Abstract
The completion-of-tumor hypothesis involved in the dynamic interplay between the initiating oncogenic event and progression is essential to better recognize the foundational framework of tumors. Here we review and extend the gametogenesis-related hypothesis of tumors, because high embryonic/germ cell traits are common in tumors. The century-old gametogenesis-related hypothesis of tumors postulated that tumors arise from displaced/activated trophoblasts, displaced (lost) germ cells, and the reprogramming/reactivation of gametogenic program in somatic cells. Early primordial germ cells (PGCs), embryonic stem (ES) cells, embryonic germ cells (EGCs), and pre-implantation embryos at the stage from two-cell stage to blastocysts originating from fertilization or parthenogenesis have the potential to develop teratomas/teratocarcinomas. In addition, the teratomas/teratocarcinomas/germ cells occur in gonads and extra-gonads. Undoubtedly, the findings provide strong support for the hypothesis. However, it was thought that these tumor types were an exception rather than verification. In fact, there are extensive similarities between somatic tumor types and embryonic/germ cell development, such as antigens, migration, invasion, and immune escape. It was documented that embryonic/germ cell genes play crucial roles in tumor behaviors, e.g. tumor initiation and metastasis. Of note, embryonic/germ cell-like tumor cells at different developmental stages including PGC and oocyte to the early embryo-like stage were identified in diverse tumor types by our group. These embryonic/germ cell-like cancer cells resemble the natural embryonic/germ cells in morphology, gene expression, the capability of teratoma formation, and the ability to undergo the process of oocyte maturation and parthenogenesis. These embryonic/germ cell-like cancer cells are derived from somatic cells and contribute to tumor formation, metastasis, and drug resistance, establishing asexual meiotic embryonic life cycle. p53 inhibits the reactivation of embryonic/germ cell state in somatic cells and oocyte-like cell maturation. Based on earlier and our recent studies, we propose a novel model to complete the gametogenesis-related hypothesis of tumors, which can be applied to certain somatic tumors. That is, tumors tend to establish a somatic asexual meiotic embryonic cycle through the activation of somatic female gametogenesis and parthenogenesis in somatic tumor cells during the tumor progression, thus passing on corresponding embryonic/germ cell traits leading to the malignant behaviors and enhancing the cells’ independence. This concept may be instrumental to better understand the nature and evolution of tumors. We rationalize that targeting the key events of somatic pregnancy is likely a better therapeutic strategy for cancer treatment than directly targeting cell mitotic proliferation, especially for those tumors with p53 inactivation.
Keywords: Gametogenesis, p53, Cancer, primordial gem cells, germ cell like cell, parthenogenesis
Gametogenesis-related theories of tumors
In mammals, the segregation and fate decision of germ cells from somatic cells are some of the earliest fundamental events during development (Fig.1)[1–3]. They are physically segregated and rigorously prevent the mutual conversion of cell fate [1–3]. The somatic cells contribute to the maintenance of the physiological integrity of individuals, whereas germ cells generate male and female gamete and transmit particular genetic information to subsequent generations through the creation of new life by fertilization (Fig.1) [1–4]. To some extent, the somatic cells are short in life-span while the germ cells are immortal [1–4].
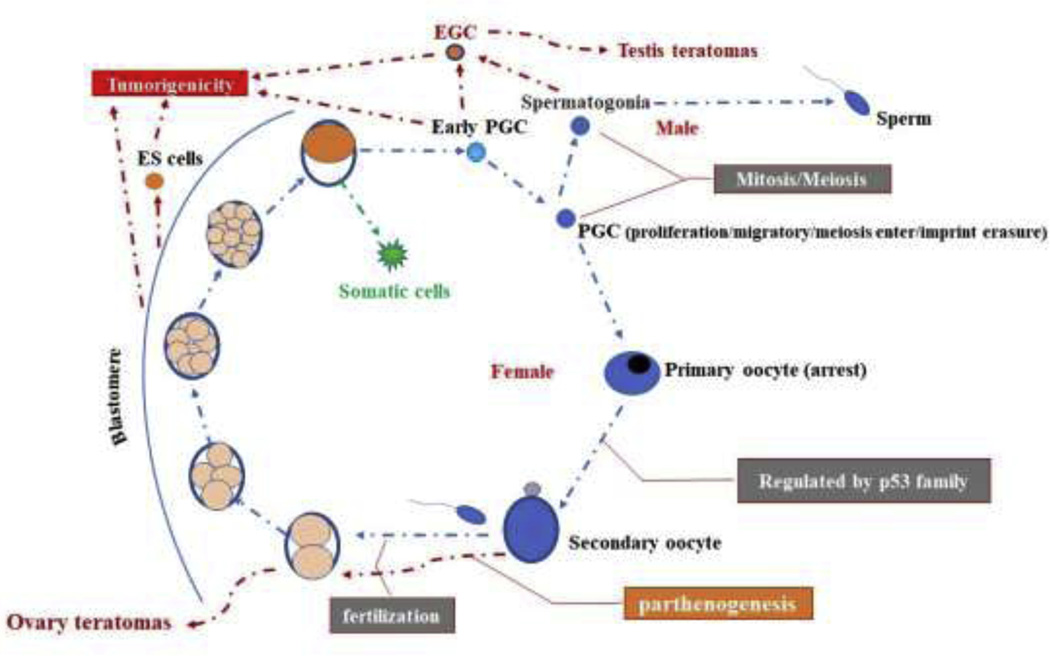
Figure 1.
Germline cycle and tumorigenicity in mammals. The segregation and fate decision of germ cells from somatic cells are some of earliest fundamental events in development. The somatic lineages generally maintain the physiological integrity of the organism while germ cells contribute to an enduring link between generations through the creation of offspring by fertilization. Pre-implantation embryo from fertilization or parthenogenesis including two-eight cell stage, morulae and blastocysts, could develop teratomas/teratocarcinomas after grafted to other extra-uterine sites. ES cells, PGCs and EGCs also have the potential to develop teratomas/teratocarcinomas.
However, the gametogenesis-related hypotheses of tumors were proposed as earlier as 19th century. The foundation establishing this link of tumor formation and gametogenesis began with the discoveries of a mammalian egg in that gametes have a capacity to exist independently of surrounding cells [5, 6]. Virchow then proposed the ’embryonal–rest hypothesis’ in 1855 because of striking similarities between teratomas and embryonic tissues [7–9]. In light of the similarity of the biological characteristics between trophoblasts and cancer, Beard (1902) introduced a ‘trophoblastic theory of cancer’, which postulates cancers are derived from germ cells that stray or are arrested in the wrong place during the migration of embryonic cells to the gonads [10–12]. Under the induction of carcinogenic stimuli, germ cells undergo a transition to malignant trophoblastic cells [10, 11]. Mintz et al (1978) postulated that embryonal somatic cells are an origin of teratocarcinomas in light of the finding that malignant teratocarcinoma was formed in mice injected with day 6 (egg-cylinder stage) mouse embryos with genetically impaired germ cell development, which failed to undergo the formation of germ cells and parthenogenetic “embryos” [12]. Vinnitsky (1993) proposed the oncogerminative hypothesis of tumor postulating that the activation of germinative life cycle endows somatic tumors with immortal and tumor initiation potential [13]. Similar to this concept, Old (2001) believed that the similarity between trophoblasts and tumor cells in antigens is better explained by the reactivation of a gametogenic program in tumor cells instead of the derivation of tumors from lost germ cells as well as the reactivation of a gametogenic program is a driving force in tumor malignant progression [10, 11]. Subsequently, the hypotheses were integrated into the gametogenesis-related theories of tumors including those postulating that tumors arise from displaced/activated trophoblasts, displaced germ cells and the reprogramming/reactivation of gametogenic programs in somatic cells [5–8, 10, 11, 13–15]. Thus, the formation of tumor cells is in some way similar to the formation of gametes and fertilization [5–8, 10, 11, 13, 15, 16].
Early evidence of gametogenesis-related theories
Over the past centuries, numerous scientists were devoted to the study of the gametogenesis-related theories. In the mid-19th century, Rudolph Virchow, the father of pathology, noted that teratocarcinomas are composed of an abnormal fetal mixture and mature tissues [7–9]. Later on, his student, Julius Conheim, recognized the tissue of teratocarcinomas resembling embryonic tissue and used this similarity to support the embryonal rest theory of cancer [8, 17]. Pathologists further observed that most teratocarcinomas consist of a mixture of mature, differentiated tissues and fetal components, such as placental elements, the yolk sac and even the embryoid body which closely resembles early embryos [18, 19]. The malignant component of a teratocarcinoma is restricted to structures that contain embryonal cells and resemble early embryos, i.e. the embryoid body [18, 19]. Further studies uncovered that the undifferentiated and transplantable features of teratocarcinomas attributed to their stem cells, embryonal carcinoma (EC) cells which displayed the capability of tumorigenicity at the single-cell level, pluripotency in vitro/in vivo as well as in chimera development (Kleinsmith and Pierce 1964) [20, 21]. Many EC cell lines were established since 1970 in distinct mammals [22, 23]. This indicates that teratomas/teratocarcinomas imitate embryonic development [18–23].
Intriguingly, numerous earlier studies revealed that disequilibrium in the surrounding tissues would allow the early embryo to resume cell proliferation, resulting in masses of cells resembling embryonic tissues in a disorganized manner [24–31]. The first study performed by Runner (1947) revealed that the injection of tubal fertilized mouse egg to the anterior chamber of the eye led to the generation of the three primary germ layers resembling teratomas [30]. Subsequently, the studies showed that pre-implantation embryos including two-eight cell stage, morulae and blastocysts, could be grafted to other extra-uterine sites such as kidney and testis (Fig. 1) [24–29, 31]. The tubal embryos (early embryos) except those at one cell stage are capable of developing teratomas or teratocarcinoma in a different frequency under distinct stages of embryogenesis, at a graft site, and based on the genetic background of mice [24–31]. Of note is that Kirby (1963) [27] and Stevens (1964) [32] found that the testicular environment offers a much better bed for the acquisition of teratomas/teratocarcinomas from blastomere. Notably, the similar developmental properties of EC cells and early embryos formulate the basis for the generation of embryonic stem (ES) cells that were first isolated from blastocytes of mice (1981) [33]. On the basis of this pioneer finding, many types of mammalian ES cells including human have been subsequently generated from morula, later blastocyst stage embryos, single blastomeres of two- to eight-cell stage embryos, and even parthenogenetic embryos (Fig. 1) [34–38]. As a counterpart of EC cells, ES cells have the ability of developing into teratomas (Fig. 1) [33–38]. These findings, therefore, establish a close link between teratomas/teratocarcinomas and embryonic development [24–38].
Roles of germ cells in teratomas/teratocarcinomas
Stevens et al. (1954) found that spontaneous testicular teratocarcinomas were developed in about 2% of certain sublines of the 129 inbred male mice [39]. The incidence of spontaneous testicular teratocarcinomas in mice is markedly influenced by their genetic backgrounds and the alteration of genetic factors. Whiles spontaneous testicular teratomas rarely develop in mice except for those in the 129 inbred male mice, the alterations of tumor suppressors or oncogenes, such as Steel, p53, Pten, and Akt, abruptly enhanced the incidence[32, 40–43]. Of note, 100% of Pten knockout mice developed bilateral testicular teratomas [42]. Steven (1962) proposed that teratomas originate from primordial germ cells (PGCs) [44]. To confirm the hypothesis, Steven grafted genital ridges to testis, spleen, and kidney and found that 82% of E12.5-day genital ridges from strain 129 fetuses developed teratomas in testis (Fig.1) [41, 45, 46]. This is a much higher incidence than that occurred spontaneously [32, 40, 41, 45, 46]. In essence, the teratomas derived from grafting genital ridges into the testes of adult mice resemble the spontaneous testicular teratomas in histology [41, 44–46]. Therefore, Steven confirmed that testicular teratocarcinomas in mice indeed originate from PGCs [45]. The concept was also confirmed in clinical samples of human testicular teratomas [47, 48]. Interestingly, it was documented that pluripotent embryonic germ cells (EGCs) could be generated from PGCs cultured in vitro in the presence of stem cell factor (SCF), leukemia inhibitory factor (LIF), and basic fibroblast growth factor (bFGF) [1, 49, 50]. The PGC to EGC conversion, which was regulated by Pten, p53, and Akt, not only enables teratoma formation (Fig. 1) but also contributes to chimeras [1, 42, 49–51].
The spontaneous formation of teratomas also occurs in the female ovary [52–57]. About 50% of female mice in LT/Sv background developed spontaneous ovarian teratomas within 90 days of birth (1974) [52]. The teratomas arise from parthenogenetic cleavage of oocytes (Fig. 1) [52, 53] [50–52], which has completed the first meiotic division and undergone metaphase I arrest [55–57][54–56]. It was documented that human ovarian teratomas showed similar origins [58] [57]. Some ovarian parthenotes could reach a developmental stage close to E7 days prior to becoming disorganized and causing teratomas [52–54]. In general, mouse parthenogenetic embryos fail to develop normally and usually die by day E10 in gestation because parthenogenesis is prevented by paternal imprinting [59, 60]. However, the parthenogenetic embryos could give birth to live mice with the ability to reproduce offspring if there is proper expression of the Igf2 and H19 genes in conjunction with other imprinted genes [60]. Notably, ES cells can be isolated from the parthenogenetic blastomeres of oocytes (Fig. 1), which are regarded as the source of human ES cells [38, 61]. Additionally, germ cell tumors found in testis, ovary and even extragonadal sites are also regarded as direct evidence of the gametogenesis-related hypothesis of tumors [47, 62, 63]. The extragonadal germ cell tumors are usually located in the midline of the body, such as the mediastinum, parapineal and sacrococcygeal regions and retroperitoneum, consistent with the pathway of migration of the PGCs from the wall of the yolk sac to the primitive gonad during embryonic development [63, 64].
In further support of the gametogenesis-related hypothesis of tumors, it was documented that part of germ cells may accidentally go astray and reside in tissues outside of the genital ridge due to abrupt failure in proper migration to the future site of the gonads under certain conditions, such as ectopic expression of Sox17, CXCR4, CXCR7B, c-Kit or Steel [1, 65], thereby leading to tumor formation potential in new locations. Consistent with this notion, teratomas/germ cells outside of genital ridges also exist in human tumors, suggesting that the teratomas/germ cells might arise from “lost” germ cells [63, 64]. The misplaced PGCs are often removed through programmed cell death in a p53-dependent manner [66]. Collectively, these findings validate that PGCs and oocytes have the potential to give rise to tumors and likely serve as original cells of testicular teratomas and ovarian teratomas, respectively [41, 45, 46, 52], thus lending strong support to the gametogenesis theory of tumors in certain cancers. However, the teratomas/ teratocarcinomas/germ cell tumors were regarded as an exception rather than the rule [8].
Embryonic/germ cell antigens of tumors
Could the rules learned from teratomas/teratocarcinomas/germ cell tumors be useful for other tumor types? As the matter of fact, the traits of embryonic/germ cells were not only restricted in teratomas/teratocarcinomas/germ cell tumors but also in somatic tumor types. It recently became clear that tumor cells display embryonic/germ cell features at a far higher rate than their primary tissues, involving the induction of embryonic/germ cell genes in somatic tumors [8]. It was proposed that embryos and tumors may share common antigens [8]. The aberrant production of embryonic development-related genes appeared in a wide range of histologically different cancers, such as α-fetoprotein (AFP), carcinoembryonic antigen (CEA), human beta chorionic gonadotropin (beta HCG), and placental alkaline phosphatase (PLAP), which usually serve as the biomarkers for assisting clinical tumor diagnosis [7, 10]. The discovery of cancer/testis (CT) antigens in cancers, which are predominantly restricted to testis while almost absent in somatic tissues, is another milestone to support the gametogenesis-related theory of cancer [10, 11, 15, 67]. In normal tissues, the expression of CT antigens is primarily restricted to immature germ cells, such as spermatogonia and oogonia/primary oocytes, and trophoblasts [10, 11, 15]. It should be noted that only a few CT antigens are involved in germ cells at late stages of sperm or oocyte maturation in the resting primordial follicles [10, 11, 15].
The first CT antigen, which serves as a target for CD8 T cell recognition, was identified in human melanoma cells [10, 11, 15]. This CT gene termed MAGE1 (melanoma antigen Expression of the MAGEA1 gene) was subsequently identified and detected in melanomas, breast carcinomas, and other tumor types, but not in any normal tissues except testis. Subsequently, a serial of CT antigens, such as BAGE, GAGE1, SCP1, NY-ESO-1, and SSX were identified [10, 11, 15]. At the moment, more than 40 CT antigens have been discovered and expressed extensively in a variety of human cancers, including melanoma and carcinomas from lung, bladder, kidney, and liver [10, 11, 15]. CT antigens can be divided into two groups based on their encoded genes, CTX antigens (encoded on the X chromosome), and non-X CT antigens (not on the X chromosome) [10, 11, 15]. The function of most CT antigens is unclear since the proposed function is mainly based on their sequence homology of proteins with known functions [10, 11, 15]. CT antigens, such as synaptonemal complex protein 1 (SCP1) and OY-TES-1, involved in meiosis and late sperm maturation, respectively, are clearly essential for gametogenesis [10, 11, 15].
Unlike typical differentiation antigens, CT antigens are highly heterogeneous in terms of their expression in individual cancers [10, 11, 15]. In principle, only a small subpopulations of cancer cells do express CT antigens in sharp contrast to non-CT expressing cells and tissues [10, 11, 15]. Interestingly, numerous CT genes are frequently co-expressed in the same tumor cells [10, 11, 15]. These findings indicate that CT antigen expression may be the result of the activation of an orchestrated gene expression program, rather than being the result of random events [10, 11, 15]. In light of the properties of CT antigens expressed in cancers, Old and his colleagues stated that ectopic expression of germline genes in cancer reflects the activation of the silenced gametogenic program in somatic cells [10, 11, 15]. They further postulated that the activation of a gametogenic program is one of the driving forces for tumorigenesis since it could give tumors with a serial of the neoplastic phenotypes, including immortality, invasiveness, immune escape, hypomethylation, and metastatic capacity [10, 11, 15]. The hypothetical concept provides a causal link between gametogenesis and cancer formation [10, 11, 15]. Finally, Old proposed an intriguing concept that cancer may be a somatic cell pregnancy in that cancer is not a disease of abnormal growth as what we usually thought, but a disease of reproduction through gametic recapitulation in somatic cancer cells [10, 11, 15]. The provocative concept provides a new way to think about cancer and its evolution during the progression of the disease.
Support from the gene level
Of note is that the high expression of the key genes involved in embryonic/germ cell development, such as Stellar, GDF3, SSEA1, IFITM3, CD117, Oct4, Sox2, and Nanog, was observed frequently in several types of human somatic cancers [10, 11, 68–74], indicative of the possibility of reactivation of germ cell programming in somatic tumor types[10, 11, 75]. However, what functions do embryonic/germ cell developmental genes display in cancer and does the expression of these genes really play a driving force for malignant progression of tumor cells as expected by Old? Synthesizing studies indeed demonstrated that embryonic/germ cell-related genes play a crucial role in tumorigenesis and metastasis. Janic et al. (2010) reported that Drosophila gene lethal (3) malignant brain tumor lost its tumorigenicity in fly after inactivation of any of the germline genes, such as nanos, vasa, piwi, and aubergine [76]. This study indicated that germline features at the gene level may be a driving force for tumorigenesis in the Drosophila model [76]. The findings from two studies revealing that soma-to-germline transformation occurred in Caenorhabditis elegans with inactivation of Rb homolog LIN-35 and long-lived C. elegans strains led to a proposed model that the soma-to-germline transformation might appear and contribute to increased fitness and survival in the Drosophila tumors through the aberrant expression of germline genes [77–79]. Further studies demonstrated that embryo/germ cell-related genes are found to be essential for tumor formation and metastasis [80–84]. Interestingly, Kaufman et al. revealed the importance of neural crest progenitor state reappearance in melanoma initiation using a zebrafish model [85]. They found that melanoma precursor cells reinitiated an embryonic neural crest feature and elicited a melanoma gene program during cancer initiation [85]. While these findings lend certain support for the gametogenesis-related hypothesis in somatic tumors, attempts to identify the germ cell-like cells in tumors would be warranted to further support the hypothesis.
Owing to the strikingly heterogeneous CT expression in tumors, Old postulated that CT antigen expression marks cancer stem cells (CSCs) and disappears as CSCs differentiate and lose clonogenicity capacity [10, 11]. Interestingly, several studies indeed showed that the genes involved in embryonic/germ cell development can be used as the markers of CSCs and play a significant role in tumorigenesis and metastasis [72, 74, 84]. The study from Son et al (2009) uncovered that SSEA-1, a marker of early germ cells, could be utilized to isolate cancer-initiating cells in human glioblastoma [72]. Boumahdi et al (2014) revealed that Sox2 plays a crucial role in CSC functions in squamous-cell carcinoma [84]. The essential roles of these germ cell-related genes in tumor malignant behaviors were attributed to the regulation of cancer stemness[72, 74, 84].
Cell reprogramming, which is regulated by oncogenes and tumor suppressors, is a way to obtain the ES-like state and tumorigenicity potential [81, 86–93]. For example, oncogene C-Myc is one of the key driving genes for generating iPS cells from the somatic cells [86, 87], while tumor suppressors, such as p53 and Rb [88–91], markedly inhibit the efficiency of generating an ES-like state. Consistent with this, lineage plasticity is involved in cancer progression and recurrence and drug resistance of tumors, while the inhibition of the pluripotency for lineage plasticity impairs these properties [81, 88–90, 92]. These studies establish a close link between pluripotency and tumor malignant behaviors.
Germ cell-like tumor cells
Although somatic tumor cells displayed high expression of embryonic/germ cell genes, it is unclear why these genes are aberrantly expressed in somatic tumor cells, how they play a crucial role in tumor progression and metastasis, and what the cellular basis of their expression is. Is it caused by the reactivation of genes or reacquisition of the cell fate in somatic tumors? In 2006, our team showed that teratoma could arise from malignant bone marrow-derived cells induced by chemical carcinogen, indicative of the possible direct link between adult somatic tumor cells and embryonic development[94]. We (2011) further observed the occurrence of cancer cells with the embryonic/germ cell characteristics in the malignant BMDCs, which may serve as the origin of teratoma [95]. Importantly, embryonic/germ cell-like cancer cells were identified in diverse tumor types by our group [95–101]. In addition to in vitro evidence using in vitro cultures from distinct cell lines and models, we also presented the in vivo evidence using p53 knockout mice demonstrated that germ cell-like cells could be acquired and present in p53 deficient tissues [98, 101]. These embryonic/germ cell-like cancer cells resemble the natural embryonic/germ cells in morphology, gene expression, the capability of teratoma formation, and the ability to undergo the process of oocyte maturation and parthenogenesis [95–100]. In some cases, the embryonic/germ cell-like cancer cells could be observed at different developmental stages including PGC and oocyte to early embryo-like stage [95–100]. Consistently, imprint erasure could be observed in some subpopulations of cancer cells [100]. Developmental events with oocyte-like cell maturation could be detected, including the expression of the markers related to late oocyte (e.g. GDF9), estrogen production, size increase, meiosis entry (expression of SCP3), oocyte with the germinal vesicle (GV)-like structures, and oocyte completing the first meiosis [96–100]. Moreover, our recent study revealed that knockout of those core genes critically involved in PGC specification and fate maintenance such as Oct4, Nanog, Prdm14, DDX4 and DAZL impairs tumorigenicity and metastasis potential of 4T1 somatic tumor cells, indicating that the PGC-like cells are likely an origin for somatic cancer initiation and metastasis[101]. Additional evidence from further studies will be needed to attest whether PGCs-like cells play a general role in all somatic tumorigenesis and metastasis. The observation that the appearance of germ cell-like tumor cells at PGC and oocyte-like stage provides a cellular basis for the gametogenesis-related hypothesis at least in certain types of somatic cancer such as breast cancer. In further support of this theory, Niu et al. uncovered that somatic tumor cells have the potential to cause germ cell tumors upon injected to mice [102].
Activation of parthenogenesis
Intriguingly, our group found that the activation of parthenogenesis from the oocyte-like cells occurred spontaneously in many tumor types (Fig. 2) [96–100]. The parthenogenetic embryo-like structures were observed at the different developmental stages including two cell-, several cell-, morula, blastocyte, and even post-implantation embryo-like structures, which are similar to cultured early embryo in morphology, gene expression, differentiation, and the formation of tumors with mature tissues [96–100]. Parthenogenesis is a form of asexual embryonic reproduction in that an oocyte can be activated without the intervention of the male gamete [103]. Somatic cells can establish an asexual cycle through the abnormal somatic female gametogenesis and subsequent formation of a parthenogenetic embryo, the latter returning back to the somatic tumor cells and PGC-like state (Fig. 2) [95, 98]. The asexual embryonic reproduction can endow tumors with powerful abilities for survival, fitness and individualism similar to the embryogenesis (Fig. 2), thus markedly enhancing the independence of tumors [98].
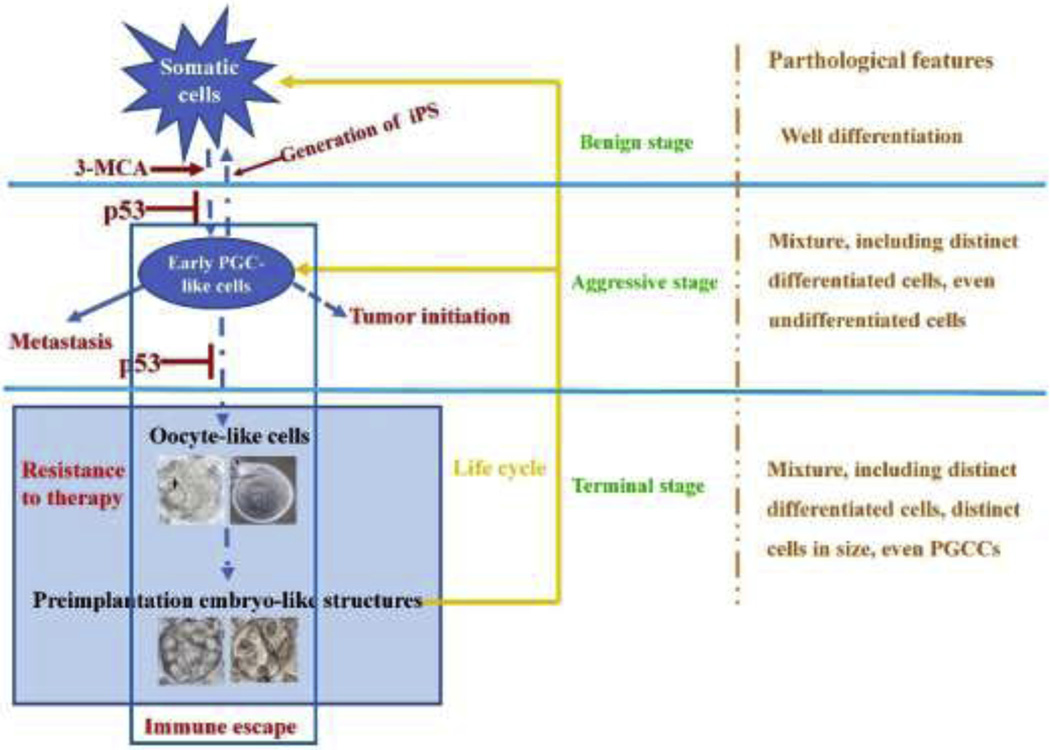
Figure 2.
Independent life cycle of tumors and tumor grade. The cycle of tumor progression may be involved in four key stages due to the genetic and epigenetic alterations: (1) Somatic cells are transformed and exhibit the traits of growth out of control, representing the benign stage; (2) Activation of somatic cell-ES/PGC-like cell conversion (including somatic cell-ES cell-like cell conversion and somatic cell-PGC-like cell conversion), which endows tumor with the capabilities of tumor initiation and metastasis, representing the entrance of malignant stage; (3) Activation of PGC-like cells undergo further development along with germ cell maturation to generate oocyte-like cells; (4) Parthenogenetic activation of oocyte-like cells generates to blastomere-like cells which can endow tumor with powerful abilities, such as asexual life cycle, pluripotency, immortality and therapeutic resistance, representing the terminal stages. Thus, the oocyte-like and preimplantation-like states allow for tumors to resist to therapy.
It is not surprising that the spontaneous activation of parthenogenesis could be observed in oocyte-like tumor cells. As mentioned above, parthenogenesis can be activated in oocytes and results in ovary teratomas [52–54]. In mammals, the derivation of parthenogenetic embryos from oocytes can be successfully induced in vitro by various physical or chemical stimuli, which leads to the intracellular calcium wave resembling that triggered by sperm at fertilization [103]. Studies in animal models showed that the incidence of parthenogenesis is dependent on the genetic background [52–54]. It has been reported that parthenogenetic embryo in mice can give rise to normal offspring when genetic imprinting was modified [60]. In culture, the parthenogenesis was frequently observed in normal oocytes, oocytes from ES cells, and oocyte-like cells.
Notably, abnormal female gametogenesis and parthenogenesis could also be induced in male (XY) gametogenesis under certain environments. It was shown that developing from PGCs to mature male germ cells strictly depends on the appropriate SRY expression [104]. In the absence of appropriate SRY expression in the gonads, male PGCs entered into the female pathway and often underwent the first step of oogenesis, which represents meiotic arrest at prophase I [104]. Male-to-female sex reversal is frequently observed in the mammalian embryo when certain genes such as Sry, Sox9, or Fgf9 are inactivated or deleted. Interestingly, the p53 tumor suppressor also regulates the male-to-female sex reversal [104–107]. Yasuda et al. showed that testis–ova transformation spontaneously occurred in p53-deficient testes and was also induced by γ- irradiation [108]. It was also documented that oocyte-like cells could be derived from male somatic cells [109]. Given the CT antigen, OY-TES-1, involved in the formation of the sperm head can be detected in various tumor types [10, 11, 15], it raises the possibility that male gametes may be generated in some somatic tumors.
Somatic pregnancy
The key question about the origin of aberrant germ cells in somatic tumors remains unclear. It has been proposed that somatic tumors are derived from either residual embryonic/germ cells or re-acquisition of the gametogenic program in somatic cells/tissues [5–8, 10, 11]. While it remains debatable, it is possible that both ways may contribute to the high embryonic/germ cell features, leading to tumor formation in light of recent discoveries. However, we believe that re-acquisition of the gametogenesis program is the main way for the derivation of most somatic tumor types. The findings that teratomas/germ cell tumors were found in genital ridges provide the direct evidence for the germ cell origin of tumors [18, 32, 39, 41, 46, 52–54, 62, 63]. Hepatoblastoma occurring in young children and teratomas/germ cell tumors out of genital ridge likely derive from residual embryonic/germ cells [63]. However, the findings that induced pluripotent stem (iPS) cells could be generated from somatic cells through genetic reprogramming highlight the possibility that somatic cells have a potential to dedifferentiate to the embryonic state for tumor formation through re-acquisition of the gametogenic program [86, 87]. The findings that tumors with strong embryonic features were generated from somatic cells in vivo lend the support of the gametogenesis-related hypothesis of tumors [86, 87].
To further validate this hypothesis, our group uncovered that teratomas and PGC-like cells could be generated in somatic cells by chemical carcinogen challenge or upon p53 deficiency [98, 100]. At a single-cell level, the somatic tumor cells could spontaneously give rise to PGC-like cells with a higher incidence in many tumor types [100]. Other studies also showed that normal cells from somatic tissues could generate a serial of germ cell-like cells including oocyte-like cells and their embryonic derivatives under certain culture conditions, albeit the incidence is relatively low [110–113]. Interestingly, these ES/PGC-like cells could trigger the female germ cell pathways, leading to the development of oocytes [112, 114] and subsequent parthenogenetic embryo under specific conditions, likely involving the proper genetic and epigenetic change and/or microenvironment change [98, 100, 112, 114]. Therefore, somatic tumor cells in some ways can establish an asexual embryonic cycle [98]. In our view, the completed asexual cycle of tumors through somatic-PGC-oocyte-cleave-parthenogenetic embryo-somatic cell conversion represents the activation of a whole embryonic/germ cell developmental axis in somatic cells (Fig. 2) [98]. This process is indeed consistent with the concept of “somatic pregnancy” defined by Old [75], which may serve as a tumor progression.
Is asexual embryonic reproduction crucial for tumor malignant traits?
As it has been well established that ES cells and early PGCs have the capability to elicit tumor initiation [19, 24–29, 33–38, 41, 45, 46], the acquisition of the ES-like or early PGC-like cells regardless of what they come from may contribute to the tumorigenicity. The concept is indirectly supported by several recent studies [86–93]. Abad et al showed that transitory induction of the four transcription factors, Oct4, Sox2, Klf4, and c-Myc, which could induce iPSs from somatic cells [86, 87], in mice led to tumors in multiple organs by inducing reprogramming of specific cells from various tissues in vivo, including epithelial cells from stomach, intestine, kidney, and pancreas as well as cells from the hematopoietic lineages [93]. Apart from teratomas, several other tumor types including urothelial carcinomas, Wilms tumors, and other tumors were observed [93]. Since iPS cells were generated upon ectopic expression of these four factors and displayed the tumorigenicity potential, it is likely that the acquisition of iPS cells may contribute to the tumor formation upon the challenge of these four factors[86, 87, 115]. We provided evidence that PGC-like cells were detected in p53−/− mice and displayed the capability to form tumors in mice [98]. Consistent with the migratory characteristic of early PGCs [1], we demonstrated that PGC-like cells, which displayed high CXCR4 and c-Kit expression, served as metastasis-initiating cells in hepatic metastasis from multiple somatic tumor types [101]. The striking similarities between our PGC-like cells and PGCs in cell properties, such as in migration and tumor metastasis potential, and gene expression profiles such as CXCR4 and c-Kit, are intriguing [1, 74, 116]. The ES/PGC-like cells are small in size (~5–15cm in diameter), round-shape, and high nuclear-to-cytoplasmic (N/C) ratio [96–101], likely representing undifferentiated cells in tumor tissues (Fig.2). We postulated that the PGC-like cells might also play a role in cancer metastasis to other organs/tissues.
Polyploid giant cancer cells (PGCCs), either mononucleated or multinucleated single cells, have been frequently described by pathologists for many years [7, 102, 117–121]. PGCCs often detected in high-grade tumors and enriched upon the therapy are considered as the common histopathological features for advanced and high grade tumors [7, 102, 119, 120]. PGCCs were traditionally viewed as a byproduct during tumor progression and likely do not play a role in tumor malignant progression because they are nonviable and stay in a terminal cell fate incapable of executing mitosis [122]. However, the evidence from several other groups indicated that PGCCs could indeed give rise to the daughter cells via a unique mode called “budding division” instead of the classic mitotic division [7, 102, 117–133]. The formation of PGCCs was up-regulated upon p53 and Rb deficiency [134–137]. The progeny cells derived from PGCCs have the capability of proliferating and forming tumors [130, 138–140]. Therefore, this process was described as “Polyploid Cycle” [7, 102, 117–120]. Of interest is that PGCCs can escape from a variety of stresses and damages, such as senescence, hypoxia, genotoxic stress, and target therapy treatment, thereby leading to multiple therapy resistance and recurrence through “Polyploid Cycle” [7, 102, 117–120].
Recently, the PGCCs, proposed from somatic cells that underwent endoreplication, are shown to be the blastomere- or blastocyst-like embryo, since they resemble the early embryo in morphology, gene expression, and the ability to generate germ cell tumors [102]. Interestingly, activation of meiosis appeared prior to the formation of PGCCs [123, 124, 141], indicating that the PGCCs, blastomere-like cells, likely arise from the cleavage of oocyte-like cells. Erenpreisa et al pointed out that polyploidy resembling the phylogenetically pre-programmed “oncofetal attractor” state could establish “asexual reproduction” through embryonic/parthenogenetic and sporogenic-like cycle [142]. In light of these findings along with our recent study [98], we proposed that PGCCs may be the counterparts of oocyte-like cancer cells (mononucleated state) and preimplantation embryo-like (multinucleated state). Similar to the PGCCs, our study indicated that the oocyte-like cells and their embryonic derivation contribute to the independent life cycle, tumorigenicity, and therapeutic resistance [98]. Moreover, the occurrence of PGCCs reflecting the activation of abnormal female gametogenesis and asexual embryonic cycle may explain why the appearance of PGCCs examined by pathological morphology occurs in late stages of tumors. In addition to the “Polyploid Cycle” model [7, 102, 117–120], our asexual embryonic cycle model showed that PGCCs may be derived from those somatic cells undergoing female gametogenesis and parthenogenesis (Fig. 2) [98]. We postulated that PGC-like cells derived from somatic cells enter meiosis and go through the pathways of oocyte-like maturation and the later cleavage through parthenogenesis to develop multinucleated PGCCs, blastomere-like cells [98].
Immune escape represents one of the key characteristics of germ cells and embryos. Old team pointed out that the capacity of immune escape in tumors is attributed to their traits of embryonic/germ cells [11]. Consistent with this hypothesis, we showed that germ cell-like cells isolated from various tumor types displayed the high expression of the immune escape genes [101], such as PDL1, FASL, TRAIL, and CD47 [143, 144]. We speculated that diverse strategies utilized by germ cells and embryos such as immune escape and therapeutic resistance were also adapted by germ cell-like cancer cells and/or blastomere-like cancer cells to evade immune cell attack and facilitate cancer progression and drug resistance. This concept may be further supported by the recent study uncovering a shared immunosuppressive onco-fetal ecosystem between fetal liver and hepatocellular carcinoma [145]. Reprogramming and embryogenesis offer the pluripotent state [24, 26–29, 31, 86, 87], allowing for establishing the suitable micro-environments and protective mechanisms to facilitate tumor cell survival and fitness. Hence, the abnormal female gametogenesis and asexual embryonic cycle not only give tumors the powerful potential of survival and fitness through diverse malignant traits but also link distinct malignant traits of tumors together (Fig. 2) [98, 101]. The germ cell arrest at different stage will lead to different types of tumors [47, 146]. Consistently, it is possible that the germ cell-like tumor cells derived from somatic cells would be arrested at different stage and cause different tumor types due to distinct genetic and epigenetic changes or microenvironment, such as PGC-like stage arrest resulting in tumors with immature tissues and parthenogenetic activation of primary oocyte at meiotic arrest causing tumors with mature tissues. The different pathways might be either co-existed or sequential in some tumors. Of note, the tumors were malignant or benign also dependent on the efficiency of somatic embryonic cycle and/or the maintenance of embryonic/PGC-like state to some extents. Collectively, the activation of embryonic/germ cell-like developmental axis might be a driving force for tumor malignant behaviors, possibly reflecting the real stemness of tumors.
Mechanisms of p53 tumor suppression
p53 is the most frequently mutated gene in the majority of human cancers. It was initially considered a cellular proto-oncogene in 1979 but was subsequently approved as a tumor suppressor gene in 1989 [147]. Over 50% of tumors harbor mutations in p53 itself and over 80% of tumors have dysfunctional p53 signaling [147–149]. Inherited heterozygous loss-of-function mutations in p53 leads to Li-Fraumeni syndrome in human, which is a familial disease that facilitates cancer predisposition in affected patients [147–150]. Earlier studies showed that mice homozygous for the germline p53 allele are developmentally normal but develop tumors in multiple tissues [43, 151]. Donehower LA and colleagues reported that all p53−/− mice develop spontaneous tumors within 10 months of age (mean time to develop a tumor is 4.5 months), while about 50% p53 + /− mice display spontaneous tumors by 18 months with over 90% incidence by two years of age [43]. However, p53 wild type mice are tumor-free until 18 months with less than 25% developing tumors by the age of two years [43]. The findings from the genetic mouse model provide direct evidence that p53 plays a critical role in suppressing tumorigenesis [43]. Subsequently, numerous studies from diverse groups revealed that p53 is a center tumor suppressor gene, which is involved in tumor growth, progression, metastasis, and drug resistance [147–149, 151–157].
Several studies focused on how p53 works biochemically. The most prominent function of p53 protein is to act as a DNA sequence-specific transcription factor [147–149, 152, 153]. p53 is induced by various stress conditions [147–149, 152, 153]. Upon genotoxic stress, p53 is stabilized and selectively induces a plethora of target genes that elicit diverse cellular processes including cell cycle arrest, apoptosis, and senescence [147–149, 152, 153]. Through these mechanisms, cells with damaged and mutated genomes are eliminated before they can become cancer cells[147–149, 152, 153]. Therefore, cell cycle arrest, apoptosis, and senescence were generally thought to be the main tumor-suppressive mechanisms for p53 against cancer [147–149, 152, 153]. However, this notion has been challenged by recent studies revealing that the induction of cell-cycle arrest, apoptosis, and senescence is insufficient for p53-mediated tumor suppression using the mice bearing specific p53 mutations in the presence or absence of p21, Puma and Noxa deficiency [156, 158], highlighting the importance of other cellular properties regulated by p53 in p53-mediated tumor suppression. In this regard, recent studies showed that p53 can regulate diverse metabolic pathways to orchestrate ferroptosis and lipid biogenesis, which may offer a potential mechanism accounting for p53-mediated tumor suppression [159, 160].
The p53 family proteins consist of p53, p63, and p73 which are evolutionarily conserved from invertebrates to mammals [161, 162]. As evolutionarily ancient transcription factors, the family proteins have a primary role in regulating DNA damage response in cells, apart from other distinct mechanisms described above [161, 162]. Although the tumor-suppressive role for p53 gene has been firmly established[147–149, 152, 153], the role in tumor suppression may not be the primary function for the p53 family genes, since homologs of the p53 family genes exist in simpler organisms, such as worms and flies, which never develop tumors due to their short life span [161–163]. In worms and flies, the functions of the p53 ancestral genes are to ensure the integrity of germline genes and the fidelity of the developmental process [161–163]. In vertebrates, the p53 family genes retain not only the functions in maintaining the integrity of germline genes but also act as “the guardian of reproduction” [161–163]. p63 is essential for oocyte maturation, whereas p73 maintains normal mitosis for developing blastocyst [161–163]. Notably, p53 deficiency in female mice leads to infertility, since it impairs the production of leukemia inhibitory factor (LIF) for maintaining the implantation of embryos [161–164].
Several studies showed that the p53 family also plays an important role in meiosis [165–173]. The expression of p53 increased in tetraploid primary spermatocytes at the meiotic pachytene stage of spermatogenesis [166]. To suppress genomic instability in meiotic germ cells, p53 works in meiosis-specific homologous recombination repair upon DNA breaks through directly or indirectly binding to RecA-like proteins Rad51 and DMC1 (meiosis-specific) [167, 168]. Oocytes harboring unrepaired DNA will be eliminated through a mechanism dependent on p53 and p63, which are induced by ATR/CHK2 [169, 170]. Upon DNA damage, secondary oocytes can survive while oocytes from primordial follicle will be eliminated at the meiotic pachytene I stage in a p53- and p63-dependent manner [169, 170]. Deletion of p53 and p63 fully rescues the oocyte meiotic elimination upon DNA damage [169, 170]. This provides the molecular basis of why cancer radiotherapy or chemotherapy induces constantly ovarian failure [173].
Of the three p53 family proteins in mammals, p53 is unique in its function as a tumor suppressor [147, 152, 161, 163, 174, 175]. Unlike p53 deficiency, p63 deficiency fails to facilitate tumor formation in animal models [147, 152, 161, 163, 174, 175]. Since p63 deficiency induces senescence, p63 deficient mice displayed severe developmental defects in the skin and limbs and died shortly after birth [174, 175]. Mice with heterozygous loss of the p63 allele displayed no obvious tumor phenotypes, albeit a small lesion was observed occasionally in some mice, accompanied by premature aging features and reduced lifespan [174, 175]. In essence, p63 does not appear to have a strong link to tumor suppression despite some conflicting studies reporting that p63 is either an oncogenic or tumor suppressor gene [147, 152, 161, 163, 174, 175].
Interestingly, reprogramming efficiency of human somatic cells to iPS cells is drastically regulated by p53 [90, 91]. Zhao et al. (2008) documented that p53 targeting with p53 siRNA enhanced the reprogramming efficiency of human iPS up to 100-fold, even though the oncogene c-Myc was removed from those reprogramming factors [91]. While somatic reprogramming to iPS cells could be achieved by introduction of 4 reprograming factors to somatic cells, the reprograming efficiency is typically low [86, 87]. Activation of p53 pathway by the reprogramming factors indeed serves as a barrier for IPS reprogramming, as p53 targeting markedly enhanced the reprograming efficiency [90, 91]. Further studies validated that enhanced reprogramming upon p53 deficiency facilitates tumor formation and metastasis[81, 88]. Collectively, these findings establish the possible role of p53 tumor suppressor in suppressing embryonic/germ cell traits of tumors, thereby leading to tumor suppression [81, 88, 90, 91].
The evidence in direct support of this theory came from our recent studies demonstrating that p53 deficiency promoted the acquisition of PGC-like cells from somatic cells, which display tumorigenicity potential and serve as liver metastasis initiating cells in various tumor types [98, 101]. Moreover, p53 deficiency also promoted oocyte-like tumor cell maturation to complete the first meiosis, therefore allowing for the activation of parthenogenesis and then establishing asexual embryonic reproduction (Fig.2) [98]. Importantly, the asexual embryonic cycle promoted upon p53 deficiency likely gives tumor cells the ability to survive under the treatment of various chemotherapy agents and γ-irradiation [98]. In addition, the enhancement of oocyte-like cell maturation and parthenogenetic activation in response to p53 deficiency could explain why PGCCs were frequently observed in tumors with p53 deficiency/inactivation and were up-regulated by p53 deficiency/inactivation [117–120, 135, 136].
The unique role of p53 in serving as a guardian to suppress asexual embryonic reproduction identified from our studies provides a plausible explanation for the evolution of the p53 gene to acquire dual roles in reproduction maintenance and tumor suppression (Fig.2), thus filling the evolutional gap of p53 gene family[98]. We speculate that inhibiting asexual embryonic reproduction by p53 may serve as a core function of p53 in tumor suppression, although further study is required to firmly validate this theory. Collectively, our findings that p53 deficiency triggering abnormal female gametogenesis and asexual reproduction may lend strong support for our gametogenesis-related concept, as least in certain somatic tumors, in that tumor is somatic pregnancy.
Evolutionary goal of tumors: somatic embryonic reproduction
Sydney Brenner wrote: “Technology gives us the tools to analyze organisms at all scales, but we are drowning in a sea of data and thirsting for some theoretical framework with which to understand it. We need theory and a firm grasp on the nature of the objects we study to predict the rest”[176].
We concur with this statement in regard to cancer and believe we need a testable theory together with concrete experimental validations in order to better understand the biology of cancer. For evolutionary dynamics of cancer, Gillies et al. pointed out is that “Nature selects for phenotype, not genotype” [177]. Although there are inevitable variations between patients (e.g. in genetic changes, epigenetic changes and inducing factors), once a tumor is initiated, the disease sticks to a carefully orchestrated and predictable progression [178] including invasion, metastasis, therapy resistance, and recurrence. In essence, tumors exhibit common hallmarks of cellular phenotypes despite their distinct tissues of origin and genetic backgrounds [177–179]. Accumulating documents pointed the atavistic origin of cancer, which speculated that the biological origin of cancer involved in atavistic process, such as the reactivation of atavistic genetic programs from unicellularity to multicellularity in cancer origin and evolution. [142, 176, 178, 179]. It is time to rethink the fundamental questions to figure out the common framework of tumors. Namely, how does a tumor come from? Why do tumors display common hallmarks? Where is a tumor real destination?
Generally speaking, reproduction is a central objective to the life and evolutionary process. The occurrence of somatic embryonic reproduction in certain tumors [95–100], which is reminiscent of meiotic embryonic reproduction processes during the embryonic development, may be the nature and goal for developing tumors. This leads us to think about the progression of tumors from the standpoint of the evolution pathway. To get full somatic pregnancy cycle, tumor cells need the following two basic evolutional events (Fig. 3): (i) somatic gametogenesis, (ii) parthenogenesis which is regarded as incomplete sexual reproduction. Both somatic gametogenesis and parthenogenesis occur naturally in eukaryotes [180–184]. In following section, we will introduce the evolution of gametogenesis and parthenogenesis to better understand the nature and progression of tumors.
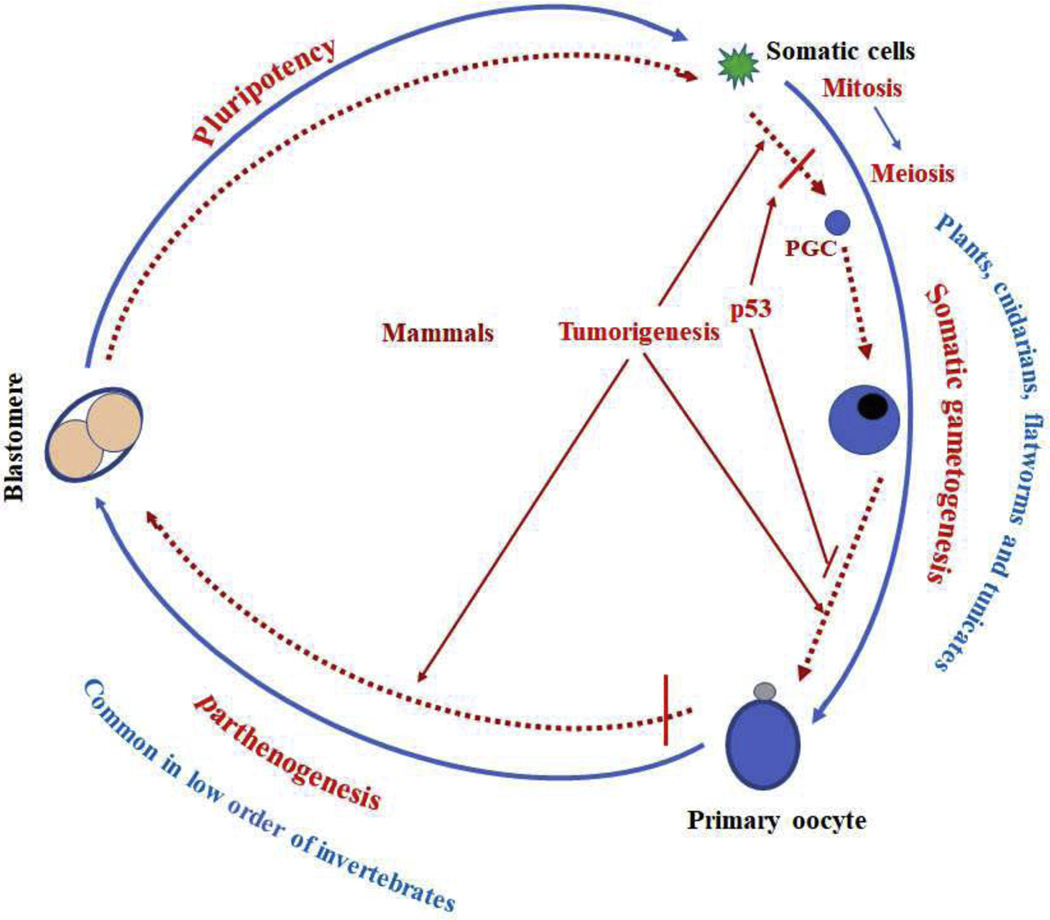
Figure 3.
Tumor progression and evolution. We propose that tumor cells get full somatic pregnancy cycle through the following two basic evolutional events, somatic gametogenesis and parthenogenesis, which are naturally occurring phenomenon in some organisms. Somatic gametogenesis occurs in the entire plant kingdom and in a few animal phyla such as cnidarians, flatworms, and tunicates. Parthenogenesis is very common among the lower order of invertebrates.
Meiosis and sexual reproduction are widely conserved throughout the evolution from all animal phyla to most eukaryotes, while clonality is an oddity, indicating that they are ancient, beneficial, and indispensable [181, 185–187]. In eukaryotic life cycles, meiosis, which is a crucial component of sexual reproduction, is a ubiquitous and highly conserved process[181, 185–187]. However, nearly all forms of uniparental reproduction do maintain meiosis, but just abandon outcrossing, leading to questioning about the purpose of meiosis [184–186]. Li et al pointed out that meiosis may have evolved by combining features of two responses in which cells need to cope with double-strand-DNA breaks, homologous recombination repair, and apoptosis [188]. It is thought that the meiosis benefits sexual reproduction through DNA restoration, genetic recombination, repair of oxidative DNA damage, ploidy reduction, and the formation of gamete. During meiosis, prophase I allows for the repair of DNA damage, while reductional division is essential for the elimination of mutations in the haploid phase [188–191]. The two RecA homologs in meiotic cells of eukaryotes, Rad51 and DMC1, which are involved in meiotic DNA damage repair, might play an important role in tumors [191–193]. As the meiotic DNA repair and checkpoint also depend on activation of p53 and p63 [169, 170], it is likely that p53 deficiency or inactivation might allow some germ cell-like tumor cells with damaged DNA to escape from the meiotic elimination leading to developing secondary oocyte-like cells.
Germ cells have two different origins, the pool of PGCs or somatic cells [1, 180, 181, 194, 195]. Fate decision of germ cells and somatic cells were determined at the very early stage of development in most animals, whereas a clear separation does not occur in the plant kingdom and in a few animal phyla such as cnidarians, flatworms and tunicates whose germ cells are not derived from a pool of PGCs but arise from somatic cells, likely representing the evolutionary method of gamete origin in unicellular organisms [1, 180, 194, 195]. Therefore, somatic gametogenesis is more ancient than advanced fate determination of germ cells and somatic cells [180, 181, 194–196]. The persistence of the advanced germ cell determination faces the competition from somatic gametogenesis and thus some specific barriers are possibly required. However, the mechanism underlying the conversion from a somatic cell fate to a germline fate in plants or other species remains unclear [180, 194, 195]. A logical guess is that mammalian p53 serves as a strong barrier for somatic gametogenesis on the basis of crucial roles of p53 in inhibiting iPS and PGC-like cell formation from somatic cells [90, 91, 98], which is instrumental to understand the evolution of the p53 family. We suspect the same with many other tumor suppressors, which also prevent the soma-to-germline fate transformation. Consistent with this, inactivation of Rb homolog LIN-35 results in soma-to-germline fate transformation in C. elegans [79]. On the contrary, oncogenes, such as c-Myc, might function as activators of this transition [86, 87]. This hypothesis is also consistent with the homolog of p53, which is undetectable in plants [197]. We proposed that the ancient somatic gametogenesis may be reactivated during tumorigenesis.
There are two ways for the activation of oocyte to occur, thus leading to embryogenesis, fertilization, and parthenogenesis [103]. Fertilization, oocyte and sperm fusion, existing in nearly all of mammals are overwhelmingly beneficial for improving fitness [181, 185–187]. In 1945, parthenogenesis was firstly found by Kosin in unfertilized eggs of Barred Plymouth Rock and White Leghorn hens [182]. Parthenogenesis includes any degree of embryonic development in unfertilized oocytes [182]. In contrast to asexual forms of reproduction (e.g. fission and budding), parthenogenesis is often considered as an incomplete form of sexual reproduction and derives from sexual reproduction through evolution [103]. Among the lower order of the animal kingdom, especially invertebrates, parthenogenesis is indeed a very common naturally occurring phenomenon (Fig. 3) [103, 182, 183]. In vertebrates, natural occurrence of parthenogenesis yielding live offspring has been documented in some animals, such as python snakes, Whiptail lizards, Komodo dragons, and even more advanced vertebrates, like birds (Fig. 3) [103, 182, 183]. However, among higher order of vertebrates, natural parthenogenesis is largely unorganized and abortive in nature because of genomic imprinting which acts as a suppressor for natural parthenogenesis [59, 103, 182–184]. In mammals, spontaneous parthenogenesis of ovarian oocytes leads to ovary teratomas/teratocarcinomas [55, 57], the most common ovarian tumors found in young women. It has been established that parthenogenetic oocytes can give rise to live offspring in mice after modification of genomic imprinting [60]. Collectively, acquisition of somatic embryonic reproduction might be the nature and ultimate goal of tumors in order to keep seeds and to improve survival, fitness, and independence.
The evolution of cancer has become increasingly intriguing and pellucid [198–204]. In light of earlier and recent studies (Table 1) in support of the gametogenesis-related hypothesis in certain somatic tumors, we proposed a novel evolutional model based on the concept of somatic pregnancy of tumors. The key events of somatic malignancy likely corresponding to the tumor progression processes contribute to malignant traits, suggesting that a tumor is a disease of abnormal reproduction rather than a disease of growth (Fig. 2). At the beginning, somatic cells only obtain the ability of uncontrolled growth under genetic and/or epigenetic changes, thus filling in a sense of immortal-like single-cell organisms by division, corresponding to the benign stage of tumors (Fig. 2). Through the evolution, the somatic tumor cells then acquire the capability of generating ES/PGC-like cells, meaning that tumors regain an ancient way to generate seeds under the hostile microenvironments. In general, tumorigenicity is an inherent ability of ES cells and early PGCs, indicating that activation of ES/PGC-like cell formation could endow tumor cells with tumor initiation potential (Fig. 2). Interestingly, the finding that the motile PGC-like cells could spread to new sites suggests that the tumor cells obtain the ability to escape from the bad soil and move to new good soil. Continuously obtaining ES/PGC-like tumor cells from somatic cells means the entry of the aggressive stage of cancers and confers the tumor heterogeneity (Fig. 2). Tumors in this stage are composed of somatic tumor cells and ES/PGC-like tumor cells, the latter of which corresponds to undifferentiated state in pathological morphology. Subsequently, the tumors obtain oogenesis and parthenogenesis-like capabilities, which endow tumors with a powerful ability of survival, fitness, and displaying independence as well as strong heterogeneity. Tumors in this stage are composed of somatic tumor cells, ES-like tumor cells and a serial germ cell-like cells at the different developmental stages, blastomere-like cells at the different developmental stages with difference in the tumor size and shape, as well as the occurrence of PGCCs (Fig. 2). This represents the advanced stage of tumors, in which tumors can survive under nearly all distinct genotoxic agents and contribute to the recurrence (Fig. 2).
Table 1.
Experimental evidence for the gametogenesis-related hypothesis.
| Core evidence | Details | Reference |
|---|---|---|
|
| ||
| Tumorigenicity of embryonic/germ cells | Preimplantation embryo, EC cells, ES cells, early PGCs, EGCs, iPS cells, Parthenogenetic oocytes | 19, 24–58, 85, 86 |
| Extensive expression of embryonic antigens, CT antigens and germ cell antigens in tumors | About 40 CT antigens, Embryonic/germ cell proteins: Oct4, Sox2, Nanog, SCYP1, SCYP3, DMC1, SSEA1, IFITM3, Stellar, CD117 | 10, 11, 67–74 |
| Isolation of cancer stem cells with embryonic/germ cell markers | SSEA1, CD117 | 72, 74 |
| Crucial role of embryonic/germ cell- related genes in tumor malignant traits | Oct4, Sox2, Nanog, DDX4, PRDM14, IFITM3, DAZL | 70–75, 78–84, 100 |
| Role of tumor suppressors/oncogenes in reprogramming and germ cell development. | P53, Rb, PTEN, C-MYc | 1, 66, 85–90, 158–170 |
| Existence of germ cell-like tumor cells in somatic tumor cells | From PGC-like cells to oocyte-like cells. | 94–100 |
| Existence of blastomere-like tumor cells in somatic tumor cells | preimplantation embryo-like cells at the different developmental stage | 95–100, 119 |
PGC-like cells have the capacity to undergo both mitosis and meiosis [1]. Some of PGC-like tumor cells enter meiosis for oocyte maturation and parthenogenesis, endowing tumors with the ability to survive in multiple genotoxic therapy treatments, particularly in the absence of p53 [98]. This is similar to the damage resistance of natural oocyte under chk2 or p53/p63 deficiency [169, 170]. The evolution might be caused by genetic and epigenetic changes associated with the changes in microenvironments and stressors [198–204]. It is possible that activation of somatic pregnancy cycle is induced upon the mutations of certain oncogenes (e.g. c-Myc) and/or tumor suppressors (e.g. p53) and serves as a key evolutional mechanism to drive cancer malignancy and drug resistance.
In the cancer field, one of the most conflicting things is that the two divided worlds one in which the molecular biologists who decode cancer pathways and the other where the pathologists who observe and describe cancer phenotypes[117]. Our evolutional model of tumors provides an explanation for the change of genes and phenotypes, thus linking the two divided worlds together.
Conclusion
In essence, our tumor’s gametogenesis-related model explains four key events of the tumor progression, at least in certain somatic cancer, likely due to the genetic and epigenetic changes (Fig. 2): (1) Somatic cells are transformed and display the traits of growth out of control, representing the benign stage; (2) Activation of somatic cell-ES/PGC-like cell conversion (including somatic cell-ES cell-like cell conversion and somatic cell-PGC-like cell conversion), which can endow tumor with the capabilities of tumor initiation and metastasis, representing the entrance of malignant stage; (3) Activation of PGC-like cells undergo further development along with germ cell maturation to give rise to oocyte-like cells; (4) Parthenogenetic activation of oocyte-like cells generates to blastomere-like cells which can endow tumor with powerful abilities, such as asexual life cycle, pluripotency, immortality and therapeutic resistance, representing the terminal stages. The oocyte-like and preimplantation-like state can allow for tumors to resist to therapy. The soma-derived ES/PGC-like cancer cells could be commonly generated, resembling their original tissues instead of germ cell tumors, likely because most ES/PGC-like cancer cells still maintain similar genetic imprinting of their original cells.
There are two compelling hypotheses proposed by Vinnitsky [13, 16] and Liu [7, 117], respectively, for cancer evolution. Dr. Vinnitsky proposed that the life cycle of somatic cells → CSC (pseudo-germline cells) → pseudo-blastula-stage embryo (endow tumors with tumor initiation and metastasis) existed in somatic tumors [13, 16]. He believes that a tumor may be resulted from the aggregation of oncogerminative cells, which, imitate the behavior of cells of the morula [13, 16]. Compared with the Oncogerminative hypothesis of tumor from Vinnitsky [13, 16], our gametogenesis-related hypothesis of tumors revealed that 1) the oncogerminative cells (germ cell-like cells) are composed of germ cell-like cells at the developmental stage such as early PGC-like cells, migratory PGC-like cells, and oocyte-like cells; 2) the embryo-like structures arise from the parthenogenesis of oocyte-like cells; 3) there are two ways for generating tumor cells with metastasis potential; PGC-like cells with metastasis ability from somatic cells or embryo-like cells, namely, preimplantation embryo-like state that is not necessary for the metastatic ability in some tumor types. Dr. Liu proposed that undifferentiated tumors arise from mature somatic cells through “somatic embryogenesis” involving the giant cell life cycle or the giant cell cycle, in which somatic tumors is reprogrammed via mononucleated or multinucleated polyploid giant cancer cells (PGCCs)[117]. That is, PGCCs mimicking the blastomere-stage embryo are taken as cancer stem like cells [117]. Distinct from our hypothesis, Liu’s “Life code theory” of cancer highlights that the fertilized embryo-like state arises directly from somatic cells under “endoreplication” instead of via the process of generating somatic derived germ cell-like cells in turn leading to the fertilized embryo-like state, which is the starting point of malignant behaviors of tumors, such as metastasis property [7, 117]. However, our hypothesis proposed that the starting point of malignant behaviors of tumors are both the obtaining of ES/PGC-like state direct from somatic cells-ES/PGC-like transformation and somatic cells → ES/PGC-like cells → oocyte-like cell → parthenogenic embryo-like structure → ES/PGC-like cells.
Our tumor’s gametogenesis-related hypothesis of somatic pregnancy points out that tumors tend to establish an independent somatic embryonic reproduction through the activation of somatic female gametogenesis and parthenogenesis in somatic tumor cells during the tumor progression. As a hidden “code” of cancer, activation of somatic embryonic reproduction reflects that the acquisition of distinct embryonic/germ cell-like developmental state contributing to the different malignant traits of tumors, representing distinct grades of tumors. The goal for acquiring the primary tumor-initiating cells seems to produce “seed” for establishing independent somatic asexual embryonic reproduction leading to cancer initiation and progression. In other words, activation of gametogenesis, which can be observed in certain somatic cancers, is a driving force of tumor malignant behaviors, although more evidence is clearly needed to support this theory for diverse somatic cancers. This concept may be instrumental to better understand the nature and evolution of tumors. Although our recent study revealing that the PGC-like cells from 4T1 cells could give rise to malignant tumors with strong metastatic potential [101] offers the strong support of our hypothesis, we recognize that further additional in vivo experiments demonstrating that isolated PGC-like cells from somatic tumors could differentiate into sperm (male) or egg (female) as well as give rise to mouse off-spring will be warranted to further validate our model.
We rationalize that targeting the key events of somatic pregnancy including somatic-ES/PGC conversion, PGC-like further development and parthenogenetic activation is likely a better therapeutic strategy for cancer treatment than directly targeting cell mitotic proliferation, especially for those tumors with p53 inactivation. In support of this notion, our recent study demonstrated that genetical silencing of PGC-specific genes or pharmacological inactivation of BMP pathways leading to deletion of PGC-like tumor cells markedly impairs cancer metastasis [101].
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of China (No. 81372351) and China Scholarship Council (201406105051) to C.L, Start-ups and Anderson Endowed Professorship fund from Wake Forest School of Medicine, and NIH grants (R01CA182424 and R01CA193813) to H.K.L.
Footnotes
Explanation of the author change
Zhan Ma has contributed to inserting some important information in the revised manuscript during the revision process. This is the reason why he is added in this revised manuscript.
Author declaration: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harbor perspectives in biology. 2012;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nikolic A, Volarevic V, Armstrong L, Lako M, Stojkovic M. Primordial Germ Cells: Current Knowledge and Perspectives. Stem cells international. 2016;2016:1741072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Strome S, Updike D. Specifying and protecting germ cell fate. Nature reviews Molecular cell biology. 2015;16:406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nicholas CR, Chavez SL, Baker VL, Reijo Pera RA. Instructing an embryonic stem cell-derived oocyte fate: lessons from endogenous oogenesis. Endocrine reviews. 2009;30:264–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brewer BG, Mitchell RA, Harandi A, Eaton JW. Embryonic vaccines against cancer: an early history. Experimental and molecular pathology. 2009;86:192–7. [DOI] [PubMed] [Google Scholar]
- [6].Bignold LP, Coghlan BL, Jersmann HP. Hansemann, Boveri, chromosomes and the gametogenesis-related theories of tumours. Cell biology international. 2006;30:640–4. [DOI] [PubMed] [Google Scholar]
- [7].Liu J. The dualistic origin of human tumors. Seminars in cancer biology. 2018;53:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sell S. On the stem cell origin of cancer. The American journal of pathology. 2010;176:2584–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].R V. Die Cellularpathologie in ihrer Begrundung auf physiologische und pathologische Gewebelehre. Berlin: August Hirschwald. 1958. [PubMed] [Google Scholar]
- [10].Old LJ. Cancer/testis (CT) antigens - a new link between gametogenesis and cancer. Cancer immunity. 2001;1:1. [PubMed] [Google Scholar]
- [11].Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nature reviews Cancer. 2005;5:615–25. [DOI] [PubMed] [Google Scholar]
- [12].J B. Embryological aspects and etiology of carcinoma. Lancet. 1902;1:1758–61. [Google Scholar]
- [13].Vinnitsky VB. Oncogerminative hypothesis of tumor formation. Medical hypotheses. 1993;40:19–27. [DOI] [PubMed] [Google Scholar]
- [14].Mintz B, Cronmiller C, Custer RP. Somatic cell origin of teratocarcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:2834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kalejs M, Erenpreisa J. Cancer/testis antigens and gametogenesis: a review and “brain-storming” session. Cancer cell international. 2005;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vinnitsky V. The development of a malignant tumor is due to a desperate asexual self-cloning process in which cancer stem cells develop the ability to mimic the genetic program of germline cells. Intrinsically disordered proteins. 2014;2:e29997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].J C. Vorlesungen uber ellgemine Pathologie. Hirschwald, Berlin. 1877. [Google Scholar]
- [18].Stevens LC. The biology of teratomas. Advances in morphogenesis. 1967;6:1–31. [DOI] [PubMed] [Google Scholar]
- [19].Pierce GB Jr., Dixon FJ Jr., Verney EL. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Laboratory investigation; a journal of technical methods and pathology. 1960;9:583–602. [PubMed] [Google Scholar]
- [20].Kleinsmith LJ, Pierce GB Jr., Multipotentiality Of Single Embryonal Carcinoma Cells. Cancer research. 1964;24:1544–51. [PubMed] [Google Scholar]
- [21].Pierce GB. Teratocarcinoma: model for a developmental concept of cancer. Current topics in developmental biology. 1967;2:223–46. [DOI] [PubMed] [Google Scholar]
- [22].Kahan BW, Ephrussi B. Developmental potentialities of clonal in vitro cultures of mouse testicular teratoma. Journal of the National Cancer Institute. 1970;44:1015–36. [PubMed] [Google Scholar]
- [23].Hogan B, Fellous M, Avner P, Jacob F. Isolation of a human teratoma cell line which expresses F9 antigen. Nature. 1977;270:515–8. [DOI] [PubMed] [Google Scholar]
- [24].Fawcett DW, Wislocki GB, Waldo CM. The development of mouse ova in the anterior chamber of the eye and in the abdominal cavity. The American journal of anatomy. 1947;81:413–43. [DOI] [PubMed] [Google Scholar]
- [25].Fawcett DW. The development of mouse ova under the capsule of the kidney. The Anatomical record. 1950;108:71–91. [DOI] [PubMed] [Google Scholar]
- [26].Kirby DR. Reciprocal transplantation of blastocysts between rats and mice. Nature. 1962;194:785–6. [DOI] [PubMed] [Google Scholar]
- [27].Kirby DR. The development of mouse blastocysts transplanted to the scrotal and cryptorchid testis. Journal of anatomy. 1963;97:119–30. [PMC free article] [PubMed] [Google Scholar]
- [28].Stevens LC. The development of teratomas from intratesticular grafts of tubal mouse eggs. Journal of embryology and experimental morphology. 1968;20:329–41. [PubMed] [Google Scholar]
- [29].Stevens LC. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Developmental biology. 1970;21:364–82. [DOI] [PubMed] [Google Scholar]
- [30].Runner MN. Development of mouse eggs in the anterior chamber of the eye. The Anatomical record. 1947;98:1–17. [DOI] [PubMed] [Google Scholar]
- [31].Kirby DR. Development of mouse eggs beneath the kidney capsule. Nature. 1960;187:707–8. [DOI] [PubMed] [Google Scholar]
- [32].Stevens LC. Experimental Production Of Testicular Teratomas In Mice. Proceedings of the National Academy of Sciences of the United States of America. 1964;52:654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. [DOI] [PubMed] [Google Scholar]
- [34].Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:7634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. [DOI] [PubMed] [Google Scholar]
- [36].Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biology of reproduction. 1996;55:254–9. [DOI] [PubMed] [Google Scholar]
- [37].Yu J, Thomson JA. Pluripotent stem cell lines. Genes & development. 2008;22:1987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim K, Lerou P, Yabuuchi A, Lengerke C, Ng K, West J, et al. Histocompatible embryonic stem cells by parthenogenesis. Science. 2007;315:482–6. [DOI] [PubMed] [Google Scholar]
- [39].Stevens LC, Little CC. Spontaneous Testicular Teratomas in an Inbred Strain of Mice. Proceedings of the National Academy of Sciences of the United States of America. 1954;40:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stevens LC. Experimental production of testicular teratomas in mice of strains 129, A/He, and their F1 hybrids. Journal of the National Cancer Institute. 1970;44:923–9. [PubMed] [Google Scholar]
- [41].Stevens LC. Experimental production of testicular teratomas in the mouse. International journal of andrology. 1981;4 Suppl s4:54–9. [DOI] [PubMed] [Google Scholar]
- [42].Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, et al. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–700. [DOI] [PubMed] [Google Scholar]
- [43].Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr., Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. [DOI] [PubMed] [Google Scholar]
- [44].Stevens LC. The biology of teratomas including evidence indicating their origin form primordial germ cells. L’ Annee biologique. 1962;1:585–610. [PubMed] [Google Scholar]
- [45].Stevens LC. Origin of testicular teratomas from primordial germ cells in mice. Journal of the National Cancer Institute. 1967;38:549–52. [PubMed] [Google Scholar]
- [46].Stevens LC. Spontaneous and experimentally induced testicular teratomas in mice. Cell differentiation. 1984;15:69–74. [DOI] [PubMed] [Google Scholar]
- [47].Oosterhuis JW, Looijenga LHJ. Human germ cell tumours from a developmental perspective. Nature reviews Cancer. 2019;19:522–37. [DOI] [PubMed] [Google Scholar]
- [48].Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Human reproduction update. 2006;12:303–23. [DOI] [PubMed] [Google Scholar]
- [49].Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–7. [DOI] [PubMed] [Google Scholar]
- [50].Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120:3197–204. [DOI] [PubMed] [Google Scholar]
- [51].Kimura T, Tomooka M, Yamano N, Murayama K, Matoba S, Umehara H, et al. AKT signaling promotes derivation of embryonic germ cells from primordial germ cells. Development. 2008;135:869–79. [DOI] [PubMed] [Google Scholar]
- [52].Stevens LC, Varnum DS. The development of teratomas from parthenogenetically activated ovarian mouse eggs. Developmental biology. 1974;37:369–80. [DOI] [PubMed] [Google Scholar]
- [53].Stevens LC. Teratocarcinogenesis and spontaneous parthenogenesis in mice. Results and problems in cell differentiation. 1980;11:265–74. [DOI] [PubMed] [Google Scholar]
- [54].Stevens LC. Animal model of human disease: benign cystic and malignant ovarian teratoma. The American journal of pathology. 1976;85:809–13. [PMC free article] [PubMed] [Google Scholar]
- [55].Eppig JJ, Kozak LP, Eicher EM, Stevens LC. Ovarian teratomas in mice are derived from oocytes that have completed the first meiotic division. Nature. 1977;269:517–8. [DOI] [PubMed] [Google Scholar]
- [56].Eppig JJ, Wigglesworth K, Hirao Y. Metaphase I arrest and spontaneous parthenogenetic activation of strain LTXBO oocytes: chimeric reaggregated ovaries establish primary lesion in oocytes. Developmental biology. 2000;224:60–8. [DOI] [PubMed] [Google Scholar]
- [57].Eppig JJ, Wigglesworth K, Varnum DS, Nadeau JH. Genetic regulation of traits essential for spontaneous ovarian teratocarcinogenesis in strain LT/Sv mice: aberrant meiotic cell cycle, oocyte activation, and parthenogenetic development. Cancer research. 1996;56:5047–54. [PubMed] [Google Scholar]
- [58].Linder D, McCaw BK, Hecht F. Parthenogenic origin of benign ovarian teratomas. The New England journal of medicine. 1975;292:63–6. [DOI] [PubMed] [Google Scholar]
- [59].Mann JR, Lovell-Badge RH. Inviability of parthenogenones is determined by pronuclei, not egg cytoplasm. Nature. 1984;310:66–7. [DOI] [PubMed] [Google Scholar]
- [60].Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, et al. Birth of parthenogenetic mice that can develop to adulthood. Nature. 2004;428:860–4. [DOI] [PubMed] [Google Scholar]
- [61].Mai Q, Yu Y, Li T, Wang L, Chen MJ, Huang SZ, et al. Derivation of human embryonic stem cell lines from parthenogenetic blastocysts. Cell research. 2007;17:1008–19. [DOI] [PubMed] [Google Scholar]
- [62].Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nature reviews Cancer. 2005;5:210–22. [DOI] [PubMed] [Google Scholar]
- [63].Talerman A. Germ cell tumours. Annales de pathologie. 1985;5:145–57. [PubMed] [Google Scholar]
- [64].Gobel U, Schneider DT, Calaminus G, Haas RJ, Schmidt P, Harms D. Germ-cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups. Annals of oncology : official journal of the European Society for Medical Oncology. 2000;11:263–71. [DOI] [PubMed] [Google Scholar]
- [65].Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nature reviews Molecular cell biology. 2010;11:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yamada Y, Davis KD, Coffman CR. Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the Outsiders monocarboxylate transporter. Development. 2008;135:207–16. [DOI] [PubMed] [Google Scholar]
- [67].Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nature genetics. 2008;40:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255–65. [DOI] [PubMed] [Google Scholar]
- [70].Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nature genetics. 2012;44:1111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, et al. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59:763–75. [DOI] [PubMed] [Google Scholar]
- [72].Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell stem cell. 2009;4:440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu X, Chen L, Fan Y, Hong Y, Yang X, Li Y, et al. IFITM3 promotes bone metastasis of prostate cancer cells by mediating activation of the TGF-beta signaling pathway. Cell death & disease. 2019;10:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, et al. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer research. 2010;70:4602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Old LJ. Cancer is a somatic cell pregnancy. Cancer immunity. 2007;7:19. [PMC free article] [PubMed] [Google Scholar]
- [76].Janic A, Mendizabal L, Llamazares S, Rossell D, Gonzalez C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science. 2010;330:1824–7. [DOI] [PubMed] [Google Scholar]
- [77].Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–3. [DOI] [PubMed] [Google Scholar]
- [78].Curran SP, Wu X, Riedel CG, Ruvkun G. A soma-to-germline transformation in long-lived Caenorhabditis elegans mutants. Nature. 2009;459:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wang D, Kennedy S, Conte D Jr., Kim JK, Gabel HW, Kamath RS, et al. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature. 2005;436:593–7. [DOI] [PubMed] [Google Scholar]
- [80].Moriya C, Taniguchi H, Miyata K, Nishiyama N, Kataoka K, Imai K. Inhibition of PRDM14 expression in pancreatic cancer suppresses cancer stem-like properties and liver metastasis in mice. Carcinogenesis. 2017;38:638–48. [DOI] [PubMed] [Google Scholar]
- [81].Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhang F, Liu R, Zhang H, Liu C, Liu C, Lu Y. Suppressing Dazl modulates tumorigenicity and stemness in human glioblastoma cells. BMC cancer. 2020;20:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, et al. NANOG Metabolically Reprograms Tumor-Initiating Stem-like Cells through Tumorigenic Changes in Oxidative Phosphorylation and Fatty Acid Metabolism. Cell metabolism. 2016;23:206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–50. [DOI] [PubMed] [Google Scholar]
- [85].Kaufman CK, Mosimann C, Fan ZP, Yang S, Thomas AJ, Ablain J, et al. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science. 2016;351:aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. [DOI] [PubMed] [Google Scholar]
- [87].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- [88].Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kareta MS, Gorges LL, Hafeez S, Benayoun BA, Marro S, Zmoos AF, et al. Inhibition of pluripotency networks by the Rb tumor suppressor restricts reprogramming and tumorigenesis. Cell stem cell. 2015;16:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhao Y, Yin X, Qin H, Zhu F, Liu H, Yang W, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell stem cell. 2008;3:475–9. [DOI] [PubMed] [Google Scholar]
- [92].Roe JS, Hwang CI, Somerville TDD, Milazzo JP, Lee EJ, Da Silva B, et al. Enhancer Reprogramming Promotes Pancreatic Cancer Metastasis. Cell. 2017;170:875–88 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Abad M, Mosteiro L, Pantoja C, Canamero M, Rayon T, Ors I, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502:340–5. [DOI] [PubMed] [Google Scholar]
- [94].Liu C, Chen Z, Chen Z, Zhang T, Lu Y. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia. 2006;8:716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Liu C, Xu S, Ma Z, Zeng Y, Chen Z, Lu Y. Generation of pluripotent cancer-initiating cells from transformed bone marrow-derived cells. Cancer letters. 2011;303:140–9. [DOI] [PubMed] [Google Scholar]
- [96].Ma Z, Hu Y, Jiang G, Hou J, Liu R, Lu Y, et al. Spontaneous generation of germline characteristics in mouse fibrosarcoma cells. Scientific reports. 2012;2:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ma Z, Liu R, Wang X, Huang M, Gao Q, Lu Y, et al. Spontaneous germline potential of human hepatic cell line in vitro. Molecular human reproduction. 2013;19:216–26. [DOI] [PubMed] [Google Scholar]
- [98].Liu C, Cai Z, Jin G, Peng D, Pan BS, Zhang X, et al. Abnormal gametogenesis induced by p53 deficiency promotes tumor progression and drug resistance. Cell discovery. 2018;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liu C, Ma Z, Hou J, Zhang H, Liu R, Wu W, et al. Germline traits of human hepatoblastoma cells associated with growth and metastasis. Biochemical and biophysical research communications. 2013;437:120–6. [DOI] [PubMed] [Google Scholar]
- [100].Liu C, Ma Z, Xu S, Hou J, Hu Y, Yu Y, et al. Activation of the germ-cell potential of human bone marrow-derived cells by a chemical carcinogen. Scientific reports. 2014;4:5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu C, Ma Z, Cai Z, Zhang F, Liu C, Chen T, et al. Identification of primordial germ cell-like cells as liver metastasis initiating cells in mouse tumour models. Cell discovery. 2020;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Niu N, Mercado-Uribe I, Liu J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene. 2017;36:4887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pennarossa G, Paffoni A, Ragni G, Gandolfi F, Brevini T. Parthenogenesis in mammals: pros and cons in pluripotent cell derivation. Open Life Sciences. 2011;6:770–5. [Google Scholar]
- [104].Tilmann C, Capel B. Cellular and molecular pathways regulating mammalian sex determination. Recent progress in hormone research. 2002;57:1–18. [DOI] [PubMed] [Google Scholar]
- [105].Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–4. [DOI] [PubMed] [Google Scholar]
- [106].Gonen N, Futtner CR, Wood S, Garcia-Moreno SA, Salamone IM, Samson SC, et al. Sex reversal following deletion of a single distal enhancer of Sox9. Science. 2018;360:1469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Harris A, Siggers P, Corrochano S, Warr N, Sagar D, Grimes DT, et al. ZNRF3 functions in mammalian sex determination by inhibiting canonical WNT signaling. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:5474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yasuda T, Oda S, L Z, Kimori Y, Kamei Y, Ishikawa T, et al. Gamma-ray irradiation promotes premature meiosis of spontaneously differentiating testis-ova in the testis of p53-deficient medaka (Oryzias latipes). Cell death & disease. 2012;3:e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Dyce PW, Shen W, Huynh E, Shao H, Villagomez DA, Kidder GM, et al. Analysis of oocyte-like cells differentiated from porcine fetal skin-derived stem cells. Stem cells and development. 2011;20:809–19. [DOI] [PubMed] [Google Scholar]
- [110].Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–14. [DOI] [PubMed] [Google Scholar]
- [112].Dyce PW, Wen L, Li J. In vitro germline potential of stem cells derived from fetal porcine skin. Nature cell biology. 2006;8:384–90. [DOI] [PubMed] [Google Scholar]
- [113].Linher K, Dyce P, Li J. Primordial germ cell-like cells differentiated in vitro from skin-derived stem cells. PloS one. 2009;4:e8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:12516. [DOI] [PubMed] [Google Scholar]
- [115].Liu SV. iPS cells: a more critical review. Stem cells and development. 2008;17:391–7. [DOI] [PubMed] [Google Scholar]
- [116].Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer metastasis reviews. 2010;29:709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].The Liu J. “life code”: A theory that unifies the human life cycle and the origin of human tumors. Seminars in cancer biology. 2020;60:380–97. [DOI] [PubMed] [Google Scholar]
- [118].Coward J, Harding A. Size Does Matter: Why Polyploid Tumor Cells are Critical Drug Targets in the War on Cancer. Frontiers in oncology. 2014;4:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chen J, Niu N, Zhang J, Qi L, Shen W, Donkena KV, et al. Polyploid Giant Cancer Cells (PGCCs): The Evil Roots of Cancer. Current cancer drug targets. 2019;19:360–7. [DOI] [PubMed] [Google Scholar]
- [120].Erenpreisa J, Salmina K, Huna A, Jackson TR, Vazquez-Martin A, Cragg MS. The “virgin birth”, polyploidy, and the origin of cancer. Oncoscience. 2015;2:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Salmina K, Huna A, Kalejs M, Pjanova D, Scherthan H, Cragg MS, et al. The Cancer Aneuploidy Paradox: In the Light of Evolution. Genes. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ganem NJ, Pellman D. Limiting the proliferation of polyploid cells. Cell. 2007;131:437–40. [DOI] [PubMed] [Google Scholar]
- [123].Kalejs M, Ivanov A, Plakhins G, Cragg MS, Emzinsh D, Illidge TM, et al. Upregulation of meiosis-specific genes in lymphoma cell lines following genotoxic insult and induction of mitotic catastrophe. BMC cancer. 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ianzini F, Kosmacek EA, Nelson ES, Napoli E, Erenpreisa J, Kalejs M, et al. Activation of meiosis-specific genes is associated with depolyploidization of human tumor cells following radiation-induced mitotic catastrophe. Cancer research. 2009;69:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wang Q, Wu PC, Dong DZ, Ivanova I, Chu E, Zeliadt S, et al. Polyploidy road to therapy-induced cellular senescence and escape. International journal of cancer. 2013;132:1505–15. [DOI] [PubMed] [Google Scholar]
- [126].Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Erenpreisa JA, Cragg MS, Fringes B, Sharakhov I, Illidge TM. Release of mitotic descendants by giant cells from irradiated Burkitt’s lymphoma cell line. Cell biology international. 2000;24:635–48. [DOI] [PubMed] [Google Scholar]
- [128].Puig PE, Guilly MN, Bouchot A, Droin N, Cathelin D, Bouyer F, et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell biology international. 2008;32:1031–43. [DOI] [PubMed] [Google Scholar]
- [129].Diaz-Carballo D, Saka S, Klein J, Rennkamp T, Acikelli AH, Malak S, et al. A Distinct Oncogenerative Multinucleated Cancer Cell Serves as a Source of Stemness and Tumor Heterogeneity. Cancer research. 2018;78:2318–31. [DOI] [PubMed] [Google Scholar]
- [130].Weihua Z, Lin Q, Ramoth AJ, Fan D, Fidler IJ. Formation of solid tumors by a single multinucleated cancer cell. Cancer. 2011;117:4092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Salmina K, Jankevics E, Huna A, Perminov D, Radovica I, Klymenko T, et al. Up-regulation of the embryonic self-renewal network through reversible polyploidy in irradiated p53-mutant tumour cells. Experimental cell research. 2010;316:2099–112. [DOI] [PubMed] [Google Scholar]
- [132].Erenpreisa J, Kalejs M, Ianzini F, Kosmacek EA, Mackey MA, Emzinsh D, et al. Segregation of genomes in polyploid tumour cells following mitotic catastrophe. Cell biology international. 2005;29:1005–11. [DOI] [PubMed] [Google Scholar]
- [133].Erenpreisa J, Salmina K, Huna A, Kosmacek EA, Cragg MS, Ianzini F, et al. Polyploid tumour cells elicit paradiploid progeny through depolyploidizing divisions and regulated autophagic degradation. Cell biology international. 2011;35:687–95. [DOI] [PubMed] [Google Scholar]
- [134].Andreassen PR, Lohez OD, Lacroix FB, Margolis RL. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Molecular biology of the cell. 2001;12:1315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–7. [DOI] [PubMed] [Google Scholar]
- [136].Margolis RL, Lohez OD, Andreassen PR. G1 tetraploidy checkpoint and the suppression of tumorigenesis. Journal of cellular biochemistry. 2003;88:673–83. [DOI] [PubMed] [Google Scholar]
- [137].Vogel C, Kienitz A, Hofmann I, Muller R, Bastians H. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene. 2004;23:6845–53. [DOI] [PubMed] [Google Scholar]
- [138].Fei F, Zhang D, Yang Z, Wang S, Wang X, Wu Z, et al. The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer. Journal of experimental & clinical cancer research : CR. 2015;34:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Qu Y, Zhang L, Rong Z, He T, Zhang S. Number of glioma polyploid giant cancer cells (PGCCs) associated with vasculogenic mimicry formation and tumor grade in human glioma. Journal of experimental & clinical cancer research : CR. 2013;32:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Illidge TM, Cragg MS, Fringes B, Olive P, Erenpreisa JA. Polyploid giant cells provide a survival mechanism for p53 mutant cells after DNA damage. Cell biology international. 2000;24:621–33. [DOI] [PubMed] [Google Scholar]
- [141].Erenpreisa J, Cragg MS, Salmina K, Hausmann M, Scherthan H. The role of meiotic cohesin REC8 in chromosome segregation in gamma irradiation-induced endopolyploid tumour cells. Experimental cell research. 2009;315:2593–603. [DOI] [PubMed] [Google Scholar]
- [142].Erenpreisa J, Salmina K, Anatskaya O, Cragg MS. Paradoxes of cancer: Survival at the brink. Seminars in cancer biology. 2020. [DOI] [PubMed] [Google Scholar]
- [143].Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27:5894–903. [DOI] [PubMed] [Google Scholar]
- [145].Sharma A, Seow JJW, Dutertre CA, Pai R, Bleriot C, Mishra A, et al. Onco-fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell. 2020. [DOI] [PubMed] [Google Scholar]
- [146].Kaku H, Usui H, Qu J, Shozu M. Mature cystic teratomas arise from meiotic oocytes, but not from pre-meiotic oogonia. Genes, chromosomes & cancer. 2016;55:355–64. [DOI] [PubMed] [Google Scholar]
- [147].Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nature reviews Cancer. 2009;9:749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Muller PA, Vousden KH. p53 mutations in cancer. Nature cell biology. 2013;15:2–8. [DOI] [PubMed] [Google Scholar]
- [149].Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170:1062–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nichols KE, Malkin D, Garber JE, Fraumeni JF Jr., Li FP. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:83–7. [PubMed] [Google Scholar]
- [151].Srivastava S, Zou ZQ, Pirollo K, Blattner W, Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990;348:747–9. [DOI] [PubMed] [Google Scholar]
- [152].Malkin D, Li FP, Strong LC, Fraumeni JF Jr., Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–8. [DOI] [PubMed] [Google Scholar]
- [153].Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nature reviews Cancer. 2014;14:359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Lu X, Magrane G, Yin C, Louis DN, Gray J, Van Dyke T. Selective inactivation of p53 facilitates mouse epithelial tumor progression without chromosomal instability. Molecular and cellular biology. 2001;21:6017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8:8921–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, et al. Integrative clinical genomics of metastatic cancer. Nature. 2017;548:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Valente LJ, Gray DH, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL, et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell reports. 2013;3:1339–45. [DOI] [PubMed] [Google Scholar]
- [159].Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Moon SH, Huang CH, Houlihan SL, Regunath K, Freed-Pastor WA, Morris JPt, et al. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell. 2019;176:564–80 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G. The p53 family: guardians of maternal reproduction. Nature reviews Molecular cell biology. 2011;12:259–65. [DOI] [PubMed] [Google Scholar]
- [162].Paskulin D, Paixao-Cortes VR, Hainaut P, Bortolini MC, Ashton-Prolla P. The TP53 fertility network. Genetics and molecular biology. 2012;35:939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Hu W. The role of p53 gene family in reproduction. Cold Spring Harbor perspectives in biology. 2009;1:a001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–4. [DOI] [PubMed] [Google Scholar]
- [165].Lu WJ, Chapo J, Roig I, Abrams JM. Meiotic recombination provokes functional activation of the p53 regulatory network. Science. 2010;328:1278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Schwartz D, Goldfinger N, Rotter V. Expression of p53 protein in spermatogenesis is confined to the tetraploid pachytene primary spermatocytes. Oncogene. 1993;8:1487–94. [PubMed] [Google Scholar]
- [167].Linke SP, Sengupta S, Khabie N, Jeffries BA, Buchhop S, Miska S, et al. p53 interacts with hRAD51 and hRAD54, and directly modulates homologous recombination. Cancer research. 2003;63:2596–605. [PubMed] [Google Scholar]
- [168].Habu T, Wakabayashi N, Yoshida K, Yomogida K, Nishimune Y, Morita T. p53 Protein interacts specifically with the meiosis-specific mammalian RecA-like protein DMC1 in meiosis. Carcinogenesis. 2004;25:889–93. [DOI] [PubMed] [Google Scholar]
- [169].Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343:533–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Rinaldi VD, Bloom JC, Schimenti JC. Oocyte Elimination Through DNA Damage Signaling from CHK1/CHK2 to p53 and p63. Genetics. 2020;215:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–8. [DOI] [PubMed] [Google Scholar]
- [172].Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction. 2008;135:3–12. [DOI] [PubMed] [Google Scholar]
- [173].Murray D, Mirzayans R. Cellular Responses to Platinum-Based Anticancer Drugs and UVC: Role of p53 and Implications for Cancer Therapy. International journal of molecular sciences. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Guo X, Mills AA. p63, cellular senescence and tumor development. Cell cycle. 2007;6:305–11. [DOI] [PubMed] [Google Scholar]
- [175].Finlan LE, Hupp TR. p63: the phantom of the tumor suppressor. Cell cycle. 2007;6:1062–71. [DOI] [PubMed] [Google Scholar]
- [176].Brenner S. Turing centenary: Life’s code script. Nature. 2012;482:461. [DOI] [PubMed] [Google Scholar]
- [177].Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nature reviews Cancer. 2012;12:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Davies P. Exposing cancer’s deep evolutionary roots. Physics World 2013;26:37–40. [Google Scholar]
- [179].Trigos AS, Pearson RB, Papenfuss AT, Goode DL. Altered interactions between unicellular and multicellular genes drive hallmarks of transformation in a diverse range of solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:6406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Wang Y, Ma H. Development: a pathway to plant female germ cells. Current biology : CB. 2011;21:R476–8. [DOI] [PubMed] [Google Scholar]
- [181].Extavour C. Oogenesis: making the mos of meiosis. Current biology : CB. 2009;19:R489–91. [DOI] [PubMed] [Google Scholar]
- [182].Ramachandran R, McDaniel CD. Parthenogenesis in birds: a review. Reproduction. 2018;155:R245–R57. [DOI] [PubMed] [Google Scholar]
- [183].Brunes TO, da Silva AJ, Marques-Souza S, Rodrigue MT, Pellegrino KCM. Not always young: The first vertebrate ancient origin of true parthenogenesis found in an Amazon leaf litter lizard with evidence of mitochondrial haplotypes surfing on the wave of a range expansion. Molecular phylogenetics and evolution. 2019;135:105–22. [DOI] [PubMed] [Google Scholar]
- [184].Neiman M, Sharbel TF, Schwander T. Genetic causes of transitions from sexual reproduction to asexuality in plants and animals. Journal of evolutionary biology. 2014;27:1346–59. [DOI] [PubMed] [Google Scholar]
- [185].Mirzaghaderi G, Horandl E. The evolution of meiotic sex and its alternatives. Proceedings Biological sciences. 2016;283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Vesteg M, Krajcovic J. On the origin of meiosis and sex. Rivista di biologia. 2007;100:147–61. [PubMed] [Google Scholar]
- [187].Holliday R. Meiosis and sex: potent weapons in the competition between early eukaryotes and prokaryotes. BioEssays : news and reviews in molecular, cellular and developmental biology. 2006;28:1123–5. [DOI] [PubMed] [Google Scholar]
- [188].Li W, Zheng GC. A resurgent phoenix--a hypothesis for the origin of meiosis. IUBMB life. 2002;54:9–12. [DOI] [PubMed] [Google Scholar]
- [189].Lenormand T, Engelstadter J, Johnston SE, Wijnker E, Haag CR. Evolutionary mysteries in meiosis. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2016;371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Loidl J. Conservation and Variability of Meiosis Across the Eukaryotes. Annual review of genetics. 2016;50:293–316. [DOI] [PubMed] [Google Scholar]
- [191].Harada H, Nagai H, Tsuneizumi M, Mikami I, Sugano S, Emi M. Identification of DMC1, a novel gene in the TOC region on 17q25.1 that shows loss of expression in multiple human cancers. Journal of human genetics. 2001;46:90–5. [DOI] [PubMed] [Google Scholar]
- [192].Klein HL. The consequences of Rad51 overexpression for normal and tumor cells. DNA repair. 2008;7:686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Raderschall E, Stout K, Freier S, Suckow V, Schweiger S, Haaf T. Elevated levels of Rad51 recombination protein in tumor cells. Cancer research. 2002;62:219–25. [PubMed] [Google Scholar]
- [194].Seipel K, Yanze N, Schmid V. The germ line and somatic stem cell gene Cniwi in the jellyfish Podocoryne carnea. The International journal of developmental biology.2004;48:1–7. [DOI] [PubMed] [Google Scholar]
- [195].Extavour CG. Evolution of the bilaterian germ line: lineage origin and modulation of specification mechanisms. Integrative and comparative biology. 2007;47:770–85. [DOI] [PubMed] [Google Scholar]
- [196].Domazet-Loso T, Tautz D. Phylostratigraphic tracking of cancer genes suggests a link to the emergence of multicellularity in metazoa. BMC biology. 2010;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ma H, Song T, Wang T, Wang S. Influence of Human p53 on Plant Development. PloS one. 2016;11:e0162840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Lichtenstein AV. On evolutionary origin of cancer. Cancer cell international. 2005;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nature reviews Cancer. 2016;16:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Vincent TL, Gatenby RA. An evolutionary model for initiation, promotion, and progression in carcinogenesis. International journal of oncology. 2008;32:729–37. [PubMed] [Google Scholar]
- [201].Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nature reviews Cancer. 2006;6:924–35. [DOI] [PubMed] [Google Scholar]
- [202].Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Baez-Ortega A, Gori K, Strakova A, Allen JL, Allum KM, Bansse-Issa L, et al. Somatic evolution and global expansion of an ancient transmissible cancer lineage. Science. 2019;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]