Abstract
Purpose:
To assess the safety and efficacy of image-guided percutaneous cecostomy/colostomy (PC) in the management of colonic obstruction in patients with cancer.
Materials and Methods:
Twenty-seven consecutive patients underwent image-guided PC to relieve large bowel obstruction at a single institution between 2000 and 2012. Colonic obstruction was the common indication. Patient demographics, diagnosis, procedural details, and outcomes including maximum colonic distension (MCD; ie, greatest transverse measurement of the colon on radiograph or scout computed tomography image) were recorded and retrospectively analyzed.
Results:
Following PC, no patient experienced colonic perforation; pain was relieved in 24 of 27 patients (89%). Catheters with tip position in luminal gas rather than mixed stool/gas or stool were associated with greater decrease in MCD (−40%, −12%, and −16%, respectively), with the difference reaching statistical significance (P = .002 and P = .013, respectively). Catheter size was not associated with change in MCD (P = .978). Catheters were successfully removed from six of nine patients (67%) with functional obstructions and two of 18 patients (11%) with mechanical obstructions. One patient underwent endoscopic stent placement after catheter removal. Three patients required diverting colostomy after PC, and their catheters were removed at the time of surgery. One major complication (3.7%; subcutaneous emphysema, pneumomediastinum, and sepsis) occurred 8 days after PC and was successfully treated with cecostomy exchange, soft-tissue drainage, and intravenous antibiotic therapy.
Conclusions:
Image-guided PC is safe and effective for management of functional and mechanical bowel obstruction in patients with cancer. For optimal efficacy, catheters should terminate within luminal gas.
Colonic obstruction occurs in as many as 24% of patients with advanced colorectal malignancies and 42% of patients with ovarian malignancies, with 30-day operative mortality rates in patients with end-stage cancer and bowel obstruction reported as high as 40% (1). Management of colonic obstruction requires consideration of the potential benefits and risks of available invasive treatments, including the patient’s life expectancy in addition to personal choices regarding the acceptability of an exteriorized catheter. The ideal treatment should provide durable palliation with minimal morbidity (2). Currently, treatment strategies are varied and include endoscopic, surgical, and interventional radiologic therapies (1–4). Percutaneous cecostomy/colostomy (PC) has been used as an alternative to open or laparoscopic surgical cecostomy for management of adult bowel obstruction (5–7) and pediatric constipation/fecal incontinence (8,9). Established indications of PC include acute colonic pseudoobstruction (ACPO), distal colonic obstruction, cecal volvulus, impending perforation, and preanastomotic decompression (10), the clinical rationale being decompression of the colon to prevent colonic perforation. Although the use of PC has demonstrated high rates of technical success, it is underused, and few studies report outcomes in terms of underlying disease (11). In addition, there are few relevant sources suggesting possible palliative benefits such as pain control. The purpose of the present study was to evaluate the safety and efficacy of PC in the treatment of colonic obstruction in patients with cancer.
MATERIALS AND METHODS
Subjects
With approval of our institutional review board, interventional radiology procedure reports between June 1, 2000, and August 26, 2012, were retrospectively queried for the keyword “cecostomy,” and 28 PC procedures were identified. Inclusion criteria consisted of any patient with known malignant diagnosis and large bowel obstruction without any clinical or radiographic signs of perforation. Patients with mechanical obstruction and functional obstruction (ie, ACPO, n = 3) were included. One patient with known cecal perforation was excluded from review, leaving 27 patients without perforation as the study group. Of these, 14 men and 13 women aged 42–83 years (median, 66 y) were included, who had the following primary malignancies: colorectal (n = 7), ovarian (n = 5), prostate (n = 3), leukemia (n = 2), pancreatic (n = 2), gastric (n = 2), hepatocellular (n = 2), cervical (n = 1), endometrial (n = 1), laryngeal (n = 1), and melanoma (n = 1). Patient demographics, clinical symptoms, etiology of colonic obstruction, consultation history, and procedural details were retrospectively reviewed in the medical record. Clinical outcomes, including pain control, toleration of diet, and whether the patient had any bowel movement(s), were also retrospectively reviewed through the medical record. Pain control was generally assessed subjectively by using the numeric rating scale for pain immediately after the procedure and at each follow-up clinic visit.
Clinical Presentation
In 18 cases (67%), colonic obstruction was mechanical, and nine others (33%) had functional obstructions (ie, ACPO). Of the 18 mechanical obstructions, 14 were caused by extraluminal masses, whereas four had intraluminal masses. Pharmacologic therapy had previously failed in three patients (eg, neostigmine, erythromycin, and/or lactulose administration). Twenty-four of 27 patients (89%) had earlier surgical consultations, and all were considered poor candidates for operative treatment in view of the extent of disease and/or operative risk. No patient in the study group underwent surgical treatment for colonic obstruction before PC. Twenty-six of 27 patients (96%) had preceding gastroenterology consultation, and, as a result, six patients underwent lower endoscopy before PC placement. Three of these patients had obstructions that were not amenable to endoscopic stent placement; three others received endoscopic colonic stents without subsequent symptomatic improvement. Other patients were deemed inappropriate for endoscopy for reasons including high risk of perforation (8), ACPO (7), and multifocal or distal (ie, near the rectosigmoid) obstructions (4). One patient refused endoscopy. The study design is shown in Figure 1.
Figure 1.
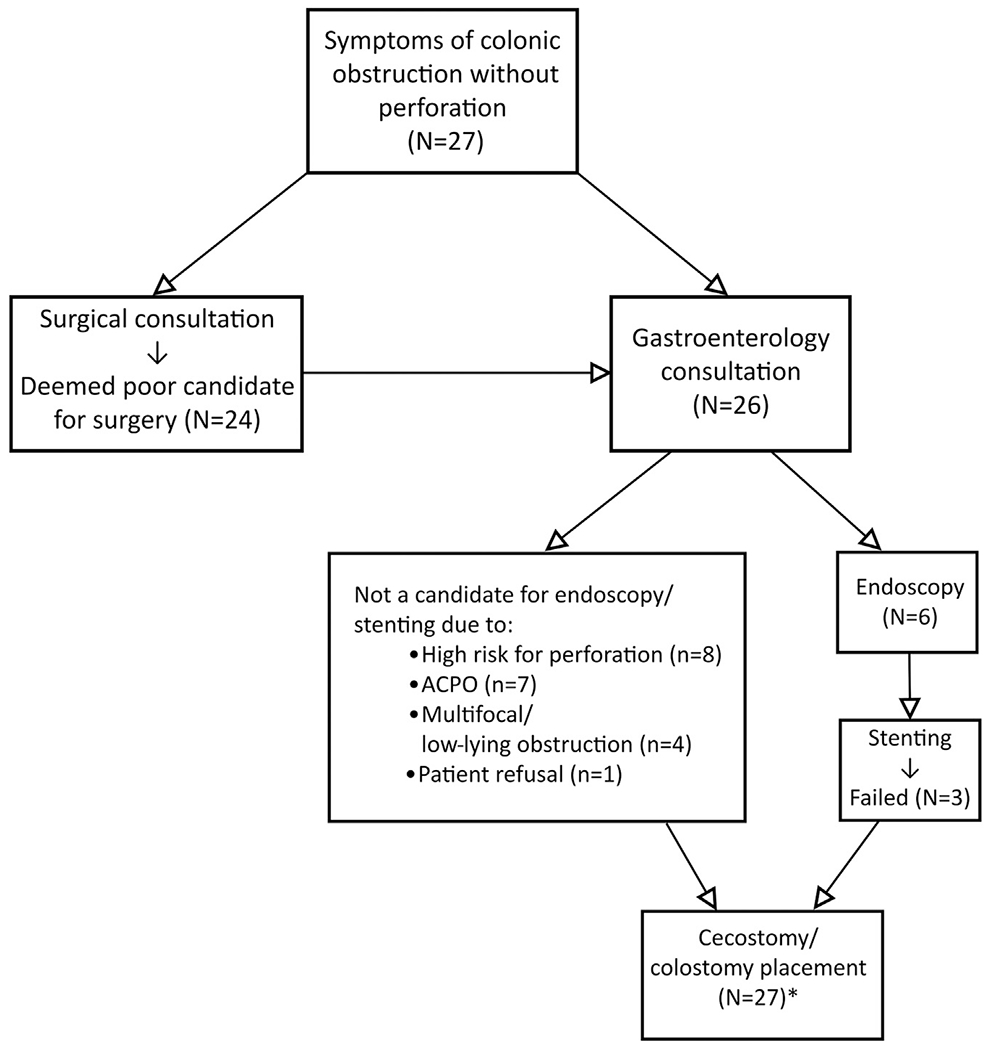
Schematic diagram of study design. *The total number includes the 26 patients who underwent prior surgical or gastroenterology consultation, or both, and one patient who had a do-not-resuscitate status but was found to have a large bowel obstruction on CT scan and underwent palliative cecostomy the same day.
Procedure
Procedural data (Table), including type of anesthetic agent, mode of imaging guidance, catheter size, and T-fastener use, were retrospectively recorded from the procedure dictation in the medical record.
Table.
Procedural Details, Changes in MCD, and Long-Term Follow-up after Percutaneous Cecostomy
| Parameter | No. of Pts. | Change in MCD |
|---|---|---|
| Image guidance | ||
| Fluoroscopy | 11 | – |
| CT | 8 | – |
| CT and fluoroscopy | 6 | – |
| US and fluoroscopy | 2 | – |
| Catheter size | ||
| 10 F | 7 | – |
| 12 F | 12 | – |
| 14 F | 3 | – |
| 16 F | 4 | – |
| 20 F | 1 | – |
| < 13 F | 19 | −23.9% (−62.4% to 10.8%) |
| > 13 F | 8 | −24.2% (−40.9% to 3.9%) |
| Catheter tip position | ||
| Air | 11 | −39.7% (−62.4% to −16.7%) |
| Mixed | 8 | −12.0% (−40.4% to 9.0%) |
| Stool | 8 | −16.1% (−34.3% to 10.8%) |
| Long term follow-up | ||
| Died with catheter in situ | 16 | – |
| Catheter removed | ||
| During secondary procedure | 3 | – |
| After symptom resolution* | 8 | – |
Values presented as mean (range) where appropriate.
MCD = maximum colonic distension; Pts = patients.
Two of 18 patients with mechanical obstruction (11%) and six of nine patients with functional obstruction (67%) had the catheter removed after resolution of symptoms.
Immediate preprocedural care always included sterile skin preparation and administration of moderate sedation or monitored anesthesia care. Antibiotic prophylaxis was routinely performed before the procedure with the use of second-generation cephalosporins administered intravenously. PC was performed by using image-guided localization of the dilated cecum (or, rarely, of another more accessible dilated colonic loop). Access to the colonic lumen was accomplished by image-guided percutaneous puncture. The procedure was performed with or without anchors at operator discretion. One to three anchors were deployed in the colonic lumen and used to pull the anterior wall of the colon to the anterior abdominal wall. An 18-gauge single wall puncture needle was then used to puncture the colon between the previously placed anchors. The needle was exchanged over a guide wire for the operator’s catheter of choice. In cases in which no anchors were used, a puncture into the colon was performed with an 18-gauge single wall puncture needle. The needle was then exchanged over a guide wire for a standard all-purpose locking-loop drainage catheter (Cook, Bloomington, Indiana). Serial dilation was not performed in any cases. When the catheters were in place, contrast agent injection or limited computed tomography (CT) was performed to document position. Contrast agent injections were performed to document final catheter position. Technical success was defined by intraluminal placement of the catheter tip and was accomplished in all 27 cases. To facilitate evacuation of gas from the closed drainage system, several venting pin holes were made in the nondependent portion of the drainage bag to which the catheter was attached.
The catheter was secured to the skin by using a catheter fixation device (Tru-Fix; UreSil, Skokie, Illinois). Postprocedural care involved daily cleaning of the cecostomy puncture site. Catheters were maintained with forward flushing with 10 mL of normal saline solution twice per day and/or as needed.
Image Analysis and Statistics
Images were reviewed to determine the position of the catheter with regard to luminal contents at the catheter termination site. Maximum colonic distension (MCD) was defined as the greatest transverse colonic diameter measured on an abdominal radiograph or CT scout image before and after PC placement. Images were first interpreted by a radiology resident physician and then an attending interventional radiologist. A couple of minor discrepancies with regard to measurements were reviewed again in tandem, and consensus was achieved. Differences among groups in MCD change after treatment were evaluated for significance by t test or χ2 test as appropriate with the use of Stata software (version 11.0; StataCorp, College Station, Texas). A P value of less than .05 was considered statistically significant.
RESULTS
Procedural Data
Nearly all PCs were performed with moderate sedation (26 of 27) with a combination of intravenous midazolam, fentanyl, and/or meperidine, while using a variety of forms of image guidance per operator preference (Table). One patient’s PC was performed under general anesthesia with endotracheal intubation to facilitate emergent secondary intervention in the event of failed PC. Catheters ranging in size from 10 to 20 F (median, 12 F) were selected per operator preference. T-fasteners were used in 21 of 27 cases (78%; range, one to three fasteners per patient; median of two). Most PCs were placed into the cecum (23 of 27); in four cases, the hepatic flexure (n = 2) or transverse colon (n = 2) was intentionally selected for access, based on operator determination of the safest loop available for drainage given the patient’s pattern of obstruction, respectively.
Outcomes
After PC, no patient showed clinical or radiographic evidence of cecal perforation, the feared complication that had precipitated all procedures. Clinical evidence of pain relief was subjectively documented in the medical records of 24 of 27 patients (89%). Eight patients (35%) had successful catheter removal after a median of 29 days (range, 7–51 d), including one patient whose symptoms resolved after PC and who underwent endoscopic management of complete obstruction at the site of a previous colostomy takedown on day 29 day after PC. Catheters could more often be removed from patients with functional (six of nine) rather than mechanical obstructions (two of 18); this difference was statistically significant (P = .006). Three patients subsequently required colostomy (1, 3, and 4 d after PC) for reasons not attributable to technical failure. Reasons for diversion included oxygen desaturation believed to be caused by abdominal distension and hypoventilation; increasing pain, and persistent obstruction. In some patients, predominantly in those with the catheter tip placed in stool, catheter obstruction occurred. In those patients in whom obstruction resolved, a capping trial of 24–48 hours was performed, followed by bedside removal of the catheter.
Twenty-four of 27 patients had medical record follow-up to the date of death, which was an average of 123.6 days after the procedure. Of the three remaining patients, two had resolution of obstruction and catheter removal 34 and 36 days after the procedure, respectively, before being lost to follow-up. The remaining patient was lost to follow-up on postprocedure day 8 with the PC still in situ.
Complications
Complications were categorized according to Society of Interventional Radiology Standards of Practice Committee classifications of complications by outcome (12). There was one major and two minor complications. Eight days after PC, one patient presented with sepsis and extensive subcutaneous emphysema extending cranially from the PC tube site (Fig 2). The cecostomy catheter was exchanged over a wire, all T-fasteners were released, and a separate drainage catheter was placed within the abdominal wall. The etiology of infection was likely secondary to catheter obstruction, with leaking of stool and air around the site. The patient subsequently showed a full recovery after 6 weeks following catheter revision and died from progression of disease 112 days after the PC procedure. The two minor complications were as follows: one patient had two episodes of catheter dislodgment treated with catheter replacement through an existing tract, and one patient had pericatheter stool leakage, which ceased after upsizing the catheter from 12 F to 20 F.
Figure 2.
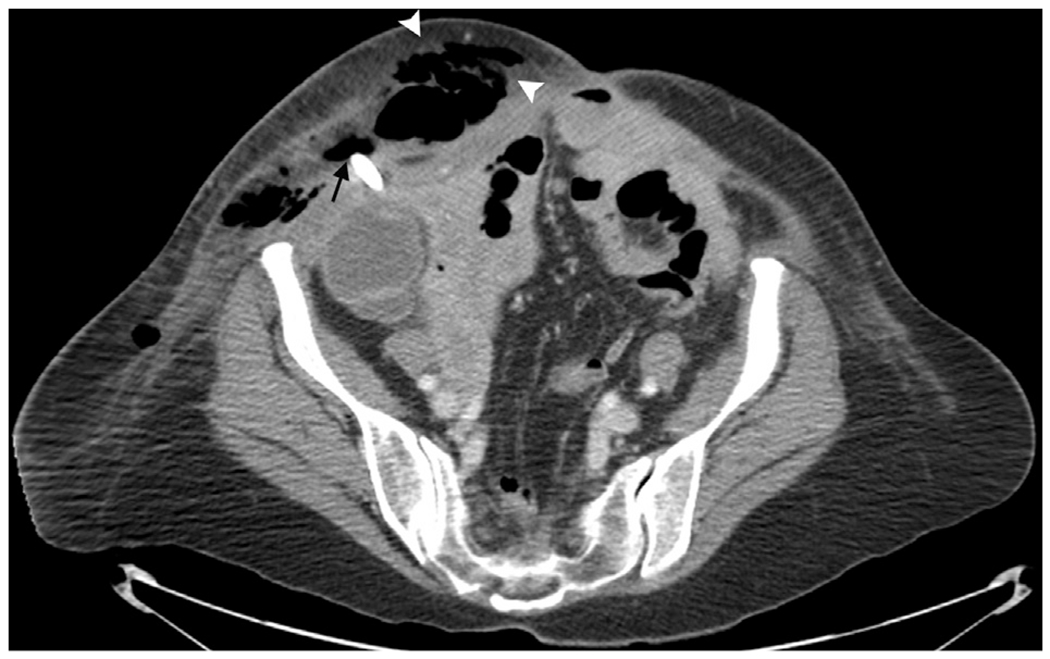
Postoperative day 8 axial CT slices of the single major complication. The patient presented with massive subcutaneous emphysema (arrowheads). Note the air adjacent to the cecostomy tract (arrow).
Radiographic Assessment
Average preprocedure MCD was 10.7 cm (range, 8.1–13.5 cm) and average postprocedure MCD was 8.0 cm (range, 4.4–13.3 cm), for a mean difference of 2.71 cm (P < .0001). On average, MCD was recorded from an abdominal radiograph or CT scout image 0.89 days before PC placement (range, 0–4 d; median, 1 d) and 2.25 days after PC placement (range, 0–12 d; median, 1 d). Most imaging assessments were performed on an abdominal radiograph rather than a CT scout image (70% and 83% before and after the procedure, respectively). Mean overall change in MCD was −24% (range, −62% to +11%; Fig 3). Change in MCD was not significantly different between patients with functional obstruction (−26%) and mechanical obstruction (−23%; P = .75).
Figure 3.
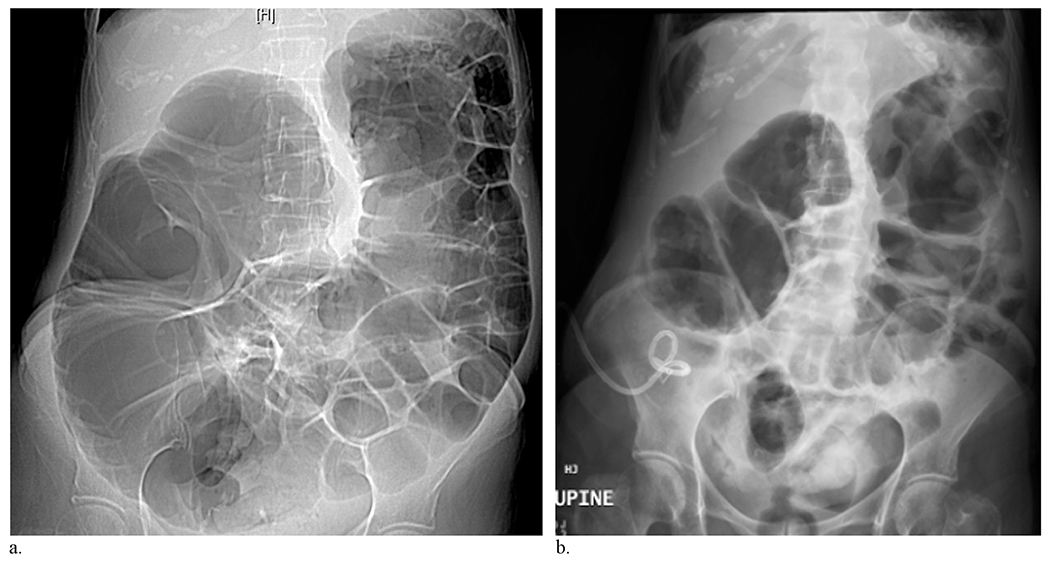
Preoperative day 1 CT scout image (a) and postoperative day 1 anteroposterior abdominal radiograph (b) of a 74-year-old female patient with mechanical obstruction secondary to peritoneal carcinomatosis. This patient showed a radiographic response to cecostomy catheter placement with change in MCD of −41%. Of note, the cecostomy catheter was placed with the tip positioned in the air.
In 11 patients, initial catheter tip position was within luminal gas. In eight, the catheter was placed in mixed stool and gas. In the remaining eight, the final catheter position appeared to be within stool (Fig 4). Catheter position in fluoroscopically performed cases was generally assessed without difficulty; however, a single case required correlation with same-day preoperative CT, which demonstrated a pan–stool-filled colon. Change in MCD stratified by catheter tip position is shown in the Table. Overall, the greatest change in MCD occurred among patients whose catheter tip terminated within luminal gas. Two of three patients who underwent diverting colostomy, the single patient who had a major complication, and one of two patients who had minor complications had PC catheters that terminated in stool.
Figure 4.
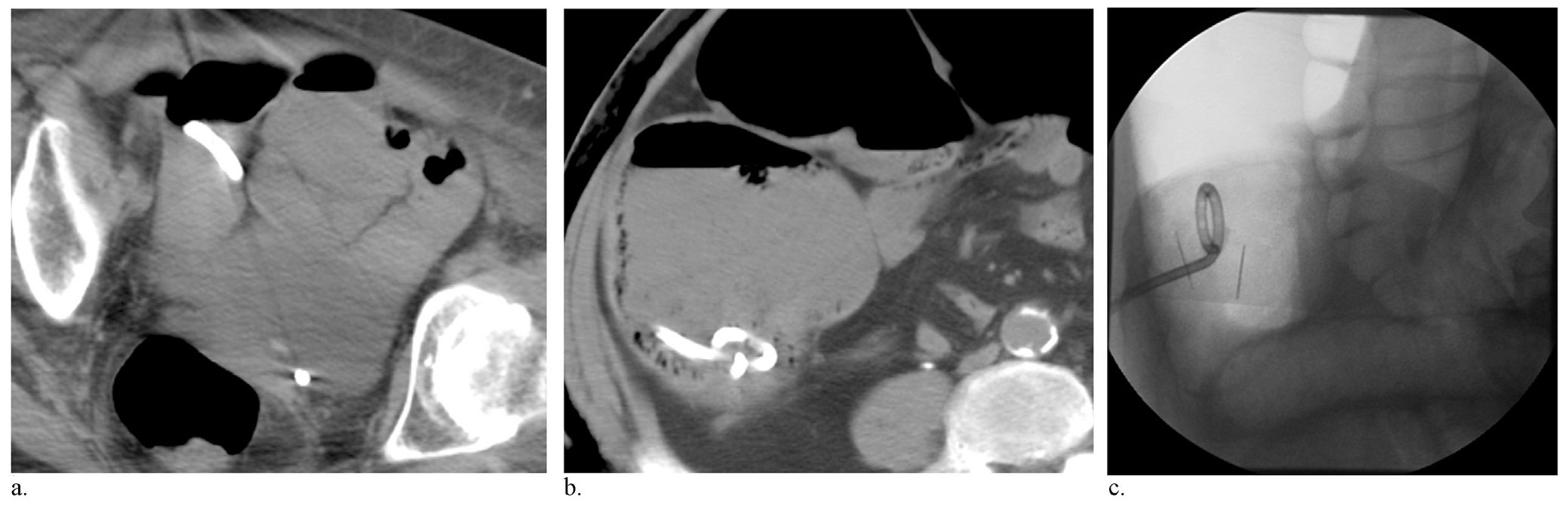
Images demonstrating different catheter tip positions. Intraoperative axial CT images (a, b) demonstrating cecostomy catheter tip in a “mixed” position along an air/fluid level (a) and with the catheter tip in stool (b). Intraoperative fluoroscopic image (c) demonstrates the catheter tip position in air.
Decompression of the colon that manifested as change in MCD was not associated with size of the drainage catheter. PCs with tubes smaller than 13 F had a mean change in MCD of −24% (range, −62% to +11%), whereas PCs performed with tubes larger than 13 F had a mean change in MCD of −24% (range, −40% to 4%; P = .98).
Long-Term Follow-up
Of the 27 patients in the present retrospective study, 16 died with their PC catheter in place, eight underwent catheter removal after relief of symptoms, and three had the catheter removed during a secondary procedure (ie, diverting colostomy). Average catheter dwell time was 41.9 days (range, 2–302 d). Nearly half the patients regained bowel function (13 of 27) and/or resumed a normal diet (14 of 27).
DISCUSSION
The present data illustrate the safety and efficacy of PC among patients with cancer who were treated for impending colonic perforation as a result of large bowel obstruction. Accumulation of gas within an obstructed colon causes increasing distension of the viscus, leading to mural ischemia and perforation (13). Clinical effectiveness of PC was indicated by a significant decrease in colonic diameter following catheter drainage and absence of colonic perforation—the undesired and feared outcome—in all treated patients. Additionally, pain relief was observed in the overwhelming majority of patients in the present cohort. Complications from PC were relatively uncommon among patients in the present study; the overall complication rate of 11%, major complication rate of 3.7%, and absence of procedural mortality stand in contrast to higher complication rates reported for surgery. Surgical outcomes in the setting of malignant bowel obstruction have been studied extensively (4,14–19), with morbidity and mortality rates as high as 42% and 32%, respectively (17). The rate of recurrent obstruction after surgical treatment is as high as 50% (20). Numerous studies have delineated criteria common to patients who may be least likely to benefit from an operative approach. Among these criteria—ascites, carcinomatosis, hypoalbuminemia, and multifocal obstruction (4,14,16,18,19)—are many conditions known to be common among patients with cancer. A retrospective series of 32 patients by Woolfson et al (4) found surgery to be valuable only among patients with benign causes of obstruction. Ample evidence therefore depicts a pressing need to report and compare the outcomes achieved by available nonsurgical alternatives. According to the present data, percutaneous catheter drainage has a desirable performance profile, including relief of patient symptoms and decompression of the dilated colon. No patient showed a progression to perforation, as was clinically feared.
Research has already accumulated to support the use of PC as a first-line intervention. A recent randomized trial by Saber and Hokkam (21) found significantly decreased morbidity in patients who underwent initial surgical tube cecostomy (12.8%) versus diverting loop colostomy (29.5%). However, rates of minor complications from surgical cecostomy placement have been reported to be as high as 45% (10), a rate substantially higher than the 11.1% overall complication rate we observed for image-guided PC. Further research is therefore indicated to directly compare PC with other accepted interventions, including surgical tube cecostomy and diverting loop colostomy. Endoscopic interventions are widely acknowledged as alternatives to surgery for colonic obstruction. A number of recent studies have established endoscopic stent placement as a valid primary or “bridging” therapy in the setting of malignant bowel obstruction (22–26). However, as evident in the present study, many patients with cancer are not appropriate candidates for endoscopic stent placement, and some who appear to be may not show any improvement after endoscopic intervention.
The single major complication in the present study—development of large subcutaneous emphysema and sepsis following PC—has been previously reported by others (27) who similarly attributed the complication to ischemic erosion of the bowel wall by T-fasteners under excess tension. Conversely, of the six PC placements in the present study performed without use of T-fasteners, none of the patients experienced any complications. There is conflicting evidence and little consensus regarding the need for such devices even during gastrostomy insertion, the procedure in which they are most commonly used. Regardless of whether T-fasteners are used during cecostomy, we have adopted the strategy of retracting the catheter after locking its loop until the loop apposes the anterior punctured wall of the viscus. This places the drainage pigtail advantageously at the nondependent aspect of the lumen, where gas is most likely to be present, and, in combination with an external silicone fixation device, holds the punctured viscus apposed to the peritoneal surface even in the absence of T-fasteners.
Several findings of the present study provide data that support statements regarding optimal technique for PC placement. Because the level of clinical concern for colonic perforation is driven in part by the radiographic measurement of MCD, we expressed the efficacy of drainage in terms of the change in this value after catheter placement. Because gas-distended bowel loops are readily apparent and characteristically measured for this purpose, it is not surprising that tubes terminating in gas rather than other bowel contents were more effective in decreasing gaseous colon distension. It is equally logical that catheters larger than 12 F evacuated gas no better than 8-F catheters in the clinical time frame. Catheters terminating in more complex, particulate colonic contents can be expected to function less well as a result of intermittent occlusion by solid elements. We do not assert that catheter size is unimportant when drainage of particulate liquids or semisolids is the clinical task. Although the need for T-fasteners during PC placement remains unclear, judgment is required when they are employed to avoid complications related to excessive tension on the bowel wall by these devices.
Limitations of the present study include retrospective analysis, a fairly small sample size of 27 patients that limits the generalization of our findings, and qualitative rather than quantitative pain assessment. In addition, further research is indicated to directly compare different treatment options including surgical tube cecostomy and diverting colostomy.
In summary, image-guided PC is an effective intervention for patients with cancer with functional and mechanical colonic obstruction, reducing pain and evacuating gas from the distended lumen. Endoscopic stent placement for malignant large bowel obstruction offers the distinct advantage of an internal appliance without an exteriorized drain, whereas traditional surgical approaches are increasingly recognized as appropriate only in specific, limited situations. When surgical and endoscopic approaches are contraindicated or unsuccessful, percutaneous drainage is most efficacious when the catheter is positioned to drain luminal gas, in which case even 8.5-F drains perform as well as larger catheters. Other key technical requirements include addition of pinpoint venting holes to a nondependent aspect of the drainage bag, and avoidance of excess tension on the bowel wall by T-fasteners when they are used.
ABBREVIATIONS
- ACPO
acute colonic pseudoobstruction
- MCD
maximum colonic distension
- PC
percutaneous cecostomy/colostomy
Footnotes
None of the authors have identified a conflict of interest.
Contributor Information
Sanjit O. Tewari, Department of Radiology, Section of Interventional Radiology, Memorial Sloan Kettering Cancer Center, New York, New York.
George I. Getrajdman, Department of Radiology, Section of Interventional Radiology, Memorial Sloan Kettering Cancer Center, New York, New York.
Elena N. Petre, Department of Radiology, Section of Interventional Radiology, Memorial Sloan Kettering Cancer Center, New York, New York.
Constantinos T. Sofocleous, Department of Radiology, Section of Interventional Radiology, Memorial Sloan Kettering Cancer Center, New York, New York.
Robert H. Siegelbaum, Department of Radiology, Section of Interventional Radiology, Memorial Sloan Kettering Cancer Center, New York, New York.
Joseph P. Erinjeri, Department of Radiology, Section of Interventional Radiology, Memorial Sloan Kettering Cancer Center, New York, New York.
Martin R. Weiser, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Raymond H. Thornton, Department of Radiology, Section of Interventional Radiology, Memorial Sloan Kettering Cancer Center, New York, New York.
REFERENCES
- 1.Ripamonti C, Twycross R, Baines M, et al. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233. [DOI] [PubMed] [Google Scholar]
- 2.Bellavance EC, Alexander HR Jr. Palliative interventions in patients with peritoneal metastases and malignant bowel obstruction. J Clin Oncol 2012; 30:4290–4291. [DOI] [PubMed] [Google Scholar]
- 3.Jain A, Vargas HD. Advances and challenges in the management of acute colonic pseudo-obstruction (Ogilvie syndrome). Clin Colon Rectal Surg 2012; 25:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097. [DOI] [PubMed] [Google Scholar]
- 5.Casola G, Withers C, vanSonnenberg E, Herba MJ, Saba RM, Brown RA. Percutaneous cecostomy for decompression of the massively distended cecum. Radiology 1986; 158:793–794. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier P, Marcy PY, Francois E, et al. Controlled transperitoneal percutaneous cecostomy as a therapeutic alternative to the endoscopic decompression for Ogilvie’s syndrome. Am J Gastroenterol 2002; 97:471–474. [DOI] [PubMed] [Google Scholar]
- 7.Haaga JR, Bick RJ, Zollinger RM Jr. CT-guided percutaneous catheter cecostomy. Gastrointest Radiol 1987; 12:166–168. [DOI] [PubMed] [Google Scholar]
- 8.Donkol RH, Al-Nammi A. Percutaneous cecostomy in the management of organic fecal incontinence in children. World J Radiol 2010; 2:463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sierre S, Lipsich J, Questa H, Bailez M, Solana J. Percutaneous cecostomy for management of fecal incontinence in pediatric patients. J Vasc Interv Radiol 2007; 18:982–985. [DOI] [PubMed] [Google Scholar]
- 10.Benacci JC, Wolff BG. Cecostomy. Therapeutic indications and results. Dis Colon Rectum 1995; 38:530–534. [DOI] [PubMed] [Google Scholar]
- 11.Chait PG, Shlomovitz E, Connolly BL, et al. Percutaneous cecostomy: updates in technique and patient care. Radiology 2003; 227:246–250. [DOI] [PubMed] [Google Scholar]
- 12.Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol 2003; 14(suppl):S293–S295. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CD, Rice RP, Kelvin FM, Foster WL, Williford ME. The radiologic evaluation of gross cecal distension: emphasis on cecal ileus. AJR Am J Roentgenol 1985; 145:1211–1217. [DOI] [PubMed] [Google Scholar]
- 14.Dalal KM, Gollub MJ, Miner TJ, et al. Management of patients with malignant bowel obstruction and stage IV colorectal cancer. J Palliat Med 2011; 14:822–828. [DOI] [PubMed] [Google Scholar]
- 15.Henry JC, Pouly S, Sullivan R, et al. A scoring system for the prognosis and treatment of malignant bowel obstruction. Surgery 2012; 152:747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs HB, Goplerud DR. Surgical management of bowel obstruction in advanced ovarian carcinoma. Obstet Gynecol 1983; 61:327–330. [PubMed] [Google Scholar]
- 17.Krouse RS. Invasive treatment options for malignant bowel obstruction. J Palliat Med 2009; 12:1152–1153. [DOI] [PubMed] [Google Scholar]
- 18.Larson JE, Podczaski ES, Manetta A, Whitney CW, Mortel R. Bowel obstruction in patients with ovarian carcinoma: analysis of prognostic factors. Gynecol Oncol 1989; 35:61–65. [DOI] [PubMed] [Google Scholar]
- 19.Miner TJ, Brennan MF, Jaques DP. A prospective, symptom related, outcomes analysis of 1022 palliative procedures for advanced cancer. Ann Surg 2004; 240:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000:CD002764. [DOI] [PubMed] [Google Scholar]
- 21.Saber A, Hokkam EN. Efficacy of protective tube cecostomy after restorative resection for colorectal cancer: a randomized trial. Int J Surg 2013; 11:350–353. [DOI] [PubMed] [Google Scholar]
- 22.Aadam AA, Martin JA. Enteral stents in malignant bowel obstruction. Gastrointest Endosc Clin N Am 2013; 23:153–164. [DOI] [PubMed] [Google Scholar]
- 23.Cirocchi R, Farinella E, Trastulli S, et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol 2013; 22:14–21. [DOI] [PubMed] [Google Scholar]
- 24.Ding XL, Li YD, Yang RM, Li FB, Zhang MQ. A temporary self-expanding metallic stent for malignant colorectal obstruction. World J Gastroenterol 2013; 19:1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghazal AH, El-Shazly WG, Bessa SS, El-Riwini MT, Hussein AM. Colonic endoluminal stenting devices and elective surgery versus emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J Gastrointest Surg 2013; 17: 1123–1129. [DOI] [PubMed] [Google Scholar]
- 26.Grundmann RT. Primary colon resection or Hartmann’s procedure in malignant left-sided large bowel obstruction? The use of stents as a bridge to surgery. World J Gastrointest Surg 2013; 5:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maginot TJ, Cascade PN. Abdominal wall cellulitis and sepsis secondary to percutaneous cecostomy. Cardiovasc Intervent Radiol 1993; 16:328–331. [DOI] [PubMed] [Google Scholar]


