This systematic review and meta-analysis describes postoperative outcomes associated with gross total resection, subtotal resection, and biopsy in children with high-grade gliomas.
Key Points
Question
Is the extent of tumor resection associated with survival in pediatric patients with high-grade gliomas?
Findings
In this systematic review and meta-analysis of 37 studies involving 1387 unique pediatric patients with high-grade gliomas, gross total resection was independently associated with better overall survival compared with subtotal resection and biopsy, especially in patients with hemispheric and infratentorial tumors.
Meaning
Findings of this study suggest that maximal safe resection should be performed when feasible to treat high-grade gliomas in pediatric patients.
Abstract
Importance
Pediatric patients with high-grade gliomas have a poor prognosis. The association among the extent of resection, tumor location, and survival in these patients remains unclear.
Objective
To ascertain whether gross total resection (GTR) in hemispheric, midline, or infratentorial pediatric high-grade gliomas (pHGGs) is independently associated with survival differences compared with subtotal resection (STR) and biopsy at 1 year and 2 years after tumor resection.
Data Sources
PubMed, EBMR, Embase, and MEDLINE were systematically reviewed from inception to June 3, 2022, using the keywords high-grade glioma, pediatric, and surgery. No period or language restrictions were applied.
Study Selection
Randomized clinical trials and cohort studies of pHGGs that stratified patients by extent of resection and reported postoperative survival were included for study-level and individual patient data meta-analyses.
Data Extraction and Synthesis
Study characteristics and mortality rates were extracted from each article. Relative risk ratios (RRs) were pooled using random-effects models. Individual patient data were evaluated using multivariate mixed-effects Cox proportional hazards regression modeling. The PRISMA reporting guideline was followed, and the study was registered a priori.
Main Outcomes and Measures
Hazard ratios (HRs) and RRs were extracted to indicate associations among extent of resection, 1-year and 2-year postoperative mortality, and overall survival.
Results
A total of 37 studies with 1387 unique patients with pHGGs were included. In study-level meta-analysis, GTR had a lower mortality risk than STR at 1 year (RR, 0.69; 95% CI, 0.56-0.83; P < .001) and 2 years (RR, 0.74; 95% CI, 0.67-0.83; P < .001) after tumor resection. Subtotal resection was not associated with differential survival compared with biopsy at 1 year (RR, 0.82; 95% CI, 0.66-1.01; P = .07) but had decreased mortality risk at 2 years (RR, 0.89; 95% CI, 0.82-0.97; P = .01). The individual patient data meta-analysis of 27 articles included 427 patients (mean [SD] age at diagnosis, 9.3 [5.9] years), most of whom were boys (169 of 317 [53.3%]), had grade IV tumors (246 of 427 [57.7%]), and/or had tumors that were localized to either the cerebral hemispheres (133 of 349 [38.1%]) or midline structures (132 of 349 [37.8%]). In the multivariate Cox proportional hazards regression model, STR (HR, 1.91; 95% CI, 1.34-2.74; P < .001) and biopsy (HR, 2.10; 95% CI, 1.43-3.07; P < .001) had shortened overall survival compared with GTR but no survival differences between them (HR, 0.91; 95% CI, 0.67-1.24; P = .56). Gross total resection was associated with prolonged survival compared with STR for hemispheric (HR, 0.29; 95% CI, 0.15-0.54; P < .001) and infratentorial (HR, 0.44; 95% CI, 0.24-0.83; P = .01) tumors but not midline tumors (HR, 0.63; 95% CI, 0.34-1.19; P = .16).
Conclusions and Relevance
Results of this study show that, among patients with pHGG, GTR is independently associated with better overall survival compared with STR and biopsy, especially among patients with hemispheric and infratentorial tumors, and support the pursuit of maximal safe resection in the treatment of pHGGs.
Introduction
Malignant brain tumors are the leading cause of cancer death in children.1 High-grade gliomas (HGGs) are among the most lethal of cancers, with a median overall survival (OS) of 14 to 20 months after optimal multimodal therapy.2,3,4 The current standard of care for HGGs in adults includes maximal safe resection, high-dose radiotherapy, and chemotherapy.5
The survival benefit of gross total resection (GTR) compared with subtotal resection (STR) for HGGs in adults is well established.6 Current data suggest incremental improvements in both survival and function with a greater extent of resection (EOR).7,8 Although there is robust evidence supporting greater EOR in adults, evidence supporting greater EOR for HGGs in children is limited.9 Furthermore, pediatric HGGs (pHGGs) are distinct from adult HGGs in molecular signature as well as clinical and radiologic presentation.10,11,12,13,14 Thus, whether the evidence for EOR in adults translates to benefits for children remains to be seen.14 Current evidence of the implications of EOR for survival in pHGGs includes prospective data demonstrating an association between greater-than-90% tumor resection and improved progression-free survival (PFS).2 Other studies have found associations among EOR, OS, and PFS and identified GTR as an independent factor in improved survival.15,16,17,18,19
However, GTR in children is often challenging because of tumors in eloquent areas or midline locations (eg, thalamus and brainstem), the most prevalent anatomical sites for pHGGs.9 The survival benefit of GTR in midline locations has not been demonstrated. Furthermore, it is unclear whether STR, compared with biopsy, confers a survivorship advantage in pHGGs and should be aggressively pursued. As a result, clinicians currently face the challenge of balancing the uncertain potential benefits of aggressive tumor resection with the nonnegligible risks of postoperative neurologic impairment and morbidity.
The need for studies elucidating the association between EOR and survival in pHGGs is therefore emphasized. To address this need, we conducted a systematic review and meta-analysis of the published literature to ascertain whether GTR in hemispheric, midline, or infratentorial pHGGs is independently associated with survival differences compared with STR and biopsy at 1 year and 2 years after tumor resection. Ultimately, these findings are intended to help guide clinicians who treat children and youth with HGGs.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) checklist.20,21 A review protocol was registered a priori through the International Prospective Register of Systematic Reviews (PROSPERO) after preliminary searches were conducted.
Eligibility Criteria
Randomized clinical trials (RCTs), cohort studies, and conference abstracts were included if the study population (1) was predominantly (>80%) younger than 21 years, the upper age limit of pediatric populations according to the American Academy of Pediatrics; (2) predominantly (>50%) had histologically confirmed intracranial HGG diagnoses (WHO [World Health Organization Classification of Tumors of the Central Nervous System] grade III-IV); (3) underwent surgical cytoreduction; (4) was stratified into 3 or more EOR categories (GTR, STR, or biopsy); and (5) reported objective data on OS and/or PFS. Studies were excluded if they did not contain EOR comparison groups or if they focused on diffuse intrinsic pontine glioma because it exhibits a distinct entity that is not amenable to tumor resection.
Search Strategy and Selection Process
We developed the search strategy with a medical librarian trained in performing systematic reviews (P.D.), using the keywords high-grade glioma, pediatric, and surgery (eMethods in the Supplement). We searched PubMed, EBMR (Evidence-Based Medicine Reviews), Embase, and MEDLINE from inception to June 3, 2022. No period or language restrictions were applied. Non-English articles were translated using online tools. The reference lists of included articles were manually searched during full-text review to capture additional articles that met the inclusion criteria but were indexed discordantly from the search strategy. Study selection occurred in 2 stages and was performed by 2 of us (R.H., P.L.). First, we independently screened titles and abstracts to identify relevant studies. Second, we independently performed full-text reviews of the screened studies using a predetermined, pilot-tested form containing all of the eligibility criteria. Disagreements (κ = 0.92) were resolved through consultation with a predetermined third author (A.G.W.).
Data Extraction
Two of us (R.H., J.-S.C.) extracted the data independently, and disagreements were resolved by discussion with a third author (A.G.W.). We defined EOR according to the authors of studies and summarized EOR as GTR, STR, or biopsy. Resection that was greater than 90% was considered to be GTR. Subtotal resection encompasses all forms of partial resection and is considered to be less-than-90% tumor removal if not explicitly defined.
For the study-level meta-analysis, the comparison of interest was OS at 1 year and 2 years after tumor resection. One-year OS was chosen because it approximates the median OS for pHGGs, thus making 2-year OS a clinically relevant comparison that only 10% to 30% of patients reach.3,22 Six-month and 1-year PFS were also collected because they represent the standard timeline of clinical progression.3,22 For studies that did not report outcomes in the text, relative risk ratios (RRs) at the aforementioned time points were extracted from their Kaplan-Meier curves using a Cochrane-approved pixel-coordinate method that maps axis intercepts and calculates the percentage of at-risk patients.23
Individual patient data (IPD) reported in included articles were collected to create a pooled cohort for the IPD meta-analysis (IPDMA). Authors of included articles were not contacted when IPD were missing or not reported. Extracted characteristics included EOR and OS and, if available, age at diagnosis, sex, tumor location (hemispheric, midline, or infratentorial), tumor histologic grade (WHO grade III, IV), and adjuvant treatments (radiotherapy with concomitant chemotherapy, radiotherapy only, chemotherapy only, or no adjuvant treatment). Tumors were considered to be midline if they affected supratentorial midline structures (notably, the thalamus), whereas tumors were considered to be infratentorial if they resided within the brainstem, cerebellum, or spinal cord.24
Quality Appraisal
The quality of each article was graded independently by one of us (R.H.) using the Newcastle-Ottawa Scale for nonrandomized observational studies25 and the Cochrane Collaboration risk-of-bias tool for RCTs.26 The Newcastle-Ottawa Scale is a method for assessing risk of bias based on appropriate participant selection, exposure measures, outcome variables, and comparability; the summary score given to studies can be categorized as poor (0-3 points), fair (4-6 points), or good (≥7 points) quality (maximum score, 9 points). The Cochrane Collaboration risk-of-bias tool covers 7 methodological domains, including selection, performance, attrition, and reporting, that can be graded as having low, moderate, high, or unclear risk of bias.
Statistical Analysis
Descriptive statistics were used to present the IPD, with continuous variables summarized using means and SDs and categorical variables described using frequencies and percentages. Continuous variables of each EOR subgroup were compared using 1-way analysis of variance with the post hoc Tukey test. Categorical variables were evaluated using χ2 tests. A multivariate mixed-effects Cox proportional hazards regression model using the included study as a random-effects variable to account for methodological and clinical variations across studies was constructed to identify independent factors in time to mortality. Variables were included in the multivariate model if they were associated with OS in the univariate analysis with P < .20. Hazard ratios (HRs), 95% CIs, and P values were reported for each covariate. Kaplan-Meier curves with log-rank test were constructed to visualize survival differences on a time-to-event basis. A 2-tailed P ≤ .05 was the threshold for statistical significance.
Aggregate data were pooled using random-effects models and the Mantel-Haenszel method to account for between-study variability. Pooled RRs of mortality and progression with 95% CIs were generated for the comparisons of interest at each time point. Heterogeneity among studies was assessed using the Cochran Q statistic to calculate the I2 statistic. P < .10 for Cochran Q and I2>50% were considered to be heterogeneous and warranted investigation to identify the source of heterogeneity.27 Publication bias was evaluated using funnel plots and the modified Egger test.28,29 Sensitivity analyses were performed to assess the robustness of the findings by removing subgroups of studies with potential bias that may distort results and then repeating pooling. The largest study based on the Surveillance, Epidemiology, and End Results (SEER) Program data was removed to gauge its role in changing pooled results. Results were also reassessed after excluding (1) studies that had RRs extracted from Kaplan-Meier curves and (2) cohort studies with a quality score lower than 6 points and RCTs with a high risk of bias, given their methodological limitations, to ascertain these studies’ impact. All analyses were performed in RStudio, version 1.2.1335 (RStudio, PBC).
Results
Study Selection
The search yielded 11 973 articles (eFigure 1 in the Supplement). After exclusion of 3120 duplicates, 8853 unique articles underwent title and abstract screening, of which 281 were full-text articles assessed for eligibility. Ultimately, 37 articles published between March 1984 and April 12, 2022, reporting on 1387 unique patients were included for data extraction.15,16,18,19,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62 Of the 37 articles, 8 (21.6%) were identified from screening the reference lists of the 29 (78.4%) initially found through the search strategy.31,32,36,40,49,56,58,59 For the IPDMA, 27 articles (73.0%) provided IPD on 427 unique patients (mean [SD] age at diagnosis, 9.3 [5.9] years; 169 of 317 [53.3%] were boys, and 148 were girls [46.7%]).18,30,31,32,33,35,36,37,38,40,41,42,43,44,45,49,50,52,53,54,55,56,57,58,59,60,62 Twenty-six articles (70.3%) were observational cohort studies, and 10 (27.0%) were prospective studies. Most of the 36 nonrandomized observational studies (33 [91.7%]) were graded as fair or good quality, and the only RCT was graded as poor quality (eTables 1 and 2 in the Supplement). Eight articles did not explicitly report RRs of mortality (6 of 37 [16.2%]) or progression (8 of 37 [21.6%]) and had them extracted from their respective Kaplan-Meier curves.16,34,39,46,47,48,51,61
Study-Level Meta-analysis
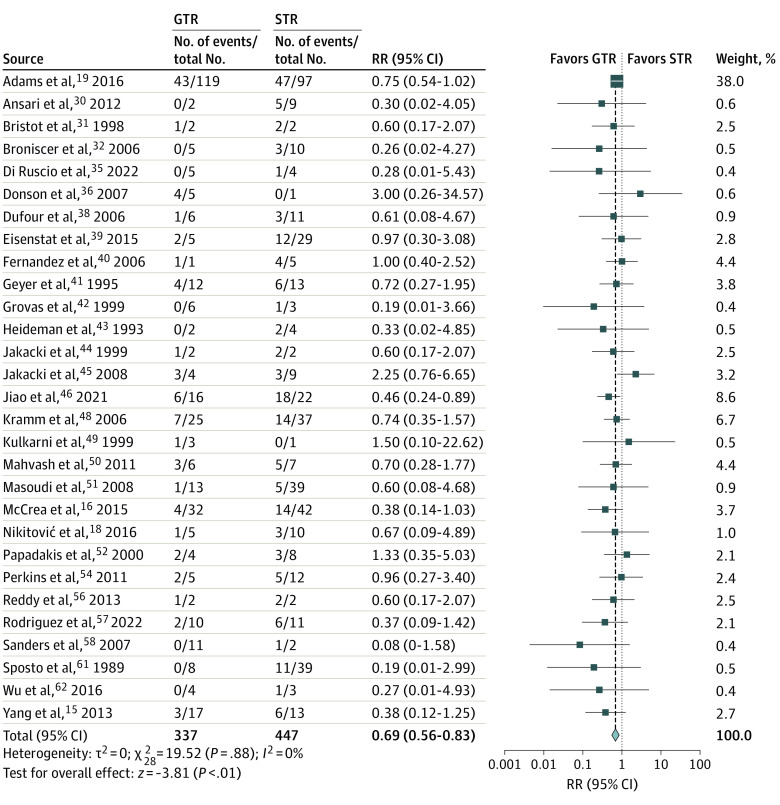
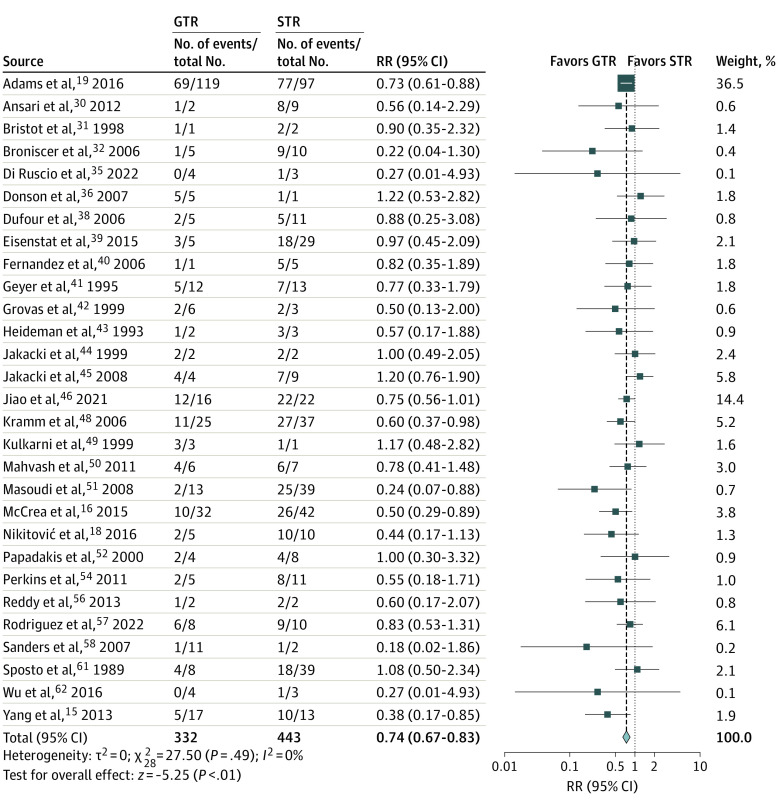
Gross total resection was associated with lower mortality risk than STR at 1 year (RR, 0.69; 95% CI, 0.56-0.83; P < .001) and 2 years (RR, 0.74; 95% CI, 0.67-0.83; P < .001) after tumor resection (Figure 1 and Figure 2). When STR was compared with biopsy, no survival advantages were observed at 1 year (RR, 0.82; 95% CI, 0.66-1.01; P = .07), but the 2-year mortality was improved (RR, 0.89; 95% CI, 0.82-0.97; P = .01) (eFigure 2 in the Supplement). The association between EOR and PFS was similar, with GTR associated with decreased progression risk at 6 months (RR, 0.62; 95% CI, 0.46-0.82; P = .001) and 1 year (RR, 0.74; 95% CI, 0.60-0.90; P = .003) (eFigure 3 in the Supplement). When PFS between STR and biopsy was compared, there were no differences in progression risk at 6 months (RR, 0.91; 95% CI, 0.65-1.28; P = .59) or 1 year (RR, 1.00; 95% CI, 0.88-1.13; P = .95) (eFigure 4 in the Supplement). Heterogeneity across publications for OS comparisons between GTR and STR as well as STR and biopsy was not significant (for all comparisons, Q statistic P > .10; I2<50%).
Figure 1. Forest Plot of Relative Risk (RR) for 1-Year Mortality for Gross Total Resection (GTR) vs Subtotal Resection (STR).
Blue squares indicate weight-adjusted relative risk ratios (RRs) of individual studies; horizontal black lines, 95% CIs; and blue diamond, the pooled relative RR estimate.
Figure 2. Forest Plot of Relative Risk (RR) for 2-Year Mortality for Gross Total Resection (GTR) vs Subtotal Resection (STR).
Blue squares indicate weight-adjusted relative risk ratios (RRs) of individual studies; horizontal black lines, 95% CIs; and blue diamond, the pooled relative RR estimate.
Funnel plots were generated along with each survival forest plot to evaluate publication bias (eFigure 5 in the Supplement). The largest funnel plot comparing the 1-year OS between GTR and STR displayed asymmetry at the level of small studies, with a modest deficit in studies favoring STR over GTR. However, the modified Egger test did not demonstrate significant publication bias (–0.38; P = .14). During sensitivity analysis, removing SEER Program data did not change the significance of results. Excluding manually extracted outcomes from Kaplan-Meier curves and studies with lower-quality grades also did not affect the results between GTR and STR. However, the survival advantage associated with STR over biopsy at 2 years was lost (eTable 3 in the Supplement).
IPD Meta-analysis
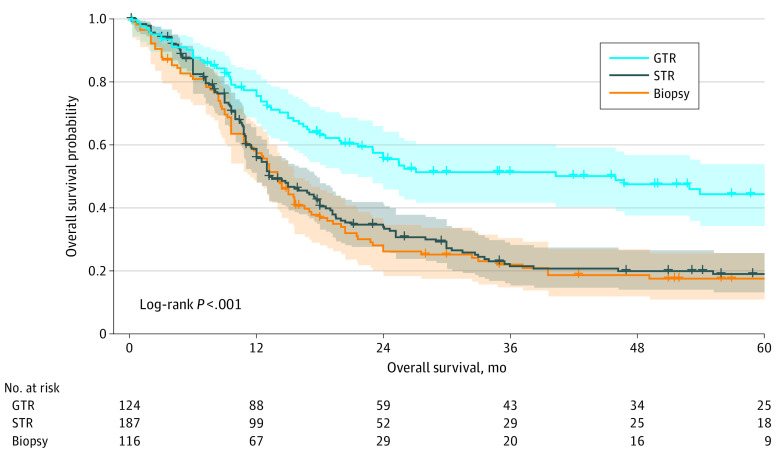
The cohort of patients with IPD (n = 427) was composed of predominantly boys (169 of 317 [53.3%]). Most patients had WHO grade IV tumors (246 of 427 [57.7%]) localized to the cerebral hemispheres (133 of 349 [38.1%]) or midline structures (132 of 349 [37.8%]). Characteristics of of patients with IPD are stratified by EOR and summarized in Table 1. The prevalence was 29.0% (n = 124) for GTR, 43.8% (n = 187) for STR, and 27.2% (n = 116) for biopsy. There were no differences in sex across the subgroups; however, there were significant differences in age at diagnosis, tumor location, tumor histologic grade, and adjuvant treatment (for all comparisons, P < .001). Patients with GTR were younger than those with STR (mean [SD] age, 7.3 [5.9] years vs 9.7 [5.7] years; P < .001) or biopsy (vs 10.8 [5.7] years; P < .001). The GTR cohort also had higher rates of infratentorial tumors, WHO grade IV tumors, and patients who did not receive both radiotherapy and chemotherapy. However, patients who underwent GTR had longer OS compared with survival of those who underwent STR and biopsy (27.1 months vs 13.2 months vs 14.0 months; log-rank P < .001) (Figure 3).
Table 1. Baseline Characteristics of 427 Patients in the Individual-Patient Data Meta-analysis.
| Characteristic | Extent of resection, No. (%) | P value | ||
|---|---|---|---|---|
| GTR (n = 124) | STR (n = 187) | Biopsy (n = 116) | ||
| Age at diagnosis, mean (SD), y | 7.3 (5.9) | 9.7 (5.7) | 10.8 (5.7) | <.001a |
| Sex (n = 317) | ||||
| Female | 35 (40.7) | 67 (48.6) | 46 (49.5) | .42 |
| Male | 51 (59.3) | 71 (51.4) | 47 (50.5) | |
| Tumor location (n = 349) | ||||
| Hemispheric | 50 (46.3) | 59 (39.9) | 24 (25.8) | <.001a |
| Midline | 23 (21.3) | 58 (39.2) | 51 (54.8) | |
| Infratentorial | 35 (32.4) | 31 (20.9) | 18 (19.4) | |
| Tumor histologic grade (n = 427) | ||||
| WHO grade III | 40 (32.3) | 73 (39.0) | 68 (58.6) | <.001a |
| WHO grade IV | 84 (67.7) | 114 (61.0) | 48 (41.4) | |
| Adjuvant treatment (n = 421) | ||||
| Radiotherapy with chemotherapy | 72 (59.0) | 130 (70.7) | 88 (76.5) | <.001a |
| Radiotherapy alone | 7 (5.7) | 22 (12.0) | 12 (10.4) | |
| Chemotherapy alone | 34 (27.9) | 29 (15.8) | 13 (11.3) | |
| None | 9 (7.4) | 3 (1.6) | 2 (1.7) | |
| Overall survival, median (95% CI), mob | 27.1 (41.7-60.3) | 13.2 (12.0-17.7) | 14.0 (11.4-15.6) | <.001a |
Abbreviations: GTR, gross total resection; STR, subtotal resection; WHO, World Health Organization Classification of Tumors of the Central Nervous System.
P < .05 indicates statistically significant difference in characteristics between groups.
Overall survival is reported as median (95% CI), both of which were derived from Kaplan-Meier analysis.
Figure 3. Kaplan-Meier Curve Comparing Overall Survival According to Extent of Resection.
GTR indicates gross total resection; STR, subtotal resection.
Univariate mixed-effects Cox proportional hazards regression modeling showed that STR (HR, 1.99; 95% CI, 1.45-2.72; P < .001) and biopsy (HR, 2.09; 95% CI, 1.48-2.95; P < .001) were associated with worse OS compared with GTR (Table 2). Midline and infratentorial tumors as well as radiotherapy alone and no adjuvant treatment were also associated with worse OS compared with hemispheric tumors and adjuvant radiotherapy with concomitant chemotherapy (eFigure 6 in the Supplement). The association between EOR and OS persisted during multivariate analysis after controlling for tumor location and adjuvant treatment. Subtotal resection (HR, 1.91; 95% CI, 1.34-2.74; P < .001) and biopsy (HR, 2.10; 95% CI, 1.43-3.07; P < .001) were independently associated with worse estimated outcome compared with GTR. However, STR had no associated survival advantage (HR, 0.91; 95% CI, 0.67-1.24; P = .56) over biopsy. Other independent associations included the association of midline (HR, 1.74; 95% CI, 1.16-2.63; P = .008) and infratentorial (HR, 2.21; 95% CI, 1.48-3.31; P < .001) tumors, radiotherapy alone (HR, 2.84; 95% CI, 1.67-4.81; P < .001), and no adjuvant treatment (HR, 4.93; 95% CI, 2.98-8.16; P < .001) with shortened survival.
Table 2. Univariate and Multivariate Mixed-Effects Cox Proportional Hazards Regression Model of Overall Survival for Patients in the Individual-Patient Data Meta-analysis.
| Factor | Univariate HR (95% CI) | P value | Multivariate HR (95% CI) | P value |
|---|---|---|---|---|
| Older publication year | 1.01 (0.98-1.04) | .54 | NA | NA |
| Older age at diagnosis | 1.00 (0.99-1.01) | .31 | NA | NA |
| Male sex | 1.01 (0.78-1.32) | .92 | NA | NA |
| Extent of resection | ||||
| GTR | 1 [Reference] | 1 [Reference] | ||
| STR | 1.99 (1.45-2.72) | <.001a | 1.91 (1.34-2.74) | <.001a |
| Biopsy | 2.09 (1.48-2.95) | <.001a | 2.10 (1.43-3.07) | <.001a |
| Tumor location | ||||
| Hemispheric | 1 [Reference] | 1 [Reference] | ||
| Midline | 1.93 (1.30-2.85) | .001a | 1.74 (1.16-2.63) | .008a |
| Infratentorial | 2.16 (1.47-3.19) | <.001a | 2.21 (1.48-3.31) | <.001a |
| Tumor histologic grade | ||||
| WHO grade III | 1 [Reference] | NA | NA | |
| WHO grade IV | 1.27 (0.95-1.69) | .11 | NA | NA |
| Adjuvant treatment | ||||
| Radiotherapy with chemotherapy | 1 [Reference] | 1 [Reference] | ||
| Radiotherapy only | 2.01 (1.27-3.17) | .003a | 2.84 (1.67-4.81) | <.001a |
| Chemotherapy only | 0.79 (0.47-1.31) | .35 | 0.77 (0.47-1.28) | .32 |
| None | 3.95 (1.61-9.67) | .003a | 4.93 (2.98-8.16) | <.001a |
Abbreviations: GTR, gross total resection; HR, hazard ratio; NA, not applicable; STR, subtotal resection; WHO, World Health Organization Classification of Tumors of the Central Nervous System.
P < .05 indicates statistically significant HR of time to mortality.
The outcome of EOR was also examined in location-specific subgroups. Gross total resection was independently associated with longer survival in hemispheric (HR, 0.29; 95% CI, 0.15-0.54; P < .001) and infratentorial (HR, 0.44; 95% CI, 0.24-0.83; P = .01) tumors compared with STR. However, GTR in patients with midline tumors (HR, 0.63; 95% CI, 0.34-1.19; P = .16) was not associated with improved survival (eFigure 7 in the Supplement).
Discussion
This systematic review and meta-analysis of RCTs and cohort studies of pHGGs found that GTR was independently associated with prolonged survival. Patients with GTR were 45% more likely to survive 1 year and 35% more likely to survive 2 years after tumor resection than patients with STR. Patients with GTR were also 61% more likely to be progression free at 6 months and 35% more likely to be progression free at 1 year after resection. Subtotal resection was associated with survival benefits over biopsy at the 2-year but not at the 1-year follow-up. The number needed to treat was 7.3 for GTR and 4.4 for STR to achieve an additional 1 or 2 years of life. Clinical trial guidelines recommend that an optimal number needed to treat for dichotomous outcomes is lower than 10, suggesting that achieving GTR may extend survival in pHGGs.63 Although the association between EOR and survival is well documented in adult HGGs,6 no meta-analysis of pHGGs has yet been conducted, to our knowledge. The superiority of GTR over STR and biopsy, lack of superiority between STR and biopsy, and median OS across the 3 EOR subgroups in this study are consistent with results of a large-scale SEER database analysis of 342 pHGGs that was conducted by Adams et al,19 whose sample was independent of the IPD cohort in the present study, thus providing quantitative validation of the recommendations for maximal safe resection when technically feasible in pHGGs.4,24,64,65 However, this study also estimated that only 29% of patients received GTR, an estimate consistent with those in previous studies (35%-45%).4,19,66 The low rate of pursuing GTR in pHGGs is commonly attributed to tumor location because of varying degrees of difficulty across localizations and an incomplete understanding of the association between EOR and tumor survival in different regions, particularly deep-seated infratentorial and midline tumors.66 Previous studies have tried to elucidate the latter issue but were limited by underpowered samples.16,17 Therefore, we attempted to address this association using a large, pooled sample of IPD.
In the IPDMA, certain subgroups within the study population responded to GTR better than others. Specifically, patients with hemispheric and infratentorial pHGGs experienced prolonged survival after GTR, whereas no survival difference was observed in those with tumors of the midline cerebrum. The classification of tumor location mirrored the post hoc analysis in the clinical trial by the Children’s Cancer Group.24 In that trial, Wisoff et al24 noted a significant difference in the frequency of radical resection across tumor sites, with the lowest GTR rate and highest biopsy rate occurring in midline tumors, which is consistent with the results of the present study and other studies on thalamic or midline pHGGs.16,67 Disparate rates of surgical management in midline pHGGs underscore the difficulty of achieving GTR because of the high risk of postoperative morbidity and mortality from proximity to or infiltration of critical structures.68 However, despite the technological advances that have made maximal resection safer and more feasible, there is still no consensus on whether GTR should be pursued in midline tumors.69,70 Findings of this study suggest that EOR has no implications for midline pHGGs, but the classification of tumors based on mutations was not captured. Thus, the lack of association between survival and EOR in midline tumors may not be attributed to a lack of efficacy in aggressive surgical management but instead may result from the pooling of clinically and biologically distinct tumors that respond differently to tumor resection.
Thalamic pHGGs are known to represent a distinct clinical subset, as reported by Kramm et al,67 and the discovery of H3K27M-altered tumors with more aggressive mechanisms of tumorigenesis and substantially worse survival has further stratified this cohort.11,71,72 Thus, future investigations of the association between EOR and survival in midline pHGGs should consider the molecular classification of tumors to further elucidate whether and when extensive surgical debulking is worth pursuing in this subgroup. However, given the overwhelming survival benefit associated with GTR in the general population of this study, maximal safe resection—when resection is indicated—should still be pursued for all pHGGs in the meantime.
Limitations and Strengths
This study has several limitations. First, the lack of differential survival between STR and biopsy may be partly a result of the smaller sample size of the biopsy cohort, suggesting a possible publication bias. Compounding this issue is that STR, for the purposes of this study, included partial tumor resections. Furthermore, we defined EOR according to the judgment of the study authors, which may create bias, imprecision, heterogeneity, and/or overlap between EOR subgroups. However, we attempted to account for these biases by performing a sensitivity analysis that excluded studies with questionable quality, and we found no significant differences in the results.
Second, IPDMAs have inherent limitations because they are still vulnerable to reviewer selection bias, data availability, and publication bias.73 The IPD did not capture postoperative functional neurologic status as an outcome because of scarce reporting. The balance between cytoreduction and risk of neurologic morbidity is an important consideration when developing EOR guidelines, especially for children undergoing continued neurodevelopment.74 Thus, future studies may evaluate the survival impact of EOR in conjunction with functional status to ensure that protocols and studies encouraging maximal safe resection do not compromise postoperative quality of life.
This study also has some strengths. Extracting IPD in addition to study-level estimates allowed us to perform 2 types of meta-analyses. The results were similar, suggesting reliable representation of the general population and low selection bias. Furthermore, the IPDMA was robust given that the mixed-effects Cox proportional hazards regression model allowed us to control for methodological and clinical variations across studies and covariates associated with survival, including location and adjuvant treatments. Consequently, we were able to mitigate the possibility of aggregation bias commonly observed in study-level meta-analyses.75
Conclusions
To our knowledge, this systematic review and meta-analysis was the first to assess the association between EOR and survival in pHGGs. The results suggest that GTR is independently associated with prolonged OS and PFS compared with STR and biopsy and that STR may not prolong survival compared with biopsy alone. These findings support the pursuit of maximal safe resection when feasible in the treatment of pHGGs. Future studies should seek to identify specific molecular subgroups that benefit most from aggressive cytoreduction.
eMethods. Complete Search Strategy
eTable 1. Characteristics of Included Studies
eTable 2. Newcastle-Ottawa Scale and Cochrane Collaboration’s Risk of Bias Tool Evaluation of Articles Included in Meta-analysis
eTable 3. Sensitivity Analysis of Meta-analysis Results After Removing Subgroups of Studies With Potential Bias
eFigure 1. PRISMA Flow Diagram
eFigure 2. Forest Plot of Relative Risk for 1-Year and 2-Year Mortality for Subtotal Resection Versus Biopsy
eFigure 3. Forest Plot of Relative Risk for 6-Month and 1-Year Progression for Gross Total Resection Versus Subtotal Resection
eFigure 4. Forest Plot of Relative Risk for 6-Month and 1-Year Progression for Subtotal Resection Versus Biopsy
eFigure 5. Funnel Plots for Forest Plots of Relative Risk for 1-Year and 2-Year Mortality Between Gross Total Resection Versus Subtotal Resection and Subtotal Resection Versus Biopsy
eFigure 6. Kaplan-Meier Curve Comparing Overall Survival According to Tumor Location and Adjuvant Treatment Regimen
eFigure 7. Kaplan-Meier Curve Comparing Overall Survival According to EOR in Tumor Location Subgroups
References
- 1.Withrow DR, Berrington de Gonzalez A, Lam CJK, Warren KE, Shiels MS. Trends in pediatric central nervous system tumor incidence in the United States, 1998-2013. Cancer Epidemiol Biomarkers Prev. 2019;28(3):522-530. doi: 10.1158/1055-9965.EPI-18-0784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finlay JL, Boyett JM, Yates AJ, et al. ; Childrens Cancer Group . Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. J Clin Oncol. 1995;13(1):112-123. doi: 10.1200/JCO.1995.13.1.112 [DOI] [PubMed] [Google Scholar]
- 3.Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9(2):197-206. doi: 10.1634/theoncologist.9-2-197 [DOI] [PubMed] [Google Scholar]
- 4.Lam S, Lin Y, Zinn P, Su J, Pan IW. Patient and treatment factors associated with survival among pediatric glioblastoma patients: a Surveillance, Epidemiology, and End Results study. J Clin Neurosci. 2018;47:285-293. doi: 10.1016/j.jocn.2017.10.041 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331(2):139-146. doi: 10.1016/j.canlet.2012.12.024 [DOI] [PubMed] [Google Scholar]
- 6.Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460-1469. doi: 10.1001/jamaoncol.2016.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almenawer SA, Badhiwala JH, Alhazzani W, et al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro Oncol. 2015;17(6):868-881. doi: 10.1093/neuonc/nou349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198. doi: 10.3171/jns.2001.95.2.0190 [DOI] [PubMed] [Google Scholar]
- 9.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17(suppl 4):iv1-iv62. doi: 10.1093/neuonc/nov189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi: 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425-437. doi: 10.1016/j.ccr.2012.08.024 [DOI] [PubMed] [Google Scholar]
- 12.Wu G, Broniscer A, McEachron TA, et al. ; St Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project . Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251-253. doi: 10.1038/ng.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjerke L, Mackay A, Nandhabalan M, et al. Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Cancer Discov. 2013;3(5):512-519. doi: 10.1158/2159-8290.CD-12-0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones C, Karajannis MA, Jones DTW, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. 2017;19(2):153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang T, Temkin N, Barber J, et al. Gross total resection correlates with long-term survival in pediatric patients with glioblastoma. World Neurosurg. 2013;79(3-4):537-544. doi: 10.1016/j.wneu.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 16.McCrea HJ, Bander ED, Venn RA, et al. Sex, age, anatomic location, and extent of resection influence outcomes in children with high-grade glioma. Neurosurgery. 2015;77(3):443-452. doi: 10.1227/NEU.0000000000000845 [DOI] [PubMed] [Google Scholar]
- 17.Walston S, Hamstra DA, Oh K, et al. A multi-institutional experience in pediatric high-grade glioma. Front Oncol. 2015;5:28. doi: 10.3389/fonc.2015.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikitović M, Stanić D, Pekmezović T, et al. Pediatric glioblastoma: a single institution experience. Childs Nerv Syst. 2016;32(1):97-103. doi: 10.1007/s00381-015-2945-6 [DOI] [PubMed] [Google Scholar]
- 19.Adams H, Adams HHH, Jackson C, Rincon-Torroella J, Jallo GI, Quiñones-Hinojosa A. Evaluating extent of resection in pediatric glioblastoma: a multiple propensity score-adjusted population-based analysis. Childs Nerv Syst. 2016;32(3):493-503. doi: 10.1007/s00381-015-3006-x [DOI] [PubMed] [Google Scholar]
- 20.Shamseer L, Moher D, Clarke M, et al. ; PRISMA-P Group . Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Thakkar JP, Garcia CR, et al. National cancer database analysis of outcomes in pediatric glioblastoma. Cancer Med. 2018;7(4):1151-1159. doi: 10.1002/cam4.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelicic Kadic A, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J Clin Epidemiol. 2016;74(74):119-123. doi: 10.1016/j.jclinepi.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 24.Wisoff JH, Boyett JM, Berger MS, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children’s Cancer Group trial No. CCG-945. J Neurosurg. 1998;89(1):52-59. doi: 10.3171/jns.1998.89.1.0052 [DOI] [PubMed] [Google Scholar]
- 25.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Accessed January 11, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 26.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 28.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443-3457. doi: 10.1002/sim.2380 [DOI] [PubMed] [Google Scholar]
- 29.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 30.Ansari M, Nasrolahi H, Kani AA, et al. Pediatric glioblastoma multiforme: a single-institution experience. Indian J Med Paediatr Oncol. 2012;33(3):155-160. doi: 10.4103/0971-5851.103142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bristot R, Raco A, Vangelista T, Delfini R. Malignant cerebellar astrocytomas in childhood. Experience with four cases. Childs Nerv Syst. 1998;14(10):532-536. doi: 10.1007/s003810050268 [DOI] [PubMed] [Google Scholar]
- 32.Broniscer A, Chintagumpala M, Fouladi M, et al. Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. J Neurooncol. 2006;76(3):313-319. doi: 10.1007/s11060-005-7409-5 [DOI] [PubMed] [Google Scholar]
- 33.Broniscer A, Baker SJ, Stewart CF, et al. Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clin Cancer Res. 2009;15(2):701-707. doi: 10.1158/1078-0432.CCR-08-1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chastagner P, Kalifa C, Doz F, et al. ; French Society of Pediatric Oncology (SFOP) Pilot Study . Outcome of children treated with preradiation chemotherapy for a high-grade glioma: results of a French Society of Pediatric Oncology (SFOP) pilot study. Pediatr Blood Cancer. 2007;49(6):803-807. doi: 10.1002/pbc.21051 [DOI] [PubMed] [Google Scholar]
- 35.Di Ruscio V, Carai A, Del Baldo G, et al. Molecular landscape in infant high-grade gliomas: a single center experience. Diagnostics (Basel). 2022;12(2):372. doi: 10.3390/diagnostics12020372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donson AM, Addo-Yobo SO, Handler MH, Gore L, Foreman NK. MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr Blood Cancer. 2007;48(4):403-407. doi: 10.1002/pbc.20803 [DOI] [PubMed] [Google Scholar]
- 37.Dorfer C, Czech T, Gojo J, et al. Infiltrative gliomas of the thalamus in children: the role of surgery in the era of H3 K27M mutant midline gliomas. Acta Neurochir (Wien). 2021;163(7):2025-2035. doi: 10.1007/s00701-020-04589-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dufour C, Grill J, Lellouch-Tubiana A, et al. High-grade glioma in children under 5 years of age: a chemotherapy only approach with the BBSFOP protocol. Eur J Cancer. 2006;42(17):2939-2945. doi: 10.1016/j.ejca.2006.06.021 [DOI] [PubMed] [Google Scholar]
- 39.Eisenstat DD, Pollack IF, Demers A, et al. Impact of tumor location and pathological discordance on survival of children with midline high-grade gliomas treated on Children’s Cancer Group high-grade glioma study CCG-945. J Neurooncol. 2015;121(3):573-581. doi: 10.1007/s11060-014-1669-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez C, Maues de Paula A, Colin C, et al. Thalamic gliomas in children: an extensive clinical, neuroradiological and pathological study of 14 cases. Childs Nerv Syst. 2006;22(12):1603-1610. doi: 10.1007/s00381-006-0184-6 [DOI] [PubMed] [Google Scholar]
- 41.Geyer JR, Finlay JL, Boyett JM, et al. Survival of infants with malignant astrocytomas: a report from the Childrens Cancer Group. Cancer. 1995;75(4):1045-1050. doi: [DOI] [PubMed] [Google Scholar]
- 42.Grovas AC, Boyett JM, Lindsley K, Rosenblum M, Yates AJ, Finlay JL. Regimen-related toxicity of myeloablative chemotherapy with BCNU, thiotepa, and etoposide followed by autologous stem cell rescue for children with newly diagnosed glioblastoma multiforme: report from the Children’s Cancer Group. Med Pediatr Oncol. 1999;33(2):83-87. doi: [DOI] [PubMed] [Google Scholar]
- 43.Heideman RL, Douglass EC, Krance RA, et al. High-dose chemotherapy and autologous bone marrow rescue followed by interstitial and external-beam radiotherapy in newly diagnosed pediatric malignant gliomas. J Clin Oncol. 1993;11(8):1458-1465. doi: 10.1200/JCO.1993.11.8.1458 [DOI] [PubMed] [Google Scholar]
- 44.Jakacki RI, Siffert J, Jamison C, Velasquez L, Allen JC. Dose-intensive, time-compressed procarbazine, CCNU, vincristine (PCV) with peripheral blood stem cell support and concurrent radiation in patients with newly diagnosed high-grade gliomas. J Neurooncol. 1999;44(1):77-83. doi: 10.1023/A:1006360222643 [DOI] [PubMed] [Google Scholar]
- 45.Jakacki RI, Yates A, Blaney SM, et al. A phase I trial of temozolomide and lomustine in newly diagnosed high-grade gliomas of childhood. Neuro Oncol. 2008;10(4):569-576. doi: 10.1215/15228517-2008-019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiao Y, Wang M, Liu X, et al. Clinical features and prognostic factors of pediatric glioblastoma: report of 38 cases. World Neurosurg. 2021;153:e105-e111. doi: 10.1016/j.wneu.2021.06.033 [DOI] [PubMed] [Google Scholar]
- 47.Jung TY, Lee JY, Kim DS, et al. Pediatric supratentorial high-grade glioma: multicenter retrospective observational study of the Korean Society for Pediatric Neuro-Oncology. J Neurooncol. 2015;121(2):413-419. doi: 10.1007/s11060-014-1653-5 [DOI] [PubMed] [Google Scholar]
- 48.Kramm CM, Wagner S, Van Gool S, et al. Improved survival after gross total resection of malignant gliomas in pediatric patients from the HIT-GBM studies. Anticancer Res. 2006;26(5B):3773-3779. [PubMed] [Google Scholar]
- 49.Kulkarni AV, Becker LE, Jay V, Armstrong DC, Drake JM. Primary cerebellar glioblastomas multiforme in children: report of four cases. J Neurosurg. 1999;90(3):546-550. doi: 10.3171/jns.1999.90.3.0546 [DOI] [PubMed] [Google Scholar]
- 50.Mahvash M, Hugo HH, Maslehaty H, Mehdorn HM, Stark AM. Glioblastoma multiforme in children: report of 13 cases and review of the literature. Pediatr Neurol. 2011;45(3):178-180. doi: 10.1016/j.pediatrneurol.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 51.Masoudi A, Elopre M, Amini E, et al. Influence of valproic acid on outcome of high-grade gliomas in children. Anticancer Res. 2008;28(4C):2437-2442. [PubMed] [Google Scholar]
- 52.Papadakis V, Dunkel IJ, Cramer LD, et al. High-dose carmustine, thiotepa and etoposide followed by autologous bone marrow rescue for the treatment of high risk central nervous system tumors. Bone Marrow Transplant. 2000;26(2):153-160. doi: 10.1038/sj.bmt.1702475 [DOI] [PubMed] [Google Scholar]
- 53.Parekh C, Jubran R, Erdreich-Epstein A, et al. Treatment of children with recurrent high grade gliomas with a bevacizumab containing regimen. J Neurooncol. 2011;103(3):673-680. doi: 10.1007/s11060-010-0444-x [DOI] [PubMed] [Google Scholar]
- 54.Perkins SM, Rubin JB, Leonard JR, et al. Glioblastoma in children: a single-institution experience. Int J Radiat Oncol Biol Phys. 2011;80(4):1117-1121. doi: 10.1016/j.ijrobp.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 55.Phuphanich S, Edwards MS, Levin VA, et al. Supratentorial malignant gliomas of childhood: results of treatment with radiation therapy and chemotherapy. J Neurosurg. 1984;60(3):495-499. doi: 10.3171/jns.1984.60.3.0495 [DOI] [PubMed] [Google Scholar]
- 56.Reddy GD, Sen AN, Patel AJ, Bollo RJ, Jea A. Glioblastoma of the cerebellum in children: report of five cases and review of the literature. Childs Nerv Syst. 2013;29(5):821-832. doi: 10.1007/s00381-012-1996-1 [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez D, Calmon R, Aliaga ES, et al. MRI and molecular characterization of pediatric high-grade midline thalamic gliomas: the HERBY phase II trial. Radiology. 2022;304(1):174-182. doi: 10.1148/radiol.211464 [DOI] [PubMed] [Google Scholar]
- 58.Sanders RP, Kocak M, Burger PC, Merchant TE, Gajjar A, Broniscer A. High-grade astrocytoma in very young children. Pediatr Blood Cancer. 2007;49(7):888-893. doi: 10.1002/pbc.21272 [DOI] [PubMed] [Google Scholar]
- 59.Shinoda J, Yamada H, Sakai N, Ando T, Hirata T, Hirayama H. Malignant cerebellar astrocytic tumours in children. Acta Neurochir (Wien). 1989;98(1-2):1-8. doi: 10.1007/BF01407169 [DOI] [PubMed] [Google Scholar]
- 60.Sirachainan N, Boongird A, Swangsilpa T, Klaisuban W, Lusawat A, Hongeng S. Reported outcomes of children with newly diagnosed high-grade gliomas treated with nimotuzumab and irinotecan. Childs Nerv Syst. 2017;33(6):893-897. doi: 10.1007/s00381-017-3409-y [DOI] [PubMed] [Google Scholar]
- 61.Sposto R, Ertel IJ, Jenkin RD, et al. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial: a report from the Childrens Cancer Study Group. J Neurooncol. 1989;7(2):165-177. doi: 10.1007/BF00165101 [DOI] [PubMed] [Google Scholar]
- 62.Wu CT, Tsay PK, Jaing TH, Chen SH, Tseng CK, Jung SM. Oligodendrogliomas in children: clinical experiences with 20 patients. J Pediatr Hematol Oncol. 2016;38(7):555-558. doi: 10.1097/MPH.0000000000000610 [DOI] [PubMed] [Google Scholar]
- 63.Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407-411. doi: 10.1111/ijcp.12142 [DOI] [PubMed] [Google Scholar]
- 64.Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;115(24):5761-5770. doi: 10.1002/cncr.24663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanan MI, Eisenstat DD. Management of high-grade gliomas in the pediatric patient: past, present, and future. Neurooncol Pract. 2014;1(4):145-157. doi: 10.1093/nop/npu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blionas A, Giakoumettis D, Klonou A, Neromyliotis E, Karydakis P, Themistocleous MS. Paediatric gliomas: diagnosis, molecular biology and management. Ann Transl Med. 2018;6(12):251. doi: 10.21037/atm.2018.05.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kramm CM, Butenhoff S, Rausche U, et al. Thalamic high-grade gliomas in children: a distinct clinical subset? Neuro Oncol. 2011;13(6):680-689. doi: 10.1093/neuonc/nor045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steiger HJ, Götz C, Schmid-Elsaesser R, Stummer W. Thalamic astrocytomas: surgical anatomy and results of a pilot series using maximum microsurgical removal. Acta Neurochir (Wien). 2000;142(12):1327-1336. doi: 10.1007/s007010070001 [DOI] [PubMed] [Google Scholar]
- 69.Steinbok P, Gopalakrishnan CV, Hengel AR, et al. Pediatric thalamic tumors in the MRI era: a Canadian perspective. Childs Nerv Syst. 2016;32(2):269-280. doi: 10.1007/s00381-015-2968-z [DOI] [PubMed] [Google Scholar]
- 70.Cinalli G, Aguirre DT, Mirone G, et al. Surgical treatment of thalamic tumors in children. J Neurosurg Pediatr. 2018;21(3):247-257. doi: 10.3171/2017.7.PEDS16463 [DOI] [PubMed] [Google Scholar]
- 71.Ryall S, Krishnatry R, Arnoldo A, et al. Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol Commun. 2016;4(1):93. doi: 10.1186/s40478-016-0353-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu VM, Alvi MA, McDonald KL, Daniels DJ. Impact of the H3K27M mutation on survival in pediatric high-grade glioma: a systematic review and meta-analysis. J Neurosurg Pediatr. 2018;23(3):308-316. doi: 10.3171/2018.9.PEDS18419 [DOI] [PubMed] [Google Scholar]
- 73.Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344:d7762. doi: 10.1136/bmj.d7762 [DOI] [PubMed] [Google Scholar]
- 74.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463-469. doi: 10.1227/01.NEU.0000349763.42238.E9 [DOI] [PubMed] [Google Scholar]
- 75.Stewart GB, Altman DG, Askie LM, Duley L, Simmonds MC, Stewart LA. Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS One. 2012;7(10):e46042. doi: 10.1371/journal.pone.0046042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Complete Search Strategy
eTable 1. Characteristics of Included Studies
eTable 2. Newcastle-Ottawa Scale and Cochrane Collaboration’s Risk of Bias Tool Evaluation of Articles Included in Meta-analysis
eTable 3. Sensitivity Analysis of Meta-analysis Results After Removing Subgroups of Studies With Potential Bias
eFigure 1. PRISMA Flow Diagram
eFigure 2. Forest Plot of Relative Risk for 1-Year and 2-Year Mortality for Subtotal Resection Versus Biopsy
eFigure 3. Forest Plot of Relative Risk for 6-Month and 1-Year Progression for Gross Total Resection Versus Subtotal Resection
eFigure 4. Forest Plot of Relative Risk for 6-Month and 1-Year Progression for Subtotal Resection Versus Biopsy
eFigure 5. Funnel Plots for Forest Plots of Relative Risk for 1-Year and 2-Year Mortality Between Gross Total Resection Versus Subtotal Resection and Subtotal Resection Versus Biopsy
eFigure 6. Kaplan-Meier Curve Comparing Overall Survival According to Tumor Location and Adjuvant Treatment Regimen
eFigure 7. Kaplan-Meier Curve Comparing Overall Survival According to EOR in Tumor Location Subgroups