Abstract
Although nano-immunotherapy has advanced dramatically in recent times, there remain two significant hurdles related to immune systems in cancer treatment, such as (namely) inevitable immune elimination of nanoplatforms and severely immunosuppressive microenvironment with low immunogenicity, hampering the performance of nanomedicines. To address these issues, several immune-regulating camouflaged nanocomposites have emerged as prevailing strategies due to their unique characteristics and specific functionalities. In this review, we emphasize the composition, performances, and mechanisms of various immune-regulating camouflaged nanoplatforms, including polymer-coated, cell membrane-camouflaged, and exosome-based nanoplatforms to evade the immune clearance of nanoplatforms or upregulate the immune function against the tumor. Further, we discuss the applications of these immune-regulating camouflaged nanoplatforms in directly boosting cancer immunotherapy and some immunogenic cell death-inducing immunotherapeutic modalities, such as chemotherapy, photothermal therapy, and reactive oxygen species-mediated immunotherapies, highlighting the current progress and recent advancements. Finally, we conclude the article with interesting perspectives, suggesting future tendencies of these innovative camouflaged constructs towards their translation pipeline.
Keywords: Biological camouflage, Immunogenic cell death, Prolonged blood circulation, Immune-regulating, Nanovaccine
Graphical abstract
Highlights
-
•
Various immune-regulating camouflaged nanoplatforms are emphasized.
-
•
Immunotherapeutic applications of camouflaged nanoplatforms are systematically summarized.
-
•
ICD-induced therapeutic modalities based on these nanoplatforms are discussed.
1. Introduction
Cancer has become one of the dreadful threats to human health globally due to high morbidity and mortality rates, accounting for millions of deaths every year [1]. Despite several therapeutic options against cancer, immunotherapy has emerged as a groundbreaking treatment strategy with numerous advantages, such as high specificity and low side effects to kill cancer cells, as well as prevent their recurrence [2,3]. Currently, available cancer immunotherapies include adoptive T-cell therapy, immune checkpoint blockade (ICB), and therapeutic cancer vaccines, exhibiting significant efficacy in clinical trials to treat different types of tumors [4,5]. In addition, some other ablative cancer therapies like chemotherapy, photothermal therapy (PTT), and some reactive oxygen species (ROS)-mediated therapies, can enhance antitumor immunity by inducing immunogenic cell death (ICD) [6,7]. In the ICD process, the dying tumor cells secrete damage-associated molecular patterns (DAMPs), such as calreticulin (CRT), adenosine triphosphate (ATP), high mobility group box 1 (HMGB1), and heat shock proteins (HSP70 and HSP90) [8]. These factors can significantly facilitate tumor antigen uptake by antigen-presenting cells (APCs) and their subsequent activation, allowing these treatment strategies for potential ICD-inducing immunotherapeutic modalities. However, the applicability is limited due to a major challenge of the accurate access of these therapeutic agents to the target site (including the tumor cells, the immune microenvironment, and the peripheral immune system) at the right time and place [9].
In recent years, several advancements in exploring the fabrication of nanomaterials have brought significant breakthroughs in cancer diagnosis, treatment, and prevention towards increasingly accelerated development of therapeutic nanoplatforms with ultrasmall size range, adjustable physiochemical properties, and flexible surface modification [10]. In terms of passive targeting, the ultrasmall particle sizes with a high surface-to-volume ratio of nanomaterials present high drug delivery efficacy in the tumor sites via the enhanced permeation and retention (EPR) effect due to the fact that vasculature within a tumorous zone is leaky and the lymphatic system is dysfunctional [11,12]. In addition, the immobilization of various targeting moieties, such as receptor ligands, on the surface of nanomaterials precisely conveys the loaded therapeutic payload to the tumor sites through active targeting [13]. The nanoplatforms for cancer therapy are designed to improve the direct eradication of tumor cells by raising the delivery of therapeutic agents to the tumor site. However, over the past few years, several nanoformulations have been applied to enhance anticancer immunity or synergize with the existing immunotherapeutics.
Despite the significant advancements in fabricating various nanoplatforms in cancer immunotherapy, a thorough cure of cancer remains challenging due to some general hurdles related to immune systems, limiting the therapeutic effects of nanomedicines. Indeed, the immune system often works by recruiting innate and adaptive immune responses for two different purposes [14]. On the one end, the innate immune responses implicate the sequestration of pathogens and efficient removal of senescent cells by some proteins and phagocytic cells without much specificity, resulting in the generation of cytokines such as interleukin-1 (IL-1) or type I interferon (IFN–I) [15]. Cytokines are the biological mediators that cause the death of infected cells and generate a further protection response against the infection [16]. On the other end, the adaptive immune system prevents the invasion of foreign pathogens from maintaining homeostasis with a more specific response and prepares for future prevention by the delayed reactions, including both cellular and humoral reactions. In the defense process of the adaptive immune system, the mature immune cells and antibodies can recognize specific antigens on the pathogens specifically. The adaptive immune responses also trigger immunological memory against infecting pathogens due to the remained clones of lymphocytes [17]. Owing to such a complex immune system, there exist two major obstacles for nanoparticle-based cancer immunotherapies.
The first extremely critical hurdle of nanoplatforms is the inevitable elimination of the nanoplatforms by the immune system. Nanoplatforms possess similar physical and chemical characteristics to pathogens, such as size that is only a tiny part of the cell diameter, a powerful liquid-solid interface, and a patterned surface [18]. Thus, some mechanisms protecting against pathogens also apply to the recognition and elimination of nanoplatforms. However, immunological memory against pathogens is helpful for the prevention of infections, while immune responses toward nanoparticles may limit their treatment efficiency or cause adverse reactions. After administration, the nanoparticles can be quickly recognized as extraneous matter and eliminated by the immune system. The second challenge is implicated in the tumor immune escape and low tumor immunogenicity. In recent years, it has been confirmed that the immune system paradoxically inhibits and promotes the survival and proliferation of cancer cells. This proposed “cancer immunoediting” for the relationship between tumor and immunity is proceeded by three stages: elimination, equilibrium, and escape [[19], [20], [21]]. In the first period, immunological surveillance occurs to recognize and eliminate most malignant cells [22]. Then, the selection of tumor variants occurs to balance the immune system and malignant cells in the “equilibrium” phase [23]. Eventually, the rapid and unrestrained growth of malignant tumor cells breaks the equilibrium state, achieving the tumor immune escape. In this context, several mechanisms of tumor immune escape include the selection of immune-resistant tumor cells, abnormalities in antitumor-related immune cells like dendritic cells (DCs) and T cells, barriers in T-cell trafficking, and formation of the immunosuppressive tumor microenvironment (TME), which are responsible for the immune tolerance in various stages of the cancer-immune cycle [24]. Due to the high adaptability of the transformed tumor cells, the insufficient antitumor response or suppressed immunocyte functions may lead to further tumor proliferation and metastasis, directing the ineffective cancer treatment outcomes of nano-immunotherapy [25,26]. Therefore, tackling these two problems induced by the immune system has attracted growing interest from researchers in developing various innovative strategies for cancer nano-immunotherapy.
Based on the technological advancements of biomaterials, immune-regulating camouflage strategies aimed at evading the immune clearance of nanoplatforms or upregulating the immune function against tumors have emerged as novel interfacing approaches for promoting cancer therapy. Before reaching the lesion site, these biomimetic systems can serve as a stealthiness to evade the surveillance of the immune system, thus minimizing the capture by the mononuclear phagocyte system (MPS) and prolonged circulation in the blood [27]. After reaching the tumor site, the camouflage coating can trigger or enhance the immune response against tumors through different mechanisms. In general, the immune-regulating camouflaged nanoplatforms have been investigated for their application in various modern cancer therapies, such as coating with distinct polymers [28,29] and camouflaging various cell membranes [[30], [31], [32]] or based on cell-derived exosomes [33,34]. Owing to the unique characteristics and particular functionalities, these immune-regulating camouflage enables the nanosystems to overcome or alleviate the limitations mentioned above induced by the immune system, presenting prolonged circulation and improved anticancer efficacy [35]. Although several reviews have been published discussing the biomimetic camouflage delivery strategies for cancer therapy over the past decade, significant attention has been barely paid to the immune-regulating function of nanoplatforms for enhanced cancer nano-immunotherapy. Therefore, a timely review of relevant research progress is of great significance for the continuous development of cancer therapy. From the unique perspective of the cancer nano-immunotherapy, this review focuses on the recent efforts made in the construction of various immune-regulating camouflaged nanoplatforms, including polymer-coated, cell membrane-camouflaged, and exosome-based nanoplatforms (Fig. 1). Further, we give a brief overview of these immune-regulating camouflaged nanoplatforms applied in the currently established immunotherapies and some potential therapeutic strategies for ICD-inducing immunotherapies. Finally, we discuss the challenges and perspectives, suggesting future tendencies of these innovative camouflaged constructs towards their clinical application.
Fig. 1.
Schematic illustrating different types of immune-regulating camouflaged nanoplatforms with evading immune clearance or enhancing immune response functions to enhance the currently established immunotherapies and some ICD-inducing immunotherapies.
2. Immune-regulating camouflaged nanoplatforms
As specified earlier, nanoplatforms intrinsically offer the unique characteristics of the immune-regulating camouflage materials (including polymers, cell membranes, and exosomes) via different camouflage approaches to ameliorate the limitations of the inevitable immune clearance of nanoscale platforms and tumor immune escape. Various polymeric materials have been used as a protective covering to provide biocompatibility and “stealth” properties on nanoplatforms, refraining from activating the immune system [29,36]. Further advancements in the interactions between tumor cells and the immune system have resulted in the fabrication of diverse polymeric materials to stimulate the antitumor immune reactivity with different functions and mechanisms. However, the bottom-up ligand conjugation methods for coating polymeric materials on nanoparticles are increasingly challenging as more surface functionalities are required. To overcome the tedious work of conjugating polymers on the surface of nanoplatforms, considerable efforts have been dedicated to fabricating biomimetic cell-based nanoplatforms, including cell membrane-cloaked and exosome-based nanoplatforms. Cloaking with cell membranes via a top-down method or encapsulating into cell-derived exosomes requires more accessible technologies to endow nanoplatforms with surfaces that directly replicate the proteins or other biologically active molecules and highly complex behaviors of the source cells, rendering the prolonged nanoparticles circulation time or the induction of antitumor immunity. Due to the various properties or the forms of these immune-camouflage materials, various immune-regulating camouflaged nanoplatforms with specialized design and functionalization have been developed.
2.1. Polymer-coated nanoplatforms
In general, various polymers have been widely used in the surface modification of nanoplatforms to promote water solubility of encapsulated therapeutic guests, biocompatibility, biodegradability, and provide other functionalities, such as targeting ability, stimuli-responsiveness, and immune modulation [29,37]. To reduce the immune elimination of nanoplatforms or upregulate the immune response against tumor cells, polymeric materials from the natural or synthetic origin are used to cloak nanomaterials.
2.1.1. Evading immune clearance
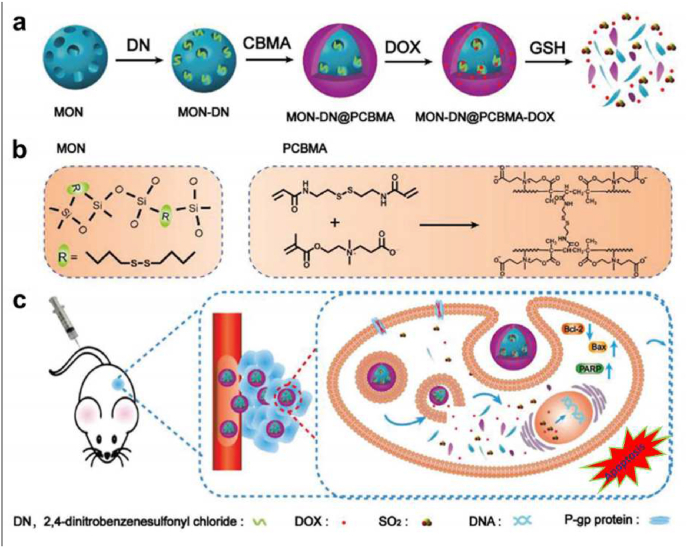
Non-reactive or inert polymeric materials that have almost no interactions with the host's immune cells are applied as stealth coatings on the nanoplatforms, making the foreign substances (nanoparticles) more amenable as “self” to refrain from activating the immune system and consequently reducing the immune clearance [30,38]. Traditionally, coating the synthetic polymer, poly(ethylene glycol) (PEG), on the surface of nanoparticles, often referred to as PEGylation, is considered an effective strategy to decrease nanoparticle elimination [30]. The hydrophilic PEG layer on the surface of nanoplatforms can provide steric stability and decrease the interactions between nanosystems and the environment [38]. For example, a novel contrast agent based on iron oxide nanoparticles (IONPs) for magnetic resonance imaging (MRI) was developed, the size of which was only 15 nm [39]. Such a tiny scale would lead to fast immune clearance and rapid elimination of IONPs from the body, resulting in weak signal intensity. To alleviate the immune clearance of IONPs, PEG was coated over IONPs to protect them from rapid clearance, in addition to improved biocompatibility and longer blood circulation time over the bare IONPs. The PEG-coated IONPs showed great potential as contrast agents for longitudinal MRI of solid cancers. Interestingly, Jutaek Nam and his colleagues found that PEG modification could abolish the cytotoxicity of polyethyleneimine (PEI)-based nanovaccines. In addition, PEG could serve as an uncharged spacer unit that provided steric stabilization, decreased nonspecific cellular uptake, and improved in vivo performance for PEI and its nano-complex [40]. However, it has been reported that repetitive administration of PEGylated treatment would induce anti-PEG antibodies, which might increase the immune elimination of nanoparticles. With the progression of the polymer coating nanotechnology, some other synthetic polymeric materials have also been investigated as cloaking materials to overcome the limit of PEGylation, including PEG-based copolymers, such as poloxamers [41] and polysorbates [42], some hydrophilic polymers like polyvinyl pyrrolidone (PVP) [43] and polyvinyl alcohol (PVA) [44], and zwitterionic polymers like poly(carboxybetaine methacrylate) (PCBMA) [45]. Among these polymers, zwitterionic polymers present a higher potential to be the alternatives to PEG. Compared to solely positively or negatively charged polymers that are easier to be eliminated by the reticuloendothelial system (RES), zwitterionic polymers possessing either zwitterionic groups or a mixture of anionic and cationic terminal groups have demonstrated high resistance to non-specific protein binding, thereby avoiding rapid immunological recognition and showing prolonged blood circulation in the body [46]. Nevertheless, the current zwitterionic polymer coatings are primarily based on linear zwitterionic polymer chains, which are probably inadequate to cover the surface of nanoplatforms entirely. Polymerizing monomers into dense polymer shells on nanoparticles seems better to provide enhanced long-term stability. A recent study covered a dense PCBMA polymer shell from polymerizing CBMA monomers on mesoporous organosilica nanoparticles (MON) via a reflux precipitation polymerization approach, providing prolonged blood circulation up to 48 h blocking the adsorption of proteins (Fig. 2) [45]. Furthermore, MON and PCBMA shell with disulfide bonds could present TME-responsive biodegradation to realize controlled release of the loaded SO2 prodrug molecules and doxorubicin (DOX).
Fig. 2.
(a) Schematic illustration of the redox-responsive sulfur dioxide‒releasing nanosystem with a prolonged circulation time for producing the synergistic effect of chemotherapy and gas therapy. (b) Fabrication of MON-DN@PCBMA-DOX and the structure of MON and PCBMA. (c) Mechanism of controlled drug release and combination therapy. Reproduced with permission from Ref. [45] Copyright 2020, John Wiley & Sons.
In addition to these synthetic polymeric materials, polymers from natural sources, such as heparin [47,48] and hyaluronic acid (HA) [49], have been explored in a wide range for cloaking foreign particle surfaces. Heparin and HA offer the combination of shielding by anti-inflammatory and anti-immunogenic function and targeting ability through binding with a cell-specific surface marker CD44, which is highly expressed in various cancer cells. In a case, Soleymani and colleagues fabricated the HA-conjugated Fe3O4 nanoparticles (Fe3O4@HA) via a facile one-pot hydrothermal method. The obtained Fe3O4@HA nanoparticles with suitable size and biocompatibility demonstrated exceptional targeting ability towards CD44-overexpressing cancerous cells and high heating efficacy for magnetic hyperthermia therapy [50].
2.1.2. Enhancing immune response against tumor
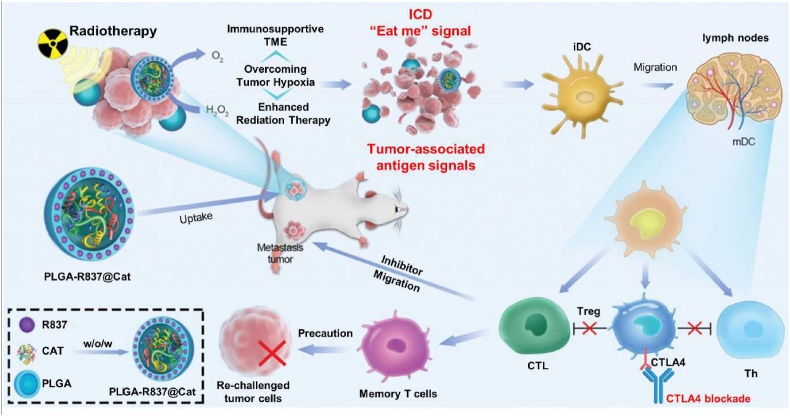
In terms of amplifying the immune reactivity to alleviate tumor immune escape, nanoplatforms are often coated with polymeric materials aiming at different targets. In this context, various synthetic polymers, such as polystyrene [51], poly(lactic-co-glycolic acid) (PLGA) [52,53], and poly(beta-amino esters) (PBAEs) [54], in the order of non-, slowly-, and rapidly-degradable, present intrinsic immunostimulatory properties via activating dendritic cells (DCs) and causing an increase of inflammatory cytokines (IL-1β) that supports early immune responses [55]. Compared to polystyrene and PBAEs, PLGA-based nanoparticles are more frequently used to serve not only as carriers but also as agents that elicit or modulate the immune response. In an instance, Chen and colleagues developed core-shell nanoparticles based on PLGA for enhanced radiotherapy (RT) via a water/oil/water (w/o/w) double emulsion approach (Fig. 3) [53]. Inside the aqueous cavity of the PLGA shell, catalase (Cat), a water-soluble enzyme that could rapidly decompose H2O2, was encapsulated as the inner core, whereas hydrophobic imiquimod (R837), a Toll-like-receptor (TLR)-7 agonist, was loaded within the PLGA shell. In the prepared PLGA-R837@Cat nanoparticles, the PLGA shell might help R837, as immune adjuvants, to amplify RT-triggered immune responses by presenting antigen-capturing functions. In addition to the direct activation of the immune system, PLGA-based nanoparticles could also act as an amplifier of ICD via controlling the release kinetics of the ICD-inducing drugs to provide a boosted immune response. With the extracellular release of DAMPs, including CRT, ATP, HMGB1, and heat shock proteins (HSP70 and HSP90) from the dying tumor cells, the engulfment of tumor antigens by DCs and the activation of CD8+ T cells were promoted. Recently, PLGA-based nanoparticles with different molecular weights (7000 g/mol and 12,000 g/mol) were applied to encapsulate DOX by oil-in-water (o/w) emulsion approach for realizing different release kinetics of DOX, eliciting the ICD of cancer cells [56]. The DOX-PLGA7K and DOX-PLGA12K nanoparticles exhibited specific cytotoxicity and HMGB1 secretion due to the different DOX release rates. Sustained release of DOX from both DOX-PLGA7K and DOX-PLGA12K could prevent tumor growth and stimulate DC maturation and tumor-infiltration of cytotoxic T-lymphocytes (CTLs), forming a tumor-specific immunological memory effect.
Fig. 3.
Schematic illustrating the mechanism of antitumor immune responses induced by PLGA-R837@Cat-based radiotherapy combined with checkpoint-blockade to inhibit cancer metastases and recurrence. Reproduced with permission from Ref. [53] Copyright 2019, John Wiley & Sons.
Polysaccharides from natural sources, including chitosan (CS) [57], dextran [58], and sodium alginate [59], have also been commonly coated over nanoparticles. These polysaccharides can promote the reactivity of non-specific immune cells (neutrophils and macrophages), inducing the elevated generation of growth factors and cytokines that can further elicit the differentiation and proliferation of CD4+ and CD8+ T cells to tune the whole immune response against the tumor. It is worth mentioning that chitosan is one of the natural positively charged polysaccharides due to its protonating amino groups, allowing mucosal adhesion of chitosan-cloaked nanoplatforms and then escaping from the RES. Utilizing the mucosal route, chitosan-cloaked nanoplatforms can be transferred into immune-competent cells to foster cellular immune responses. By tuning the size of chitosan nanoparticles, Walter and coworkers explored the potential of chitosan nanoparticles as antigen vehicles to deliver ovalbumin-derived peptide SIINFEKL (OVA 257–264) into tumor tissue for a potent immune response [60]. It was observed that DCs could effectively take up the chitosan-based nanoparticles with small particle sizes, generally regarded as the most potent APCs to induce activation and proliferation of tumor antigen-specific CD8+ T cells. Despite the advances of these polymers applying as the immune camouflage over nanoplatforms, there are a few limitations that remain to be solved, such as the quantitative control of their molecular weight and density, the tedious work of conjugating polymeric materials on the surface of nanoparticles, and rational ligand modifications of polymers for more surface functionalities [61].
2.2. Cell membrane-camouflaged nanoplatforms
To overcome the challenges faced by the polymer-coated nanoplatforms for better delivery and therapeutic effects, considerable efforts have been dedicated to exploring biomimetic nanotechnology. Utilizing naturally-occurring strategies improved by the evolutionary processes, biomimetic nanotechnology has emerged as a reasonable method for efficient nanoparticle design that considers the inherent biological nature of interactions of nanoparticles in the body [62]. Notably, the biomimetic nanoplatforms mimicking cellular components have been widely investigated by researchers due to their capability of inheriting the physicochemical properties and defined biological functions, such as interacting with diverse proteins and other different cells in the complicated extracellular matrix (ECM) environment [63]. Cell membranes and natural components derived from cells can be cloaked over nanoplatforms to obtain cell membrane-camouflaged nanoplatforms with cell-like activities. Cell membrane-camouflaged nanoparticles provide an easy top-down approach for entrapping nanoplatforms in cell membranes extracted from erythrocytes, platelets, cancer cells, stem cells, immune cells, and bacteria [64]. Thus, the intrinsic characteristics of cell membranes can be transferred to the nanoplatform surface for various source cell-inherited functions, such as “self” markers, targeting or homing to specific areas and communication with the immunologic system.
2.2.1. Evading immune clearance
Inspired by the role of erythrocytes as the most abundant circulating cells in the blood for delivering oxygen to tissues, red blood cell (RBC) membranes have attracted wide attention in coating over nanomaterials for potently escaping the immunological clearance. The reasons behind the long-term circulation of RBCs (average life span of 120 days) are due to the expression of immunomodulatory glycans and proteins on their surfaces, such as sialic acid moieties, “don't eat me” marker CD47, CD59, complement factor 1, decay-accelerating factor, C8 binding protein, among others [65,66]. Owing to the inherited features from the original cells, RBC membrane-camouflaged nanoplatforms effectively evade the immune attack, presenting extended survival in the systemic circulation [67]. In a recent study, Li and colleagues constructed an erythrocyte-camouflaged mesoporous titanium dioxide nanoplatform loaded with hypoxia-activated prodrug AQ4N. By inheriting the specific features from RBC, the obtained RBC-mTNPs@AQ4N presented an excellent ability to escape immune clearance, resulting in the efficient tumoral distribution to effectively kill tumor cells [68]. Apart from erythrocytes, platelets stemming from the mature megakaryocytes in the bone marrow are another significant non-nucleated blood cell [69]. They are essential for thrombosis and homeostasis in vessel injuries, as well as involved in developing lymphatic vasculature and mediating immune response [70]. Similar to RBCs, there are some vital surface proteins expressed on the surface of platelets to suppress the immunologic system, such as CD47, CD55, and CD59. These support platelets to evade macrophage phagocytosis, while CD55 and CD59 can restrain the activation of complement-mediated immune activation. Moreover, the biotropism to vasculature trauma allows platelets to naturally adhere to the leaky tumor vasculature, contact the tumor, and then extravasate into the TME [71]. Surface-expressed p-selectin can bind with the adhesion molecules CD44, rendering high affinity to circulating tumor cells [72,73]. Therefore, nanoplatforms camouflaged by platelet membranes offer enormous potential in realizing prolonged circulation time, avoidance of clearance by RES, and targeting small lesions [74]. In one case, a platelet-engineered nanoplatform was designed via uniquely co-assembling DOX and cyclin-dependent kinase 5 inhibitor roscovitine (Rosco) with platelet membrane fragment (PMF) as the particulate stabilizer, to effective drug delivery to residual micro-tumors after surgical resection [75].
Compared to RBC and platelet membranes, membranes extracted from immune cells, cancer cells, and stem cells can protect nanoplatforms from immune clearance and target the solid tumor. As a second candidate for the cell membrane-camouflaged nanoplatforms after the RBC membrane, cancer cell membrane-camouflaged nanoplatforms have been constructed to mimic cancer cells, which can avoid the immune attacks against tumors on the above-mentioned mechanism of immune escape of tumor. Moreover, the membrane-expressed proteins CD47 are responsible for preventing phagocytic uptake by macrophages and immune tolerance [76]. In addition, cancer cell membrane-coated nanoparticles reportedly demonstrate homotypic aggregation into the tumor site, depending on the specific interactions of identical surface adhesion molecules as the source of cancer cells, such as epithelial cell adhesion molecule (EpCAM), galectin-3, and N-cadherin [27,76]. A nanoplatform based on the 2D MnO2 nanosheets wrapped over gold nanorods (loading with DOX), and subsequent cancer cell membranes, was recently developed [77]. The prepared nanoplatform was endowed with homotypic tumor targeting and immune clearance evading abilities from cancer cell membranes. Under endogenous TME stimuli, the membrane could be disrupted to release the drug load to realize the remarkable treatment effects of dual-imaging-guided synergistic cancer therapy.
Stem cells or mesenchymal stem cells (MSCs) can differentiate into various cell types and have long-term proliferation via self-renewal division [78]. In the bloodstream, MSC membrane-camouflaged nanoparticle systems exhibit a considerable circulating duration and active attraction to neoplastic cells, as tumor-specific cell adhesion molecules on MSC surfaces (such as CXCR4, epidermal growth factor, EGF, and integrins) can be attracted by tumoral chemoattractants (stromal cell-derived factor 1) or tumor-targeted ligands (human epidermal growth factor receptor-2 (HER2)) [79,80]. Compared with RBC or cancer cell membranes, MSC membranes possess a stronger clearance evading ability than tumor cell membranes. Despite the similarities in terms of long-circulation ability, MSC membranes can target tumor sites actively, which is lacking in the RBC membranes. Thus, in a recent case, a biomimetic vehicle was established by integrating MSC membranes with mesoporous silica nanoparticles (MSN@M). The MSC membrane-coated nanoplatform acquired the ability to escape immunosurveillance via CD47 on the membrane surface and reduced distribution in normal tissues with increased residence in tumor tissue, enhancing the therapeutic outcomes with alleviated side effects (Fig. 4) [81].
Fig. 4.
In vitro immune clearance evasion, pharmacokinetics, and biodistribution of MSN@M. (a) MSN@M could avoid phagocyte clearance through CD47 and target tumor sites through their homing ability. (b) RAW 264.7 cell uptake of MSN and MSN@M at different time points captured by CLSM, scale bar = 5 μm, and (c) flow cytometry analyzing of the fluorescence intensity of FITC-MSN@M and FITC-MSN in RAW 264.7 cells at 2 h and (d) 4 h (n = 3, *p < 0.05, **p < 0.01). (e) Blood retention of MSN@M, MSN, and free FITC (dosage of FITC at 1 mg/kg) in HepG2 xenograft mice after one dose intravenous injection to analyze the pharmacokinetics of MSN@M (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001). (f) Distribution of ICG-MSN@M, ICG-MSN, and free ICG in HepG2 xenograft nude mice at 4, 8, 12, and 24 h after tail vein injection of 0.8 mg/mL ICG-MSN@M, ICG-MSN, and free ICG. The tumor size of HepG2 xenograft mice was approximately 150 mm3. (g) Distribution of ICG-MSN@M, ICG-MSN, and free ICG in tumor site of HepG2 xenograft nude mice at 24 h and (h) fluorescence quantitative analysis of signal intensity in tumor site of three groups at 24 h (n = 3, *p < 0.05, ***p < 0.001). (i) Ratio of the fluorescence signal at major organs and tumor site to the total fluorescence signal in the ICG-MSN@M, ICG-MSN, and free ICG groups at 24 h (n = 3, *p < 0.05, ***p < 0.001). (j) CLSM image of the distribution of MSN@M and MSN in tumors after tail vein injection of 1 mg/mL FITC-MSN@M, FITC-MSN, and free FITC. Left scale bar = 750 μm, right scale bar = 100 μm. Reproduced with permission from Ref. [81] Copyright 2020, Elsevier.
Typically, immune cells include APCs (e.g., macrophages, DCs), natural killer (NK) cells, and cytotoxic T lymphocytes, among others [69]. A wide range of surface markers responsible for immune recognition and response, including receptors, antigens, and adhesion molecules, are expressed on the immune cell surfaces. Due to the homotypic recognition, membranes isolated from immune cells can serve as stealth coatings to assist nanoplatforms in escaping immune surveillance and clearance. Immune cell membrane-camouflaged nanoplatforms present the targeting ability to tumor cells since immune cells show a natural and high tumor affinity via specifically recognizing correlated molecules on the tumor surface [82]. Membranes derived from macrophage and T cells are mainly applied just to enable the nanoplatforms immune evasion and targeting towards tumor tissues. In contrast, membranes derived from DCs and NK cells are commonly used not only for prolonging nanoplatforms circulation time but also for enhancing tumor immunity (see discussions later in section 2.2.2). As a vital cell subset of the APCs, macrophages play an important role in the mononuclear phagocytic system, homing to chronic inflammatory tumor sites and presenting the innate inflammation-directed chemotactic ability via vascular cell adhesion molecule-1 (VCAM1) and α4 integrins [30,69]. Duplicating these characteristics, foreign nanoparticles coated with macrophage membranes can escape the MPS and drive the vector to accumulate into tumor tissues. In a recent effort to delay the elimination of MPS, macrophage membranes were covered on the nanoparticles loaded with DOX, chlorin e6 (Ce6) and indoleamine 2,3-dioxygenase 1 inhibitor (IDO1), providing an excellent drug delivery system (DDS) to solve the low cargo delivery efficiency into the solid tumor [83]. CTLs, or T cells, are antigen-specific lymphocytes, which can be triggered by encountering antigens to mature and become functional, subsequently secreting the corresponding antibodies and posing a cytotoxic effect [27]. T cells can exhibit a high tumor affinity via the binding of related molecules on tumor cell membranes and the specific immune recognition protein complex T-cell receptors (TCRs) on the T-cell surface [84,85]. TCRs can recognize debris of antigens as peptides bound to a major histocompatibility complex (MHC), allowing for the targeting ability of T cells towards both surface and intracellular tumor neoantigens [86,87]. Taking advantage of antigen-specific recognition of T cells, T-cell membrane-coated nanoplatforms have been developed for evading immune clearance and cancer-targeted treatment. For instance, membranes extracted from gp100-specific T-lymphocyte hybridoma targeted against melanoma cancer were applied to camouflage PLGA nanoparticles with encapsulated dyes (coumarin-6/lipophilic DiD) or drugs (Trametinib) for diagnosis or treatment, respectively. The coated membranes presented a significant increase in the aggregation and stabilities of the nanoparticles [88].
2.2.2. Enhancing immune response against tumor
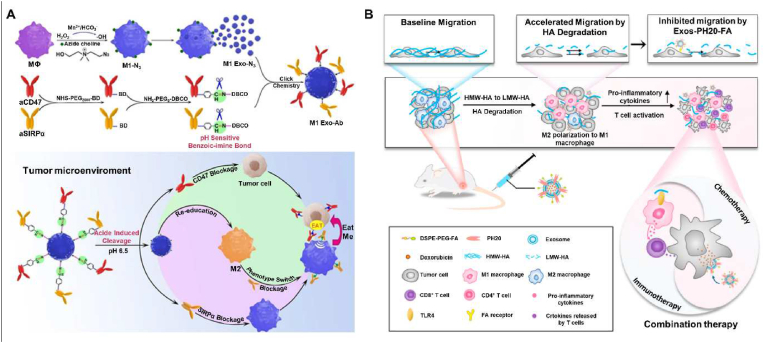
In addition to evading the clearance of nanoparticles by the immune system, some types of cell membranes coated over nanoparticles enable triggering or enhancing the immune response against tumors through different mechanisms. Firstly, membranes extracted from immune cells like tumor-associated macrophages (TAMs), DCs, and NK cells have been demonstrated to showcase their immune-camouflage effects after coating nanoparticles. Unlike regular macrophages, TAMs containing myeloid-derived macrophages and tissue-resident macrophages are significant components in TME, which can interact with tumor cells to promote tumor growth, progression, and metastasis, rendering the immunosuppressive TME and resistance to cancer treatments [89]. In general, TAMs tend to be polarized towards the immunosuppressive M2 phenotype after the binding of macrophage colony-stimulating factor 1 (CSF1) secreted from tumor cells with the receptor CSF1R on the macrophage membrane [30,90]. Because of the crucial role of the CSF1-CSF1R axis in forming immunosuppressive TME, preventing the binding of CSF1 and CSF1R seems an excellent way to block TAMs trafficking and alleviate the tumor immune escape [91,92]. Chen et al. constructed TAM-like upconversion nano-photosensitizers (NPR@TAMM) by coating TAM membranes on photosensitizer-loaded upconversion nanoparticles [93]. These TAM-mimic NPR@TAMMs could selectively accumulate in the tumor sites and bind with the immunoregulatory molecule CSF1. These consequences reduced the binding of CSF1 with the endogenous TAMs, thus alleviating the immunosuppression in TME for improved therapeutic efficacy (Fig. 5A).
Fig. 5.
(A) Schematic illustration of the tumor-associated-macrophage-membrane-coated upconversion nanoparticles for improved photodynamic immunotherapy. Reproduced with permission from Ref. [93] Copyright 2021, American Chemical Society. (B) Generation and characterization of DCNV-rAd-Ag. a) Generation of DCNVs derived from adenovirus-infected mature dendritic cells. (1) The genes of tumor-specific antigen were genetically engineered into the adenovirus vector. (2) Recombinant adenovirus infected the immature DC2.4 cells to express the modified antigen on the cell surface and stimulate it. (3) Differentiation, maturation, and antigen presentation. (4) Harvesting of the induced mature cell membrane and preparation of DCNV-rAd-Ag. b) Schematic illustration of the generation of DCNV-rAd-Ag. c,d) Cryo-electron microscopy (c) and dynamic light scattering analyses (d) showed uniform DCNV-rAd-Ag (approximately 108 nm average diameter, polydispersity index = 0.14) with a vesicle-like morphology. Scale bar, 50 nm. e) The Western blot on membrane proteins from DCNV-rAd-GFP demonstrates a similar protein content on the surface compared to that of the parental cells. Panels (c–e) show representative results of two independent experiments with similar results. f) Comparison of upregulated immune-response-related proteins in NVs and DCs. g) The relative abundance of antigen presentation and migration-related proteins on DCNV-rAd-GFP. r.p.m., revolutions per minute. CCR - CC chemokine receptor; CXCR - C-X-C chemokine receptor; EpCAM - epithelial cellular adhesion molecule; ICAM 1 - intercellular adhesion molecule 1; pMHC-I - peptide-major histocompatibility complex class I. Reproduced with permission from Ref. [98] Copyright 2022, Springer Nature.
As the most potent APCs, DCs can motivate both resting helper T cells, as well as memory and naive T cells, which play a crucial role in activating and modulating adaptive immune responses [94,95]. Tolerogenic DCs can induce anergy and further lead to immune escape of tumor cells, so artificial manipulation of DCs to promote T cells is of great significance. DC membrane-camouflaged nanoplatforms emerge with great potential to more efficiently activate T cells, realizing antitumor immunity for potential cancer treatment. To further amplify the immune response, membranes isolated from hybrid cells fused by two types of cells into a unified cytoplasm with the maintained identity of dual nuclei were explored to camouflage therapeutic nanoparticles [96]. Because of the close interrelation of cancer cells and DCs, the cytomembrane of their fused cells (FCs) carrying highly expressed tumor antigens and DC-inherited stimulatory molecules (B7 family members) was developed. Liu and coworkers used such cytomembranes to coat metal-organic frameworks (MOFs) containing photosensitizers to induce innate immunity and adaptive immunity, as well as to trigger more cancer cell death, providing more tumor antigens to the immune system [97]. Recently, a genetically engineered cell membrane nanoplatform was constructed by integrating antigen self-presentation and immunosuppression reversal (ASPIRE) for cancer immunotherapy [98]. The ASPIRE nanovaccine was based on artificial cytomembrane nanovesicles (NVs) derived from DCs that featured the directional presentation of specific antigen epitopes by major histocompatibility complex class I (MHC-I) molecules, as well as the co-delivery of anti-PD1 antibody and B7 co-stimulatory molecules via a programmed process (Fig. 5B) [98]. The generated nanoscale platform featured good stability and a homing effect that was mediated by surface adhesion molecules. The ASPIRE nanovaccine showed the ability to present neoantigens to CD8+ T-cells directly and thus stimulating strong CTL responses. In addition, it acted as a new enhanced type of checkpoint inhibitor, which strengthened the immunosuppression reversal function of the anti-PD-1 antibody via CD28/B7 co-stimulation and maintained a more sustained CTL response. The ASPIRE nanovaccine showed promising effects in terms of activating strong antitumor immune responses and overcoming persistent immune tolerance.
NK cells, the central effector cells in innate immunity, can contribute to immune surveillance and present cytotoxic effects against tumor cells without specific antigen stimulation [89]. Through a unique set of receptors expressed on the surface (NKp30, NKp44, NKp46, DNAM-1 (CD226), and NKG2D), NK cells can induce cell lysis via the interaction with ligands on abnormal cells [85,99]. Moreover, some other NK cell membrane proteins, including RAB-10, IRGM1, RANKL Galectin-12, and CB1, can bind with receptors on macrophage membranes like tumor necrosis factor (TNF) or TLR 4, promoting pro-inflammatory M1 phenotype polarization to directly eliminate cancer cells via the produced reactive oxygen species (ROS) and nitrogen radicals [85,100]. Inspired by these properties, there have been efforts to mask nanoplatforms by NK cell membranes to mimic NK cells, targeting tumor tissues and effectively killing tumor cells. For example, photosensitizer 4,4′,4″,4‴-(porphine-5,10,15,20-tetrayl) tetrakis (benzoic acid) (TCPP)-loaded PLGA nanoparticles were coated with extracted NK cell membrane by extrusion approach. The nanoparticles-mediated PDT could eradicate tumor cells and subsequently induce ICD to promote the NK cell-membranes immunotherapy [100].
In addition to these immune cell membranes, bacterial membranes have also been investigated to generate engineered nanoparticle surfaces. Bacteria are single-celled organisms lacking organelles and membrane-bound nuclei. Though there are many bacteria in the human body, vital for metabolism and other biological activities, some bacteria are pathogenic [101]. Bacterial-derived substances can be recognized as exogenous “danger signals” by the immune system to elicit immune responses, while they may also cause some toxic effects, limiting their applications [102]. Nonetheless, some bacteria naturally possess tumor-targeting features mediated by adhesion proteins, antigens, or other molecules on the surface of the protein shell. Moreover, the bacterial cytoplasmic membrane can be isolated from the organism's cell wall spatially and easily separated from bacteria, removing lipopolysaccharide (LPS) and other cell wall components, which may induce severe side effects. Therefore, similar to mammalian cell membranes, various properties of bacterial membranes represent a promising functionality to explore and develop them as coating materials for synthetic nanoparticles. In the latest work, a hybrid-membrane vaccine based on E. coli cytoplasmic membranes (EMs) and autologous cancer cell membranes from resected tumor tissue were constructed by membrane fusion technology to deliver the bacteria-based adjuvants and tumor antigens into DCs, safely amplifying both innate and adaptive immune response [101].
2.3. Exosome-based nanoplatforms
Exosomes, a subtype of extracellular vesicles (EVs), are often called phospholipid bilayer and spherical membrane vectors with a diameter of 50–150 nm naturally secreted from various cells [103]. By serving as nanocarriers of biological components from the parental cells, such as intercellular mediators of protein, nucleic acid, and other molecules, exosomes have been reported to play significant roles in mediating intracellular communications, enhancing cell proliferation, maintaining body function, and indicating disease emergence [104]. Due to the contents of signal molecules, membrane coatings, and ideal sizes, exosomes display great potential as a novel nanovehicle for drug delivery. In this context, therapeutic cargos can be encapsulated within the exosomes via several methods, either by incubating with isolated exosomes to shape hybrid nanostructures or being incorporated into exosomes during exosome formation after introducing parental cells firstly via cellular uptake [105]. As a naturally occurring nanoplatform, the inner space of exosomes can be the room for a wide range of contents loading, which includes genetic materials, proteins, drugs, or therapeutic nanoparticles, overcoming the above-discussed shortcomings of the foreign artificial nanoplatforms, like the immune elimination or limited therapeutic effects in immunosuppressive TME.
2.3.1. Evading immune clearance
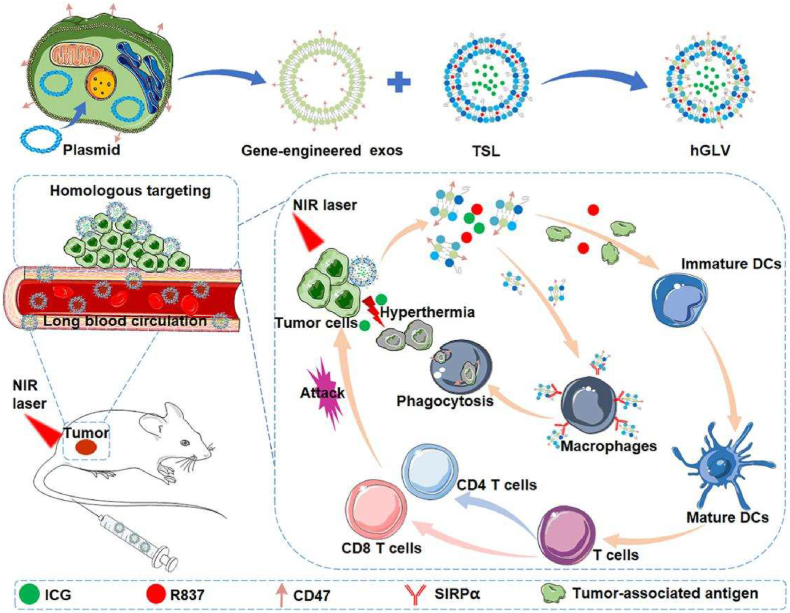
Exosomes are commonly secreted by the fusion of external film of multi-vesicular bodies with the cell membrane, meaning that the membrane coatings of exosomes possess almost the same properties as the parental cell membrane. Therefore, exosomes released from cells like RBCs, platelets, MSCs, and immature DCs, can shield the loads against immune clearance from the body owing to their bilayer membranes with the inherited surface proteins like CD47, CD55, CD59, etc., consequently prolonging their existence period and improving their biological behaviors [[106], [107], [108]]. Exosomes from RBCs were selected to act as the proper carrier for efficient RNA delivery in an RNA DDS. Due to the lack of nuclear and mitochondrial DNA, RBC-derived exosomes would barely cause any risk of horizontal gene transfer [108]. In addition to the exosomes directly derived from natural human cells, engineered exosomes have been further exploited to function as more efficient nanocarriers. To enhance the encapsulating ability, CD47-overexpressed gene-engineered-exosomes derived from parent CT26 cells were fused with the thermosensitive liposomes (TSL) (hGLV), followed by loading ICG and an immune adjuvant R837 to achieve efficient PTT and immunotherapy (Fig. 6) [109].
Fig. 6.
The design principle of hGLV and the antitumor mechanism of hGLV through PTT combined with immunotherapy. Abbreviation: exos, exosomes; TSL, thermosensitive liposomes; hGLV, gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles; DCs, dendritic cells. Reproduced with permission from Ref. [109] Copyright 2021, Elsevier.
Although human cell-secreted exosomes present several advantages as delivery vehicles, scaling up for mass production remained a significant challenge. Exosome-like vesicles from plants like grapefruit, grapes, ginger, sunflowers, carrots, etc., hold similar properties as mammalian EVs, emerging to be a substitute with lifted EV yield. For instance, ginger-derived exosome-like nanovesicles (GDENs) were engineered to display specific tumor-targeting ligands on the surface via the post-biogenesis method of RNA nanotechnology for intravenously delivering the small interfering RNA (siRNA) of tumor suppression [110]. Nevertheless, there are rare reports of plant-derived exosome-like vesicles for intravenous administration due to the impurity and biocompatibility regarding the particle size, requiring further development.
2.3.2. Enhancing immune response against tumor
Exosomes were initially regarded as aggregates of cellular waste or by-products of cellular metabolism. However, the subsequent investigations on exosomes uncovered their essential roles in direct cell-to-cell communication [111,112]. These functionalities made exosomes a vital component in immunomodulation and other biotic processes [113]. Recently, exosomes have been explored in the application to regulate the adaptive immune system and alleviate immune escape. Immune cells like macrophages, DCs, NK cells, and T cells can release exosomes that involve multiple physiological and pathological processes. Therefore, these immune cell-derived exosomes engaging in both activation and inhibition of the immune system have been explored as potential tools for cancer treatment. Exosomes from professional APCs, such as DCs and macrophages, express functional proteins modulating immune systems, containing MHC class-I and/or MHC class-II. These immune-modulating proteins are preferential to activate T cells to attack infected cells or abnormal cells like cancer cells. DCs-derived exosomes (Dexs) can stimulate NK cells owing to their surface-expressed NK cell lectin-like subfamily K and B-cell lymphoma-2 (BCL-2) associated athanogene 6 [114]. Moreover, it was proposed that T-cell responses could be amplified by Dexs containing IFN-γ, which could suppress tumor growth by promoting the proliferation of IFN-γ-producing CD8+ T cells, reducing regulatory T cells, and elevating the expression of IL-2 [115]. A recent study proposed that exosomes were produced from activated DCs pulsed with ovalbumin (OVA), followed by modification with anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) monoclonal antibody (mAb) via a post-insertion technique. The obtained nanoplatform EXO-OVA-mAb showed a more potent bond with T cells in tumor-draining lymph nodes, effectively stimulated T cells, and promoted the tumor homing of effector T cells, eventually suppressing tumor growth [116]. Exosomes derived from another APC macrophage can also modulate the immune system and elicit macrophage polarization. Since M1 macrophages exhibit efficient anticancer effect while M2 phenotype always leads to immune-suppressive TME, azide-modified M1 macrophage exosomes (M1 Exo-Ab) conjugated with immune-stimulatory antibodies (CD47 and SIRPα) via pH-sensitive linkers were constructed. It was demonstrated that the M1 Exo effectively reprogramed the pro-tumoral M2 toward antitumor M1, which further enhanced the anticancer effects (Fig. 7A) [117]. As other promising candidates for cancer treatment, NK cell-derived exosomes possessed typical NK markers like CD56 and cytotoxic molecules like Fas ligand, which could augment the number of NK cells and promote NK-mediated cytotoxicity [118]. In a recent instance, BCL-2 targeting siRNA was loaded in exosomes secreted from lentivirally-modified NK cells (siBCL-2 NKExos). Anti-apoptotic protein BCL-2 consistently overexpressed in the patients with estrogen receptor-positive (ER+) breast cancer, which was deemed as an estrogen-responsive gene facilitating cancer cell growth by allowing programmed-cell-death evasion of breast cancer cells. In this study, NKExos acted as suitable vectors to deliver siRNA targeting BCL-2 into tumor sites with high efficiency and amplified the eradication of cancer cells by enhancing the immune response [119]. Lastly, T cell-derived exosomes carrying TCR/CD3 complex from parental activated T cells can present antitumor responses. At the same time, there are still few studies on them in cancer immunotherapy compared to the widely studied Dexs, which required further exploration in the future researches [120].
Fig. 7.
(A) Schematic of Mn2+-induced M1 macrophages polarization and the synergistic anticancer effect of M1 Exo-engineered with aCD47 and aSIRPα. Reproduced with permission from Ref. [117] Copyright 2020, John Wiley & Sons. (B) Scheme illustration of the effects of DOX@Exos-PH20-FA on the modulation of the TME, which leads to enhanced DDS uptake by the tumor and conversion of the immune microenvironment from immunosuppressive to immunosupportive to favor cancer therapy. Furthermore, Exos-PH20-FA directly reduces the accelerated migration of tumor cells triggered by HA degradation. Reproduced with permission from Ref. [122] Copyright 2021, Elsevier.
In addition to naturally-derived exosomes, diverse varieties of chemically or biologically modified exosomes have been generated to broaden, alter, or enhance their therapeutic capabilities. In this framework, various surface functionalization approaches have been widely explored, such as genetic or metabolic engineering, covalent surface chemistry, and hydrophobic insertion, among others [121]. In a case, Feng and coworkers developed a new exosome-based approach for improving cancer treatment by modulating the TME via hyaluronidase and folic acid (FA) modification [122]. The HA content was usually higher in malignant tumors than in the corresponding benign tumors or normal tissues. A high accumulation of HA in the TME often leads to an increase in the interstitial pressure and reduced perfusion of drugs. Furthermore, HMW-HA acts by suppressing M1 macrophage polarization, enhancing M2 polarization, and inducing immunosuppression. Owing to these aspects, a novel exosome-based DDS, named Exos-PH20-FA, was designed, using genetic engineering to express human hyaluronidase (PH20) and self-assembly techniques to modify the exosomes with FA (Fig. 7B). The results showed that Exos-PH20-FA degraded HMW-HA to low-molecular-weight (LMW)-HA. Moreover, LMW-HA polarized macrophages to the M1 phenotype and reduced the number of relevant immunosuppressive immunocytes, which changed the immune microenvironment from an immunosuppressive to an immunosupportive phenotype. In addition, several reports on some specially-engineered exosomes demonstrated the assembly of multivalent antibodies into one nanoplatform to regulate cellular immunity. In a case, SMART-Exos were designed with anti-human CD3 and anti-human HER2 antibodies displaying on the surface of exosomes derived from Expi293 cells. By recognizing T cell surface CD3 and cancer cell-associated EGFR, SMART-Exos acted as an artificial modulator to control the immunoreactivity of immune effector cells, stimulating potent antitumor immunity both in vitro and in vivo [123].
3. Applications in cancer nano-immunotherapy
Owing to the abilities to evade immune clearance or enhance immune responses, immune-regulating camouflaged nanoplatforms, including polymer-coated, cell membrane-camouflaged, and exosome-based nanoplatforms, have been widely exploited in immunotherapy, and some ICD-inducing immunotherapeutic modalities including chemotherapy, PTT, photodynamic therapy (PDT), sonodynamic therapy (SDT), chemodynamic therapy (CDT), and RT. Firstly, it is of great significance to improve therapeutic efficiency by protecting the loaded therapeutic agents like immunotherapeutics, chemotherapy drugs, and molecules medicine or inorganic nanoparticles such as PTT, PDT, SDT, CDT, and RT agents from immune clearance by realizing immune clearance evasion of the immune-regulating camouflaged nanoplatforms. In addition, with the ability to enhance the immune response, the immune-regulating camouflaged nanoplatforms may be helpful in enhancing anticancer immunity and synergizing with these immune-related treatment methods for better therapeutic effects. The recent progress in this field is summarized in Table 1.
Table 1.
Summary of typical immune-regulating camouflaged nanoplatforms towards cancer nano-immunotherapy.
| Immune-regulating camouflaged nanoplatforms | Construction | Function | Applications | Outcomes/Merit | Ref. |
|---|---|---|---|---|---|
| Polymer-coated nanoplatforms | PEG-modified PEI-based nanovaccine consists of PEI-antigen conjugates and CpG adjuvants formed through electrostatic interaction. | Alleviating immune clearance | Immunotherapy | PEG conjugation completely abolished the cytotoxicity of PEI and reduced the nonspecific cellular uptake. | [40] |
| SO2 prodrug molecules and DOX were co-loaded on MON covered with a dense PCBMA polymer shell via a reflux precipitation polymerization approach. | Prolonging blood circulation/controlled release of drug | Chemotherapy and ICD-elicited immunotherapy | The obtained nanoplatform presented an increased accumulation of nanomedicine in the tumor site. The produced SO2 molecules could downregulate the P-glycoprotein expression, overcoming MDR with effective chemotherapy. | [45] | |
| A water-soluble enzyme Cat was encapsulated in the aqueous cavity of an R837-loaded PLGA shell via a water/oil/water (w/o/w) double emulsion approach. | Amplifying immune response | RT and ICD-induced immunotherapy | The prepared nanoplatforms raised RT effects by alleviating hypoxic conditions and modulating the immune-suppressive TME. | [53] | |
| PLGA-based NPs with different molecular weights (7000 g/mol and 12,000 g/mol) were applied to encapsulate DOX by an oil-in-water (o/w) emulsion approach. | Enhancing immune response | Chemotherapy and ICD-elicited immunotherapy | PLGA-based NPs realized sustained release of DOX to prevent tumor growth and stimulate DC maturation and tumor-infiltration of CTLs, forming a tumor-specific immunological memory effect. | [56] | |
| OVA 257–264 was loaded on chitosan nanoparticles of three different sizes and qualities (90/10, 90/20, and 90/50). | Eliciting an antitumor immune response | Immunotherapy | Chitosan nanoparticles of small size were confirmed to act as suitable antigen vehicles to induce activation and proliferation of tumor antigen-specific CD8+ T cells. | [60] | |
| A core-shell gold nanocage coated with manganese dioxide and HA (AMH). | Evading immune elimination/tumor targeting |
PDT and ICD-elicited immunotherapy | AMH nanoparticles with biocompatibility demonstrated targeting ability towards CD44-overexpressing cancerous cells and oxygenation-boosted immunogenic phototherapy in situ. | [153] | |
| Cell membrane-camouflaged nanoplatforms | Hypoxia-activated prodrug AQ4N was loaded on mesoporous titanium dioxide nanoparticles with red blood cell membranes coated on the surface (RBC-mTNPs@AQ4N). | Escaping immune clearance | Combined SDT and chemotherapy. | RBC-mTNPs@AQ4N exhibited efficient distribution in tumor tissue because of the immune escape ability and presented good antitumor results both in vitro and in vivo. | [68] |
| IR780 and DOX were firstly loaded on the PLGA NPs, followed by wrapping platelet membranes onto the NPs. | Prolonging circulation/evading immune clearance | Synergistic PTT and chemotherapy | The constructed NPs were investigated to show a prolonged internal circulation time, as well as strong treatment effects. | [74] | |
| 2D MnO2 nanosheets were firstly wrapped with gold nanorod and then loaded with DOX. Cancer cell membranes were subsequently introduced to coat the nanoplatform. | Homotypic cancer-targeting/immune escape | MRI/PTI-guided PTT and chemotherapy | The nanoplatforms with tumor targeting and immune escape abilities were disrupted to release the loads that afforded a robust result of the imaging-guided PTT/chemotherapy. | [77] | |
| The biomimetic vehicle was established by integrating MSC membranes with DOX-loaded mesoporous silica NPs. | Escaping immunosurveillance/active tumor targeting | Chemotherapy | With the acquired immunosurveillance escape and self-position abilities, the MSC membrane-coated NPs showed enhanced therapeutic outcomes with alleviated side effects. | [81] | |
| NPs covered with macrophage membranes were loaded with DOX, Ce6 and IDO1. | Delaying MPS clearance/tumor targeting | Chemotherapy, PDT, and ICD-induced immunotherapy | The DDS coated with macrophage membranes promoted the delivery efficiency of cargos, alleviated the suppressive TME, and further activated T cells. | [83] | |
| PLGA NPs with encapsulated dyes (coumarin-6/lipophilic DiD) or drugs (Trametinib) were camouflaged with gp100-specific hybridoma T-cell membranes. | Extending circulation time/specific tumor targeting | Chemotherapy | The nanoplatform with specific T-cell membranes presented not only significantly increased aggregation and stabilities and alleviated systemic clearance but also enhanced therapeutic ability. | [88] | |
| Photosensitizer-loaded upconversion nanoparticles were coated with TAM membranes. | Alleviate the immunosuppression | PDT and immunotherapy | The obtained TAM-mimic nanosystems selectively accumulated in the tumor site and bound with CSF1, reducing the binding of CSF1 with the endogenous TAMs, thus alleviating the immunosuppression in TME for better treatment. | [93] | |
| FC (DCs/cancer cells) cytomembranes coated on the surface of MOFs containing photosensitizers. | Amplifying immune response | PDT and ICD-induced immunotherapy | The final nanoplatform was successful in inducing innate immunity and adaptive immunity, posing a more inflammatory TME, as well as causing more cancer cell death. | [97] | |
| The nanovaccine was based on artificial cytomembrane NVs derived from DCs that directional presentation of specific antigen epitopes by MHC-I molecules, as well as the co-delivery of anti-PD1 antibody and B7 co-stimulatory molecules via a programmed process. | Antigen self-presentation and immunosuppression reversal | Immunotherapy | The nanovaccine platform can markedly improve antigen delivery to lymphoid organs and generate broad-spectrum T-cell responses that eliminate established tumors. | [98] | |
| Photosensitizer TCPP-loaded PLGA NPs were coated with extracted NK cell membrane by extrusion. | Eliciting immune response and M1 macrophage polarization | PDT and immunotherapy | The results demonstrated that the nanoplatforms with selective accumulation in the tumor site effectively inhibited both primary tumor growth and distant untreated tumors. | [100] | |
| PD-1-expressing cancer cell membranes were extracted to encapsulate the photosensitizer DVDMS-binding HSA-PFTBA nanoemulsion. | Boosting antitumor immune response | PDT/Immunotherapy | The expressed PD-1 protein on the coated cell membrane directly blocked PD-L1 to boost the immune response against tumors via rejuvenating exhausted T cells. | [130] | |
| Sulfasalazine was loaded on Fe3O4 NPs and then covered by platelet membranes. | Prolonging circulation/evading immune clearance | CDT | With the assistance of sulfasalazine, Fe2+ released from Fe3O4 activated Fenton reaction to generate an excessive accumulation of ·OH, leading to oxidative damage to cancer cells | [148] | |
| Exosome-based nanoplatforms | BPQDs were encapsulated in serum exosomes. | Eliciting immune response | PTT and immunotherapy | After hyperthermia treatment, serum exosomes-encapsulated BPQDs displayed a series of patient-specific TAAs, boosting the T-cell infiltration into the tumor site. | [103] |
| Curcumin was encapsulated on bovine milk-derived exosomes (ExoCUR). | Immune clearance evasion | Chemotherapy | ExoCUR showed increased biological efficacy in antiproliferative, anti-inflammatory, and antitumor activities. | [107] | |
| CD47-overexpressed gene-engineered exosomes derived from CT26 cells were fused with thermosensitive liposomes, followed by loading ICG and R837. | More prolonged circulation time/homologous targeting | PTT and immunotherapy | The nanoplatforms successfully avoided the clearance by MPS and realized PTT-induced ICD to motivate DCs maturation with the assistance of R837, further eradicating cancer cells completely. | [109] | |
| Ginger-derived exosome-like nanovesicles engineered with specific surface ligands encapsulated the siRNA for tumor suppression. | Escaping immune clearance/tumor targeting | Immunotherapy | The engineered nanovesicles showed economic advantages and significantly increased productivity, and provided a proper delivery vector for intravenous administration. | [110] | |
| Exosomes produced from activated DCs pulsed with OVA were modified with anti-CTLA-4 monoclonal antibodies via a post-insertion technique. | Inducing immune response/T-cell targeting | Immunotherapy | The constructed exosomes significantly facilitated T-cell targeting, effectively stimulated T cells, and ultimately inhibited tumor growth appreciably. | [116] | |
| Azide-modified M1 macrophage exosomes were conjugated with immune-stimulatory antibodies (CD47 and aSIRPα) via pH-sensitive linkers. | Enhancing immune response/active tumor targeting | Immunotherapy | The azide-modified M1 macrophage exosomes effectively reprogramed the pro-tumoral M2 toward antitumor M1, which could further enhance the anticancer effects. | [117] | |
| BCL-2 targeting siRNA was loaded in exosomes secreted from lentivirally modified NK cells (siBCL-2 NKExos). | Enhancing immune response/tumor targeting | Immunotherapy | siBCL-2 NKExos demonstrated specific targeting of BCL-2 and significant antitumor efficacy against ER+ breast cancer. | [119] | |
| Human hyaluronidase (PH20) was expressed on the surface of exosomes derived from 293T cells using genetic engineering and modifying the exosomes with FA by self-assembly techniques (Exos-PH20-FA) | Inducing immune response/tumor targeting | Immunotherapy | Exos-PH20-FA modulated the TME via polarized macrophages to the M1 phenotype and reduced the number of relevant immunosuppressive immunocytes. | [122] | |
| Anti-human CD3 and anti-human HER2 antibodies were displayed on the surface of exosomes derived from Expi293 cells (SMART-Exos). | Amplifying antitumor immunity | Immunotherapy | SMART-Exos acted as an artificial modulator to control the immunoreactivity of immune effector cells, stimulating potent antitumor immunity both in vitro and in vivo. | [123] |
Abbreviations: BCL-2 - B-cell lymphoma-2; BPQDs - Black phosphorus quantum dots; Ce6 - Chlorin e6; CTLA-4 - Cytotoxic T-lymphocyte antigen 4; CTLs - Cytotoxic T lymphocytes; CSF1 - Colony-stimulating factor 1; DCs - Dendritic cells; DDS - Drug delivery system; DOX - Doxorubicin; DVDMS - Sinoporphyrin sodium; ER+ - Estrogen receptor positive; FA - Folic acid; FC - Fused cell; HA - Hyaluronic acid; HER2 - Human epidermal growth factor receptor-2; HSA - Human serum albumin; ICD - Immunogenic cell death; IDO1 - Indoleamine 2,3-dioxygenase 1 inhibitor; MDR - Multiple drug resistance; MOFs - Metal-organic frameworks; MPS - Mononuclear phagocyte system; MRI - Magnetic resonance imaging; MSCs - Mesenchymal stem cells; NK - Natural killer; NPs - Nanoparticles; NVs - Nanovaccines; OVA - Ovalbumin; PCBMA - Poly(carboxybetaine methacrylate); PD-1 - Programmed death 1; PD-L1 - Programmed death ligand 1; PDT - Photodynamic therapy; PEG - Poly(ethylene glycol); PEI - Polyethyleneimine; PFTBA - Perfluorotributylamine; PLGA - Poly(lactic-co-glycolic acid); pMHC-I - Peptide-major histocompatibility complex class I; PTI - Photothermal imaging; PTT - Photothermal therapy; RT - Radiotherapy; SDT - Sonodynamic therapy; siRNA - Small interfering RNA; TAAs - Tumor-associated antigens; TAMs - Tumor-associated macrophages; TCPP - 4,4′,4″,4‴-(porphine-5,10,15,20-tetrayl) tetrakis (benzoic acid); TME - Tumor microenvironment.
3.1. Immunotherapy
By eliciting or strengthening the host's immune response against cancer cells via stimulating immune system activities directly or blocking immunosuppressive signals generated by cancer cells, cancer immunotherapy emerges as a revolutionary treatment regimen, mainly including adoptive T cell therapy, immune checkpoint blockade (ICB), and therapeutic cancer vaccines [124]. Cancer immunotherapy aims to not only damage or kill both primary and distant metastatic tumors but also trigger further long-term immune memory that can prevent cancer recurrence [125].
The commonly used immune checkpoint inhibitor and cancer vaccines are usually prescribed in the form of mRNA, proteins, and peptides, which are easily eliminated in the delivery process to the tumor site. To reduce the in vivo clearance, immune-regulating camouflaged nanoplatforms have been recently applied as protective carriers to deliver these immunotherapeutic agents [126]. By loading on the hybrid biomimetic membrane (from RAW264.7 macrophages and 4T1 breast cancer cells)-coated PLGA nanoparticles, immuno-metabolic modulator metformin (Met), and siRNA targeting fibrinogen-like protein 1 mRNA (siFGL1) were co-delivered at the tumor site. The effective co-delivery of both immuno-metabolic adjuvant and immune checkpoint inhibitors was proven to present potent inhibition of tumor growth and invasion [127].
The immune-regulating camouflaged nanoplatforms, particularly some cell membrane-camouflaged and exosome-based nanoplatforms, can also directly act as the immune checkpoint inhibitor or cancer vaccines to activate or amplify immune response in cancer immunotherapy [128,129]. Recently, the cell membranes of monoclonal programmed death 1 (PD-1)-expressing HEK293T cells were extracted to encapsulate the photosensitizer sinoporphyrin sodium (DVDMS)-binding human serum albumin (HSA)-perfluorotributylamine (PFTBA) nanoemulsion [130]. The expressed PD-1 protein on the coated cell membrane could block programmed death-ligand 1 (PD-L1) to directly boost the immune response against the tumor via rejuvenating exhausted T cells (Fig. 8A). In another case, Xiong and his colleagues developed an R837-loaded PLGA nanovaccine coated with a CRT-expressed cancer cell membrane antigen for immunotherapy [131]. To obtain CRT-expressed tumor cell membrane antigens, Luc-4T1 cells were incubated with a DOX-containing medium to induce the ICD of tumor cells. The coating surface provided the whole cancer cell membrane antigen array and exposed CRT to increase the uptake of nanovaccine by DCs. Subsequently, R837 could be continuously released from the vaccine to excite toll-like receptor 7, further activating DCs with antigens, consequently enhancing the antitumor effect (Fig. 8B).
Fig. 8.
(A) Schematic of synergistic photodynamic-immunotherapy mediated by PHD@PM. (a) The PHD@PM preparation procedure. (b) The mechanism underlying PDT-induced ICD and simultaneous PD-L1 blocking is mediated by PHD@PM. Reproduced with permission from Ref. [130] Copyright 2021, John Wiley & Sons. (B) Schematic illustration of the (a) preparation of the R@P-IM nanovaccine and (b) CRT exposed on the surface of the intratumoral-injected nanovaccine communicates an “Eat Me” sign to induce DCs to take up the nanovaccine. Reproduced with permission from Ref. [131] Copyright 2021, American Chemical Society.
3.2. ICD-inducing immunotherapeutic modalities
3.2.1. Chemotherapy
Chemotherapy, one of the traditional cancer treatment methods, interferes with tumor cell growth via various chemotherapeutic drugs. The applicability of chemotherapy is always limited due to poor specificity and systemic toxicity. Moreover, many chemotherapeutic drugs are hydrophobic, resulting in poor absorption and low bioavailability. Therefore, diverse immune-regulating camouflaged nanoplatforms have been constructed to effectively deliver chemotherapeutic drugs in recent studies, which significantly alleviated immune elimination and improved the treatment effects [81].
Chemotherapeutic drugs, such as DOX, oxaliplatin, cyclophosphamide, and some other chemotherapy drugs, can induce ICD reportedly [132,133]. In addition to directly triggering DCs engulfment, NK cell proliferation, and T cells activation by the released DAMPs, chemotherapy can enhance tumor cell immunogenicity via eliciting the expression of MHC-I molecules and tumor-specific antigens on the tumor cell surface [134]. To further amplify the chemotherapy-induced immune response, immune-regulating camouflaged nanoplatforms have been used. As mentioned earlier, PLGA nanoparticles loaded with DOX were prepared, in which PLGA nanoparticles could act as an amplifier of chemotherapy-induced ICD via controlling the release kinetics of DOX. Then, the ICD-elicited tumor-specific immune responses could further establish the immunological memory effect to ultimately obtain an enhanced immune response [56]. IFN-γ plays an essential role in antitumor immunity and is remarkably down-regulated after surgical resection of the tumor, thus weakening the antitumor immunity against cancer proliferation. Recent studies have shown that chemotherapy-induced ICD can significantly up-regulate IFN-γ expression by initiating the antitumor immune response, which can precisely be utilized to restore IFN-γ levels, relieve the immunosuppression caused by surgery, and effectively regulate postoperative tumor recurrence and metastasis [75].
3.2.2. Photothermal therapy
PTT is a treatment using photothermal agents (PTAs) to absorb and convert the laser energy into localized heat at the tumor site, damaging or killing cancer cells at a temperature over 41 °C [135,136]. Several reports on the immune-regulating camouflaged nanoplatforms loaded with PTAs have been proposed to reduce the immune clearance of PTAs for more effective PTT [74,109]. It is worth noting that PTT is also a method to elicit ICD. In addition to direct cell damage, hyperthermia can also lead to the variation of cytokine expression and immune response, which may promote the DCs activation and boost the delivery of tumor-specific agents to the lymph nodes to further activate T cells [137]. Immune-regulating camouflaged nanoplatforms loaded with PTAs have been exploited to achieve PTT and amplify antitumor immunity. In a case, black phosphorus quantum dots (BPQDs), as an effective PTA, were encapsulated in serum exosomes (hEX) that had been investigated to display a series of patient-specific tumor-associated antigens (TAAs) after hyperthermia treatment. Thus, hEX-encapsulated BPQDs exhibited potent immune-PTT outcomes by significantly elevating the temperature of the tumor area and boosting the T-cell infiltration into the tumor site [103]. In another case, Xu and colleagues reported a polymeric multicellular nanoengager (SPNE) for synergistic second-near-infrared-window (NIR-II) photothermal immunotherapy [138]. The designed nanoengager of a NIR-II absorbing polymer as the photothermal core was camouflaged with the fused membranes derived from immunologically-engineered tumor cells and dendritic cells (DCs) as the cancer vaccine shell. Due to their high accumulation in the lymph nodes and tumors, the multicellular engagement ability of the SPNE enabled effective cross-interactions among tumor cells, DCs, and T cells, leading to augmented T-cell activation relative to bare or tumor-cell-coated nanoparticles. In the presence of deep-tissue penetrating NIR-II photoirradiation, SPNE substantially eradicated the tumor and induced ICD, eliciting further antitumor T-cell immunity (Fig. 9). Together, these shreds of evidence indicate that the synergistic photothermal immunotherapeutic effect substantially inhibits the growth of tumors and prevents further metastasis, as well as procures immunological memory.
Fig. 9.
Fused membranes of 4T1 tumor cells and dendritic cells (DCs) camouflaged nanoplatforms for synergistic NIR-II photothermal immunotherapy. a) Preparation of SPNU, SPNT, and SPNE. b) SPNE mediated multicellular engagement, immune activation, and NIR-II photothermal effects. DAMPs, damage-associated molecular patterns. TLR, Toll-like receptor. MHC-I, major histocompatibility complex class I molecule. TCR, T-cell receptor. c) SPNE-induced immune activation and systemic immune responses for NIR-II photothermal immunotherapy. Reproduced with permission from Ref. [138] Copyright 2021, John Wiley & Sons.
3.2.3. ROS-mediated therapy
ROS, a series of reactive molecules containing singlet oxygen (1O2), superoxide , hydroxyl (·OH), and hydrogen peroxide (H2O2) act as significant regulators in a variety of cellular biological processes [139,140]. Nevertheless, the accumulation of ROS to an excessive degree in cells can cause uncontrollable stress, leading to the oxidative damage of the intracellular biomacromolecules, such as DNA, lipids, and proteins, thus inducing the eventual cellular apoptosis or growth arrest [141,142]. Furthermore, it has been proposed that ROS-based endoplasmic reticulum (ER) stress is essential for triggering the intracellular danger signaling pathways that govern ICD. ROS generation is regarded as a crucial role in the release of DAMPs, and the ICD-induced immunogenicity has shown diminishment in the presence of antioxidants. Recently, immune-regulating camouflaged nanoplatforms with immune clearance evading or enhancing antitumor immunity functions in several potent ROS-mediated treatment strategies, including PDT, SDT, CDT, and RT, have been developed to assist the generation of ROS in the TME.
PDT and SDT are both treatments transforming the molecular oxygen into cytotoxic 1O2 in tumor area towards the ablation of tumor cells, in which the photosensitizers or sonosensitizers react with the endogenous O2 in the presence of appropriate light illumination or ultrasound treatment, respectively [2,143]. However, limited to the hypoxia condition in the tumor area, PDT or SDT could be combined with other treatment strategies for superior anticancer efficacy than a single treatment [144]. A synergistic strategy of combining PTT and SDT was proposed by integrating iridium complexes into black-titanium nanoparticles camouflaged with cancer cell membranes. Upon exposure to a laser in the near-infrared II (NIR-II) region (1000–1700 nm), the prepared nanoplatforms presented good photothermal efficacy, which expedited the blood flow to deliver more oxygen into the tumor site, further augmenting the therapeutic efficiency of oxygen-dependent SDT [145].
In CDT, metal ions (e.g., Fe, Mn, Co, Cu, and Ag)-based Fenton nanomaterials are introduced to trigger the Fenton-like reaction in which the overexpressed intracellular H2O2 degrades into ·OH species, rendering specific damage of the cancer cells but negligible destruction to the normal tissues [146,147]. For example, sulfasalazine (SAS) was encapsulated in magnetic nanoparticles (Fe3O4) and then covered by platelet membranes. With the assistance of SAS, Fe2+ released from Fe3O4 activated the Fenton reaction to generate an excessive accumulation of ·OH, leading to oxidative damage to cancer cells (Fig. 10) [148].
Fig. 10.
In vitro cytotoxicity and mechanism of ferroptosis induced by Fe3O4-SAS@PLT. (a) Cell viability of 4T1 cells treated with different concentrations of free SAS, Fe3O4, Fe3O4-SAS, and Fe3O4-SAS@PLT, respectively; n = 6. (b) Cell viability of Fe3O4-SAS@PLT-treated 4T1 cells in the presence of Fer-1 and DFO, respectively; n = 6. (c) Representative CLSM images of 4T1 cells stained with DCFH-DA in different groups (SAS, Fe3O4, Fe3O4-SAS, v-SAS@PLT, Fe3O4-SAS@PLT + Fer-1, and Fe3O4-SAS@PLT + DFO groups). (d) Quantification of total ROS, superoxide, hydroxyl peroxide, and hydroxyl radical by using appropriate fluorescent probes; n = 3. (e) Flow cytometry analysis of lipid peroxidation in different formulation-treated 4T1 cells by using a C11-BODIPY fluorescent probe. (f) Intracellular GSH levels in 4T1 cells treated with different formulations (SAS, Fe3O4, Fe3O4-SAS, Fe3O4-SAS@ PLT, Fe3O4-SAS@PLT + Fer-1, and Fe3O4-SAS@PLT + DFO groups); n = 3. (g) Intracellular XcT and GPX4 expression in 4T1 cells treated with different formulations including (1) Control, (2) SAS, (3) Fe3O4, (4) Fe3O4-SAS, (5) Fe3O4-SAS@PLT, (6) Fe3O4-SAS@PLT + Fer-1, and (7) Fe3O4-SAS@PLT + DFO; n = 3. Untreated 4T1 cells were taken as a control. ns represented no significance, *p < 0.001. Reproduced with permission from Ref. [148] Copyright 2020, John Wiley & Sons.
As a broadly applied clinical treatment method for solid tumors, RT uses ionizing radiation (such as high-energy X-rays or γ-rays) to kill tumor cells without depth restriction via complicated mechanisms [149]. On the one hand, double-stranded deoxyribonucleic acid (DNA) can be directly destroyed by the interaction of the radiation beam with DNA strands [149,150]. On the other hand, ROS generated from the reaction of water radiolysis with oxygen can further pose DNA lesions with steady DNA peroxide, accounting for 80% of DNA damage. Recently, to simultaneously realize RT, PTT, and exogenous oxygen supplying, Wu et al. co-loaded RBC contents and CuS on dendritic large pore MSNs, subsequently coated with RBC membranes [151]. CuS endowed the nanoplatform with photothermal property and radiosensitization ability. Meanwhile, RBC contents acted as an oxygen reservoir, causing increased tumor oxygenation. The final results of in vitro study indicated that this multifunctional nanoplatform might have great potential in the field of cancer treatment.
Extensive research confirmed that these ROS-mediated treatment strategies could elicit ICD to further activate the immune responses. ROS produced from these methods could disrupt the integrity of the nucleus and release HMGB1. Furthermore, the elevated level of ROS in the ER would lead to CRT exposure [152]. In a recent study, core-shell gold nanocages coated with manganese dioxide and HA (AMH) were developed for targeted delivery to colorectal tumors, and oxygenation boosted immunogenic phototherapy in situ. The AMH nanoparticles exhibited high tumor-targeted delivery efficiency and generated abundant oxygen in a mild acidic/H2O2 microenvironment to relieve the tumor hypoxia and further improve the PDT killing effect. Furthermore, AMH-mediated oxygen-boosted PDT induced ICD with DAMPs release, promoting DC maturation and facilitating the activation of systematic antitumor immunity [153]. In another case, photosensitizer TCPP was first loaded on porphyrin-based Zr-MOF (PCN-224), followed by the camouflage of fused cell membrane (cancer cells and DCs). With the laser irradiation, the generated cytotoxic ROS combined with ICD-induced immunotherapy posed effective cell eradication towards primary and distant tumors (Fig. 11) [97]. However, few studies exist on utilizing immune camouflage to directly enhance the immune response against the tumor. Thus, there is great potential for applying immune-regulating camouflaged nanoplatforms in future research studies to amplify the insufficient systemic immune responses induced by ROS-mediated treatment strategies to meet clinical requirements.
Fig. 11.
Schematic illustration of PCN@FM for combined tumor therapy. (a) The preparation process of PCN@FM; and (b) combination between irradiation-mediated PDT and FM- and ICD-induced immunotherapy toward primary tumor and distant tumor therapy. Reproduced with permission from Ref. [97] Copyright 2019, John Wiley & Sons.
4. Conclusion and perspectives
In summary, this article has emphasized the state-of-the-art of various immune-regulating camouflaged nanoplatforms to alleviate the immune-related limitations of conventional nanoplatforms for cancer nano-immunotherapy. These revolutionary nanosystems, coating with specific polymeric materials, cell membranes, or based on exosomes, presented great potential in various cancer immune-related therapies, including immunotherapy, chemotherapy, PTT, and several ROS-related therapies due to their abilities to escape immune clearance or enhance the immune response. Although the development of immunotherapy has brought hope to tumor patients, the clinical data show that immunotherapy alone has a low effective rate. For example, the effective rate of PD-1 antibody in treating liver cancer patients is only 20%. The main reason for this phenomenon is that tumors are classified into “cold” tumors and “hot” tumors according to the characteristics of the immune microenvironment. “Hot” tumor means that the number of immune cells around the tumor tissue is significant; therefore, the immunomodulator is easy to play an efficient role. In contrast, a “cold” tumor refers to the absence or very few immune cells in the tumor tissue, and the cancer cells respond poorly to immunotherapy. Cancers, such as liver cancer, pancreatic cancer, and breast cancer, are all typical “cold” tumors, such that the effective rate of immunotherapy is very low. Immune-regulating camouflaged nanoplatforms can activate the immune system by enabling tumor cells to release relevant antigens through chemotherapy, PTT, and several ROS-related therapies, thereby recruiting immune cells, transforming “cold” tumors into “hot” tumors, thereby enhancing the performance of immunomodulators, enabling efficient treatment of “cold” tumors. In addition, many immunotherapies would simultaneously activate immune cells in other parts of the body during the treatment process, thereby indiscriminately attacking normal tissues and causing side effects. These immune-regulating camouflaged nanoplatforms can specifically target cancer foci by designing rational targeting groups. Another application is to use the immune cell membrane camouflage strategy to disguise as immune cells and be actively recognized by tumor cells with immune evasion properties so as to achieve precise activation of the tumor immune microenvironment, thereby avoiding the problem of significant side effects of immunotherapy.
Despite current progress in the field of immune-regulating camouflaged nanoplatforms for cancer nano-immunotherapy, there are still many challenges that remain before their translation to clinics. Firstly, there is a considerable gap between lab studies and clinical investigations. It's required to further study the biology of these immune-regulating camouflaged nanoplatforms, as well as evaluate their biosafety and efficacy by complete clinical trials. For instance, exosomes are complex entities of biological sources, which are yet remained to well understand their biogenesis, composition, and roles in physiological and pathological conditions, along with addressing the associated formidable barriers. Currently, the fundamental knowledge of exosome biology remains unexplored completely for the successful formulation of exosome-like nanoplatforms. As a competing therapeutic agent and drug delivery tool in a clinical setting, it is necessary to highlight the need for more basic research dedicated to elucidating the structural, compositional, and functional characteristics of exosomes. By understanding the influence of factors that contribute to the free circulation of exosomes, intrinsic targeting, and the ability to overcome biological barriers, advanced exosome-like nanoplatforms that are closer to natural exosomes with higher efficiency and safety will become available [33]. Secondly, the large-scale production of such immune-regulating camouflaged nanoplatforms may be limited by their current preparation approaches due to many issues, like the tedious work of conjugating polymeric materials on the surface of NPs, the insufficient sources of various cell membranes or cell-derived exosomes, the low yield, and the challenges in preservation of the obtained immune-regulating camouflaged nanoplatforms. As the currently used membranes or exosomes are mainly derived from laboratory cell culture and do not reflect the real cell phenotypes changes or membrane proteins expressed in the disease condition, these may impair their targeting ability, requiring them to regulate the culture conditions to conform to the actual situation. Therefore, translating these biomimetic systems from the bench to the bedside requires the development of standard, scalable, and cost-effective production methods. Finally, different from synthetic nanoplatforms, the quality and purity of immune-regulating camouflaged nanoplatforms are not easy to control, such as the quantitative control of polymers molecular weight and density and the integrity of cell-membrane coatings or exosomes. Several characterization techniques on quality control should be developed in the future. For example, the pure tumor cell membrane can hardly maintain the same integrity as the whole tumor cell during membrane extraction. Incomplete tumor cell membranes may lose some proteins that help avoid immune surveillance, posing safety and efficacy risks. Moreover, once the camouflaged nanoplatforms enter the blood vessel, a protein corona will be attached to the surface of nanoparticles. The compositions of the protein corona and its role in the biological events of camouflaged nanoplatforms, such as immune response, toxicity, biodistribution, metabolism, and clearance, need to be carefully identified to optimize the theranostic efficacy. Hence, there is still a long way to translate them into clinical applications. Together, we believe that, once these intractable issues are successfully solved, these immune-regulating camouflaged nanoplatforms can provide a new direction for the clinical use of therapeutic nanotechnology and pave the way for many novel cancer treatment strategies that are based on nanosystems.
Ethical statement for solid state ionics
Hereby, I Prof.Ai-Zheng Chen, consciously assure that for the manuscript entitled “Immune-regulating camouflaged nanoplatforms: A promising strategy to improve cancer nano-immunotherapy” the following is fulfilled:
-
1)
This material is the authors' own original work, which has not been previously published elsewhere.
-
2)
The paper is not currently being considered for publication elsewhere.
-
3)
The paper reflects the authors' own research and analysis in a truthful and complete manner.
-
4)
The paper properly credits the meaningful contributions of co-authors and co-researchers.
-
5)
The results are appropriately placed in the context of prior and existing research.
-
6)
All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
-
7)
All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
Declaration of competing interest
We clarify that this publication has no such conflicts of interest from either of the authors.
Acknowledgments
Financial support from the National Key Research & Development Program of China (2019YFE0113600), National Natural Science Foundation of China (NSFC, 81971734, and 32071323), the Program for Innovative Research Team in Science and Technology in Fujian Province University, and the Scientific Research Funds of Huaqiao University (20BS104).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Ranjith Kumar Kankala, Email: ranjithkankala@hqu.edu.cn.
Ai-Zheng Chen, Email: azchen@hqu.edu.cn.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021, CA-Cancer. J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y., Chen B.Q., Kankala R.K., Wang S.B., Chen A.Z. Recent advances in combination of copper chalcogenide-based photothermal and reactive oxygen species-related therapies. ACS Biomater. Sci. Eng. 2020;6(9):4799–4815. doi: 10.1021/acsbiomaterials.0c00830. [DOI] [PubMed] [Google Scholar]
- 3.Yang R., Xu J., Xu L., Sun X., Chen Q., Zhao Y., Peng R., Liu Z. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12(6):5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 4.Li Q., Shi Z., Zhang F., Zeng W., Zhu D., Mei L. Symphony of nanomaterials and immunotherapy based on the cancer-immunity cycle. Acta Pharm. Sin. B. 2022;12(1):107–134. doi: 10.1016/j.apsb.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y.X., Wang Y., Ding J., Jiang A., Wang J., Yu M., Blake S., Liu S., Bieberich C.J., Farokhzad O.C., Mei L., Wang H., Shi J. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 2021;13(599) doi: 10.1126/scitranslmed.aba9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan X., Chan C., Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew. Chem., Int. Ed. 2019;58(3):670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou S., Shang Q., Wang N., Li Q., Song A., Luan Y. Rational design of a minimalist nanoplatform to maximize immunotherapeutic efficacy: four birds with one stone. J. Contr. Release. 2020;328:617–630. doi: 10.1016/j.jconrel.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Irvine D.J., Dane E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020;20(5):321–334. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y., Lammers T. Combining nanomedicine and immunotherapy. Acc. Chem. Res. 2019;52(6):1543–1554. doi: 10.1021/acs.accounts.9b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kankala R.K., Han Y.H., Na J., Lee C.H., Sun Z.Q., Wang S.B., Kimura T., Ok Y.S., Yamauchi Y., Chen A.Z., Wu K.C.W. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020;32(23) doi: 10.1002/adma.201907035. [DOI] [PubMed] [Google Scholar]
- 11.Narain A., Asawa S., Chhabria V., Patil-Sen Y. Cell membrane coated nanoparticles: next-generation therapeutics. Nanomedicine. 2017;12(21):2677–2692. doi: 10.2217/nnm-2017-0225. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Wang X., Chu C., Zhou Z., Chen B., Pang X., Lin G., Lin H., Guo Y., Ren E., Lv P., Shi Y., Zheng Q., Yan X., Chen X., Liu G. Genetically engineered magnetic nanocages for cancer magneto-catalytic theranostics. Nat. Commun. 2020;11(1):5421. doi: 10.1038/s41467-020-19061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai S., Lu Z., Jiang Y., Shi X., Xu D., Shi Y., Lin G., Liu C., Zhang Y., Liu G. Nanotransferrin-based programmable catalysis mediates three-pronged induction of oxidative stress to enhance cancer immunotherapy. ACS Nano. 2021;16(1):997–1012. doi: 10.1021/acsnano.1c08619. [DOI] [PubMed] [Google Scholar]
- 14.Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., Riksen N.P., Schlitzer A., Schultze J.L., Stabell Benn C., Sun J.C., Xavier R.J., Latz E. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello C.A., Simon A., van der Meer J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poon I.K., Lucas C.D., Rossi A.G., Ravichandran K.S. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 2014;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viana I.M.O., Roussel S., Defrêne J., Lima E.M., Barabé F., Bertrand N. Innate and adaptive immune responses toward nanomedicines. Acta Pharm. Sin. B. 2021;11(4):852–870. doi: 10.1016/j.apsb.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nel A.E., Madler L., Velegol D., Xia T., Hoek E.M., Somasundaran P., Klaessig F., Castranova V., Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell J.S., Teng M.W.L., Smyth M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019;16(3):151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 21.Kunimasa K., Goto T. Immunosurveillance and immunoediting of lung cancer: current perspectives and challenges. Int. J. Mol. Sci. 2020;21(2) doi: 10.3390/ijms21020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okazaki T., Chikuma S., Iwai Y., Fagarasan S., Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013;14(12):1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 23.Chen B., Dai W., He B., Zhang H., Wang X., Wang Y., Zhang Q. Current multistage drug delivery systems based on the tumor microenvironment. Theranostics. 2017;7(3):538–558. doi: 10.7150/thno.16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang S., Ning Q., Yang L., Mo Z., Tang S. Mechanisms of immune escape in the cancer immune cycle. Int. Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106700. [DOI] [PubMed] [Google Scholar]
- 25.Seliger B., Massa C. Immune therapy resistance and immune escape of tumors. Cancers. 2021;13(3) doi: 10.3390/cancers13030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M., Qin X., Zhao Z., Du Q., Li Q., Jiang Y., Luan Y. A self-amplifying nanodrug to manipulate the Janus-faced nature of ferroptosis for tumor therapy. Nanoscale Horiz. 2022;7(2):198–210. doi: 10.1039/d1nh00506e. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H.Y., Dong S.J., Li Z.M., Feng X.R., Xu W.G., Tulinao C.M.S., Jiang Y., Ding J.X. Biointerface engineering nanoplatforms for cancer-targeted drug delivery. Asian J. Pharm. Sci. 2020;15(4):397–415. doi: 10.1016/j.ajps.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Li L., Wang J., Radford D.C., Gu Z., Kopeček J., Yang J. Dendronized polymer conjugates with amplified immunogenic cell death for oncolytic immunotherapy. J. Contr. Release. 2021;329:1129–1138. doi: 10.1016/j.jconrel.2020.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Zheng C., Wang Q., Wang Y., Zhao X., Gao K., Liu Q., Zhao Y., Zhang Z., Zheng Y., Cao J., Chen H., Shi L., Kang C., Liu Y., Lu Y. In situ modification of the tumor cell surface with immunomodulating nanoparticles for effective suppression of tumor growth in mice. Adv. Mater. 2019;31(32) doi: 10.1002/adma.201902542. [DOI] [PubMed] [Google Scholar]
- 30.Dash P., Piras A.M., Dash M. Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy. J. Contr. Release. 2020;327:546–570. doi: 10.1016/j.jconrel.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Li S., Liu J., Sun M., Wang J., Wang C., Sun Y. Cell membrane-camouflaged nanocarriers for cancer diagnostic and therapeutic. Front. Pharmacol. 2020;11:24. doi: 10.3389/fphar.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xuan M., Shao J., Li J. Cell membrane-covered nanoparticles as biomaterials. Natl. Sci. Rev. 2019;6(3):551–561. doi: 10.1093/nsr/nwz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu M., Huang Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials. 2020;242 doi: 10.1016/j.biomaterials.2020.119925. [DOI] [PubMed] [Google Scholar]
- 34.Barjesteh T., Mansur S., Bao Y. Inorganic nanoparticle-loaded exosomes for biomedical applications. Molecules. 2021;26(4) doi: 10.3390/molecules26041135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Zhang Y., Zhang Y., Lv P., Zhang P., Chu C., Mao J., Wang X., Li W., Liu G. Bio-engineered cell membrane nanovesicles as precision theranostics for perihilar cholangiocarcinoma. Biomater. Sci. 2020;8(6):1575–1579. doi: 10.1039/c9bm02088h. [DOI] [PubMed] [Google Scholar]
- 36.Fang R.N.H., Hu C.M.J., Chen K.N.H., Luk B.T., Carpenter C.W., Gao W.W., Li S.L., Zhang D.E., Lu W.Y., Zhang L.F. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale. 2013;5(19):8884–8888. doi: 10.1039/c3nr03064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J.Y., Zhao D.D., Li D., Wang X.H., Jin Z., Zhao K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers. 2018;10(1) doi: 10.3390/polym10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99(Pt A):28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazaro-Carrillo A., Filice M., Guillén M.J., Amaro R., Viñambres M., Tabero A., Paredes K.O., Villanueva A., Calvo P., del Puerto Morales M., Marciello M. Tailor-made PEG coated iron oxide nanoparticles as contrast agents for long lasting magnetic resonance molecular imaging of solid cancers. Mater. Sci. Eng. C. 2020;107 doi: 10.1016/j.msec.2019.110262. [DOI] [PubMed] [Google Scholar]
- 40.Nam J., Son S., Park K.S., Moon J.J. Modularly programmable nanoparticle vaccine based on polyethyleneimine for personalized cancer immunotherapy. Adv. Sci. 2021;8(5) doi: 10.1002/advs.202002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albano J.M.R., Ribeiro L.N.d.M., Couto V.M., Barbosa Messias M., Rodrigues da Silva G.H., Breitkreitz M.C., de Paula E., Pickholz M. Rational design of polymer-lipid nanoparticles for docetaxel delivery. Colloids Surf. B Biointerfaces. 2019;175:56–64. doi: 10.1016/j.colsurfb.2018.11.077. [DOI] [PubMed] [Google Scholar]
- 42.Roacho-Pérez J.A., Ruiz-Hernandez F.G., Chapa-Gonzalez C., Martínez-Rodríguez H.G., Flores-Urquizo I.A., Pedroza-Montoya F.E., Garza-Treviño E.N., Bautista-Villareal M., García-Casillas P.E., Sánchez-Domínguez C.N. Magnetite nanoparticles coated with PEG 3350-Tween 80: in vitro characterization using primary cell cultures. Polymers. 2020;12(2):300. doi: 10.3390/polym12020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sui Y., Gu Y., Lu Y., Yu C., Zheng J., Qi H. Fucoxanthin@polyvinylpyrrolidone nanoparticles promoted oxidative stress-induced cell death in Caco-2 human colon cancer cells. Mar. Drugs. 2021;19(2):92. doi: 10.3390/md19020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yousefi E., Javadpour S., Ansari M., Eslami H. Sonodynamic therapy of cancer using a novel TiO2-based nanoparticles. Mater. Technol. 2021;36(9):521–528. [Google Scholar]
- 45.Yao X., Ma S., Peng S., Zhou G., Xie R., Jiang Q., Guo S., He Q., Yang W. Zwitterionic polymer coating of sulfur dioxide-releasing nanosystem augments tumor accumulation and treatment efficacy. Adv. Healthc. Mater. 2020;9(5) doi: 10.1002/adhm.201901582. [DOI] [PubMed] [Google Scholar]
- 46.Hoang Thi T.T., Pilkington E.H., Nguyen D.H., Lee J.S., Park K.D., Truong N.P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers. 2020;12(2) doi: 10.3390/polym12020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie M., Wang Z., Lu Q., Nie S., Butch C.J., Wang Y., Dai B. Ultracompact Iron oxide nanoparticles with a monolayer coating of succinylated heparin: a new class of renal-clearable and nontoxic T1 agents for high-field MRI. ACS Appl. Mater. Interfaces. 2020;12(48):53994–54004. doi: 10.1021/acsami.0c12454. [DOI] [PubMed] [Google Scholar]
- 48.Sun X., Li Y., Xu L., Shi X., Xu M., Tao X., Yang G. Heparin coated meta-organic framework co-delivering doxorubicin and quercetin for effective chemotherapy of lung carcinoma. J. Int. Med. Res. 2020;48(2) doi: 10.1177/0300060519897185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun W., Du Y., Liang X., Yu C., Fang J., Lu W., Guo X., Tian J., Jin Y., Zheng J. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119264. [DOI] [PubMed] [Google Scholar]
- 50.Soleymani M., Velashjerdi M., Shaterabadi Z., Barati A. One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 2020;237 doi: 10.1016/j.carbpol.2020.116130. [DOI] [PubMed] [Google Scholar]
- 51.Chen B.W., He Y.C., Sung S.Y., Le T.T.H., Hsieh C.L., Chen J.Y., Wei Z.H., Yao D.-J. Synthesis and characterization of magnetic nanoparticles coated with polystyrene sulfonic acid for biomedical applications. Sci. Technol. Adv. Mater. 2020;21(1):471–481. doi: 10.1080/14686996.2020.1790032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu T., Tong L., Ao Y., Zhang G., Liu Y., Zhang H. NIR triggered PLGA coated Au-TiO2 core loaded CPT-11 nanoparticles for human papillary thyroid carcinoma therapy. Drug Deliv. 2020;27(1):855–863. doi: 10.1080/10717544.2020.1775723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Q., Chen J.W., Yang Z.J., Xu J., Xu L.G., Liang C., Han X., Liu Z. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv. Mater. 2019;31(10) doi: 10.1002/adma.201802228. [DOI] [PubMed] [Google Scholar]
- 54.Alejandro Á.O., Leydi Maribel C.C., Christofer Enmanuel O.N., Geovanny I.N.C., Alfredo Rafael V.N., William Alejandro T.P. Increased toxicity of doxorubicin encapsulated into pH-responsive poly(β-Amino Ester)-functionalized MCM-41 silica nanoparticles. Curr. Drug Deliv. 2020;17(9):799–805. doi: 10.2174/1567201817999200728123915. [DOI] [PubMed] [Google Scholar]
- 55.Andorko J.I., Hess K.L., Pineault K.G., Jewell C.M. Intrinsic immunogenicity of rapidly-degradable polymers evolves during degradation. Acta Biomater. 2016;32:24–34. doi: 10.1016/j.actbio.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi Y., Yoon H.Y., Kim J., Yang S., Lee J., Choi J.W., Moon Y., Kim J., Lim S., Shim M.K., Jeon S., Kwon I.C., Kim K. Doxorubicin-loaded PLGA nanoparticles for cancer therapy: molecular weight effect of PLGA in doxorubicin release for controlling immunogenic cell death. Pharmaceutics. 2020;12(12):1165. doi: 10.3390/pharmaceutics12121165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jogdand A., Alvi S.B., Rajalakshmi P.S., Rengan A.K. NIR-dye based mucoadhesive nanosystem for photothermal therapy in breast cancer cells. J. Photochem. Photobiol., B. 2020;208 doi: 10.1016/j.jphotobiol.2020.111901. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Q., Liu Q., Du M., Vermorken A., Cui Y., Zhang L., Guo L., Ma L., Chen M. Cetuximab and doxorubicin loaded dextran-coated Fe3O4 magnetic nanoparticles as novel targeted nanocarriers for non-small cell lung cancer. J. Magn. Magn Mater. 2019;481:122–128. [Google Scholar]
- 59.Zhao J., Yao L., Nie S., Xu Y. Low-viscosity sodium alginate combined with TiO2 nanoparticles for improving neuroblastoma treatment. Int. J. Biol. Macromol. 2021;167:921–933. doi: 10.1016/j.ijbiomac.2020.11.048. [DOI] [PubMed] [Google Scholar]
- 60.Walter F., Winter E., Rahn S., Heidland J., Meier S., Struzek A.M., Lettau M., Philipp L.M., Beckinger S., Otto L., Möller J.L., Helm O., Wesch D., Scherließ R., Sebens S. Chitosan nanoparticles as antigen vehicles to induce effective tumor specific T cell responses. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng Y., Xiang Y., Sheng R., Tomás H., Rodrigues J., Gu Z., Zhang H., Gong Q., Luo K. Polysaccharide-based nanomedicines for cancer immunotherapy: a review. Bioact. Mater. 2021;6(10):3358–3382. doi: 10.1016/j.bioactmat.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv. Mater. 2018;30(23) doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang P., Chen H., Liu J., Liu G. Genetically engineered plasma membrane nanovesicles for cancer-targeted nanotheranostics. Methods Mol. Biol. 2019;2054:283–294. doi: 10.1007/978-1-4939-9769-5_18. [DOI] [PubMed] [Google Scholar]
- 64.Wang P., Kankala R.K., Chen B., Zhang Y., Zhu M., Li X., Long R., Yang D., Krastev R., Wang S., Xiong X., Liu Y. Cancer cytomembrane-cloaked prussian blue nanoparticles enhance the efficacy of mild-temperature photothermal therapy by disrupting mitochondrial functions of cancer cells. ACS Appl. Mater. Interfaces. 2021;13(31):37563–37577. doi: 10.1021/acsami.1c11138. [DOI] [PubMed] [Google Scholar]
- 65.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. vol. 108. 2011. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform; pp. 10980–10985. (Proceedings of the National Academy of Sciences of the United States of America). 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu C.M., Fang R.H., Copp J., Luk B.T., Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013;8(5):336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang X., Ye X.Y., Wang C., Xing C.Y., Miao Q.W., Xie Z.J., Chen X.L., Zhang X.D., Zhang H., Mei L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Contr. Release. 2019;296:150–161. doi: 10.1016/j.jconrel.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 68.Li Q.Y., Lin B., Li Y.Z., Lu N. Erythrocyte-camouflaged mesoporous titanium dioxide nanoplatform for an ultrasound-mediated sequential therapies of breast cancer. Int. J. Nanomed. 2021;16:3875–3887. doi: 10.2147/IJN.S301855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zou S., Wang B., Wang C., Wang Q., Zhang L. Cell membrane-coated nanoparticles: research advances. Nanomedicine. 2020;15(6):625–641. doi: 10.2217/nnm-2019-0388. [DOI] [PubMed] [Google Scholar]
- 70.Zhang M., Du Y., Wang S., Chen B. A review of biomimetic nanoparticle drug delivery systems based on cell membranes. Drug Des. Dev. Ther. 2020;14:5495–5503. doi: 10.2147/DDDT.S282368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y., Sun Y., Dong X., Wang Q.S., Zhu D., Mei L., Yan H., Lv F. A platelet intelligent vehicle with navigation for cancer photothermal-chemotherapy. ACS Nano. 2022;16:6359–6371. doi: 10.1021/acsnano.2c00453. [DOI] [PubMed] [Google Scholar]
- 72.Li Z., Hu S., Cheng K. Platelets and their biomimetics for regenerative medicine and cancer therapies. J. Mater. Chem. B. 2018;6(45):7354–7365. doi: 10.1039/C8TB02301H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moghimi S.M., Hunter A.C., Peer D. Platelet mimicry: the emperor's new clothes? Nanomedicine. 2016;12(1):245–248. doi: 10.1016/j.nano.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Pei W., Huang B., Chen S., Wang L., Xu Y., Niu C. Platelet-mimicking drug delivery nanoparticles for enhanced chemo-photothermal therapy of breast cancer. Int. J. Nanomed. 2020;15:10151–10167. doi: 10.2147/IJN.S285952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q., Zhou Y., He W., Ren X., Zhang M., Jiang Y., Zhou Z., Luan Y. Platelet-armored nanoplatform to harmonize janus-faced IFN-γ against tumor recurrence and metastasis. J. Contr. Release. 2021;338:33–45. doi: 10.1016/j.jconrel.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 76.Sun H., Su J., Meng Q., Yin Q., Chen L., Gu W., Zhang P., Zhang Z., Yu H., Wang S., Li Y. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv. Mater. 2016;28(43):9581–9588. doi: 10.1002/adma.201602173. [DOI] [PubMed] [Google Scholar]
- 77.Zhang D., Ye Z., Liu H., Wang X., Hua J., Ling Y., Wei L., Xia Y., Sun S., Xiao L. Cell membrane coated smart two-dimensional supraparticle for in vivo homotypic cancer targeting and enhanced combinational theranostics. Nanotheranostics. 2021;5(3):275–287. doi: 10.7150/ntno.57657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y., Luo J., Chen X., Liu W., Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett. 2019;11(1):100. doi: 10.1007/s40820-019-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toledano Furman N.E., Lupu-Haber Y., Bronshtein T., Kaneti L., Letko N., Weinstein E., Baruch L., Machluf M. Reconstructed stem cell nanoghosts: a natural tumor targeting platform. Nano Lett. 2013;13(7):3248–3255. doi: 10.1021/nl401376w. [DOI] [PubMed] [Google Scholar]
- 80.Roorda B.D., Elst A., Boer T.G., Kamps W.A., de Bont E.S. Mesenchymal stem cells contribute to tumor cell proliferation by direct cell-cell contact interactions. Cancer Invest. 2010;28(5):526–534. doi: 10.3109/07357900903179625. [DOI] [PubMed] [Google Scholar]
- 81.Li Y.S., Wu H.H., Jiang X.C., Zhang T.Y., Zhou Y., Huang L.L., Zhi P., Tabata Y., Gao J.Q. Active stealth and self-positioning biomimetic vehicles achieved effective antitumor therapy. J. Contr. Release. 2021;335:515–526. doi: 10.1016/j.jconrel.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 82.Raza F., Zafar H., Zhang S., Kamal Z., Su J., Yuan W.E., Mingfeng Q. Recent advances in cell membrane-derived biomimetic nanotechnology for cancer immunotherapy. Adv. Healthc. Mater. 2021;10(6) doi: 10.1002/adhm.202002081. [DOI] [PubMed] [Google Scholar]
- 83.Hu C., Lei T., Wang Y., Cao J., Yang X., Qin L., Liu R., Zhou Y., Tong F., Umeshappa C.S., Gao H. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120159. [DOI] [PubMed] [Google Scholar]
- 84.Raskov H., Orhan A., Christensen J.P., Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021;124(2):359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gong P., Wang Y., Zhang P., Yang Z., Deng W., Sun Z., Yang M., Li X., Ma G., Deng G., Dong S., Cai L., Jiang W. Immunocyte membrane-coated nanoparticles for cancer immunotherapy. Cancers. 2021;13(1):77. doi: 10.3390/cancers13010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rudolph M.G., Stanfield R.L., Wilson I.A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 87.Katz S.G., Rabinovich P.M. In: Cell Reprogramming for Immunotherapy: Methods and Protocols. Katz S.G., Rabinovich P.M., editors. Springer US; New York, NY: 2020. T cell reprogramming against cancer; pp. 3–44. [Google Scholar]
- 88.Yaman S., Ramachandramoorthy H., Oter G., Zhukova D., Nguyen T., Sabnani M.K., Weidanz J.A., Nguyen K.T. Melanoma peptide MHC specific TCR expressing T-Cell membrane camouflaged PLGA nanoparticles for treatment of melanoma skin cancer. Front. Bioeng. Biotechnol. 2020;8(943) doi: 10.3389/fbioe.2020.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen X., Liu B., Tong R., Zhan L., Yin X., Luo X., Huang Y., Zhang J., He W., Wang Y. Orchestration of biomimetic membrane coating and nanotherapeutics in personalized anticancer therapy. Biomater. Sci. 2021;9(3):590–625. doi: 10.1039/d0bm01617a. [DOI] [PubMed] [Google Scholar]
- 90.Dong X., Chu D., Wang Z. Leukocyte-mediated delivery of nanotherapeutics in inflammatory and tumor sites. Theranostics. 2017;7(3):751–763. doi: 10.7150/thno.18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mantovani A., Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015;212(4):435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009;86(5):1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 93.Chen C., Song M., Du Y., Yu Y., Li C., Han Y., Yan F., Shi Z., Feng S. Tumor-associated-macrophage-membrane-coated nanoparticles for improved photodynamic immunotherapy. Nano Lett. 2021;21(13):5522–5531. doi: 10.1021/acs.nanolett.1c00818. [DOI] [PubMed] [Google Scholar]
- 94.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20(1):7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 95.Lee H.K., Iwasaki A. Innate control of adaptive immunity: dendritic cells and beyond. Semin. Immunol. 2007;19(1):48–55. doi: 10.1016/j.smim.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 96.Liao Y., Zhang Y., Blum N.T., Lin J., Huang P. Biomimetic hybrid membrane-based nanoplatforms: synthesis, properties and biomedical applications. Nanoscale Horiz. 2020;5(9):1293–1302. doi: 10.1039/d0nh00267d. [DOI] [PubMed] [Google Scholar]
- 97.Liu W.L., Zou M.Z., Liu T., Zeng J.Y., Li X., Yu W.Y., Li C.X., Ye J.J., Song W., Feng J., Zhang X.Z. Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells. Adv. Mater. 2019;31(18) doi: 10.1002/adma.201900499. [DOI] [PubMed] [Google Scholar]
- 98.Liu C., Liu X., Xiang X., Pang X., Chen S., Zhang Y., Ren E., Zhang L., Liu X., Lv P., Wang X., Luo W., Xia N., Chen X., Liu G. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat. Nanotechnol. 2022;17:531–540. doi: 10.1038/s41565-022-01098-0. [DOI] [PubMed] [Google Scholar]
- 99.Puxeddu I., Bongiorni F., Chimenti D., Bombardieri S., Moretta A., Bottino C., Migliorini P. Cell surface expression of activating receptors and co-receptors on peripheral blood NK cells in systemic autoimmune diseases. Scand. J. Rheumatol. 2012;41(4):298–304. doi: 10.3109/03009742.2011.648657. [DOI] [PubMed] [Google Scholar]
- 100.Deng G., Sun Z., Li S., Peng X., Li W., Zhou L., Ma Y., Gong P., Cai L. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 101.Chen L., Qin H., Zhao R., Zhao X., Lin L., Chen Y., Lin Y., Li Y., Qin Y., Li Y., Liu S., Cheng K., Chen H., Shi J., Anderson G.J., Wu Y., Zhao Y., Nie G. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. 2021;13(601) doi: 10.1126/scitranslmed.abc2816. [DOI] [PubMed] [Google Scholar]
- 102.Fitzgerald K.A., Kagan J.C. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Q., Fan T., Zheng Y., Yang S.L., Yu Z., Duo Y., Zhang Y., Adah D., Shi L., Sun Z., Wang D., Xie J., Wu H., Wu Z., Ge C., Qiao L., Wei C., Huang L., Yan Q., Yang Q., Bao S., Liu L.P., Zhang H. Immunogenic exosome-encapsulated black phosphorus nanoparticles as an effective anticancer photo-nanovaccine. Nanoscale. 2020;12(38):19939–19952. doi: 10.1039/d0nr05953f. [DOI] [PubMed] [Google Scholar]
- 104.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. : CM. 2018;75(2):193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fu S., Wang Y., Xia X., Zheng J.C. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20 [Google Scholar]
- 106.Liu H., Deng S., Han L., Ren Y., Gu J., He L., Liu T., Yuan Z.-x. Mesenchymal stem cells, exosomes and exosome-mimics as smart drug carriers for targeted cancer therapy. Colloids Surf. B Biointerfaces. 2022;209 doi: 10.1016/j.colsurfb.2021.112163. [DOI] [PubMed] [Google Scholar]
- 107.Aqil F., Munagala R., Jeyabalan J., Agrawal A.K., Gupta R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017;19(6):1691–1702. doi: 10.1208/s12248-017-0154-9. [DOI] [PubMed] [Google Scholar]
- 108.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B., Chan Y.S., Wei L., Chin S.M., Azad A., He A.B.L., Leung A.Y.H., Yang M., Shyh-Chang N., Cho W.C., Shi J., Le M.T.N. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018;9(1):2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng L.L., Zhang X.G., Tang J.J., Lv Q.J., Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021:275. doi: 10.1016/j.biomaterials.2021.120964. [DOI] [PubMed] [Google Scholar]
- 110.Li Z., Wang H., Yin H., Bennett C., Zhang H.-g., Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-32953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sancho-Albero M., Encabo-Berzosa M., Beltran-Visiedo M., Fernandez-Messina L., Martin-Duque P. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11(40):18825–18836. doi: 10.1039/c9nr06183e. [DOI] [PubMed] [Google Scholar]
- 112.Bang C., Thum T. Exosomes: new players in cell-cell communication. Int. J. Biochem. Cell Biol. 2012;44(11):2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 113.Baquir B., Hancock R.E. Exosomes, your body's answer to immune health. Ann. Transl. Med. 2017;5(4):81. doi: 10.21037/atm.2017.01.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Munich S., Sobo-Vujanovic A., Buchser W.J., Beer-Stolz D., Vujanovic N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. OncoImmunology. 2012;1(7):1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simhadri V.R., Reiners K.S., Hansen H.P., Topolar D., Simhadri V.L., Nohroudi K., Kufer T.A., Engert A., von Strandmann E.P. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Phung C.D., Pham T.T., Nguyen H.T., Nguyen T.T., Ou W., Jeong J.-H., Choi H.-G., Ku S.K., Yong C.S., Kim J.O. Anti-CTLA-4 antibody-functionalized dendritic cell-derived exosomes targeting tumor-draining lymph nodes for effective induction of antitumor T-cell responses. Acta Biomater. 2020;115:371–382. doi: 10.1016/j.actbio.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 117.Nie W.D., Wu G.H., Zhang J.F., Huang L.L., Ding J.J., Jiang A.Q., Zhang Y.H., Liu Y.H., Li J.C., Pu K.Y., Xie H.Y. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew. Chem., Int. Ed. 2020;59(5):2018–2022. doi: 10.1002/anie.201912524. [DOI] [PubMed] [Google Scholar]
- 118.Jong A.Y., Wu C.H., Li J.B., Sun J.P., Fabbri M., Wayne A.S., Seeger R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles. 2017;6:1–12. doi: 10.1080/20013078.2017.1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaban K., Hinterleitner C., Zhou Y., Salva E., Kantarci A.G., Salih H.R., Märklin M. Therapeutic silencing of BCL-2 using NK cell-derived exosomes as a novel therapeutic approach in breast cancer. Cancers. 2021;13(10):2397. doi: 10.3390/cancers13102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blanchard N., Lankar D., Faure F., Regnault A., Dumont C., Raposo G., Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 2002;168(7):3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 121.Armstrong J.P., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11(1):69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feng C., Xiong Z., Wang C., Xiao W., Xiao H., Xie K., Chen K., Liang H., Zhang X., Yang H. Folic acid-modified Exosome-PH20 enhances the efficiency of therapy via modulation of the tumor microenvironment and directly inhibits tumor cell metastasis. Bioact. Mater. 2021;6(4):963–974. doi: 10.1016/j.bioactmat.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi X.J., Cheng Q.Q., Hou T.L., Han M.L., Smbatyan G., Lang J.E., Epstein A.L., Lenz H.J., Zhang Y. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol. Ther. 2020;28(2):536–547. doi: 10.1016/j.ymthe.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mi Y., Hagan C.T., Vincent B.G., Wang A.Z. Emerging nano-/microapproaches for cancer immunotherapy. Adv. Sci. 2019;6(6) doi: 10.1002/advs.201801847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ye X., Liang X., Chen Q., Miao Q., Chen X., Zhang X., Mei L. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 2019;13(3):2956–2968. doi: 10.1021/acsnano.8b07371. [DOI] [PubMed] [Google Scholar]
- 126.Li Q., Zhao Z., Qin X., Zhang M., Du Q., Li Z., Luan Y. A checkpoint‐regulatable immune niche created by injectable hydrogel for tumor therapy. Adv. Funct. Mater. 2021;31(37) [Google Scholar]
- 127.Gong C.A., Yu X.Y., Zhang W., Han L., Wang R., Wang Y.J., Gao S., Yuan Y.F. Regulating the immunosuppressive tumor microenvironment to enhance breast cancer immunotherapy using pH-responsive hybrid membrane-coated nanoparticles. J. Nanobiotechnol. 2021;19(1) doi: 10.1186/s12951-021-00805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li X., Zhang W., Lin J., Wu H., Yao Y., Zhang J., Yang C. T cell membrane cloaking tumor microenvironment-responsive nanoparticles with a smart “membrane escape mechanism” for enhanced immune-chemotherapy of melanoma. Biomater. Sci. 2021;9(9):3453–3464. doi: 10.1039/d1bm00331c. [DOI] [PubMed] [Google Scholar]
- 129.Liu W.L., Zou M.Z., Liu T., Zeng J.Y., Li X., Yu W.Y., Li C.X., Ye J.J., Song W., Feng J., Zhang X.Z. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-11157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y.F., Liao Y.Y., Tang Q.N., Lin J., Huang P. Biomimetic nanoemulsion for synergistic photodynamic-immunotherapy against hypoxic breast tumor. Angew. Chem., Int. Ed. 2021;60(19):10647–10653. doi: 10.1002/anie.202015590. [DOI] [PubMed] [Google Scholar]
- 131.Xiong X., Zhao J., Pan J., Liu C., Guo X., Zhou S. Personalized nanovaccine coated with calcinetin-expressed cancer cell membrane antigen for cancer immunotherapy. Nano Lett. 2021;21(19):8418–8425. doi: 10.1021/acs.nanolett.1c03004. [DOI] [PubMed] [Google Scholar]
- 132.Duan X., Chan C., Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew. Chem., Int. Ed. 2019;58(3):670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 134.Wu J.J., Waxman D.J. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018;419:210–221. doi: 10.1016/j.canlet.2018.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen B.Q., Kankala R.K., Zhang Y., Xiang S.T., Tang H.X., Wang Q., Yang D.Y., Wang S.B., Zhang Y.S., Liu G., Chen A.Z. Gambogic acid augments black phosphorus quantum dots (BPQDs)-based synergistic chemo-photothermal therapy through downregulating heat shock protein expression. Chem. Eng. J. 2020;390 [Google Scholar]
- 136.Zhang F., Lu G., Wen X., Li F., Ji X., Li Q., Wu M., Cheng Q., Yu Y., Tang J., Mei L. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J. Contr. Release. 2020;326:131–139. doi: 10.1016/j.jconrel.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 137.Sun Y.J., Feng X.R., Wan C., Lovell J.F., Jin H.L., Ding J.X. Role of nanoparticle-mediated immunogenic cell death in cancer immunotherapy. Asian J. Pharm. Sci. 2021;16(2):129–132. doi: 10.1016/j.ajps.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu C., Jiang Y., Han Y., Pu K., Zhang R. A polymer multicellular nanoengager for synergistic NIR-II photothermal immunotherapy. Adv. Mater. 2021;33(14) doi: 10.1002/adma.202008061. [DOI] [PubMed] [Google Scholar]
- 139.Zhou Z.J., Song J.B., Nie L.M., Chen X.Y. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016;45(23):6597–6626. doi: 10.1039/c6cs00271d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu Q.H., He C.L., Xiao C.S., Chen X.S. Reactive oxygen species (ROS) responsive polymers for biomedical applications. Macromol. Biosci. 2016;16(5):635–646. doi: 10.1002/mabi.201500440. [DOI] [PubMed] [Google Scholar]
- 141.Ferreira C.A., Ni D.L., Rosenkrans Z.T., Cai W.B. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018;11(10):4955–4984. doi: 10.1007/s12274-018-2092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mu X.Y., He H., Wang J.Y., Long W., Li Q.F., Liu H.L., Gao Y.L., Ouyang L.F., Ren Q.J., Sun S., Wang J.Y., Yang J., Liu Q., Sun Y.M., Liu C.L., Zhang X.D., Hu W.P. Carbogenic nanozyme with ultrahigh reactive nitrogen species selectivity for traumatic brain injury. Nano Lett. 2019;19(7):4527–4534. doi: 10.1021/acs.nanolett.9b01333. [DOI] [PubMed] [Google Scholar]
- 143.Celli J.P., Spring B.Q., Rizvi I., Evans C.L., Samkoe K.S., Verma S., Pogue B.W., Hasan T. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem. Rev. 2010;110(5):2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ou M., Lin C., Wang Y., Lu Y., Wang W., Li Z., Zeng W., Zeng X., Ji X., Mei L. Heterojunction engineered bioactive chlorella for cascade promoted cancer therapy. J. Contr. Release. 2022;345:755–769. doi: 10.1016/j.jconrel.2022.03.059. [DOI] [PubMed] [Google Scholar]
- 145.Shen J.C., Karges J., Xiong K., Chen Y., Ji L.N., Chao H. Cancer cell membrane camouflaged iridium complexes functionalized black-titanium nanoparticles for hierarchical-targeted synergistic NIR-II photothermal and sonodynamic therapy. Biomaterials. 2021:275. doi: 10.1016/j.biomaterials.2021.120979. [DOI] [PubMed] [Google Scholar]
- 146.Tang Z.M., Liu Y.Y., He M.Y., Bu W.B. Chemodynamic therapy: tumour microenvironment-mediated Fenton and fenton-like reactions. Angew. Chem., Int. Ed. 2019;58(4):946–956. doi: 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- 147.Han Y.H., Liu C.G., Chen B.Q., Fu C.P., Kankala R.K., Wang S.B., Chen A.Z. Orchestrated tumor apoptosis (Cu2+) and bone tissue calcification (Ca2+) by hierarchical Copper/Calcium-ensembled bioactive silica for osteosarcoma therapy. Chem. Eng. J. 2022;435 [Google Scholar]
- 148.Jiang Q., Wang K., Zhang X.Y., Ouyang B.S., Liu H.X., Pang Z.Q., Yang W.L. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. 2020;16(22) doi: 10.1002/smll.202001704. [DOI] [PubMed] [Google Scholar]
- 149.Srinivas U.S., Tan B.W.Q., Vellayappan B.A., Jeyasekharan A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. doi: 10.1016/j.redox.2018.101084. 101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baskar R., Dai J., Wenlong N., Yeo R., Yeoh K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014;1:24. doi: 10.3389/fmolb.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu L.T., Xin Y.J., Guo Z.Y., Gao W., Zhu Y.P., Wang Y.S., Ran R.X., Yang X.Y. Cell membrane-camouflaged multi-functional dendritic large pore mesoporous silica nanoparticles for combined photothermal therapy and radiotherapy of cancer. Chem. Res. Chin. Univ. 2022;38:562–571. [Google Scholar]
- 152.Li W., Yang J., Luo L.H., Jiang M.S., Qin B., Yin H., Zhu C.Q., Yuan X.L., Zhang J.L., Luo Z.Y., Du Y.Z., Li Q.P., Lou Y., Qiu Y.Q., You I. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019;10:3349. doi: 10.1038/s41467-019-11269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.He H., Liu L., Liang R., Zhou H., Pan H., Zhang S., Cai L. Tumor-targeted nanoplatform for in situ oxygenation-boosted immunogenic phototherapy of colorectal cancer. Acta Biomater. 2020;104:188–197. doi: 10.1016/j.actbio.2020.01.012. [DOI] [PubMed] [Google Scholar]