Abstract
Background:
High-intensity focused ultrasound (HIFU) is a noninvasive thermal ablation technique. High-intensity focused ultrasound has been used in small-animal models to lesion neural tissue selectively. This study aimed to evaluate the efficacy of HIFU in a large-animal model for ablation of nerves similar in size to human nerves.
Methods:
Twelve acute magnetic resonance–guided HIFU ablation lesions were created in intercostal nerves in a swine model. In a second pig, as a control, 4 radiofrequency ablation and 4 alcohol lesions were performed on intercostal nerves under ultrasound guidance. Preprocedural and postprocedural magnetic resonance imaging was then performed to evaluate radiologically the lesion size created by HIFU. Animals were euthanized 1 hour postprocedure, and necropsy was performed to collect tissue samples for histopathologic analysis.
Results:
On gross and histological examination of the intercostal nerve, acute HIFU nerve lesions showed evidence of well-demarcated, acute, focally extensive thermal necrosis. Four intercostal nerves ablated with HIFU were sent for histopathologic analysis, with 2 of 4 lesions showing pathologic damage to the intercostal nerve. Similar results were shown with radiofrequency ablation technique, whereas the intercostal nerves appeared histologically intact with alcohol ablation.
Conclusions:
High-intensity focused ultrasound may be used as a noninvasive neurolytic technique in swine. High-intensity focused ultrasound may have potential as a neuroablation technique for patients with chronic and cancer pain.
Neuroablative techniques allow physicians to disrupt neural pathways involved in the transmission of pain.1 Our current methods of ablation—cryotherapy, radiofrequency ablation (RFA), and chemical neurolysis—are commonly used for the improvement of pain in patients with chronic or cancer pain.1 Each method, however, requires percutaneous deployment of the neurolytic device or agent,2-4 making regional bleeding and infection a concern.
Newer techniques involving high-intensity focused ultrasound (HIFU) may prove to be a useful adjunct in the treatment of neuropathic pain and other neurologic disorders, especially in patients for whom percutaneous approaches are undesirable (eg, anticoagulated patients). Current HIFU technology uses concave probes to direct ultrasound waves to a specified focal distance from the probe, allowing for the ultrasound waves to heat tissue at a predetermined point. For 20 years, the use of HIFU for the treatment of tumors has been investigated.5 High-intensity focused ultrasound has been shown to treat prostate cancer, uterine fibroids, hepatic tumors, renal tumors, breast cancer, and pancreatic cancer.6-8
Although HIFU has the potential to be a neurolytic modality with a lower risk of complications, to our knowledge, its use to ablate peripheral nerves has been studied only in small-animal models. Recent work by Foley et al,9-11 for example, has demonstrated the efficacy of using HIFU for ablation, both temporarily and permanent, of peripheral nerves in a rat model. We aimed to investigate HIFU in a larger mammalian species to better approximate human nerves and surrounding anatomy. We believe that successful HIFU lesions produced in a swine model will contribute to an understanding of how HIFU lesions could be used on human tissue, potentially as a neurolytic modality. We describe the use of HIFU ablative techniques on an intercostal nerve in a swine model and discuss how our findings may be applied to human trials.
METHODS
The Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee approved the use of HIFU for the creation of peripheral nerve lesions. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. This study was approved for the use of 2 Yorkshire pigs weighing 30 to 40 kg (Archer Farms, Darlington, Maryland).
Protocol
The procedure was divided into 2 arms. Preliminary data regarding lesioning of the sciatic nerve yielded our initial settings for HIFU technique for thermal destruction of the intercostal nerve (described later). The first arm of the study was to optimize the settings for lesioning the intercostal nerve. This differs from the sciatic nerve protocol because of the smaller size and difficulty in visualizing the intercostal nerve with magnetic resonance imaging (MRI) relative to the sciatic nerve. Our sciatic nerve data showed a minimum acoustic power of 60 W (with a minimal temperature of 48°C under real-time MR thermal imaging), with 20 lesions (or sonications) being required for adequate thermal destruction of the swine sciatic.12 It was thought that a lower acoustic intensity and number of sonications would be sufficient to lesion an intercostal nerve. However, both lower and higher intensities were studied in the first arm to ensure that an appropriate intensity was chosen for the study’s second arm.
The second arm of the study was designed to test the feasibility of lesioning the intercostal nerve with the optimal settings obtained in the first arm of the study. The lesions were then compared with thermal lesioning by bipolar RFA (a known and accepted thermal technique) and chemical neurolysis. Because this was regarded as a feasibility data set, a sample size was not calculated. Our goal was to see if neural lesioning with these techniques was similar.
Swine Model
For the intercostal nerve lesions, the night before the procedure, the pigs were fasted for 12 hours and allowed water ad libitum. Twelve hours before the HIFU procedure, a 25-μg/h fentanyl patch was placed on each study pig. All pigs were sedated with tiletamine/zolazepam (4.4 mg/kg) and given glycopyrrolate (0.007 mg/kg) and an epidural injection of preservative-free morphine (Duramorph) 0.1 mg/kg. The animals were intubated and maintained on isoflurane 1.5% to 4% with 100% oxygen on respirators with an MRI-compatible pressure-cycled ventilator. To improve acoustic coupling and prevent the development of any skin burns, we removed all traces of hair on the back of each animal through shaving and the application of a chemical depilatory cream (Nair; Church & Dwight Company Inc, Ewing, New Jersey). Furthermore, when the coupling gel was applied, the skin was evaluated for bubbles (under direct visual inspection), and air was removed before MRI.
Each animal was then placed supine and feet first into the MRI machine (3.0 Tesla Excite HD, GE MRI system; General Electric Company, Fairfield, Connecticut), and a preprocedure MR image of the thorax was obtained. While under anesthesia 1 hour after the procedure, as per the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association, each pig was euthanized with an intravenous injection of pentobarbital (1 mL/4.5 kg, concentration of 390 mg/mL). Posteuthanasia, tissue samples were collected from each sciatic and intercostal nerve at necropsy, processed routinely for histology, and hematoxylin and eosin–stained sections were examined.
HIFU Ablation
High-intensity focused ultrasound ablation was performed using the InSightec MR–guided focused ultrasound system (ExAblate; InSightec, Haifa, Israel). Each sonication was 20 seconds in duration, creating a focal spot (in the shape of a cylinder) of 5 to7 mm in diameter and 20 mm in length (the smallest length possible with our device), with MR thermometric readings ranging from 50 to 78°C. Eight intercostal nerves were targeted on the inferior edge of the corresponding rib about 5 cm lateral from the spinous process. For Pig 1, each rib intercostal nerve was targeted with 20 sonications. Two intercostal nerves were lesioned at each of the following acoustic powers: 35, 60, 90, and 115 W. Because the surrounding anatomy of the intercostal nerve (rib and pleura) was different from the sciatic nerve anatomy, we selected acoustic powers above and below the 60 to 80 W required to lesion the sciatic nerve to determine an appropriate acoustic power to lesion the intercostal nerve.
After the results from the first arm of the study, we selected an acoustic power of 35 W and reduced the number of sonications, as it was noted that 20 sonications create large lesions that were encompassing the entire internal intercostal muscle in addition to the presumed location of the intercostal nerve (histopathology had not been seen at this time). The number of sonications was decreased to 6, controlled by MR evaluation of the ongoing lesion. Four intercostal nerves were targeted with these settings. Four intercostal nerves were also targeted with alcohol (EtOH) and thermal ablation (RFA) as discussed below.
Alcohol and Radiofrequency Ablation
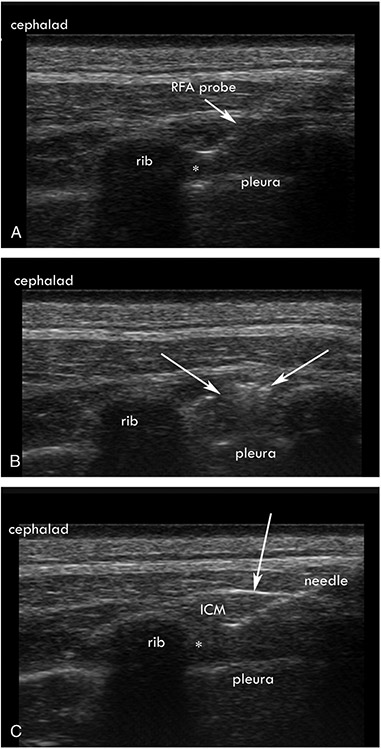
Four of the left intercostal nerves were ablated under ultrasound guidance with chemical neurolysis (EtOH). Under ultrasound guidance, a 25-gauge, 1.5-inch needle was introduced to the inferior border of the rib, 2 inches lateral to the spinous process (Fig. 1). After negative aspiration, 1 mL of 1% lidocaine was given to confirm spread in the internal intercostal muscle. After 2 minutes, 2 mL of 90% alcohol was injected under visual guidance. The needle was then removed.
FIGURE 1.
A, Ultrasound-guided radiofrequency ablation (RFA) showing placement of the tip (*) at the inferior border of the corresponding rib within the internal intercostal muscle (ICM) and adjacent to innermost intercostal muscle. B, Ultrasound image of the muscle after RFA, with the arrows showing hyperechoic changes in the ICM. C, Ultrasound-guided alcohol neurolysis, with a 25-gauge needle (*) showing the location of the needle tip and injection of local anesthetic. Arrows point to the tenting of the ICM, indicating spread of the alcohol into the ICM and innermost intercostal muscle.
Four intercostal nerves were also targeted with a bipolar radiofrequency probe. Using ultrasound guidance, after 1 mL of 1% lidocaine was given for local anesthesia, the probe was guided to the inferior border of the corresponding rib 2 inches lateral to the spinous process (Fig. 1). The probe was placed in the internal intercostal musculature under ultrasound guidance, and once confirmed, a thermal lesion of 80°C was performed for 60 seconds, and the needle was removed.
Statistics
For comparison of the 3 neurolytic techniques, a 2 × 3 contingency table was produced. The Freeman-Halton extension of the Fisher exact test was used to compute a 2-tailed probability. The data are presented in Table 1.
TABLE 1.
Each ICN Was Lesioned by HIFU, RFA, or EtOH
| ICN | Neurolytic Technique |
Pathology Results |
Pathology Nerve Lesioned |
|---|---|---|---|
| Right ICN 1 | HIFU | Muscle necrosis, nerve intact | No |
| Right ICN 2 | HIFU | Muscle necrosis, nerve intact | No |
| Right ICN 3 | HIFU | Muscle and nerve necrosis with hyperemia and stromal hyalinization | Yes |
| Right ICN 4 | HIFU | Muscle and nerve necrosis with hyperemia | Yes |
| Left ICN 1 | RFA | Muscle necrosis and nerve stromal hyalinization | Yes |
| Left ICN 2 | RFA | Muscle necrosis without nerve damage | No |
| Left ICN 3 | RFA | Muscle and nerve necrosis with hyperemia and stromal hyalinization | Yes |
| Left ICN 4 | RFA | Muscle necrosis without nerve damage | No |
| Left ICN 5 | EtOH | Muscle necrosis without nerve damage | No |
| Left ICN 6 | EtOH | Muscle necrosis without nerve damage | No |
| Left ICN 7 | EtOH | Muscle necrosis with no nerve present | No |
| Left ICN 8 | EtOH | Muscle necrosis without nerve damage | No |
The pathology results are described for each lesion. Note that the pig was euthanized 1 hour postprocedure. No significant difference (P = 0.41) was seen between each neurolytic technique, comparing nerve damage on a 3 × 2 contingency table.
ICN indicates intercostal nerve.
RESULTS
Twelve intercostal HIFU, 4 intercostal RFA, and 4 intercostal alcohol neurolytic procedures were performed.
Eight intercostal nerves were ablated in the first arm of the study. As greater acoustic power (and resulting increase in temperature) was used, an increased amount of surrounding musculature and vasculature was damaged, without a distinct difference in neural thermal damage, as shown in our histopathology (Figs. 2, 3). To reduce the likelihood of damage of the surrounding tissue, we selected an acoustic power of 35 W (low compared with our protocol for larger nerves, 60 W for sciatic nerve lesions).
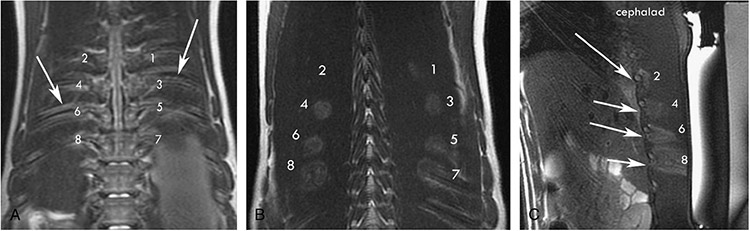
FIGURE 2.
A, Eight rib lesions were performed with T1W MR guidance. Each lesion was performed with the following acoustic power (1–2 at 35 W, 3–4 at 65 W, 5–6 at 90 W, and 7–8 at 115 W). The arrows point to corresponding ribs. B, A greater area of thermal changes can be seen surrounding the rib in T1W MR coronal images as the acoustic power is increased. C, A sagittal section of the HIFU lesion showing extension of the thermal injury into greater parts of the muscle as the acoustic power increases. The arrows point to the inferior border of the rib that shows thermal change in the area of the intercostal nerve.
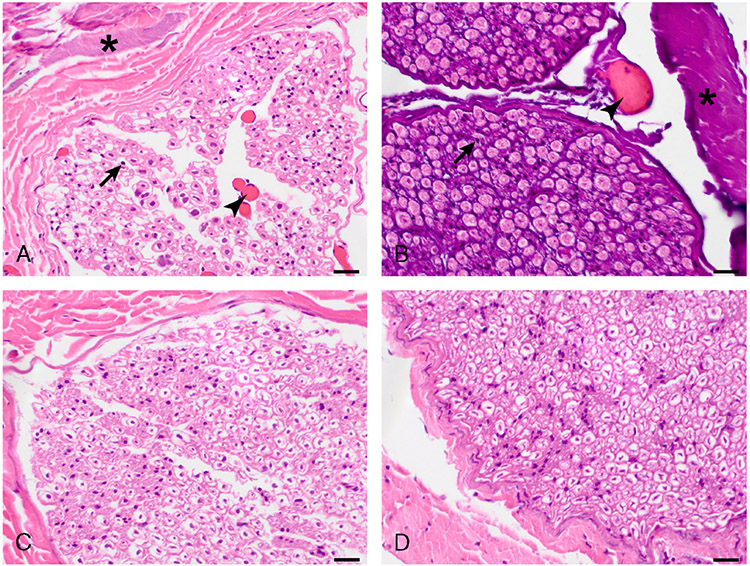
FIGURE 3.
Histopathologic findings in ablated intercostal nerve (ICN). Right ICN 3 ablated with HIFU (A) and left ICN 3 ablated with RFA (B) display nuclear pyknosis (arrows), vascular hyperemia (arrowheads), and stromal hyalinization (asterisks, more intense in B). Left ICN 6 ablated with EtOH (C) does not display any histological change. Untreated control ICN (D) is histologically normal. Hematoxylin and eosin stain. Original magnification: 600×. Scale bar, 20 μm.
We focused our lesions at the inferior border of each rib in the second arm of the study (Fig. 4). Four intercostal nerves were lesioned with the HIFU protocol established from the first arm of the study. Furthermore, 4 intercostal nerves were lesioned with RFA technique and 4 intercostal nerves were lesioned with alcohol neurolysis. Both the HIFU and RFA technique showed histologic evidence of neural damage in 50% of the lesions, whereas no neural damage was seen in the EtOH lesions (Fig. 3). One EtOH lesion site did not include an intercostal nerve. No significant differences in pathology results were detected between each neurolytic technique (P = 0.41). The results are summarized in Table 1.
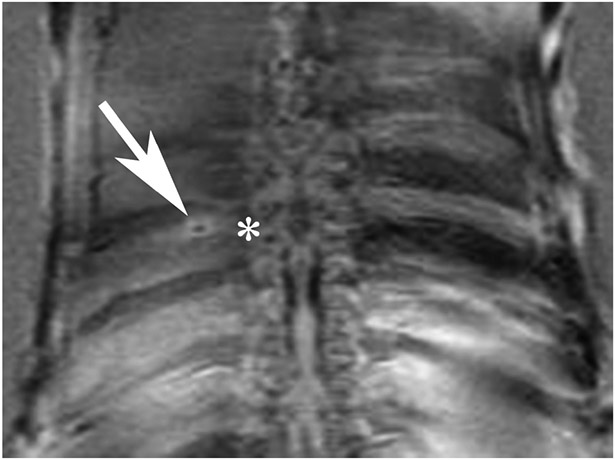
FIGURE 4.
After optimal settings were obtained, the goal lesions were inferior to the rib and discrete around the intercostal nerve, as shown in T2W MR coronal images. The arrow points at the discrete lesion at the inferior border of the rib. The * marks the corresponding rib and vertebral level.
DISCUSSION
Although the ablation of nerve tissue using HIFU has been performed successfully in small-animal studies (rat and rabbit), no studies to date have attempted to use HIFU in the ablation of nerves in a large-animal model. In an earlier study, we lesioned the sciatic nerve in a porcine model to illustrate that neural tissue could be lesioned without significant surrounding soft tissue damage.12 However, in targeting smaller, primarily sensory, nerves, we achieved mixed results, with 50% of the intercostal nerves being successfully lesioned and showing histologic changes.
The concept of using HIFU as a therapeutic tool was developed in the 1940s as a possible method for creating damage in the brain for neurological research, with later research suggesting HIFU damage primarily resulted from thermal lesions.13-15 We aimed to create discrete lesions along a nerve while reducing surrounding tissue damage. Although our lowest acoustic power setting of 35 W was sufficient to produce a neurolytic lesion with minimal damage to surrounding tissue, we were unable to consistently lesion the intercostal nerve. Each HIFU sonication is a cylinder 2 cm in length and 0.5 cm in diameter. This sonication size is small enough to target the intercostal nerve with 1 or 2 sonications; however, the intercostal nerve is not visualized regularly in either MR or ultrasound guidance, making accurate lesioning more difficult. We chose to perform more sonications in the area where the intercostal nerve is likely to be. Future animal studies must improve targeting of the HIFU lesion on the intercostal nerve before human trials can be considered.
It is noteworthy that neither the RFA nor EtOH neurolytic technique resulted in consistent thermal or neural damage immediately postprocedure. Whereas this was expected with alcohol neurolysis, which depends on Wallerian degeneration as its primary mechanism for neurolysis, we believed that RFA would lead to consistent neurolysis.16 It is possible that thermal lesions may evolve over time. Furthermore, anatomy may prevent thermal lesions from reliably creating neurolysis of the intercostal nerve. Precise probe placement between the internal intercostal muscle and innermost intercostal muscle may improve results.17 This may require ultrasound guidance over MR guidance because the muscles are easier to delineate in the ultrasound image in real time.
The ability to ablate intercostal nerves using HIFU highlights an advantage of this therapeutic modality over interventional nerve blocks. High-intensity focused ultrasound nerve lesions were not associated with the development of uncontrolled bleeding or a hematoma when reviewing the histopathologic slides. Thus, HIFU has the potential to lesion peripheral nerve structures thermally without puncture of the skin. The pigs did have transient skin changes at the point of contact of the introducer (postprocedure erythema of the skin was observed at 2 of 8 HIFU lesion sites on 1 pig), suggesting that care to eliminate air pockets at the skin contact point is paramount.
This study shows that small peripheral nerves can be neurolyzed with a thermal lesion using HIFU without causing skin puncture. The technology has the potential to create discrete lesions of a nerve with minimal damage to surrounding tissue. We saw minimal postprocedure complications (superficial skin lesions from poor HIFU contact with the skin), although it is possible that errors in targeting may cause thermal damage in unintended areas. Although we did not see obvious damage to the intercostal vein and artery on histopathology, damage is possible to these structures. However, similar damage could be expected with RFA and needling before EtOH ablation.
One potential use of HIFU is the possibility of partial conduction blockade. Early studies have shown that HIFU can selectively lesion C-fibers while leaving A-fibers relatively unaffected in small animal models.18,19 Lele20 selected temperatures between 41 and 45°C to lesion sciatic nerves, showing disruption of C-fibers. In this study, the average ablative temperature ranged from 55 to 78°C, which allows for complete thermal destruction of the intercostal nerve. At lower acoustic intensities, reversal of conduction blockade may be possible, with recovering neural tissue at different time points.8
This study has several limitations. Because it was not a survival study, we could not determine the long-term effects of HIFU on neuronal function. Analyzing nerve structure and function at 14 and 28 days after HIFU ablation would help determine temperatures and, more importantly, acoustic power used to selectively lesion C-fiber nerves and, perhaps, temporary neuronal disruption. Whereas both pathologic and histologic studies show neuronal disruption, further research incorporating electromyography, or evoked potentials, and nerve conduction velocities would help elucidate nerve function after HIFU ablation. Also, because animals were sacrificed within a few hours of alcohol injection, thermal- and alcohol-induced neurolytic damage might not be apparent in the pathology slides. It is possible that, in all lesions, damage to the neural tissue could continue to evolve for several hours to days after the time of ablation, resulting from ongoing apoptosis or tissue damage induced by inflammation. We suggest that the pathology to review these lesions be taken 24 hours to a few days after the lesion.
Further studies could compare all 3 neurolytic techniques with increased precision and accuracy to determine the most effective method for neurolysis. We suggest that the pathologic specimen to review these lesions be taken a few days after the lesion. Further work analyzing the long-term effects of MR-guided HIFU is needed. In particular, studies showing the ability of HIFU to selectively lesion C-fibers are necessary to help validate the utility of HIFU in chronic pain patients.
CONCLUSIONS
High-intensity focused ultrasound can be used as a noninvasive neurolytic technique in a swine model. Further research in HIFU may lead to a new therapeutic tool for noninvasive creation of peripheral nerve lesions in real time.
Acknowledgments
This study was funded by the Departments of Radiology and Anesthesiology and Critical Care Medicine at Memorial Sloan-Kettering Cancer Center.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Gulati A, Joshi J, Baqai A. An overview of treatment strategies for cancer pain with a focus on interventional strategies and techniques. Pain Management. 2012;2:569–580. [DOI] [PubMed] [Google Scholar]
- 2.Rauchwerger JJ, Zoarski GH, Waghmarae R, et al. Epidural abscess due to spinal cord stimulator trial. Pain Pract. 2008;8:324–328. [DOI] [PubMed] [Google Scholar]
- 3.Smith CC, Lin JL, Shokat M, Dosanjh SS, Castheyl D. A report of paraparesis following spinal cord stimulator trial, implantation and revision. Pain Physician. 2010;13:357–363. [PubMed] [Google Scholar]
- 4.Narouze SN, Yonana S, Kapural L, Malak O. Erosion of the inferior epigastric artery: a rare complication of intrathecal drug delivery. Pain Med. 2007;8:468–470. [DOI] [PubMed] [Google Scholar]
- 5.Kopelman D, Papa M. Magnetic resonance-guided focused ultrasound surgery for the noninvasive curative ablation of tumors and palliative treatments: a review. Ann Surg Oncol. 2007;14:1540–1550. [DOI] [PubMed] [Google Scholar]
- 6.Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high-intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009;10:123–129. [PubMed] [Google Scholar]
- 7.Jang HY, Lee JY, Lee DH, Kim WH, Hwang JH. Current and future clinical applications of high-intensity focused for pancreatic cancer. Gut Liver. 2010;4 suppl 1:S57–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou YF. High-intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol. 2011;2:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foley JL, Little JW, Starr FL 3rd, Frantz C, Vaezy S. Image-guided HIFU neurolysis of peripheral nerves to treat spasticity and pain. Ultrasound Med Biol. 2004;30:1199–1207. [DOI] [PubMed] [Google Scholar]
- 10.Foley JL, Little JW, Vaezy S. Image-guided high-intensity focused ultrasound for conduction block of peripheral nerves. Ann Biomed Eng. 2007;35:109–119. [DOI] [PubMed] [Google Scholar]
- 11.Foley JL, Little JW, Vaezy S. Effects of high-intensity focused ultrasound on nerve conduction. Muscle Nerve. 2008;37:241–250. [DOI] [PubMed] [Google Scholar]
- 12.Loh J, Gulati A, Gutta N, et al. The use of high-intensity focused ultrasound (HIFU) to create controlled thermal ablative lesions of the sciatic nerve in a pig model. Poster and abstract presented at the American Society of Regional Anesthesia and Pain Medicine 10th Annual Meeting and Workshops, New Orleans, LA, November 17–20, 2011. [Google Scholar]
- 13.ter Haar G Ultrasound focal beam surgery. Ultrasound Med Biol. 1995;21:1089–1100. [DOI] [PubMed] [Google Scholar]
- 14.Frizell LA, Linke CA, Carstensen EL, Fridd CS. Thresholds for focal ultrasonic lesions in rabbit kidney, liver, and testicle. IEEE Trans Biomed Eng. 1977;24:393–396. [DOI] [PubMed] [Google Scholar]
- 15.Hill CR, Rivens I, Vaughan MG, ter Haar GR. Lesion development in focused ultrasound surgery: a general model. Ultrasound Med Biol. 1994;20:259–269. [DOI] [PubMed] [Google Scholar]
- 16.Molloy RE, Benzon HT. Neurolytic blocking agents: uses and complications. In: Benzon HT, Rathmell JP, Wu CL, Turk DC, Argoff CE, eds. Raj’s Practical Management of Pain. 4th ed. St. Louis, MO: Mosby Inc.; 2008. [Google Scholar]
- 17.Davies F, Gladstone RJ, Stibbe EP. The anatomy of the intercostal nerve. J Anat 1932;66(pt 3):323–333. [PMC free article] [PubMed] [Google Scholar]
- 18.Young RR, Henneman E. Reversible block of nerve conduction by ultrasound. Arch Neurol. 1961;4:83–89. [DOI] [PubMed] [Google Scholar]
- 19.Young RR, Henneman E. Functional effects of focused ultrasound on mammalian nerves. Science. 1961;134:1521–1522. [DOI] [PubMed] [Google Scholar]
- 20.Lele PP. Effects of focused ultrasonic radiation on peripheral nerve, with observations on local heating. Exp Neurol. 1963;8:47–83. [Google Scholar]