Abstract
Mitochondria-targeted H2S donors are thought to protect against acute ischemia-reperfusion (IR) injury by releasing H2S that decreases oxidative damage. However, the rate of H2S release by current donors is too slow to be effective upon administration following reperfusion. To overcome this limitation here we develop a mitochondria-targeted agent, MitoPerSulf that very rapidly releases H2S within mitochondria. MitoPerSulf is quickly taken up by mitochondria, where it reacts with endogenous thiols to generate a persulfide intermediate that releases H2S. MitoPerSulf is acutely protective against cardiac IR injury in mice, due to the acute generation of H2S that inhibits respiration at cytochrome c oxidase thereby preventing mitochondrial superoxide production by lowering the membrane potential. Mitochondria-targeted agents that rapidly generate H2S are a new class of therapy for the acute treatment of IR injury.
Keywords: Hydrogen sulfide donors, Mitochondria, Ischemia-reperfusion injury, Mitochondria targeting, Reverse electron transport (RET)
1. Introduction
Hydrogen sulfide (H2S) and H2S releasing compounds are protective against ischemia-reperfusion (IR) injury [[1], [2], [3]] in the liver [4,5], kidney [6], lung [7] and heart [[8], [9], [10], [11]] and against IR injury during organ transplantation [12,13]. The H2S donors used so far include simple hydrosulfide, disulfide and trisulfide salts that spontaneously hydrolyse to release H2S [[14], [15], [16]], as well as H2S donors such as GYY 4137 [[17], [18], [19]], HS-NSAIDs [20], S-diclofenac [21], DATS-MSN [22] and ammonium tetrathiomolybdate [23].
The production of superoxide by the mitochondrial respiratory chain upon reperfusion of ischemic tissue is a key initiator of the oxidative damage that underlies IR injury [[24], [25], [26]]. Consequently, there is considerable interest in developing H2S-donors that protect against IR injury by decreasing mitochondrial oxidative damage [[27], [28], [29], [30]]. Candidate protective mechanisms include free-radical scavenging by H2S [[31], [32], [33], [34]] or via the reversible S-thiolation of protein cysteine residues to form a persulfide (r-SPSH) [35,36] that can prevent irreversible oxidative damage to cysteine residues and may enhance the protective activity of some proteins [37,38]. Alternatively, H2S is a reversible inhibitor of cytochrome c oxidase [39]. Thereby, H2S may lower the proton motive force, a major driver of mitochondrial superoxide production upon reperfusion following ischemia [24,25], but whether this contributes to its protection against IR injury is not known.
The mitochondria-targeted H2S donors AP39 and AP123 have also been developed [6,[40], [41], [42], [43]] (Fig. 1A-B). These compounds comprise the mitochondria-targeting lipophilic triphenylphosphonium (TPP) cation [44] coupled via a ten-carbon aliphatic linker to either an anethole dithiolethione moiety in AP39 [41,42] (Fig. 1A), or an hydroxythiobenzamide moiety for AP123 [45] (Fig. 1B). These AP39 and AP123 moieties spontaneously hydrolyse to release H2S [[46], [47], [48], [49], [50]]. Furthermore, the initial AP39 hydrolysis product RT01 hydrolyzes further to generate more H2S [43] (Fig. 1A). Due to the TPP component these molecules are rapidly concentrated several hundred-fold within mitochondria potentially leading to the local generation of H2S. These data were interpreted to suggest that the protective effects against IR injury of AP39, AP123 and RT01 are due to H2S release within mitochondria. However, to be effective, mitochondria-targeted H2S donors have to be taken up and deliver H2S rapidly and selectively within mitochondria during the first few minutes of reperfusion to counteract the oxidative damage caused by the burst of superoxide that occurs at the onset of reperfusion [24,25]. Thus, the time available clinically to reperfuse the ischemic tissue to treat heart attack or stroke is short. As rapid release of H2S in vivo within this timeframe was never confirmed [51], any acute protective effects of AP39 and AP123 against IR injury may be unrelated to H2S release.
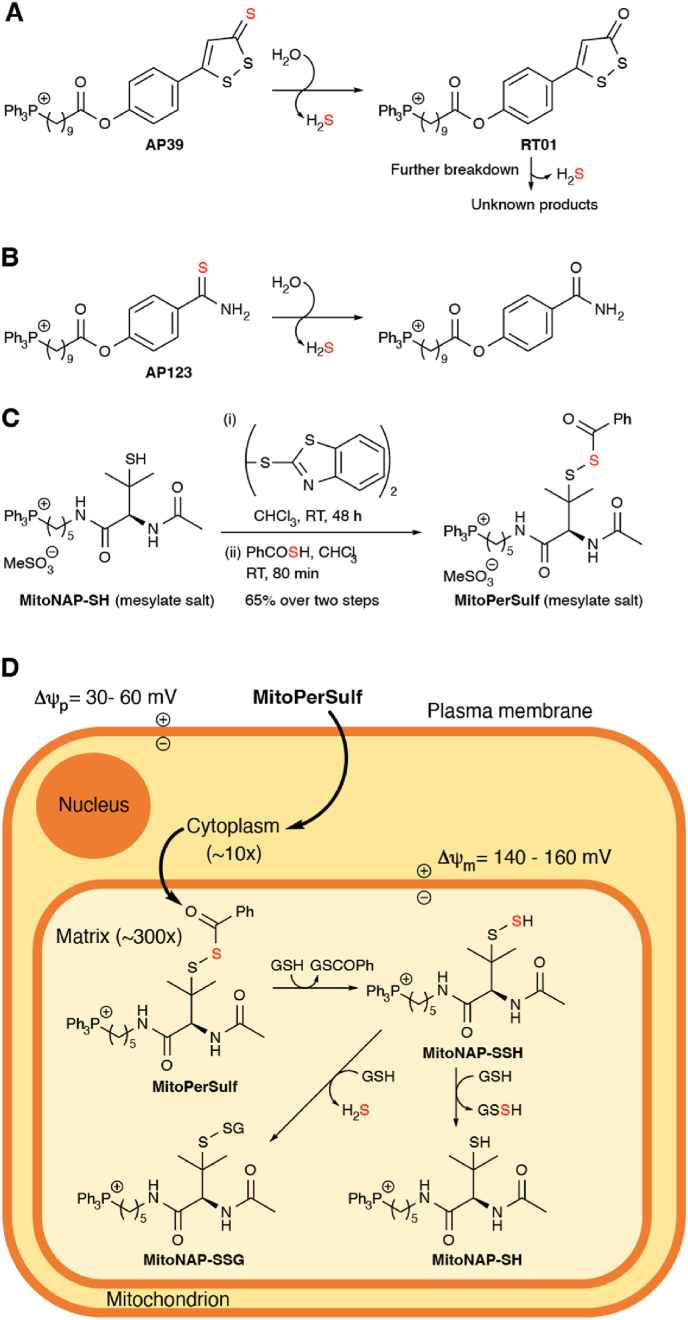
Fig. 1.
Mitochondria-Targeted H2S Donors and MitoPerSulf. (A) Mechanism of H2S release by AP39. The thiocarbonyl group of the 1,2-dithiole-3-thione hydrolyzes to form the corresponding 1,2-dithiole-3-one (RT01) and release H2S. RT01 undergoes further hydrolysis to release H2S and generate unknown products. (B) Mechanism of H2S release by AP123. The thiocarbonyl group of the thiobenzamide hydrolyzes to form the corresponding amide and release H2S. (C) Synthesis of MitoPerSulf. (D) Mitochondria-selective H2S generation by MitoPerSulf. The mitochondria-targeting triphenylphosphonium group (TPP) leads to uptake of MitoPerSulf into mitochondria where the benzoyl thioester is cleaved by reaction with thiols to generate the unstable persulfide, MitoNAP-SSH that forms persulfides with mitochondrial thiols. These persulfides will then rapidly generate H2S and disulfides by reaction with other thiols. For simplicity, reactions with mitochondrial protein thiols are omitted and only reactions with GSH are shown.
Therefore, here we set out to develop a mitochondria-targeted agent that rapidly and selectively released H2S solely within mitochondria and could thus be administered upon reperfusion to prevent IR injury. Here we describe the development, assessment and mechanism of action of MitoPerSulf, a mitochondria-targeted molecule that rapidly releases H2S within mitochondria in vivo and is protective against cardiac IR injury when administered at reperfusion.
2. Results
2.1. Design and synthesis of the rapid H2S releasing agent MitoPerSulf
To generate a molecule that rapidly and selectively releases H2S within mitochondria, we exploited the mitochondrial membrane potential-dependent accumulation of TPP cations, the chemistry of persulfides and the high mitochondrial concentration of protein and glutathione (GSH) thiols, which are particularly reactive due to the elevated matrix pH [52]. A mitochondria-targeted persulfide should react rapidly with intramitochondrial thiols to generate persulfides that react further with thiols to generate H2S and disulfides [10]. Due to its instability, we protected the persulfide by synthesizing it as a stable thioester with a benzoyl group, that will be rapidly removed by reacting with thiols within mitochondria. The rapid deprotection of the persulfide in vivo is essential for the timely generation of H2S. The persulfide benzoyl thioester enables this because the low pKa of the persulfide (∼5.45) [53] makes it a good leaving group [54,55], as has been demonstrated previously [10]. To ensure rapid deprotection of the persulfide by thiol attack at the thioester carbonyl, rather than at the α-sulfur atom to form thiobenzoate and a mixed disulfide, we chose a penicillamine-based substituted tertiary persulfide that is sterically constrained at the α-sulfur atom [10]. By conjugating this moiety to a TPP cation via a five-carbon aliphatic linker we constructed a mitochondria-targeted penicillamine-based protected persulfide, MitoPerSulf (Fig. 1C). The synthesis of MitoPerSulf involved modifying MitoNAP-SH, a late-stage intermediate used in the synthesis of MitoSNO [56] by converting it to a mixed disulfide with 2,2ʹ-dithiobis(benzothiazole) and then displacing the 2-mercaptobenzothiazole with thiobenzoic acid [10] (Fig. 1C). It is anticipated that the benzoyl thioester would first be rapidly cleaved by thiols within mitochondria, thus generating the unstable persulfide MitoNAP-SSH that should then transiently persulfidate mitochondrial thiols which then react further with other thiols to release H2S (Fig. 1D).
2.2. Activation of MitoPerSulf by glutathione in vitro
As GSH is the most abundant small molecule thiol within mitochondria, we assessed the activation of MitoPerSulf in vitro by reacting it with a 2-fold excess of GSH. This should be sufficient to activate MitoPerSulf, while still allowing MitoNAP-SSH to persist for analysis (Fig. 2, S2). We also used a 10-fold excess of GSH to better mimic the thiol concentration within mitochondria in vivo [57] (Fig. 2, S2). To trap the unstable thiol intermediates such as MitoNAP-SSH, we quenched the reaction with excess iodoacetamide (IAM) [58,59], followed by LC-MS/MS analysis to detect the carbamidomethylated (CAM) thiol adducts and other reaction products (Fig. S1). This analysis revealed the rapid formation of a benzoyl thioester of GSH that was complete within 1 min (GSCOPh; Fig. S2A). We also detected the uncapped persulfide MitoNAP-SSH as MitoNAP-SS-CAM, which was rapidly formed within 1 min and subsequently declined over time (Fig. S2B). These findings are consistent with the rapid activation of MitoPerSulf by thiols cleaving the benzoyl thioester to generate MitoNAP-SSH (Fig. 1D). Once formed, reaction of MitoNAP-SSH with other thiols (in this case GSH) could in principle occur at the α-sulfur to generate the disulfide MitoNAP-SSG with H2S release, or at the β-sulfur to generate MitoNAP-SH and glutathione persulfide (GSSH) (Fig. 2A). Formation of GSSH, detected as the GSS-CAM adduct, was rapidly generated in the presence of GSH and then declined over time (Fig. S2D), consistent with the initial formation of GSSH from MitoNAP-SSH that subsequently reacts with GSH to generate GSSG and H2S (Fig. 1D). The MitoNAP-SSG adduct also increased, albeit more slowly, over time (Fig. S2C), consistent with the subsequent disulfide exchange of MitoNAP-SH and GSSG. We also observed a slight increase in the MitoNAP-S-CAM adduct over time (Fig. S2E), while the GS-CAM adduct only decreased at the lower GSH concentration (Fig. S2F). The lag in formation of IAM adducts of GSSH relative to those of MitoNAP-SSH (Fig. S2G), upon reaction of MitoPerSulf with GSH are consistent with the early formation of MitoNAP-SSH, followed later by the formation of GSSH. Incubation of MitoPerSulf, with a 2-fold excess of GSH generated a little of the GSS-CAM adduct over time, measured as the GSS-CAM/GS-CAM ratio (Fig. S2H), but with a 10-fold excess of GSH there was no increase in GSS-CAM over time, consistent with the rapid reaction of GSSH with thiols. Only GS-CAM, and MitoNAP-S-CAM were observed when MitoNAP-SH was incubated with different concentrations of GSH (data not shown). The relative changes in all these species over time are shown in Fig. 2B and C. Together these data indicate that steric hindrance of the methyl groups prevents GSH reaction at the α-sulfur of MitoNAP-SSH, and that the main pathway is via attack of GSH on the β-sulfur (Fig. 2A) [10].
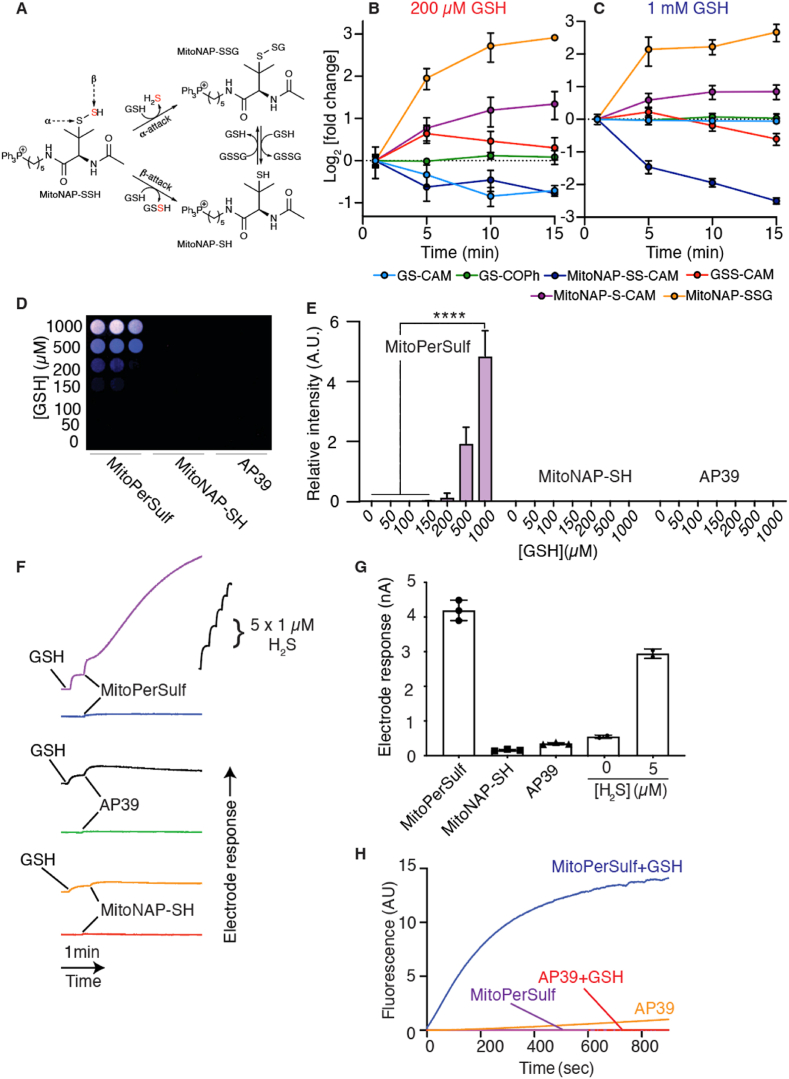
Fig. 2.
Characterization of Reaction Products of MitoPerSulf and GSH. (A) Schematic of the reaction of GSH with MitoNAP-SSH at the α− or β−sulfur. (B, C) Time course of all intermediary species analyzed in Fig. S2. Data are represented as log2 of the fold-change relative to the level at t = 1 min for reaction with 200 μM (B) or 1 mM GSH (C). (D, E) Lead acetate detection of free H2S production from reaction of MitoPerSulf (100 μM) and various concentration of GSH in 25 mM HEPES buffer (pH 7.4) at 37 °C. (D) shows a typical result using a false colour scale. (E) Shows the relative intensity (mean ± s.e.m., n = 3, ****p < 0.001, one-way ANOVA). (F, G) Detection of free H2S production from reaction of MitoPerSulf, MitoNAP-SH or AP39 (70 μM each) and excess GSH (700 μM) in 25 mM HEPES buffer (pH 7.4) at 23 °C performed using H2S-selective micro electrode. (F) Representative traces of electrode response against time. (G) Calibrating the electrode response using anaerobically prepared Na2S enabled the [H2S] produced after 30 min to be determined. All experiments were performed in triplicates and results are represented as the mean ± s.e.m., n = 3 (****p < 0.001, one-way ANOVA). (H) Fluorescent detection of H2S using WSP-5. MitoPerSulf or AP39 (20 μM) were incubated in 25 mM HEPES buffer (pH 7.4) with WSP-5 (10 μM). Where indicated 200 μM GSH was added. The traces are from a typical experiment performed in triplicate. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Our hypothesis was that MitoNAP-SSH should react with thiols to generate free H2S. This was confirmed by assessing H2S diffusion through air to a lead acetate impregnated filter paper to form lead sulfide (Fig. 2D and E). In contrast, the production of H2S by AP39, even in the presence of GSH, was negligible over this time scale (Fig. 2D and E). Generation of H2S by MitoPerSulf in the presence of GSH was further demonstrated using an H2S electrode (Fig. 2F and G). Again, the production of H2S by AP39 over this time scale was negligible, even in the presence of GSH (Fig. 2F and G), consistent with its proposed mechanism as a slow-release H2S donor activated by hydrolysis [60]. Finally, we used the fluorescent probe WSP-5, in which a disulfide undergoes nucleophilic attack by HS− followed by cyclization to a fluorescent product [61]. Neither MitoPerSulf nor AP39 showed initial generation of H2S, but upon addition of GSH MitoPerSulf rapidly generated H2S, while AP39 did not (Fig. 2H).
The proposed reaction scheme for MitoPerSulf with thiols, illustrated using GSH, is shown (Fig. 3). In summary, the spontaneous production of H2S by MitoPerSulf and AP39 is very low, but in the presence of excess thiols, as occurs in vivo, MitoPerSulf rapidly generates H2S, while AP39 does not.
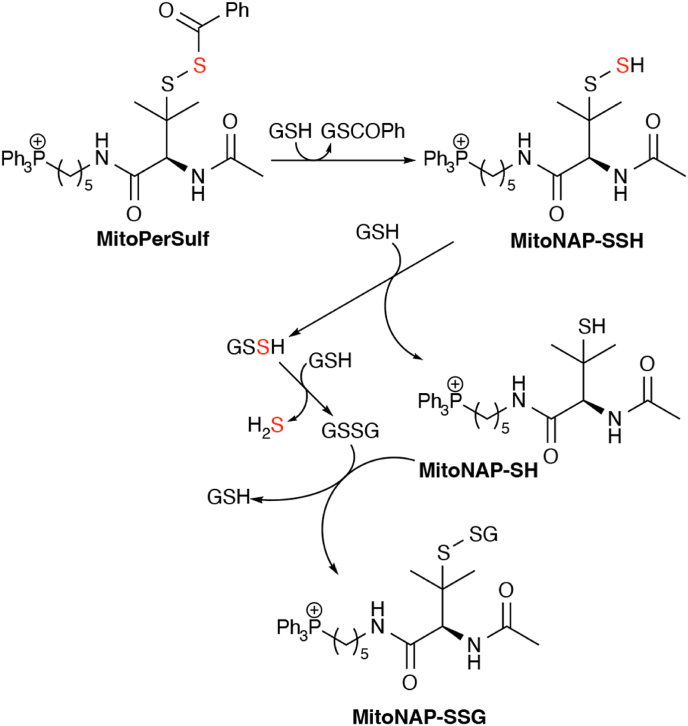
Fig. 3.
Reaction Mechanism of Thiol-dependent H2S Release by MitoPerSulf. MitoPerSulf reacts with thiols (illustrated here solely with GSH) to rapidly form the persulfide, MitoNAP-SSH and GSCOPh. MitoNAP-SSH is further transformed by reacting with GSH to form GSSH and MitoNAP-SH. In the presence of excess GSH, GSSH forms H2S via formation of GSSG which can react with MitoNAP-SH to form the MitoNAP-SSG.
2.3. MitoPerSulf is taken up by mitochondria and cells rapidly forming H2S
To be an effective mitochondrial H2S-generating agent, MitoPerSulf has to be accumulated by mitochondria in response to the membrane potential (Δψ). Using a TPP-selective electrode we showed that MitoPerSulf was accumulated by energized mitochondria and that the dissipation of Δψ with the uncoupler FCCP released the TPP-containing moiety (MitoNAP-SH) from the mitochondria (Fig. 4A). The Δψ-dependent uptake of MitoPerSulf by mitochondria was further confirmed by RP-HPLC analysis of mitochondria pelleted after incubation with MitoPerSulf (Fig. 4B). Only MitoNAP-SH was detected by HPLC following incubation of energized mitochondria with MitoPerSulf, consistent with reduction of MitoPerSulf to MitoNAP-SH by thiols within mitochondria (Fig. 4B). The effect of MitoPerSulf on respiration of isolated mitochondria showed that at high concentrations MitoPerSulf inhibited respiration, while the same concentration of MitoNAP-SH did not (Fig. S3A), suggesting that the effect of MitoPerSulf on respiration was most likely due to the generation of H2S, as is explored in detail later.
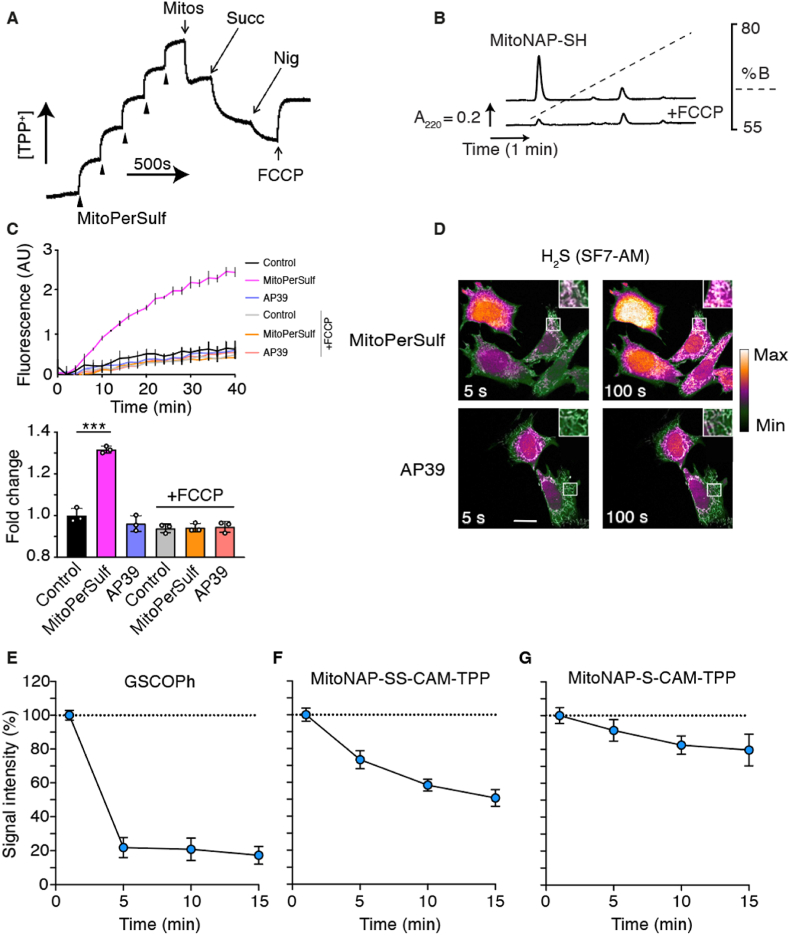
Fig. 4.
Accumulation and Metabolism of MitoPerSulf in Mitochondria and Cells. (A) Accumulation of MitoPerSulf by energized mitochondria. MitoPerSulf was assessed using a TPP-selective electrode, calibrated by additions of 5 × 1 μM MitoPerSulf followed by rat liver mitochondria (Mitos) (1 mg protein/ml) in the presence of rotenone (4 μg/ml), and then by succinate (Succ; 10 mM), followed by nigericin (Nig; 0.5 μM) and FCCP (0.5 μM) as indicated. (B) Uptake of MitoPerSulf into mitochondria analyzed by RP-HPLC. Mitochondria were incubated with succinate and rotenone as in (A) and MitoPerSulf (5 μM) ± FCCP (0.5 μM) in KCl buffer (pH 7.4) for 3 min at 37°C. Panels (A) and B) are typical results of experiments performed in triplicate. Peak identities were confirmed by use of MitoNAP-SH and MitoPerSulf standards. (C) Effect of MitoPerSulf, and AP39 on H2S generation within isolated mitochondria. Rat heart mitochondria (0.5 mg protein/ml) were incubated with 20 μM MitoPerSulf, AP39 or EtOH (vehicle) in KCl buffer as above in the presence of WSP-5 (20 μM) ± FCCP (0.5 μM). The upper panel shows the development of WSP-5 fluorescence over time using a fluorescent plate reader. The lower bar chart shows the fold change of the fluorescence signal at 20 min compared to control. Data are mean ± s.e.m. (n = 3) (***p < 0.001 by Student's t-test). (D) Representative confocal microscopy live cell imaging of mitochondrial H2S formation by MitoPerSulf using the SF7-AM probe. Mouse embryonic fibroblasts stably expressing the mitochondrial matrix-targeted derivative of the red fluorescent protein (mScarlet) were stained with SF7-AM (2.5 μM) for 40 min, washed and placed in imaging medium. After obtaining the base line signal the indicated compounds were added (20 μM) and imaging was continued. Representative images are shown at indicative time points. Heat map-based green to white H2S fluorescence (SF7-AM; λem = 526 nm emission) and mitochondrial fluorescence (grey; mScarlet λem 647 nm), are overlaid. Co-localized signal of the formation of H2S in functional mitochondria is highlighted in zoom insets (n = 3, scale bar = 20 μm). (E, F, G) MitoPerSulf metabolism within mitochondria. RHM (1 mg protein/mL) were incubated ± MitoPerSulf (10 μM) in KCl buffer at 37°C supplemented with succinate (10 mM) and rotenone (4 μg/ml). Aliquots were centrifuged (1 min at 17 000×g) at the indicated times and precipitated mitochondrial pellets were rapidly resuspended in 50 μL of 40 mM TPP-IAM (200 mM stock solution in MeOH or DMSO) in 100 mM HEPES buffer (pH 7.8). Samples were vortexed and sonicated in a sonic bath at RT in dark for 20 min. Next, 200 μL of ACN were added and samples were placed at −20 °C for 5 min. Samples were centrifuged (10 min at 17 000×g) to pellet proteins and 100 μL of supernatant were retrieved and combined with 400 μl of MS-grade H2O containing FA (0.1%). Subsequently, samples were diluted (in 20 % ACN 0.1% FA) as required and analyzed by LC-MS/MS to to assess levels of GSCOPh (E), MitoNAP-SS-CAM-TPP (F) and MitoNAP-S-CAM-TPP (G). Data are mean ± s.e.m. (n = 3). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To investigate the generation of H2S within mitochondria, we next measured H2S release by MitoPerSulf when incubated with mitochondria in the presence of the fluorescent H2S sensor WSP-5 (Fig. 4C). This showed that when succinate was added to drive MitoPerSulf accumulation within mitochondria H2S production rapidly increased, but that addition of FCCP to prevent MitoPerSulf uptake blocked H2S generation. In contrast, AP39 did not generate H2S within mitochondria over this time scale. To examine whether MitoPerSulf can induce the formation of H2S within cells, we stably transfected mouse embryonic fibroblasts with a mitochondria-targeted version of the red fluorescent protein mScarlet and used the fluorescent H2S sensor SF7-AM, that tends to distribute evenly throughout the cell [62] (Fig. S3B). This showed the rapid and time-dependent formation of H2S from MitoPerSulf (Fig. S3B), but limited formation from AP39 over this time scale (Fig. 4D). Colocalization of the SF7-AM and mitochondrial matrix-targeted mScarlet signals showed that the H2S signal from MitoPerSulf was present in mitochondria (Fig. 4D, inset), but also diffused throughout the cell (Fig. 4D). Together these data are consistent with rapid accumulation of MitoPerSulf within mitochondria where it generates H2S some of which may diffuse out to the rest of the cell.
2.4. MitoPerSulf metabolism within mitochondria
To analyze the interaction of MitoPerSulf with mitochondrial thiols we incubated isolated mitochondria with MitoPerSulf and then analyzed extracts by LC-MS/MS. This demonstrated the initial formation of the benzoylated GSH, GSCOPh, which then rapidly decreased (Fig. 4E). In order to increase the sensitivity of the LC-MS/MS detection for the lower amounts of MitoPerSulf metabolites being analyzed, we replaced IAM as the quenching reagent with IAM-TPP [63], an IAM derivative modified to incorporate a TPP cation. Trapping these species as X-CAM-TPP derivatives will introduce a fixed positive charge via the TPP moiety greatly enhancing detection sensitivity by MS (Fig. S1). Using this strategy, we demonstrated the initial formation of MitoNAP-SSH (detected as MitoNAP-SS-CAM-TPP) (Fig. 4F) and MitoNAP-SH (detected as MitoNAP-S-CAM-TPP) (Fig. 4G) within mitochondria. We also attempted to use IAM-TPP to detect GSSH (detected as GSS-CAM-TPP) within mitochondria incubated with MitoPerSulf, but the amounts detected were not significantly above baseline, consistent with the rapid metabolism of GSSH to H2S.
MitoNAP-SSH may also directly persulfidate protein thiols. To assess this possibility, we used recombinant Cofilin-1 protein in vitro, which contains 4 Cys residues (Fig. S4A), and is known to be persulfidated under certain conditions within cells [64]. We incubated Cofilin-1 with MitoPerSulf and GSH to generate MitoNAP-SSH and then assessed protein persulfidation by trapping with IAM, followed by trypsin digestion and LC-MS analysis to detect the persulfidated peptides (Fig. S4B). We detected two persulfidated peptides at Cys residues C39 and C139 in response to MitoPerSulf (Figs. S4C and D). We were not able to reliably detect persulfidation of cysteine residues C80 and C147. By comparing the relative amounts of the persulfidated Cys residues with those that were free to react with IAM we could estimate the extent of persulfidation as between 10 and 20% under these conditions (Figs. S4C and D). This suggests that MitoPerSulf can potentially lead to protein persulfidation. To assess if MitoPerSulf could lead to protein persulfidation within mitochondria, we incubated heart mitochondria with MitoPerSulf under the same conditions as in Fig. 4 and then analyzed for protein persulfidation using a fluorescence tag switch method [38] followed by analysis of incorporated fluorescence after separation of proteins by SDS-PAGE. However, we did not find consistent increases in fluorescent labelling of individual protein bands on the gels above control (Fig. S5). Furthermore, the negligible amounts of GSSH found when MitoPerSulf was incubated in vitro with excess GSH (Fig. 2E) and the lack of detection of GSSH within mitochondria incubated with MitoPerSulf make it likely that the majority of protein persulfides formed by MitoPerSulf are transient and react further to generate H2S. Together these data are consistent with the rapid but transient formation of persulfides from MitoPerSulf within mitochondria that rapidly react further with thiols to form H2S.
2.5. Distribution and cardioprotective effects of MitoPerSulf on acute IR injury in vivo
We next used an in vivo mouse model of cardiac IR injury to investigate the potential protective effects of MitoPerSulf. First, we analyzed the cardiac uptake of MitoPerSulf in vivo in mice following a bolus, intravenous tail vein injection of MitoPerSulf (0.2 mg/kg) with the tissue distribution analyzed by LC-MS/MS spectrometry. Tissues were reduced by addition of dithiothreitol (DTT) during extraction to convert any residual MitoPerSulf derivatives to MitoNAP-SH, thus data are reported as MitoNAP-SH content. As expected from similar TPP-based compounds [65], MitoPerSulf and any derivatives formed over this time scale were rapidly cleared from the plasma (Fig. S6A), leading to their rapid accumulation in the heart (Fig. S6B) as well as into the kidney and liver, with less penetration into the brain, followed by their gradual clearance from these tissues over time (Figs. S6B and C). Therefore, MitoPerSulf is taken up rapidly into the heart (Fig. S6B) following i.v. injection, making it suitable as a potential protective agent against cardiac IR injury for administration upon reperfusion.
Next, we assessed the protective effects of MitoPerSulf against cardiac IR injury by performing left anterior descending (LAD) coronary artery ligation in mice, followed by reperfusion and assessment of infarct size (Fig. 5A). Infusion of MitoPerSulf for 20 min starting 5 min before reperfusion resulted in a dose-dependent reduction of infarct size that reached a maximum at 10 μg/kg/min (Fig. S6D). Comparison of the most effective dose with the same concentration of MitoNAP-SH showed that MitoPerSulf was protective while MitoNAP-SH was not (Fig. 5B). As MitoNAP-SH is structurally very similar to MitoPerSulf and it is produced upon metabolism of MitoPerSulf within mitochondria this suggests that the protection against cardiac IR injury by MitoPerSulf is due to its rapid generation of H2S within mitochondria. Furthermore, as the uptake of MitoNAP-SH into mitochondria in vivo will be to a very similar extent as for MitoPerSulf, the protective effects of MitoPerSulf are not due to the disruption of mitochondrial function by the alkylTPP molecule.
Fig. 5.
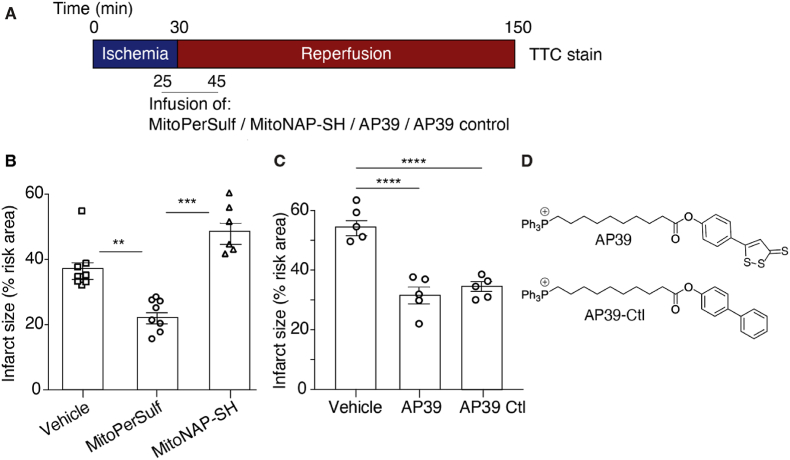
Cardioprotective effect of MitoPerSulf on cardiac IR. (A) Schematic of cardiac IR injury experiments. Mice were subjected to 30 min ischemia by ligation of the left anterior descending coronary artery (LAD) ligation followed by 120 min of reperfusion. Compounds (10 μg/kg) or vehicle (0.5% DMSO) were infused into the tail vein at 5 μL/min for 20 min starting 10 min before reperfusion. Myocardial infarct size was then determined as a percentage of the area at risk quantified from a single mouse by triphenyl tetrazolium chloride (TTC) stain. (B, C). Effect of compounds on infarct size. (B) MitoPerSulf or MitoNAP-SH. (C) AP39 or AP39 control compound. *p < 0.05, **p < 0.01, one-way ANOVA, n = 5–8 ± s.e.m. (D) Structures of AP39 and the AP39 control compounds.
Acute protection against cardiac IR injury has been reported when AP39 is administered upon reperfusion [40,41,66]. We confirmed this protection here (Fig. 5C). The tacit assumption in these earlier publications was that the mode of action of AP39 was through H2S release in vivo, but this was not demonstrated. AP39 releases H2S far more slowly than MitoPerSulf within mitochondria (Fig. 4) making it unlikely that the protection against acute cardiac IR injury by AP39 is due to rapid H2S release and may instead be due to off-target effects. To explore this possibility, we made a chemically similar control version of AP39 that does not release H2S (Fig. 5D). AP39's reactive group is comprised of two planar highly conjugated rings capable of conjugation to each other at the oxygen atom of the ester. These rings are linked by a rotatable bond allowing other conformations (Fig. S7A). The planar 1,2-dithio-3-thione is weakly aromatic [67,68] and carbon and sulfur have very similar electronegativities. Therefore, we reasoned that a planar aromatic phenyl ring with the same number of heavy atoms would mimic its size, shape, and overall lipophilicity well (Fig. S7A). To confirm this the logP was calculated for the reactive head group of AP39 and the corresponding phenyl analogue using a consensus model built on Chemaxon and Klopman et al. [69] models using the PHYSPROP database (Fig. S7A). Calculating only the head group simplifies the calculation and avoids complications associated with the modelling of logPs of single ions [70,71]. The similarity of the logPs calculated for the head groups gave confidence that a control with the same TPP targeting group and alkyl linker would have similar physicochemical properties and thus uptake into mitochondria in vivo (Fig. S7B). RP-HPLC confirmed this similarity (Fig. S7C). The AP39 control compound was indeed as protective against cardiac IR injury as AP39 in the LAD model (Fig. 5C), further confirming that the protection afforded by AP39 is not due to the release of H2S, but to off-target effects, which may be due to accumulation of the hydrophobic alkylTPP molecule within mitochondria affecting organelle function. Of course, the slow release of H2S by AP39 may protect against tissue damage that occurs in the hours following reperfusion, but this was not explored here. In contrast, the protection against cardiac IR injury by MitoPerSulf, which rapidly releases H2S, but not by the chemically closely related compound MitoNAP-SH which does not release H2S, suggests that the rapid release of H2S within mitochondria in the heart is protective against IR injury.
2.6. Mechanism of protection by MitoPerSulf against acute cardiac IR injury
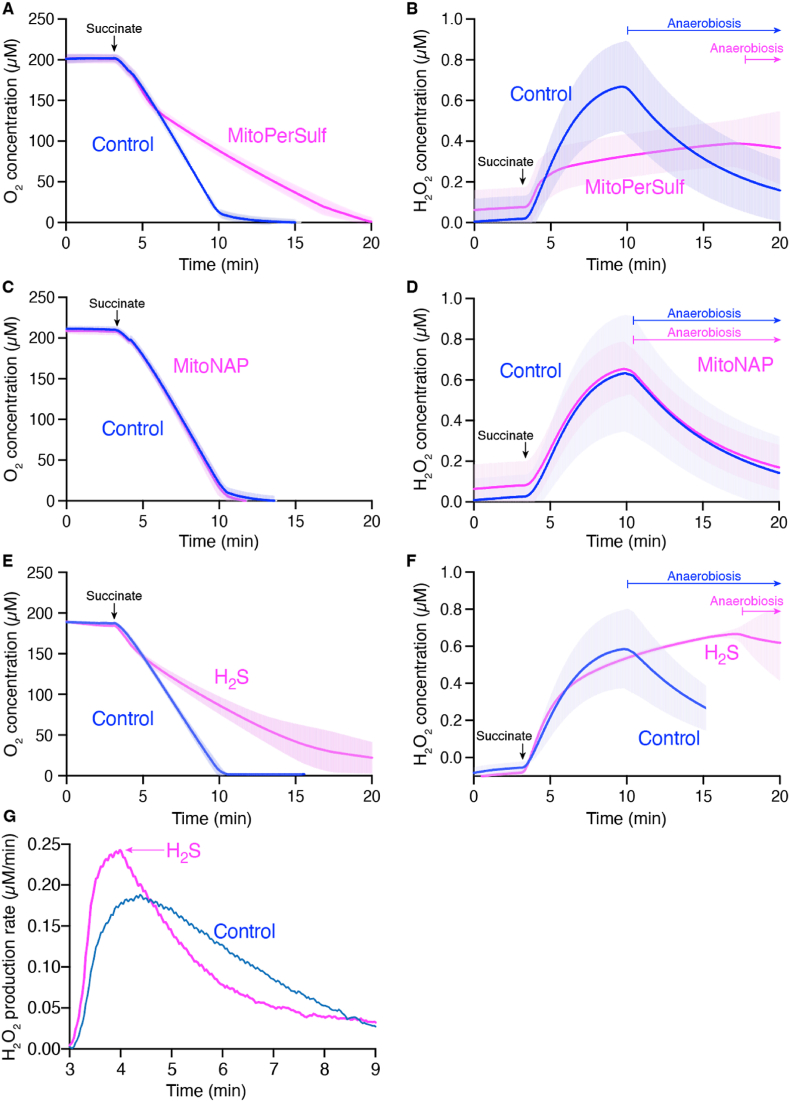
We next explored the mechanism of protection against cardiac IR injury by the rapid burst of H2S generation produced by MitoPerSulf within mitochondria. Mitochondrial superoxide production by reverse electron transport (RET) upon reperfusion is thought to initiate the damaging cycle that leads to tissue damage [24,25]. To explore whether H2S could alter this process, we investigated the effect of MitoPerSulf on superoxide production by RET in isolated mitochondria (Fig. 6). Addition of MitoPerSulf decreased respiration compared to control and this inhibitory effect of MitoPerSulf increased as the oxygen concentration diminished, thereby extending the time taken to remove all the oxygen from the incubation (Fig. 6A). In parallel, we measured the extent of superoxide production by RET through the generation of H2O2. In control mitochondria there was considerable H2O2 generation that slowed as the oxygen level fell (Fig. 6B). Following anaerobiosis the fluorescence due to Resorufin decreased, due to its enzymatic reduction to dihydroresorufin upon anaerobic conditions [72], that is likely to disrupted by the presence of H2S [73]. In contrast, addition of MitoPerSulf greatly decreased H2O2 generation, in parallel with its effect of on respiration (Fig. 6A). The control compound MitoNAP-SH had no effect on respiration (Fig. 6C), or on the generation of H2O2 (Fig. 6D). Thus, the effect of MitoPerSulf on respiration and on the generation of H2O2 is not due to any non-specific effects of the accumulation of the TPP cation on the mitochondria but instead is due to the generation of H2S within mitochondria. To investigate this further, we incubated mitochondria in the presence of H2S by adding Na2S, which had a very similar effect on mitochondrial respiration (Fig. 6E). The addition of H2S also slowed the rate of H2O2 generation (Fig. 6F), compared to the control incubation. To better illustrate the effect of adding H2S on H2O2 generation, we plotted the slope of the data in Fig. 6F against time, which showed that the rate of H2O2 generation decreased immediately upon addition of H2S (Fig. 6G), while in contrast in the control incubation the rate of H2O2 generation decreased gradually as the O2 concentration decreased. These data suggest that the generation of H2S from MitoPerSulf within mitochondria disrupts respiration and thereby prevents mitochondrial superoxide production by RET.
Fig. 6.
Effect of MitoPerSulf on mitochondrial respiration and superoxide production by RET in vitro. Rat heart mitochondria RHM (1 mg protein/2 mL) were resuspended in KCl buffer in an Oroboros Oxygraph-2k system, and respiration and H2O2 generation by RET were initiated by adding 10 mM succinate followed 1 min later by vehicle, or 10 μM MitoPerSulf, MitoNAP or Na2S. Oxygen consumption (A, C, E) and H2O2 formation by RET (B, D, F) were recorded. The effect of vehicle on these was compared with that of 10 μM MitoPerSulf (A, B), MitoNAP (C, D) or Na2S (E, F). The first derivative of the data in panel F are replotted in panel G. Data are represented as means ± s.e.m. (shading) of n = 3 of one mitochondrial preparation, which was repeated with the same outcome on 4 independent mitochondrial preparations.
3. Conclusions
The role of H2S donors as potential therapies has attracted considerable interest. In particular, it has been proposed that these donors could be used to prevent the damage associated with IR injury in heart attack and stroke by selective targeting to mitochondria. However, for the clinical treatment of IR injury it is necessary to add the protective agent upon reperfusion. While the targeting of compounds to mitochondria by conjugation to the lipophilic TPP cation is well established [44], the mitochondria-targeted H2S donors developed to date such as AP39 release H2S slowly, suggesting that any acute protective effects are not due to H2S release. Thus, the potential therapeutic utility of acute release of H2S within mitochondria remains unexplored. Here we addressed this by developing MitoPerSulf, a mitochondria-targeted H2S donor. We used a TPP cation to target MitoPerSulf to mitochondria in vivo, following intravenous administration. By adapting persulfide chemistry we were able to mask a reactive persulfide moiety that then rapidly releases H2S within mitochondria. This development opens the way for the development of further donors designed to rapidly release H2S within mitochondria.
Most importantly, we showed that MitoPerSulf was acutely protective in the in vivo LAD model of cardiac IR injury. In doing this, we utilized appropriate control compounds to show that the protective effects of MitoPerSulf were due to rapid H2S release and not to off-target effects of the mitochondria targeting TPP moiety. We also demonstrated, through the use of an appropriate control compound, that the reported protective effects of AP39 against IR injury were due to off-target effects resulting from the physicochemical properties of molecules that have a targeting TPP moiety linked by a long alkyl chain to a nonpolar biaryl system. Thus, for the first time we have demonstrated that the acute generation of H2S within mitochondria is a viable therapeutic strategy against IR injury.
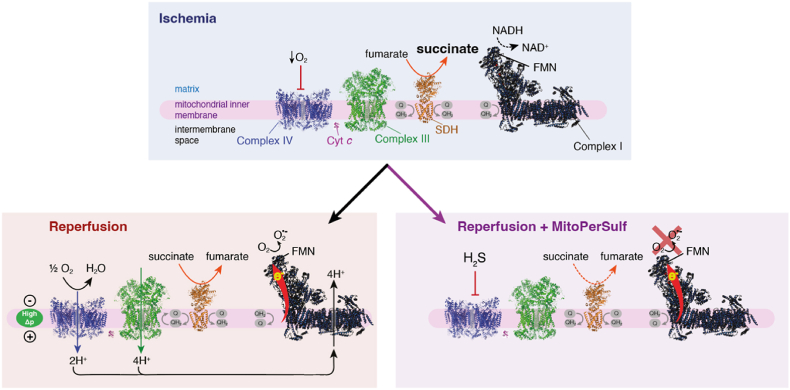
The mechanism of protection by acute H2S generation within mitochondria was also determined. H2S is well established to bind selectively and reversibly to cytochrome c oxidase and thereby to inhibit mitochondrial respiration. We showed that MitoPerSulf acted in this way by rapidly inhibiting respiration and that its inhibitory potency increased as the oxygen concentration decreased. This is consistent with the well-established competition between O2 and H2S at cytochrome c oxidase. This inhibition of respiration will lower the mitochondrial protonmotive force and should thereby prevent the ability of mitochondrial complex I to generate superoxide by RET. We demonstrated this in isolated mitochondria with both MitoPerSulf and with pure H2S. Thus, we suggest that the protective effects of acute generation of H2S within mitochondria against IR injury is largely by preventing the burst of superoxide production by complex I upon reperfusion (Fig. 7). Even so, it is important to note that additional protective effects of H2S, such as by preventing overoxidation of protein thiols, are not excluded. The reversible inhibition of cytochrome c oxidase by H2S is similar to that by nitric oxide (NO) [56] and suggests that acute generation of NO within mitochondria may also be protective against IR injury by a similar mechanism. Indeed, in earlier work we developed a mitochondria-targeted NO donor (MitoSNO) which was acutely protective against IR injury [56]. While we interpreted this as being due to the selective S-nitrosation of Cys 39 on complex I, thereby preventing RET, the degree of exposure of this Cys residue in vivo has been reassessed [63]. Thus, the protection against IR injury by MitoSNO may have been, at least in part, due to the reversible inhibition of cytochrome c oxidase decreasing respiration and thereby decreasing mitochondrial superoxide production at complex I upon RET.
Fig. 7.
Schematic of effect of rapid H2S generation by MitoPerSulf on cardiac IR injury in vivo. The upper panel shows the accumulation of succinate during ischemia. The lower left panel shows that this succinate then drives mitochondrial superoxide production by reverse electron transport (RET) upon reperfusion that depends on the development of a high proton motive force (Δp) upon reperfusion. The lower right panel shows that the H2S generated by MitoPerSulf reversibly inhibits complex IV/cytochrome c oxidase thereby preventing the build-up of Δp upon reperfusion and thus blocking superoxide production by RET.
In summary, we have developed the first approach to rapidly and selectively generate H2S within mitochondria in vivo. Using this approach, we were able to demonstrate that H2S is acutely protective against IR injury by reversibly inhibiting respiration at cytochrome oxidase and thereby preventing superoxide production at complex I.
4. Materials and methods
4.1. Animals
All procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and the University of Cambridge Animal Welfare Policy. Procedures were approved to be carried out under the Project Licenses: 70/7963, 70/8238. Female Wistar rats, or male or female C57BL/6J mice (both Charles River Laboratories, UK) were maintained in pathogen-free facilities with ad libitum chow and water until being 8–20 weeks of age for experimental use.
4.2. Chemicals
All buffers used in this study were prepared with salts of highest purity using MiliQ water (18.2 μ ), supplemented with Chelex-100 resin. All chemicals were obtained from commercial sources, except TPP-IAM, MitoNAP-SH, MitoPerSulf, AP39 control and (9-carboxynonyl)triphenylphosphonium bromide (Table S1). TPP-IAM and MitoNAP-SH (MitoNAP) were prepared as described previously [56,63]. MitoPerSulf was prepared by converting MitoNAP-SH to a mixed disulfide by reaction with 2,2-dithiobis(benzothiazole) and then displacing the 2-mercaptobenzothiazole with thiobenzoic acid. In brief, AP39 control was prepared by coupling (9-carboxynonyl)triphenylphosphonium bromide, prepared by the method of Thurnhofer et al. [74], to 4-phenylphenol using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI) and 4-dimethylaminopyridine (DMAP). Synthetic procedures for MitoPerSulf and AP39 control are given below. NMR data are deposited at: https://doi.org/10.5525/gla.researchdata.1304.
4.2.1. 5-(2′-Acetylamino-3′-benzoyldisulfanyl-3′-methylbutyrylamino)pent-1-yl]-triphenylphosphonium methanesulfonate MitoPerSulf
MitoNAP mesylate [200 mg, 0.320 mmol, 1 eq., prepared by the method described previously [56], was added to a solution of 2,2′-dithiobis(benzothiazole) (144 mg, 0.432 mmol, 1.35 eq.) in CHCl3 (12 mL). The solution was stirred at RT for 48 h then concentrated under vacuum. Automated flash chromatography [SiO2, dichloromethane-MeOH (99:1) to (70:30)] then gave the mixed disulfide intermediate as a white solid (165 mg, 65 %). The mixed disulfide (120 mg, 0.146 mmol, 1 eq.) was dissolved in CDCl3 (1.9 mL) and thiobenzoic acid (75 μL, 0.59 mmol, 4 eq.) was added. The reaction mixture was stirred at RT for 80 min and the crude mixture was purified by automated flash chromatography [SiO2, dichloromethane-MeOH (99:1) increasing to (70:30)] to provide MitoPerSulf as an oil (115 mg, 100 %). Rf [SiO2, CH2Cl2: MeOH (99:1)]: 0.17. ʋmax (ATR): 3279 (NH), 2931 (CH), 2870 (CH), 1651 (C=O) cm−1. δH (400 MHz, CDCl3): 8.16 (1H, t, J = 5.4 Hz, CH2NH), 8.01–7.99 (2H, m, 2 × ortho-H of benzoyl group), 7.82–7.66 (16H, m, Ph3P and CHNH), 7.59 (1H, broad t, J = 7.5 Hz, para-H of benzoyl group), 7.45 (2H, broad t, J = 7.7 Hz, 2 × meta-H of benzoyl group), 4.68 (1H, d, J = 9.4 Hz, CH), 3.58–3.51 (2H, m, CH2P+), 3.35–3.19 (2H, m, CH2NH), 2.74 (3H, s, SO3CH3), 2.12 (3H, s, COCH3), 1.74–1.61 (6H, m, 3 × CH2), 1.49 (3H, s, CH3), 1.42 (3H, s, CH3). δC (126 MHz, CDCl3): 191.81 (C), 170.69 (C), 169.51 (C), 135.88 (C), 135.16 (d, J = 2.8 Hz, CH), 134.09 (CH), 133.63 (d, J = 10.0 Hz, CH), 130.60 (d, J = 12.6 Hz, CH), 128.91 (CH), 127.96 (CH), 118.46 (d, J = 86.3 Hz, C), 60.21 (CH), 54.37 (C), 39.74 (CH3), 38.50 (CH2), 27.85 (CH2), 27.22 (d, J = 16.9 Hz, CH2), 25.86 (CH3), 25.35 (CH3), 23.33 (CH3), 22.10 (d, J = 43.9 Hz, CH2), 21.88 (d, J = 1.4 Hz, CH2). δP (162 MHz, CDCl3): 24.25 (1P, s). LRMS (ESI+): 657 [M+, 100%], 625 (51), 536 (50), 413 (35). HRMS (ESI+): 657.2350. C37H42N2O3PS2 requires M+ 657.2369.
4.2.2. Synthesis of [9-(4′-phenylphenoxycarbonyl)nonyl]triphenylphosphonium chloride
EDCI (63 mg, 0.330 mmol, 1.5 eq.) was added to a solution of (9-carboxynonyl)triphenylphoshonium bromide (113 mg, 0.220 mmol 1.0 eq.), DMAP (2.0 mg, 0.02 mmol, 0.1 eq.) and 4-phenylphenol (56 mg, 0.330 mmol, 1.5 eq.) in dry dichloromethane (2 mL). After stirring overnight at RT under an atmosphere of argon the solution was diluted with dichloromethane and washed with 1 M hydrochloric acid, brine and then NaHCO3 solution. The organic layer was dried over magnesium sulfate and concentrated under vacuum. Automated flash chromatography [dichloromethane-MeOH (100:0) to (91:9)] gave the phosphonium salt as a white solid. (102 mg, 75%). δH (400 MHz, CDCl3): 7.84–7.72 (9H, m, 6 × ortho and 3 × para H's of PPh3), 7.71–7.62 (6H, m, 6 × meta H's of PPh3), 7.53 (2H, d, J = 8.5 Hz, H-3′ and H-5′), 7.53–7.49 (2H, m, H-2″ and H-6″), 7.43–7.36 (2H, m, H-3″ and H-5″), 7.34–7.27 (1H, m, H-4″), 7.10 (2H, d, J = 8.6 Hz, H-2′ and H-6′), 3.78–3.64 (2 H, m, PCH2), 2.51 (2H, t, J = 7.4 Hz, CH2CO2), 1.68 (2 H, qn, J = 7.4 Hz, CH2CH2CO2), 1.63–1.53 (4H, m, 2 × CH2), 1.40–1.20 (8H, m, 4 × CH2). δC (101 MHz, CDCl3): 172.43 (C), 150.21 (C), 140.39 (C), 138.85 (C), 135.06 (d, J = 3.0 Hz, CH), 133.68 (d, J = 9.9 Hz, CH), 130.55 (d, J = 12.6 Hz, CH), 128.83 (CH), 128.13 (CH), 127.37 (CH), 127.12 (CH), 121.90 (CH), 118.46 (d, J = 85.7 Hz, C), 34.38 (CH2), 30.41 (d, J = 15.6 Hz, CH2), 29.09 (CH2), 29.08 (CH2), 28.99 (CH2), 28.95 (CH2), 24.86 (CH2), 22.68 (d, J = 4.6 Hz, CH2), 22.56 (d, J = 49.8 Hz, CH2). δP (162 MHz, CDCl3) 24.28. HRMS (ESI+): 585.2912. C40H42O2P requires M+ 585.2917.
4.3. RP-HPLC analysis
Reverse phase HPLC (RP-HPLC) on a C18 column (Jupiter 300 Å, Phenomenex) with a Widepore C18 guard column (Phenomenex) and a Gilson 321 pump was used for the separation of MitoPerSulf, MitoNAP-SH and their derivatives. Samples were injected through a 0.22 μm PVDF filter (Millipore) and buffer A (0.1 % trifluoracetic acid (TFA) in water (v/v)) and B (0.1 % TFA/acetonitrile (v/v)) was run with the gradient: 0–2 min, 5 %B; 2–17 min, 5–100 % B; 17–19 min, 100 % B; 19–22, 100-5 % B. Peaks at 220 nm were detected with a Gilson UV/VIS 151 spectrophotometer. MitoPerSulf and MitoNAP-SH stock solution in ethanol were used to identify peak elution times. To assess the stability in aqueous buffer and the reaction with glutathione (GSH), MitoPerSulf was incubated in KCl buffer (120 mM KCl, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM MgCl2 and 5 mM KH2PO4, pH 7.4) ± GSH at room temperature in the dark, and then mixed with buffer B and analyzed by RP-HPLC.
4.4. Detection of H2S by fluorescence sensors
To access the H2S formation from MitoPerSulf, the fluorescent-based sensors WSP-5 was used. Initially, the spectral properties of WSP-5 (10 μM) were analyzed in 25 mM HEPES buffer (pH 7.4) by exposing it to various concentration of MitoPerSulf, MitoNAP-SH, AP39 (0–25 μM) or GSH (0–1 mM) using high precision spectrofluorometer equipped with stirring and heating block (Shimadzu). For quantification studies, experiments with various concentration of MitoPerSulf and GSH in 25 mM HEPES buffer (pH 7.4) were performed in glass bottom 96 well plate by using SpectraMAX platereader (Molecular Devices). WSP-5 was excited at 502 nm and emission recorded at 525 nm.
4.5. Detection of H2S by amperometry
H2S release from MitoPerSulf and GSH was accessed by using H2S sensitive electrode. 5 mm fibre wire H2S microelectrode (WPI) was connected to Apollo 4000 free radical analyzer (WPI) and polarized overnight in 10 mM phosphate buffer saline (Gibco) under the 150 mV until reaching the stabile baseline. Amperometric traces in time were obtained by performing the reaction in 3 mL of 25 mM HEPES (pH 7.8) or 10 mM PBS buffer (pH 7.8) under constant and stable stirring and temperature in a multi-port reaction chamber (WPI). Reaction was performed by injecting the various concentration of GSH (0–1 mM) followed by injecting boluses of different concentrations of MitoPerSulf, MitoNAP-SH or AP39 (0–100 μM) from DMSO-based stock solutions into the reaction chamber. Results were obtained by measuring the difference of maximum signal obtained before and after the injections (p(A)max - p(A)min = Δp(A)) for each experimental condition. The H2S electrode was calibrated using 25 mM HEPES buffer (pH 7.8) and anaerobically prepared solutions of anhydrous and ultra-pure Na2S (Sigma Aldrich Product. Code. 407410) in Chelex-100 treated and argon-purged MiliQ dH2O prepared and used at the same day.
4.6. Detection of diffusible H2S by the lead acetate assay
Release of hydrogen sulfide in the gas phase was assessed using lead (II) acetate [75]. Lead (II) acetate-impregnated filter paper was prepared by soaking clean sheets of Whatman filter paper (# 3030-917) in 20 mM lead (II) acetate in dH2O for 20 min and drying them for 2 h at 50°C. Upon drying, lead acetate impregnated paper was stored protected from light at room temperature in a dry and sealed glass container. In brief, 100 μL of reaction mixture containing 100 μM of MitoPerSulf, MitoNAP-SH or vehicle (EtOH) and different concentrations of GSH ranging from 0 to 1 mM in 25 mM HEPES buffer (pH 7.8) was placed in 96-well plate and covered with lead (II) acetate-impregnated filter paper leaving approximately 5 mm of head space between liquid phase and the filter paper. 96-well plate with samples was incubated at 50°C in the oven for 2 h to allow efficient evaporation and accumulation of H2S in the head space of well plate and after the incubation the filter paper containing developed lead (II) sulfide spots was immediately scanned using bio scanner (HP) and analyzed by densitometry (ImageJ).
4.7. Generation of lentiviral particles and transduction of MEFs
MTS-Scarlet was amplified by PCR with specific oligonucleotides using pMTS_mScarlet_N1(Addgene; #85057) plasmid. This insert was introduced into the pWPXLd-IRES-HygroR lentiviral expression vector, modified versions of pWPXLd (Addgene; #12258), by restriction enzyme digestion with PmeI and BamHI and ligation with T4 DNA ligase (New England Biolabs). Lentiviral particles were generated in HEK293T packaging cells by co-transfection of the lentiviral expression vector with the packaging psPAX2 (Addgene; # 12260) and envelope pMD2.G (Addgene; # 12259) vectors with FuGENE HD (Promega) according to manufacturer's instructions. Mouse embryonic fibroblast cells (MEFs) were transduced with previously generated lentiviral particles with Polybrene (Merck, TR-1003) for 24 h. Transduced cells were then selected for resistance using hygromycin B (Roche, 10843555001) at 50 μg/mL.
4.8. Detection of H2S by fluorescent microscopy
MEFs stably expressing the fluorescent mitochondrial matrix red protein, MTS-mScarlet were grown in high glucose glutaMAX containing DMEM medium supplemented with 10 % FBS, 1 % Streptomycin-Penicillin solution and at 37°C under the atmosphere of 5 % CO2. Upon reaching the 80 % confluency, cells were detached using 0.25 % trypsin and plated in glass bottom 35-mm high μ-Dish (ibidi, Germany) at 3 × 104 cells per dish. After attachment cells were stained with 2.5 μM SF7-AM in complete cell medium for 40 min in dark at 37 °C under the atmosphere of 5 % CO2. After staining, cells were washed three times with phenol red-free full DMEM and mounted on the microscope stage. For some experiments cells were washed and imaged using phosphate buffer saline (PBS). 180 images per sample were obtained during the 900 s of live cell imaging (integration time: 5 s) at 37oC and stimulation of H2S production was initiated by adding the boluses of 20 μM MitoPerSulf or AP39 directly into dishes 10 s upon starting the time-lapse video recording. Fluorescence values were collected every 5 s for 15 min. Images were acquired using a 100x objective of the Nikon Eclipse Ti-E microscope, coupled to an Andor Dragonfly spinning disk confocal system equipped with an Andor Ixon camera, and 488 nm and 561 nm excitation lasers were used for SF7-AM and MTS-mScarlet, respectively. All images were postprocessed under the same parameters using ImageJ software (NIH) and for enhanced visualisation the original SF7-AM fluorescence was presented using the specific heat map projection of signal (ImageJ).
4.9. Mitochondria preparations
Rat liver and heart mitochondria (RLM and RHM respectively) were prepared by homogenization of heart tissue obtained from 10 to 12 weeks old Female Wistar rats (Charles River, UK) that were killed by stunning and cervical dislocation, in STEB buffer (250 mM sucrose, 5 mM Tris-HCl and 1 mM EGTA, pH 7.4). Following homogenization, mitochondria were isolated by differential centrifugation (2 x 2450 × g for 5 min, 2 x 9150 × g for 10 min at 4°C). STE buffer was supplemented with 0.1% fatty acid–free BSA for isolation of RHM. Protein concentration was determined by the bicinchoninic acid (BCA) assay using BSA as a standard.
4.10. Mitochondrial uptake of MitoPerSulf
Mitochondrial uptake of MitoPerSulf was assessed using TPP-selective electrode. The electrode was calibrated with five boluses of 1 μM MitoPerSulf followed by 1 mg/mL of RLM in KCl buffer (120 mM KCl, 10 mM HEPES, 1 mM EGTA, 1 mM MgCl2 and 5 mM KH2PO4, pH 7.4). 5 mM succinate was then added to energize RLM after which, the H+/K+ ionophore nigericin (0.5 μM) and the uncoupler carbonyl cyanide p-trifluoromethoxy- phenylhydrazone (FCCP, 0.5 μM) were added to maximize and collapse the mitochondrial membrane potential, respectively. The uptake was also analyzed with RP-HPLC analysis. RLM were incubated with 5 mM succinate and 4 μg/mL rotenone and 5 μM MitoPerSulf in KCl buffer ± 0.5 μM FCCP to collapse the membrane potential respectively. Compounds in the mitochondria were extracted from mitochondrial pellet with mixture of 20 % Acetonitrile/0.1 % TFA in water (v/v) after 3 min incubation period and detected by RP-HPLC as described above.
4.11. Detection of H2S release from mitochondria
Freshly isolated RHM (0.5 mg protein/sample) were resuspended in KCL buffer containing 10 mM succinate, 20 μM WSP-5 and/or 0.5 μM FCCP and aliquoted at 180 μL/well in glass-bottom black 96-well plate (Greiner, USA). Immediately upon distribution in well plate, samples were supplemented with 20 μM of MitoPerSulf, AP39 or EtOH (vehicle), and transferred in to a platereader. WSP-5-based fluorescence was measured at various time points using 502 nm excitation and 525 nm emission wavelength in SpectraMAX plate reader (Molecular Devices) at 37°C. Each experimental condition is performed in triplicate.
4.12. Mitochondrial respiration and superoxide production in vitro
Oxygen consumption and superoxide production were determined using the high resolution O2k oxygraph (Oroboros Instruments). Freshly isolated RHM (1 mg protein) were resuspended in 2 mL of KCL buffer supplemented with 17.6 U SOD, 8.76 U HRP, 12.5 μM Amplex Red and 3 μM BSA and oxygen consumption and superoxide production were induced by simultaneous addition of 10 mM of succinate in each chamber under the constant stirring and constant temperature (T = 37°C). After 1 min, the indicated compounds and control (EtOH) were added and recording of amperometric and fluorescence changes was continued for 25 min. Obtained results of all measurements are presented as means ± s.e.m. of n = 3, repeated on 4 different occasions.
4.13. Pharmacokinetics analysis
200 μg/kg MitoPerSulf in 100 μL of saline was administered by tail-vein injection in Wild-type male C57BL/6 mice. Tissues were collected after respective time periods, frozen in liquid nitrogen and then stored at −80°C. MitoPerSulf and its derivatives inside the tissues were reduced to MitoNAP-SH by addition of 0.3 M DTT during the procedure and then MitoNAP-SH, in homogenate was extracted with 0.1 % TFA/acetonitrile and its amount was analyzed by LC-MS/MS as described.
4.14. MS method development for detection of reaction metabolites
The mass spectrometric fragmentation patterns for reaction intermediates/metabolites of MitoPerSulf were determined in samples from in vitro kinetic experiments of MitoPerSulf in the presence of GSH. Samples were quenched with IAM or TPP-IAM and prepared as follows. IAM quenched samples were prepared by incubating 100 μM of MitoPerSulf with 1 mM GSH in 25 mM HEPES buffer (pH 7.8) at 37°C for 15 min. Fractions of 50 μL were taken after 1, 5, 10 and 15 min and were immediately mixed with 20 mM iodoacetamide (from 200 mM fresh stock solution in dH2O) and incubated at RT in dark for 20 min. TPP-IAM quenched samples were prepared by incubating 100 μM of MitoPerSulf with 1 mM GSH in 25 mM HEPES buffer (pH 7.8) at 37°C for 15 min. Fractions of 50 μL were taken after 1, 5, 10 and 15 min and were immediately mixed with 20 mM TPP-IAM (from 200 mM fresh stock solution in MeOH) and incubated at RT in dark for 20 min. Fragmentation patterns were determined by direct infusion of appropriately diluted samples (in 20% ACN, 0.1 % FA) at 2 μL/min into a triple quadrupole mass spectrometer (Waters Xevo TQ-S). Electrospray ionisation in positive ion mode was used with the following settings: capillary voltage – 3.0 kV; cone voltage – 30 V; ion source temperature – 100°C; collision energy – 20 V. Nitrogen and argon were used as the curtain and the collision gases, respectively.
4.15. LC-MS/MS analysis of TPP-IAM or IAM quenched samples
IAM-quenching. For MS/MS analysis of samples quenched with IAM, a triple-quadrupole mass spectrometer was used (Waters Xevo TQ-S under positive ion mode: source spray voltage, 3.4 kV; ion source temperature, 150°C). Nitrogen and argon were used as curtain and collision gas, respectively. For LC-MS/MS analyses the mass spectrometer was connected in series to an I-Class ACQUITY UPLC system (Waters). Samples were stored in an autosampler at 8°C and 2 μL samples were injected via a 15 μL flow-through needle and RP-UPLC at 40°C using an I-Class ACQUITY UPLC BEH C18 column (1 × 50 mm, 130 Å, 1.7 μm: Waters) with a Waters UPLC filter (0.2 μm. Waters). MS buffers A (95 % water, 5% ACN, 0.1% FA) and B (90% ACN, 10 % water, 0.1% FA) were infused at 200 μL/min using the following gradient (the proportion of MS solvent B is given in %): 0–0.3 min, 5 %; 0.3–2 min, 5–100 %; 2–2.5 min, 100 %, 2.5–2.8, 100–5 %; 2.8–3.0 min, 5 %. Compounds were detected in multiple reaction monitoring (MRM) in positive ion mode. The peak areas of the molecules were quantified using the MassLynx 4.1 or 4.2 software.
The following MS settings were used for the MRM detection of the individual compounds.
| Molecule | MRM transition | Cone voltage (V) | Collision energy (V) |
|---|---|---|---|
| GS-CAM | 365.1596 > 290.0879 | 32 | 10 |
| GSS-CAM | 397.1596 > 322.0562 | 26 | 12 |
| GS-benzoyl | 412.1596 > 283.0566 | 18 | 6 |
| MitoNAP-SSG (M2+) | 413.8404 > 183.0482 | 32 | 70 |
| MitoNAP-SSG (M+) | 826.3936 > 262.0814 | 2 | 46 |
| MitoNAP-S-CAM | 578.3298 > 262.0817 | 26 | 38 |
| MitoNAP-SS-CAM | 610.2660 > 262.0873 | 2 | 30 |
TPP-IAM quenching. For MS/MS analysis, a triple-quadrupole mass spectrometer was used (Waters Xevo TQ-S under positive ion mode: source spray voltage, 2.6 kV; ion source temperature, 150°C). Nitrogen and argon were used as curtain and collision gas, respectively. For LC-MS/MS analyses the mass spectrometer was connected in series to an I-Class ACQUITY UPLC system (Waters). Samples were stored in an autosampler at 8°C and 0.5–5 μL samples were injected via a 15 μL flow-through needle and RP-UPLC at 40°C using an I-Class ACQUITY UPLC BEH C18 column (1 × 50 mm, 130 Å, 1.7 μm; 0.2 μm; Waters Waters) with a Waters UPLC filter (0.2 μm; Waters). MS buffers A (95% water, 5% ACN, 0.1 % FA) and B (90 % ACN, 10 % water, 0.1 % FA) were infused at 200 μL/min using either of the following gradients (the proportion of MS solvent B is given in %): 3 min gradient: 0–0.3 min, 5 %; 0.3–2 min, 5–100 %; 2–2.5 min, 100%, 2.5–2.8, 100–5 %; 2.8–3.0 min, 5 %. 5 min gradient: 0–0.3 min, 5 %; 0.3–3.0 min, 5–100 %; 3.0–4.0 min, 100 %, 4.0–4.1, 100–5 %; 4.1–5.0 min, 5 %. Compounds were detected in multiple reaction monitoring (MRM) in positive ion mode. The peak areas of the molecules were quantified using the MassLynx 4.1 or 4.2 software.
The following MS settings were used for the MRM detection of the individual compounds.
| Molecule | MRM transition | Cone voltage (V) | Collision energy (V) | UPLC |
|---|---|---|---|---|
| GS-CAM-TPP | 695.4149 > 422.2755 | 38 | 40 | 3 or 5 min |
| GSS-CAM-TPP | 727.4149 > 262.1422. | 98 | 40 | |
| GS-benzoyl | 412.1596 > 283.0566 | 18 | 6 | |
| MitoNAP-SSG (M2+) | 413.8404 > 183.0482 | 32 | 70 | |
| MitoNAP-CAM-TPP | 454.9681 > 262.1792 | 52 | 32 | |
| MitoNAP-SS-CAM-TPP | 470.9681 > 262.0474 | 50 | 40 |
4.16. LC-MS/MS characterization of in vitro reaction products
To analyze the reaction in time, the reaction mixture of 100 μM of MitoPerSulf, MitoNAP-SH and different concentration of GSH (0.2 or 1 mM) in 25 mM HEPES buffer (pH 7.8) was incubated at 37°C for 15 min and 50 μL fractions taken after 1, 5, 10 and 15 min and were immediately mixed with 20 mM iodoacetamide (5.5 μL from 200 mM fresh stock solution in water) and incubated at RT in dark for 20 min. Blocked samples were diluted 1:200 with 20% acetonitrile in 0.1 % formic acid and immediately analyzed by LC-MS/MS.
4.17. LC-MS/MS characterization of reaction products-in organelle
MitoPerSulf metabolism within mitochondria. RHM (1 mg protein/mL) were incubated with MitoPerSulf in KCl buffer (120 mM KCl, 10 mM HEPES, 1 mM EGTA, 1 mM MgCl2 and 5 mM KH2PO4, pH 7.4) at 37°C, supplemented with succinate (10 mM) and rotenone (4 μg/mL). Aliquots were centrifuged (1 min at 17 000×g at RT) at the indicated times and precipitated mitochondrial pellets were rapidly resuspended in 50 μL of 40 mM TPP-IAM (200 mM stock solution in MeOH or DMSO) in 100 mM HEPES buffer (pH 7.8). Samples were vortexed and sonicated in a sonic bath at RT in dark for 20 min. Next, 200 μL of ACN were added and samples were placed at −20°C for 5 min. Samples were centrifuged (10 min at 17 000×g) to pellet proteins and 100 μL of supernatant were retrieved and combined with 400 μl of MS-grade H2O containing FA (0.1 %). Subsequently, samples were diluted (in 20 % ACN 0.1 % FA) as required and analyzed by LC-MS/MS to assess levels of TPP-IAM quenched reaction products. All experiments were performed in triplicates and data are mean ± s.e.m.
4.18. Determining persulfidation of Cofilin-1 by MitoPerSulf via LC-MS
Cysteines (∼160 μM of total thiols) of his-tagged human recombinant cofilin-1 (∼118 μg) were reduced with TCEP (200 μM) for 30 min at 37°C and TCEP was removed by desalting the sample with BioSpin 6 columns (pre-equilibrated with Chelex-100 treated 25 mM HEPES pH 7.4) prior to distribution of the equal amount of desalted protein into individual tubes (calculated: 11 μg/sample). Samples were incubated with 100 μM MitoPerSulf or AP39 (control received ethanol) and 1 mM GSH for 7.5 min in 25 mM HEPES at 37°C in the total volume of 50 μL of 25 mM HEPES pH 7.4 using the 200 μL PCR-grade test tubes to minimize the gas head space volume. Upon incubation, samples were alkylated by addition of 20 mM IAM (from 200 mM stock solution in Chelex-100 treated water) in dark at room temperature for 20 min. After the treatment, samples were precipitated by addition of 50 μl cold MeOH (−25°C) and 12.5 μL of cold CHCl3 and centrifugated for 10 min at 4°C. Both MeOH and CHCl3 layers were removed from protein precipitates (protein disc in between two liquid phases) and the residual liquid was evaporated on air leaving the precipitated and labelled proteins at the bottom of the tubes. Precipitated proteins were dissolved in 50 μL of 50 mM ammonium bicarbonate buffer pH 7.8 containing 1 mM CaCl2 and 12.5 ng/μL trypsin and digested overnight at 37°C.
Peptides were resuspended in 3 % ACN, 0.1 % TFA buffer and portions were fractionated by liquid chromatography on a Biosphere C18 reversed-phase column, 75 μm inner diameter, 100 mm length (Nanoseparations, Nieukoop, Netherlands) in a Proxeon EASY-nLC II system using Buffer A (0.1 % formic acid, 2 % acetonitrile) and Buffer B (98% acetonitrile, 0.1 % formic acid) and a gradient of 2–35 % B over 84 min at a flow rate of 300 nL/min, followed by an increase in acetonitrile concentration to 90 % B over 5 min and re-equilibration with 2 % B within a total time of 102 min. The eluate was transferred in-line to a LTQ Orbitrap XL ETD mass spectrometer (Thermo Scientific, UK).
Peptides were analyzed by positive ion electrospray mass spectrometry in a data-dependent acquisition mode. Up to ten of the most abundant precursor ions with multiple charge states, were selected and fragmented by CID each second. The m/z values of precursor and up to 10 fragment ions were measured simultaneously in the Orbitrap (400–2000 m/z scan, resolution of 60 000) and ion-trap analyzers, respectively. A lock mass ion (polysiloxane, m/z = 445.1200) was used for internal MS calibration. For protein identification the fragment patterns were compared to the UniProt database using the Mascot search engine with Proteome Discoverer (v1.4) software (Thermo Scientific). Relative quantification was performed by comparing the peak area of XICs (extracted ion chromatograms) for the monoisotopic peak using Xcalibur software (Thermo Scientific).
4.19. Tag switch assay
Detection of protein persulfidation was performed by using the dimedone-based tag-switch method as reported previously [38] with modifications. In brief, 1 mg of RHM proteins were incubated in 2 mL of KCl buffer with 10 μM MitoPerSulf, MitoNAP or vehicle (EtOH) in the presence of 10 mM succinate and 4 μg/mL rotenone for 5 min at 37 °C. Subsequently, pelleted mitochondria (1 min at 17 000 g) were resuspended in 50 μL of HENS buffer (50 mM HEPES, 1 mM EDTA, 0.1 mM Neocuproine, 1 % NP-40, 2% SDS and protease inhibitor cocktail, pH 7.4) supplemented with 5 mM 4-chloro-7-nitrobenzofurazan (NBF–Cl, from 500 stock solution in DMSO) and incubated at 37°C for 30 min in dark. Proteins were retrieved using methanol/chloroform precipitation (H2O/MeOH/CHCl3: 4/4/1) and obtained protein pellets were resuspended using ultrasonication in 50 mM HEPES buffer (pH 7.4) containing 1 % SDS. Protein concentration was determined by BCA assay and 1 mg of protein were labelled with 25 μM of Daz-2-Cy5 alkyne click master mix [38] for 30 min at room temperature in the dark. After labelling, protein pellets obtained using methanol/chloroform were resuspended using ultrasonication in 50 mM HEPES buffer (pH 7.4) containing 1 % SDS and equal amount of protein (approximately 50 μg/sample) were resolved using standard Laemmli reducing 10% SDS PAGE. After electrophoresis, gel was fixed in the dark for 30 min, washed and equilibrate with dH2O and scanned using Typhoon FLA 9500 fluorescent scanner (Cy3 and Cy5 fluorescence was recorded using 473 and 635 nm filter sets). Obtained raw images were post processed using ImageJ software.
4.20. LAD ligation model
We used an open-chest, in situ mouse cardiac infarction model as recently described (Prag et al., 2022). Briefly, Wild-type male C57BL/6J mice (8–10 weeks of age; Charles River Laboratories, UK) were anethetized with sodium pentobarbital (70 mg per kg of body weight intraperitoneally (i.p.)), intubated endotracheally and ventilated with 3 cm H2O positive-end expiratory pressure. We monitored the adequacy of the anesthesia using corneal and withdrawal reflexes, and additional anesthesia was administered as needed throughout the experiment. We kept the ventilation frequency at 240 breaths per minute with a tidal volume between 125 μL and 150 μL. We performed a small thoracotomy, and the heart was exposed by stripping of the pericardium. All hearts underwent 30 min of regional ischemia by ligation of a main branch of the left coronary artery. We introduce MitoPerSulf or MitoNAP-SH (100 ng per kg body weight each) 10 min before reperfusion as a slow infusion intravenously into a tail vein over 20 min.
We assessed infarct size after 120 min of reperfusion using triphenyltetrazolium chloride (TTC) staining and expressed it as a percentage of the risk zone as described previously (Prag et al., 2022). For various experiments on treated tissues, we removed the left ventricle at various time points after reperfusion, as indicated in the corresponding Fig. legends.
4.21. Statistical analyses
Error bars represent the s.e.m. from at least three replicates unless otherwise stated. We quantified P values using Student's t-test or one-way ANOVA. Values of P < 0.05 was considered as statistically significant.
Author contributions
M. P. M., R. C. H., T. K., A. M. J., and J. Lj. M. carried out study conception and design. J. Lj. M. and N. B. designed, performed, and analyzed most experiments. A. L. helped in method development. J. F. M., D. A., T. N., H. A. P., and O. S. carried out in vivo and ex vivo experiments and tissue sampling. T. K. supervised mouse experiments. T. A. P., T. N. and G. R. B., carried out in vitro experiments. N. B. and J. Lj. M. developed, optimized, and performed MRM analysis. J. Lj. M. and J. L. M. generated corresponding fluorescently labelled cell line and performed all microscopy analysis that was supervised by J. P.. J. M. G., S. T. C., A. A. I. N., S. W. and R. C. H. designed and synthesized compounds. The manuscript was written by J. Lj. M and M. P. M. with assistance from all other authors. The study was directed by M. P. M., T. K., and R. C. H.
Declaration of competing interest
Authors have no conflict of interest to declare.
Acknowledgments
This research was funded by the Medical Research Council (MC_UU_00028/4) [M.P.M.], (MC_UU_00015/7 and MC_UU_00028/5) [J.P], and by the Wellcome Trust (220257/Z/20/Z [M.P.M.], WT 220257/B/20/Z and WT WT110158/Z/15/Z [R.C.H.]), by the Biotechnology and Biological Sciences Research Council (BB/I012826/1) and by studentships to A.A.I.N. and J.M.G. from University of Glasgow. For the purpose of open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102429.
Contributor Information
Richard C. Hartley, Email: Richard.Hartley@glasgow.ac.uk.
Michael P. Murphy, Email: mpm@mrc-mbu.cam.ac.uk.
Abbreviations
- IR
ischemia-reperfusion
- H2S
hydrogen sulfide
- RET
reverse electron transport
- GYY4137
4-Methoxyphenyl(morpholino)phosphinodithioate morpholinium salt
- AP123
(10-(4-carbamothioylphenoxy)-10- oxodecyl)triphenylphosphonium bromide)
- AP39
(10-Oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5- yl)phenoxy)decyl)triphenylphosphonium bromide
- HS-NSAIDs
hydrosoluble non-steroidal anti-inflammatory drugs
- DATS-MSN
diallyl trisulfide-loaded mesoporous silica nanoparticles
- r-SPSH
reversible S-thiolation of protein cysteine residues
- TPP
triphenylphosphonium cation
- GSH
Glutathione
- MitoPerSulf
5-(2′-Acetylamino-3′-benzoyldisulfanyl-3′- methylbutyrylamino)pent-1-yl]- riphenylphosphonium methanesulfonate
- MitoNAP-SSH
MitoNAP persulfide
- MitoNAP-SS-CAM
carbamidomethylated MitoNAP persulfide
- Iodoacetamide
IAM
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- CAM
carbamidomethylated
- GSCOPh
benzoylated glutathione
- MitoNAP-SSG
glutathionylated MitoNAP
- GSSH
glutathione persulfide
- GSS-CAM
carbamidomethylated glutathione persulfide
- GS-CAM
carbamidomethylated glutathione
- MitoNAP-S-CAM
carbamidomethylated glutathione MitoNAP
- Δψ
membrane potential
- IAM-TPP
triphenylphosphonium iodoacetamide
- MitoNAP-SS-CAM-TPP
TPP-carbamidomethylated MitoNAP persulfide
- MitoNAP-S-CAM-TPP
TPP-carbamidomethylated MitoNAP
- GSS-CAM-TPP
TPP-carbamidomethylated glutathione persulfide
- LAD
left anterior descending
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Nicholson C.K., Calvert J.W. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol. Res. 2010;62:289–297. doi: 10.1016/j.phrs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu D., Wang J., Li H., Xue M., Ji A., Li Y. Role of hydrogen sulfide in ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2015 doi: 10.1155/2015/186908. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citi V., Piragine E., Testai L., Breschi M.C., Calderone V., Martelli A. The role of hydrogen sulfide and H2S-donors in myocardial protection against ischemia/reperfusion injury. Curr. Med. Chem. 2018;25:4380–4401. doi: 10.2174/0929867325666180212120504. [DOI] [PubMed] [Google Scholar]
- 4.Kang K., Zhao M., Jiang H., Tan G., Pan S., Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transplant. 2009;15:1306–1314. doi: 10.1002/lt.21810. [DOI] [PubMed] [Google Scholar]
- 5.Tan G., Pan S., Li J., Dong X., Kang K., Zhao M., Jiang X., Kanwar J.R., Qiao H., Jiang H., et al. Hydrogen sulfide attenuates carbon tetrachloride-induced hepatotoxicity, liver cirrhosis and portal hypertension in rats. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad A., Olah G., Szczesny B., Wood M.E., Whiteman M., Szabo C. AP39, A mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock. 2016;45:88–97. doi: 10.1097/SHK.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Z., Liu X., Geng B., Fang L., Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008;82:1196–1202. doi: 10.1016/j.lfs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y.Z., Wang Z.J., Ho P., Loke Y.Y., Zhu Y.C., Huang S.H., Tan C.S., Whiteman M., Lu J., Moore P.K. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J. Appl. Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 9.Elrod J.W., Calvert J.W., Morrison J., Doeller J.E., Kraus D.W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y., Bhushan S., Yang C., Otsuka H., Stein J.D., Pacheco A., Peng B., Devarie-Baez N.O., Aguilar H.C., Lefer D.J., et al. Controllable hydrogen sulfide donors and their activity against myocardial ischemia-reperfusion injury. ACS Chem. Biol. 2013;8:1283–1290. doi: 10.1021/cb400090d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y.-H., Zhang N., Li L.-F., Zhang Q.-Z., Xie L.-J., Jiang H., Li L.-P., Hao N., Zhang J.-X. Hydrogen sulfide reduces regional myocardial ischemia injury through protection of mitochondrial function. Mol. Med. Rep. 2014;10:1907–1914. doi: 10.3892/mmr.2014.2391. [DOI] [PubMed] [Google Scholar]
- 12.Hauet T., Thuillier R. Protecting the mitochondria against ischemia reperfusion: a gassy solution? Am. J. Transplant. 2017;17:313–314. doi: 10.1111/ajt.14150. [DOI] [PubMed] [Google Scholar]
- 13.Juriasingani S., Vo V., Akbari M., Grewal J., Zhang M., Jiang J., Haig A., Sener A. Supplemental hydrogen sulfide in models of renal transplantation after cardiac death. Can. J. Surg. 2022;65:E193–E202. doi: 10.1503/cjs.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvert J.W., Elston M., Nicholson C.K., Gundewar S., Jha S., Elrod J.W., Ramachandran A., Lefer D.J. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamsath A., Wang Y., Yang C., Xu S., Cañedo D., Chen W., Xian M. Acyl selenyl sulfides as the precursors for reactive sulfur species (hydrogen sulfide, polysulfide, and selenyl sulfide) Org. Lett. 2019;21:5685–5688. doi: 10.1021/acs.orglett.9b02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu S., Hamsath A., Neill D.L., Wang Y., Yang C., Xian M. Strategies for the design of donors and precursors of reactive sulfur species. Chemistry. 2019;25:4005–4016. doi: 10.1002/chem.201804895. [DOI] [PubMed] [Google Scholar]
- 17.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., Zhao Y., Baskar R., Tan C.-H., Moore P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 18.Lilyanna S., Peh M.T., Liew O.W., Wang P., Moore P.K., Richards A.M., Martinez E.C. GYY4137 attenuates remodeling, preserves cardiac function and modulates the natriuretic peptide response to ischemia. J. Mol. Cell. Cardiol. 2015;87:27–37. doi: 10.1016/j.yjmcc.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Karwi Q.G., Whiteman M., Wood M.E., Torregrossa R., Baxter G.F. Pharmacological postconditioning against myocardial infarction with a slow-releasing hydrogen sulfide donor, GYY4137. Pharmacol. Res. 2016;111:442–451. doi: 10.1016/j.phrs.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y., Yang C., Organ C., Li Z., Bhushan S., Otsuka H., Pacheco A., Kang J., Aguilar H.C., Lefer D.J., et al. Design, synthesis, and cardioprotective effects of N-Mercapto-Based hydrogen sulfide donors. J. Med. Chem. 2015;58:7501–7511. doi: 10.1021/acs.jmedchem.5b01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L., Rossoni G., Sparatore A., Lee L.C., Del Soldato P., Moore P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Sun X., Wang W., Dai J., Jin S., Huang J., Guo C., Wang C., Pang L., Wang Y. A long-term and slow-releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Sci. Rep. 2017;7:3541. doi: 10.1038/s41598-017-03941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyson A., Dal-Pizzol F., Sabbatini G., Lach A.B., Galfo F., Dos Santos Cardoso J., Pescador Mendonça B., Hargreaves I., Bollen Pinto B., Bromage D.I., et al. Ammonium tetrathiomolybdate following ischemia/reperfusion injury: chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N.J., Smith A.C., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chouchani E.T., Pell V.R., James A.M., Work L.M., Saeb-Parsy K., Frezza C., Krieg T., Murphy M.P. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabol. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Yin Z., Burger N., Kula-Alwar D., Aksentijević D., Bridges H.R., Prag H.A., Grba D.N., Viscomi C., James A.M., Mottahedin A., et al. Structural basis for a complex I mutation that blocks pathological ROS production. Nat. Commun. 2021;12:707. doi: 10.1038/s41467-021-20942-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goubern M., Andriamihaja M., Nübel T., Blachier F., Bouillaud F. Sulfide, the first inorganic substrate for human cells. Faseb. J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 28.Lagoutte E., Mimoun S., Andriamihaja M., Chaumontet C., Blachier F., Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim. Biophys. Acta. 2010;1797:1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Fu M., Zhang W., Wu L., Yang G., Li H., Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo C., Ransy C., Módis K., Andriamihaja M., Murghes B., Coletta C., Olah G., Yanagi K., Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteman M., Armstrong J.S., Chu S.H., Jia-Ling S., Wong B.-S., Cheung N.S., Halliwell B., Moore P.K. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite “scavenger”. J. Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 32.Nagy P., Winterbourn C.C. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem. Res. Toxicol. 2010;23:1541–1543. doi: 10.1021/tx100266a. [DOI] [PubMed] [Google Scholar]
- 33.Filipovic M.R., Miljkovic J., Allgäuer A., Chaurio R., Shubina T., Herrmann M., Ivanovic-Burmazovic I. Biochemical insight into physiological effects of H₂S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem. J. 2012;441:609–621. doi: 10.1042/BJ20111389. [DOI] [PubMed] [Google Scholar]
- 34.Carballal S., Trujillo M., Cuevasanta E., Bartesaghi S., Möller M.N., Folkes L.K., García-Bereguiaín M.A., Gutiérrez-Merino C., Wardman P., Denicola A., et al. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med. 2011;50:196–205. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- 35.Paul B.D., Snyder S.H. H 2 S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015;40:687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul B.D., Snyder S.H. H₂S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 37.Vandiver M.S., Paul B.D., Xu R., Karuppagounder S., Rao F., Snowman A.M., Ko H.S., Lee Y. Il, Dawson V.L., Dawson T.M., et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zivanovic J., Kouroussis E., Kohl J.B., Adhikari B., Bursac B., Schott-Roux S., Petrovic D., Miljkovic J.L., Thomas-Lopez D., Jung Y., et al. Selective persulfide detection reveals evolutionarily conserved antiaging effects of S-sulfhydration. Cell Metabol. 2019;30:1152–1170. doi: 10.1016/j.cmet.2019.10.007. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls P., Marshall D.C., Cooper C.E., Wilson M.T. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem. Soc. Trans. 2013;41:1312–1316. doi: 10.1042/BST20130070. [DOI] [PubMed] [Google Scholar]
- 40.Karwi Q.G., Bornbaum J., Boengler K., Torregrossa R., Whiteman M., Wood M.E., Schulz R., Baxter G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2 S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017;174:287–301. doi: 10.1111/bph.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Trionnaire S., Perry A., Szczesny B., Szabo C., Winyard P.G., Whatmore J.L., Wood M.E., Whiteman M. The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor, (10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)decyl)triphenylphosphonium bromide (AP39) Med. Chem. Commun. 2014;5:728–736. [Google Scholar]
- 42.Szczesny B., Módis K., Yanagi K., Coletta C., Le Trionnaire S., Perry A., Wood M.E., Whiteman M., Szabo C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide Biol. Chem. 2014;41:120–130. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latorre E., Torregrossa R., Wood M.E., Whiteman M., Harries L.W. Mitochondria-targeted hydrogen sulfide attenuates endothelial senescence by selective induction of splicing factors HNRNPD and SRSF2. Aging (Albany. NY) 2018;10:1666–1681. doi: 10.18632/aging.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith R.A.J., Hartley R.C., Murphy M.P. Mitochondria-targeted small molecule therapeutics and probes. Antioxidants Redox Signal. 2011;15:3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 45.Qandil A.M. Prodrugs of nonsteroidal anti-inflammatory drugs (NSAIDs), more than meets the eye: a critical review. Int. J. Mol. Sci. 2012;13:17244–17274. doi: 10.3390/ijms131217244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levitt M.D., Furne J., Springfield J., Suarez F., DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J. Clin. Invest. 1999;104:1107–1114. doi: 10.1172/JCI7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picton R. Mucosal protection against sulphide: importance of the enzyme rhodanese. Gut. 2002;50:201–205. doi: 10.1136/gut.50.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dali M.-M., Dansette P.M., Mansuy D., Boucher J.-L. Comparison of various aryl-dithiolethiones and aryl-dithiolones as hydrogen sulfide donors in the presence of rat liver microsomes. Drug Metab. Dispos. 2020;48:426–431. doi: 10.1124/dmd.119.090274. [DOI] [PubMed] [Google Scholar]
- 49.Carey K.A., Kensler T.W., Fishbein J.C. Kinetic constraints for the thiolysis of 4-methyl-5-(pyrazin-2-yl)-1,2-dithiole-3-thione (oltipraz) and related dithiole-3-thiones in aqueous solution. Chem. Res. Toxicol. 2001;14:939–945. doi: 10.1021/tx0100340. [DOI] [PubMed] [Google Scholar]
- 50.Kensler T.W., Groopman J.D., Sutter T.R., Curphey T.J., Roebuck B.D. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem. Res. Toxicol. 1999;12:113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- 51.Gerő D., Torregrossa R., Perry A., Waters A., Le-Trionnaire S., Whatmore J.L., Wood M., Whiteman M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016;113:186–198. doi: 10.1016/j.phrs.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Requejo R., Hurd T.R., Costa N.J., Murphy M.P. Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J. 2010;277:1465–1480. doi: 10.1111/j.1742-4658.2010.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benchoam D., Cuevasanta E., Möller M.N., Alvarez B. Persulfides, at the crossroads between hydrogen sulfide and thiols. Essays Biochem. 2020;64:155–168. doi: 10.1042/EBC20190049. [DOI] [PubMed] [Google Scholar]
- 54.Bracher P.J., Snyder P.W., Bohall B.R., Whitesides G.M. The relative rates of thiol-thioester exchange and hydrolysis for alkyl and aryl thioalkanoates in water. Orig. Life Evol. Biosph. 2011;41:399–412. doi: 10.1007/s11084-011-9243-4. [DOI] [PubMed] [Google Scholar]
- 55.Hupe D.J., Jencks W.P. Nonlinear structure-reactivity correlations. Acyl transfer between sulfur and oxygen nucleophiles. J. Am. Chem. Soc. 1977;99:451–464. [Google Scholar]
- 56.Prime T.A., Blaikie F.H., Evans C., Nadtochiy S.M., James A.M., Dahm C.C., Vitturi D.A., Patel R.P., Hiley C.R., Abakumova I., et al. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10764–10769. doi: 10.1073/pnas.0903250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy M.P. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxidants Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 58.Bostelaar T., Vitvitsky V., Kumutima J., Lewis B.E., Yadav P.K., Brunold T.C., Filipovic M., Lehnert N., Stemmler T.L., Banerjee R. Hydrogen sulfide oxidation by myoglobin. J. Am. Chem. Soc. 2016;138:8476–8488. doi: 10.1021/jacs.6b03456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuevasanta E., Möller M.N., Alvarez B. Biological chemistry of hydrogen sulfide and persulfides. Arch. Biochem. Biophys. 2017;617:9–25. doi: 10.1016/j.abb.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Y., Biggs T.D., Xian M. Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem. Commun. (Camb). 2014;50:11788–11805. doi: 10.1039/c4cc00968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng B., Chen W., Liu C., Rosser E.W., Pacheco A., Zhao Y., Aguilar H.C., Xian M. Fluorescent probes based on nucleophilic substitution-cyclization for hydrogen sulfide detection and bioimaging. Chemistry. 2014;20:1010–1016. doi: 10.1002/chem.201303757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin V.S., Lippert A.R., Chang C.J. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl. Acad. Sci. U.S.A. 2013;110:7131–7135. doi: 10.1073/pnas.1302193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burger N., James A.M., Mulvey J.F., Hoogewijs K., Ding S., Fearnley I.M., Loureiro-López M., Norman A.A.I., Arndt S., Mottahedin A., Sauchanka O., Hartley R.C., Krieg T., Murphy M.P. ND3 Cys39 in complex I is exposed during mitochondrial respiration. Cell Chem. Biol. 2022;29:636–649. doi: 10.1016/j.chembiol.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu L., Liu K., He J., Tian C., Yu X., Yang J. Direct proteomic mapping of cysteine persulfidation. Antioxidants Redox Signal. 2020;33:1061–1076. doi: 10.1089/ars.2019.7777. [DOI] [PubMed] [Google Scholar]
- 65.Porteous C.M., Logan A., Evans C., Ledgerwood E.C., Menon D.K., Aigbirhio F., Smith R.A.J., Murphy M.P. Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: implications for mitochondria-specific therapies and probes. Biochim. Biophys. Acta. 2010;1800:1009–1017. doi: 10.1016/j.bbagen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 66.Whiteman M., Karwi Q.G., Wood M.E., Baxter G.F. Mitochondria-targeted hydrogen sulfide (H 2 S), but not untargeted H 2 S, reverses ventricular arrhythmia at reperfusion. Free Radic. Biol. Med. 2017;112:124–125. [Google Scholar]
- 67.Botrel A., Illien B., Rajczy P., Ledoux I., Zyss J. Intramolecular charge transfer in 5-phenyl-3H-1,2-dithiole-3-thione and 5-phenyl-3H-1,2-dithiole-3-one derivative molecules for quadratic nonlinear optics. Theor. Chim. Acta. 1993;87:175–194. [Google Scholar]
- 68.Pedersen C.T. 1,2-dithiole-3-thiones and 1,2-dithiol-3-ones. Adv. Heterocycl. Chem. 1982;31:63–113. [Google Scholar]
- [69.Klopman G., Li J.-Y., Wang S., Dimayuga M. Computer automated log P calculations based on an extended group contribution approach. J. Chem. Inf. Comput. Sci. 1994;34:752–781. [Google Scholar]
- 70.Bittermann K., Spycher S., Goss K.-U. Comparison of different models predicting the phospholipid-membrane water partition coefficients of charged compounds. Chemosphere. 2016;144:382–391. doi: 10.1016/j.chemosphere.2015.08.065. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y.H., Abraham M.H. Octanol/water partition of ionic species, including 544 cations. J. Org. Chem. 2005;70:2633–2640. doi: 10.1021/jo048078b. [DOI] [PubMed] [Google Scholar]
- 72.Nims R.W., Prough R.A., Lubet R.A. Cytosol-mediated reduction of resorufin: a method for measuring quinone oxidoreductase. Arch. Biochem. Biophys. 1984;229:459–465. doi: 10.1016/0003-9861(84)90176-0. [DOI] [PubMed] [Google Scholar]
- 73.Zhao B., Ranguelova K., Jiang J., Mason R.P. Vol. 51. 2011. pp. 153–159. (Studies on the Photosensitized Reduction of Resorufin and Implications for the Detection of Oxidative Stress with Amplex Red). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thurnhofer S., Vetter W. Synthesis of (S)-(+)-enantiomers of food-relevant (n-5)-monoenoic and saturated anteiso-fatty acids by a Wittig reaction. Tetrahedron. 2007;63:1140–1145. [Google Scholar]
- 75.Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B.C., Brace L., Longchamp A., Treviño-Villarreal J.H., Mejia P., Ozaki C.K., et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.