Abstract
Introduction
We aimed to characterize the clinical impact of amyloid PET (APET) in a veteran population with cognitive decline by comparing differences in management between those who did and did not have an APET.
Methods
This was a retrospective observational study. Poisson regressions and logistic regression were used for comparisons.
Results
Out of 565 veterans, 197 underwent APET; positivity rate was 36.55%. Having an APET was associated with longer follow‐up, and increased diagnostic variability; it was not associated with number of additional studies, cholinesterase inhibitors prescription, or referrals to research. A positive APET was associated with less diagnostic variability, fewer additional tests, greater cholinesterase inhibitor prescriptions, and more research referrals.
Discussion
In a medically complex, real‐world population, APET yielded lower positivity rates and was not associated with classical clinical utility variables when comparing patients with and without an APET. APET may be used more to “rule out” rather than to confirm Alzheimer's disease.
Highlights
Amyloid PET was associated with longer follow‐up, and higher diagnostic variability.
No association was seen with cholinesterase inhibitors prescription, or referrals to research.
In complex patients, expected amyloid PET positivity rates are lower than previously described.
Amyloid PETs were used to “rule out” AD than to confirm the diagnosis of AD.
Keywords: amyloid PET, Alzheimer's disease, cognitive decline, clinical impact
1. BACKGROUND
The field of cognitive‐behavioral neurology has dramatically changed in the last decades with the introduction of reliable biomarkers to assess Alzheimer's disease (AD) pathology in vivo. Since then, there has been a shift in research from a definition of a disease founded on clinical symptoms to a biologically‐based definition of AD. 1 , 2 , 3 , 4 Given that 10% to 20% of patients clinically diagnosed with AD do not have AD pathology 5 , 6 , 7 and more than 40% of patients clinically diagnosed with non‐AD dementias end up having AD pathology on autopsy, 7 , 8 there is a push from clinicians to use available biomarkers to aid with the accurate diagnosis of AD in clinical practice. Biomarkers could improve clinical confidence, increase early diagnosis, and assist with counseling and medical management of patients. However, translating the use of biomarkers from research to clinical practice has been challenging due to limited access, and just initial studies assessing their performance and clinical utility in real‐world settings. 1 , 2 , 9
Prior studies assessing the use of amyloid PET (APET) scans in clinical settings have reported a significant impact on the number of diagnostic tests ordered, medication management, diagnosis certainty, and patient counseling. 7 , 10 , 11 , 12 , 13 However, most studies have been limited to isolated assessments shortly before and after the scan, and information on its clinical impact at longer follow‐up is generally not available. In addition, only limited comparisons exist between patients with and without an APET as part of their diagnostic work‐up. 11 The final phase of the Imaging Dementia‐Evidence for Amyloid Scanning (IDEAS) Study aimed to address this question by comparing participants with an APET with unscanned matched MEDICARE participants. However, these results have not been published in a peer reviewed journal.
The Veterans Affairs Boston Healthcare System (VA Boston) provides a unique opportunity because APET imaging is available for clinical purposes and has been used for years. In this retrospective study, we aimed to characterize the clinical impact of APET in the follow up of patients with cognitive decline by comparing differences in clinical management with 2 years of follow up between patients who had an APET scan as part of their workup and those in whom an APET was not ordered.
2. METHODS
2.1. Patient population
Veterans Health Administration (VHA) is the largest integrated health care system in the United States, providing care at 1,293 health care facilities, and serving 9 million veterans each year.
The VA Boston Memory disorders clinic is a tertiary outpatient clinic that receives consults from VHA facilities throughout New England. Its primary focus is the diagnosis and treatment of patients with neurodegenerative disorders. APETs have been available at VA Boston for the last 5 years. The current study includes all patients seen for an initial evaluation related to cognitive complaints in the Memory Disorders Clinic from October 2016 to January 2020. The study was approved by the Institutional Review Board, which granted a waiver of informed consent since the data was collected only from medical records and the study was considered to involve no more than minimal risk.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using traditional (e.g., PubMed) sources. Prior studies assessing the use of amyloid PET scans in clinical settings have reported a significant impact, shortly after the scan, on medication management, diagnosis certainty, additional diagnostic studies, and counseling. These relevant citations are appropriately cited.
Interpretation: Our findings document that, in a medically complex, real‐world population, APET yielded lower positivity rates and was not associated with classical clinical utility variables when comparing patients with and without an APET. APET may be used more to “rule out” rather than to confirm Alzheimer's disease.
Future Directions: Impact variables are likely an oversimplification of medical management and patient care. Future studies should focus on a broader definition of clinically meaningful impact including patient‐centered outcomes such as patients’ perspective regarding education, prognosis, and management of cognitive decline after amyloid PET.
2.2. Clinical evaluation and decision to order an APET scan
Three cognitive‐behavioral neurologists are in charge of the initial evaluation and follow‐up of patients with cognitive complaints. Assessment involves clinical history and exam, brain imaging (magnetic resonance imaging [MRI] or computed tomography [CT]), blood work (including TSH, vitamin B12, and vitamin D), and a cognitive battery that includes the Montreal cognitive assessment (MoCA), Mini‐mental Status Exam (MMSE), the verbal learning task from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) neuropsychological battery, 14 F‐A‐S Phonemic Verbal Fluency Test 15 and category fluency (including animals, fruits, and vegetables), Trail Making Test part A and B 16 , and the Boston Naming test short form (BNT). 14 We also include the Geriatric Depression Scale 17 and the Geriatric Anxiety Inventory 18 to measure mood and anxiety components. After this evaluation, the cognitive neurologist orders additional testing in selected cases, including APET, fluorodeoxyglucose PET (FDG‐PET), Dopamine transporter scan (DaTscan), or lumbar puncture, depending on the diagnostic suspicion and indication. In our facility, due to the high cost of APETs, the ordering of these scans is restricted for cognitive neurologists working in the Memory disorders clinic. Appropriate Use Criteria (AUC) for APET proposed by the Amyloid Imaging Taskforce (AIT) are considered as guidelines but is ultimately a decision of the treating clinician to order the test. 19
2.3. (18)F‐florbetapir imaging
Description of the imaging protocol used in our facility can be found in the supplementary material.
2.4. Clinical data collection for the analysis of clinical impact
Clinical details were retrospectively collected using electronic medical records, including demographic characteristics, comorbidities, disease course, cognitive syndrome (i.e., subjective cognitive decline [SCD], mild cognitive impairment [MCI], and dementia), clinical diagnosis based on National Institute of Aging‐Alzheimer's Association (NIA‐AA) diagnostic guidelines and other international consortium guidelines 1 (e.g., AD, dementia with Lewy bodies, vascular dementia, etc.), cognitive testing, structural imaging, and treatment. Information from the first assessment and follow‐up visits for the next 2 years were included. For the APET group, all etiological diagnoses were collected both pre‐ and post‐APET and on each follow‐up visit. For the group without APET, etiological diagnoses were collected after the initial evaluation and for each follow‐up visit.
For the analysis of clinical impact, on each follow‐up visit, we collected data on etiological diagnosis, additional diagnostic studies ordered (i.e., additional neuropsychological testing, fluorodeoxyglucose PET (FDG‐PET), genetic testing, lumbar puncture, or repeat structural imaging), social work consultations, and referrals for research and clinical trials. To document the clinicians’ change in diagnostic impression at follow‐up, we used the variability of clinical diagnosis (i.e., total number of changes in etiological diagnosis from one visit to the next). For later analysis, these variables were transformed to count variables by adding them and adjusting for the total follow‐up time for each patient. Cholinesterase inhibitor prescription was also collected at each visit and converted to a binary variable for the analysis (i.e., initiation within the 1st year of follow‐up).
2.5. Statistical analysis
Data analyses were completed in Stata (Version IC 16.1). Bivariate analyses were performed using t‐test, Mann‐Whitney (for non‐parametric data), and chi‐square tests to evaluate differences between patients who had an APET scan as part of their workup and those in whom an APET was not ordered (significance level of 0.05).
Data from follow‐up visits were compared between patients with and without APET to analyze clinical impact outcomes. Patients who only were seen once in the clinic were excluded from this analysis (n = 55). Poisson regressions and negative binomial regressions (when overdispersion was observed) were used for count variables. Binomial logistic regression was used to assess differences in cholinesterase inhibitors prescription between groups. Multiple linear regression was used to evaluate differences in follow‐up among the two groups (in months). Age, cognitive syndrome (i.e., MCI vs. dementia), MoCA scores, clinical etiological diagnosis were used as covariates to control for confounding. To be able to control for clinicians’ diagnostic certainty after the routine evaluation, we determined the number of patients who met AUC for the whole sample and used it as a covariate (for further details see the supplementary material). We performed a complete case analysis of the clinical outcomes variables and added follow‐up time as a covariate to control for differences in follow‐up among the individuals. To further understand the clinical impact outcomes in the APET group, multiple regressions were performed within the group with an APET, using the test result as a predictor and age, MoCA score, cognitive syndrome, clinical etiological diagnosis after initial evaluation, and follow‐up time as covariates. Propensity score (PS) matching and sensitivity analysis were performed to balance confounders between patients with and without an APET. More details can be found in the supplementary material.
3. RESULTS
3.1. Baseline characteristics
From October 2016 to January 2020, 570 patients were evaluated, a total of 565 patients were included in the analysis as five patients were excluded due to not having clinical information available (Table 1). The mean age was 73.78 ± 8.93, and the mean initial MoCA score was 20.02 ± 4.74. Our population was characterized by a high prevalence of psychiatric comorbidity with mood disorders, substance use disorders, and post‐traumatic stress disorder (PTSD) being the most prevalent diagnoses (38.8%, 35.9%, and 30.3%, respectively). There was also a high prevalence of traumatic brain injury (TBI) which was reported in 45.9% of patients. Most patients had a cognitive syndrome of MCI (n = 308, 56.0%) followed by a diagnosis of dementia (n = 200, 36.4%). The most frequent clinical etiological diagnosis was AD, followed by vascular dementia and Lewy body disease (50.3%, 9.0%, and 6.9%, respectively).
TABLE 1.
Baseline demographics of the studied population (n = 565)
| Characteristics | Total sample | Patients with an amyloid PET scan n = 197 | Patients without an amyloid PET scan n = 368 | p‐value |
|---|---|---|---|---|
| Age in years | 73.78 ± 8.93 | 69.57 ± 0.42 | 76.04 ± 0.49 | <0.001* |
| Male gender | 549 (97.17) | 190 (96.4) | 359 (97.6) | 0.41 |
| Years of education | 13.80 ± 2.63 | 13.85 ± 2.54 | 13.77 ± 2.68 | 0.69 |
| MoCA initial visit | 20.02 ± 4.74 | 20.29 ± 0.35 | 19.88 ± 0.25 | 0.33 |
| Family history of dementia | 191 (35.9) | 66 (36.1) | 125 (35.8) | 0.95 |
| Psychiatric history | ||||
| History of PTSD | 170 (30.3) | 72 (36.4) | 98 (26.7) | 0.02 |
| Substance abuse | 202 (35.9) | 75 (37.9) | 127 (34.6) | 0.12 |
| Depression | 188 (33.5) | 74 (37.4) | 114 (31) | 0.44 |
| History of TBI | 206 (45.9) | 78 (50.0) | 128 (43.7) | 0.20 |
| Vascular risk factors | ||||
| Hypertension | 400 (70.8) | 129 (65.2) | 271 (73.8) | 0.03 |
| Hyperlipidemia | 390 (69.0) | 135 (23.9) | 255 (45.1) | 0.85 |
| Diabetes | 165 (29.2) | 56 (28.3) | 109 (29) | 0.86 |
| Coronary artery disease | 137 (24.3) | 39 (19.7) | 98 (26.7) | 0.06 |
| Stroke | 70 (12.5) | 25 (12.6) | 45 (12.2) | 0.90 |
| Cognitive syndrome | n = 550 | n = 192 | n = 358 | <0.001* |
| Unimpaired | 10 (1.8) | 0 (0) | 10 (2.8) | 0.02 |
| SCD | 32 (5.8) | 4 (2.1) | 28 (7.8) | 0.01 |
| MCI | 308 (56.0) | 124 (64.6) | 184 (51.4) |
<0.005 |
| Dementia | 200 (36.4) | 64 (33.3) | 136 (38.0) | 0.28 |
| Clinical diagnosis | n = 435 | n = 174 | n = 261 | <0.001* |
| Alzheimer disease | 219(50.3) | Prior scan: | After initial evaluation: | |
| Vascular | 39 (9.0) | AD: 107 (61.5) | AD: 112 (42.9) | |
| Lewy body diseases | 30 (6.9) | Non‐AD: 57 (32.8) | Non‐AD: 136 (52.1) | |
| Psychiatric disorders | 28 (6.4) | Unclear dx: 10 (5.7) | Unclear: 13 (5.0) | |
| FTD | 14 (3.2) | |||
| CTE | 10 (2.3) | |||
| Atypical parkinsonism | 10 (2.3) | |||
| PPA | 7 (1.6) | |||
| Other | 55 (12.6) | |||
| Unclear† | 23 (5.3) | |||
| Met AUC | 403/523 (77.0) | 193/197 (98.0) | 210/326(64.4) | <0.001* |
| Cognitive testing | ||||
| CERAD | ||||
| Encoding totala | 13.94 ± 4.69 | 13.84 ± 0.35 | 14.00 ± 0.24 | 0.70 |
| Delayed recallb | 3 (IQR 1‐5) | 4 (IQR 1‐5) | 3 (IQR 1‐5) | 0.79 |
| Corr. recognitionb | 8 (IQR 6‐10) | 8 (IQR 7‐10) | 8 (IQR 6‐10) | 0.95 |
| TMT part A timeb | 52 (IQR 38‐77.5) | 47 (IQR 37‐71) | 54 (IQR 40‐80) | 0.05 |
| TMT part B timeb | 127 (IQR 87‐204) | 114 (IQR 77‐192) | 136 (IQR 90‐216) | 0.05 |
| FASb | 27 (IQR 20‐36) | 27 (IQR 21‐35) | 27 (IQR 20‐36) | 0.26 |
| Total categoriesb | 28 (IQR 21‐36) | 29 (IQR 20‐38) | 28 (IQR 21‐35) | 0.60 |
| FAS/CATb | 0.96 (IQR 0.76‐1.29) | 0.92 (IQR 0.72‐1.25) | 0.98 (IQR 0.77‐1.30) | 0.12 |
| Boston naming testb | 13 (IQR 11‐14) | 13 (IQR 12‐14) | 13 (IQR 11‐14) | 0.52 |
| GDSb | 4 (IQR 2‐7) | 4 (IQR 2‐7) | 4 (IQR 2‐7) | 0.92 |
| GAIb | 4 (IQR 1‐10) | 4 (IQR 1‐11) | 3.5 (IQR 1‐10) | 0.52 |
| MRI | ||||
| Pattern of atrophy | ||||
| Anterior temporal | 194 (35.9) | 66 (33.3) | 128 (34.9) | 0.70 |
| Medial temporal | 294 (54.4) | 108 (54.5) | 186 (50.7) | 0.39 |
| Parietal | 219 (40.6) | 78 (39.4) | 141 (38.0) | 0.74 |
| Frontal | 149 (27.70) | 52 (26.0) | 97 (26.0) | 1.00 |
| Small vessel disease | 261 (48.4) | 86 (43.4) | 175 (47.7) | 0.32 |
| Lacunar strokes | 54 (10.04) | 12 (6.0) | 42 (11.4) | 0.04 |
| Microhemorrhages | 40 (7.48) | 7 (3.5) | 33 (8.9) | 0.02 |
| Amyloid PET completed | 197 (34.87) | |||
| Number of visit the PET was ordered | ||||
| First | 85 (43.2) | |||
| Second | 85 (43.2) | |||
| Third | 22 (11.2) | |||
| Fourth | 5 (2.5) | |||
| Positive studies | 72 (36.5) |
Values represent number (percentage) and means with standard deviation (SD) unless specified. IQR = interquartile range; MoCA = Montreal Cognitive assessment; TBI = traumatic brain injury; SCD = subjective cognitive decline; MCI = mild cognitive impairment; FTD = frontotemporal dementia; CTE = chronic traumatic encephalopathy; PPA = primary progressive aphasia; AUC = Appropriate use criteria; CERAD = Consortium to Establish a Registry for Alzheimer's Disease. †Unclear category: etiological process of the cognitive decline was unclear after the initial evaluation a t‐test, bMann Whitney test. * were significant after correcting for multiple comparisons using Bonferroni correction at adjusted cut off of p < 0.001
3.1.1. APET ordering and performance
Of the 565 patients, 197 patients (34.9%) underwent APET imaging in addition to routine diagnostic workup. APET imaging was ordered early in the clinical assessment for most patients, with 86% of patients having the study ordered in the first or second visit.
When comparing patients with and without APET ordered, patients with an APET were younger (69.57± 0.42 vs. 76.04 ± 0.49) and were more frequently in a MCI stage (64.6% vs. 51.4%) as opposed to dementia (33.3% vs. 38.0%). AD as the etiological clinical diagnosis was more prevalent in the APET group as compared to the group without this study (61.5% vs. 42.9%). No statistically significant differences were seen on MoCA scores, neuropsychological testing, imaging, or other baseline demographics. Regarding diagnostic certainty, 77% of our sample had a high level of diagnostic uncertainty per AUC (98.0% in the APET group vs. 64.4% in the group without APET, p <0.001; Table S1).
The study was positive in 72 patients (36.5%). When ordered, an APET yielded a change in diagnosis in 84 cases (52.8%). Figure 1 describes the diagnostic trajectories before and after the scan for the patients with APET. Of the 197 patients who underwent APET, 107 patients had a pre‐scan diagnosis of AD, 57 patients had a suspected non‐AD etiology, 10 patients were documented as having an unclear etiology, and 23 patients did not have information on file regarding suspected etiology. In the subgroup with a suspected AD diagnosis pre‐scan (n = 107), a positive scan yielded a diagnosis confirmation of AD in 42 out of 44 cases (no post‐scan diagnosis documented in 2 cases). In contrast, patients with suspected AD pre‐scan with a negative scan, had a diagnostic change to a non‐AD etiology in 49 out of 63 cases (77.8%). In the subgroup with a suspected non‐AD diagnosis pre‐scan (n = 57), a positive scan yielded a diagnostic change to AD in 14 out of 19 cases (73.7%). Patients with a negative scan in this subgroup (n = 38) remained with a non‐AD etiological diagnosis post‐scan in 33 out of 38 cases (86.8%).
FIGURE 1.
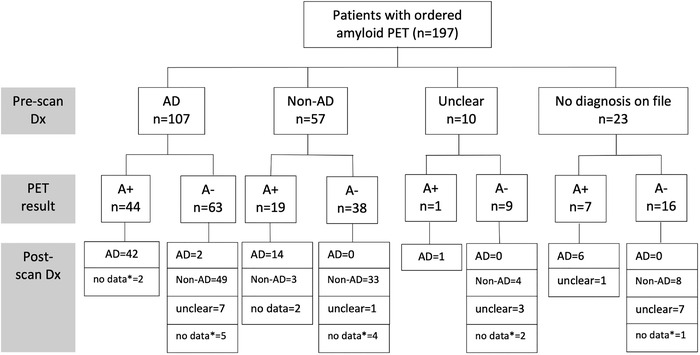
Diagnostic pathways of patients who underwent amyloid PET. A+ = amyloid PET positive; A‐ = amyloid PET negative. *no data available on file
3.1.2. Other biomarkers used
Other biomarkers were ordered for a minority of patients in the sample (7.4%). Further descriptive information is provided in the supplementary material (Figure S1 and S2).
3.2. Comparison between groups with and without APET at follow‐up
A total of 510 patients returned after the initial evaluation for follow‐up visits (190 patients with an APET and 320 with routine evaluation only). The median follow‐up time was 7 months (IQR: 3‐14). When comparing the groups with and without APET, there was a significant difference in follow‐up with a longer follow‐up time seen in the APET group (Mann‐Whitney U = ‐6.32, p < 0.001). After controlling for age, MoCA score, baseline cognitive syndrome, and clinical etiological diagnosis (Table 2), having an APET was still associated with longer follow‐up (coef: 2.76; SE: 0.85; p = 0.001). No differences were seen in the number of additional diagnostic studies ordered among the two groups. Only age, clinical etiological diagnosis, and AUC criteria were significantly associated with the number of additional studies subsequently ordered, with older patients, AD diagnosis, and lower level of diagnostic uncertainty per AUC undergoing less additional testing (coef: ‐0.04; SE: 0.02; p = 0.03; coef: ‐0.84; SE: 0.32; p = 0.009; coef: 0.81; SE: 0.41; p = 0.048, respectively). Regarding diagnostic variability at each follow‐up visit, the APET ordering was associated with an increased change in diagnosis from one visit to the next (coef: 0.45; SE: 0.14; p = 0.001). A clinical diagnosis of AD and a stage of dementia were associated with less diagnostic variability over time (coef: ‐0.44; SE: 0.13; p = 0.001; coef: ‐0.34; SE: 0.16; p = 0.03, respectively).
TABLE 2.
Association between having an amyloid PET as part of initial workup and clinical management parameters at follow‐up (n = 352)
| Variable | Follow‐up time a | Tests ordered b | DX variability c | |||
|---|---|---|---|---|---|---|
| Coef (SE) | p | Coef (SE) | p | Coef (SE) | p | |
| Intercept | 6.91 (4.62) | 0.13 | −2.40 (1.74) | 0.48 | −2.27 (0.77) | 0.003 |
| Amyloid PET ordered* | 2.76 (0.85) | 0.001 | 0.17 (0.33) | 0.59 | 0.45 (0.14) | 0.001 |
| Age | 0.05 (0.05) | 0.33 | −0.04 (0.02) | 0.03 | −0.01 (0.01) | 0.32 |
| MoCA | −0.06 (0.09) | 0.48 | 0.04 (0.04) | 0.26 | 0.02 (0.02) | 0.17 |
| Cognitive syndrome ** | −0.74 (0.91) | 0.41 | 0.19 (0.36) | 0.60 | −0.34 (0.16) | 0.03 |
| Clinical diagnosis *** | 1.76 (0.82) | 0.03 | −0.84 (0.32) | 0.009 | −0.44 (0.13) | 0.001 |
| Met AUC criteria**** | −1.01 (0.88) | 0.25 | 0.81 (0.41) | 0.048 | 0.04 (0.14) | 0.80 |
| Variable | ChEI prescriptions d | Social work referrals c | Research referrals c | |||
|---|---|---|---|---|---|---|
| Coef (SE) | p | Coef (SE) | p | Coef (SE) | p | |
| Intercept | 0.06 (0.10) | 0.07 | −6.87 (1.79) | <0.001 | −5.88 (1.76) | 0.001 |
| Amyloid PET ordered* | 1.24 (0.36) | 0.46 | 0.21 (0.31) | 0.50 | 0.001 (0.32) | 0.99 |
| Age | 1.05 (0.02) | 0.005 | 0.04 (0.02) | 0.05 | 0.006 (0.02) | 0.77 |
| MoCA | 0.93 (0.03) | 0.01 | −0.03 (0.03) | 0.33 | 0.03 (0.03) | 0.41 |
| Cognitive syndrome** | 2.20 (0.65) | 0.008 | 0.86 (0.31) | 0.005 | 0.17 (0.31) | 0.59 |
| Clinical diagnosis *** | 0.89 (0.24) | 0.67 | −0.05 (0.27) | 0.86 | 0.91 (0.34) | 0.007 |
| Met AUC criteria**** | 1.05 (0.30) | 0.87 | −0.07 (0.28) | 0.78 | 0.17 (0.31) | 0.59 |
Abbreviations: DX: diagnosis, ChEI: cholinesterase inhibitors, Coef: coefficient, SE: standard error, MoCA: Montreal Cognitive Assessment, AUC: Appropriate Use Criteria
linear regression.
negative binomial regression.
Poisson regression adjusted for difference in follow‐up time.
logistic regression.
* Referent: Ordered versus not ordered, ** Referent: Dementia versus mild cognitive impairment, *** Referent: Alzheimer's disease (AD) versus Non‐AD, ****Referent: yes versus no
Regarding clinical management outcomes, no difference in early cholinesterase inhibitor prescription was observed between groups with and without an APET ordered (coef: 1.24, SE: 0.36; p = 0.46). Older age, follow‐up time, a diagnosis of dementia, and a lower MoCA score at baseline were significantly associated with cholinesterase inhibitors prescribing in this cohort. The presence of an APET was not associated with a greater number of referrals to social work. A clinical diagnosis of dementia was associated with more social work referrals (coef: 0.86; SE: 0.31; p = 0.005). Finally, no differences were seen in the number of research and clinical trial referrals between the groups with and without an APET. The only factor associated with a higher number of research referrals was a clinical diagnosis of AD (coef:0.91; SE: 0.34; p = 0.007).
To confirm the robustness of our results, propensity score matching, to balance known confounders between groups, and subgroup sensitivity analysis were performed. Details can be found in the supplementary material (TableS2).
3.3. Comparisons of clinical impact within the APET group
When analyzing the same outcomes within the group with APET, we found that a positive result was significantly associated with fewer number of additional diagnostic tests, less diagnostic variability at follow‐up visits, a greater likelihood of having a cholinesterase inhibitors prescription, and more research referrals (Table 3).
TABLE 3.
Association between a positive amyloid PET result and clinical management parameters at follow up (n = 154)
| Variable | Follow‐up time a | Tests ordered b | DX variability b | |||
|---|---|---|---|---|---|---|
| Coef (SE) | p | Coef (SE) | p | Coef (SE) | p | |
| Intercept | 6.71 (7.79) | 0.39 | −1.03 (1.99) | 0.60 | −2.67 (1.05) | 0.01 |
| Amyloid PET result* | 4.41 (1.20) | <0.001 | −2.58 (0.73) | <0.001 | −1.37 (0.20) | <0.001 |
| Age | 0.04 (0.10) | 0.96 | −0.04 (0.03) | 0.09 | 0.01 (0.14) | 0.43 |
| MoCA | −0.01 (0.13) | 0.67 | 0.05 (0.04) | 0.27 | −0.001(0.02) | 0.97 |
| Cognitive syndrome ** | −1.49 (1.34) | 0.27 | 0.37 (0.40) | 0.36 | −0.05 (0.19) | 0.98 |
| Clinical diagnosis *** | 2.09 (1.21) | 0.09 | −0.86 (0.34) | 0.01 | −0.07 (0.17) | 0.67 |
| Variable | ChEI prescriptions c | Social work referrals b | Research trials referrals b | |||
|---|---|---|---|---|---|---|
| Coef (SE) | p | Coef (SE) | p | Coef (SE) | p | |
| Intercept | 0.03 (0.08) | 0.19 | −8.79 (2.53) | 0.001 | −6.13 (2.20) | 0.005 |
| Amyloid PET result* | 6.29 (2.94) | <0.001 | 0.40 (0.37) | 0.28 | 3.00 (0.73) | <0.001 |
| Age | 1.06 (0.04) | 0.12 | 0.07 (0.03) | 0.04 | −0.02 (0.03) | 0.55 |
| MoCA | 0.94 (0.04) | 0.19 | −0.05 (0.04) | 0.26 | 0.04 (0.05) | 0.44 |
| Cognitive syndrome ** | 2.65 (1.24) | 0.04 | 0.97 (0.43) | 0.03 | −0.09 (0.41) | 0.83 |
| Clinical diagnosis *** | 0.71 (0.31) | 0.43 | 0.25 (0.42) | 0.001 | 0.42 (0.48) | 0.38 |
Abbreviations: DX: diagnosis, ChEI: cholinesterase inhibitors, Coef: coefficient, SE: standard error, MoCA: Montreal Cognitive Assessment
linear regression.
Poisson regression adjusted for difference in follow‐up time.
logistic regression.
* Referent: positive versus negative, ** Referent: Dementia versus mild cognitive impairment, *** Referent: Alzheimer's disease (AD) versus Non‐AD
4. DISCUSSION
This was a study of 565 veterans with cognitive decline followed at a tertiary memory disorders clinic. In this cohort, having an APET study ordered in addition to the usual workup, was associated with longer follow‐up time in the clinic, and a higher level of diagnostic variability at follow‐up. We did not find an association between APET ordering and the number of additional diagnostic studies ordered, early cholinesterase inhibitors prescription patterns, social work referrals, or referrals to clinical trials. Longer follow‐up time could be associated with the time involved completing this additional imaging study.
At first glance, these findings seem discordant with prior published studies assessing the clinical impact of APET. The largest prospective study published in the subject is the Imaging Dementia‐Evidence for Amyloid Scanning (IDEAS) Study. 13 This was a multicenter study aimed at assessing the utility of APET scans in Medicare beneficiaries by evaluating the association between APET and subsequent change in management. This study reported a 60% change in the composite management endpoint, which included changes in drug therapy and counseling about future planning and safety. They also reported a 43% change in AD drug use, a 56% decrease in diagnostic uncertainty, and a 4% decrease in clinical trial referrals before and after APET. Other prospective and retrospective studies have reported 40% to 80% changes in management and a significant reduction in the number of additional cognitive investigations. 11 , 12
In the present study, when analyzing the same clinical outcomes within the APET group, we were able to confirm that a positive APET result was associated with the expected and previously described impact: fewer additional diagnostic tests, less diagnostic variability at follow‐up visits, more cholinesterase inhibitors prescriptions, and more referrals to research. However, there was a relatively decreased rate of positive APET results in our cohort compared to that of other studies (37% compared to 49‐64% in prior studies) 7 , 10 , 11 , 12 that influenced the outcome comparisons between the groups with and without APET. Even though we were able to exclude AD pathology in patients with a negative APET result, a high level of diagnostic variability regarding the exact etiology often remained present at follow‐up.
The divergence of findings between the present study and prior investigations of the clinical impact of APET are explained by two key differences: (1) the baseline characteristics of our patient population, (2) significant difference in the study design and group comparisons with a more naturalistic assessment of APET use in the current study.
Our veteran population is medically complex. As reported in previous studies, the risk of mental health disorders is approximately doubled in the military population, and PTSD, substance use disorder, and TBI are also reported at higher rates in military personnel. 20 , 21 , 22 This increased level of neuropsychiatric comorbidity potentiates the diagnostic uncertainty in assessing cognitive impairment in this population, which ultimately affects the way diagnostic biomarkers are used in clinical practice. AD was the suspected prePET‐etiology in 73% of MCI patients and 82% of patients with dementia in the IDEAS study. 13 Other studies assessing the clinical significance of APET have excluded patients with MCI, and thus have reported high rates of AD as the primary pre‐PET diagnosis (around 70% to 80%) consistent with high diagnostic confidence pre‐PET. 7 , 12 , 23 –25 In contrast, in this study, only 50.3% of patients in the total memory disorders clinic population and 61.5% in the APET subgroup had a primary suspected diagnosis of AD. APET has been used in our clinic more as a tool to exclude AD pathology in highly complex cases with multiple neuropsychiatric comorbidities and a possible diagnosis of AD, and for prognostic considerations in patients with MCI, rather than to confirm a diagnosis of AD dementia. This difference translates into relatively increased levels of negative APET results in our study compared to prior studies.
Another critical aspect to consider is the difference in our study design. Prior studies provided only a snapshot in time regarding the changes in management before and after APET rather than longitudinal follow up data, a strength of the current study. Additionally, most other studies give a somewhat artificial assessment of the clinician's intended change in plan by completing a before and after survey regarding diagnosis impression, qualitative level of diagnostic certainty, and proposed treatment plan. Finally, the vast majority of studies assessing clinical utility of an APET have included only patients who undergo these scans and no comparison is made with patients with only a clinical diagnosis. Our study provides a more naturalistic evaluation of APET's impact on diagnosis and management by offering information on clinical management for the entire group of patients seen in our clinic and making comparisons among the patients with and without APET.
The second phase of the IDEAS study aimed to compare hospitalizations and emergency departments visits between participants with an APET and unscanned matched MEDICARE participants. Final results have not been published in a peer‐reviewed journal. However, preliminary insights were presented at the 2020 Alzheimer's Association International Conference. 26 The authors reported a failure of the primary outcome to meet clinically meaningful criterion. However, among the IDEAS participants, having a positive amyloid scan was associated with better health outcomes than having a negative scan. A possible explanation, exposed by the authors, is that a confirmation of AD by a positive scan led to an improved care management in this group. In contrast, participants with a negative scan, continued without an etiological diagnosis which could lead to suboptimal management. Even though these are preliminary results, they mirror our findings. It seems that the true value of the APET lies upon the possibility of giving the clinician a diagnostic certainty of AD. If the diagnosis uncertainty remains, the scan will not change management significantly.
This study has several limitations. The observational nature of the design raises the possibility of residual and unknown confounding variables. However, we controlled for the known confounders between the two groups during the construction of regression models. Also, 9% of the initial cohort was lost to follow up after the first assessment. However, the baseline characteristics of these patients did not differ significantly from the analyzed group. Finally, this is a study of an all‐veteran, mostly male population with a high prevalence of psychiatric comorbidity and TBI, so the results may not be easily generalizable to other civilian memory disorders clinics. However, the results of the present study contribute to generate initial insights about how biomarkers are being used and how they can perform in medically‐complex populations like this one. Additionally, prior studies describing the prevalence of dementia among US veterans have described similar prevalence of AD compared to the general US population. 27
Another limitation of the present study was the fact that we were not able to measure other outcomes that are of great value to patients, such as potential quality of life gains associated with reassuring patients that they do not have AD, counseling regarding the prognosis of cognitive decline in patients with MCI, and an increase in diagnostic certainty of an AD diagnosis in patients with a positive result. Finally, in patients with a high prevalence of neuropsychiatric comorbidities, excluding a diagnosis of AD with a negative APET might facilitate the reorientation of pharmacologic and nonpharmacologic therapy to the underlying diagnoses. Future studies should focus on a broader definition of clinical utility.
In conclusion, in medically‐complex populations, APET positivity rates may be lower than prior studies have reported. This lower positivity rate results in less robust differences in classically explored clinical utility variables when comparing patients with and without an APET. However, these impact variables are likely an oversimplification of medical management and patient care. Further studies are needed to assess the additional value of biomarkers in diverse clinical settings.
CONFLICT OF INTEREST
A.L.V.‐R. has no disclosures to report. K.S. has no disclosures to report. A.M. has no disclosures to report. R.W. has no disclosures to report. P.H. has no disclosures to report. R.P. has no disclosures to report. R.D. has no disclosures to report. A.B. has been a consultant for Eli Lilly, Corium, Cognito, and Sage, and a clinical trial investigator for Biogen, Eli Lilly, vTv therapeutics, and Cognito. He is also the PI on investigator initiated trials for Biogen, Cyclerion, and Bristol Myers Squibb. Katherine Turk has no disclosures to report. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
Andrew E. Budson was supported by a VA Merit Award (CX001698), Andrew E. Budson and Katherine W. Turk were investigators on NIH/NIA P30‐AG072978. Katherine W. Turk was supported by a VA Career Development Award (CX002065).
Vives‐Rodriguez AL, Schiloski KA, Marin A, et al. Impact of amyloid PET in the clinical care of veterans in a tertiary memory disorders clinic. Alzheimer's Dement. 2022;8:e12320. 10.1002/trc2.12320
REFERENCES
- 1. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, Carri, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR, Therneau TM, Weigand SD, et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the national institute on aging‐Alzheimer's association research framework. JAMA Neurol. 2019;76(10):1174‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dodich A, Mendes A, Assal F, et al. The A/T/N model applied through imaging biomarkers in a memory clinic. Eur J Nucl Med Mol Imaging. 2020;47(2):247‐255. [DOI] [PubMed] [Google Scholar]
- 5. Lim A, Tsuang D, Kukull W, et al. Clinico‐neuropathological correlation of Alzheimer's disease in a community‐based case series. J Am Geriatr Soc. 1999;47(5):564‐569. [DOI] [PubMed] [Google Scholar]
- 6. Ranginwala NA, Hynan LS, Weiner MF, White CL. Clinical criteria for the diagnosis of Alzheimer disease: still good after all these years. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2008;16(5):384‐388. [DOI] [PubMed] [Google Scholar]
- 7. Grundman M, Pontecorvo MJ, Salloway SP, et al. Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord. 2013;27(1):4‐15. [DOI] [PubMed] [Google Scholar]
- 8. Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005‐2010. J Neuropathol Exp Neurol. 2012;71(4):266‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotta Ramusino M, Perini G, Altomare D, Barbarino P, et al. Outcomes of clinical utility in amyloid‐PET studies: state of art and future perspectives. Eur J Nucl Med Mol Imaging. 2021;48(7):2157‐2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pontecorvo MJ, Siderowf A, Dubois B, et al. Effectiveness of florbetapir PET imaging in changing patient management. Dement Geriatr Cogn Disord. 2017;44(3‐4):129‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carswell CJ, Win Z, Muckle K, et al. Clinical utility of amyloid PET imaging with (18)F‐florbetapir: a retrospective study of 100 patients. J Neurol Neurosurg Psychiatry. 2018;89(3):294‐299. [DOI] [PubMed] [Google Scholar]
- 12. Ceccaldi M, Jonveaux T, Verger A, et al. Added value of 18F‐florbetaben amyloid PET in the diagnostic workup of most complex patients with dementia in France: a naturalistic study. Alzheimers Dement J Alzheimers Assoc. 2018;14(3):293‐305. [DOI] [PubMed] [Google Scholar]
- 13. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 15. Machado TH, Fichman HC, Santos EL, et al. Normative data for healthy elderly on the phonemic verbal fluency task ‐ FAS. Dement Neuropsychol. 2009;3(1):55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2004;19(2):203‐214. [DOI] [PubMed] [Google Scholar]
- 17. Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD‐10 and DSM‐IV. Int J Geriatr Psychiatry. 1999;14(10):858‐865. [DOI] [PubMed] [Google Scholar]
- 18. Pachana NA, Byrne GJ, Siddle H, Koloski N, Harley E, Arnold E. Development and validation of the geriatric anxiety inventory. Int Psychogeriatr. 2007;19(1):103‐114. [DOI] [PubMed] [Google Scholar]
- 19. Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement J Alzheimers Assoc. 2013;9(1):e‐1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rafferty LA, Cawkill PE, Stevelink SAM, Greenberg K, Greenberg N. Dementia, post‐traumatic stress disorder and major depressive disorder: a review of the mental health risk factors for dementia in the military veteran population. Psychol Med. 2018;48(9):1400‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodwin L, Wessely S, Hotopf M, et al. Are common mental disorders more prevalent in the UK serving military compared to the general working population? Psychol Med. 2015;45(9):1881‐1891. [DOI] [PubMed] [Google Scholar]
- 22. Hunt EJF, Wessely S, Jones N, Rona RJ, Greenberg N. The mental health of the UK Armed Forces: where facts meet fiction. Eur J Psychotraumatology. 2014;5:23617. doi: 10.3402/ejpt.v5.23617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zwan MD, Bouwman FH, Konijnenberg E, et al. Diagnostic impact of [18F]flutemetamol PET in early‐onset dementia. Alzheimers Res Ther. 2017;9(1):2. doi: 10.1186/s13195-016-0228-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ossenkoppele R, Prins ND, Pijnenburg YAL, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement J Alzheimers Assoc. 2013;9(4):414‐421. [DOI] [PubMed] [Google Scholar]
- 25. Boccardi M, Altomare D, Ferrari C, et al. Assessment of the incremental diagnostic value of florbetapir F 18 imaging in patients with cognitive impairment: the incremental diagnostic value of amyloid PET With [18F]‐Florbetapir (INDIA‐FBP) Study. JAMA Neurol. 2016 Dec 1;73(12):1417‐1424. [DOI] [PubMed] [Google Scholar]
- 26. IDEAS Finds Small Drop in Hospitalizations, Missing Goal | ALZFORUM [Internet]. [cited 2022 Jan 26]. Available from: https://www.alzforum.org/news/conference‐coverage/ideas‐finds‐small‐drop‐hospitalizations‐missing‐goal
- 27. Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among veterans affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20(4):245‐253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


