Abstract
Aim
The Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) showed that tafamidis reduced all-cause mortality and cardiovascular-related hospitalizations in patients with transthyretin amyloid cardiomyopathy (ATTR-CM). This study aimed to estimate the impact of tafamidis on survival and quality-adjusted life-years (QALYs).
Methods and results
A multi-state, cohort, Markov model was developed to simulate the disease course of ATTR-CM throughout a lifetime. For survival extrapolation, survival curves were fitted by treatment arm and New York Heart Association (NYHA) Class I/II (68% of patients) and NYHA Class III (32% of patients) cohorts using the individual patient-level data from both the ATTR-ACT and the corresponding long-term extension study. Univariate and multivariate sensitivity analyses were conducted. The predicted mean survival for the total population (NYHA Class I/II + III) was 6.73 years for tafamidis and 2.85 years for the standard of care (SoC), resulting in an incremental mean survival of 3.88 years [95% confidence interval (CI) 1.32–5.66]. Of the 6.73 life-years, patients on tafamidis spend, on average, 4.82 years in NYHA Class I/II, while patients on SoC spend an average of 1.60 life-years in these classes. The combination of longer survival in lower NYHA classes produced a QALY gain of 5.39 for tafamidis and 2.11 for SoC, resulting in 3.29 incremental QALYs (95% CI 1.21–4.74) in favour of tafamidis.
Conclusion
Based on the disease simulation model results, tafamidis is expected to more than double the life expectancy and QALYs of ATTR-CM patients compared to SoC. Longer-term follow-up data from the ATTR-ACT extension study will further inform these findings.
Clinical trials.gov identifier
NCT01994889 (date of registration: 26 November 2013).
Keywords: Amyloidosis, Mortality, Tafamidis, Transthyretin, Cardiomyopathy
Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a progressive infiltrative cardiomyopathy in which amyloid fibrils composed of misfolded transthyretin protein accumulate in the heart, impairing myocardial function over time and leading to progressive heart failure (HF) and death.1,2 ATTR-CM is classified as a rare disease, and traditionally, treatments have been limited to supportive care and, in rare cases, heart transplant.3,4
Prior to 2019, there were no proven pharmacotherapies for the treatment of ATTR-CM, and prognosis has been typically poor, with a median survival of 2–6 years.5–8 Tafamidis was recently approved and received orphan designation in several countries, including the USA, Japan, and the European Union (EU) for the treatment of ATTR-CM, based on the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT).9–11
ATTR-ACT was a global, phase III, placebo-controlled, randomized clinical trial assessing patients with hereditary and wild-type ATTR-CM.12 The ATTR-ACT showed that tafamidis was associated with a reduction in all-cause mortality [hazard ratio (HR) 0.70, 95% confidence interval (CI) 0.51–0.96], decrease in cardiovascular (CV)-related hospitalizations (relative risk ratio 0.68, 95% CI 0.56–0.81), and reductions in the decline of functional capacity and quality of life when compared with standard of care (SoC).13 After 30 months, all trial participants were allowed to continue or start tafamidis in an open-label extension study.14
Preliminary data from the ongoing open-label extension study showed continued survival improvements at 49 months in patients treated with tafamidis who had initiated treatment from ATTR-ACT [HR 0.64 (95% CI 0.47–0.85)].
Considering that the median overall survival has not been reached for the tafamidis arm and that the ATTR-ACT and extension studies showed continued separation of the overall survival curves for the two treatment arms, the shape of the survival curve predicting the full survival benefit for tafamidis is still unknown.13–15 To examine the potential long-term outcomes associated with tafamidis treatment, we used a disease simulation model that was informed by patient-level data from ATTR-ACT and the open-label extension study to estimate the impact of tafamidis treatment (mean difference from SoC) over a patient's lifetime.13,14
Methods
Model structure
A flexible probabilistic multi-state Markov model was developed in Microsoft Excel to simulate the disease course and survival associated with tafamidis vs. SoC treatment in patients with ATTR-CM in the USA over a lifetime horizon (maximum of 30 years). The model was probabilistic such that it runs multiple iterations of a cohort (1000 patients) with randomly selected baseline characteristics to define the ‘average’ characteristics of that cohort. The model structure was developed in line with previously published reviews and models in the HF indication, which modelled different rates of progression by the New York Heart Association (NYHA) functional classification—a clinician-rated assessment intended to evaluate the impact of patient symptoms on functional capacity.16–18 The prognosis of HF and ATTR-CM patients has been shown to worsen with higher NYHA functional classification stages.13,19 As such, the NYHA functional classification is considered a predictor of health-related quality of life and survival, making it an excellent patient-centric measure of disease burden.18,20,21
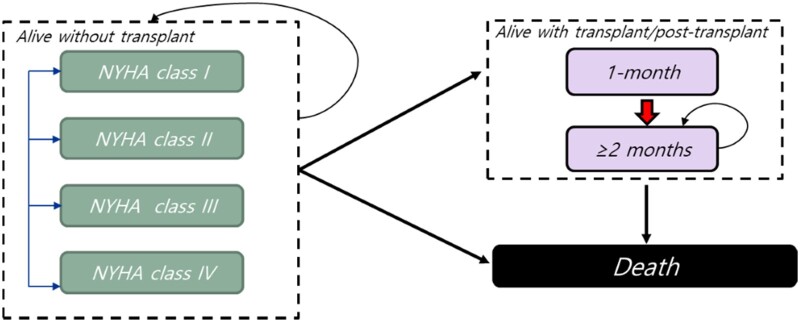
The model simulates patients' transitions to three main disease-specific health states: (i) alive without (heart) transplant, (ii) alive with (heart) transplant, and (iii) dead. The ‘alive without (heart) transplant’ state is subdivided into the four NYHA class stages to model disease progression. In contrast, the ‘alive with (heart) transplant’ state is subdivided into the first month and subsequent months following a heart transplant (Figure 1).22 Given the rarity of heart transplants in patients with ATTR-CM in the USA, the base case analysis did not allow patients to transition to the ‘alive with (heart) transplant’ state. Instead, the transplant probabilities were set to zero in the base case to reflect the real-world circumstances.23 To test the impact of this assumption on outcomes, various probability values of receiving a transplant were assessed as part of the sensitivity analyses.
Figure 1.
Model structure. NYHA, New York Heart Association Functional Classification.
Patient characteristics
The characteristics of the modelled patient cohort reflect the average patient characteristics of those included in the ATTR-ACT.13 An overview of patient characteristics is shown in Table 1.
Table 1.
Overview of patient and model characteristics
| Model parameter | Base case value | Source(s) |
|---|---|---|
| Model characteristics | ||
| Setting | USA | NA |
| Time horizon | 30 years | Caro et al. 201247 |
| Cycle length | 1 month | Caro et al. 201247 |
| Patient characteristics | ||
| % NYHA Class I/II at baseline | 68% | ATTR-ACT13 |
| % NYHA Class III at baseline | 32% | ATTR-ACT13 |
| % NYHA Class IV at baseline | 0.00% | ATTR-ACT13 |
| Age at model entry | 74 years | ATTR-ACT13 |
| Efficacy data | ||
| All-cause mortality extrapolation function—tafamidis |
NYHA Class I/II: Gompertz NYHA Class III: Weibull |
ATTR-ACT13 |
| All-cause mortality extrapolation function—SoC |
NYHA Class I/II: Gompertz NYHA Class III: Weibull |
ATTR-ACT13 |
| NYHA class transition probabilities (by treatment) | Based on ATTR-ACT for 0–30 months, for extrapolations based on data from Months 24–30 for SoC or Months 30–48 for tafamidis | ATTR-ACT13 |
| Utilities, mean (SE) | ||
| Tafamidis |
NYHA Class I: 0.874 (0.01) NYHA Class II: 0.832 (0.01) NYHA Class III: 0.707 (0.01) NYHA Class IV: 0.558 (0.06) |
ATTR-ACT13 and US value set30 |
| SoC |
NYHA Class I: 0.893 (0.02) NYHA Class II: 0.802 (0.01) NYHA Class III: 0.706 (0.01) NYHA Class IV: 0.406 (0.06) |
ATTR-ACT13 and US value set30 |
ATTR-ACT, Tafamidis in Transthyretin Cardiomyopathy Clinical Trial; NA, not applicable; NYHA, New York Heart Association Functional Classification; SE, standard error; SoC, standard of care.
Clinical data and survival modelling
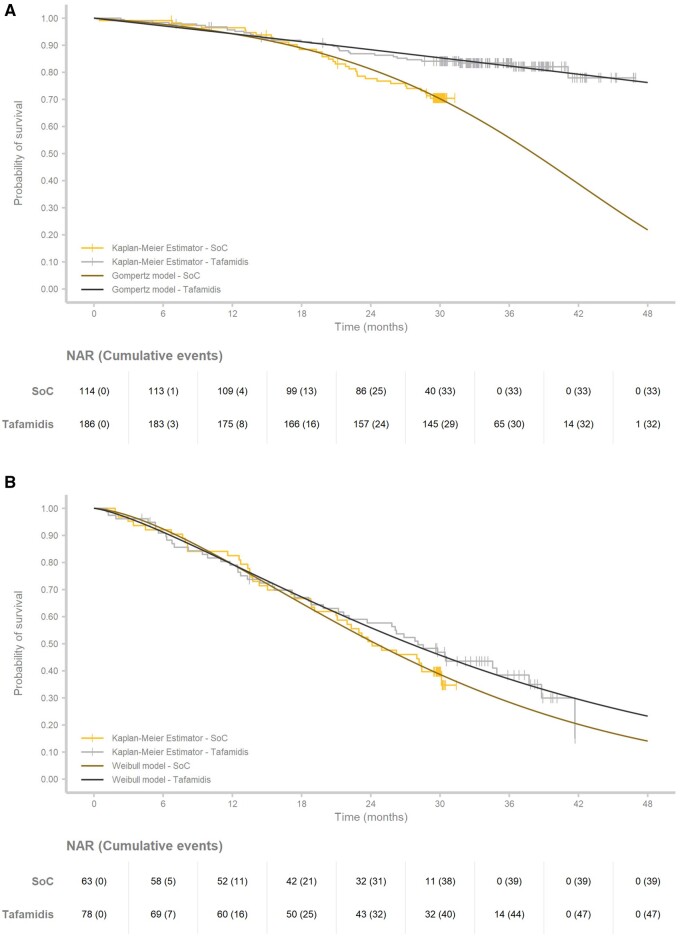
Clinical inputs from two sources were included: (i) the 30-month follow-up data from ATTR-ACT assessing SoC (n = 177) vs. the pooled tafamidis cohorts (20 mg dose and 80 mg dose; n = 264) and (ii) the 49-month follow-up data from the open-label extension study of participants who continued tafamidis treatment since the start of ATTR-ACT (n = 264).13,14 Since the research question concerned a lifetime survival comparison of tafamidis vs. SoC, using the SoC data from the extension study would produce biased results due to the crossover of all SoC-treated patients to tafamidis at Month 30.14 Therefore, only data up to 30 months from the intent-to-treat analysis of ATTR-ACT were used for the SoC arm, and the 49-month extension study data were used for tafamidis.13,14 Furthermore, given that NYHA classification is prognostic for survival, and previous HF models modelled disease progression based on NYHA classifications, the survival outcomes for NYHA Class I/II and NYHA Class III patients were assessed separately (Figure 2).
Figure 2.
Overview of observed and predicted survival for standard of care and tafamidis for (A) New York Heart Association I/II patients and (B) New York Heart Association III. NAR, number at risk; SoC, standard of care.
In-trial overall survival Kaplan–Meier (KM) curves from patient-level data for the tafamidis and SoC arms were extrapolated beyond observed time points from ATTR-ACT and its extension study by following international extrapolation guidelines.24 To mimic reality, patients undergoing a transplant or receiving a left ventricular assist device (LVAD) were treated as censored at the time of the procedure, rather than treated as death as in the clinical trial statistical analysis. As noted earlier, patients undergoing transplants were considered separately in the model to capture transplant outcomes. The proportional hazard (PH) assumption was tested to determine whether independent survival models should be applied in each treatment arm.25 Based on the scaled Schoenfeld residuals, evidence of a violation of the PH assumption was found in the most prevalent NYHA Class I/II cohort; consequently, individual parametric models were fitted by treatment and NYHA class cohort for extrapolation (Supplementary material online, Figure S5).
For extrapolation purposes, the following seven commonly used parametric survival models were considered and fitted to the clinical data following guidelines: exponential, generalized gamma, gamma, Gompertz, log-logistic, log-normal, and Weibull.24 The parametric distribution analyses were conducted using the ‘Survival’ and ‘Flexsurv’ packages in R for Statistical Computing version 3.5.0.26–28 The best fitting parametric distributions were selected based on visual inspection, goodness-of-fit statistics for survival analyses, including Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), as well as clinical plausibility.24 The lifetime outcomes, in terms of survival and quality of life, were first estimated per NYHA Class I/II and NYHA Class III stratified cohorts, after which the weighted mean outcomes were calculated for the entire population of ATTR-ACT based on the underlying baseline NYHA class distribution in ATTR-ACT.
Utility estimates
The ATTR-ACT collected quality of life data from both the Kansas City Cardiomyopathy Questionnaire (KCCQ), a functional and clinical assessment tool for patients with HF, as well as the three-level version of the EQ-5D (EQ-5D-3L), a self-administered generic health status instrument.13,29,30 Since the latter is a preferred tool by various health technology assessment bodies, the utility values with the EQ-5D-3L value set for the USA was used in the model.13,30 The utility weights were stratified by treatment arm and NYHA class. As part of the sensitivity analyses, alternative utility assumptions were assessed. Table 1 presents an overview of the model characteristics and parameters.
Outcomes
The primary outcomes of interest were (i) the mean and incremental survival in life-years gained and (ii) the mean and incremental quality-adjusted life-years (QALYs) gained. The means and their 95% CIs were estimated by bootstrapping from the distributions defined over the following parameters: utilities by health state and treatment; parametric survival extrapolation distributions by NYHA cohorts and treatment; the treatment-specific transition probabilities over the NYHA cohorts; and the distribution of mortality parameter alongside the transition probabilities to distribute alive patients into NYHA classes after Month 30.31
Sensitivity analyses
The effect of various parametric distributions, incremental mean life-years, and QALYs were assessed. Parametric distributions that increased the AIC/BIC for the SoC arm by more than 2 points compared to the best fitting distribution were disregarded from the analyses, as models with <2 points difference are well supported as suitable model selections.32 The same distribution was applied for both treatment arms per NYHA cohort to limit the number of scenarios. If the predicted mortality hazards in the probabilistic sensitivity analysis were lower than the general population hazards, the general population hazards were applied in the model to avoid biased predictions.33,34 Uncertainties regarding the impact of transplant rates were assessed through scenario analyses. The 30-month probabilities of transplant per treatment and NYHA stage cohort were set to reflect the rates observed in ATTR-ACT, which were 2.3% for SoC and 2.7% for tafamidis.13
With the introduction of tafamidis, it is expected that patients will be identified at earlier NYHA functional classification stages due to the increased awareness and adoption of non-invasive imaging modalities, such as bone scintigraphy and cardiac magnetic resonance imaging (MRI). In ATTR-ACT, 31.97% of the patients were categorized as NYHA Class III.35–38 To assess the impact of earlier diagnosis, the percentage of NYHA Class I/II patients at model entry (base case 68.03%) ranged from 0% to 100% to assess the mean expected (incremental) survival and QALY.
Additional sensitivity analyses assessed the impact of utility values. In one scenario, we assumed no difference between treatments by applying the SoC arm's utility value from ATTR-ACT to both arms in the model.13 In a second scenario, the utility used in the post-transplant setting was set at 0.76 in line with what has been reported for late-stage HF.22
Besides the tremendous burden for patients, there is also a burden for caregivers of patients with ATTR-CM.39 In this scenario, a disutility of 0.01 was applied to the NYHA Class IV health state utility to consider the caregiver burden.40
Results
Extrapolations
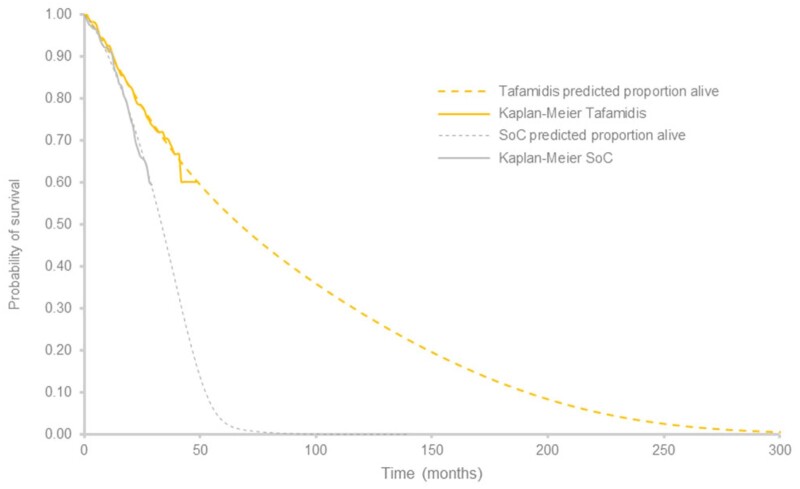
In the base case, the Gompertz and the Weibull distributions were selected to extrapolate overall survival for the NYHA I/II and the NYHA III cohorts, respectively. The parametric survival distributions for the NYHA I/II and NYHA III cohorts fitted to the trial data are presented in Figure 2. Statistically, these distributions provided the best fits (Supplementary material online, Tables S3–S6) and clinically plausible estimates for both treatment groups. As determined by visual inspection, the survival extrapolations fit the overall survival KM plots observed in ATTR-ACT and extension study for both tafamidis and SoC (Figure 3).
Figure 3.
Predicted extrapolation and overall survival Kaplan–Meier for tafamidis and standard of care arms from Tafamidis in Transthyretin Cardiomyopathy Clinical Trial. SoC, standard of care.
Base case results
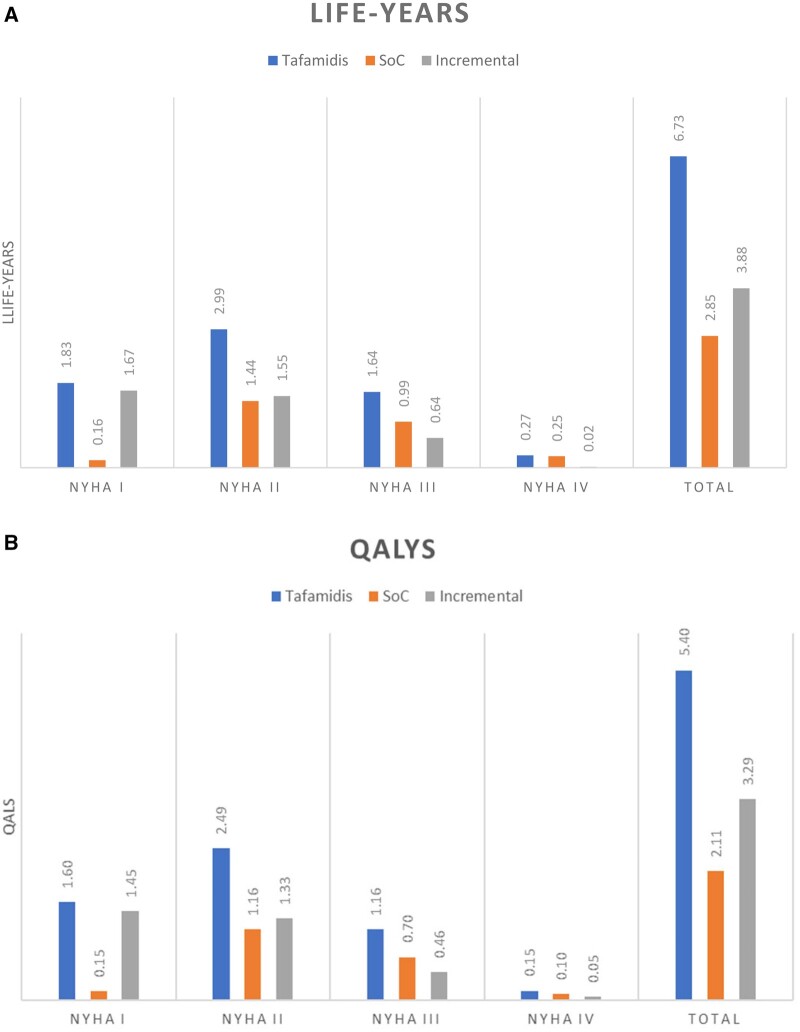
The model predicts a higher mean life expectancy for patients with ATTR-CM treated with tafamidis [6.73 years (95% CI 4.21–8.34)] compared to those treated with SoC [2.85 (95% CI 2.5–3.34)], resulting in an incremental life expectancy of 3.88 years (95% CI 1.32–5.66) in favour of tafamidis (Figure 4A). Of the total life-years, patients treated with tafamidis remain longer in the early NYHA stages (i.e. Class I and II), with an estimated life expenditure of 1.83 years (27.2%) in NYHA Class I, 2.99 years (44.4%) in NYHA Class II, 1.64 years (24.4%) in NYHA Class III, and 0.27 years in NYHA Class IV (4.0%) (Figure 4A). Compared to those receiving SoC, patients on tafamidis increased the total amount of time spent in NYHA Class I/II by 201% from 1.60 to 4.82 life-years.
Figure 4.
Overview of (A) the mean and incremental mean survival and (B) quality-adjusted life-years by health state as predicted by the model. NYHA, New York Heart Association Functional Classification; QALYs, quality-adjusted life-years; SoC, standard of care.
Similarly, tafamidis provides more QALYs [5.39 QALYs (95% CI 3.35–6.79)] compared to the SoC arm [2.11 QALYs (95% CI 1.88–2.42)] in patients with ATTR-CM, resulting in an increment of 3.29 QALYs (95% CI 1.21–4.74). The QALYs spent in the NYHA Class I/II cohorts increased from 1.31 to 4.09 QALYs when comparing SoC with tafamidis (Figure 4B).
Sensitivity analyses
Based on the probabilistic sensitivity analyses conducted, the impact of different parametric distributions on the results was moderate. The most substantial reduction of incremental QALYs and life-years was observed when the gamma (NYHA Class I/II) and the Weibull (NYHA Class III) distributions were selected, which resulted in a 29.4% reduction in incremental life-years and 15.8% reduction in incremental QALYs when compared to the base case. The largest increase in incremental QALYs and life-years was observed after selecting the Gompertz (NYHA Class I/II) combined with the generalized gamma (NYHA Class III), which resulted in an 11.6% increase in incremental life-years and a 12.5% increase in incremental QALYs when compared to the base case. Table 2 presents the results of the alternative parametric distributions tested in the probabilistic sensitivity analyses.
Table 2.
Overview of the impact of the base case and scenario analyses on the outcomes
| Life-years (95% CI) | QALYs (95% CI) | |||||
|---|---|---|---|---|---|---|
| Base case | Tafamidis | SoC | Incremental | Tafamidis | SoC | Incremental |
| NYHA I/II—Gompertz | 6.73 | 2.85 | 3.88 | 5.39 | 2.11 | 3.29 |
| NYHA III—Weibull | (4.21–8.34) | (2.5–3.34) | (1.32–5.66) | (3.35–6.79) | (1.88–2.42) | (1.21–4.74) |
| Sensitivity analyses: parametric distributions by NYHA cohorts | ||||||
| NYHA I/II—log-logistic | 7.52 | 4.3 | 3.22 | 6.03 | 2.84 | 3.19 |
| NYHA III—Weibull | (6.08–8.3) | (3.27–5.4) | (1.44–4.64) | (4.85–6.76) | (2.27–3.5) | (1.85–4.1) |
| NYHA I/II—Weibull | 7.09 | 3.4 | 3.69 | 5.69 | 2.41 | 3.27 |
| NYHA III—Weibull | (4.87–8.24) | (2.75–4.33) | (1.37–5.12) | (3.87–6.67) | (2.02–2.94) | (1.47–4.36) |
| NYHA I/II—gamma | 6.95 | 4.2 | 2.74 | 5.57 | 2.81 | 2.77 |
| NYHA III—Weibull | (3.08–8.69) | (1.78–8.31) | (−2.21 to 6.43) | (2.45–7.1) | (1.32–5.03) | (−0.67 to 5.32) |
| NYHA I/II—Gompertz | 6.65 | 2.78 | 3.87 | 5.33 | 2.06 | 3.27 |
| NYHA III—Gompertz | (4.15–8.24) | (2.47–3.28) | (1.34–5.61) | (3.32–6.71) | (1.84–2.39) | (1.21–4.7) |
| NYHA I/II—Gompertz | 7.04 | 3.11 | 3.93 | 5.64 | 2.24 | 3.4 |
| NYHA III—log-logistic | (4.5–8.75) | (2.69–3.65) | (1.32–5.78) | (3.6–7.07) | (1.97–2.57) | (1.3–4.86) |
| NYHA I/II—Gompertz | 6.82 | 2.96 | 3.87 | 5.47 | 2.16 | 3.31 |
| NYHA III—gamma | (4.18–8.93) | (2.29–4.12) | (0.86–6.18) | (3.34–7.19) | (1.72–2.84) | (0.94–5.11) |
| NYHA I/II—Gompertz | 7.4 | 3.07 | 4.33 | 5.92 | 2.22 | 3.7 |
| NYHA III—G. gamma | (3.87–10.86) | (2.52–4.69) | (0.49–8.02) | (3.13–8.7) | (1.88–3.07) | (0.79–6.56) |
| Sensitivity analyses: transplant rate | ||||||
| Transplants as observed in the trial | 7.1 (4.62–8.7) | 3.28 (2.84–3.97) | 3.82 (1.24–5.6) | 5.7 (3.67–7.03) | 2.45 (2.13–2.91) | 3.25 (1.19–4.68) |
| Sensitivity analyses: utility estimates | ||||||
| Equal utilities SoC and tafamidis | 6.73 (4.21–8.34) | 2.85 (2.5–3.34) | 3.88 (1.32–5.66) | 5.3 (3.3–6.7) | 2.11 (1.88–2.42) | 3.19 (1.15–4.66) |
| Considering caregiver disutility | 6.73 (4.21–8.34) | 2.85 (2.5–3.34) | 3.88 (1.32–5.66) | 5.39 (3.35–6.79) | 2.11 (1.88–2.42) | 3.29 (1.21–4.74) |
| Sensitivity analyses: NYHA class at diagnoses | ||||||
| 100% NYHA I/II | 8.57 (4.95–10.91) | 3.08 (2.64–3.78) | 5.49 (1.75–8.04) | 6.95 (4.01–8.97) | 2.32 (2.02–2.75) | 4.62 (1.62–6.7) |
| 100% NYHA III | 2.8 (2.19–3.57) | 2.36 (1.86–2.97) | 0.44 (−0.41 to 1.34) | 2.09 (1.62–2.7) | 1.64 (1.31–2.01) | 0.45 (−0.15 to 1.11) |
CI, confidence interval; G. gamma, generalized gamma; NYHA, New York Heart Association Functional Classification; SoC, standard of care; QALY, quality-adjusted life-years.
When assuming heart transplant probabilities of 2.3% for SoC and 2.7% for tafamidis in line with the 30-month data from ATTR-ACT, there was a decrease in incremental life-years gained from 3.88 to 3.82 (1.5% decrease) and in QALYs from 3.54 to 3.51 (0.85% decrease).13 Assuming equal utilities by health state for tafamidis and SoC reduced the incremental QALYs from 3.29 to 3.19 (3.1% decrease). The impact of caregiver disutility did not affect incremental life-years or QALYs.
When assuming that 100% of patients would be diagnosed at NYHA Class I/II stages (vs. 68% in ATTR-ACT), the predicted incremental life-years for tafamidis improved from 3.88 to 5.49 years (41.5% increase), as well as the incremental QALYs from 3.29 to 4.62 (40.4% increase). Even if all patients were diagnosed and initiated treatment in NYHA Class III, tafamidis was still associated with a life-year gain of 0.44 (18.6% increase compared to SoC) and a corresponding gain in QALYs of 0.45, reflecting a 27.4% increase compared to SoC. Table 2 presents the results of the alternative values tested in the scenario analyses.
Discussion
Tafamidis is the first-in-class transthyretin stabilizer approved for the treatment of ATTR-CM.41 The pivotal study, ATTR-ACT, showed tafamidis improved survival and decreased CV-related hospitalizations.13 In the present study using a flexible disease simulation model based on data from ATTR-ACT and its extension study, we show that tafamidis was associated with a mean survival of 6.73 years as compared to 2.85 years for SoC, resulting in an incremental mean survival of 3.88 years (95% CI 1.32–5.66).13,14 Of the 6.73 life-years, patients treated with tafamidis spend an average of 4.82 years in NYHA Class I/II, while patients on SoC spend an average of only 1.60 life-years in these early NYHA stages. The combination of longer survival in NYHA classes I/II for tafamidis compared to SoC resulted in a QALY gain of 5.39 and 2.11, respectively, resulting in 3.29 incremental QALYs (95% CI 1.21–4.74) in favour of tafamidis.
Sensitivity analyses demonstrated moderate impact on the results based on the various parametric distributions selected for both SoC and tafamidis, with variations ranging from a reduction of 29.4% and an increase of 11.6% in incremental life-years, and a reduction of 15.8% to an increase of 12.5% for incremental QALYs when compared to the base case results. Similar conclusions were derived for scenario analyses with differing utility estimates and heart transplantation rates.
With the increased awareness of ATTR-CM and the adoption of non-invasive imaging modalities such as bone scintigraphy and cardiac MRI, the percentage of patients diagnosed in NYHA Class I/II will likely increase in the future. The scenario analysis showed that if 100% of the patients were detected in NYHA Class I/II and subsequently treated with tafamidis instead of SoC, the incremental survival would improve by 41.5%. We assumed that the age of treatment initiation would be unchanged for these analyses, and only the proportion of patients by NYHA class varied. Of course, this simplifies the clinical reality, as one would expect that earlier diagnosis would also result in an earlier age of diagnosis. We hypothesize that if the average age of diagnosis decreased, then the health benefits of tafamidis would probably increase; however, further research is needed to confirm these potential benefits.
In a recent publication by Kazi et al.42, a cost-effectiveness model estimated mean survival for SoC and tafamidis at 3.46 and 5.43 years, respectively, resulting in an incremental mean survival of 1.97 years in favour of tafamidis. Although their survival estimate favours tafamidis, the lower incremental gain compared to our model can be explained by the different model structures, assumptions, and inputs. First, Kazi et al.42 used the 30-month data-cut of ATTR-ACT for survival extrapolation, while our model benefitted from the availability of the open-label extension study data, which showed a continued overall survival divergence between both arms. Using survival data from a longer follow-up period reduced the uncertainty in the extrapolations for the tafamidis arm. The sensitivity analyses tested different extrapolation models, resulting in similar outcomes as those in the base case. Unlike our approach, Kazi et al.42 assumed constant HRs to extrapolate survival of tafamidis, which likely underestimated the effect of tafamidis considering the observed separation of the overall survival curves for tafamidis and placebo in ATTR-ACT and its long-term extension studies. Furthermore, Kazi et al.42 considered heart transplants and implantation of LVAD as deaths in their analyses, which may have underestimated survival in their extrapolations.
Another significant difference of the model by Kazi et al. is the model structure, which does not take NYHA classes into account and inadequately captures all potential benefits of the tafamidis treatment, such as delayed disease progression. Tafamidis is a stabilizer of ATTR-CM disease, and the timing of its initiation is crucial in determining its lifetime efficacy for the individual patient. In our model, the NYHA Class I/II and NYHA Class III patients were separately modelled since they are two distinct cohorts in ATTR-ACT and the extension study, whereas the Kazi et al. study pooled all patients regardless of NYHA class. There were two reasons for applying this approach. First and most importantly, a substantial difference was observed in the survival and disease progression of the NYHA Class I/II cohort at baseline compared to the NYHA Class III cohort at baseline. The present model simulated NYHA subgroups separately in line with ATTR-ACT findings and several published literature reviews on the HF modelling and health technology assessment survival extrapolation guidance.17,24,43 Therefore, the present model should be considered a more comprehensive analysis of the impact of tafamidis treatment. Second, the proportion of patients in NYHA Class III was slightly lower for the tafamidis treatment arm compared to the SoC arm in ATTR-ACT.13 Applying the weighted modelling approach resulted in an equal proportion of patients in NYHA Class III for both the SoC and tafamidis arm. Therefore, the subgroup analyses can be considered as a conservative approach for tafamidis.8
Our study has strengths and limitations. One advantage of the current model is the lifetime prediction of outcomes based on a ‘hard’ clinical endpoint, such as overall survival.44–46 Another advantage of the disease simulation model is its structure, which explicitly modelled disease progression. This structure is in line with previously published HF models that have explicitly considered ‘alive’ and ‘dead’ and recommended using NYHA classes as health states when feasible.16,17 Using different NYHA classes is a logical choice for utility purposes, given that utilities vary by classification and the effect of tafamidis on disease progression by NYHA class.
Despite these strengths, some limitations to the model should be emphasized. In the model base case, we did not include heart transplant rates as these are rare in ATTR-CM patients in the USA.3,4 However, a scenario analysis including heart transplant rates showed results were not sensitive to this assumption. Moreover, the model relies on parametric extrapolations on data derived from tafamidis patients who had not reached the median survival for the NYHA I/II cohort. Consequently, several different parametric distributions were tested as part of the sensitivity analyses, demonstrating that the base case results were relatively stable across the various parametric distributions. In general, the limited availability of data is a primary challenge to ATTR-CM modelling, as it is a rare, infrequently diagnosed condition. As such, the natural history of the condition has not been studied in large, longitudinal datasets. This poses limitations on the complexity of the model structure and the prediction of future events. A future research area will be the development of patient simulation models, which could better consider patient heterogeneity and predict the probability of events based on individual patient characteristics and history. Such a model structure requires comprehensive data sources not currently available, although possible in the future, as ATTR-CM is more frequently diagnosed and more considerable, long-term datasets are collected. Moreover, ATTR-ACT included a smaller percentage of patients with hereditary ATTR-CM (24%), limiting our ability to build separate models for each ATTR-CM genotype.
Finally, the current model used the pooled efficacy data from the two tafamidis arms (20 mg dose and 80 mg dose) vs. SoC as assessed in ATTR-ACT.13,14 The extension study of ATTR-ACT showed good performance of both the 20 mg and the 80 mg dose, but overall, the 80 mg dose was found to be superior with reduced all-cause mortality without added side effects.15 Using the pooled data of the 20 mg and 80 mg arms of ATTR-ACT to inform the model was considered a conservative approach.15
Conclusion
Based on the disease simulation model results, tafamidis is expected to more than double the life expectancy and QALYs of ATTR-CM patients compared to SoC. Longer-term follow-up data from the extension study to ATTR-ACT will further inform these findings.
Funding
This work was financially supported by Pfizer Inc.
Conflict of interest: D.T. and D.G. are employees of EVERSANA, who were paid consultants for Pfizer Inc. in connection with the development of the model. B.L., R.B., M.S., and M.H.R. are employees of Pfizer and hold Pfizer stock and/or stock options. B.H. and A.G. are employees of Ingress-health and have received personal consulting fees from Pfizer Inc. in connection with the development of the model and manuscript. M.P. is an employee of the University of Groningen and director/sole stockholder of Pharmacoeconomics Advice Groningen and reports grants and honoraria for both entities. A.M. received research grants (paid to the Oregon Health & Science University) from Pfizer and Akcea Therapeutics and serves on a scientific advisory board with Ionis and Eidos.
Data availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results (accessed 20 January 2021) for more information.
Supplementary Material
Contributor Information
Mark H Rozenbaum, Pfizer Inc., Rivium Westlaan 142, 2909 LD, Capelle aan den IJssel, The Netherlands.
Andrea Garcia, Ingress-health, Weena 316-318 3012 NJ, Rotterdam, The Netherlands.
Daniel Grima, Eversana Life Science Services, 204-3228 South Service Road, Burlington L7N 3H8 ON, Canada.
Diana Tran, Eversana Life Science Services, 204-3228 South Service Road, Burlington L7N 3H8 ON, Canada.
Rahul Bhambri, Pfizer Inc., 235 E 42nd St, New York, NY, USA.
Michelle Stewart, Pfizer Inc., 235 E 42nd St, New York, NY, USA.
Benjamin Li, Pfizer Inc., 235 E 42nd St, New York, NY, USA.
Bart Heeg, Ingress-health, Weena 316-318 3012 NJ, Rotterdam, The Netherlands.
Maarten Postma, Unit of PharmacoEpidemiology & PharmacoEconomics, Department of Pharmacy, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands; Unit of Economics, Econometrics & Finance, Faculty of Economics & Business, University of Groningen, Nettelbosje 2, 9747 AE, Groningen, The Netherlands; Department of Health Sciences, University of Groningen, University Medical Center Groningen, Antonius Deusinglaan 1, 9713 AV Groningen NL, The Netherlands.
Ahmad Masri, The Amyloidosis Center, Knight Cardiovascular Institute, Oregon Health & Science University, 3303 S Bond Ave Building 1, 9th Floor, Portland, OR 97239, USA.
References
- 1. Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M. et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol 2010;7:398–408. [DOI] [PubMed] [Google Scholar]
- 2. Arbustini E, Merlini G.. Early identification of transthyretin-related hereditary cardiac amyloidosis. JACC Cardiovasc Imaging 2014;7:511–514. [DOI] [PubMed] [Google Scholar]
- 3. Barrett CD, Alexander KM, Zhao H, Haddad F, Cheng P, Liao R. et al. Outcomes in patients with cardiac amyloidosis undergoing heart transplantation. JACC Heart Fail 2020;8:461–468. [DOI] [PubMed] [Google Scholar]
- 4. Ericzon B-G, Wilczek HE, Larsson M, Wijayatunga P, Stangou A, Pena JR. et al. Liver transplantation for hereditary transthyretin amyloidosis: after 20 years still the best therapeutic alternative? Transplantation 2015;99:1847–1854. [DOI] [PubMed] [Google Scholar]
- 5. Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY. et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). American Heart Journal 2012;164:222–228. [DOI] [PubMed] [Google Scholar]
- 6. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G. et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016;68:1014–1020. [DOI] [PubMed] [Google Scholar]
- 7. Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL. et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation 2016;133:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B. et al. ; THAOS Investigators. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 2016;68:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grogan M, Dispenzieri A, Carlsson M, Stewart M, Schumacher J.. A Survival analysis of subjects with transthyretin amyloid cardiomyopathy from the Transthyretin Amyloidosis Outcomes Survey. J Card Fail 2017;23:S41. [Google Scholar]
- 10. Pfizer. 2020. http://www.pfizer.com/health-and-wellness/health-topics/rare-diseases/areas-of-focus (20 January 2021).
- 11. Pharmaceutical Safety and Environmental Health Bureau Ministry of Health LaW. Report on the Deliberation Results for Tafamidis Meglumine. 2019. https://www.pmda.go.jp/files/000237869.pdf (20 January 2021).
- 12. Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M. et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail 2019;12:e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M. et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 14. Elliott P, Drachman BM, Gottlieb SS, Hoffman JE, Hummel SL, Lenihan DJ, Ebede B. et al. Interim analysis of data from a long-term, extension trial of tafamidis meglumine in patients with transthyretin amyloid cardiomyopathy. Eur Heart J 2019;40: [Google Scholar]
- 15. Damy T, Garcia‐Pavia P, Hanna M, Judge DP, Merlini G, Gundapaneni B. et al. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR‐ACT) and long‐term extension study. Eur J Heart Fail 2021;23:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goehler A, Geisler BP, Manne JM, Jahn B, Conrads-Frank A, Schnell-Inderst P. et al. Decision-analytic models to simulate health outcomes and costs in heart failure. Pharmacoeconomics 2011;29:753–769. [DOI] [PubMed] [Google Scholar]
- 17. Di Tanna GL, Bychenkova A, O'Neill F, Wirtz HS, Miller P, Ó Hartaigh B. et al. Evaluating cost-effectiveness models for pharmacologic interventions in adults with heart failure: a systematic literature review. PharmacoEconomics 2019;37:359–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Göhler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WS. et al. Utility estimates for decision–analytic modeling in chronic heart failure—health states based on New York Heart Association classes and number of rehospitalizations. Value Health 2009;12:185–187. [DOI] [PubMed] [Google Scholar]
- 19. Madsen BK, Hansen JF, Stokholm KH, Brøns J, Husum D, Mortensen LS.. Chrome congestive heart failure: description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J 1994;15:303–310. [DOI] [PubMed] [Google Scholar]
- 20. Chen J, Normand S-LT, Wang Y, Krumholz HM.. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA 2011;306:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dunlay SM, Redfield MM, Weston SA, Therneau TM, Long KH, Shah ND. et al. Hospitalizations after heart failure diagnosis: a community perspective. J Am Coll Cardiol 2009;54:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Long EF, Swain GW, Mangi AA.. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail 2014;7:470–478. [DOI] [PubMed] [Google Scholar]
- 23. U.S. Department of Health and Human Services. Adult Heart Allocation. Richmond, VA: Organ Procurement and Transplantation Network; 2019. [Google Scholar]
- 24. Latimer N. NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations Alongside Clinical Trials-Extrapolation with Patient-Level Data. Sheffield, UK: National Institute for Health and Clinical Excellence Decision Support Unit; 2011. [PubMed] [Google Scholar]
- 25. Stensrud MJ, Hernán MA.. Why test for proportional hazards? JAMA 2020;323:1401–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. R Core Team. R: A Language and Environment for Statistical Computing Computer Program, Version 3.5. 0. Vienna, Austria: R Core Team; 2018. [Google Scholar]
- 27. Jackson C. flexsurv: A Platform for Parametric Survival Modeling in R. J Stat Softw 2019;70:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Therneau TM, Lumley T.. Package ‘survival’. Survival Analysis Published on CRAN. 2014;2:3. [Google Scholar]
- 29. Maurer MS, Elliott P, Merlini G, Shah SJ, Cruz MW, Flynn A. et al. Design and rationale of the phase 3 ATTR-ACT Clinical Trial (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial). Circ Heart Fail 2017;10:e003815. [DOI] [PubMed] [Google Scholar]
- 30. Shaw JW, Johnson JA, Coons SJ.. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–220. [DOI] [PubMed] [Google Scholar]
- 31. Efron B, Tibshirani RJ.. An Introduction to the Bootstrap. New York, NY: CRC Press; 1994. [Google Scholar]
- 32. Burnham KP, Anderson DR.. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Vol. 2). New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 33. Burnham KP, Anderson DR.. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 2004;33:261–304. [Google Scholar]
- 34. Arias E, Xu J.. United States Life Tables, 2017. National Vital Statistics Report; vol 68 no 7. Hyattsville, MD: National Center for Health Statistics; 2019. [PubMed] [Google Scholar]
- 35. Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A. et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019;140:16–26. [DOI] [PubMed] [Google Scholar]
- 36. Ladefoged B, Dybro A, Povlsen JA, Vase H, Clemmensen TS, Poulsen SH.. Diagnostic delay in wild type transthyretin cardiac amyloidosis—a clinical challenge. Int J Cardiol 2020;304:138–143. [DOI] [PubMed] [Google Scholar]
- 37. Canepa M, Tini G, Musumeci B, Cappelli F, Milandri A, Mussinelli R. et al. Real-world versus trial patients with transthyretin amyloid cardiomyopathy. Eur J Heart Fail 2019;21:1479–1481. [DOI] [PubMed] [Google Scholar]
- 38. Sultan MB, Gundapaneni B, Schumacher J, Schwartz JH.. Treatment with tafamidis slows disease progression in early-stage transthyretin cardiomyopathy. Clin Med Insights Cardiol 2017;11:1179546817730322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stewart M, Shaffer S, Murphy B, Loftus J, Alvir J, Cicchetti M. et al. Characterizing the high disease burden of transthyretin amyloidosis for patients and caregivers. Neurol Ther 2018;7:349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. National Institute for Health and Clinical Excellence. Donepezil, Galantamine, Rivastigmine (Review) and Memantine for the Treatment of Alzheimer's Disease (Amended). London, UK: National Institute for Health and Clinical Excellence; 2007. [Google Scholar]
- 41. U.S. Food and Drug Administration. FDA Approves New Treatments for Heart Disease Caused by a Serious Rare Disease, Transthyretin Mediated Amyloidosis. 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatments-heart-disease-caused-serious-rare-disease-transthyretin-mediated#:∼:text=On%20May%203%2C%20the%20U.S.,approved%20treatments%20for%20ATTR%2DCM (21 January 2021).
- 42. Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA. et al. Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation 2020;141:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Latimer NR, Abrams KR. NICE DSU Technical Support Document 16: Adjusting Survival Time Estimates in the Presence of Treatment Switching. Sheffield, UK: National Institute for Health and Clinical Excellence Decision Support Unit;2014. [PubMed] [Google Scholar]
- 44. Nicod E, Annemans L, Bucsics A, Lee A, Upadhyaya S, Facey K.. HTA programme response to the challenges of dealing with orphan medicinal products: process evaluation in selected European countries. Health Policy 2019;123:140–151. [DOI] [PubMed] [Google Scholar]
- 45. Augustine EF, Adams HR, Mink JW.. Clinical trials in rare disease: challenges and opportunities. J Child Neurol 2013;28:1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nestler-Parr S, Korchagina D, Toumi M, Pashos CL, Blanchette C, Molsen E. et al. Challenges in research and health technology assessment of rare disease technologies: report of the ISPOR rare disease special interest group. Value Health 2018;21:493–500. [DOI] [PubMed] [Google Scholar]
- 47. Caro JJ, Briggs AH, Siebert U, Kuntz KM; ISPOR-SMDM Modeling Good Research Practices Task Force-1. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force. Med Decis Making 2012;32:667–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results (accessed 20 January 2021) for more information.