Abstract
Purpose
To describe the tolerability and efficacy of neratinib as a monotherapy and in combination with capecitabine in advanced HER2-positive breast cancer in a real-world setting.
Methods
Patients who received neratinib for advanced HER2-positive at the Royal Marsden Hospital NHS Trust between August 2016 and May 2020 were identified from electronic patient records and baseline characteristics, previous treatment and response to treatment were recorded. The primary endpoint of the study was progression-free survival (PFS). Secondary endpoints included overall survival (OS) and safety.
Results
Seventy-two patients were eligible for the analysis. Forty-five patients received neratinib in combination with capecitabine and 27 patients received monotherapy. After a median duration of follow-up of 38.5 months, the median PFS for all patients was 5.9 months (95% confidence interval (CI) 4.9–7.4 months) and median OS was 15.0 months (95% Cl 10.4–22.2 months). Amongst the 52.7% (38/72) patients with confirmed brain metastases at baseline, median PFS was 5.7 months (95% CI 2.9–7.4 months) and median OS was 12.5 months (95% CI 7.7–21.4 months). Despite anti-diarrhoeal prophylaxis, diarrhoea was the most frequent adverse event, reported in 64% of patients which was grade 3 in 10%. There were no grade 4 or 5 toxicities. Seven patients discontinued neratinib due to toxicity.
Conclusions
Neratinib monotherapy or in combination with capecitabine is a useful treatment for patients with and without brain metastases. PFS and OS were found to be similar as previous trial data. Routine anti-diarrhoeal prophylaxis allows this combination to be safely delivered to patients in a real-world setting.
Keywords: HER2-positive, Advanced breast cancer, Brain metastases, Tyrosine kinase inhibitor
Background
Approximately, 15–20% of metastatic breast cancers (MBCs) are characterised by overexpression or amplification of human epidermal growth factor receptor 2 (HER2), a biomarker that was historically associated with aggressive disease and poor overall survival [1, 2]. Novel therapeutics, in particular anti-HER2 monoclonal antibodies, have transformed outcomes for these patients, although the majority of patients with advanced disease will develop resistance to therapy and eventually succumb to their disease [3]. Intracerebral metastases, which affect up to 50% of patients with advanced HER2-positive breast cancer, cause considerable morbidity and mortality and the efficacy of anti-HER2 antibodies in this scenario is limited [4].
Tyrosine kinase inhibitors (TKIs) targeting the HER2 receptor have shown activity in the metastatic setting leading to the licenced approval of three agents [5–7]. Lapatinib, a TKI targeting both EGFR and HER2 was the first TKI to be approved for HER2-positive MBC in combination with the oral chemotherapy, capecitabine based on the results of a phase 3 study demonstrating benefit from the combination [8]. However, lapatinib is not a potent inhibitor of HER2 [9], leading to the development of a second generation of small-molecule HER2 inhibitors.
Neratinib is a highly potent, oral irreversible small-molecule tyrosine kinase inhibitor of EGFR, HER2 and HER4 [5] that has activity as both a single agent [10–12] and in combination with chemotherapy [13–16]. In women with MBC previously treated with chemotherapy and trastuzumab, the overall response rates range from 29 to 40% with neratinib alone [10–12]. Response rates of up to 64% have been observed in patients receiving neratinib in combination with capecitabine [15] and the phase 3 NALA trial established the combination’s superiority over capecitabine/lapatinib [16]. Importantly, neratinib is able to cross the brain barrier and has useful activity in patients with brain metastasis, with a CNS response rate of 49% reported in a phase 2 study in combination with capecitabine [17, 18]. These findings led to regulatory approval in the USA of neratinib both as monotherapy and in combination with capecitabine for treatment of metastatic or advanced HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting [19].
The most commonly reported side effect of neratinib is diarrhoea. In the extended adjuvant phase 3 ExteNET study over 95% of patients reported any grade of diarrhoea, with 40% of patient reporting grade 3 and < 1% grade 4 diarrhoea [20]. Subsequently, the CONTROL trial has shown that the use of routine anti-diarrhoeal prophylaxis improves tolerability [21], a finding confirmed in the phase 3 NALA trial [16].
To date, there are no published studies detailing the activity and toxicity of neratinib in advanced HER2-positive setting in a real-world setting. We present the experience of tolerability and efficacy of neratinib as monotherapy and in combination with capecitabine patients with advanced HER2-positive breast cancer at the Royal Marsden Hospital as part of the PUMA then Pierre Fabre pre-licence patient access scheme.
Methods
The study was approved by the Royal Marsden NHS Trust committee for clinical research as a service evaluation (Ref. SE768).
Eighty-seven patients were identified from the pharmacy database as having started neratinib between 31/08/2016 and 05/05/2020 and their electronic medical records were retrospectively reviewed. Patients were eligible for analysis if they had a diagnosis of advanced H2.7 ER2-positive breast cancer and received neratinib 240 mg/day either as monotherapy or in combination with capecitabine (1500 mg/m2/day on a 2 week on, 1 week off or 1 week on, 1 week off schedule). Neratinib was not initiated with a dose escalation approach. Routine testing for HER2 mutations was not performed during the study period; however, patients known to have HER2 mutations were excluded from the study. Patients receiving neratinib in the extended adjuvant setting for early HER2-positive breast cancer were also ineligible.
Patient, tumour and treatment characteristics were obtained from electronic patient records including time of diagnosis, previous treatment lines and sites of metastatic disease. Toxicity was graded by CTCAE version 4.0. Diarrhoeal prophylaxis with loperamide (4 mg four times a day on day 1; three times a day from day 2–14; twice daily for days 15–28 then as required) and budesonide 9 mg daily for 28 days on cycle 1 only was prescribed for all patients.
The primary endpoint of the study was progression-free survival (PFS) defined from the time of commencement of treatment until progression or death. A PFS event was recorded as the date of the response assessment. Patients free from progression were censored at the last follow-up cut-off date (14/12/2021) for the analysis. Secondary objectives were overall survival (OS), overall response rate (ORR), clinical benefit rate (CBR) and safety. OS was calculated from the time of commencement of neratinib until death from any cause. Surviving patients were censored at the last follow-up date. ORR was defined as having either stable partial or complete response radiologically. CBR was defined as partial or complete response or stable disease as best response for at least 24 weeks.
Descriptive analysis was used to summarise data using counts and percentages for categorical variables, and continuous non-normal variables using a median, range and interquartile range (IQR). Kaplan–Meier method was used for the calculation of PFS and OS time. Median time to event reported with 95% confidence interval (CI). Cox proportional hazard model was used in an exploratory comparison analysis to calculate hazard ratio with 95% confidence interval. STATA version 13.1 was used was used to undertake statistical analysis.
Results
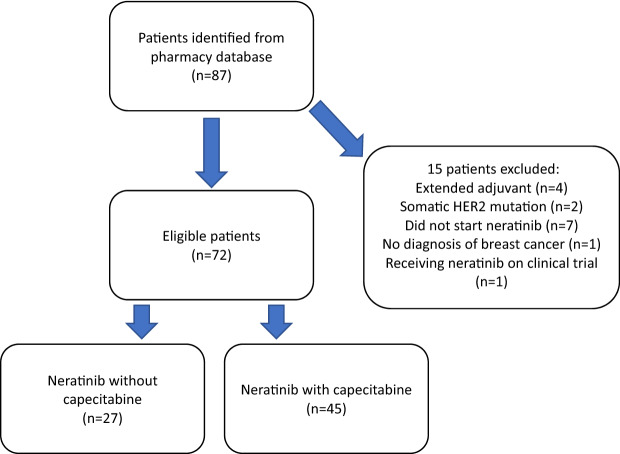
Seventy-two patients were identified as eligible (Fig. 1). Patient characteristics are outlined in Table 1. All patients in the study were female with a median age of 55 years (IQR 49–61; range 37–82 years). A median of 3 prior lines of treatment had been given in the advanced setting (IQR 2–4; range 0–7). Forty-five patients were treated with neratinib in combination with capecitabine and 27 received neratinib without capecitabine. Three patients were co-prescribed trastuzumab and 4 patients were co-prescribed endocrine therapy. Neratinib monotherapy was prescribed due to prior progression on capecitabine in 78% (21/27) monotherapy patients. At the time of treatment initiation 89% (64/72) patients had ECOG performance status of 0–1.
Fig.1.
CONSORT diagram
Table 1.
Patient and disease characteristics
| Variable | Total cohort (n = 72) | Neratinib monotherapy n = 27) | Neratinib with capecitabine (n = 45) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Age | |||
| < 65 | 58 (81) | 19 (70) | 39 (87) |
| ≥ 65 | 14 (19) | 8 (30) | 6 (13) |
| ECOG Performance status | |||
| 0 | 15 (21) | 3 (11) | 12 (27) |
| 1 | 49 (68) | 19 (70) | 30 (67) |
| 2 | 6 (8) | 3 (11) | 3 (7) |
| 3 | 2 (3) | 2 (7) | 0 (0) |
| Cutaneous disease | |||
| Yes | 14 (19) | 6 (22) | 8 (18) |
| Bone | |||
| Yes | 46 (64) | 17 (63) | 29 (64) |
| Visceral | |||
| Yes | 53 (74) | 21 (78) | 32 (71) |
| Brain metastasis | |||
| Yes | 38 (53) | 15 (56) | 18 (40) |
| Leptomeningeal disease | |||
| Yes | 7 (10) | 5 (19) | 4 (9) |
| Measurable disease | |||
| Yes | 63 (88) | 23 (85) | 40 (89) |
| Histology | |||
| IDC | 61 (85) | 25 (93) | 36 (80) |
| ILC | 6 (8) | 2 (7) | 4 (9) |
| Mixed IDC/ILC | 2 (3) | 0 (0) | 2 (4) |
| Unknown | 3 (4) | 0 (0) | 3 (7) |
| Grade | |||
| 1 | 2 (3) | 1 (4) | 1 (2) |
| 2 | 19 (26) | 8 (30) | 11 (24) |
| 3 | 48 (67) | 18 (67) | 40 (89) |
| Unknown | 3 (4) | 0 (0) | 3 (7) |
| ER status | |||
| Negative | 29 (40) | 11 (41) | 18 (40) |
| Positive | 42 (58) | 16 (59) | 26 (58) |
| Unknown | 1 (1) | 0 (0) | 1 (2) |
| ER Allred score: median (IQR), (range) | 6 (0–8), (0–8) | 7 (0–8), (0–8) | 5 (0–8), (0–8) |
| PgR status | |||
| Negative | 37 (51) | 12 (44) | 25 (56) |
| Positive | 26 (36) | 12 (44) | 14 (31) |
| Unknown | 9 (13) | 3 (11) | 6 (13) |
| PgR Allred score: median (IQR), (range) | 0 (0–5), (0–8) | 1 (0–5), (0–8) | 1 (0–4), (0–8) |
| HER2 status | |||
| IHC3 + | 58 (81) | 22 (81) | 36 (80) |
| IHC2 + /ISH+ | 11 (15) | 3 (11) | 8 (18) |
| HER2 Positive but IHC unknown | 3 (4) | 2 (7) | 1 (2) |
| Prior lines of treatment: median (IQR), (range) | 3 (2–4) (1–7) | 3 (3–5), (1–7) | 2 (2–3), (1–6) |
| Prior anti-HER2 TKI | |||
| Yes | 22 (31) | 13 (48) | 9 (20) |
| Prior pertuzumab | |||
| Yes | 35 (49) | 12 (44) | 23 (51) |
| Prior T-DM1 | |||
| Yes | 62 (86) | 24 (89) | 38 (84) |
| Prior Fulvestrant | 4 (6) | 4 (15) | 0 (0) |
| Prior Trastuzumab | 3(6) | 2 (7) | 1 (2) |
IDC Invasive ductal carcinoma, ILC Invasive lobular carcinoma
After a median follow-up of 38.5 months (95% CI 29.3–51.8 months), the median PFS was 5.9 months (95% CI 4.9–7.4 months). The PFS rate at 12 and 24 months were 19.4% (95% CI 11.3–29.3%) and 12.5% (CI 95% 6.1–21.2%), respectively. Patients receiving neratinib in combination with capecitabine had longer median PFS of 7.2 months (95% CI 5.8–10.0 months) compared with patients receiving neratinib alone with a PFS of 2.9 months (95% CI 2.3–5.8 months), a difference which was statistically significant (hazard ratio 0.38 (95% CI 0.23–0.65); p < 0.001) (Fig. 2).
Fig. 2.
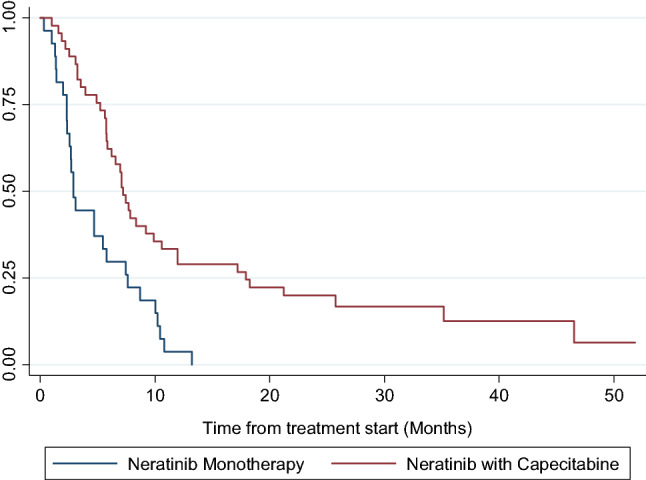
Kaplan Meier curve of PFS by treatment with monotherapy or combination with capecitabine for overall cohort. Neratinib in combination with capecitabine cohort had a median PFS of 7.2 months (95% CI 5.8–10.0). Neratinib monotherapy had median PFS 2.9 months (95% CI 2.3–5.8)
The median OS for the cohort was 15.0 months (95% CI 10.4–22.2 months). The OS rate at 12 and 24 months were 58.3% (95% CI 46.6–68.7%) and 30.0% (95% CI 19.3–41.4%) respectively. Patients receiving neratinib in combination with capecitabine had a longer median OS of 18.9 months (95% CI 12.5–27.6 months) compared to 7.7 months (95% CI 3.2–15.2 months) in those receiving neratinib as monotherapy, which was statistically significant (hazard ratio 0.42 (95% CI: 0.24 – 0.72); p = 0.001) (Fig. 3).
Fig. 3.
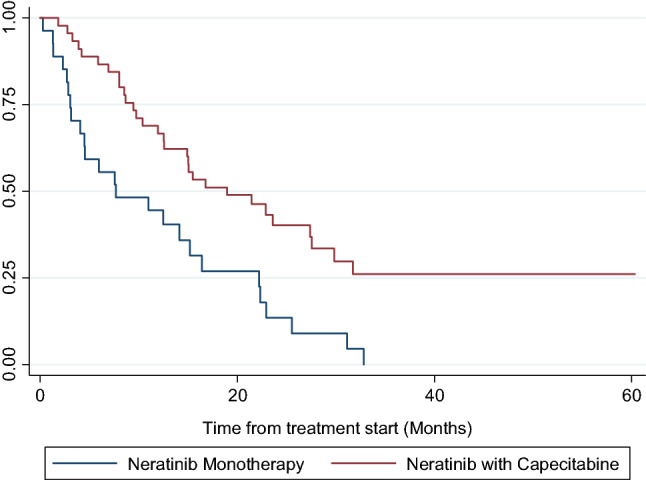
Kaplan–Meier curves of OS between patient groups treated with Neratinib monotherapy vs Neratinib with Capecitabine for overall cohort. Neratinib and capecitabine cohort had median OS of 18.9 months (95% CI 12.5–27.6). Neratinib monotherapy cohort had median OS of 7.7 months (95% CI 3.2–15.2)
The response rates for all patients can be seen in Table 2. The ORR in patients receiving a combination of neratinib and capecitabine was 47% (21/45) compared with 26% (7/27) in patients receiving monotherapy. Three patients receiving neratinib and capecitabine achieved complete radiological responses, two of whom remain on treatment at 22 months follow–up, the third relapsed after 50 months on treatment.
Table 2.
Response to treatment
| Response | Total cohort | Neratinib monotherapy | Neratinib with capecitabine |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| SD | 14 (19) | 4 (15) | 10 (22) |
| PR | 26 (36) | 7 (26) | 19 (42) |
| CR | 3 (4) | 0 (0) | 3 (7) |
| PD | 18 (25) | 7 (26) | 11 (24) |
| Non evaluable ψ | 11 (15) | 9 (33) | 2 (4) |
| ORR | 29 (40) | 7 (26) | 21(47) |
| CBR | 38 (64) | 7 (26) | 31 (69) |
Defined in the method section
SD stable disease, PR partial response, CR complete response, PD progressive disease, ORR objective response rate, CBR clinical benefit rate
Response radiologically as per reporting radiologist
ψ2 patients not evaluable radiologically due to cutaneous only disease; the other 9 patients progressed clinically before radiological assessment undertaken
The median PFS in the sub-group of 38 patients with confirmed brain metastases at initiation of neratinib was 5.7 months (95% CI 2.9–7.4 months) and median OS was 12.5 months (95% CI 7.7–21.4 months). The median PFS for patients without brain metastases was similar, although numerically longer at 7.2 months (95% CI 4.9–9.9 months) and median OS was 15.5 months (95% CI 11.9–30.9 months).
Adverse events are outlined in Table 3. Any grade of diarrhoea was reported in 64% of patients and 7 patients experienced grade 3 diarrhoea (10%). There were no recorded cases of grade 4 diarrhoea. Non-haematological grade 3–4 adverse events were documented in 18% (13/72) of patients and included diarrhoea (n = 6), vomiting (n = 4), transaminitis (n = 2) and pneumonitis (n = 1). The single case of pneumonitis was in a patient taking neratinib monotherapy and occurred following treatment with nitrofurantoin for a urinary tract infection.
Table 3.
Adverse events
| Total cohort | Neratinib monotherapy | Neratinib with capecitabine | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Grade 3–4 haematological AEs | 2 (3) | 0 (0) | 2 (4) |
| Grade 3–4 non-haematological AEs | 13 (18) | 7 (26) | 6 (13) |
| Any grade of diarrhoea | 46 (64) | 18 (67) | 28 (62) |
| Grades of diarrhoea | |||
| 1 | 27 (38) | 12 (44) | 15 (33) |
| 2 | 12 (17) | 4 (15) | 8 (18) |
| 3 | 7 (10) | 2 (7) | 5 (11) |
| Dose reduction | 17 (24) | 4 (15) | 13 (29) |
| Dose delaysa | 34 (47) | 13 (48) | 21 (47) |
AE Adverse event
aDose delay on at least one occasion
Forty seven percent of patients (34/72) required at least 1 dose delay, 22 of whom were receiving combination of neratinib and capecitabine. Of the 24% (17/72) patients requiring a dose reduction, 13 were receiving neratinib in combination with capecitabine. Dose reduction due to diarrhoea was required for 7 patients. The most common reason for discontinuation was progression of disease (Table 4). Discontinuation due to toxicity occurred in 7 patients due to diarrhoea (n = 1), nausea and vomiting (n = 2), combination of vomiting and diarrhoea (n = 3) or pneumonitis (n = 1). Of these patients 6 were receiving a combination of neratinib with capecitabine.
Table 4.
Reasons for discontinuation of treatment
| Reason for treatment discontinuation | Total n = 67 |
|---|---|
| n (%) | |
| PD | 55 (82) |
| Toxicity | 7 (10) |
| Patient choice | 1 (2) |
| Non cancer related deaths | 1 (2) |
| Other | 3 (4) |
Toxicity diarrhoea (n = 1), nausea and vomiting (n = 2), combination of vomiting and diarrhoea (n = 3) or pneumonitis (n = 1). Non cancer related deaths include 1 patient who died of COVID-19 and 2 patients admitted to local hospital with infection. Other: patient stopped due to discitis
Discussion
Although the current standard of care for the first-line treatment for metastatic HER2 breast cancer is clearly defined as dual anti-HER2 monoclonal antibodies in combination with a taxane chemotherapy on the basis of the CLEOPATRA data [22], there have been a number of recent developments which have provided novel options beyond the first-line setting. The antibody–drug conjugate (ADC) T-DM1 previously superseded oral chemotherapy with capecitabine in combination with the first anti-HER2 TKI, lapatinib based on the results of the EMILIA trial [23] but the recently presented DESTINY-BREAST-03 trial has defined a new standard of care in the novel ADC, trastuzumab deruxtecan (T-DXd) [24]. With increasing number of therapeutic options available, the optimal sequence of treatments beyond second-line is unclear however; two novel anti-HER2 TKIs have proven benefit in this setting. The HER2CLIMB study demonstrated a significant improvement in PFS, OS and response rate from the addition of the selective HER2 TKI, tucatinib, to trastuzumab and capecitabine in a large randomised phase 2 study, leading to regulatory approvals around the world. All patients were required to have received prior trastuzumab, pertuzumab, taxane and T-DM1, but none had received previous T-DXd. Notably, the study included patients with active or untreated CNS disease, demonstrating a similar magnitude of benefit in these patients, presenting this as a favourable second- or third-line option, in particular for patients with brain metastases, especially as the combination was very well-tolerated [25]. The results of the phase 3 NALA trial of neratinib in combination with capecitabine, compared to lapatinib and capecitabine also reported a statistically and clinically significant benefit from the pan-HER TKI, in patients who had received at least two lines of therapy for metastatic HER2-positive breast cancer, although neither pertuzumab nor T-DM1 was mandated. The median PFS was 5.6 months (95% CI 4.9–6.9 months), median OS was 21 months (95% CI 17.7–23.8 months) and the overall response rate was 32.8% [16].
In our study, the observed median PFS of 5.9 months (95% CI 4.7–7.4 months) for patients receiving the combination of capecitabine and neratinib was comparable to the median PFS reported the NALA trial (5.6 months; 95% CI 4.9–6.9 months) [16]. The ORR of 47% we report here for patients receiving the combination therapy is higher than reported in NALA (32.8%; 95% CI 27.1–38.9), likely a product of the RECIST definition being applied more strictly in the context of a phase 3 clinical trial. Of interest however, the rate is comparable to the CNS ORR of 49% reported with the doublet in the TBCRC 020 Trial and over half of our patients had brain metastases [17]. We report here a similar median PFS or OS for patients with or without brain disease, in a population not screened for brain metastases, which further supports this as a useful option for patients with as well as without brain disease. Consistent with previous reports [17] single agent neratinib was less effective in our cohort, with an ORR of 26%, median PFS of 2.9 months and median OS of 7.7 months.
The development of neratinib has been hampered by gastrointestinal side effects, in particular grade 3 diarrhoea. Grade 3 diarrhoea rates of 28–40% were reported in trials of monotherapy [12, 20], leading to the use of loperamide prophylaxis in combination with capecitabine in NALA, which reduced the rate to 24.4% [16]. In our study, all patients received loperamide and budesonide prophylaxis, and the rate of grade 3 diarrhoea was 10%. The regimen was otherwise generally well tolerated, with no grade 4 adverse events, but discontinuation due to toxicity in 10% patients; most commonly due to gastrointestinal adverse events.
One of the limitations of our study is the use of observational retrospective data in which the timing of response assessment was not uniform. The grading of adverse events was also dependent on clinicians’ contemporaneous grading of the adverse event or accurate recording of toxicity, and as such, it was not possible to assess duration of adverse events. Furthermore, this is a relatively small study from a single centre; however, this study provides real-world evidence that neratinib in combination with capecitabine can be an effective treatment for patients with advanced HER2-positive breast cancer.
Conclusion
This single-centre retrospective study demonstrates that neratinib as a monotherapy or in combination with capecitabine is well tolerated by the majority of patients when prescribed with appropriate anti-diarrhoeal prophylaxis, with a similar response rate, median PFS and median OS in the real-world setting as reported in previous clinical trials. Neratinib in combination with capecitabine should be considered a useful alternative for the treatment of metastatic HER2-positive disease in the third-line setting or beyond, in particular for patients with brain metastasis.
Author contributions
AO: Initiated the study, which was designed by AO, SS and KM. Material preparation, data collection and analysis were performed by NC, SS, KM and KL. The first draft of the manuscript was written by Niamh Cunningham and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors wish to acknowledge the support of the Royal Marsden NIHR Biomedical Research Centre for Cancer
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality, but are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Financial interests: Alicia Okines has received research funding from Pfizer and Roche; speakers fees from Pfizer, Lilly, Seagen, Gillead and Astra Zeneca; advisory boards for Roche, Seagen and Astra Zeneca and travel support from Lilly and Astra Zeneca. Alistair Ring has received research funding from PUMA and honoraria for advisory boards and speaker fees from Roche, Seagen, AZ-Daiichi-Sankyo. Stephen Johnston has received advisory board honoraria and research funding from PUMA. Nicholas Turner has received advisory board honoraria from Astra Zeneca, Bristol-Myers Squibb, Lilly, Merck Sharpe and Dohme, Novartis, Pfizer, Roche/Genentech, GlaxoSmithKline, Zentalis pharmaceuticals, Repare therapeutics, Arvinas and research funding from Astra Zeneca, BioRad, Pfizer, Roche/Genentech, Merck Sharpe and Dohme, Guardant Health, Invitae, Inivata, Personalis, Natera.
Ethical approval
The study was approved by the Royal Marsden NHS Trust committee for clinical research as a service evaluation (Ref. SE768).
Consent to participate
No consent was required for the use of link-anonymised patient data.
Consent to publish
Not relevant.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Cronin KA, Harlan LC, Dodd KW, Abrams JS, Ballard-Barbash R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. 2010;28(9):963–968. doi: 10.3109/07357907.2010.496759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luque-Cabal M, García-Teijido P, Fernández-Pérez Y, Sánchez-Lorenzo L, Palacio-Vázquez I. Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome It. Clin Med Insights Oncol. 2016;10(Suppl 1):21–30. doi: 10.4137/CMO.S34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 5.Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64(11):3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 6.Moulder SL, Borges VF, Baetz T, Mcspadden T, Fernetich G, Murthy RK, et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2+-advanced solid tumors, with an expansion cohort in HER2+ Metastatic Breast Cancer (MBC) Clin Cancer Res. 2017;23(14):3529–3536. doi: 10.1158/1078-0432.CCR-16-1496. [DOI] [PubMed] [Google Scholar]
- 7.Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1(2):85–94. [PubMed] [Google Scholar]
- 8.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 9.Kulukian A, Lee P, Taylor J, Rosler R, de Vries P, Watson D, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with Trastuzumab or Docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–987. doi: 10.1158/1535-7163.MCT-19-0873. [DOI] [PubMed] [Google Scholar]
- 10.Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28(8):1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 11.Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro G, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15(7):2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 12.Martin M, Bonneterre J, Geyer CE, Ito Y, Ro J, Lang I, et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer. 2013;49(18):3763–3772. doi: 10.1016/j.ejca.2013.07.142. [DOI] [PubMed] [Google Scholar]
- 13.Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2(12):1557. doi: 10.1001/jamaoncol.2016.0237. [DOI] [PubMed] [Google Scholar]
- 14.Awada A, Dirix L, Manso Sanchez L, Xu B, Luu T, Diéras V, et al. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2- positive metastatic breast cancer pretreated with anti- HER2 therapy. Ann Oncol. 2013;24(1):109–116. doi: 10.1093/annonc/mds284. [DOI] [PubMed] [Google Scholar]
- 15.Saura C, Garcia-Saenz JA, Xu B, Harb W, Moroose R, Pluard T, et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2014;32(32):3626–3633. doi: 10.1200/JCO.2014.56.3809. [DOI] [PubMed] [Google Scholar]
- 16.Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–3149. doi: 10.1200/JCO.20.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. doi: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurvitz SA, Saura C, Oliveira M, Trudeau ME, Moy B, Delaloge S, et al. Efficacy of neratinib plus capecitabine in the subgroup of patients with central nervous system involvement from the NALA trial. Oncologist. 2021;26(8):e1327–e1338. doi: 10.1002/onco.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Drug Administration (FDA). FDA approves neratinib for metastatic HER2-positive breast cancer [Internet]. 2020 [cited 2021 Sep 20]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neratinib-metastatic-her2-positive-breast-cancer. Accessed 20 Sept 2021
- 20.Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 21.Barcenas CH, Hurvitz SA, Di Palma JA, Bose R, Chien AJ, Iannotti N, et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann Oncol. 2020;31(9):1223–1230. doi: 10.1016/j.annonc.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 23.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab Emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortes J, Kim S-B, Chung W-P, Im S-A, Park Y, Hegg R, et al. LBA1 Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): Results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;1(32):S1287–S1288. doi: 10.1016/j.annonc.2021.08.2087. [DOI] [Google Scholar]
- 25.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality, but are available from the corresponding author on reasonable request.