Abstract
Background
Diabetic retinopathy (DR) is a common complication of diabetes mellitus (DM). Systemic inflammation is intimately associated with DR. The neutrophil-to-lymphocyte ratio (NLR) index is a relatively new indicator of inflammation.
Methods
This cross-sectional study was carried out among adults with DM based on the National Health and Nutrition Examination Survey (NHANES) from 2009 to 2016. NLR was presented as absolute neutrophil counts/ absolute lymphocyte counts. The relationship of NLR levels to DR was analyzed using multivariable logistic regression.
Results
There were 2772 eligible subjects extracted from the NHANES. In the multivariate analysis, NLR was related to the risk of DR after adjustment for potential confounders. The association between NLR levels and DR was nonlinear, with an inflection point of 4.778. Compared with the baseline values, NLR was not statistically significant on the right side of the inflection point (1.000, 0.914 to 1.094, 0.9974) but was positively associated with DR on the left side (1.236, 1.132 to 1.349, < 0.0001).
Conclusions
NLR reflects systemic inflammation that may increase the risk of DR. NLR positively correlates with DR when its value is less than 4.778.
Keywords: Neutrophil-to-lymphocyte ratio, Diabetic retinopathy, Diabetes mellitus, NHANES
Introduction
Diabetic retinopathy (DR) is a microvascular complication of diabetes mellitus (DM) and is a primary cause of acquired blindness among working-age individuals [1]. Various mechanisms and factors mediate DR development, including pregnancy, diabetic nephropathy, obesity, family history, blood glucose fluctuations, hyperlipidemia, chronic diabetes, hypertension, and hyperglycemia [2–4]. The pathogenesis of DR is not fully understood; however, several studies suggested that inflammation plays an important role [5–7]. Many epidemiological studies highlighted the association between chronic inflammation and DM [8, 9]. Chronic inflammation may contribute to the development of microangiopathy and macroangiopathy in patients with diabetes [10].
Several lines of evidence suggest that routine blood tests might provide adequate information to perform risk stratification [11, 12]. Specifically, peripheral blood leukocytes such as lymphocytes, neutrophils, basophils, eosinophils, and monocytes all have unique biological functions in systemic inflammation [13]. The neutrophil-to-lymphocyte ratio (NLR) can indicate systemic inflammation [14]. NLR represents the ratio of neutrophils to lymphocytes in peripheral blood, which integrates different but complementary immune pathways in circulating blood. Increased NLR may be the result of increased neutrophils, which can adhere to endothelial cells, resulting in vascular endothelial damage and widespread chronic inflammation [12, 15]. Furthermore, lymphocytes are the main cells of the body’s immune response and have the ability to regulate inflammatory responses [16].
NLR has been used for mortality stratification in major cardiac events [17, 18], indicating infectious and inflammatory status or postoperative complications [19, 20]. NLR was also considered a prognostic and predictive factor for DM and its complications [21, 22]. Wang et al. found that NLR was related to DR in patients with diabetes who had no associated family history in China [23]. Nevertheless, further research is required to determine whether this is the same in different regions, populations, and types of diabetes. Therefore, our study further evaluated the relationship between NLR and DR through a larger sample data from the United States (US).
Methods
Sources of the data and samples
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional, stratified, multistage probability cluster survey conducted in the US and the details of NHANES have been reported elsewhere [24]. The data for this study was derived from the NHANES between 2009 and 2016, including potential risk factors and nutrition in the civilian, non-institutionalized, American population. The National Center for Health Statistics Institutional Review Board (NCHS IRB/ERB) approved the protocol (NCHS ERB protocols #2011–17). All participants provided informed consent. After they were interviewed at home, the participants underwent health examinations in mobile examination centers. Subjects who were aged less than 18 years, pregnant or lactating, were excluded. The physiological and medical statuses of the participants were evaluated and required laboratory tests were completed. Four cycles of NHANES surveys were used to evaluate the relationship between DR and NLR. All adult patients (≥18 y) with type 1 and type 2 DM were included. The exclusion criteria were: (a) missing neutrophil or lymphocyte data, (b) incomplete Patient 90 Health Questionnaire-9 (PHQ-9) [25], and (c) the use of nonsteroidal anti-inflammatories or corticosteroids.
Variables
Using automated hematology analyzing devices, neutrophil and lymphocyte counts were measured and expressed as × 103 cells/μl. NLR was expressed as absolute neutrophil count/lymphocyte counts. The selected indicators included age, gender, marital status, systolic blood pressure (SBP), waist circumference, triglycerides (TC), body mass index (BMI), high-density lipoprotein (HDL), diastolic blood pressure (DBP), low-density lipoprotein (LDL), C-reactive protein (CRP), glycosylated hemoglobin A1c (HbA1c), hemoglobin, neutrophil count, lymphocyte count, NLR, the ratio of family income to poverty (PIR), stroke, coronary heart disease (CHD), and heart failure (HF).
Statistical analyses
The χ2 test and independent-sample t-test were used to compare the differences in features at baseline in the non-DR and DR groups for categorical and continuous variables, respectively. Multivariate logistic regression analysis was conducted to evaluate the relationship between DR and NLR. Odds ratio (OR) and 95% confidence intervals (CI) were calculated. Based on the STROBE statement [26], the fully-adjusted, minimally adjusted, and unadjusted analyses were demonstrated. The matched odds ratio was changed by more than 10% when the covariances were added to this model [27]. Non-linear relationships were identified using generalized additive models (GAM). If the correlation was non-linear, a two-piecewise linear regression model was established based on the smoothing plot. When the ratio of DR and NLR fell on a smooth curve, automatic calculation of the inflection point was achieved using the recursive method, in which the maximum model likelihood estimation was applied [28]. Subgroup analysis of the associations between NLR and DR was carried out by applying the stratified linear regression model. P < 0.05 (two-sided) was considered statistically significant. R language (http://www.R-project.org) along with EmpowerStats software (http://www.empowerstats.com/en/, X&Y solutions, Inc., Boston, MA) were employed for all the analyses.
Results
Characteristics of the subjects
A total of 2772 patients had DM and met the inclusion criteria, including 1348 females and 1424 males. The average age was 61.3 ± 13.2 years. There were 637 (23.0%) patients suffering from DR. Characteristics at baseline are displayed in Table 1. Participants with DR had elevated CRP, HbA1c, neutrophil counts, NLR, diabetes duration, and lower lymphocyte counts. Male sex, stroke history, CHD, and HF were more common in DR patients (p < 0.05). Age, years, SBP, DBP, BMI, waist circumference, TC, HDL, LDL, triglycerides, hemoglobin, PIR, and marital status showed no significant differences between diabetic patients with DR and those without.
Table 1.
Characteristics of participants in the NHANES (2009—2016) by diabetic retinopathy
| Characteristics | Diabetes Mellitus (n = 2135) |
Diabetic Retinopathy (n = 637) |
P value |
|---|---|---|---|
| Age, years | 61.1 ± 13.3 | 61.9 ± 13.0 | 0.199 |
| SBP, mmHg | 131.6 ± 19.6 | 132.1 ± 20.5 | 0.642 |
| DBP, mmHg | 68.5 ± 14.2 | 66.9 ± 14.3 | 0.075 |
| BMI, kg/m2 | 32.3 ± 7.4 | 32.9 ± 8.2 | 0.098 |
| Waist circumference, cm | 109.1 ± 15.6 | 110.9 ± 17.8 | 0.081 |
| TC, mg/dl | 180.8 ± 45.2 | 177.0 ± 45.0 | 0.063 |
| HDL, mg/dl | 48.4 ± 14.4 | 47.5 ± 14.9 | 0.198 |
| LDL, mg/dl | 101.8 ± 35.9 | 97.7 ± 34.5 | 0.085 |
| Triglycerides, mg/dl | 148.7 ± 110.8 | 149.3 ± 116.4 | 0.940 |
| CRP, mg/dl | 0.5 ± 0.7 | 0.6 ± 0.8 | 0.047* |
| HbA1c, % | 7.4 ± 1.8 | 7.9 ± 1.9 | < 0.001** |
| Hemoglobin, g/dl | 13.6 ± 1.6 | 13.4 ± 1.6 | 0.544 |
| Neutrophil count, 109 /l | 4.6 ± 1.7 | 4.8 ± 1.8 | 0.018* |
| Lymphocyte count, 109 /l | 2.2 ± 0.9 | 2.1 ± 0.8 | 0.002** |
| NLR | 2.4 ± 1.5 | 2.7 ± 1.7 | < 0.001** |
| PIR | 2.2 ± 1.5 | 2.1 ± 1.5 | 0.202 |
| Diabetes duration, years | 10.1 ± 4.9 | 12.9 ± 3.0 | < 0.001** |
| Gender, n (%) | < 0.001** | ||
| Male | 1056 (49.5) | 368 (57.8) | |
| Female | 1079 (50.5) | 269 (42.2) | |
| Marital Status | 0.348 | ||
| Married, % | 1237 (58.2) | 372 (58.6) | |
| Widowed/divorced, % | 676 (31.8) | 211 (33.2) | |
| Never married, % | 214 (10.1) | 52 (8.2) | |
| Stroke, n (%) | < 0.001** | ||
| Yes | 173 (8.1) | 80 (12.6) | |
| No | 1954 (91.9) | 555 (87.4) | |
| CHD, n (%) | 0.012* | ||
| Yes | 213 (10.1) | 86 (13.7) | |
| No | 1898 (89.9) | 544 (86.3) | |
| HF, n (%) | < 0.001** | ||
| Yes | 175 (8.3) | 92 (14.6) | |
| No | 1945 (91.7) | 538 (85.4) |
SBP Systolic blood pressure, DBP Diastolic blood pressure, BMI Body mass index, TC Total cholesterol, HDL High density lipoprotein, LDL Low density lipoprotein, CRP C-reactive protein, HbA1c Glycosylated hemoglobin A1c, NLR Neutrophil to lymphocyte ratio, PIR Poverty income ratio, CHD Coronary heart disease, HF Heart failure
*P < 0.05, **P < 0.01
NLR was related to the risk of DR
A variety of models were constructed to evaluate the relationship of NLR with DR (Table 2) after potential confounding factors were adjusted. An increased NLR level was related to the elevated risk of DR in the model (OR = 1.112, 95% CI: 1.054–1.173, P = 0.0001). After several factors were adjusted (age, gender, BMI, PIR, diabetes duration, marital status, stroke, CHD, and HF), increased NLR levels were shown to be related to the elevated risk of DR (OR = 1.076, 95% CI: 1.015–1.142, P = 0.0146). The same trend was observed in sensitivity analysis when NLR was considered a categorical variable (tertiles) (P = 0.0116).
Table 2.
Association between NLR and diabetic retinopathy
| Variable | Crude Model | Model I | Model II | |||
|---|---|---|---|---|---|---|
| OR (95%CIs) | P value | OR (95%CIs) | P value | OR (95%CIs) | P value | |
| NLR | 1.112 (1.054, 1.173) | 0.0001** | 1.098 (1.040, 1.159) | 0.0007** | 1.076 (1.015, 1.142) | 0.0146* |
| NLR (Tertiles) | ||||||
| < 1.76 | 1.0(ref) | 1.0(ref) | 1.0(ref) | |||
| ≥ 1.76, < 2.57 | 1.204 (0.962, 1.507) | 0.1047 | 1.162 (0.927, 1.457) | 0.1924 | 1.111 (0.862, 1.433) | 0.4164 |
| ≥ 2.57 | 1.520 (1.222, 1.891) | 0.0002** | 1.438 (1.152, 1.796) | 0.0013** | 1.371 (1.066, 1.765) | 0.0141* |
| P trend | 0.0002** | 0.0012** | 0.0116* | |||
OR Odds ratio, CI Confidence interval
Models were derived from logistic multivariate regression models
Crude model adjust for: none
Adjust I model adjust for: age, gender
Adjust II model adjust for: age, gender, BMI; poverty income ratio; diabetes duration; marital status; stroke; coronary heart disease; heart failure
*P < 0.05, **P < 0.01
Non-linear relationship analyses
The non-linear relationship between NLR and DR was also explored (Fig. 1). A two-piecewise linear regression model was used. The inflection point was 4.778. On the right side of the inflection point, P-value, 95% CI, and the effect size were 0.9974, 1.000, and 0.914–1.094, respectively. On the left side, a positive association between NLR and DR in the inflection point was found (1.236, 1.132–1.349, < 0.0001), and there was no significant difference in the inflection point on the right side (1.000, 0.914–1.094, 0.9974).
Fig. 1.
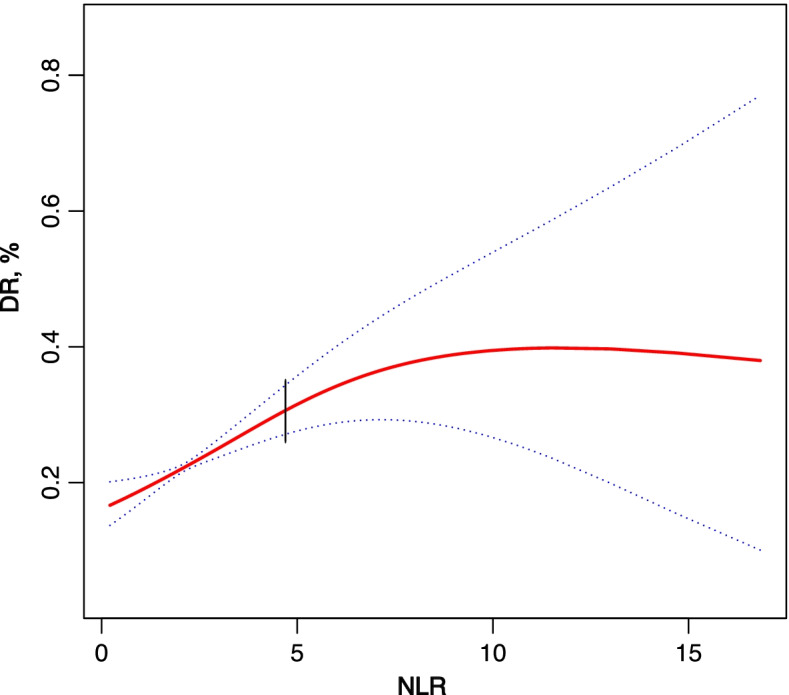
The relationship between NLR and DR. Black vertical lines: inflection point 4.778; Solid line: occurrence probability of DR; Dotted line: 95% confidence interval curve
Subgroup analysis
The results of the subgroup analysis are displayed in Table 3. There was no difference in NLR levels among most pre-specified subgroups in DR participants, except for hemoglobin and waist circumference. A high NLR level had an independent relationship with DR in high waist circumference (≥107.6 cm), and low hemoglobin (< 13.6 g/dl), and the corresponding ORs (95% CIs) were 1.459 (1.054, 2.020) and 1.699 (1.252, 2.304), respectively.
Table 3.
Subgroup analysis of the associations between NLR and diabetic retinopathy
| NLR | P for interaction | |||
|---|---|---|---|---|
| < 1.76 | ≥1.76, < 2.57 | ≥2.57 | ||
| Gender | 0.2452 | |||
| Male | 1.0(ref) | 1.259 (0.922, 1.719) | 1.361 (1.005, 1.843) | |
| Female | 1.0(ref) | 1.046 (0.750, 1.458) | 1.606 (1.166, 2.213) | |
| Marital Status | 0.1623 | |||
| Married | 1.0(ref) | 1.120 (0.828, 1.515) | 1.721 (1.291, 2.294) | |
| Widowed/divorced | 1.0(ref) | 1.199 (0.820, 1.753) | 1.183 (0.809, 1.729) | |
| Never married | 1.0(ref) | 1.833 (0.872, 3.854) | 1.609 (0.747, 3.462) | |
| Age, years | 0.2286 | |||
| < 63 | 1.0(ref) | 1.293 (0.946, 1.767) | 1.823 (1.332, 2.495) | |
| ≥ 63 | 1.0(ref) | 1.103 (0.798, 1.524) | 1.278 (0.941, 1.737) | |
| SBP, mmHg | 0.4644 | |||
| < 130 | 1.0(ref) | 1.138 (0.811, 1.596) | 1.593 (1.145, 2.216) | |
| ≥ 130 | 1.0(ref) | 1.229 (0.887, 1.702) | 1.225 (0.891, 1.683) | |
| DBP, mmHg | 0.7890 | |||
| < 68 | 1.0(ref) | 1.269 (0.888, 1.812) | 1.411 (1.001, 1.988) | |
| ≥ 68 | 1.0(ref) | 1.091 (0.797, 1.493) | 1.332 (0.975, 1.819) | |
| BMI, kg/m2 | 0.1015 | |||
| < 31.2 | 1.0(ref) | 1.019 (0.735, 1.413) | 1.520 (1.112, 2.078) | |
| ≥ 31.2 | 1.0(ref) | 1.308 (0.952, 1.796) | 1.364 (0.996, 1.867) | |
| Waist circumference, cm | 0.0241* | |||
| < 107.6 | 1.0(ref) | 1.010 (0.726, 1.406) | 1.365 (0.982, 1.899) | |
| ≥ 107.6 | 1.0(ref) | 1.386 (0.991, 1.938) | 1.459 (1.054, 2.020) | |
| Hemoglobin, g/dl | 0.0085** | |||
| < 13.6 | 1.0(ref) | 1.217 (0.881, 1.679) | 1.699 (1.252, 2.304) | |
| ≥ 13.6 | 1.0(ref) | 1.198 (0.876, 1.639) | 1.352 (0.988, 1.849) | |
| PIR | 0.2918 | |||
| < 1.7 | 1.0(ref) | 1.326 (0.958, 1.835) | 1.588 (1.152, 2.190) | |
| ≥ 1.7 | 1.0(ref) | 1.120 (0.791, 1.586) | 1.540 (1.105, 2.145) | |
| TC, mg/dl | 0.8835 | |||
| < 174 | 1.0(ref) | 1.235 (0.897, 1.699) | 1.325 (0.970, 1.809) | |
| ≥ 174 | 1.0(ref) | 1.112 (0.802, 1.541) | 1.629 (1.189, 2.232) | |
| HDL, mg/dl | 0.3069 | |||
| < 46 | 1.0(ref) | 1.302 (0.946, 1.793) | 1.478 (1.077, 2.027) | |
| ≥ 46 | 1.0(ref) | 1.082 (0.781, 1.497) | 1.492 (1.095, 2.031) | |
| LDL, mg/dl | 0.9532 | |||
| < 96 | 1.0(ref) | 1.148 (0.700, 1.883) | 1.285 (0.804, 2.053) | |
| ≥ 96 | 1.0(ref) | 1.486 (0.908, 2.431) 1 | 1.595 (0.968, 2.631) | |
| Triglycerides, mg/dl | 0.6060 | |||
| < 120 | 1.0(ref) | 1.391 (0.852, 2.274) | 1.468 (0.919, 2.343) | |
| ≥ 120 | 1.0(ref) | 1.187 (0.737, 1.911) | 1.428 (0.889, 2.293) | |
| HbA1c, % | 0.4348 | |||
| < 7 | 1.0(ref) | 1.265 (0.886, 1.806) | 1.403 (0.988, 1.993) | |
| ≥ 7 | 1.0(ref) | 1.147 (0.855, 1.540) | 1.605 (1.207, 2.133) | |
| CHD | 0.4167 | |||
| Yes | 1.0(ref) | 1.325 (0.683, 2.571) | 0.930 (0.486, 1.781) | |
| No | 1.0(ref) | 1.149 (0.903, 1.463) | 1.621 (1.283, 2.047) | |
| Stroke | 0.6780 | |||
| Yes | 1.0(ref) | 1.569 (0.741, 3.320) | 2.026 (0.991, 4.141) | |
| No | 1.0(ref) | 1.151 (0.908, 1.458) | 1.446 (1.148, 1.823) | |
| HF | 0.4004 | |||
| Yes | 1.0(ref) | 1.349 (0.651, 2.796) | 1.088 (0.552, 2.145) | |
| No | 1.0(ref) | 1.148 (0.904, 1.458) | 1.513 (1.197, 1.913) | |
NLR Neutrophil to lymphocyte ratio, SBP Systolic blood pressure, DBP Diastolic blood pressure, BMI Body mass index, PIR Poverty income ratio, TC Total cholesterol, HDL High density lipoprotein, LDL Low density lipoprotein, HbA1c Glycosylated hemoglobin A1c, CHD Coronary heart disease, HF Heart failure
*P < 0.05, **P < 0.01
Discussion
This study is the first to identify the relationship between DR and NLR levels in the US population to the best of our knowledge. NLR was related to DR, as demonstrated by the adjusted model. The same trend was seen when NLR was set as a categorical variable. Using the regression model and GAM, we found that the association between NLR and DR was non-linear. The correlations were different on different sides of the inflection point (NLR = 4.778). Compared with the NLR at baseline, there was no significant difference in the inflection point on the right side. However, NLR was positively correlated with DR on the left side.
The association of systemic inflammation and progression of DR is a research hotspot. It has been reported that bacterial infections and high levels of lipopolysaccharide activity are associated with increased risk of severe DR in type 1 diabetes [29]. Chronic inflammation is a pivotal factor in initiating and promoting diabetes and may contribute to the development of microangiopathy and macroangiopathy in diabetic patients [10]. Leukocytes and the subgroups in peripheral blood correlated with microvascular and macrovascular complications in patients with diabetes [30]. DR is a common microangiopathic complication in diabetes. Several studies found that inflammation had an essential role in different stages of DR [31, 32]. DR, a microvascular complication commonly seen in diabetes, is characterized by the formation of macular edema and new blood vessels [33] and disturbed crosstalk between glial cells and the loss of neurons [34].
NLR can indicate the status of systemic inflammation according to complete blood count. Neutrophil counts generally increase as the inflammatory disease progresses. However, the count does not increase in some diseases, such as cachexia, which leads to a “false negative” result during the evaluation of disease progression. Lymphocyte counts decrease when inflammatory disease deteriorates. However, a decreased lymphocyte cannot precisely indicate the actual status of the disease because this kind of change is relatively delayed [35, 36]. Recent research has demonstrated that NLR was a more reliable parameter in diagnosing or predicting survival than using lymphocyte or neutrophil count alone [37]. As the disease progresses, NLR increases simultaneously, especially in inflammation-related diseases [38].
Using two factors simultaneously, NLR is considered a new marker for evaluating systemic inflammation [22]. An elevated level of neutrophils may contribute to an increase in NLR, leading to damages in vascular endothelium, and in turn, causing severe chronic inflammation [12, 15]. Lymphocytes work as an essential part of systemic immune responses. A relatively higher level of CD4 T cells was shown to ameliorate atherosclerosis [16]. Thus, NLR may indicate systemic inflammation in patients with DR.
The relationship between DR and blood inflammation indexes has been widely discussed [39–41]; the present study further investigated the relationship, and the application of NLR in patients with DR. Our findings are partially similar to those of Wang et al. [23], but they are also somewhat different. We both showed that NLR was associated with DR; however, there was an inflection point in this index. NLR positively correlated with DR within a specific range as the value increased; beyond this range, there was no correlation. A high NLR indicated that the patient was in the acute phase of the disease with acute changes such as an acute infection [14, 42]. Therefore, it may not have a high correlation with the occurrence or development of DR. Moreover, unlike the former study, ours include a larger sample size.
There were some limitations in our study. First, causality cannot be established in a cross-sectional study; this would require prospective studies. Second, data were obtained from only one blood test. Serial testing provides more information because of the short life of blood cells. Third, the lack of information about DR severity and some other important indicators may have affected our conclusions. However, these limitations may be balanced by our strengths, including a large sample size, which represents the population in the US, the inclusion of ethnic/racial minorities, a wide range of ages, precise data and information of covariates, and a sufficient number of events.
Conclusion
NLR levels correlated with an elevated risk of DR. NLR positively correlated with DR when its value was less than 4.778. However, these should be further confirmed by conducting prospective studies.
Acknowledgements
Not applicable.
Authors’ contributions
X.J.H. and S.S.Q. designed the study and collected, analyzed and interpreted the data. X.Z. collected and analyzed data and drafted the manuscript. J.D.P. designed and supervised the study, obtained funding, and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Scientific Research Foundation of Wenzhou (grant No. Y20170773).
Availability of data and materials
All data were downloaded from the NHANES website (http://www.cdc.gov/nchs/Nhanes).
Declarations
Ethics approval and consent to participate
The protocols for conduct of NHANES were approved by the NCHS institutional review board. The Johns Hopkins Medical Institutions Institutional Review Board reviewed and approved the study (NCHS IRB/ERB Protocol Number: #2005–06, #2011–17). Informed written consent was obtained from all participants. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaojie He and Shanshan Qi contributed equally to this work.
References
- 1.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusuhara S, Fukushima Y, Ogura S, Inoue N, Uemura A. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. 2018;42(5):364–376. doi: 10.4093/dmj.2018.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wat N, Wong RL, Wong IY. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med J. 2016;22(6):589–599. doi: 10.12809/hkmj164869. [DOI] [PubMed] [Google Scholar]
- 4.Kuo JZ, Wong TY, Rotter JI. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014;132(1):96–107. doi: 10.1001/jamaophthalmol.2013.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein BE, Knudtson MD, Tsai MY, Klein R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol (Chicago, Ill : 1960) 2009;127(9):1175–1182. doi: 10.1001/archophthalmol.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robles-Rivera RR, Castellanos-González JA, Olvera-Montaño C, Flores-Martin RA, López-Contreras AK, Arevalo-Simental DE, Cardona-Muñoz EG, Roman-Pintos LM, Rodríguez-Carrizalez AD. Adjuvant therapies in diabetic retinopathy as an early approach to delay its progression: the importance of oxidative stress and inflammation. Oxidative Med Cell Longev. 2020;2020:3096470. doi: 10.1155/2020/3096470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016;117(11):2443–2453. doi: 10.1002/jcb.25575. [DOI] [PubMed] [Google Scholar]
- 8.Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835. doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70(6):10.26402. doi: 10.26402/jpp.2019.6.01. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T, Hemmi S, Kajiwara M, Yabuki M, Fuke Y, Satomura A, Soma M. Complement-mediated chronic inflammation is associated with diabetic microvascular complication. Diabetes Metab Res Rev. 2013;29(3):220–226. doi: 10.1002/dmrr.2380. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Tan S, Chen S, Qin S, Chen H, Qin S, Huang Z, Zhou F, Qin X. Diagnostic value of hematological parameters platelet to lymphocyte ratio and hemoglobin to platelet ratio in patients with colon cancer. Clin Chim Acta. 2020;501:48–52. doi: 10.1016/j.cca.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, Gobunsuy R, Jadonath S, Baldari D, McCord D, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106(4):470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Nahrendorf M, Swirski FK. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: An expanded "cardiovascular continuum". J Am Coll Cardiol. 2016;67(9):1091–1103. doi: 10.1016/j.jacc.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):12. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sala A, Folco G. Neutrophils, endothelial cells, and cysteinyl leukotrienes: a new approach to neutrophil-dependent inflammation? Biochem Biophys Res Commun. 2001;283(5):1003–1006. doi: 10.1006/bbrc.2001.4865. [DOI] [PubMed] [Google Scholar]
- 16.Wang RT, Zhang JR, Li Y, Liu T, Yu KJ. Neutrophil-lymphocyte ratio is associated with arterial stiffness in diabetic retinopathy in type 2 diabetes. J Diabetes Complicat. 2015;29(2):245–249. doi: 10.1016/j.jdiacomp.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Weedle RC, Da Costa M, Veerasingam D, Soo AWS. The use of neutrophil lymphocyte ratio to predict complications post cardiac surgery. Ann Transl Med. 2019;7(23):778. doi: 10.21037/atm.2019.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azab B, Chainani V, Shah N, McGinn JT. Neutrophil-lymphocyte ratio as a predictor of major adverse cardiac events among diabetic population: a 4-year follow-up study. Angiology. 2013;64(6):456–465. doi: 10.1177/0003319712455216. [DOI] [PubMed] [Google Scholar]
- 19.Kahramanca S, Ozgehan G, Seker D, Gökce EI, Seker G, Tunç G, Küçükpınar T, Kargıcı H. Neutrophil-to-lymphocyte ratio as a predictor of acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2014;20(1):19–22. doi: 10.5505/tjtes.2014.20688. [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka M, Shimizu T, Kubota K. Neutrophil-to-lymphocyte ratio has a close association with gangrenous appendicitis in patients undergoing appendectomy. Int Surg. 2012;97(4):299–304. doi: 10.9738/CC161.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Liu X, Li Y, Quan J, Wei S, An S, Yang R, Liu J. The association of neutrophil to lymphocyte ratio, mean platelet volume, and platelet distribution width with diabetic retinopathy and nephropathy: a meta-analysis. Biosci Rep. 2018;38(3):BSR20180172. doi: 10.1042/BSR20180172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mertoglu C, Gunay M. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndrome. 2017;11(Suppl 1):S127–s131. doi: 10.1016/j.dsx.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Wang JR, Chen Z, Yang K, Yang HJ, Tao WY, Li YP, Jiang ZJ, Bai CF, Yin YC, Duan JM, et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol Metab Syndrome. 2020;12:55. doi: 10.1186/s13098-020-00562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, et al. National Health and Nutrition Examination Survey: sample design, 2007-2010. Vital Health Stat. 2013(160):1–23. [PubMed]
- 25.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the patient health questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22(11):1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg (London, England) 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Agoritsas T, Merglen A, Shah ND, O'Donnell M, Guyatt GH. Adjusted analyses in studies addressing therapy and harm: Users' guides to the medical literature. Jama. 2017;317(7):748–759. doi: 10.1001/jama.2016.20029. [DOI] [PubMed] [Google Scholar]
- 28.Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, Morgenstern LB, Wilterdink JL, Horwitz RI. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343(25):1826–1832. doi: 10.1056/NEJM200012213432501. [DOI] [PubMed] [Google Scholar]
- 29.Simonsen JR, Järvinen A, Hietala K, Harjutsalo V, Forsblom C, Groop PH, Lehto M. Bacterial infections as novel risk factors of severe diabetic retinopathy in individuals with type 1 diabetes. Br J Ophthalmol. 2021;105(8):1104–1110. doi: 10.1136/bjophthalmol-2020-316202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong PC, Lee KF, So WY, Ng MH, Chan WB, Lo MK, Chan NN, Chan JC. White blood cell count is associated with macro- and microvascular complications in chinese patients with type 2 diabetes. Diabetes Care. 2004;27(1):216–222. doi: 10.2337/diacare.27.1.216. [DOI] [PubMed] [Google Scholar]
- 31.Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19(4):942. doi: 10.3390/ijms19040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forrester JV, Kuffova L, Delibegovic M. The role of inflammation in diabetic retinopathy. Front Immunol. 2020;11:583687. doi: 10.3389/fimmu.2020.583687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange C, Storkebaum E, de Almodóvar CR, Dewerchin M, Carmeliet P. Vascular endothelial growth factor: a neurovascular target in neurological diseases. Nat Rev Neurol. 2016;12(8):439–454. doi: 10.1038/nrneurol.2016.88. [DOI] [PubMed] [Google Scholar]
- 35.Vidal AC, Howard LE, de Hoedt A, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, Amling CL, Taioli E, Fowke JH, et al. Neutrophil, lymphocyte and platelet counts, and risk of prostate cancer outcomes in white and black men: results from the SEARCH database. Cancer Causes Control. 2018;29(6):581–588. doi: 10.1007/s10552-018-1031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taneja R, Parodo J, Jia SH, Kapus A, Rotstein OD, Marshall JC. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med. 2004;32(7):1460–1469. doi: 10.1097/01.CCM.0000129975.26905.77. [DOI] [PubMed] [Google Scholar]
- 37.Kumarasamy C, Sabarimurugan S, Madurantakam RM, Lakhotiya K, Samiappan S, Baxi S, Nachimuthu R, Gothandam KM, Jayaraj R. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-a protocol for systematic review and meta-analysis. Medicine. 2019;98(24):e14834. doi: 10.1097/MD.0000000000014834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang J, Liu R, Yu X, Yang R, Xu H, Mao Z, Wang Y. The neutrophil-lymphocyte count ratio as a diagnostic marker for bacteraemia: a systematic review and meta-analysis. Am J Emerg Med. 2019;37(8):1482–1489. doi: 10.1016/j.ajem.2018.10.057. [DOI] [PubMed] [Google Scholar]
- 39.Luo WJ, Zhang WF. The relationship of blood cell-associated inflammatory indices and diabetic retinopathy: a Meta-analysis and systematic review. Int J Ophthalmol. 2019;12(2):312–323. doi: 10.18240/ijo.2019.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demirtas L, Degirmenci H, Akbas EM, Ozcicek A, Timuroglu A, Gurel A, Ozcicek F. Association of hematological indicies with diabetes, impaired glucose regulation and microvascular complications of diabetes. Int J Clin Exp Med. 2015;8(7):11420–11427. [PMC free article] [PubMed] [Google Scholar]
- 41.Yue S, Zhang J, Wu J, Teng W, Liu L, Chen L. Use of the monocyte-to-lymphocyte ratio to predict diabetic retinopathy. Int J Environ Res Public Health. 2015;12(8):10009–10019. doi: 10.3390/ijerph120810009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarı R, Karakurt Z, Ay M, Çelik ME, Yalaz Tekan Ü, Çiyiltepe F, Kargın F, Saltürk C, Yazıcıoğlu Moçin Ö, Güngör G, et al. Neutrophil to lymphocyte ratio as a predictor of treatment response and mortality in septic shock patients in the intensive care unit. Turkish J Med Sci. 2019;49(5):1336–1349. doi: 10.3906/sag-1901-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were downloaded from the NHANES website (http://www.cdc.gov/nchs/Nhanes).


