Folch et al., 1957).
2.6. Total RNA extraction, cDNA synthesis and quantitative real-time PCR
Six fish were sampled for brain, heart, muscle, liver, kidney, spleen, gill, intestinal and adipose tissues collection for total RNA extraction using RNAiso Plus (Takara, Japan) according to the manufacturer's instructions. The quality and quantity of RNA were determined by agarose gel electrophoresis and Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, USA), respectively. The RNA was reverse transcribed to cDNA by using the PrimeScript Reagent kit (Takara, Japan). It was subsequently used to analyze the tissue expression specificity of PDK1/2/3/4 genes. Similarly, the liver and muscle tissues of 6 fish from each treatment were also collected for total RNA extraction, cDNA synthesis and quantitative real-time PCR (qRT-PCR). The elongation factor 1 alpha (ef1α) and β-actin were used as reference genes due to their stability for Nile tilapia (Limbu et al., 2018). The primers used in the present study are showed in Table 2. The expression of genes was quantified by using qRT-PCR (Bio-rad, USA) with the SYBR qPCR reagent (Vazyme Biotech CO., Ltd. Nanjing, China). The qRT-PCR results were estimated by the 2−ΔΔCt method (Livak and Schmittgen, 2001). In the present study, the qRT-PCR efficiency was between 95% and 105% for all analyses, and the correlation coefficient was over 0.96 for each gene. Each qRT-PCR run was performed in triplicate with negative control (no cDNA) also included.
Table 2.
Primer sequences for qRT-PCR analysis in Nile tilapia.
| Gene name | Sense and antisense primer (5′–3′) | GenBank no. |
|---|---|---|
| ef1α | F: CTACGTGACCATCATTGATGCC R: AACACCAGCAGCAACGATCA |
AB075952 |
| β-actin | F: AGCCTTCCTTCCTTGGTATGGAAT R: TGTTGGCGTACAGGTCCTTACG |
KJ126772 |
| pparα | F: CTGATAAAGCTTCGGGCTTCCA R: CGCTCACACTTATCATACTCCAGCT |
KF871430 |
| cpt1b | F: AAGGGACGTTACTTCAAGGTG R: TCCGACTTGTCTGCCAAGAT |
GQ395696 |
| accβ | F: ACATGCAGTCCATGCTGCGT R: AAATGCCTCTCAAGCCACTCAA |
XM_003451659 |
| srebp1 | F: TGCAGCAGAGAGACTGTATCCGA R: ACTGCCCTGAATGTGTTCAGACA |
XM_005457771 |
| accα | F: TAGCTGAAGAGGAGGGTGCAAGA R: AACCTCTGGATTGGCTTGAACA |
XM_005471970 |
| fas | F: TTGAGGATGTGACTATCCACAGGG R: GTCAGGTTTCCGTTCTCCGAAA |
XM_003454056 |
| dgat | F: GCTTGAATTCTGTCACCCTGAAGA R: ACCTGCTTGTAGGCGTCGTTCT |
XM_003458972 |
| mtco1 | F: CTGTTTATCCCCCACTCGCA R: AATAGATGACACCCCGGCCA |
LC189956.1 |
| sdha | F: GGTATTCCGTACCGGCTCTG R: GTCGGTGTTCCACACAATGC |
XM_003443687.5 |
| ndufa9 | F: ACCTTTTGTGCCCTACCCTC R: TTTGTCTGGGGTTGTCCAGG |
XM_003447056.4 |
| gk | F: GACATGAGGACATTGACAAGGGAA R: CTTGATGGCGTCTCTGAGTAAACC |
XM_003451020.2 |
| pfk | F: AACCTGTGTGTGATTGGAGGTGAT R: CGTGATCTTACCGGCTTTAACAAG |
XM_003441476.2 |
| pk | F: CAGCATAATCTGCACCATCGGT R: ATGAGAGAAGTTAAGACGGGCGA |
XM_005472621.3 |
| pepck | F: TGGAAGAACAAACCTTGGCG R: TGGGTCAATAATGGGACACTGTCT |
XM_003448375 |
| g6pase | F: AGACCTTATTGGTGGGTTCACGA R: CTGAAGGACTTCCTGGTCCAGTTT |
XM_003448671.4 |
| fbpase | F: ACCGGACAATAGCGGAAAATACA R: TGGCGAATATTGTTCCTATGGAGA |
XM_003449650.4 |
| idh | F: ACGCATCGCTGAGTACGCCTT R: AGACCGTCTGACATCCGCATGA |
XM_003437590.5 |
| cs | F: AGCACCACAGTTTACCAG R: AGTGTTGACAAACCCAGA |
XM_003438897 |
| glut2 | F: CATTGGCATTCTAATCAGCCAGGT R: TTGTAATATTGCTGGCGCTCCA |
XM_003442884.5 |
| glut4 | F: GCAGGAGGAAAGCCATGCTTATA R: ATCATTTCAAAGGAGCGGCAGA |
XM_003458705.4 |
| gs | F: CCTCACTCTGCGCTGTTATTC R: CAGCGGCATGCCTTCAGTTT |
XM_013276796.3 |
| pdk1 | F: GAGGAGCAGCGTGTCCATAG R: AGGTAACTCCTGTCAAAATCCAGA |
XM_003447311.5 |
| pdk2 | F: GCAGAGTTCATCCAGACAA R: GACCTGTAGTGCTTATCTGAT |
XM_003448725.5 |
| pdk3 | F: GTCATGTCATTGCGAAGGGC R: CAGAGCCAGCTCCATAAGGTT |
XM_005471769.4 |
| pdk4 | F: AATCCACAGCCAGTCACT R: GCAGAGTTCATCCAGACAA |
XM_003457260.5 |
| pdhe1α | F: AATCCACAGCCAGTCACT R: GCAGAGTTCATCCAGACAA |
XM_013264731.3 |
ef1α = elongation factor 1 alpha; pparα = peroxisome proliferator activated receptor α; cpt1b = carnitine palmitoyl transferase 1b; accβ = acetyl-coa carboxylase β; srebp1 = sterol regulatory element binding transcription factor 1; accα = acetyl-coa carboxylase α; fas = fatty acid synthase; dgat = diacylglycerol o-acyltransferase; mtco1 = mitochondrial cytochrome c oxidase 1; sdha = succinate dehydrogenase complex subunit A; ndufa9 = NADH dehydrogenase [ubiquinone] 1a subcomplex subunit 9; gk = glucokinase; pfk = phosphofructokinase; pk = pyruvate kinase; pepck = phosphoenolpyruvate carboxykinase; g6pase = glucose-6-phosphatase; fbpase = fructose-1,6-bisphosphatase; idh = isocitrate dehydrogenase; cs = citrate synthase; glut2 = glucose transporter 2; glut4 = glucose transporter 4; gs = glycogen synthase; pdk1/2/3/4 = pyruvate dehydrogenase kinase 1/2/3/4; pdhe1α = pyruvate dehydrogenase E1α subunit.
2.7. Western blot analysis
The liver tissues from 9 fish from each treatment for Western blot assays were prepared as reported by Luo et al. (2020). The liver protein concentrations were measured by using a bicinchoninic acid assay protein kit (Beyotime Biotechnology, China) to determine the loading volume. Express cast PAGE gel (New Cell & Molecular Biotech, China) was run using 50 μg of protein lysate per lane. The gels obtained were then transferred into nitrocellulose (NC) membranes for 90 min at 90 mV, and the NC membranes were blocked with 5% bovine serum albumin. The information on antibodies used for this study is provided in Table 3. The glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as a reference protein. The detection and quantification were performed by using the Odyssey CLX Imager (LI-COR, Inc., USA).
Table 3.
Antibodies used for western blotting assay.
| Antibodies name | Source | Identifier |
|---|---|---|
| Pdk2 | Abways | CY7193 |
| Pdk4 | Proteintech | 12949-1-AP |
| Pdhe1α | Abways | AB3131 |
| p-Pdhe1α (Ser293) | Abways | CY7247 |
| Irβ | Cell signaling technology | Cat #3020 |
| p-Irβ (Tyr1345) | Cell signaling technology | Cat #3026 |
| Irs1 | Abways | CY3428 |
| p-Irs1 (Ser636) | Abways | CY6308 |
| Pi3k | Abways | AB0036 |
| p-Pi3k (Tyr458/199) | Cell signaling technology | Cat #4228 |
| Akt | Cell signaling technology | Cat #4691 |
| p-Akt (Ser473) | Cell signaling technology | Cat #4060 |
| Gsk3β | Abways | AB3168 |
| p-Gsk3β (Ser9) | Abways | CY6248 |
| Gapdh | Huabio | M1310-2 |
Pdk2 = pyruvate dehydrogenase kinase 2; Pdk4 = pyruvate dehydrogenase kinase 4; Pdhe1α = pyruvate dehydrogenase E1α subunit; Irβ = insulin receptor β; Irs1 = insulin receptor substrate 1; Pi3k = phosphatidylinositol 3-kinase; Akt = serine/threonine kinase; Gsk3β = glycogen synthase kinase 3β; Gapdh = glyceraldehyde-3-phosphate dehydrogenase.
2.8. Histological analysis
Small pieces (about 1 cm3) of liver and muscle tissues were fixed in 4% paraformaldehyde (Servicebio Technology Co., Ltd. Wuhan, China) for 48 h at room temperature. The fixed tissues were then embedded in paraffin wax, and were cut into 5 μm slices for periodic acid-Schiff (PAS) staining. The liver tissue was incubated by using 30% sucrose at 4 °C for 72 h. The liver tissue was then embedded at optimum cutting temperature compound (Sakura, Japan), and was immediately frozen at −80 °C for oil red O (Sigma–Aldrich, USA) staining. The histological features were observed and photographed by using a light microscope (Nikon Ds-Ri2, Japan).
2.9. Glucose tolerance test (GTT)
The GTT was performed as described in our previous studies (Liu et al., 2018; Li et al., 2020a). After the feeding trial, 96 fish (24 fish per treatment) were fasted for 12 h. The fish were then intraperitoneally (i.p.) injected with 500 mg/kg of glucose. The fish from each treatment were then sampled at 0, 30, 60 and 180 min after the glucose injection (6 fish per time point) for tail vein blood collection and the serum was immediately obtained as described in the previous section. Glucose and insulin levels in serum samples were determined by using specific kits as shown above.
2.10. Metabolic tracking test of [1-14C]-glucose
After the feeding experiment and overnight fasting, 6 fish from each treatment were randomly collected and anesthetized with tricaine methane sulfonate (Western Chemicals, Inc., Ferndale, USA) at 20 mg/L). The fish were i.p. injected with saline containing [1-14C]-glucose (500 mg/kg body weight and 0.1 MBq per 50 g body weight). The injected fish were moved immediately into a closed glass jar containing the oxygen-saturated water, which was connected to another glass bottle containing the 1 mol/L potassium hydroxide (KOH) solution. The details of the experimental process on metabolic tracking test of [1-14C]-glucose were as previously described (Younghoon et al., 2016). Briefly, 1 mol/L KOH solution absorbs 14CO2 released from the oxidation of the [1-14C]-glucose. The 14CO2 released was collected during the 90 min release period. The liver and muscle samples (0.5 g) were digested by using a digestion solvent (30% H2O2/HClO4, 1:2, vol/vol) (1:5, wt/vol) at 60 °C in a water bath for 6 h. The 14C-protein (the 1 mmol/L NaCl-Tris-HCl/10% HClO4 extraction method), 14C-lipid (the chloroform-methanol extraction method, 2:1, vol/vol) and 14C-glycogen (the 70% ethanol precipitation method) were extracted as described previously (Challiss et al., 1983; Chan and Krebs, 1985; Li et al., 2020a, 2020b, 2021). After the extraction, the 14C-labeled macronutrients were dissolved into 0.5 mL of strong lysate (30% H2O2/HClO4, 1:2, vol/vol). The radioactivity of 200 μL KOH solution and nutrient lysate were measured after mixing with 2 mL scintillation fluid (Ultima Gold XR, Packard, USA) by using the Tri-Carb 4910TR Liquid Scintillation Analyzer (PerkinElmer, USA).
2.11. Statistical analyses
All data were tested for normality by using the Kolmogorov–Smirnov test while homogeneity of variances was determined by using the Levene's test. The significant differences on final body weight (FBW), WG, SR, FCR, FI, PER, CF and CR among the DCA0, DCA3.75, DCA7.50 and DCA11.25 treatments were all tested by using one-way analysis of variance (ANOVA) followed by the Tukey's multiple comparison test for specific differences. The significant differences of the remaining measured parameters between the DCA0 and DCA3.75 treatments were all evaluated by using the independent t-test. Results with P < 0.05 were considered statistically significant. All results are reported as means ± standard error of the mean (SEM) as indicated in figure legends. All statistical analyses were performed by using the GraphPad Prism 7.0 software (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. The effects of DCA on growth performance and feed efficiency
After the 6 wk feeding trial, the Nile tilapia fed on the DCA3.75, DCA7.50 and DCA11.25 diets had significantly higher FBW, WG, PER and CR than those fed on the DCA0 diet (P < 0.05) (Fig. 1A, B, F, H). In addition, the Nile tilapia fed on the DCA3.75, DCA7.50 and DCA11.25 diets had significantly lower FCR than those fed on the DCA0 diet (P < 0.05) (Fig. 1D). However, feeding the Nile tilapia with all the experimental diets did not affect the SR, FI and CF (P > 0.05) (Fig. 1C, E, G). Therefore, we selected the DCA3.75 treatment, which contained the lowest dose of DCA for subsequent analysis.
Fig. 1.
The effects of dichloroacetate (DCA) on growth performance of Nile tilapia. (A) Body weight increase during the 6 wk feeding trial (n = 3); (B) weight gain (n = 3); (C) survival rate (n = 3); (D) feed conversion ratio (n = 3); (E) feed intake (n = 3); (F) protein efficiency ratio (n = 3); (G) condition factor (n = 9); (H) carcass ratio (n = 9). Values are means ± SEM. Statistical differences in mean values of all indexes were evaluated by using one-way analysis of variance (ANOVA) followed by Tukey test. a, b Different letters indicate a significant difference (P < 0.05).
3.2. The effects of DCA on lipid deposition
In the present study, we analyzed lipid deposition-related parameters. The results showed that, feeding the Nile tilapia with the DCA3.75 diet significantly reduced the mesenteric fat index, TG concentration in the serum, total lipid in the whole fish, lipid droplets amount in the liver, and TG level in the liver (P < 0.05) (Fig. 2B–F). However, feeding the Nile tilapia with the DCA0 and DCA3.75 diets did not affect the HSI (P > 0.05) (Fig. 2A). Interestingly, Nile tilapia fed on the DCA3.75 diet significantly reduced the expressions of genes related to lipid synthesis, such as sterol regulatory element binding transcription factor 1 (srebp1), fatty acid synthase (fas), diacylglycerol o-acyltransferase (dgat) and acetyl-coa carboxylase α (accα) (P < 0.05) (Fig. 2G). However, the DCA0 and DCA3.75 diets had no significant effect on the expressions of key genes involved in lipid catabolism in Nile tilapia, such as peroxisome proliferator activated receptor α (pparα), carnitine palmitoyl transferase 1b (cpt1b) and acetyl-coa carboxylase β (accβ) (P > 0.05) (Fig. 2G).
Fig. 2.
The effects of dichloroacetate (DCA) on conversion of glucose to lipid in Nile tilapia. (A) Hepatosomatic index (n = 9); (B) mesenteric fat index (n = 9); (C) serum triglyceride (n = 9); (D) total lipid in the whole fish (n = 9); (E) oil red staining of liver tissues (n = 3); (F) liver triglyceride (n = 9); (G–H) the mRNA expression of lipogenesis and lipolysis-related genes in the liver (srebp1 = sterol regulatory element binding transcription factor 1; fas = fatty acid synthase; dgat = diacylglycerol o-acyltransferase; accα = acetyl-coa carboxylase α; pparα = peroxisome proliferator activated receptor α; cpt1b = carnitine palmitoyl transferase 1b; accβ = acetyl-coa carboxylase β) (n = 9). Values are means ± SEM. Statistical differences in mean values of all indexes were evaluated by using independent t-test. ∗ P < 0.05, ∗∗ P < 0.01.
3.3. The effects of DCA on glucose metabolism
The Nile tilapia fed on the DCA3.75 diet had significantly lower glucose in the serum compared to those fed on the DCA0 diet (P < 0.05) (Fig. 3A). Feeding the fish with the DCA3.75 and DCA0 diets did not affect insulin concentration (P > 0.05) (Fig. 3B). The results for the GTT test showed that the DCA3.75-fed fish had a faster glucose clearance rate and a lower insulin concentration in the serum than those fed on the DCA0 diet (P < 0.05) (Fig. 3C, D). To gain insight into the role of DCA in the overall regulation of glucose metabolism in Nile tilapia, we tracked the use of [1-14C]-glucose that had been i.p. injected (Fig. 3E). The results for the metabolic tracking test showed that the fish fed on the DCA3.75 diet had significantly higher 14CO2 release (Fig. 3F) and 14C-glycogen deposition (Fig. 3H) in the liver than those fed on the DCA0 diet (P < 0.05). However, the fish fed on the DCA3.75 diet had significantly lower 14C-lipid content (P < 0.05) (Fig. 3G) in the liver than those fed on the DCA0 diet. The fish fed on the DCA3.75 diet had similar 14C-protein deposition with those fed on the DCA0 diet (Fig. 3I). In the muscle, the deposition of 14C-lipid (Fig. 3J), 14C-glycogen (Fig. 3L) and 14C-protein (Fig. 3K) were not significantly different between the fish fed on both diets (P > 0.05). The results for the PAS analysis showed that the fish fed on the DCA3.75 diet had higher glycogen content in the liver (Fig. 3M, N) accompanied with higher mRNA expression of glycogen synthase (gs) (P < 0.05) (Fig. 3O). However, glycogen content (Fig. 3P, Q) and mRNA expression of gs (Fig. 3R) in the muscle of Nile tilapia were not significantly different between the DCA3.75 and DCA0 treatments (P > 0.05).
Fig. 3.
The effects of dichloroacetate (DCA) on the glucose oxidation utilization in Nile tilapia. (A) Serum glucose (n = 9); (B) serum insulin (n = 9); (C-D) serum glucose and insulin during glucose tolerance test (GTT) (n = 6); (E) schematic diagram of 14C-labelled glucose tracking test in Nile tilapia. (F) carbon dioxide radioactivity released from [1-14C]-glucose oxidation of Nile tilapia (n = 6); (G-I) lipid, glycogen and protein radioactivity of liver during [1-14C]-glucose tracking test of Nile tilapia (n = 6); (J-L) lipid, glycogen and protein radioactivity of muscle during [1-14C]-glucose tracking test of Nile tilapia (n = 6); periodic acid-Schiff (PAS) staining in the liver (M) and muscle (P); glycogen content in the liver (N) and muscle (Q); and the mRNA expression of glycogen synthase in liver (O) and muscle (R) (gs = glycogen synthase). Values are means ± SEM. Statistical differences in mean values of all indexes were evaluated by using independent t-test. ∗ P < 0.05, ∗∗ P < 0.01.
3.4. The effects of DCA on the PDK2/4-PDHE1α axis and expression of genes related to glucose metabolism
The Nile tilapia fed on the DCA3.75 diet up-regulated the expressions of genes related to glucose transport (glucose transporter 2, glut2; glucose transporter 4, glut4) (P < 0.05) (Fig. 4A). Furthermore, the fish fed on the DCA3.75 diet up-regulated the expressions of glycolysis-related genes, including glucokinase (gk), phosphofructokinase (pfk) and pyruvate kinase (pk) (Fig. 4B), as well as citrate synthase (cs) (Fig. 4D) than those fed on the DCA0 diet (P < 0.05). However, the Nile tilapia fed on the DCA3.75 diet significantly down-regulated the mRNA expression of the gluconeogenesis related gene-phosphoenolpyruvate carboxykinase (pepck) than those fed on the DCA0 diet (P < 0.05) (Fig. 4C). The Nile tilapia fed on the DCA3.75 diet significantly increased the expressions of mitochondrial cytochrome c oxidase 1 (mtco1), succinate dehydrogenase complex subunit A (sdha) and NADH dehydrogenase [ubiquinone] 1a subcomplex subunit 9 (nduta9) (P < 0.05) (Fig. 4E).
Fig. 4.
The effects of dichloroacetate (DCA) on the PDK2/4-PDHE1α axis and expression of genes related to glucose metabolism in Nile tilapia. (A) The mRNA expression of glucose transport-related genes in the liver (glut2 = glucose transporter 2; glut4 = glucose transporter 4) (n = 9); (B) the mRNA expression of glycolysis-related genes in the liver (gk = glucokinase; pfk = phosphofructokinase; pk = pyruvate kinase) (n = 9); (C) the mRNA expression of gluconeogenesis-related genes in the liver (pepck = phosphoenolpyruvate carboxykinase; g6pase = glucose-6-phosphatase; fbpase = fructose-1,6-bisphosphatase) (n = 9); (D) the mRNA expression of tricarboxylic acids (TCA) cycle-related genes in the liver (idh = isocitrate dehydrogenase; cs = citrate synthase) (n = 9); (E) the mRNA expression of oxidative phosphorylation-related genes in the liver (mtco1 = mitochondrial cytochrome c oxidase 1; sdha = succinate dehydrogenase complex subunit A; ndufa9 = NADH dehydrogenase [ubiquinone] 1a subcomplex subunit 9) (n = 9); (F-G) mRNA and protein expression patterns of pdk1, pdk2, pdk3 and pdk4 in different tissues (pdk1/2/3/4 = pyruvate dehydrogenase kinase 1/2/3/4) (n = 6); (H–I) the protein concentrations of Pdk2, Pdk4, Pdhe1α and p-Pdhe1α in the liver (Pdk2 = pyruvate dehydrogenase kinase 2; Pdk4 = pyruvate dehydrogenase kinase 4; Pdhe1α = pyruvate dehydrogenase E1α subunit) (n = 9); and (J) the mRNA expression of pdk2, pdk4 and pdhe1α genes in the liver (pdhe1α = pyruvate dehydrogenase E1α subunit) (n = 9). Values are means ± SEM. Statistical differences in mean values were evaluated by using either one-way analysis of variance (ANOVA) followed by Tukey test (G), or the independent t-test (others except for G). a, b, c Different letters indicate a significant difference (P < 0.05). ∗ P < 0.05, ∗∗ P < 0.01.
We further analyzed the effects of DCA on the PDKs-PDHE1α axis, because it plays an important role in regulating the entry of glucose-derived pyruvate into the TCA cycle to generate acetyl-CoA. The results showed that the PDKs (pdk1, pdk2, pdk3 and pdk4) were expressed widely in many organs, including brain, heart, muscle, liver, kidney, spleen, gill, intestine and the adipose tissue of Nile tilapia. In the liver, an important target organ of glucose metabolism, the mRNA and protein expressions of pdk2 and pdk4 gene were significantly higher than pdk1 and pdk3 (P < 0.05) (Fig. 4F, G). The Nile tilapia fed on the DCA3.75 diet had lower protein concentrations of Pdk2 and Pdk4 (Fig. 4H) with low mRNA expressions (Fig. 4 J) in the liver compared to those fed on the DCA0 diet (P < 0.05). Likewise, the Nile tilapia fed on the DCA3.75 diet had lower protein concentration of p-Pdhe1α (Fig. 4H) than those fed on the DCA0 diet (P < 0.05). However, the total protein concentration of Pdhe1α and its mRNA expression were not significantly affected between the fish fed on the DCA3.75 and DCA0 diets in the liver (P > 0.05).
3.5. The effects of DCA on the insulin signaling and glycogen synthase kinase 3 beta (Gs3kβ) expression
The fish fed on the DCA3.75 diet significantly increased the phosphorylated protein levels of insulin receptor beta [Irβ (Tyr1345)], insulin receptor substrate 1 [Irs1 (Ser636/639)], phosphatidylinositol 3-kinase [Pi3k (Tyr458/198)] and serine/threonine kinase [Akt (Ser473)] compared with those fed on the DCA0 diet (P < 0.05) (Fig. 5A, B). However, the fish fed with the DCA3.75 diet did not significantly affect the total protein levels of Irβ, Irs1, Pi3k and Akt in the liver (P > 0.05). The fish fed on the DCA3.75 diet significantly increased the phosphorylated level of Gs3kβ at the site of ser9 (P < 0.05), but did not change its total protein content (P > 0.05) (Fig. 5C, D).
Fig. 5.
The effects of dichloroacetate (DCA) on the insulin signaling and glycogen synthase kinase 3 beta (GS3Kβ) in Nile tilapia. (A and B) The protein expression of insulin pathway in the liver of Nile tilapia (Irβ = insulin receptor β; Irs1 = insulin receptor substrate 1; Pi3k = phosphatidylinositol 3-kinase; Akt = serine/threonine kinase) (n = 9); (C and D) the protein expression of Gs3kβ and p-Gs3kβ in liver of Nile tilapia (n = 9). Values are means ± SEM. Statistical differences in mean values of all indexes were evaluated by using independent t-test. ∗ P < 0.05.
4. Discussion
4.1. Dietary DCA improves glucose oxidation by regulating the PDK2/4-PDHE1α axis
A previous study found that an imbalance between hepatic glucose consumption (glycolysis) and production (gluconeogenesis) in fish limits the efficiency of glucose utilization for energy production (Kamalam et al., 2017). PDKs-PDHE1α is an important enzyme system, which determines the overall rate of glucose disposal, and links the processes of glycolysis with oxidative phosphorylation (Jaswal et al., 2011; Patel et al., 2014; Wu et al., 2000). In the present study, we found that the 4 PDKs (PDK1-4) isoforms depicted tissue expression specificity, similar to results in mammals (Stacpoole, 2017; Wu et al., 2000) and common killifish (Richards et al., 2008). The mRNA and protein expressions of PDK2 and PDK4 were higher than those of PDK1 and PDK3 in the liver, which is an important glucose metabolism organ, in agreement with the results in rat (Wu et al., 2000), killifish (Richards et al., 2008) and zebrafish (Fukuda et al., 2020). Interestingly, DCA inhibited the mRNA expression and protein concentrations of PDK2 and PDK4, and decreased the phosphorylated level of PDHE1α in Nile tilapia liver in the present study. Earlier studies in mammals indicated that DCA specifically inhibited PDKs by binding to the allosteric sites of PDK1-4 (Kato et al., 2007), and increased the activity of PDHE1α through dephosphorylation, which in turn promoted the oxidative utilization of glucose (Klyuyeva et al., 2019; Thoudam et al., 2019). Therefore, the present study showed that DCA also inhibited PDK2 and 4, and increased the content of 14CO2 release from [1-14C]-glucose oxidation in Nile tilapia fed on a high carbohydrate diet.
Glucose catabolism is linked to its transport and the glycolysis process in mammals hepatocytes (Enes et al., 2009). Accordingly, the Mrna expressions of glut2, glut4, gk, pk and pfk were also up-regulated in fish fed on the DCA3.75 diet in the present study. Furthermore, the DCA3.75 diet also up-regulated the genes related to the TCA cycle (namely cs) and the oxidative phosphorylation process (namely mtcd1, sdha and nduta9). These results verified that DCA accelerated the glucose uptake and oxidation in the liver of fish. It should be noted that, the DCA3.75 diet improved growth performance-related indexes with enhanced WG, PER and CR and reduced FCR in Nile tilapia fed on the DCA0 diet in the present study, probably owing to the increased energy supply from glucose oxidation. Taken together, our results indicate that DCA improves the carbohydrate utilization efficiency and growth performance of Nile tilapia by regulating the PDK2/4-PDHE1α axis. These results provide strong evidence that the PDK2/4-PDHE1α axis plays an important regulatory role in glucose oxidation. Therefore, the PDK2/4-PDHE1α axis can be used as a potential regulatory target for improving the carbohydrate utilization in farmed fish.
4.2. Dietary DCA alleviates the high carbohydrate diet-induced glucose intolerance by enhancing insulin sensitivity
Insulin regulates glucose homeostasis by suppressing gluconeogenesis and stimulating glucose utilization (Clemmons, 2006). Accordingly, an impaired insulin function leads to the pathological disorders in the glucose homeostasis in fish (Caruso and Sheridan, 2011). Previous studies in fish have indicated that a long-term intake of the high carbohydrate diets led to insulin resistance in blunt snout bream (Xu et al., 2018) and Nile tilapia (Boonanuntanasarn et al., 2018a; Li et al., 2021). In the present study, the DCA3.75 diet reduced glucose level in the serum of Nile tilapia fed on 450 g/kg carbohydrate (often considered as a high carbohydrate level for Nile tilapia). These results indicate that DCA alleviates the hyperglycemia induced by the intake of high carbohydrate diet. However, the DCA3.75 diet did not increase insulin content in the serum in the normal state and during the GTT test. These results suggest that DCA accomplishes glucose clearance by increasing the insulin sensitivity, rather than promoting insulin secretion. Further evidences indicated that the DCA3.75 diet up-regulated the phosphorylated protein levels of Irβ, Irs1, Pi3k and Akt, which are all involved in the insulin signaling pathway. These results further confirmed our conclusion that DCA improves the insulin sensitivity in Nile tilapia. Similarly, the inhibition or deletion of PDKs in mice also improved glucose tolerance and insulin sensitivity (Thoudam et al., 2019; Wu et al., 2018a, 2018b; Younghoon et al., 2016). Therefore, our results suggest that PDK inhibition by using DCA promotes the insulin sensitivity in fish. Fish nutritionists should target the PDK2/4 as a key metabolic regulator for improving the insulin function in farmed fish.
4.3. Dietary DCA increases glycogen synthesis, and inhibits glycolipid conversion
Excess carbohydrate intake in fish usually causes high lipid deposition by increasing the lipogenic activity, subsequently elicits oxidative stress, inflammation and finally impairs health (Qiang et al., 2016; Rawles et al., 2008). In the present study, the DCA3.75 diet lowered lipid deposition in Nile tilapia, and also reduced the conversion of 14C-glucose to 14C-lipid. Accordingly, the hepatic mRNA expressions of srebp1, fas, dgat and accα genes were all down-regulated in Nile tilapia fed on the DCA3.75 diet, verifying the lipogenesis inhibition. Previous studies in mammals reported that increasing hepatic PDH activity by PDK2 inhibition ameliorated hepatic steatosis, and decreased the lipogenesis capacity by regulating the TCA cycle anaplerosis and ketogenesis (Olaniyi and Olatunji, 2019; Younghoon et al., 2016). The inhibitory effects of DCA on hepatic fat accumulation in the present study can be explained by 2 mechanisms. First, DCA promotes glucose towards oxidative catabolism rather than acting as the substrate for lipid synthesis. Secondly, DCA attenuates lipid synthesis by improving insulin sensitivity. Therefore, DCA reduces high lipid deposition in fish by promoting the glucose oxidative catabolism and improving insulin sensitivity.
A previous study found that fish respond to high carbohydrate intakes by increasing glycogen synthesis in the liver and muscle (Shi et al., 2018). In the present study, the DCA3.75 diet also increased the conversion of 14C-labled glucose to 14C-labled glycogen, and caused high glycogen deposition in the liver. Accordingly, the Nile tilapia fed on the DCA3.75 diet up-regulated the hepatic mRNA of gs. In addition, a previous study reported that glycogen synthase was inhibited by the increased phosphorylated Gs3kβ protein content (King et al., 2020). Gs3kβ was also inactivated by insulin signaling through phosphorylation of an N-terminal domain serine residue (Patel et al., 2008). In the present study, we found that the DCA3.75 diet activated insulin signaling pathway, and then increased the phosphorylation level of Gs3kβ. This inhibited Gs3kβ, stimulated glycogen synthase activity. These results indicate that DCA stimulates the conversion of glucose into glycogen synthesis, but not into lipid deposition.
5. Conclusion
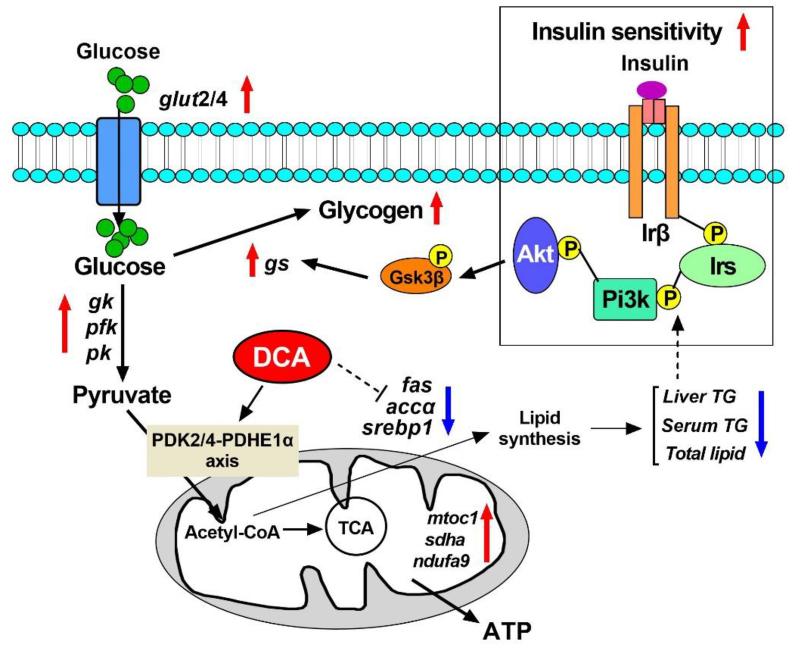
The present study demonstrated that dietary DCA treatment promotes efficient glucose oxidation and insulin sensitivity in Nile tilapia via inhibiting PDK2/4 and activating PDHE1α. Moreover, dietary DCA administration also improves the ability of Nile tilapia to synthesize glycogen, and inhibits glucose conversion into lipid. The underlying mechanisms are summarized in Fig. 6. This study provides new understandings on the regulatory effects of the PDK2/4-PDHE1α axis in carbohydrate utilization and remodeling the metabolic balance between glucose and lipid in fish. Our study brings forth new nutritional strategies for improving the adaptation of farmed fish towards high carbohydrate diets.
Fig. 6.
Summary of the results showing dichloroacetate (DCA) promotes the oxidation utilization of glucose and glycogen synthesis, improves insulin sensitivity, and inhibits glycolipid conversion by regulating the PDK2/4-PDHα axis in Nile tilapia. gk = glucokinase; pfk = phosphofructokinase; pk = pyruvate kinase; fas = fatty acid synthase; accα = acetyl-coa carboxylase α; srebp1 = sterol regulatory element binding transcription factor 1; mtco1 = mitochondrial cytochrome c oxidase 1; sdha = succinate dehydrogenase complex subunit A; ndufa9 = NADH dehydrogenase [ubiquinone] 1a subcomplex subunit 9; gs = glycogen synthase; Pdk = pyruvate dehydrogenase kinase; Pdhe1α = pyruvate dehydrogenase E1α subunit; Irβ = insulin receptor β; Irs1 = insulin receptor substrate 1; Pi3k = phosphatidylinositol 3-kinase; Akt = serine/threonine kinase; Gsk3β = glycogen synthase kinase 3β; TG = triglyceride; TCA = tricarboxylic acids.
Author contributions
Zhenyu Du and Yuan Luo designed the experiments; Yuan Luo carried out the experimental work. Yuan Luo wrote the manuscript under the direction of Zhenyu Du. Wenhao Zhou, Ruixin Li and Fang Qiao assisted with the experimental work. Samwel M. Limbu, Liqiao Chen and Meiling Zhang contributed to critical revision of the manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
The authors appreciate the financial support provided by the National Key R & D Program of China (2018YFD0900400).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- An R., Tang Z., Li Y., Li T., Xu Q., Zhen J., et al. Activation of pyruvate dehydrogenase by sodium dichloroacetate shifts metabolic consumption from amino acids to glucose in ipec-j2 eclls and intestinal bacteria in pigs. J Agric Food Chem. 2018;66:3793–3800. doi: 10.1021/acs.jafc.7b05800. [DOI] [PubMed] [Google Scholar]
- Boonanuntanasarn S., Jangprai A., Kumkhong S., Plagnes-Juan E., Veron V., Burel C., et al. Adaptation of Nile tilapia (Oreochromis niloticus) to different levels of dietary carbohydrates: new insights from a long term nutritional study. Aquaculture. 2018;496:58–65. [Google Scholar]
- Boonanuntanasarn S., Kumkhong S., Yoohat K., Plagnes-Juan E., Burel C., Marandel L., et al. Molecular responses of Nile tilapia (Oreochromis niloticus) to different levels of dietary carbohydrates. Aquaculture. 2018;482:117–123. [Google Scholar]
- Caruso M.A., Sheridan M.A. New insights into the signaling system and function of insulin in fish. Gen Comp Endocrinol. 2011;173:227–247. doi: 10.1016/j.ygcen.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Challiss R.A.J., Espinal J., Newsholme E.A. Insulin sensitivity of rates of glycolysis and glycogen synthesis in soleus, stripped soleus, epitrochlearis, and hemi-diaphragm muscles isolated from sedentary rats. Biosci Rep. 1983;3:675–679. doi: 10.1007/BF01172878. [DOI] [PubMed] [Google Scholar]
- Chan C., Krebs E.G. Epidermal growth factor stimulates glycogen synthase activity in cultured cells. Proc Natl Acad Sci USA. 1985;82:4563. doi: 10.1073/pnas.82.14.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D.R. Involvement of insulin-like growth factor-Ⅰ in the control of glucose homeostasis. Curr Opin Pharmacol. 2006;6:620–625. doi: 10.1016/j.coph.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Enes P., Panserat S., Kaushik S., Oliva-Teles A. Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem. 2009;35:519–539. doi: 10.1007/s10695-008-9259-5. [DOI] [PubMed] [Google Scholar]
- FAO . Sustainability in action. Food and Agriculture Organization; Rome: 2020. The state of world fisheries and aquaculture 2020; p. 206. [Google Scholar]
- Folch J., Lees M., Stanley G.S. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fukuda R., Marin-Juez R., El-Sammak H., Beisaw A., Ramadass R., Kuenne C., et al. Stimulation of glycolysis promotes cardiomyocyte proliferation after injury in adult zebrafish. EMBO Rep. 2020;21 doi: 10.15252/embr.201949752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal K., Saleme B., Al Batran R., Aburasayn H., Eshreif A., Ho K.L., et al. Foxo1 regulates myocardial glucose oxidation rates via transcriptional control of pyruvate dehydrogenase kinase 4 expression. Am J Physiol Heart Circ Physiol. 2017;313:H479–H490. doi: 10.1152/ajpheart.00191.2017. [DOI] [PubMed] [Google Scholar]
- Harris R.A., Melissa M., Huang B., Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzym Regul. 2002;42:249–259. doi: 10.1016/s0065-2571(01)00061-9. [DOI] [PubMed] [Google Scholar]
- Hassoun E., Kariya C., Williams F.E. Dichloroacetate-induced developmental toxicity and production of reactive oxygen species in zebrafish embryos. J Biochem Mol Toxicol. 2005;19:52–58. doi: 10.1002/jbt.20051. [DOI] [PubMed] [Google Scholar]
- Hemer G.I., Mommsen T.P., Krogdahl A. Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquacult Nutr. 2002;8:175–194. [Google Scholar]
- Jaswal J.S., Keung W., Wang W., Ussher J.R., Lopaschuk G.D. Targeting fatty acid and carbohydrate oxidation-a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Jeoung N.H., Harris R.A. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:46–54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Medale F., Kamalam B.S., Aguirre P., Veron V., Panserat S. Comparison of glucose and lipid metabolic gene expressions between fat and lean lines of rainbow trout after a glucose load. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalam B.S., Medale F., Panserat S. Utilisation of dietary carbohydrates in farmed fishes: new insights on influencing factors, biological limitations and future strategies. Aquaculture. 2017;467:3–27. [Google Scholar]
- Kato M., Li J., Chuang J.L., Chuang D.T. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by azd7545, dichloroacetate, and radicicol. Structure. 2007;15:992–1004. doi: 10.1016/j.str.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Li S., Liu C., Kim S.J., Sim C. Suppression of glycogen synthase expression reduces glycogen and lipid storage during mosquito overwintering diapause. J Insect Physiol. 2020;120 doi: 10.1016/j.jinsphys.2019.103971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyuyeva A., Tuganova A., Kedishvili N., Popov K.M. Tissue-specific kinase expression and activity regulate flux through the pyruvate dehydrogenase complex. J Biol Chem. 2019;294:838–851. doi: 10.1074/jbc.RA118.006433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X., Liu C., Fang J., Ma W., Zhang J., Cui S. The tumor suppressor gene lkb1 is essential for glucose homeostasis during zebrafish early development. FEBS Lett. 2016;590:2076–2085. doi: 10.1002/1873-3468.12237. [DOI] [PubMed] [Google Scholar]
- Li L., Li J., Ning L., Lu D., Luo Y., Ma Q., et al. Mitochondrial fatty acid β-oxidation inhibition promotes glucose utilization and protein deposition through energy homeostasis remodeling in fish. J Nutr. 2020;150:2322–2335. doi: 10.1093/jn/nxaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lu D., Jiang Z., Limbu S.M., Qiao F., Chen L., et al. Dietary l-carnitine improves glycogen and protein accumulation in Nile tilapia via increasing lipid-sourced energy supply: an isotope-based metabolic tracking. Aquacul Rep. 2020;17 [Google Scholar]
- Li L., Wang Y., Limbu S.M., Li J., Qiao F., Chen L., et al. Reduced fatty acid β-oxidation improves glucose catabolism and liver health in Nile tilapia (Oreochromis niloticus) juveniles fed a high-starch diet. Aquaculture. 2021;535 [Google Scholar]
- Limbu S.M., Zhang H., Luo Y., Chen L., Zhang M., Du Z. High carbohydrate diet partially protects Nile tilapia (Oreochromis niloticus) from oxytetracycline-induced side effects. Environ Pollut. 2020;256 doi: 10.1016/j.envpol.2019.113508. [DOI] [PubMed] [Google Scholar]
- Limbu S.M., Zhou L., Sun S., Zhang M., Du Z. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ Int. 2018;115:205–219. doi: 10.1016/j.envint.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Liu C., He A., Ning L., Luo Y., Li D., Zhang M., et al. Leptin selectively regulates nutrients metabolism in Nile tilapia fed on high carbohydrate or high fat diet. Front Endocrinol (Lausanne) 2018;9:574. doi: 10.3389/fendo.2018.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Y., Hu C., Qiao F., Wang X., Qin J.G., Du Z., et al. Gemfibrozil improves lipid metabolism in Nile tilapia Oreochromis niloticus fed a high-carbohydrate diet through peroxisome proliferator activated receptor-alpha activation. Gen Comp Endocrinol. 2020;296 doi: 10.1016/j.ygcen.2020.113537. [DOI] [PubMed] [Google Scholar]
- Olaniyi K.S., Olatunji L.A. Inhibition of pyruvate dehydrogenase kinase-4 by l-glutamine protects pregnant rats against fructose-induced obesity and hepatic lipid accumulation. Biomed Pharmacother. 2019;110:59–67. doi: 10.1016/j.biopha.2018.11.038. [DOI] [PubMed] [Google Scholar]
- Patel M.S., Nemeria N.S., Furey W., Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem. 2014;289:16615–16623. doi: 10.1074/jbc.R114.563148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Doble B.W., MacAulay K., Sinclair E.M., Drucker D.J., Woodgett J.R. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28:6314–6328. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pithukpakorn M. Disorders of pyruvate metabolism and the tricarboxylic acid cycle. Mol Genet Metabol. 2005;85:243–246. doi: 10.1016/j.ymgme.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Prisingkorn W., Prathomya P., Jakovlic I., Liu H., Zhao Y., Wang W. Transcriptomics, metabolomics and histology indicate that high-carbohydrate diet negatively affects the liver health of blunt snout bream (Megalobrama amblycephala) BMC Genom. 2017;18:856. doi: 10.1186/s12864-017-4246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang J., Yang H., Ma X., He J., Wang H., Kpundeh M.D., et al. Comparative studies on endocrine status and gene expression of hepatic carbohydrate metabolic enzymes in juvenile gift tilapia (Oreochromis niloticus) fed high-carbohydrate diets. Aquacult Res. 2016;47:758–768. [Google Scholar]
- Rawles S.D., Smith S.B., Gatlin D.M. Hepatic glucose utilization and lipogenesis of hybrid striped bass (Morone chrysops × Morone saxatilis) in response to dietary carbohydrate level and complexity. Aquacult Nutr. 2008;14:40–50. [Google Scholar]
- Richards J.G., Sardella B.A., Schulte P.M. Regulation of pyruvate dehydrogenase in the common killifish, fundulus heteroclitus, during hypoxia exposure. Am J Physiol Regul Integr Comp Physiol. 2008;295:R979–R990. doi: 10.1152/ajpregu.00192.2008. [DOI] [PubMed] [Google Scholar]
- Schafer C., Young Z.T., Makarewich C.A., Elnwasany A., Kinter C., Kinter M., et al. Coenzyme a-mediated degradation of pyruvate dehydrogenase kinase 4 promotes cardiac metabolic flexibility after high-fat feeding in mice. J Biol Chem. 2018;293:6915–6924. doi: 10.1074/jbc.RA117.000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Xu C., Liu M., Wang B., Liu W., Chen D., et al. Resveratrol improves the energy sensing and glycolipid metabolism of blunt snout bream Megalobrama amblycephala fed high-carbohydrate diets by activating the ampk-sirt1-pgc-1alpha network. Front Physiol. 2018;9:1258. doi: 10.3389/fphys.2018.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha M.K., Sharma R.L., Gharti K., Diana J.S. Polyculture of sahar (Tor putitora) with mixed-sex Nile tilapia. Aquaculture. 2011;319:284–289. [Google Scholar]
- Stacpoole P.W. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (pdc/pdk) axis in cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx071. [DOI] [PubMed] [Google Scholar]
- Su J., Mei L., Xi L., Gong Y., Yang Y., Jin J., et al. Responses of glycolysis, glycogen accumulation and glucose-induced lipogenesis in grass carp and Chinese longsnout catfish fed high-carbohydrate diet. Aquaculture. 2021;533 [Google Scholar]
- Takubo K., Nagamatsu G., Kobayashi C.I., Nakamura-Ishizu A., Kobayashi H., Ikeda E., et al. Regulation of glycolysis by pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q., Wang F., Xie S., Zhu X., Lei W., Shen J. Effect of high dietary starch levels on the growth performance, blood chemistry and body composition of gibel carp (Carassius auratus var. Gibelio) Aquacult Res. 2009;40:1011–1018. [Google Scholar]
- Thoudam T., Ha C.M., Leem J., Chanda D., Park J.S., Kim H.J., et al. Pdk4 augments er-mitochondria contact to dampen skeletal muscle insulin signaling during obesity. Diabetes. 2019;68:571–586. doi: 10.2337/db18-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Ekberg K. Splanchnic regulation of glucose production. Annu Rev Nutr. 2007;27:329–345. doi: 10.1146/annurev.nutr.27.061406.093806. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu Y., Tian L., Du Z., Wang J., Wang S., et al. Effects of dietary carbohydrate level on growth and body composition of juvenile tilapia, Oreochromis niloticusxo×O-aureus. Aquacult Res. 2005;36:1408–1413. [Google Scholar]
- Wu C., Satapati S., Gui W., Wynn R.M., Sharma G., Lou M., et al. A novel inhibitor of pyruvate dehydrogenase kinase stimulates myocardial carbohydrate oxidation in diet-induced obesity. J Biol Chem. 2018;293:9604–9613. doi: 10.1074/jbc.RA118.002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Tso S.C., Chuang J.L., Gui W., Lou M., Sharma G., et al. Targeting hepatic pyruvate dehydrogenase kinases restores insulin signaling and mitigates chrebp-mediated lipogenesis in diet-induced obese mice. Mol Metabol. 2018;12:12–24. doi: 10.1016/j.molmet.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Blair P., Sato J., Jaskiewicz J., Popov K.M., Harris R.A. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000;381:1–7. doi: 10.1006/abbi.2000.1946. [DOI] [PubMed] [Google Scholar]
- Xu C., Liu W., Zhang D., Cao X., Shi H., Li X. Interactions between dietary carbohydrate and metformin: implications on energy sensing, insulin signaling pathway, glycolipid metabolism and glucose tolerance in blunt snout bream Megalobrama amblycephala. Aquaculture. 2018;483:183–195. [Google Scholar]
- Xu R., Li M., Wang T., Zhao Y., Shan C., Qiao F., et al. Bacillus amyloliquefaciens ameliorates high-carbohydrate diet-induced metabolic phenotypes by restoration of intestinal acetate-producing bacteria in Nile tilapia. Br J Nutr. 2021:1–13. doi: 10.1017/S0007114521001318. [DOI] [PubMed] [Google Scholar]
- Younghoon G., Jeong J.Y., Jeoung N.H., Jeon J.H., Park B.Y., Kang H.J., et al. Inhibition of pyruvate dehydrogenase kinase 2 protects against hepatic steatosis through modulation of tricarboxylic acid cycle anaplerosis and ketogenesis. Diabetes. 2016;65:2876–2887. doi: 10.2337/db16-0223. [DOI] [PubMed] [Google Scholar]
- Zhang S., Hulver M.W., McMillan R.P., Cline M.A., Gilbert E.R. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab. 2014;11:10. doi: 10.1186/1743-7075-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]