Abstract
Calcium, as a second messenger, has an important role in a variety of cellular functions. However, disruption of intracellular calcium homeostasis leads to cytotoxicity and cell death. Excessive calcium release from intracellular stores, via the calcium channel ryanodine receptor, contributes to cell damage. Dysfunction of calcium homeostasis is established in tissue culture and animal models of ischemia, hypoxia, seizure, trauma, anesthesia, and neurodegenerative diseases. Dantrolene, the primary drug to treat malignant hyperthermia, is a ryanodine receptor antagonist. Dantrolene inhibits abnormal calcium release from the sarco-endoplasmic reticulum, which is the primary intracellular calcium store. Dantrolene has been investigated widely for its possible cytoprotective effects against cell damage in different tissue culture or animal models of diseases involving cytotoxicity induced by disruption of intracellular calcium homeostasis in pathogenesis. In this review, we summarize the role of the disruption of intracellular calcium homeostasis on cell death, the pharmacologic and pharmaco-kinetic features of dantrolene, and the cytoprotective effects and potential application of dantrolene for the inhibition of cell damage in a wide variety of models of stress and disease.
Dantrolene sodium, a ryanodine receptor (RYR) antagonist, is very well known in anesthesiology practice. The RYR is one of 2 major calcium release channels; the other is the inositol-1,4,5-trisphosphate receptor (InsP3R). Dantrolene has been in clinical use since the 1980s for treating malignant hyperthermia (MH) and more recently for neuroleptic malignant syndrome,1 spasticity,2,3 heat stroke,4 and ecstasy intoxication.5 The cytoprotective effects of dantrolene are currently being investigated. This review summarizes the pharmacology of dantrolene, the role of calcium in cytotoxicity, and the cytoprotective role of dantrolene on cell damage induced by a wide variety of stress factors.
Dantrolene was synthesized by Snyder et al.6 in 1967 as a new class of skeletal muscle relaxant. Animal studies showed that dantrolene inhibited the twitch response by direct action on the muscle. It dissociates the excitation-contraction coupling in muscle by inhibiting the release of calcium (Ca2+) from the sarcoplasmic reticulum (SR) but has no effect on the electrical excitability of the muscle, or the neuromuscular junction.7,8
The effectiveness of dantrolene on MH was reported first in swine that were susceptible to halothane anesthesia and presented clinical symptoms similar to human MH.9,10 Results of a multicenter study performed between 1977 and 1979 in patients showed that the use of dantrolene for treatment of MH decreased the mortality rate significantly11 and, since then, the mortality rate has decreased from 80% to <5% today.12
PHARMACOLOGY OF DANTROLENE
Dantrolene sodium (1-[[[5-(4-nitrophenyl)-2-furanyl]-methylene]amino]-2,4-imidazolidinedione sodium salt), a hydantoin derivative, is highly lipophilic and poorly soluble in water. Vials include 20 mg dantrolene and 3000 mg mannitol for IV administration. A vial is dissolved in 60 mL water and pH is adjusted to 9.5 using sodium hydroxide. The solution is light sensitive, so it should be protected from light in room temperature and should be used within 6 hours.
Dantrolene inhibits RYRs. These receptors are expressed on the surface of the endoplasmic reticulum (ER) and SR.13,14 RYRs release Ca2+ from intracellular stores in the ER and SR.15,16 Three different isoforms of RYRs are identified in mammalian tissues. RYR1 is mainly expressed on the terminal cisternae of SR in skeletal muscle.13 RYR2 is primarily expressed in cardiac muscle14 and dantrolene has no marked effect on this receptor.17 In cardiac muscle, membrane depolarization causes opening of Cav1.2 L-type calcium channels, which leads to influx of Ca2+ from extracellular space. As a result of Ca2+ influx, RYR2 is stimulated and Ca2+ is released from the SR.18
RYR3 is expressed in low levels in most tissues; however, it is found most abundantly in the brain.19,20 All 3 RYR isoforms are expressed in the central nervous system. RYR1 is found in cerebellar Purkinje cells and RYR2 is the predominant form in the olfactory nerve layer, cerebral cortex, dentate gyrus, cerebellar granule cells, the motor trigeminal nucleus, and the facial nucleus. RYR3 is high in the hippocampal CA1 pyramidal layer, caudate putamen, and dorsal thalamus.19,20 Dantrolene acts directly on RYR1 and RYR3 to inhibit the extent of channel activation by calmodulin (CaM) and decreases the Ca2+ sensitivity of channel activation.21
In healthy volunteers, a 5-hour IV administration of dantrolene with an accumulative dose of 2.4 mg/kg caused a 75% depression in muscle twitch response. The plasma dantrolene concentration was 4.2 μg/mL and the elimination half-life was 12 hours. The plasma concentration was maintained in the therapeutic range for approximately 5 hours.22 After oral administration of dantrolene, 70% is absorbed, peak plasma levels are obtained 4 to 6 hours later, and the elimination half-life is 8.7 hours.2
Dantrolene is metabolized in the liver by oxidative and reductive pathways. Through the oxidative pathway, the hydantoin ring is hydroxylated and forms 5-hydroxydantrolene, and through the reductive pathway, nitro-moiety and acetylation of the benzene ring forms the reduced acetylated derivative of dantrolene. Dantrolene is excreted in both urine and bile; 79% as 5-hydroxydantrolene, 17% as reduced acetylated derivative, and 4% as the main compound.23
Dantrolene is the primary drug used for the treatment of MH. MH is a pharmacogenetic disorder of skeletal muscle in humans and other vertebrates. Halogenated or depolarizing anesthetics trigger dysregulated release of Ca2+ from the SR and cause sustained activation of the contractile apparatus, muscular rigidity, and hyperthermia. Mutations in RYR1 result in an excessive Ca2+ release from the SR by direct activation of the channel by halogenated volatile anesthetics in MH. Halothane selectively activates mutated but not wild-type RYR1.24,25 Halothane (as a trigger for Ca2+ release) is used to test MH susceptibility for an accepted, standard caffeine-halothane contracture test for the laboratory diagnosis of MH.26,27 During excitation-contraction coupling in skeletal muscle, an action potential initiated at the neuromuscular junction rapidly transmits down the surface and T tubule membranes, which causes voltage-driven conformational changes in the T tubule dihydropyridine or voltage sensor L-type calcium channel (Cav1.1). This results in a direct mechanical interaction between L-type calcium channel and RYR1 and releases Ca2+ from the SR.18,28 A recent study showed that within the clinical range, halothane did not induce Ca2+ release from SR in fibers obtained from normal human skeletal muscle. However, halothane elicited Ca2+ release from the SR, led to depletion of Ca2+ in the store, and triggered store-operated calcium influx in MH-susceptible human skeletal muscle fibers.29 Depletion of Ca2+ from intracellular stores activates store-operated calcium influx or capacitative Ca2+ entry and causes Ca2+ entry across plasma membrane.30 These results emphasize the importance of RYR1 receptor antagonists for treatment of MH.
Azumolene (1-[[[5-(4-bromophenyl)-2-oxazoly]methylene] amino]-2,4-imidazolidinedione), an analog of dantrolene, is a potential alternative drug for treating MH. It is 30-fold more water soluble than dantrolene.31 Azumolene was synthesized by replacing the para-nitro-phenyl group in dantrolene sodium with a para-bromo-phenyl group.32 Chemical structures of dantrolene and azumolene are shown in Figure 1. Azumolene suppresses the opening rate of RYR Ca2+ release channels within skeletal fibers.33 Azumolene reverses MH episodes induced by halothane in susceptible swine31,34 and inhibits caffeine-induced contractions in normal and human MH-susceptible skeletal muscle with a potency similar to dantrolene.35
Figure 1.
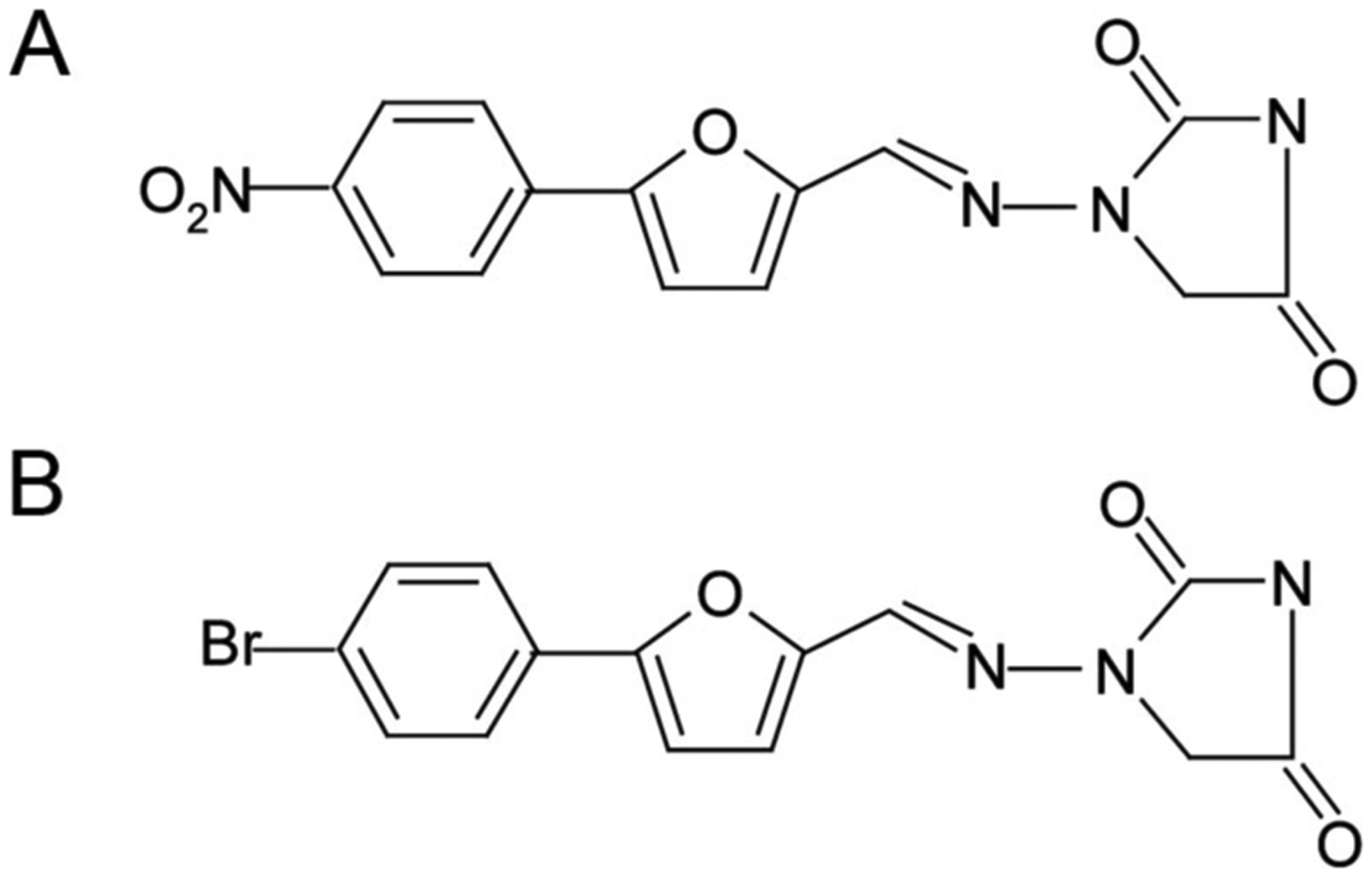
Chemical structures of dantrolene (A) and azumolene (B).
The most common side effects of dantrolene with IV and chronic oral administration are dizziness, drowsiness, light headedness, headaches, anorexia, diarrhea, nausea, and vomiting.2,22 Chronic oral use can be associated with liver dysfunction.36 Rarely observed side effects are fatigue, weakness, rash, and acne-like dermatosis.2,37 Chronic pleural effusion was also reported during dantrolene use in patients.38 During IV administration, extravasation of dantrolene can cause thrombophlebitis (due to basic pH); therefore, it is suggested to use large veins for injection.
Coadministration of dantrolene with other drugs also produces side effects. Dantrolene given with verapamil leads to an increase in cardiac dysfunction in swine and dogs.39,40 Although this effect has not been described in humans, it is recommended not to use this combination during MH in humans; correction of acidosis and hyperkalemia will be more helpful for treating arrhythmias. Driessen et al.41 reported that recovery time from neuromuscular blockade induced by vecuronium was longer in patients treated with dantrolene for MH prophylaxis compared with normal patients.
Although the common side effects of dantrolene originate in the central nervous system, it is still controversial whether dantrolene passes through the blood-brain barrier (BBB). Wuis et al.42 reported a high accumulation of radioactivity in the intestines, liver, gall bladder, kidneys, and urinary bladder but not in the brain of marmoset monkeys treated with 14C-dantrolene. Enokizono et al.43 showed that breast cancer resistance protein (Bcrp), a member of the adenosine triphosphate (ATP)-binding cassette protein transporter family, limits the tissue penetration of dantrolene, as well as some other xenobiotic compounds in the BBB in mice. Distribution and brain uptake of dantrolene were significantly increased in Bcrp knockout mice compared with wild-type littermates. There is also evidence to support the penetration of dantrolene across the BBB. First, dantrolene is a small molecule (molecular weight: 399) and has high lipid solubility. Second, it has central side effects (drowsiness and dizziness), and alters neurotransmitter levels in the cerebrospinal fluid.44,45 A dose sufficient to cause muscle relaxation may be inadequate to produce a sufficiently high concentration of active agent in the brain, because the pharmacologic effects of dantrolene are dose dependent in most models.45
ROLE OF CALCIUM IN CYTOTOXICITY AND NEURODEGENERATION
Ca2+, as a second messenger, has an important role in a variety of cellular functions such as control of cell growth and differentiation, membrane excitability, exocytosis, synaptic activity, apoptosis, and autophagy.46 – 48 The intracellular free Ca+2 is highly regulated and its concentration is maintained at approximately 100 nM. Under normal conditions, there is a 10,000-fold Ca2+ gradient between the intra- and extracellular space.46 Thus, small or localized increases in intracellular Ca2+ will quickly trigger physiologic events such as the activation of enzymes or ion channels.
An increase in intracellular Ca2+ can arise from influx from the extracellular space through voltage-dependent calcium channels (VDCCs), receptor-operated calcium channels, and store-operated calcium channels, which are located on the plasma membrane or from the release of Ca2+ from intracellular stores such as the ER, SR, or mitochondria. Ca2+ levels in the ER are regulated by sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pumps; inositol-1,4,5-triphosphate (InsP3) and InsP3Rs; RYRs; and Ca+2-binding proteins (calreticulin and calsequestrin). In unstimulated cells, normal cytosolic Ca+2 is regulated (to concentrations <100 nm) by uptake into the ER by SERCA and by efflux into the extracellular space either through the plasma-membrane Ca2+-ATPase or the Na+/Ca2+ exchanger, both of which are located on plasma membrane.46 Ca2+ release from the ER is triggered via agonist activation of InsP3 Rs by InsP3, which is generated through the phospholipase C pathway49 and by activation of RYRs. Mitochondria store Ca2+ electrophoretically by a uniport transporter and release Ca2+ via reversal of the uniporter, Na+/H+-dependent Ca2+ exchange, or the permeability transition pore. Mitochondria rapidly take up Ca2+ released from the ER. Close contact between the ER/SR and mitochondria has been observed by electron microscopy in several fixed cell types.50 The ER and mitochondria are linked through ER-mitochondrial–associated membranes. This area is rich in enzymes and proteins that are involved in lipid biosynthesis and Ca2+ signaling. Mitochondria preferentially accumulate Ca2+in cytosolic areas rich in Ca2+, called microdomains.51 Furthermore, enriched InsP3R immunoreactivity has been found in ER regions close to mitochondria.50
The ER has 2 major functions in the cell: Ca2+ storage and the facilitation of the proper folding of newly synthesized proteins destined for secretion to the cell surface or other intracellular organelles. ER stress occurs in a variety of physiologic and pharmacologic situations, for example, when the capacity of the ER to fold proteins becomes saturated as a result of expression of folding-incompetent or aggregation-prone proteins, when there is overload or depletion of the ER Ca2+ pool, and with glucose starvation and hypoxia.47 During ER stress, 2 pathways are activated to remove the incorrectly folded and/or accumulated proteins. Stimulation of the unfolding protein response pathway induces ER chaperones, such as immunoglobulin heavy-chain binding protein, glucose-regulated protein-78, calreticulin, protein disulfide isomerase, and the transcription factor CHOP/GADD153. Stimulation of the ER overload response pathway causes the production of cytokines and interferons through activation of nuclear factor-κB.46 Prolonged ER stress can induce apoptosis to eliminate the damaged cells.
One of the consequences of Ca2+ overload is neurodegeneration, a progressive loss of structure and function as well as death of neurons. As early as the 1970s, researchers reported an association between an increase in intracellular Ca2+ concentration [Ca2+] and neurotoxicity. Schlaepfer and Bunge52 investigated the degenerative changes in amputated nerve fibers in cultured rat sensory ganglia in the presence of media containing different concentrations of Ca2+. They observed loss of neurofilaments and microtubules in association with high concentrations of Ca2+. Choi53 reported that glutamate, the major excitatory amino acid in the brain, induced toxicity as a result of increased [Ca2+] in neurons in cell culture. Glutamate is released from presynaptic vesicles and binds to postsynaptic glutamate receptors. Three classes of glutamate ionotropic receptors are N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate (KA). The opening of glutamate-gated channels leads to Ca2+ influx from the extracellular space into the cytosol, which in turn stimulates Ca2+ release (calcium-induced calcium release [CICR]) from intracellular stores. CICR is also activated by other voltage-gated calcium channel–induced Ca2+ influx into the cytosol. CICR is primarily mediated by RYR and Ca2+ release from the ER and SR.54 Furthermore, NMDA and KA caused reversible and irreversible neuronal damage55 and these neurotoxic effects depended on [Ca2+] in rat cerebellar slices.56
Increased cytosolic Ca2+ also induces a secondary Ca2+-dependent phenomena, which contributes to neurotoxicity and cell death through apoptosis by activating proteases, lipases, and endonucleases. When Ca2+ binds to CaM, it activates nitric oxide synthase and increases the formation of nitric oxide. Free oxygen radicals react with nitric oxide and form peroxynitrite, a highly toxic molecule that damages DNA and proteins. DNA cleavage activates the DNA repair enzyme, poly (ADP-ribose) polymerase, which requires ATP for functioning. Decreased ATP levels cause the formation of oxygen radicals in mitochondria and increases cellular damage. Mitochondrial permeability is increased via activation of the mitochondrial permeability transition pore and results in osmolar load inside the mitochondria, causing mitochondrial swelling and rupture as well as the release of cytochrome c (CytC). CytC activates pro-apoptotic factors. Protease activation also leads to cytoskeletal breakdowns. The stimulation of phospholipase A2 releases arachidonic acid and related polyunsaturated fatty acids. During fatty acids metabolism, reactive oxygen species are also generated.46,57,58 This cascade of events leads to apoptosis and neurotoxicity.
Recent studies also indicate that cytosolic Ca2+ is involved in autophagy, a lysosomal degradation pathway to eliminate cellular proteins and organelles. Høyer-Hansen et al.48 demonstrated that different Ca2+ mobilizing agents such as vitamin D3, ionomycin, ATP, and thapsigargin induce autophagy by inhibiting the autophagy inhibitory activity of rafamycin via activation of Ca2+/CaM-dependent kinase kinase-β and AMP-activated protein kinase. Inhibition of InsP3Rs using a pharmacologic inhibitor (xestospongin B), siRNA depletion, or lithium-mediated inhibition of InsP3 synthesis triggers autophagosome formation, suggesting that stimulation of InsP3Rs inhibits autophagy.59,60
CYTOPROTECTIVE EFFECTS OF DANTROLENE
Effects of Dantrolene on Ischemia-Induced Cytotoxicity
Excessive presynaptic glutamate release occurs during hypoxia and ischemia.61,62 Cerebral ischemia can significantly increase the extracellular concentration of excitatory amino acids and cause neuronal membrane depolarization, which results in Ca2+ influx from the extracellular space into the cytosol.63,64 Mitani et al.65 demonstrated that approximately one-third of the increased [Ca2+] after ischemic stimulation was derived from an extracellular component and the remaining two-thirds was derived from intracellular stores in hippocampal slices. They also showed that administration of dantrolene diminished by half the [Ca2+] mobilization from intracellular stores. Zhang et al.66 reported that intracerebroventricular injection of dantrolene protected neurons from ischemic, delayed neuronal death induced by bilateral occlusion of the common carotid artery in the gerbil brain. Wei and Perry45 demonstrated that pretreating hypothalamic neurosecretory cells with dantrolene significantly reduced the increase in cytosolic [Ca2+] and neuronal death observed after those cells were exposed to thapsigargin (an ER Ca2+-ATPase) alone. Furthermore, the authors reported that dantrolene administered systemically before induction of global cerebral ischemia in gerbils significantly increased the number of intact CA1 pyramidal neurons in a dose-dependent manner. Dantrolene ameliorates neuronal cell death induced by transient ischemia in rats67,68 as well as in neuronal cell lines exposed to hypoxia and glucose deprivation.69 Moreover, infarct size after ischemia was also reduced by injection of dantrolene during reperfusion in adult70 and neonatal rats.71 Kocogullari et al.72 studied whether dantrolene would be protective against neuronal injury during aortic ischemia/reperfusion (mimicking spinal cord injury after aortic surgical intervention) in rabbits. Dantrolene administered intraperitoneally (IP) just 30 minutes before the surgery significantly improved neurologic deficits and decreased vascular proliferation, hemorrhage, edema, and neuron loss in spinal cord sections. Dantrolene has been shown to have synergistic effects with nimodipine, a voltage-dependent L-type Ca2+ channel blocker, against serotonin-induced vasoconstriction in isolated cerebral arteries of rats.73 Also, dantrolene inhibited presser responses induced by noradrenaline, angiotensin, and endothelin-1 in isolated arterial preparations from rodents.74–76
However, Martínez-Sánchez et al.77 found that dantrolene was not effective in preventing neuronal loss in hippocampal slice cultures exposed to oxygen-glucose deprivation. They concluded that oxygen-glucose deprivation–induced cell death is mediated by activation of ionotropic glutamate receptors, voltage-dependent Na+ channels, and both plasma membrane and mitochondrial Na+/Ca2+ exchangers. In in vivo and in vitro models of myocardial reperfusion injury, dantrolene reduced creatinine kinase release (measured as an index of cell death) during reperfusion of isolated rat hearts, but did not have any effect on infarct size or hemodynamics during reperfusion after myocardial ischemia in rabbits.78 Another study, reported by Wu et al.79 also demonstrated that dantrolene did not protect renal function against ischemia/reperfusion injury in cell cultures and in rats. Both nicardipine, an L-type calcium channel inhibitor, and TMB-8, an InsP3R antagonist, inhibited CytC release and caspase-3 activation, and decreased the apoptotic cell number suggesting an important role of the InsP3R on ER or SR membrane cytotoxicity. In addition, pretreating rats with these 2 compounds, but not dantrolene, significantly prevented an increase in serum creatinine levels during the ischemia/reperfusion injury.
Effects of Dantrolene on Glutamate-Induced Cytotoxicity
Pretreating guinea pigs with intravitreal dantrolene and nimodipine protected retinal ganglion cells against intravitreal NMDA-induced retinal injury.80 Dantrolene has also been shown to prevent KA-induced81 and NMDA-induced82 neuronal cell death in cerebellar granule cell cultures. After KA-induced seizures, RyR3 mRNA upregulation was observed in the hippocampal CA3 region and striatum, and c-fos mRNA expression increased in the hippocampus, dentate gyrus, and deeper layer of the neocortex.83 Furthermore, IP administration of dantrolene before induction of seizures by IP injection of KA reduced the apoptotic cell death in the hippocampal CA1 region and parietal cortex in rats.81 Makarewicz et al.84 studied the mechanism behind this protection using radioactive Ca2+. They demonstrated that dantrolene inhibited the NMDA-evoked 45Ca uptake in cerebellar granule cell cultured neurons in a dose-dependent manner.
Ca2+ influx is essential for the first stage (intrinsic burst firing) of epileptic neuronal events.85 Results from animal models of epilepsy have introduced the possibility that Ca2+ antagonists may be a new class of anticonvulsant agents.86 Seizures induced by a metabotropic receptor agonist, 1S,3R-ACPD, were completely abolished with a high dose of dantrolene but not by MK-801 in rats. Dantrolene also significantly diminished 1S,3R-ACPD–induced increase in brain volume.87 Niebauer and Gruenthal88 reported that when dantrolene was administered just 30 minutes after electrogenic-induced status epilepticus, it was more protective to the entire hippocampus than when injected 140 minutes after seizures in rats. Both dantrolene and thapsigargin prevented seizure-induced cell death in hippocampal slices suggesting that Ca2+ release from ER stores also contributes to seizure-induced cell death.89 Furthermore, dantrolene significantly reduced seizure activity in the EL mouse, a mutant strain susceptible to convulsive seizures.90
Dantrolene combined with LY 300164, an AMPA/kainate receptor antagonist, to treat electroshock-induced seizures in mice, impaired motor performance.91 Conversely, dantrolene had no marked effect against seizures induced by electroshock92 and those induced by 3,5-DHPG, a group I metabotropic glutamate receptor agonist,93 in mice.
Effects of Dantrolene on Neurodegeneration in Neurodegenerative Diseases
Ca2+ signaling is crucial for maintaining normal neuronal functions such as membrane excitability, neurotransmitter release, cellular growth, differentiation, and cell death. Under resting conditions, cytosolic [Ca2+] in neurons is maintained at approximately 200 nM.94 Disruptions in Ca2+ homeostasis have been reported in neurodegenerative diseases including Alzheimer disease (AD),95–97 Parkinson disease (PD),98,99 Huntington disease (HD),94,100 amyotrophic lateral sclerosis (ALS),101,102 as well as spinocerebellar ataxias (SCAs).103,104 Calcium homeostasis can be disrupted by alterations in Ca2+ buffering capacity, increased sensitivity to excitotoxicity, functional changes in both plasma and ER Ca2+ channels, as well as mitochondrial Ca2+ homeostasis. The models of cytotoxicity induced by Ca2+ dysregulation during excitotoxicity, neurodegenerative diseases, and general anesthesia are summarized in Figure 2.
Figure 2.
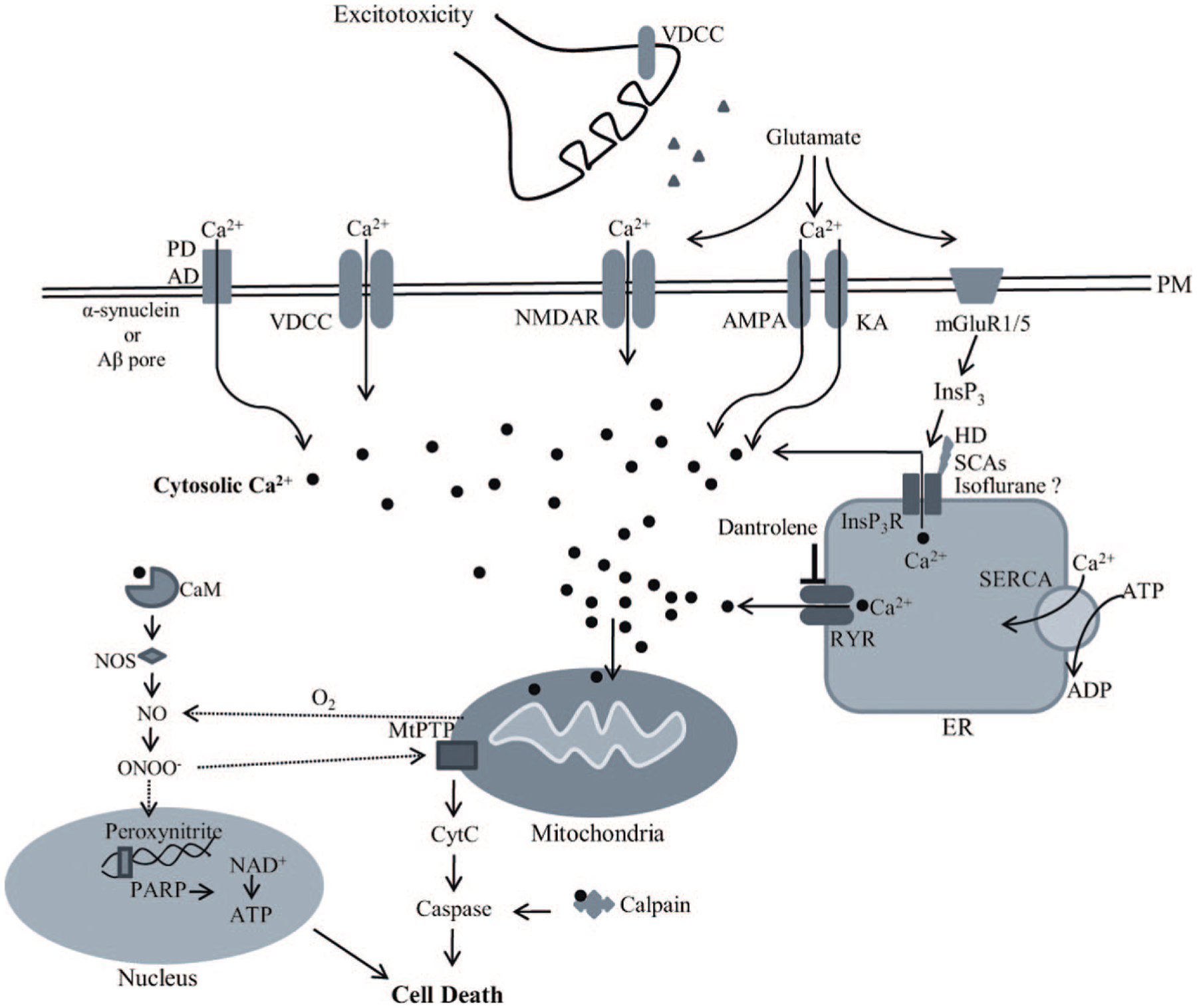
The models of cytotoxicity induced by Ca2+ dysregulation in excitotoxicity (i.e., sepsis, seizure, trauma), neurodegenerative diseases, and general anesthesia. Under normal conditions, [Ca2+] in the extracellular space is 10,000 times higher than the cytosolic Ca2+ concentration ([Ca2+]c). Upon electrical or receptor-mediated stimulation, [Ca2+]c is increased by extracellular Ca2+ influx via specific ion channels on the plasma membrane including voltage-dependent calcium channels (VDCCs) and ligand-gated calcium channels or by Ca2+ release from intracellular stores. The main intracellular Ca2+ store in neurons is the endoplasmic reticulum (ER) and Ca2+ is released into the cytosol via activation of ryanodine receptors (RYRs) and inositol-1,4,5-triphosphate receptors (InsP3Rs). Basal [Ca2+]c is maintained through calcium binding and calcium buffering proteins or uptake into internal stores by the energy-dependent sarco-ER calcium pump (SERCA) at the ER membrane or by the mitochondrial uniporter. Disturbance in Ca2+ homeostasis leads to cytotoxicity. Abnormal increase in intracellular [Ca2+] is the result of either increased extracellular Ca2+ influx via Ca2+ channels or increased release from the ER by either calcium-induced calcium release (CICR) or overactivation of RYRs and InsP3Rs. During excitotoxic conditions (i.e., ischemia, sepsis, seizure, trauma), glutamate release is increased. Glutamate stimulates N-methyl-d-aspartate (NMDA), kainate (KA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and metabotropic glutamate mGluR1/5 receptors. Amyloid-β peptide (Aβ) synthesis is increased in Alzheimer disease (AD) and forms oligomers. Both Aβ oligomers and α-synuclein, which aggregates in Parkinson disease (PD), can form Ca2+ permeable pores in the plasma membrane and facilitates the increase of [Ca2+]c. Aβ oligomers also activate NMDA and KA receptors as well as VDCCs and cause Ca2+ influx. Activation of mGluR1/5 increases inositol-1,4,5-triphosphate (InsP3), which activates the InsP3R and causes Ca2+ release from the ER. Evidence suggests that the InsP3R is sensitized to InsP3 during Huntington disease (HD), spinothalamic cerebellar ataxies (SCAs), and general anesthesia by isoflurane. Dantrolene is cytoprotective via inhibiting RYRs and preventing excessive Ca2+ release from the ER, especially during CICR. Reduction in ER Ca2+ stores results in the misfolding of proteins, which stimulates the unfolding protein response as a cellular stress response. Influx of Ca2+ into mitochondria causes the formation of oxygen radicals and energy failure as a consequence of decreased adenosine triphosphate (ATP) production. When cytosolic Ca2+ binds to calmodulin (CaM), nitric oxide synthase (NOS) is activated and nitric oxide (NO) is produced. Oxygen radicals react with NO and forms peroxynitrite, which damages DNA and proteins. DNA cleavage activates the DNA-repair enzyme poly (ADP-ribose) polymerase (PARP), which requires energy for its activation. PARP-induced energy depletion worsens cellular stress. Increased [Ca2+] in mitochondria increases mitochondrial permeability via the mitochondrial permeability transition pore (MtPTP), which causes mitochondrial swelling, outer mitochondrial membrane rupture, and release of cytochrome c (CytC). CytC activates pro-apoptotic factors (caspases) by reacting with Ca2+-activated calpain and induces apoptosis via intrinsic pathways.
Alzheimer Disease
AD is the most common idiopathic, progressive, neurodegenerative disease characterized by a progressive and irreversible loss of neurons in specific brain areas involved in learning and memory processes. The disorder gradually affects memory, learning abilities, and language skills; causes behavioral and personality changes; interferes with an individual’s ability to achieve daily activities, and finally leads to death. AD is characterized by extracellular deposits of amyloid-β peptide (Aβ) that result from altered proteolytic processing of amyloid precursor protein, intracellular neurofibrillary tangles composed of hyper-phosphorylated tau protein deposits and the reduced number of synapses and neuronal loss.105
There is growing evidence suggesting that disruption of ER Ca2+ signaling has a role in the pathogenesis of AD. PC12 cells expressing mutant human presenilin-1 (PSEN1) are more susceptible to oxidative stress and changes in intracellular Ca2+ levels, as well as more vulnerable to the general anesthetic isoflurane-induced Ca2+ release from the ER and cell apoptosis.106,107 The increase of Ca2+ in response to thapsigargin in mutated cells was prevented by pretreating cells with nifedipine and dantrolene.108 MacManus et al.95 reported that Aβ40 significantly increases 45Ca2+ influx into rat cortical synaptosomes via activation of L- and N-type VDCCs, and also increases the amplitude of N- and P-type Ca2+ channel currents in rat cultured cortical neurons. However, Rovira et al.96 found that, using the whole-cell patch-clamp recording in hippocampal CA1 pyramidal cells of mice, Aβ(25–35) acts on L-type Ca2+ channels but not Aβ40. All amyloids, and particularly Aβ42, increased intracellular Ca2+ in fluo-3-loaded SH-SY5Y cells, which lasted after the depletion of intracellular Ca2+ stores, indicating that both extracellular and intracellular Ca2+ sources contribute to this effect.97 In human cortical neurons, Aβ oligomers and Aβ-derived diffusible ligands have high affinity to synaptic contacts and cellular membranes. Aβ oligomers caused cellular changes and activated mitochondrial death via the apoptotic pathway.109 Using multiphoton imaging, Kuchibhotla et al.110 measured Ca2+ levels in cortical neurons in several transgenic mouse models of AD and found Ca2+ overload in neurites in older transgenic mice associated with proximity to plaques as well as loss of spinodendritic Ca2+ compartmentalization, which is critical for synaptic integration.
Theoretically, compounds that prevent or correct Ca2+ dysregulation would be useful for the treatment of AD and other neurodegenerative disorders. An NMDA receptor antagonist, memantine, has been approved by the Food and Drug Administration for the treatment of AD.99 Orally administered memantine to triple-transgenic (3xTg-AD) mice for 3 months significantly ameliorated cognitive dysfunction and reduced the levels of insoluble Aβ and prefibrillar soluble oligomers.111 RYRs are of interest in regulating Ca2+ dysregulation in in vivo and in vitro models for AD. Exposure of Aβ42 to rabbit skeletal SR vesicles resulted in RYR-mediated Ca2+ release, and exposure of lipid bilayers to Aβ42 resulted in an increased probability of channel openings.112 Intracellular Ca2+ levels were increased in cells expressing the human PSEN1 L286V mutation. Aβ induced cell death in these cells but both dantrolene and nifedipine protected the cells against these adverse effects.108 Imaizumi et al.113 showed that Aβ induced DP5, a neuronal apoptosis-inducing gene, expression in cultured rat cortical neurons, and also demonstrated that both nifedipine and dantrolene blocked the Aβ-induced DP5 expression.
It has been suggested that the PSEN1 mutation increases vulnerability of neurons to cerebral ischemia and oxidative stress. This hypothesis was examined in PSEN1 mutant knockin mice and in neuronal cultures from PSEN1-deficient mice, respectively.114,115 The size of cerebral infarct, caused by middle cerebral artery occlusion, was bigger in PSEN1 knockin mice compared with wild-type littermates. Researchers also showed that cultured cortical neurons from PSEN1 mutant mice were more sensitive to glucose deprivation and chemical hypoxia, had a more prominent increase of intracellular Ca2+, and that pretreating cells with dantrolene prevented this cell damage.114 Nakajima et al.115 also demonstrated that neurons cultured from PSEN1-deficient mice were more vulnerable to hydrogen peroxide (H2O2) treatment compared with wild-type controls. However, they reported that whereas antioxidants, BAPTA AM (an intracellular Ca2+ chelator), and nifedipine protected cells against H2O2-induced death, an N-type VDCC blocker ω-conotoxin or dantrolene did not prevent cell death. Moreover, Lopez et al.116 stated that cultured neurons from 3xTg-AD mice had increased intracellular Ca2+ levels and that the application of nifedipine and xestospongin C partially blocked this increase but blocking RYRs had no effect. Even though there are conflicting results, compounds that affect intracellular Ca2+ stores, particularly dantrolene, look promising for the future treatment of AD.
Huntington Disease
HD is an autosomal dominant, progressive neurodegenerative disorder. It is caused by an expansion of the polyglutamine tract in the N-terminal region of the protein huntingtin. This defect leads to loss of medium-sized spiny GABAergic projection neurons of the caudate nucleus and putamen of the basal ganglia.117,118
The type 1 isoform of the InsP3R is the major member of the InsP3R family in the central nervous system and it is mainly expressed in cerebellar Purkinje cells, the hippocampal CA1 region, caudate-putamen, and cerebral cortex.31 InsP3R1-deficient mice have severe ataxia, tonic-clonic seizures, and die by the weaning period.119 Tang et al.100 reported that huntingtin-associated protein-1 binds to InsP3R1 and forms a complex. This complex facilitates Ca2+ release in medium spiny striatal neurons in response to 3,5-DHPG, a selective mGluR1/5 agonist, suggesting an explanation for the dysfunction of cytosolic Ca2+ signaling in HD patients. In their later report, Tang et al.120 demonstrated that the binding site is located on the C-terminal cytosolic region of the InsP3R1 and the introduction of GFP-IC10 protein, using a viral vector, stabilizes Ca2+ signaling and protects medium spiny neurons from glutamate-induced apoptosis. Tang et al.121 reported that membrane-permeable InsP3R blockers, 2-APB and enoxaparin, are neuroprotective in medium spiny neurons in HD mice. Mutated huntingtin protein in mouse striatal neurons renders these cells vulnerable to isoflurane-mediated Ca2+ release from the ER via the InsP3R and cell apoptosis.107 To our knowledge, there has been no investigation of the possible cytoprotective effects of RYR antagonists on HD.
Parkinson Disease
PD is characterized by rigidity, resting tremor, bradykinesia, and postural instability. Pathologic findings are loss of dopaminergic neurons in the substantia nigra pars compacta and the formation of Lewy bodies, which are intracytoplasmic inclusion bodies composed of aggregates of α-synuclein.99,122 It has been suggested that mitochondrial dysfunction is crucial in the pathogenesis of PD122 as well as α-synuclein–induced excitotoxicity. Danzer et al.123 showed that α-synuclein oligomers increased [Ca2+] and caspase-3 activity in primary cell cultures. Furthermore, Furukawa et al.124 reported that whereas BAPTA AM protects cells, neither nifedipine nor ω-conotoxin protected cells against excitotoxicity induced by an α-synuclein mutation. Memantine and isradipine, an L-type Ca2+ channel inhibitor, are in phase II clinical trials for the treatment of PD.99 No study has directly investigated the possible cytoprotective effects of dantrolene on PD.
Amyotrophic Lateral Sclerosis
ALS is characterized by progressive loss of spinal and cortical motor neurons. Clinical findings are progressive loss of muscle force, breathing capacity, swallowing difficulties, and limb spasticity. ALS is largely a sporadic disease and only 5% to 10% of patients have an autosomal dominant inheritance of mutations in the enzyme superoxide dismutase 1.102 Excitotoxicity and Ca2+ dysregulation are factors in the pathogenesis of ALS. Activated microglia release proinflammatory factors and large amounts of glutamate, which activate the AMPA and NMDA receptors. Ca2+ influx increases and results in mitochondrial Ca2+ overload, mitochondrial swelling, and apoptosis. Mutant superoxide dismutase 1 also impairs mitochondrial function and handling Ca2+ overload.99,102 Memantine and riluzole, antiglutamate drugs, are in phase II/III clinical trials for the treatment of ALS.99 Rothstein and Kuncl101 showed that dantrolene provided partial motor neuron protection in organotypic cultures after non-NMDA receptor stimulation with threo-hydroxyaspartate, selectively inhibited glutamate transport, and induced glutamate toxicity.
Spinocerebellar Ataxia
SCAs are autosomal dominant neurodegenerative diseases caused by an expansion of polyglutamine tracts either in the nuclear protein ataxin-1 (ATx1) or the cytosolic protein ataxin-2 (ATx2) or ataxin-3 (ATx3). SCA1 and SCA2 mainly affect cerebellar Purkinje cells. Patients usually present with progressive ataxia, dysarthria, ophthalmoplegia, slow eye movements, and peripheral neuropathy.104 The areas affected in SCA3 are the dentate gyrus, pontine nuclei, globus pallidus, subthalamic nucleus, substantia nigra, and spinocerebellar tract. Chen et al.103 showed that mutant ATx3 binds to the InsP3R1, sensitizes to InsP3, and increases cytosolic Ca2+ levels in HEK293 cells compared with control cells. They further reported that oral administration of dantrolene to SCA3-YAC-84Q transgenic mice improved motor coordination and increased neuronal cell counts. A similar mechanism was shown for SCA2. Mutant ATx2 to 58Q associates with the InsP3R1, and sensitizes to InsP3 in planar lipid bilayers. Increased Ca2+ release induced by DHPG and more prominent cell death induced by glutamate were observed in 58Q Purkinje cell cultures compared with wild-type cell cultures. Furthermore, pretreating cells with dantrolene protected 58Q Purkinje cells against glutamate-induced cell death. Similarly, oral administration of dantrolene to transgenic mice reduced age-dependent motor deficits and Purkinje cell loss.104
Other Cytoprotective Effects of Dantrolene
Intracellular Calcium Dysregulation and Anesthesia Neurotoxicity
Previous and recent studies suggest that inhaled anesthetics, especially isoflurane, induce neurotoxicity via disruption of intracellular Ca2+ homeostasis. In brain cortical slices and in cell cultures, application of inhaled anesthetics induces Ca2+ release from the ER, increases cytosol and mitochondrial [Ca2+], depletes ER Ca2+ stores, and elicits apoptosis.125–128 Wei et al.126 reported that dantrolene suppresses cytotoxicity induced by isoflurane in cells.
Recent studies from our laboratory demonstrated that inhaled anesthetic–induced neurotoxicity is a consequence of overactivation of InsP3Rs by anesthetics.127,128 Using DT40 chicken B lymphocytes with total InsP3Rs knockout and their wild-type control cells, Yang et al.127 showed that whereas isoflurane, sevoflurane, and desflurane induced cell damage in control cells, with isoflurane being most potent, they had no effect on InsP3Rs knockout cells. Furthermore, Zhao et al.128 reported that (a) the isoflurane-induced increase in cytosolic [Ca2+] and cell damage was diminished in InsP3Rs knockdown primary cortical and hippocampal neurons; (b) isoflurane activated β-site amyloid β precursor protein-cleaving enzyme by activating InsP3Rs; and (c) early postnatal exposure of isoflurane caused transient memory and learning impairment in neonatal rats. Anesthesia-induced neurodegeneration was also shown in pups exposed to isoflurane during delivery.129 General anesthetics at low concentration or for short duration may be neuroprotective via preconditioning,130,131 whereas general anesthetics at high concentration or for prolonged exposure may be neurotoxic.129,131 Both neuroprotective and neurotoxic effects of general anesthetics may be exerted via activation of the InsP3R with differential degrees.106,107,127,128,132 Additionally, there is growing concern that anesthesia may contribute to delirium and postoperative cognitive dysfunction as well as the onset and progression of AD,133,134 although further clinical studies are needed to confirm these initial findings.
Sepsis
Increase of intracellular [Ca2+] is a critical event in the pathophysiology of endotoxemia and sepsis.135–137 Using the cecal ligation and perforation (CLP) sepsis model in rats, Song et al.135 demonstrated that perfused aortic strips from septic animals released more than 2 times the amount of Ca2+ compared with strips from control rats and that dantrolene inhibited this Ca2+ release. Dantrolene also decreased metabolic disruptions during CLP-induced sepsis in rats and increased survival rate in Escherichia coli–induced sepsis in mice.136 Furthermore, whereas dantrolene inhibited lipopolysaccharide-elicited production of interleukin-12 and interferon γ levels, neither verapamil nor diltiazem had a marked effect.137
Williams et al.138 investigated the underlying mechanism for the sepsis-induced catabolic response in skeletal muscle using CLP-induced sepsis in rats. They found that sepsis increased myofilament and calpain release and dis-integrated Z bands. Pre- or postsurgery administration of dantrolene significantly inhibited myofilament release and Z band disruption. In a following study, sepsis induced the ubiquitin-proteosome proteolytic pathway in skeletal muscle, and treatment with dantrolene prevented activation of this pathway and reduced protein degradation in rats.139 Moreover, Hassoun et al.140 demonstrated that the disruption of the SR to store Ca2+ and an increase in intracellular [Ca2+] and mitochondrial dysfunction were a consequence of Ca2+ overload in lipopolysaccharide-induced sepsis in rats. Treatment with dantrolene decreased the Ca2+ overload and mitochondrial dysfunction.
Trauma
Aslan et al.141 investigated the protective effects of dantrolene on a surgical spinal cord injury model in rabbits. Systemically administered dantrolene not only improved motor paralysis (at 24 hours) but also augmented antioxi-dative defense systems and decreased injury-induced apoptosis in spinal cord.
CONCLUSIONS
This review summarizes scientific studies investigating the possible cytoprotective effects of dantrolene on cytotoxicity mainly induced by deregulation in Ca2+ homeostasis. More studies are required to determine whether dantrolene is an effective cytoprotective drug in humans. Nevertheless, dantrolene has an advantage over other experimental compounds that regulate Ca2+ homeostasis, because it has long been in clinical use.
ACKNOWLEDGMENTS
The authors thank Drs. Roderic Eckenhoff and Maryellen Eckenhoff from the Department of Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, for valuable comments and editing of this review.
STUDY FUNDING
Supported by the National Institute of General Medical Science, National Institutes of Health, Baltimore, MD, K08 grant (1-K08-GM-073224, to HW) and R01 grant (1-R01GM084979-01, 3R01GM084979-02S1 to HW); and March of Dimes Birth Defects Foundation research grants (12-FY05-62 and 12-FY08-167 to HW), White Plains, NY.
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Tsutsumi Y, Yamamoto K, Matsuura S, Hata S, Sakai M, Shirakura K. The treatment of neuroleptic malignant syndrome using dantrolene sodium. Psychiatry Clin Neurosci 1998;52: 433–8 [DOI] [PubMed] [Google Scholar]
- 2.Dykes MH. Evaluation of a muscle relaxant: dantrolene sodium (Dantrium). JAMA 1975;231:862–4 [PubMed] [Google Scholar]
- 3.Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene: a review of its pharmacology, therapeutic use and new developments. Anaesthesia 2004;59:364–73 [DOI] [PubMed] [Google Scholar]
- 4.Hadad E, Cohen-Sivan Y, Heled Y, Epstein Y. Clinical review: treatment of heat stroke—should dantrolene be considered? Crit Care 2005;9:86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth 2006;96:678–85 [DOI] [PubMed] [Google Scholar]
- 6.Snyder HR, Davis CS, Bickerton RK, Halliday RP. 1-[(5-Arylfurfurylidene)amino]hydantoins: a new class of muscle relaxant. J Med Chem 1967;10:807–10 [DOI] [PubMed] [Google Scholar]
- 7.Ellis KO, Bryant SH. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol 1972;274:107–9 [DOI] [PubMed] [Google Scholar]
- 8.Ellis KO, Carpenter JF. Studies on the mechanism of action of dantrolene sodium: a skeletal muscle relaxant. Naunyn Schmiedebergs Arch Pharmacol 1972;274:83–94 [DOI] [PubMed] [Google Scholar]
- 9.Harrison GG. Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium. Br J Anaesth 1975;47:62–5 [DOI] [PubMed] [Google Scholar]
- 10.Hall GM, Lucke JN, Lister D. Treatment of porcine malignant hyperpyrexia: the successful use of dantrolene in the Pietrain pigs. Anaesthesia 1977;32:472–4 [DOI] [PubMed] [Google Scholar]
- 11.Kolb ME, Horne ML, Martz R. Dantrolene in human malignant hyperthermia: a multicenter study. Anesthesia 1982;56:254–62 [DOI] [PubMed] [Google Scholar]
- 12.Rosero EB, Adesanya AO, Timaran CH, Joshi GP. Trends and outcomes of malignant hyperthermia in the United States, 2000–2005. Anesthesiology 2009;110:89–94 [DOI] [PubMed] [Google Scholar]
- 13.Inui M, Saito A, Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem 1987;262:1740–7 [PubMed] [Google Scholar]
- 14.Lai FA, Anderson K, Rousseau E, Liu Q, Meissner G. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun 1988;151:441–9 [DOI] [PubMed] [Google Scholar]
- 15.Endo M Calcium release from the sarcoplasmic reticulum. Physiol Rev 1977;57:71–108 [DOI] [PubMed] [Google Scholar]
- 16.Fleischer S, Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem 1989;18:333–64 [DOI] [PubMed] [Google Scholar]
- 17.Zhao F, Li P, Chen SRW, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels: molecular mechanism and isoform selectivity. J Biol Chem 2001;276:13810–6 [DOI] [PubMed] [Google Scholar]
- 18.Essin K, Gollasch M. Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle. J Biomed Biotech 2009:135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differently expressed in rabbit brain. J Neurosci 1994;8:4794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol 1995;128:893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fruen BR, Mickelson JR, Louis CF. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J Biol Chem 1997;272:26965–71 [DOI] [PubMed] [Google Scholar]
- 22.Flewellen EH, Nelson TE, Jones WP, Arens JF, Wagner DL. Dantrolene dose response in awake man: implications for management of malignant hyperthermia. Anesthesia 1983;59:275–80 [DOI] [PubMed] [Google Scholar]
- 23.Harrison GG. Malignant hyperthermia: dantrolene—dynamics and kinetics. Br J Anaesth 1988;60:279–86 [DOI] [PubMed] [Google Scholar]
- 24.Nelson TE. Halothane effects on human malignant hyperthermia skeletal muscle single calcium-release channels in planar lipid bilayers. Anesthesiology 1992;76:588–95 [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Sylvester PL, Porta M, Copella JA. Halothane modulation of skeletal muscle ryanodine receptors: dependence on Ca2+, Mg2+, and ATP. Am J Physiol Cell Physiol 2008;294:C1103–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen GC, Larach MG, Kunselman AR. The sensitivity and specificity of the caffeine-halothane contracture test: a report from the North American Malignant Hyperthermia Registry. Anesthesiology 1998;88:579–88 [DOI] [PubMed] [Google Scholar]
- 27.Litman RS, Rosenberg HR. Malignant hyperthermia update on susceptibility testing. JAMA 2005;293:2918–24 [DOI] [PubMed] [Google Scholar]
- 28.Dirksen RT. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol 2009;587:3139–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duke AM, Hopkins PM, Calaghan SC, Halsall JP, Steele DS. Store operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J Biol Chem 2010;285:25645–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurebayashi N, Ogawa Y. Depletion of Ca2+ in sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol 2001;533:185–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derhwitz M, Sréter FA. Azumolene reverses episodes of malignant hyperthermia in susceptible swine. Anesth Analg 1990;70:253–5 [DOI] [PubMed] [Google Scholar]
- 32.White RL, Wessels FL, Schwan TJ, Ellis KO. 1-[[[5-(substituted phenyl)-2-oxazolyl]methylene]amino]-2,4-imidazolidinediones, a new class of skeletal muscle relaxants. J Med Chem 1987;2:263–6 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Rodney GG, Schneider MF. Effects of azumolene on Ca2+ sparks in skeletal muscle fibers. J Pharmacol Exp Ther 2005;314:94–102 [DOI] [PubMed] [Google Scholar]
- 34.do Carmo PL, Zapata-Sudo G, Trachez MM, Antunes F, Guimarães SE, Debom R, Rizzi MD, Sudo RT. Intravenous administration of azumolene to reverse malignant hyperthermia in swine. J Vet Intern Med (in press) [DOI] [PubMed] [Google Scholar]
- 35.Sudo RT, do Carmo PL, Trachez MM, Zapata-Sudo G. Effects of azumolene on normal and malignant hyperthermia-susceptible skeletal muscle. Basic Clin Pharmacol Toxicol 2008;102:308–16 [DOI] [PubMed] [Google Scholar]
- 36.Faling LJ, Petusevsky ML, Snider GL. Nitrofrontain and dantrolene; liver and lung. Ann Intern Med 1980;93:151. [DOI] [PubMed] [Google Scholar]
- 37.Mowbray M, Sinclair SA, Allan SJ. Severe acneiform eruption exacerbated by dantrolene sodium. Clin Exp Dermatol 2009;34:248–9 [DOI] [PubMed] [Google Scholar]
- 38.Petusevsky ML, Faling LJ, Rocklin RE, Snider GL, Merliss AD, Moses JM, Dorman SA. Pleuropericardial reaction to treatment with dantrolene. JAMA 1979;242:2772–4 [PubMed] [Google Scholar]
- 39.Saltzman LS, Kates RA, Corke BC, Norfleet EA, Heath KR. Hyperkalemia and cardiovascular collapse after verapamil and dantrolene administration in swine. Anesth Analg 1984;63:473–8 [PubMed] [Google Scholar]
- 40.Lynch C, Durbin CG, Fisher NA, Veselis RA, Althaus JS. Effects of dantrolene and verapamil on atrioventricular conduction and cardiovascular performance in dogs. Anesth Analg 1986;65:252–8 [PubMed] [Google Scholar]
- 41.Driessen JJ, Wuis EW, Gielen MJ. Prolonged vecuronium neuromuscular blockade in a patient receiving orally administered dantrolene. Anesthesiology 1985;62:523–4 [DOI] [PubMed] [Google Scholar]
- 42.Wuis EW, Rijntjes NVM, Kleijn V. Whole-body autoradiography of 14C-dantrolene in the marmoset monkey. Pharmacol Toxicol 1989;64:156–8 [DOI] [PubMed] [Google Scholar]
- 43.Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab Dispos 2008;36:995–1002 [DOI] [PubMed] [Google Scholar]
- 44.Patti F, Maccagnano C, Panico AM, Giammona G, Rampello L, Reggio A, Di Giorgio RM, Nicoletti F. Effects of dantrolene sodium on gabaergic activity in spinal cord, corpus striatum, substantia nigra and cerebral cortex in rat. Acta Neurol (Napoli) 1981;3:384–8 [PubMed] [Google Scholar]
- 45.Wei H, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. J Neurochem 1996;67:2390–8 [DOI] [PubMed] [Google Scholar]
- 46.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 2003;4:552–65 [DOI] [PubMed] [Google Scholar]
- 47.Høyer-Hansen M, Jäättelä M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 2007;14:1576–82 [DOI] [PubMed] [Google Scholar]
- 48.Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaä¨ttelä M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 2007;25:193–205 [DOI] [PubMed] [Google Scholar]
- 49.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature 1993;361:315–25 [DOI] [PubMed] [Google Scholar]
- 50.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Science’s STKE 2004; www.stke.org/cgi/content/full/sigtrans;2004/215/re1 [DOI] [PubMed] [Google Scholar]
- 51.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+ molecular determinants and functional consequences. Physiol Rev 2006;86:369–408 [DOI] [PubMed] [Google Scholar]
- 52.Schlaepfer WW, Bunge RP. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J Cell Biol 1973;59:456–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett 1985;58:293–7 [DOI] [PubMed] [Google Scholar]
- 54.Verkhratsky A, Shmigol A. Calcium-induced calcium release in neurones. Cell Calcium 1996;19:1–14 [DOI] [PubMed] [Google Scholar]
- 55.Hajos F, Garthwaite G, Garthwaite J. Reversible and irreversible neuronal damage caused by excitatory amino acid analogues in rat cerebellar slices. Neuroscience 1986;18:417–36 [DOI] [PubMed] [Google Scholar]
- 56.Garthwaite G, Garthwaite J. Neurotoxicity of excitatory amino acid receptor agonists in rat cerebellar slices: dependence on calcium concentration. Neurosci Lett 1986;66:193–8 [DOI] [PubMed] [Google Scholar]
- 57.Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med 2000;78:3–13 [DOI] [PubMed] [Google Scholar]
- 58.Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care 2009;10:103–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Criollo A, Vicencio JM, Tasdemir E, Maiuri MC, Lavandero S, Kroemer G. The inositol trisphosphate receptor in the control of autophagy. Autophagy 2007;3:350–3 [DOI] [PubMed] [Google Scholar]
- 60.Sarkar S, Korolchuk V, Renna M, Winslow A, Rubinsztein DC. Methodological considerations for assessing autophagy modulators: a study with calcium phosphate precipitates. Autophagy 2009;5:307–13 [DOI] [PubMed] [Google Scholar]
- 61.Katchman AN, Hershkowitz N. Early anoxia-induced vesicular glutamate release results from mobilization of calcium from intracellular stores. J Neurophysiol 1993;70:1–7 [DOI] [PubMed] [Google Scholar]
- 62.Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem 1984;43:1369–74 [DOI] [PubMed] [Google Scholar]
- 63.Benveniste H, Jorgensen MB, Diemer NH, Hansen AJ. Calcium accumulation by glutamate receptor activation is involved in hippocampal cell damage after ischemia. Acta Neurol Scand 1988;78:529–36 [DOI] [PubMed] [Google Scholar]
- 64.Choi DW. Possible mechanisms limiting N-methyl-D-aspartate receptor overactivation and the therapeutic efficacy of N-methyl-D-aspartate antagonists. Stroke 1990;21:III20–2 [PubMed] [Google Scholar]
- 65.Mitani A, Yanase H, Sakai K, Wake Y, Kataoka K. Origin of intracellular Ca2+ elevation induced by in vitro ischemia-like condition in hippocampal slices. Brain Res 1993;601:103–10 [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Andou Y, Masuda S, Mitani A, Kataoka K. Dantrolene protects against ischemic, delayed neuronal death in gerbil brain. Neurosci Lett 1993;158:105–8 [DOI] [PubMed] [Google Scholar]
- 67.Yano T, Nakayama R, Imaizumi T, Terasaki H, Ushijima K. Dantrolene ameliorates delayed cell death and concomitant DNA fragmentation in the rat hippocampal CA1 neurons subjected to mild ischemia. Resuscitation 2001;50:117–25 [DOI] [PubMed] [Google Scholar]
- 68.Nakayama R, Yano T, Ushijima K, Abe E, Terasaki H. Effects of dantrolene on extracellular glutamate concentration and neuronal death in the rat hippocampal CA1 region subjected to transient ischemia. Anesthesiology 2002;96:705–10 [DOI] [PubMed] [Google Scholar]
- 69.Wang C, Nguyen HN, Maguire JL, Perry DC. Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in a neuronal cell line. J Cereb Blood Flow Metab 2002;22:206–14 [DOI] [PubMed] [Google Scholar]
- 70.Li F, Hayashi T, Jin G, Deguchi K, Nagotani S, Nagano I, Shoji M, Chan PH, Abe K. The protective effect of dantrolene on ischemic neuronal cell death is associated with reduced expression of endoplasmic reticulum stress markers. Brain Res 2005;1048:59–68 [DOI] [PubMed] [Google Scholar]
- 71.Gwak M, Park P, Kim K, Lim K, Jeong S, Baek C, Lee J. The effects of dantrolene on hypoxic-ischemic injury in the neonatal rat brain. Anesth Analg 2008;106:227–33 [DOI] [PubMed] [Google Scholar]
- 72.Kocogullari CU, Emmiler M, Cemek M, Sahin O, Aslan A, Ayva E, Tur L, Buyukokuroglu ME, Demirkan I, Cekirdekci A. Can dantrolene protect spinal cord against ischemia/reperfusion injury? An experimental study. Thorac Cardiovasc Surg 2008;56:406–11 [DOI] [PubMed] [Google Scholar]
- 73.Salomone S, Soydan G, Moskowitz MA, Sims JR. Inhibition of cerebral vasoconstriction by dantrolene and nimodipine. Neurocrit Care 2009;10:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ally A, Horrobin DF, Manku MS, Morgan RO, Karmazyn M, Karmali RA, Cunnane SC. Dantrolene blocks intracellular calcium release in smooth muscle: competitive antagonism of thromboxane A2. Can J Physiol Pharmacol 1978;56:520–3 [DOI] [PubMed] [Google Scholar]
- 75.Savineau JP, Gonzalez Da La Fuente P, Marthan R. Effect of modulators of tyrosine kinase activity on agonist-induced contraction in the rat pulmonary vascular smooth muscle. Pulm Pharmacol 1996;3:189–95 [DOI] [PubMed] [Google Scholar]
- 76.Giulumian AD, Meszaros LG, Fuchs LC. Endothelin-1-induced contraction of mesenteric small arteries is mediated by ryanodine receptor Ca2+ channels and cyclic ADP-ribose. J Cardiovasc Pharmacol 2000;36:758–63 [DOI] [PubMed] [Google Scholar]
- 77.Martínez-Sánchez M, Striggow F, Schroder UH, Kahlert S, Reymann KG, Reiser G. Na(+) and Ca(2+) homeostasis pathways, cell death and protection after oxygen-glucose-deprivation in organotypic hippocampal slice cultures. Neuroscience 2004; 128:729 – 40 [DOI] [PubMed] [Google Scholar]
- 78.Preckel B, Schlack W, Comfere T, Thamer V. Effect of dantrolene in an in vivo and in vitro model of myocardial reperfusion injury. Acta Anaesthesiol Scand 2000;44:194–201 [DOI] [PubMed] [Google Scholar]
- 79.Wu D, Chen X, Ding R, Qiao X, Shi S, Xie Y, Hong Q, Feng Z. Ischemia/reperfusion induce renal tubule apoptosis by inositol 1,4,5-trisphosphate receptor and L-type Ca2+ channel opening. Am J Nephrol 2008;28:487–99 [DOI] [PubMed] [Google Scholar]
- 80.Kaya M, Tunc M, Ozdemir T, Altuntas I. Calcium antagonists in N-methyl d-aspartate-induced retinal injury. Graefes Arch Clin Exp Ophthalmol 2003;241:418–22 [DOI] [PubMed] [Google Scholar]
- 81.Popescu BO, Oprica M, Sajin M, Stanciu CL, Bajenaru O, Predescu A, Vidulescu C, Popescu LM. Dantrolene protects neurons against kainic acid induced apoptosis in vitro and in vivo. J Cell Mol Med 2002;6:555–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duzenli S, Bakuridze K, Gepdiremen A. The effects of ruthenium red, dantrolene and nimodipine, alone or in combination, in NMDA induced neurotoxicity of cerebellar granular cell culture of rats. Toxicol In Vitro 2005;19:589–94 [DOI] [PubMed] [Google Scholar]
- 83.Mori F, Okada M, Tomiyama M, Kaneko S, Wakabayashi K. Effects of ryanodine receptor activation on neurotransmitter release and neuronal cell death following kainic acid-induced status epilepticus. Epilepsy Res 2005;65:59–70 [DOI] [PubMed] [Google Scholar]
- 84.Makarewicz D, Zieminska E, Lazarewicz JW. Dantrolene inhibits NMDA-induced 45Ca uptake in cultured cerebellar granule neurons. Neurochem Int 2003;43:273–8 [DOI] [PubMed] [Google Scholar]
- 85.Sanabria ERG, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol 2001;532:205–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kulak W, Sobaniec W, Wojtal K, Czuczwar SJ. Calcium modulation in epilepsy. Pol J Pharmacol 2004;56:29–41 [PubMed] [Google Scholar]
- 87.McDonald JW, Fix AS, Tizzano JP, Schoepp DD. Seizures and brain injury in neonatal rats induced by 1S,3R-ACPD, a metabotropic glutamate receptor agonist. J Neurosci 1993;13:4445–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niebauer M, Gruenthal M. Neuroprotective effects of early vs. late administration of dantrolene in experimental status epilepticus. Neuropharmacology 1999;38:1343–8 [DOI] [PubMed] [Google Scholar]
- 89.Pelletier MR, Wadia JS, Mills LR, Carlen PL. Seizure-induced cell death produced by repeated tetanic stimulation in vitro: possible role of endoplasmic reticulum calcium stores. J Neurophysiol 1999;81:3054–64 [DOI] [PubMed] [Google Scholar]
- 90.Nagatomo I, Hashiguchi W, Tominaga M, Akasaki Y, Uchida M, Takigawa M. Effects of MK-801, dantrolene, and FK506 on convulsive seizures and brain nitric oxide production in seizure-susceptible EL mice. Brain Res 2001;888:306–10 [DOI] [PubMed] [Google Scholar]
- 91.Swiader M, Borowicz KK, Porebiak J, Kleinrok Z, Czuczwar SJ. Influence of agents affecting voltage-dependent calcium channels and dantrolene on the anticonvulsant action of the AMPA/kainate receptor antagonist LY 300164 in mice. Eur Neuropsychopharmacol 2002;12:311–9 [DOI] [PubMed] [Google Scholar]
- 92.Borowicz KK, Gasior M, Kleinrok Z, Czuczwar SJ. Influence of isradipine, niguldipine and dantrolene on the anticonvulsive action of conventional antiepileptics in mice. Eur J Pharmacol 1997;323:45–51 [DOI] [PubMed] [Google Scholar]
- 93.Barton ME, Shannon HE. Behavioral and convulsant effects of the (S) enantiomer of the group I metabotropic glutamate receptor agonist 3,5-DHPG in mice. Neuropharmacology 2005;48:779–87 [DOI] [PubMed] [Google Scholar]
- 94.Marambaud P, Dreses-Werringloer U, Vingtdeux V. Calcium signaling in neurodegeneration. Mol Neurodegener 2009;4:20–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacManus A, Ramsden M, Murray M, Henderson Z, Pearson HA, Campbell VA. Enhancement of (45)Ca(2+) influx and voltage-dependent Ca(2+) channel activity by beta-amyloid-(1–40) in rat cortical synaptosomes and cultured cortical neurons: modulation by the proinflammatory cytokine interleukin-1beta. J Biol Chem 2000;275:4713–8 [DOI] [PubMed] [Google Scholar]
- 96.Rovira C, Arbez N, Mariani J. Abeta(25–35) and Abeta(1–40) act on different calcium channels in CA1 hippocampal neurons. Biochem Biophys Res Commun 2002;296:1317–21 [DOI] [PubMed] [Google Scholar]
- 97.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 2005;280:17294–300 [DOI] [PubMed] [Google Scholar]
- 98.Egea J, Rosa AO, Cuadrado A, Garcia AG, Lopez MG. Nicotinic receptor activation by epibatidine induces heme oxygenase-1 and protects chromaffin cells against oxidative stress. J Neurochem 2007;102:1842–52 [DOI] [PubMed] [Google Scholar]
- 99.Bezprozvanny I Calcium signaling and neurodegenerative diseases. Trends Mol Med 2009;15:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron 2003;39:227–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rothstein JD, Kuncl RW. Neuroprotective strategies in a model of chronic glutamate-mediated motor neuron toxicity. J Neurochem 1995;65:643–51 [DOI] [PubMed] [Google Scholar]
- 102.Von Lewinski F, Keller BU. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci 2005;28:494–500 [DOI] [PubMed] [Google Scholar]
- 103.Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, Nukina N, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci 2008;28:12713–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci 2009;29:9148–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 2007;8:101–12 [DOI] [PubMed] [Google Scholar]
- 106.Liang G, Wang Q, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei H. A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg 2008;106:492–500 [DOI] [PubMed] [Google Scholar]
- 107.Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-triphosphate receptors. Anesthesiology 2008;108:251–60 [DOI] [PubMed] [Google Scholar]
- 108.Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci 1997;17:4212–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci 2006;26:6011–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 2008;59:214–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, Parsons CG, Gupta S, Banerjee P, LaFerla FM. Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. Am J Pathol 2010;176:870–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shtifman A, Ward CW, Laver DR, Bannister ML, Lopez JR, Kitazawa M, LaFerla FM, Ikemoto N, Querfurth HW. Amyloid-β protein impairs Ca2+ release and contractility in skeletal muscle. Neurobiol Aging 2008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Imaizumi K, Morihara T, Mori Y, Katayama T, Tsuda M, Furuyama T, Wanaka A, Takeda M, Tohyama M. The cell death-promoting gene DP5, which interacts with the BCL2 family, is induced during neuronal apoptosis following exposure to amyloid beta protein. J Biol Chem 1999;274:7975–81 [DOI] [PubMed] [Google Scholar]
- 114.Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J Neurosci 2000;20:1358–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakajima M, Miura M, Aosaki T, Shirasawa T. Deficiency of presenilin-1 increases calcium-dependent vulnerability of neurons to oxidative stress in vitro. J Neurochem 2001;78:807–14 [DOI] [PubMed] [Google Scholar]
- 116.Lopez JR, Lyckman A, Oddo S, LaFerla FM, Querfurth HW, Shtifman A. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J Neurochem 2008;105:262–71 [DOI] [PubMed] [Google Scholar]
- 117.Wexler NS, Rose EA, Housman DE. Molecular approaches to hereditary diseases of the nervous system: Huntington’s disease as a paradigm. Annu Rev Neurosci 1991;14:503–29 [DOI] [PubMed] [Google Scholar]
- 118.DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, Martin E, Vonsattel JP, Carraway R, Reeves SA, Boyce FM, Aronin N. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 1995;14:1075–81 [DOI] [PubMed] [Google Scholar]
- 119.Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, Minowa O, Kuno J, Sakakibara S, Yamada M, Yoneshima H, Miyawaki A, Fukuuchi Y, Furuichi T, Okano H, Mikoshiba K, Noda T. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature 1996;379:168–71 [DOI] [PubMed] [Google Scholar]
- 120.Tang TS, Guo C, Wang H, Chen X, Bezprozvanny I. Neuroprotective effects of inositol 1,4,5-trisphosphate receptor C-terminal fragment in a Huntington’s disease mouse model. J Neurosci 2009;29:1257–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, Kristal BS, Hayden MR, Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci USA 2005;102:2602–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci 2006;7:207–19 [DOI] [PubMed] [Google Scholar]
- 123.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci 2007;27:9220–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Sugeno N, Itoyama Y, Wang Y, Yao PJ, Bushlin I, Takeda A. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J Neurochem 2006;97:1071–7 [DOI] [PubMed] [Google Scholar]
- 125.Kindler CH, Eilers H, Donohoe P, Ozer S, Bickler PE. Volatile anesthetics increase intracellular calcium in cerebrocortical and hippocampal neurons. Anesthesiology 1999;90:1137–45 [DOI] [PubMed] [Google Scholar]
- 126.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res 2005;1037:139–47 [DOI] [PubMed] [Google Scholar]
- 127.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei HF. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 2008;109:243–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao Y, Liang G, Chen Q, Joseph DJ, Meng Q, Eckenhoff RG, Eckenhoff MF, Wei H. Anesthetic induced neurodegeneration mediated via inositol 1,4,5-trisphosphate receptors. J Pharmacol Exp Ther 2010;333:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang S, Peretich K, Zhao Y, Liang G, Meng Q, Wei H. Anesthesia-induced neurodegeneration in fetal rat brains. Pediatr Res 2009;66:435–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H. Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology 2007;53:942–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei H, Liang G, Yang H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci Lett 2007;425:59–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology 2005;103:532–9 [DOI] [PubMed] [Google Scholar]
- 133.Baranov D, Bickler PE, Crosby GJ, Culley DJ, Eckenhoff MF, Eckenhoff RG, Hogan KJ, Jevtovic-Todorovic V, Palotas A, Perouansky M, Planel E, Silverstein JH, Wei H, Whittington RA, Xie Z, Zuo Z. Consensus statement: First International Workshop on Anesthetics and Alzheimer’s Disease. Anesth Analg 2009;108:1627–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wei H, Xie Z. Anesthesia, calcium homeostasis and Alzheimer’s disease. Curr Alzheimer Res 2009;6:30–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song SK, Karl IE, Ackerman JJ, Hotchkiss RS. Increased intracellular Ca2+: a critical link in the pathophysiology of sepsis? Proc Natl Acad Sci 1993;90:3933–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hotchkiss RS, Karl IE. Dantrolene ameliorates the metabolic hallmarks of sepsis in rats and improves survival in a mouse model of endotoxemia. Proc Natl Acad Sci 1994;91:3039–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nemeth ZH, Hasko G, Szabo C, Salzman AL, Vizi ES. Calcium channel blockers and dantrolene differentially regulate the production of interleukin-12 and interferon-gamma in endotoxemic mice. Brain Res Bull 1998;46:257–61 [DOI] [PubMed] [Google Scholar]
- 138.Williams AB, Decourten-Myers GM, Fischer JE, Luo G, Sun X, Hasselgren PO. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J 1999;13:1435–43 [DOI] [PubMed] [Google Scholar]
- 139.Wray CJ, Sun X, Gang GI, Hasselgren PO. Dantrolene down-regulates the gene expression and activity of the ubiquitinproteasome proteolytic pathway in septic skeletal muscle. J Surg Res 2002;104:82–7 [DOI] [PubMed] [Google Scholar]
- 140.Hassoun SM, Marechal X, Montaigne D, Bouazza Y, Decoster B, Lancel S, Neviere R. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Crit Care Med 2008;36:2590–6 [DOI] [PubMed] [Google Scholar]
- 141.Aslan A, Cemek M, Buyukokuroglu ME, Altunbas K, Bas O, Yurumez Y, Cosar M. Dantrolene can reduce secondary damage after spinal cord injury. Eur Spine J 2009;18:1442–51 [DOI] [PMC free article] [PubMed] [Google Scholar]


