Abstract
Objective:
Bipolar disorder (BD) is highly heritable. Neuroimaging studies comparing unaffected youth at high familial risk for BD (i.e., those with a first-degree relative with the disorder; termed “high-risk” [HR]) to “low-risk” (LR) youth (i.e., those without a first-degree relative with BD) and to patients with BD may help identify potential brain-based markers associated with risk (i.e., regions where HR+BD≠LR), resilience (HR≠BD+LR), or illness (BD≠HR+LR).
Method:
During functional magnetic resonance imaging (fMRI), 99 youths (i.e., adolescents and young adults) aged 9.8 to 24.8 years (36 BD, 22 HR, 41 LR) performed a task probing face emotion labeling, previously shown to be impaired behaviorally in youth with BD and HR youth.
Results:
We found three patterns of results. Candidate risk endophenotypes (i.e., where BD and HR shared deficits) included dysfunction in higher-order face processing regions (e.g., middle temporal gyrus, dorsolateral prefrontal cortex). Candidate resilience markers and disorder sequelae (where HR and BD, respectively, show unique alterations relative to the other two groups) included different patterns of neural responses across other regions mediating face processing (e.g., fusiform), executive function (e.g., inferior frontal gyrus), and social cognition (e.g., default network, superior temporal sulcus, temporoparietal junction).
Conclusion:
If replicated in longitudinal studies and with additional populations, neural patterns suggesting risk endophenotypes could be used to identify individuals at risk for BD who may benefit from prevention measures. Moreover, information about risk and resilience markers could be used to develop novel treatments that recruit neural markers of resilience and attenuate neural patterns associated with risk.
Keywords: bipolar, brain, adolescence, risk, endophenotype
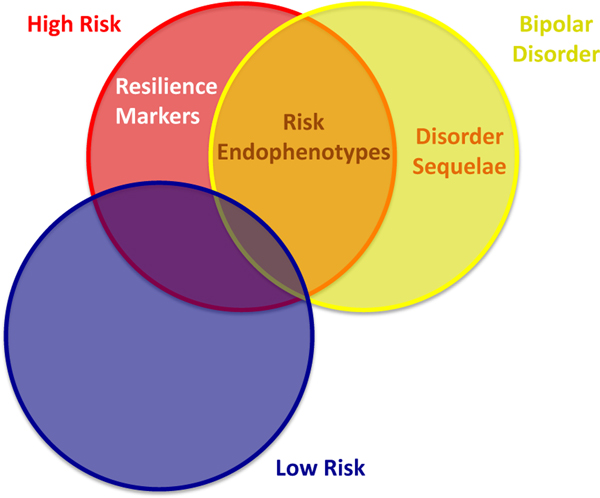
Bipolar disorder (BD), 1 of the 10 leading causes of disability (per The Global Burden of Disease, 2004 update of the World Health Organization), is highly heritable, with estimates ranging from 59% to 85%.1,2 Neuroimaging studies comparing youth at high familial risk for BD (i.e., those with a first-degree relative with the disorder; “high-risk” [HR]) to “low-risk” (LR) youth (i.e., those without a first-degree relative with BD) and to youth with BD can help to identify potential brain-based markers associated with risk, resilience, or illness (Figure 1).
FIGURE 1.
Identifying neural markers associated with risk, resilience, or disorder sequelae. Note: Having all three of these groups (high- and low-risk youths and those with bipolar disorder [BD]) is necessary to disentangle these markers. Brain activation patterns (a) shared by high-risk (HR) and BD (but not low-risk [LR]) youths (HR+BD≠LR) may indicate potential risk endophenotypes; (b) unique to high-risk youths (HR≠BD+LR) may indicate potential resilience markers; (c) and unique to youths with BD (BD≠HR+LR) may indicate potential disorder sequelae.
Previous work has defined risk endophenotypes as biomarkers that are associated with illness, are familial, are state independent, and are found more commonly in unaffected family members of patients than in the general population.3 Brain-based measures found in HR youth and youth with BD, but not in LR youth, may reflect neural markers of risk for BD (potential risk endophenotypes, HR+BD≠LR).4 Comparison among BD, HR, and LR groups can also identify potential biomarkers for resilience to BD.5 Specifically, regions where HR youth show differences in brain activity, relative to BD and LR youth, may reflect potential compensatory, protective, or resilience markers (HR≠BD+LR). Finally, regions where youth with BD show dysfunction relative to HR and LR youth may reflect potential illness-related “scars” (disorder sequelae, BD≠HR+LR). Of note, in cross-sectional designs such as this, it is only possible to identify associations, not causality. Therefore we cannot conclude that these brain profiles definitively lead to risk or resilience or result from disorder, but only that they are associated with these outcomes. Identifying causality requires longitudinal studies with the ability to rule out all alternative explanations. Thus, these potential associations should be interpreted tentatively.
We used a face emotion labeling paradigm to investigate neural mechanisms in these populations, because both HR youth and youth with BD make more errors labeling emotions on faces,6,7 particularly ambiguous faces.8 A recent meta-analysis suggests that during face processing, pediatric BD is marked by alterations in both emotion processing (i.e., limbic, dorsolateral prefrontal cortex) and visual perception (i.e., occipital) regions.9 The few face processing studies in HR youth also demonstrate alterations in limbic, dorsolateral prefrontal cortex, and occipital function.10–15 However, with few exceptions,10,14 and in two additional studies not using face stimuli,16,17 studies have not included both HR youth and youth with BD, possibly because of the difficulties involved in recruiting such samples. As discussed above, such direct comparisons are important to disentangle risk factors for, versus consequences of, BD.
Moreover, although previous studies in HR or BD populations used paradigms in which participants rated aspects of face stimuli, no study yet has used a task that involved face emotion labeling per se to identify risk and resilience markers and disorder sequelae in BD. Such a study would be important because behavioral deficits in facial emotion labeling have been documented in both youth with BD and HR youth. Recently, we used a face emotion labeling paradigm in an overlapping sample of youth with BD or disruptive mood dysregulation disorder (DMDD) to test whether the neural mechanisms of irritability, a symptom dimension common to both disorders, differ in BD versus DMDD.18 Although the goal of that paper18 was to differentiate bipolar versus DMDD (two disorders often conflated with one another), the current study investigates risk, resilience, and sequelae in youth with BD or at familial risk for the illness.
Thus, here we address gaps in the literature by identifying shared and unique neural alterations in BD and HR youth, relative to LR youth, during face emotion labeling. We compare HR and LR youth and youth with BD to identify potential risk and resilience endophenotypes as well as disorder sequelae. We are interested in identifying regions that fit into one of three patterns: patterns of potential risk endophenotypes (HR+BD≠LR); resilience markers (HR≠BD+LR); and disorder sequelae (BD≠HR+LR). Of note, these group difference patterns represent a heuristic that can lead to identifying potential risk and resilience endophenotypes and disorder sequelae but should be interpreted tentatively. Overall, we expect to find these patterns (i.e., HR+BD≠LR; HR≠BD+LR; and BD≠HR+LR) in limbic, dorsolateral prefrontal, and occipital regions, consistent with prior studies. However, as this study, unlike most prior studies, directly compares HR youth and youth with BD, we will be better equipped to separate patterns relating to potential risk vs. resilience markers and risk endophenotypes versus disorder sequelae.
METHOD
Participants
Data from 99 individuals (i.e., older children, adolescents, and young adults) aged 9.8 to 24.8 years were included (36 BD, 22 HR, 41 LR). Thirteen additional participants were excluded due to poor data quality, and 10 pairs and 1 trio within the dataset were biologically related (see Supplement 1, Methods, available online). BD was diagnosed using the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS)19 in youths less than 18 years of age or the Structured Clinical Interview for DSM Disorders (SCID)20 in youths more than 18 years of age. Inclusion in the HR group required a first-degree relative with BD. Exclusion criteria consisted of any bipolar spectrum disorder, pervasive developmental disorder, or schizophrenia; other disorders were included to avoid recruiting a particularly resilient group. LR youth were free of all psychopathology and did not have any first-degree relatives with BD. Exclusion criteria for all groups included orthodontic braces, other magnetic resonance imaging (MRI) contraindications, history of neurological or other significant medical disorders, and IQ < 80. Participants with BD and HR participants were recruited from across the United States and LR participants from the Washington, DC metropolitan area via advertisements and received monetary compensation. Participants more than 18 years of age and parents of minor participants gave written informed consent after receiving complete description of the study; minors gave written assent. Procedures were approved by the institutional review board of the National Institute of Mental Health (NIMH)/National Institutes of Health (NIH). Data from 22 of 41 LR youths and 24 of 36 youths with BD included in the current report have been previously published.18,21 No imaging data in the 22 HR youths have been published.
Face Emotion Labeling Task
Participants performed a jittered, event-related task during functional MRI (fMRI) acquisition in which they labeled the emotion on angry, fearful, and happy faces morphed with neutral faces to create 0% (i.e., neutral), 50%, 75%, and 100% intensity faces presented for 4,000 milliseconds total (2,000 milliseconds of face only, 2,000 milliseconds of face with options to label the emotion on the face). Before each face presentation, a fixation cross appeared for a variable amount of time (mean = 1,800 milliseconds, range = 5007,000 milliseconds). Across four 8.5-minute runs, there were 28 trials per emotion intensity condition (e.g., angry 50%, angry 75%, etc.), except for neutral faces (i.e., 0% intensity of each angry, fearful, and happy), of which there were 84 trials (28 trials × 3). Details on this task, which has been used with an overlapping sample of healthy (LR) youth and youth with BD, as well as healthy adults and youth with DMDD, are provided elsewhere.18,21
Behavioral Data Analysis
To examine whether face emotion labeling accuracy differs by diagnostic group, emotion, or intensity level, we conducted a linear mixed effects model with diagnostic group (LR versus HR versus BD) as between-subjects and emotion (fearful versus happy versus angry) and intensity (0%, 50%, 75%, and 100%) as within-subjects factors weighted linearly, quadratically, and cubically. False discovery rate (FDR)corrected post hoc comparisons were performed.
fMRI Acquisition and Preprocessing
Parameters for MRI data acquisition and preprocessing steps are available in Supplement 1, Methods, available online.
fMRI Data Analysis
Individual-Level Models.
Conditions were modeled as regressors convolved with AFNI’s BLOCK basis function over 4,000 milliseconds of face presentation for each trial, and incorrect trials were removed with a nuisance regressor. Details on individual level models are provided in Supplement 1, Methods, available online. These analyses produced β images, representing the estimated activation in each condition for each participant, for use in group-level analyses.
Group-Level Models.
AFNI’s 3dLME was used to create a whole-brain linear mixed effects model with diagnostic group (LR versus HR versus BD) as a between-subjects factor, and emotion (fearful versus happy versus angry) and intensity (0%, 50%, 75%, and 100%) as within-subjects factors. We weighted intensity quadratically and cubically in addition to linearly (as previous work has modeled morphed faces18) to allow us to detect nonlinear patterns of activation across intensities, that is, U- or ∩-shaped activation across intensities for the quadratic model, and cubic-shaped activation across intensities for the cubic model. This model yielded group main effect, group × emotion, and group × emotion × intensity (with intensity modeled to detect linear, quadratic, or cubic trends across intensity values), each contrast controlling for the other contrasts. In other words, this analysis examined how the groups differed on brain activation, taking into account stimulus qualities (emotion, intensity). The contrasts identified group differences: across all stimuli (group main effect); dependent on emotion (group × emotion); and dependent on both emotion and intensity, testing for linear and nonlinear (quadratic, cubic) trends of activation across intensity values (group × emotion × intensity, modeled linearly, quadratically, and cubically). The specific ways in which the groups differed were then identified using post hoc analyses and compared with heuristics (Figure 1) for potential risk endophenotypes, resilience markers, and disorder sequelae.
First, to characterize candidate risk endophenotypes, we identified brain regions with significant group main effects, where post hoc contrasts indicate that, regardless of stimulus, HR youth and youth with BD differ from LR youth; group × emotion interactions, where post hoc analyses indicate that HR youth and youth with BD share the same neural alterations, elicited by the same emotion category stimuli; and group × emotion × intensity interactions, where post hoc analyses show that HR youth and youth with BD share the same neural alterations, elicited by stimuli of the same emotion and intensity, relative to LR youth.
Second, to characterize potential markers of resilience, we identified brain regions with significant group main effects, where post hoc analyses indicate that, regardless of stimulus, HR differ from youth with BD and LR youth; group × emotion interactions, where post hoc analyses indicate that the pattern of activation in HR youth, dependent on the emotion of the stimulus, is not shown in either LR youth or youth with BD; and group × emotion × intensity interactions, where post hoc analyses demonstrate that the HR group shows unique neural alterations, relative to the LR and BD groups, dependent on stimulus qualities (both face emotion and intensity of emotion).
Third, to characterize candidate disorder sequelae, we identified brain regions with significant group main effects, where post hoc analyses show neural dysfunction in youth with BD relative to HR and LR youth; group × emotion interactions, where post hoc analyses indicate that the pattern of activation in youth with BD, dependent on the emotion of the stimulus, is not shown in either LR or HR youth; and group × emotion × intensity interactions, where post hoc analyses demonstrate that the group with BD shows unique neural alterations, relative to the LR and HR groups, dependent on stimulus qualities (face emotion and intensity of emotion).
A whole-brain approach (as opposed to choosing a priori regions of interest) was used to include potential markers in all brain regions. This voxelwise approach results in fewer false-positive results than a clusterwise approach.22 For all contrasts, the cluster extent threshold was set to k ≥ 39 (609 mm3) with a height threshold of p < .005, equivalent to a whole-brain corrected false-positive probability of p < .05, as calculated by 3dClustSim, using blur estimates averaged across participants. Activation maps were masked to include only those areas of the brain for which 90% of participants had valid data. To characterize significant group differences and interactions, post hoc analyses were performed in SPSS statistical software (version 22; SPSS Inc., Chicago, IL) using values extracted and averaged from the clusters. Significance values from post hoc analyses were corrected for multiple comparisons using FDR.
RESULTS
Behavior
Sample characteristics are available in Table 1. The group × emotion interaction predicted accuracy to label the face emotion (F4,1056 = 2.59, p= .035). Specifically, BD, HR, and LR groups differ on accuracy to label the face, depending on the emotion (Figure S1, available online). However, post hoc comparisons among groups were not significant, precluding the identification of specific group differences giving rise to the significant interaction.
TABLE 1.
Participant Characteristics
| Characteristic | Low-Risk (n = 41) | High-Riska (n = 22) | Bipolarb (n = 36) | χ2 | df | P |
|---|---|---|---|---|---|---|
| Sex (% female) | 49 | 41 | 42 | 0.54 | 2 | .77 |
| Age, y | F | |||||
| Mean (SD) | 17.31 (4.2) | 15.7 (3.6) | 17.9 (3.3) | 2.38 | 2,96 | .1 |
| Range | 9.8–24.8 | 9.5–22.8 | 10.1–22.6 | F | ||
| Overall task accuracy, percent correct | 74.0% (12%) | 75.5% (16%) | 71% (11%) | 1.01 | 2,96 | .37 |
| IQ, mean (SD) | 113 (11.7) | 110 (10.6) | 109 (13.1) | 1.18 | 2,92 | .31 |
| ARI, mean (SD) | 0.78 (1.3) | 2.5 (3.4) | 4.4 (3.2) | 16.87 | 2,87 | <.001c |
| SCARED, mean (SD) | 6.7 (6.4) | 21.1 (14.3) | 23.3 (12.1) | 14.46 | 2,55 | <.001d |
| Non-euthymic mood state (total), n | 1 | 7 | ||||
| Depressed | 1 | 2 | ||||
| Manic | 0 | |||||
| Hypomanic | 5 | |||||
| Mixed | 0 | t | df | P | ||
| CGAS/GAF, mean (SD) | 75.5 (16.3) | 52.1 (14.3) | 5.29 | 46 | <.001 | |
| CDRS, mean (SD) | 21.2 (4.8) | 27.5 (6.9) | 2.96 | 30 | .006 | |
| SIGH-SAD, mean (SD) | 5.8 (9.7) | 24.6 (35.0) | 1.17 | 17 | .26 | |
| YMRS, mean (SD) | 1.6 (2.3) | 6.3 (6.0) | 3.07 | 42 | .004 | |
| Average number of medications | 0.4 (1.0) | 2.8 (1.7) | 6.04 | 54 | <.001 | |
| On psychotropic medication (any), n | 4 | 27 | ||||
| Antidepressants | 3 | 15 | ||||
| Stimulants | 1 | 9 | ||||
| Nonstimulant anti-ADHD | 1 | 8 | ||||
| Antiepileptics | 1 | 16 | ||||
| Atypical antipsychotics | 1 | 23 | ||||
| Lifetime comorbidity (any disorder), n | 6 | 22 | ||||
| Anxiety disorders (DSM-5) | 2 | 13 | ||||
| ODD | 1 | 3 | ||||
| ADHD | 4 | 12 | ||||
| MDD | 1 | |||||
| DMDD | 0 |
Note: Mood state info for 7 youths with bipolar disorder (BD) and 5 high-risk (HR) youths was missing; other missing data noted through degrees of freedom. ADHD = attention-deficit/hyperactivity disorder; ARI = Affective Reactivity Index, mean parent- and child-report; CDRS = Children’s Depression Rating Scale; CGAS/GAF = Childrens Global Assessment Scale/Global Assessment of Functioning; DMDD = disruptive mood dysregulation disorder; IQ = full scale Wechsler Abbreviated Scale of Intelligence score; LR = low risk; MDD = major depressive disorder; ODD = oppositional defiant disorder; SCARED = Screen for Child AnxietyRelated Disorders, mean parent- and child-report; SIGH-SAD = Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder; YMRS = Young Mania Rating Scale.
Of the HR youths, 32% had a sibling with BD; 55% had a parent with BD; and 13% had both. Thirteen of the 22 HR youths had a relative with pediatric onset BD (i.e., age of onset < 18 years). (Three youths were missing age of onset data for their first-degree relative.)
Of the youths with BD, 55% had bipolar disorder I and 45% had bipolar disorder II. Of 36 youths with BD, 34 had pediatric onset of BD. Mean age of onset = 10.1 years, SD = 3.8 years. (One youth with BD was missing age of onset datum.)
Post hoc analyses indicate that LR<HR<BD.
LR<HR+BD. Mood state at time of scan determined with CDRS (< 18 years old) or SIGH-SAD (> 18 years old; 14 youths with BD and 5 HR youths) and YMRS: Depressed = YMRS < 12 and CDRS > 40 or SIGH-SAD > 20, Hypomanic = YMRS > 12 and < 26 and CDRS < 40 or SIGH-SAD < 20, Manic = YMRS > 26 and CDRS < 40 or SIGH-SAD < 20, Mixed = YMRS > 12 and CDRS > 40 or SIGH-SAD > 20, Euthymic = does not meet criteria for other mood states.
Candidate Risk Endophenotypes (Bipolar + High-Risk ≠ Low-Risk)
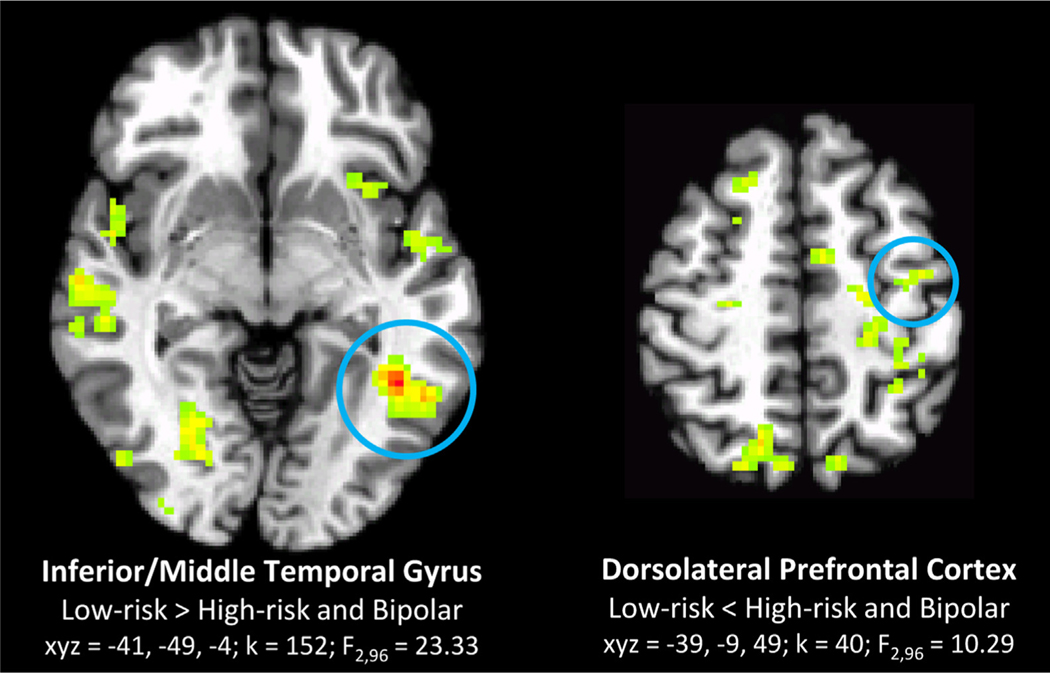
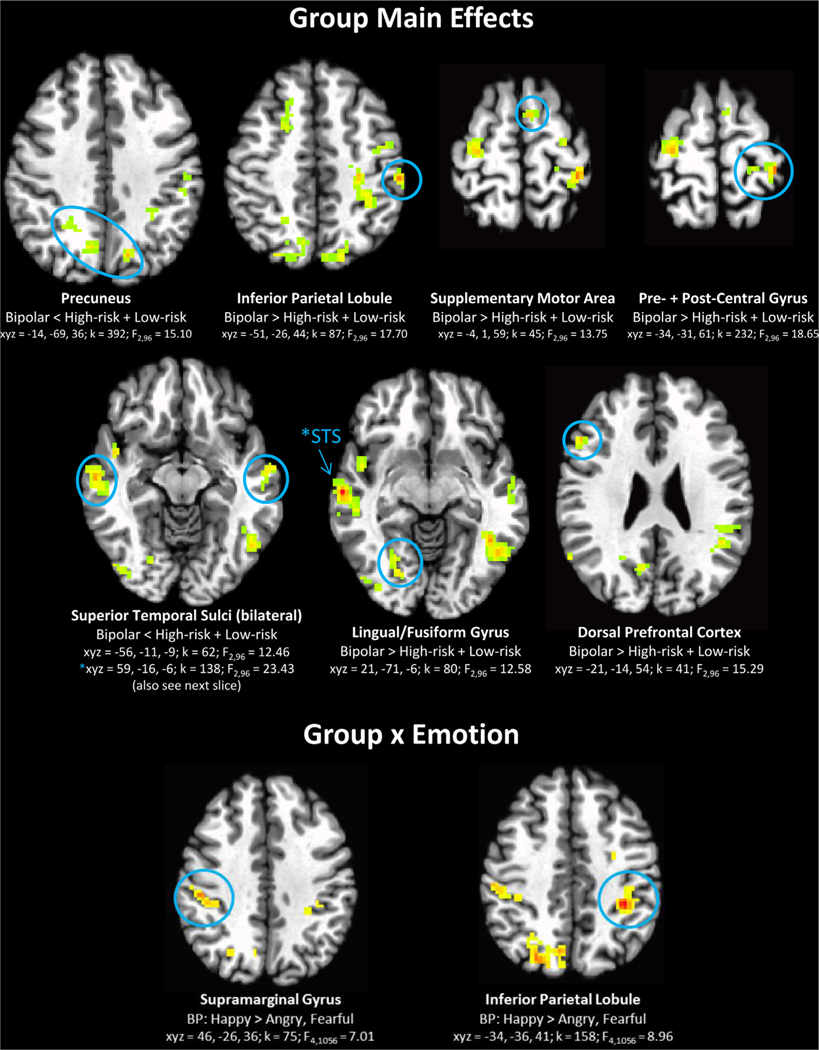
Across all stimulus types, left inferior/middle temporal gyrus and dorsolateral prefrontal cortex show neural alterations in the HR and BD groups, relative to the LR group (Table 2, Figure 2). Specifically, the inferior/middle temporal gyrus shows reduced activation to all faces, whereas the dorsolateral prefrontal cortex shows hyperactivation in the HR and BD groups relative to the LR group.
TABLE 2.
Brain Regions in Candidate Risk and Resilience Markers and Scars
| k | F 2,96 | x | y | z | BA | Region | Post hoc | |
|---|---|---|---|---|---|---|---|---|
| Candidate Risk Endophenotypes (HR+BD≠LR) | ||||||||
| Group Main Effects | ||||||||
| 152 | 23.33 | −41 | −49 | −4 | 37,19 | Inferior/middle temporal gyrus | HR+BD<LR | |
| 40 | 10.29 | −9 | −9 | 49 | 6,4 | Dorsolateral prefrontal cortex (left) | HR+BD>LR | |
| Candidate Resilience Markers (HR≠BD+LR) | ||||||||
| GroupMain Effects | ||||||||
| 69 | 13.2 | −41 | −46 | 21 | 13 | Supramarginal/angular gyrus (left) | HR<BD+LR | |
| 80 | 12.15 | −34 | −41 | −16 | 20 | Fusiform gyrus (left) | HR>BD+LR | |
| 57 | 14.4 | −16 | −56 | 14 | 30 | Posterior cingulate/precuneus | HR>BD+LR | |
| 49 | 13.35 | 19 | 14 | 41 | 32 | Superior frontal gyrus | HR>BD+LR | |
| 47 | 11.25 | 54 | −49 | 16 | 39 | Temporo-parietal junction | HR>BD+LR | |
| 41 | 14.83 | 49 | 21 | 24 | 46,9 | Inferior frontal gyrus | HR>BD+LR | |
| 39 | 13.42 | 46 | 1 | −9 | 38,13 | Temporal pole/Insula | HR>BD+LR | |
| Group × Emotion | Post hoc comparisons significant in... | |||||||
|
|
|
|||||||
| k | F 4,1056 | x | y | z | BA | Region | Group | Direction of Effects |
| 247 | 8.12 | 19 | −69 | 46 | 7 | Superior parietal lobule | HR | Angry<Happy, Fearful |
| 42 | 7.5 | 31 | −9 | 59 | 6 | Dorsolateral prefrontal cortex (right) | HR | Angry<Happy, Fearful |
| Group × Emotion × Intensity | ||||||||
|
|
||||||||
| k | F 4,1056 | x | y | z | BA | Region | Post hoc | |
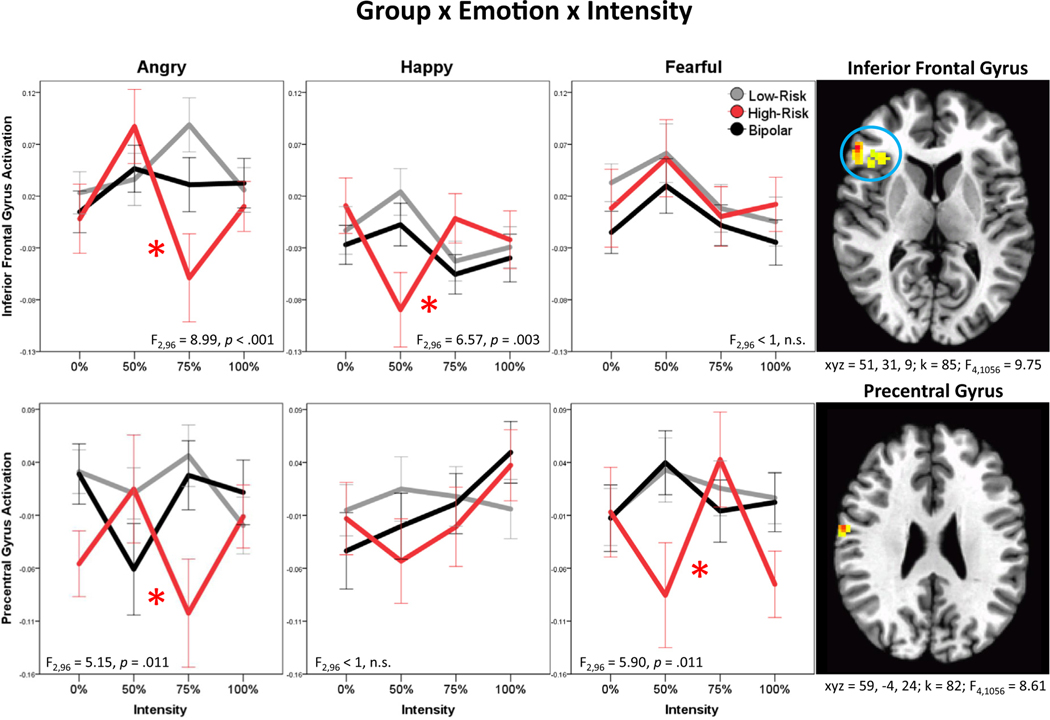
| 85 | 9.75 | 51 | 31 | 9 | 45 | Inferior frontal gyrus | HR curve across intensities differs from LR+BD for Angry and Happy faces | |
| 82 | 8.61 | 59 | −4 | 24 | 6 | Precentral gyrus | HR curve across intensities differs from LR+BD for Angry and Fearful faces | |
| k | F 2,96 | x | y | z | BA | Region | Post hoc | |
| Candidate Disorder Sequelae (BD≠HR+LR) | ||||||||
| Group Main Effects | ||||||||
| 392 | 15.1 | −14 | −69 | 36 | 7 | Precuneus | BD<HR+LR | |
| 138 | 23.43 | 59 | −16 | −6 | 21 | Superior temporal sulcus | BD<HR+LR | |
| 62 | 12.46 | −56 | −11 | −9 | 22 | Superior temporal sulcus | BD<HR+LR | |
| 232 | 18.65 | −34 | −31 | 61 | 3,40 | Pre- + postcentral gyrus | BD>HR+LR | |
| 87 | 17.7 | −51 | −26 | 44 | 2 | Inferior parietal lobule | BD>HR+LR | |
| 80 | 12.58 | 21 | −71 | −6 | 19 | Lingual/fusiform gyrus (right) | BD>HR+LR | |
| 45 | 13.75 | −4 | 1 | 59 | 6 | Supplementary motor area | BD>HR+LR | |
| 41 | 15.29 | −21 | −14 | 54 | 6 | Dorsolateral prefrontal cortex (left) | BD>HR+LR | |
| Group × Emotion | Post hoc comparisons significant in... | |||||||
|
|
|
|||||||
| k | F 4,1056 | X | y | z | BA | Region | Group | Direction of Effects |
| 158 | 8.96 | −34 | −36 | 41 | 40 | Inferior parietal lobule | BD | Happy>angry, fearful |
| 75 | 7.01 | 46 | −26 | 36 | 40 | Supramarginal gyrus (right) | BD | Happy>angry, fearful |
Note: “Group main effects” indicate simple main effects in youth with bipolar disorder (BD) vs. high-risk (HR) vs. lowrisk(LR) youth. “Group x emotion” interactions indicate regions where youth with BD and HR and LR youth significantly differ on activation, depending on emotion stimulus category (happy, angry, fearful). “Group x emotion x intensity” interactions indicate regions where youth with BD and HR and LR youth significantly differ on activation, depending on both emotion and intensity of the emotion (modeled as a cubic curve). See Table SI, available online, for other patterns of group differences. In all figures and tables, regions are significant at a whole-brain corrected p < .05. BA = Brodmann area.
FIGURE 2.
Candidate risk endophenotypes: regions where activation related to face emotion labeling in low-risk (LR) youth differ from those of high-risk (HR) youth and youth with bipolar disorder (BD), across all stimuli (group main effect). Note: Circled clusters correspond to statistical information below brain image; other clusters are significant group differences that do not fit the pattern of a risk endophenotype (i.e., LR≠HR+BP). Axial sections shown in radiological view (left = right) in all figures. Clusters for all figures significant at whole-brain corrected p < .05.
Candidate Resilience Markers (High-Risk ≠ Low-Risk + Bipolar)
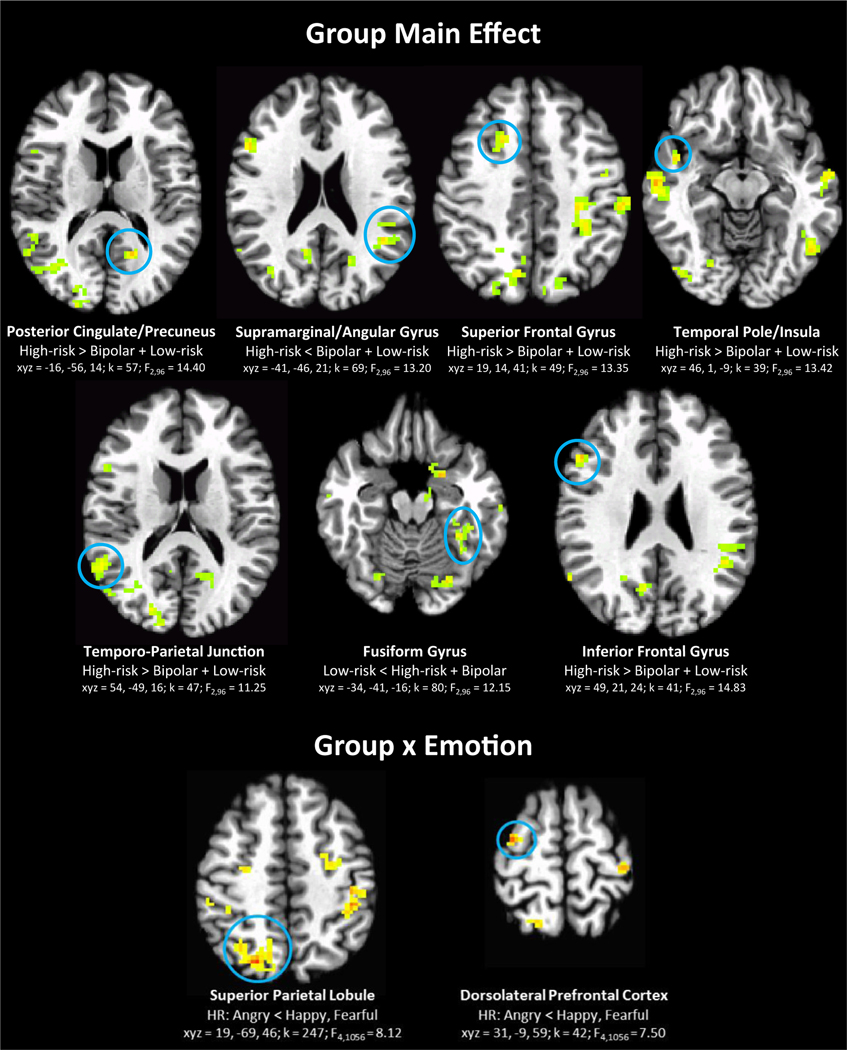
Across all stimulus types, HR youth show hyperactivation, relative to both LR youth and youth with BD, in multiple regions, including posterior cingulate/precuneus, superior and inferior frontal gyri, temporo-parietal junction, temporal pole/insula, and fusiform gyrus. One region, supramarginal/angular gyrus, exhibits hypoactivation in HR youth, relative to LR youth and youth with BD (group main effects; Table 2). Moreover, the HR group is characterized by reduced activation to angry faces, relative to happy and fearful faces, in the superior parietal lobule and superior frontal gyrus (group × emotion; Table 2); this pattern was not found in the LR or BD groups. Finally, in the inferior frontal gyrus and precentral gyrus, the group × emotion × intensity interaction was significant. Specifically, the HR group shows unique neural alterations, relative to the LR and BD groups, dependent on stimulus qualities (both face emotion and intensity of emotion, with intensity modeled as a cubic curve) (Figure 3). In the inferior frontal gyrus, the group × emotion × intensity interaction is driven by greater variation in the HR youth response curve across intensities of angry (HR versus LR, p < .001; HR versus BD, p = .028) and happy (HR versus LR, p = .004, HR versus BD, p = .009) faces. In the precentral gyrus, however, the interaction is driven by greater variation in the HR youth response curve across intensities of angry (HR versus LR, p = .017, HR versus BD, p = .009) and fearful (HR versus LR, p = .015, HR versus BD, p = .013) faces.
FIGURE 3.
Candidate resilience markers: regions where the high-risk (HR) group shows unique neural alterations, relative to the low-risk (LR) and bipolar disorder (BD) groups across all stimuli (group main effect), dependent on face emotion (group × emotion), and dependent on both face emotion and intensity of emotion (group × emotion × intensity). Note: Intensity modeled cubically in the group × emotion × intensity interaction. F and p values on plots reflect post hoc analyses comparing groups for each emotion separately. All p values reflect false discovery rate (FDR) correction. Asterisks on plots indicate that HR cubic response curve significantly differs from those of LR youth and youth with BD in FDR-corrected post hoc comparisons. See Figure 2 for information on brain images.
Candidate Disorder Sequelae (Bipolar ≠ High- and Low-Risk)
Youth with BD show widespread neural alterations, relative to HR and LR youth, in parietal, temporal, temporo-occipital, and dorsal frontal regions, during the general process of face emotion labeling (group main effect; Table 2, Figure 4). Specifically, the group with BD demonstrates hypoactivation in the bilateral superior temporal sulci and precuneus, yet hyperactivation in the frontal and parietal areas in the left hemisphere (dorsal prefrontal, supplementary motor area, pre- and postcentral gyrus, inferior parietal lobule) and in the right lingual/fusiform gyrus. In addition, the group with BD is characterized by increased activation to happy faces, relative to angry and fearful faces, in the bilateral parietal lobe (significant group × emotion interaction in left inferior parietal lobule and right supramarginal gyrus; Table 2, Figure 4). This pattern was not found for the LR or HR groups.
FIGURE 4.
Candidate disorder sequelae: activation specific to the bipolar disorder (BD) group, relative to the low-risk (LR) and high-risk (HR) group, across all stimuli types (group main effect) and in specific emotions (group × emotion). Note: See Figure 2 for information on brain images.
Other significant clusters that do not fit the pattern as candidate risk or resilience markers or sequelae of BD are shown in Table S1, available online.
Additional Analyses
Additional analyses were conducted to address the potential impact of biological relatedness among participants, mood state, comorbid anxiety disorders, age, medication, accuracy to label emotion, whether high-risk youths’ first-degree relatives with BD were siblings or parents, and irritability (see Supplement 1, Results, and Table S3, available online, for details). We repeated the primary analyses after removing individuals who were related, currently noneuthymic, or with an anxiety disorder, and after covarying for age and medication status. After taking into account each of these factors, the same patterns were found, and almost all of the findings remained significant, although some candidate disorder sequelae (BD≠HR+LR) regions became nonsignificant after covarying for medication, likely due to heavy medication use in BD. Additional analyses were also run with only the HR youth to investigate whether HR youth with versus without comorbid diagnoses, and with siblings versus parents with BD, differ on brain activation in clusters identified as candidate resilience markers; they do not (Table S2, available online). Moreover, to compare our findings with previous work on BD and DMDD,18 an analysis including irritability was performed. Findings were somewhat consistent with previous work.18 Namely, as in the prior report, irritability is significantly associated with brain response to fearful faces in the BD group. However, in the current report, this interaction manifests in inferior frontal gyrus, different from the regions reported previously. In the current data set, we also found associations in HR youth between irritability and activation in frontal, temporo-parietal, and cerebellar clusters; HR youth were not included in the prior report (Supplement 1, Results, available online). Most importantly for the current, novel findings, there is no main effect of irritability or irritability × group interaction in any of the multiple regions of association cortex that emerged as candidate endophenotype, resilience, or disorder markers.
DISCUSSION
We compared youth at familial risk for BD and youth with BD on brain activation during a face emotion labeling task. Most broadly, we found three patterns of results: regions where BD and HR share deficits (potential risk endophenotypes); regions where HR show unique alterations (potential resilience markers); and regions where alterations are specific to BD (potential disorder sequelae). Because this is a cross-sectional study, however, it is important to note that it is not possible to conclude causality between brain profiles and disorder outcomes, only associations.
First, we found that, when labeling faces of any emotion or intensity, HR youth and youth with BD share neural alterations in higher-order face processing regions (inferior/middle temporal gyrus and dorsolateral prefrontal cortex). These alterations may indicate candidate risk endophenotypes for BD (BD+HR≠LR). Second, potential resilience markers (neural alterations specific to the HR group, HR≠BD+LR) are apparent in multiple default network (medial prefrontal, posterior cingulate, and temporo-parietal regions) and face processing regions (lateral prefrontal and fusiform gyrus). Finally, we found neural alterations specific to the BD group, which may reflect disorder sequelae (BD≠HR+LR) in multiple social cognition and face processing regions (bilateral superior temporal sulci, precuneus, fusiform gyrus, dorsal prefrontal cortex). Of note, in social cognition regions, the BD group shows increased reactivity to happy faces relative to angry and fearful faces. Increased reactivity to happy faces has been linked to mania symptoms23 and may differentiate bipolar from unipolar depression24; our data suggest that this abnormality is associated with BD itself, rather than risk for the illness.
A significant advantage of this study is that we included both HR and BD in addition to LR groups, which allowed us to examine potential markers of bipolar risk and resilience as well as sequelae of BD. Comparing BD to LR groups, as is done in the vast majority of BD studies, neural alterations in participants with BD could be a cause or a consequence of illness. Comparing HR and LR groups, as is done in most familial bipolar risk studies, neural alterations in HR participants could be risk or resilience markers. Thus, having all three groups, HR, BD, and LR, increases one’s ability to study these questions.
Although our paradigm was not an executive functioning task per se, accurate face emotion labeling requires the engagement of a number of attentional and semantic processes. Executive functioning deficits have been implicated in both patients with BD and those at risk for the illness.25 (Of note, in this study, groups differ on accuracy to label the emotion, in line with prior work,6,7 although post hoc comparisons did not reach significance). Consistent with brain regions in other face processing studies on HR youth or youth with BD,10–14 we found alterations associated with risk, resilience, and sequelae in executive functioning regions as well as default network regions; the latter are typically suppressed during demanding executive functioning.26 However, whereas both the HR and BD groups show alterations in regions mediating executive function, the precise nature of the alterations differed between groups. Specifically, alterations specific to the HR group (resilience markers) include more variable responses in inferior and superior frontal gyri to different types of stimuli, compared to BD and LR groups (group × emotion × intensity interaction) and overactivation to angry, but not happy or fearful, faces (group × emotion interaction). In contrast, alterations in the dorsolateral prefrontal cortex are shared by the HR and BD groups (risk endophenotypes) and are pervasive across all types of face stimuli. The HR group’s aberrant activation in executive functioning regions in response to subtle differences in stimuli may reflect a resilience mechanism in which increased sensitivity to social cues in the environment compensates for other executive function deficits. Additional research, however, will be necessary to probe this possibility.
In addition to adaptive executive function, face emotion labeling also requires social cognition skills, which are diminished in BD.27 Consistent with this, markers of resilience and sequelae of BD include alterations in temporo-parietal and default network regions, which are involved in social cognition.26 In particular, altered recruitment of the default network (increased activation in medial and reduced activation in posterior lateral default network) may act as a protective factor, but the opposite pattern (reduced activation in medial and increased activation in posterior lateral default network) was found to be associated with disorder sequelae.
The current findings complement previous research using this paradigm to study neural correlates of irritability.18 Specifically, our prior work demonstrated that irritability severity is associated with different amygdala and ventral visual stream response in DMDD versus BD during the face emotion labeling task. However, irritability did not affect the main results in the present study on BD-related phenotypes. This suggests that irritability plays a different role in DMDD compared to BD, and it does not identify BD risk-related endophenotypes. Of note, although the most important findings in our prior paper were in the DMDD group,18 we also previously identified associations between irritability and temporal activation in the BD group, which were attenuated to trends or not significant with the larger sample of the present paper (Supplement 1, Results, available online). This could be due to a number of potential factors, including the possibility of multiple subgroups within the sample. Future replication attempts with a large, independent sample will be necessary.
Of note, questions have been raised in the literature recently regarding the appropriate methods for cluster-based thresholding in fMRI studies.22 We used a cluster-defining threshold of p < .005, which, while relatively conservative, may nonetheless be associated with type I error. Most of our findings are considerably larger than the minimum cluster size of k = 39 required for a whole-brain, cluster-corrected p < .05. However, some of our smaller findings in frontal regions (dorsolateral prefrontal, inferior frontal gyrus, insula) are close to this threshold; these findings require replication and should be interpreted with caution.
This study has several limitations. First, although our study includes data from three groups (N = 99), cell sizes are modest (n = 41 LR, n = 22 HR, and n = 36 BD).However, these sample sizes are larger than most fMRI face processing studies with HR youth (i.e., N = 13,11 N = 13,10 and N = 1513) and youth with BD (mean N = 19 [SD = 6.8] in a recent meta-analysis, sample sizes ranging from 10 to 3228). Nevertheless, results will need to be replicated with larger samples.
Second, although all HR youth have increased genetic risk of BD, most will never develop a manic episode. As individuals are unlikely to develop BD after age 25 years, we limited the HR sample to those less than 25 years of age to maximize the proportion who may ultimately develop BD. Our results remained after covarying age, suggesting that a subgroup of older, resilient participants was not influencing the analyses unduly (Supplement 1, Results, available online). Moreover, HR youths ranged in age from 9.8 to 24.8 years; however, no clusters had a significant group × age interaction effect, which suggests that age is not primarily driving our findings. Finally, HR youth with (n = 6) and without (n = 16, Table 1) psychiatric diagnoses do not differ on activation in any of the candidate resilience marker clusters (Table S2, available online). A design primarily focused on resilience markers would benefit from including only HR individuals past the age of risk for BD. Alternatively, future studies could address the inherent heterogeneity in HR outcomes by following a large sample of HR youth longitudinally. A longitudinal study, potentially with additional comparison groups, would also help to elucidate the specificity of these neural markers to BD versus depression, anxiety, or other disorders.
Finally, treatment with psychotropic medication is common, especially in BD.29 Of note, when covarying the number of medications, we found the same pattern of results as in the main analyses, although effects in some candidate disorder sequelae regions were attenuated (Supplement 1, Results, available online). Taken together, this suggests that drug treatment is not primarily driving our identification of risk and resilience markers, although medication may contribute to disorder sequelae. Studies of medication-naive individuals with BD might provide a more robust test of this but may not be feasible, as medication is the first-line treatment for BD.
By including BD, HR, and LR youth, we were able to examine potential risk and resilience markers and disorder sequelae, using an fMRI paradigm to probe the neural circuitry mediating face emotion labeling, previously shown to be impaired in youth with BD and HR youth. It is important to note that cross-sectional, correlational designs, such as the present study, cannot definitively indicate causality, only associations. Clearly, longitudinal research is needed, but our findings may have clinical implications in the future. Specifically, neural patterns that may be risk endophenotypes could potentially identify individuals at risk for BD and encourage them to receive prevention measures. Although the current study could be seen as a first step in developing a bipolar risk neural screening, fMRI would, of course, represent a very costly approach, and issues of sensitivity and specificity would need to be carefully considered. Longitudinal work and careful integration of clinical and neuroimaging data are needed to elucidate the best approach to early identification of at-risk individuals who will go on to develop BD. In any case, information about neural risk and resilience markers could increase our knowledge about the pathophysiology of BD—specifically, in helping to differentiate causes from effects of the illness—and in so doing, could perhaps help to identify novel approaches to prevention. &
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH)/the National Institutes of Health (NIH), conducted under NIH Clinical Study Protocols 02-M-0021 (ClinicalTrials.gov ID: NCT00025935) and 00-M-0198 (NCT00006177). Authors are U.S. federal government employees (NIMH IRP).
Drs. Wiggins, Chen, and Mr. Reynolds served as the statistical experts for this research.
The authors thank the Functional Magnetic Resonance Imaging Facility and Allison Oakes, BA, Elizabeth Harkins, BS, and Derek Hsu, BS, and other members of the Emotion and Development Branch at NIMH/NIH, for assistance with data collection and processing, as well as the families who participated.
Footnotes
Clinical trial registration information—Studies of Brain Function and Course of Illness in Pediatric Bipolar Disorder and Child and Adolescent Bipolar Disorder Brain Imaging and Treatment Study; http://clinicaltrials.gov/; NCT00025935 and NCT00006177.
Disclosure: Drs. Wiggins, Brotman, Adleman, Kim, Chen, Towbin, Pine, Leibenluft, Ms. Wambach, and Mr. Reynolds report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Lichtenstein P, Yip BH, Bjork C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60: 497–502. [DOI] [PubMed] [Google Scholar]
- 3.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. [DOI] [PubMed] [Google Scholar]
- 4.Glahn DC, Knowles EE, McKay DR, et al. Arguments for the sake of endophenotypes: examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson BS, Wang Z, Horga G, et al. Discriminating risk and resilience endophenotypes from lifetime illness effects in familial major depressive disorder. JAMA Psychiatry. 2014;71:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165:385–389. [DOI] [PubMed] [Google Scholar]
- 7.Seidel EM, Habel U, Finkelmeyer A, Hasmann A, Dobmeier M, Derntl B. Risk or resilience? Empathic abilities in patients with bipolar disorders and their first-degree relatives. J Psychiatr Res. 2012;46: 382–388. [DOI] [PubMed] [Google Scholar]
- 8.Brotman MA, Skup M, Rich BA, et al. Risk for bipolar disorder is associated with face-processing deficits across emotions. J Am Acad Child Adolesc Psychiatry. 2008;47:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegbreit E, Cushman GK, Puzia ME, et al. Developmental meta-analyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry. 2014;71:926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsavsky AK, Brotman MA, Rutenberg JG, et al. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51: 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng WL, Bones BL, Kayser RR, et al. An fMRI study of emotional face encoding in youth at risk for bipolar disorder. Eur Psychiatry. 2015; 30:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mourao-Miranda J, Almeida JR, Hassel S, et al. Pattern recognition analyses of brain activation elicited by happy and neutral faces in unipolar and bipolar depression. Bipolar Disord. 2012;14:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladouceur CD, Diwadkar VA, White R, et al. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev Cogn Neurosci. 2013;5: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breakspear M, Roberts G, Green MJ, et al. Network dysfunction of emotional and cognitive processes in those at genetic risk of bipolar disorder. Brain. 2015;138:3427–3439. [DOI] [PubMed] [Google Scholar]
- 15.Manelis A, Ladouceur CD, Graur S, et al. Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain. 2015;138:2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim P, Jenkins SE, Connolly ME, et al. Neural correlates of cognitive flexibility in children at risk for bipolar disorder. J Psychiatr Res. 2012;46:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deveney CM, Connolly ME, Jenkins SE, et al. Striatal dysfunction during failed motor inhibition in children at risk for bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiggins JL, Brotman MA, Adleman NE, et al. Neural correlates of irritability in disruptive mood dysregulation and bipolar disorder. Am J Psychiatry. 2016;173:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. [DOI] [PubMed] [Google Scholar]
- 21.Wiggins JL, Adleman NE, Kim P, et al. Developmental differences in the neural mechanisms of facial emotion labeling. Soc Cogn Affect Neurosci. 2016;11:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournier JC, Keener MT, Mullin BC, et al. Heterogeneity of amygdala response in major depressive disorder: the impact of lifetime subthreshold mania. Psychol Med. 2013;43:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida JR, Versace A, Mechelli A, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fears SC, Service SK, Kremeyer B, et al. Multisystem component phenotypes of bipolar disorder for genetic investigations of extended pedigrees. JAMA Psychiatry. 2014;71:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 27.Bora E, Bartholomeusz C, Pantelis C. Meta-analysis of Theory of Mind (ToM) impairment in bipolar disorder. Psychol Med. 2016;46:253–264. [DOI] [PubMed] [Google Scholar]
- 28.Lee MS, Anumagalla P, Talluri P, Pavuluri MN. Meta-analyses of developing brain function in high-risk and emerged bipolar disorder. Front Psychiatry. 2014;5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafeman DM, Bebko G, Bertocci MA, et al. Abnormal deactivation of the inferior frontal gyrus during implicit emotion processing in youth with bipolar disorder: attenuated by medication. J Psychiatr Res. 2014;58: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.