Abstract
Purpose
The combination of laser and ultrasound can significantly improve the efficiency of thrombolysis through an enhanced cavitation effect. We developed a fiber optics-based laser-ultrasound thrombolysis device and tested the feasibility and efficiency of this technology for restoring blood flow in an in vitro blood clot model.
Methods
An in vitro blood flow-clot model was set up, and then an endovascular laser thrombolysis system was combined with high-intensity focused ultrasound to remove the clot. The laser and ultrasound pulses were synchronized and delivered to the blood clot concurrently. The laser pulses of 532 nm were delivered to the blood clot endovascularly through an optical fiber, whereas the ultrasound pulses of 0.5 MHz were applied noninvasively to the same region. Effectiveness of thrombolysis was evaluated by the ability to restore blood flow, which was monitored by ultrasound Doppler.
Results
As laser powers increased, the ultrasound threshold pressures for effective thrombolysis decreased. For laser fluence levels of 0 mJ/cm2, 2 mJ/cm2 and 4 mJ/cm2, the average negative ultrasound threshold pressures were 1.26 ± 0.114 MPa, 1.05 ± 0.181 MPa, and 0.59 ± 0.074 MPa, respectively. The periods of time needed to achieve effective thrombolysis were measured at 0.8 mJ/cm2, 2 mJ/cm2 and 4 mJ/cm2 laser fluence levels and 0.42 MPa, 0.70 MPa and 0.98 MPa negative ultrasound pressures. In general, thrombolysis could be achieved more rapidly with higher laser powers or ultrasound pressures.
Conclusions
Effective thrombolysis can be achieved by combining endovascular laser with non-invasive ultrasound at relatively low power and pressure levels, which can potentially improve both the treatment efficiency and safety.
Introduction
Deep vein thrombosis (DVT), characterized by excessive blood clot (thrombus) formation in veins, is a major disease affecting more than 10 million people worldwide each year.1 Medical complications associated with DVT include pulmonary embolism (PE) and postthrombotic syndrome (PTS).2–5 PE is an acute life-threatening complication, and is known to be induced by the debris of the blood clots when they break off from the central clot, and block smaller blood vessel flowing into the lungs. The blockage of blood supply can cause severe damages to the lungs, resulting in breathing difficulties, and ultimately leading to death. Annually, as many as 100,000 patients die from PE in the United States. PTS is another costly chronic condition that develops in 30% to 75 % of patients with DVT. PTS includes redness, swelling, ulcers and chronic leg pain, and it can lead to life-long suffering and potentially disability. The annual costs for DVT-related complications are $7 to $10 billion in the United States. Worldwide, the total cost can be as high as $69 billion annually.6
Among the current standards of care, anticoagulants can prevent thrombus propagation; however, they do not dissolve existing thrombi and re-canalize vessels. Thrombolytic therapy that has been historically performed to dissolve clots may greatly increase the risk of bleeding, and their introduction may require hospitalization.7 Ultrasound-based treatment techniques have been evaluated as methods to induce effective thrombolysis.8–14 The advantage of ultrasound-based techniques is that they can dissolve blood clots quickly and re-canalize vessels noninvasively through cavitation. While ultrasound-based techniques may quickly remove blood clots noninvasively, these techniques require high acoustic peak negative pressure (as high as 19 MPa as shown in the previous study15) at relatively low ultrasound frequencies such as 500 kHz or 1 MHz for high efficiency. In order to achieve high ultrasound pressure and deliver treatment to a blood clot, focused ultrasound is employed. However, at such low ultrasound frequencies, the focal spot of the ultrasound field is usually larger than 10 mm in length, which is greater than the diameters of most veins. As a result, severe damage can occur to the surrounding tissue and vessel walls.15 This is especially problematic in areas with delicate structures that have limited surgical options, such as retina vein occlusions where the delicate structure precludes most existing non-pharmacological treatments, and renal vein thrombus where vein access and removal is highly invasive. Although the size of the focal zone may be reduced by using transducers with small f-numbers,16 it will reduce the depth of treatment and is also limited by the available acoustic window. Alternatively, higher frequency ultrasound may be used to produce a small focal size, but it will reduce the efficiency of thrombolysis because the cavitation threshold is relatively high as higher frequency. To increase the efficiency and safety of ultrasound-based thrombolysis, microbubbles can be used;17 however, it requires systemic injection and may cause unwanted vascular and tissue damages at high dosage.18–20
Laser thrombolysis is an interventional procedure to re-canalize occluded arteries using light wavelengths that are highly absorbed by the blood clots.21–26 Laser light is generally directed to the blood clot through a thin laser fiber. Once laser light is absorbed and the blood clot is heated up, cavitation can occur in the blood clot through vaporization. Then, similar as ultrasound thrombolysis, the expansion and collapse of cavitation can break down the blood clot. Its advantages include low cost, short recovery time and it is generally safe. Laser energy can induce cavitation precisely in blood clots due to the high optical absorption of blood clots and the precision at which the energy can be delivered using the fiber optics. However, the produced cavitation expansion and collapse often are not strong enough and require high laser power. As a result, laser thrombolysis often cannot completely clear thrombotic occlusions in blood vessels, typically leaving residual thrombus on the blood vessel walls,27 and its efficiency is also questionable in removing residues with high calcium content.
We have developed a novel hybrid technique, based on the combination of light and ultrasound, to improve both ultrasound-based and laser-based thrombolysis by overcoming the deficiencies of the current approaches. The main mechanism underlying combined laser and ultrasound therapy is the highly efficient and better controlled acoustic cavitation around blood clots.12,15,28–36 The significance of this technique lies in the fact that cavitation bubbles can be induced at much lower peak negative ultrasound pressures in highly optically absorptive blood clots (not the vessel wall). As a result, highly precise treatment can be achieved and only blood clots will be removed while the damage to the vessel wall can be avoided.
In a previous study, we demonstrated combined laser and ultrasound energy can significantly improve the efficiency of thrombolysis.37 Our previous study, however, focused on the development of a totally non-invasive laser and ultrasound technique for thrombolysis. While a non-invasive technique has significant advantages in safety and reducing medical care expense, a laser-based non-invasive technique has limited penetration depth because of the strong optical scattering in soft tissues. Hence, our previous system had significant limitations in removing blood clots from deep veins. In the current study, we combined an endovascular laser fiber catheter with non-invasive ultrasound for highly efficient thrombolysis. Endovascular laser fiber has been widely used and proven to be reliable and safe. The combination of an endovascular laser with ultrasound for thrombolysis allows blood clots in deep vessels to be removed. The feasibility and efficiency of the newly designed system were tested with an in vitro blood flow-blood clot system.
Materials and Methods
The detailed schematic of the endovascular laser thrombolysis-ultrasound system is shown in Fig. 1. A Q-switched diode pumped solid state laser (SPOT-10-100-532, Elforlight, Daventry, UK) with a pulse repetition rate of 10 kHz (~1.8 ns pulse width), was employed as the irradiation source for the treatment system. The laser system produced 2-ns 532-nm wavelength light with a pulse energy between 0 – 5 μJ. The laser power was measured by an optical power meter and adjusted to the desired level before each experiment. The produced laser light was coupled into an optical fiber (1.25 mm OD, M63L01, Thorlabs, Newton, NJ) and delivered to the target through a fiber optic cannula (400 μm, CFML14L20, Thorlabs), resulting in a laser fluence of 0 to 4 mJ/cm2 for each laser pulse at the fiber tip.
Figure 1.

(a) Schematic of the experimental setup for endovascular laser thrombolysis enhanced by high-intensity focused ultrasound. (b) Photograph of the experimental setup. (c) Schematic showing the treatment area with laser and ultrasound (red dashed box in Fig 1(a)).
A custom-made filter was installed on the optical fiber behind the fiber tip. The filter was made of porous soft sponge materials with ~100 μm pores. When placed in a blood vessel, the filter would be squeezed and the sizes of pores could be smaller than 100 μm. When the filter is placed downstream, it can trap large debris of blood clots that broken away during thrombolysis procedure, further improving the safety potential of this technique. Filters with similar pore sizes have been used in commercial embolic protection system such as FilterWire, Boston Scientific, and proven to be effective.
For the ultrasound system, a high-intensity focused ultrasound (HIFU) transducer (center frequency 0.5 MHz, H-107, Sonic Concepts, Bothell, WA) with a focal distance of 62.6 mm, a focal zone of 3.9 mm in lateral direction and 19.1 mm in axial direction was used to provide 0.5-MHz therapeutic ultrasound bursts, and its focal negative pressure was calibrated with a standard needle hydrophone (Onda HNC-1500, Sunnyvale, CA). The original 0.5 MHz signal was generated by a function generator (33250A, Agilent Technologies, Santa Clara, CA), and then transmitted to an RF amplifier (2100L, ENI, Rochester, NY). After passing through a matching network (Impedance Matching Network H-107, Sonic Concepts), the 0.5 MHz signal was directed to the HIFU transducer. The therapeutic ultrasound burst rate was also 10 kHz (same as the laser repetition rate) with a 2% duty cycle (5 cycles per trigger). The synchronization between the laser and the therapeutic ultrasound system was controlled by a delay/pulse generator (DG355, Stanford Research Systems, Sunnyvale, CA, USA). The delay/pulse generator triggered both the laser system and the function generator with a pre-determined trigger delay between two systems to ensure concurrent laser and therapeutic ultrasound energy were applied on the target. Each laser pulse was matched with the first negative peak of each 5 cycles of the ultrasound burst.38–40 A custom-built, 3D printed cone was designed and attached to the HIFU transducer, and filled with couplant that is the custom-made from agar gelatin and porcine skin powders to provide acoustic coupling. The couplant was prepared by mixing 8 gram of agar and 5 gram of porcine skin powder in 1000 mL 90 °C distilled water, and then pour to a mold to cool to room temperature.
For the blood flow and clot system, we used a two-branch silicone tubing (3 mm inner diameter) system to circulate human whole blood. One branch was clogged by a blood clot, and the other branch served as a bypass for the circulating blood flow. Blood clots were prepared by mixing whole blood with CaCl2 in a silicone test tube (6.4 mm inner diameter) in a 37 °C water bath for 2 h and then stored at 4 °C for up to 3 days until use.37,41 Before each experiment, a blood clot of 0.02 mL (around 3 mm length in the tubing) was carefully pushed into one end of a 3 mm diameter tubing. The tubing was then connected into the flow system as shown in Figure 1. Human whole blood was then injected into the silicone tubing using a syringe, and circulated by a peristaltic pump to simulate the bloodstream. The tubing system was immersed in a water tank, which was filled with degassed water to avoid gas bubbles. The transducer was positioned so that the focal zone of the transducer covered the entire blood clot (the focal zone of the transducer was 3.9 mm based on our measurement while the blood clot was 3 mm in length). The optical fiber was inserted into the silicone tubing from the downstream direction through a small opening and the optical fiber tip touched the blood clot and slightly moved into the blood clot during the treatment. Then the HIFU transducer delivered ultrasound bursts to the blood clot while the laser energy was delivered at a properly triggered delay time.
The blood flow speed was monitored by doppler ultrasound. The Doppler mode of a commercial ultrasound imaging unit (Z.One PRO, Mindray, Mahwah, NJ, USA) with a linear probe (L14–5W, Mindray) was used with a pulse repetition frequency of 1500 Hz and a continuous doppler frequency of 5.5 MHz. Figure 2 shows the ultrasound Doppler images when the blood flow was scanned along the tube longitudinal direction. In Figure 2(a), the color Doppler image, which is overlaid on the ultrasound image, indicates the blood flow speed in the tubing without a blood clot. Whereas, Doppler indicates no blood flow, as indicated by Figure 2(b), when the tube was occluded with a clot. In this case, blood flowed through the bypass branch of the tubing system.
Figure 2.

(a) Ultrasound Doppler image of the blood flow without a blood clot. (b) Ultrasound Doppler image of the blood flow when a blood clot blocked the silicone tubing. (Color scale was 7.5 to −7.5 cm∕s.).
To evaluate the efficiency of the thrombolysis procedure, blood volume velocity was used. The volume velocity was calculated by multiplying the flow speed and the cross-sectional area where the flow speed was measured. To be consistent, we measured the blood flow speed at an upstream location with respect to the clot location. Because there was no blood clot in the upstream location, the cross-sectional area remained constant; therefore, the blood volume velocity is proportional to the blood flow speed at that location.
Results
Throughout the study, we used a constant setting for the peristaltic pump to provide a stable flowrate and pressure. Additionally, blood clots of 0.02 mL were used for all the experiments, which were under different conditions of laser powers and therapeutic ultrasound intensities.
The therapeutic ultrasound pressure threshold for an effective thrombolysis treatment with a treatment duration of 60-second was first measured at different laser power levels. The thresholding pressure was defined as the pressure needed to restore the blood flow to at least 60% of the original flow speed, which was measured by Doppler ultrasound after 60 seconds of combined laser and ultrasound treatment. Figure 3 shows the measured ultrasound pressure threshold values where the laser fluence levels were at 0 mJ/cm2, 2 mJ/cm2 and 4 mJ/cm2. The average ultrasound pressure threshold decreased when the laser power level increased. An average negative ultrasound pressure threshold of 0.59 ± 0.074 MPa (all pressures reported are peak negative pressure unless otherwise indicated) was measured when the laser power was 4 mJ/cm2 with 10 kHz pulse repetition rate, whereas the average ultrasound pressure threshold was 1.26 ± 0.114 MPa when the laser power was 0 mJ/cm2. When the laser power level was 2 mJ/cm2, the ultrasound pressure threshold was 1.05 ± 0.181 MPa. With ANOVA test (Matlab R2018b), the significance in differences between groups was calculated. The p-value was 0.00014 among the three groups. Between two groups, t-test was used to calculate the p-values. The p-value between the group of 4 mJ/cm2 laser fluence and the group of 2 mJ/cm2 was 0.03, whereas the p-value between the group of 4 mJ/cm2 laser fluence and the group of 0 mJ/cm2 was 0.005. Both of them were statistically significant. However, the p-value between the two groups of 0 mJ/cm2 and 2 mJ/cm2 laser fluence was 0.102.
Figure 3.

Ultrasound pressure threshold for effective thrombolysis at 0, 2, and 4 mJ/cm2 of laser fluence after 60 seconds of treatment (n=4).
Figure 4a shows strong echoes produced at the tip of the optical fiber when therapeutic ultrasound waves were applied during an effective thrombolysis procedure, indicating that possible cavitation has been produced during this process.12,15,24–32 No echo on ultrasound image was observed when the therapeutic ultrasound was not applied (Figure 4b). Additionally, the signal was not observed with only ultrasound pressure applied without laser firing. The signal only was only observed when both ultrasound pressure and laser energy were applied. So it was not generated by acoustic interference between the therapy device and imaging device.
Figure 4.
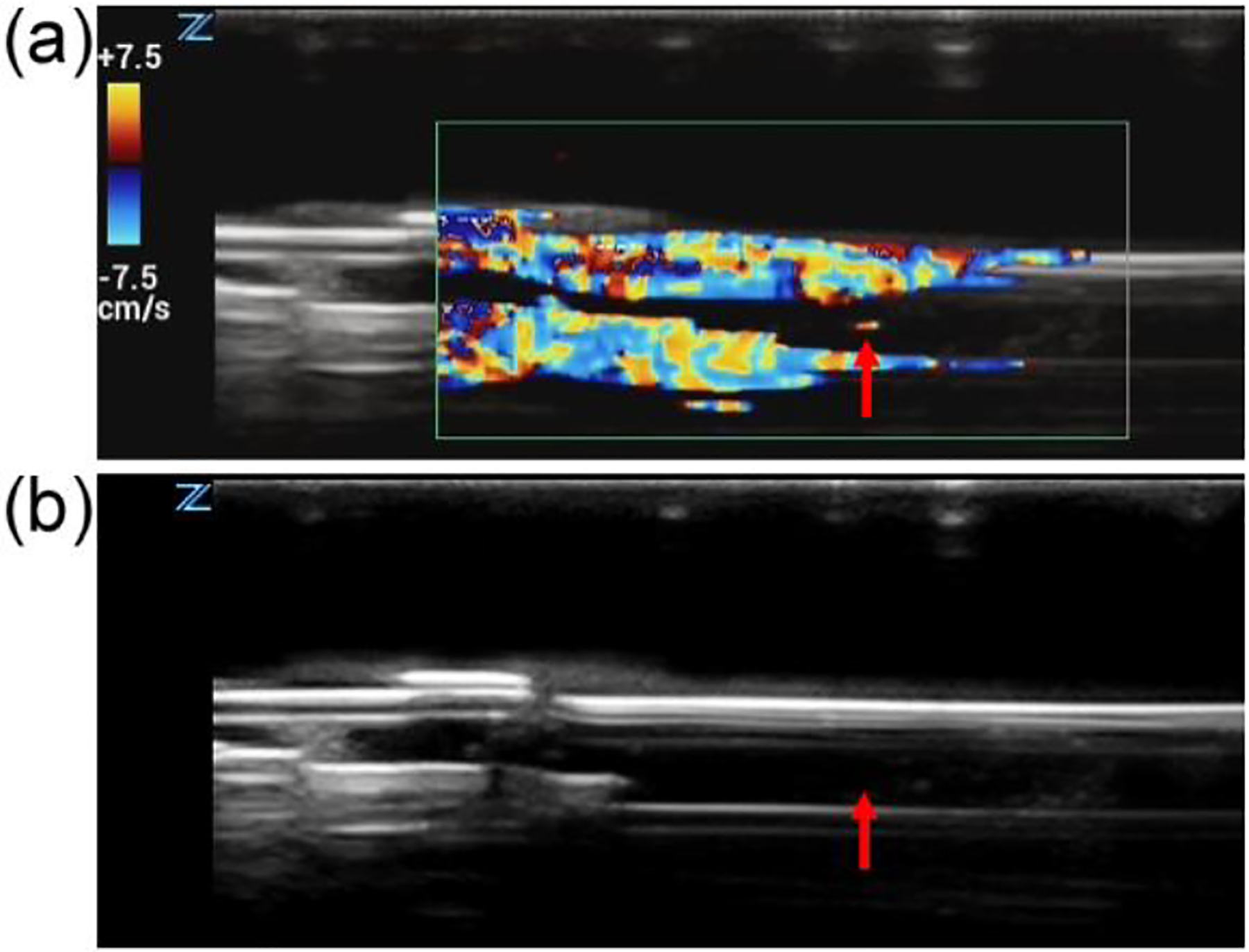
Ultrasound Doppler image showing the cavitation signal during the treatment. a) Concurrently applied ultrasound and laser induced strong echoes at the tip of the laser fiber (indicated by the red arrow). b) Ultrasound image when the therapeutic ultrasound was not applied.
The period of time needed for an effective thrombolysis treatment, which was defined as the pressure needed to restore the blood flow to at least 60% of the original flow speed, was measured at different ultrasound pressures (0.42 MPa, 0.70 MPa, and 0.98 MPa) and different laser fluence (0.8 mJ/cm2, 2 mJ/cm2 and 4 mJ/cm2) . Figure 5 shows the required period of time for each combination of therapeutic ultrasound and laser parameters to achieve effective thrombolysis. The shortest period of time needed for an effective thrombolysis was 23.3 ± 6.8 seconds with 0.98 MPa ultrasound pressure and 4 mJ/cm2 laser fluence. At 0.42 MPa ultrasound pressure, the effective thrombolysis could be achieved only when 4 mJ/cm2 laser fluence was used, whereas 0.8 and 2 mJ/cm2 laser fluences could not result in an effective thrombolysis when combined with 0.42 MPa ultrasound pressure. With ANOVA test (Matlab R2018b), the significance between groups was calculated. The p-values among the three groups were 0.00088, 0.00035 and 0.00068 for Figure 5a, 5b and 5c, respectively.
Figure 5.

The period of time needed for effective thrombolysis treatment with different laser fluences (0.8, 2, and 4 mJ/cm2) and ultrasound pressures (0.42 MPa, 0.70 MPa, and 0.98 MPa). (* : no effect of thrombolysis, n=4)
With 0.98 MPa ultrasound pressure, the average periods of time for effective thrombolysis were 95.7 s, 41.0 s and 23.3 s at 0.8 mJ/cm2, 2 mJ/cm2 and 4 mJ/cm2 laser fluence, respectively. When the ultrasound pressure was 0.7 MPa, the average times were 161 s, 62 s and 49.3 s with the laser fluence at 0.8 mJ/cm2, 2 mJ/cm2 and 4 mJ/cm2 respectively. The average time was 88.7 s at 0.42 MPa ultrasound pressure and 4 mJ/cm2 laser fluence. The Doppler ultrasound was used to monitor the blood flow speed in real-time during thrombolysis procedures. A sample video (Supp. 1) shows the result of an ultrasound Doppler at the upstream location with respect to the blood clot. During the combined laser and ultrasound treatment, although there was some noise on the Doppler image due to the applied therapeutic ultrasound, we could clearly observe the blood flow, which is displayed as a pseudo colored image in the tubing.
Using the ultrasound Doppler, we measured the blood flow speed after the 60-second PUT treatment. In Figure 6, the flow speeds are shown as a function of different laser powers and therapeutic ultrasound pressures. In these results, the Doppler signal intensity in the center area of tubing was collected and averaged. The blood flow speed was almost zero when the laser fluence was 0.8 mJ/cm2. However, the flow speed increased as either the applied ultrasound pressure or laser power increased.
Figure 6.

(a) Thrombolysis treatment time with different laser fluences (0.8, 2 and 4 mJ/cm2) and high-intensity focused ultrasound pressures (0.42 MPa, 0.70 MPa, and 0.98 MPa). (n=4) (b) Doppler images of blood flows with different laser powers and ultrasound pressures.
Discussion
The combined laser and ultrasound technique has significant advantages over the pure ultrasound-based and laser-based thrombolysis. Our results clearly demonstrated that there was a great enhancement in the efficiency of thrombolysis when the combined laser and ultrasound technique was used in comparison with ultrasound-only or laser-only. We have shown that with ultrasound only (0 mJ/cm2 laser fluence), the pressure threshold value for effective thrombolysis is much higher than that when combined with laser energy. The laser-only technique, with the energy level used in the current study, could not induce any effective thrombolysis. We have also shown that there were strong cavitation activities during the application of the combined laser and ultrasound for thrombolysis, further confirming the previous studies regarding the enhanced cavitation during concurrently applied laser and ultrasound.
The selection of the proper laser wavelength can have a significant impact on the efficiency and safety of the treatment. The combined laser and ultrasound therapy depend on optical absorption to produce cavitation. In the current study, we have used 532 nm laser light, at which hemoglobin has strong optical absorptions; hence, this optical wavelength is very effective to produce cavitation and achieve thrombolysis. Longer wavelength light such as 650-nm may also be used to induce cavitation when combined with ultrasound. However, the produced cavitation activity will not be as strong as that at 532 nm because the optical absorption of hemoglobin is relatively weak at 650 nm.40 As a result, it could take a long period of time or higher ultrasound pressure to achieve effective thrombolysis. On the other hand, hemoglobin absorption at shorter wavelength such as 400 nm is much stronger than that at 532 nm. Hence, the use of a short wavelength light for thrombolysis may improve the efficiency. In addition, because of the strong optical absorption, short wavelength light cannot penetrate deep into a blood clot. Consequently, cavitation can only be induced on a thin surface layer of the blood clot, resulting in the removal of the clot in a layer-by-layer manner; thereby, the possible damage to the blood vessel wall that is underneath the clot may be minimized. The drawback of using short wavelength light is that the volume of effective cavitation will be reduced because of less penetration. This problem may be solved by using an optical diffuser tip at the treatment end of the fiber. A diffuser tip may not only expand the light illumination area, but also reduce the likelihood of laser-caused charring near the tip, which could otherwise significantly reduce the efficiency of light delivery to the blood clot.
We have used 0.5-MHz ultrasound frequency, which is a relatively low frequency for medical ultrasound applications. At this ultrasound frequency, inducing cavitation is relatively easy, and at the same time, sufficient ultrasound intensity can be produced in the focal zone to achieve effective therapy. Low frequency ultrasound is preferred for cavitation purposes as long as adequate intensities can be achieved at the point of interest.
The novel design of the laser fiber catheter with a soft expandable filter potentially can significantly improve the safety of the thrombolysis procedure, and reduce the threats of PE. In our experiment, after each thrombolysis test, we visually inspected the filter to identify the trapped debris of blood clots in the filter. In the absence of filtering, residual particles that break off from the primary blood clot would mix into the blood flow. The added filter on the laser fiber at the downstream direction demonstrated it could effectively trap the residual debris of blood clots that remain suspended in the bloodstreams. The result is not a surprise because filters with similar pore sizes have been used in commercial embolic protection system and proven to be effective. The addition of a filter in our system is precautious and may facilitate the future translation to the clinic. We noticed that many previous studies of ultrasound thrombolysis8–14 did not need a filter. Hence, it is possible that, by selecting proper parameters, the filter may not be needed in our system.
The safety of thrombolysis treatment of the combined endovascular laser and non-invasive ultrasound was further improved because of the use of the low levels of laser power and ultrasound pressure. The applied laser powers were much lower than that of conventional laser therapies, which are generally above 1J. The applied ultrasound pressures were also low, resulting in a Mechanical Index (MI) that was much less than 1.9, the safety limit for ultrasound imaging. Consequently, the applied ultrasound pulse should not cause any collateral damage on the vessel wall where the ultrasound pulse is applied alone because laser light only illuminates blood clot.
Technically, the current technique removes blood clots through mechanical force, which is likely produced by the induced micro or nano-size bubbles in the blood clot. A big advantage of the current technique is that the produced mechanical force is not necessary to be exerted on the blood vessel wall. Hence the damage to the blood vessel wall is minimized. Many traditional mechanical thrombectomy devices generally exert a force on the inside of the blood vessel wall to “scrape” a blood clot off. The inside surface of a vein is not a smooth surface, and has venous valves to prevent the backflow of blood. Scaping off a blood clot inside a vein always has the potential to damage the venous valvular function.42,43 A few devices utilize suction force to remove blood clots. They are, however, in general limited to treatment of acute blood clots. The current technique has the potential to reduce the potential damage to venous valves.
The current technique is an upgrade for endovascular laser thrombolysis. Hence the advantages of endovascular laser thrombolysis will be retained, and laser light can be delivered to the blood clot using an optical fiber as the same matter for endovascular laser therapy, while ultrasound can be applied noninvasively. One major advantage of endovascular laser thrombolysis is that the size of a laser fiber can be very small. This small size provides great flexibility for endovascular laser therapy and allow it to be used to recanalize small blood vessels. On the other side, if needed, multiple laser fibers can be bundled together to treat blood clots in large size vessels.
Further, both endovascular laser therapy and non-invasive ultrasound therapy have been used in the clinic. The combination of these two techniques to achieve better therapeutic outcome appears to be low in risk and technical difficulty. We expect this technique can enter clinical trials in the very near future. The successful development of the technique can also have great application potential in treatment for stroke, which the current therapeutic methods are limited.
To successfully translate the technology to the clinic, studies with more complicated physiological conditions should be performed in the future. Particularly, an acute blood clot was used in the current study. In practice, DVT is usually a chronical condition that develops over a long period of time. While DVT begins as a red thrombus, it rapidly organizes to fibrous and then collagenous architecture over days or weeks. As the clot grows older and stiffer, its optical and acoustic properties will change and may require different optical and acoustic parameters for effective thrombolysis. The relative proportions of materials that make up the clot (e.g. red blood cells, platelets, fibrous tissue) will also contribute to changes in mechanical properties and the threshold for generating cavitation.
Conclusion
In conclusion, we have demonstrated that the combination of low optical power and ultrasound pressure could enhance thrombolysis effect in vitro by using a newly designed endovascular laser-based system that was enhanced by ultrasound. The combined laser and ultrasound treatment can quickly remove blood clots at relatively low ultrasound pressures by enhancing cavitation activities. In this study, we used a 532 nm wavelength laser for the optical source. However, we can consider other wavelengths with higher optical absorptions to increase the cavitation effect. Accordingly, PUT treatment can be used for DVT as a minimal invasive therapy with a high efficiency without damaging the surrounding tissues.
Supplementary Material
Acknowledgments
This study is supported in part by NIH 1R43HL147783-01.
References
- 1.Jha AK, Larizgoitia I, Audera-Lopez C, Prasopa-Plaizier N, Waters H, Bates DW. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Quality & Safety. 2013;22(10):809–815. [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M, van Es N, Buller HR. Deep vein thrombosis and pulmonary embolism [published online ahead of print 2016/07/05]. Lancet. 2016;388(10063):3060–3073. [DOI] [PubMed] [Google Scholar]
- 3.Augustinos P, Ouriel K. Invasive approaches to treatment of venous thromboembolism. Circulation. 2004;110(9):I27–I34. [DOI] [PubMed] [Google Scholar]
- 4.Johnson BF, Manzo RA, Bergelin RO, Strandness DE. Relationship between changes in the deep venous system and the development of the postthrombotic-syndrome after an acute episode of lower-limb deep-vein thrombosis - a one-year to 6-year follow-up. Journal of Vascular Surgery. 1995;21(2):307–313. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Archives of Internal Medicine. 2004;164(1):17–26. [DOI] [PubMed] [Google Scholar]
- 6.Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thrombosis research. 2016;137:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke - Final results of the multi MERCI trial. Stroke. 2008;39(4):1205–1212. [DOI] [PubMed] [Google Scholar]
- 8.Luo H, Steffen W, Cercek B, Arunasalam S, Maurer G, Siegel RJ. Enhancement of thrombolysis by external ultrasound. American Heart Journal. 1993;125(6):1564–1569. [DOI] [PubMed] [Google Scholar]
- 9.Wright C, Hynynen K, Goertz D. In Vitro and In Vivo High-Intensity Focused Ultrasound Thrombolysis. Investigative Radiology. 2012;47(4):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone MJ, Frenkel V, Dromi S, et al. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thrombosis Research. 2007;121(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92(5):1148–1150. [DOI] [PubMed] [Google Scholar]
- 12.Unger EC, Matsunaga TO, McCreery T, Schumann P, Sweitzer R, Quigley R. Therapeutic applications of microbubbles. European Journal of Radiology. 2002;42(2):160–168. [DOI] [PubMed] [Google Scholar]
- 13.Parikh S, Motarjeme A, McNamara T, et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: Initial clinical experience. Journal of Vascular and Interventional Radiology. 2008;19(4):521–528. [DOI] [PubMed] [Google Scholar]
- 14.ter Haar G Therapeutic ultrasound. European journal of ultrasound : official journal of the European Federation of Societies for Ultrasound in Medicine and Biology. 1999;9(1):3–9. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD Jr., Xu Z. Noninvasive Treatment of Deep Venous Thrombosis Using Pulsed Ultrasound Cavitation Therapy (Histotripsy) in a Porcine Model. Journal of Vascular and Interventional Radiology. 2011;22(3):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosnitskiy PB, Yuldashev PV, Sapozhnikov OA, et al. Design of HIFU Transducers for Generating Specified Nonlinear Ultrasound Fields. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2017;64(2):374–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta S, Coussics C-C, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity (R) as a cavitation nucleation agent. Ultrasound in Medicine and Biology. 2008;34(9):1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Main TMKML. Safety And Risk-Benefit Profile Of Microbubble Contrast Agents In Echocardiography. Asia Pacific Cardiology. 2008;2(1):47–49. [Google Scholar]
- 19.Paradossi G, Oddo L, Cerroni B, et al. In Vivo Toxicity Study of Engineered Lipid Microbubbles in Rodents. ACS Omega. 2019;4(3):5526–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang EH. An Introduction to Contrast-Enhanced Ultrasound for Nephrologists. Nephron. 2018;138(3):176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogueira RG, Schwamm LH, Hirsch JA. Endovascular Approaches to Acute Stroke, Part 1: Drugs, Devices, and Data. American Journal of Neuroradiology. 2009;30(4):649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlis A, Lutsep H, Barnwell S, et al. Mechanical thrombolysis in acute ischemic stroke with endovascular photoacoustic recanalization. Stroke. 2004;35(5):1112–1116. [DOI] [PubMed] [Google Scholar]
- 23.Shah R, Martin RE, Topaz O. Laser angioplasty and laser-induced thrombolysis in revascularization of anomalous coronary arteries. The Journal of invasive cardiology. 2002;14(4):180–186. [PubMed] [Google Scholar]
- 24.Topaz O, Vetrovec G. Laser for optical thrombolysis and facilitation of balloon angioplasty in acute myocardial infarction following failed pharmacologic thrombolysis. Catheterization and Cardiovascular Diagnosis. 1995;36(1):38–42. [DOI] [PubMed] [Google Scholar]
- 25.Shangguan HQ, Gregory KW, Casperson LW, Prahl SA. Enhanced laser thrombolysis with photomechanical drug delivery: An in vitro study. Lasers in Surgery and Medicine. 1998;23(3):151–160. [DOI] [PubMed] [Google Scholar]
- 26.Denheijer P, Vandijk RB, Pentinga ML, Hillege HL, Lie KI. Laser thrombolysis in acute myocardial infarction: results of a clinical feasibility study. Journal of Interventional Cardiology. 1994;7(6):525–534. [DOI] [PubMed] [Google Scholar]
- 27.Shangguan HQ, Gregory KW, Casperson LW, Prahl SA. Enhanced laser thrombolysis with photomechanical drug delivery: an in vitro study [published online ahead of print 1998/10/21]. Lasers Surg Med. 1998;23(3):151–160. [DOI] [PubMed] [Google Scholar]
- 28.Holland CK, Vaidya SS, Datta S, Coussios C-C, Shaw GJ. Ultrasound-enhanced tissue plasminogen activator thrombolysis in an in vitro porcine clot model. Thrombosis Research. 2008;121(5):663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel RJ, Luo H. Ultrasound thrombolysis. Ultrasonics. 2008;48(4):312–320. [DOI] [PubMed] [Google Scholar]
- 30.Tsivgoulis G, Alexandrov A. Ultrasound-enhanced thrombolysis from bedside to bench. Stroke. 2008;39(5):1404–1405. [DOI] [PubMed] [Google Scholar]
- 31.Tsivgoulis G, Culp WC, Alexandrov AV. Ultrasound enhanced thrombolysis in acute arterial ischemia. Ultrasonics. 2008;48(4):303–311. [DOI] [PubMed] [Google Scholar]
- 32.Molina CA, Ribo M, Rubiera M, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37(2):425–429. [DOI] [PubMed] [Google Scholar]
- 33.Coussios CC, Roy RA. Applications of acoustics and cavitation to noninvasive therapy and drug delivery. Annual Review of Fluid Mechanics. 2008;40:395–420. [Google Scholar]
- 34.Maxwell AD, Cain CA, Duryea AP, Yuan L, Gurm HS, Xu Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy - histotripsy. Ultrasound in Medicine and Biology. 2009;35(12):1982–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker A, Marxer E, Bruessler J, et al. Ultrasound active nanoscaled lipid formulations for thrombus lysis. European Journal of Pharmaceutics and Biopharmaceutics. 2011;77(3):424–429. [DOI] [PubMed] [Google Scholar]
- 36.Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound in Medicine and Biology. 2000;26(7):1153–1160. [DOI] [PubMed] [Google Scholar]
- 37.Cui H, Yang X. Laser enhanced high-intensity focused ultrasound thrombolysis: an in vitro study [published online ahead of print 2013/02/01]. J Acoust Soc Am. 2013;133(2):EL123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Xie X, Li J, et al. Removal of choroidal vasculature using concurrently applied ultrasound bursts and nanosecond laser pulses. Scientific Reports. 2018;8(1):12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Zhang H, Li J, Paulus Y, Wang X. The application of antivascular photo-mediated ultrasound therapy in removing microvessels in the eye. Paper presented at: 2017 IEEE International Ultrasonics Symposium (IUS); 6–9 Sept. 2017, 2017. [Google Scholar]
- 40.Hu Z, Zhang H, Mordovanakis A, et al. High-precision, non-invasive anti-microvascular approach via concurrent ultrasound and laser irradiation. Scientifict Report. 2017;7:40243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutton JT, Ivancevich NM, Perrin SR Jr., Vela DC, Holland CK. Clot retraction affects the extent of ultrasound-enhanced thrombolysis in an ex vivo porcine thrombosis model [published online ahead of print 2013/03/05]. Ultrasound Med Biol. 2013;39(5):813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohi MP, Kohlbrenner R, Kolli KP, Lehrman E, Taylor AG, Fidelman N. Catheter directed interventions for acute deep vein thrombosis. Cardiovasc Diagn Ther. 2016;6(6):599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharafuddin MJ, Gu X, Han YM, Urness M, Gunther R, Amplatz K. Injury potential to venous valves from the Amplatz thrombectomy device [published online ahead of print 2000/06/29]. J Vasc Interv Radiol. 1999;10(1):64–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


