Abstract
The EFSA Panel on Food Additives and Flavourings (FAF) was requested to evaluate the genotoxic potential of five flavouring substances from subgroup 3.3 of FGE.19, in the Flavouring Group Evaluation 216 (FGE.216). In FGE.216 and in FGE.216Rev1, the CEF Panel requested additional genotoxicity data on 2‐phenylcrotonaldehyde [FL‐no: 05.062], the representative for these five substances. New experimental data on [FL‐no: 05.062] were provided and are evaluated in the present revision of FGE.216 (FGE.216Rev2). Based on the new data, the Panel concluded that, for all the five substances, the concerns for gene mutations and clastogenicity are ruled out by the negative results observed in an in vivo gene mutation assay and in an in vivo comet assay, respectively. In vitro, [FL‐no: 05.062] induced micronuclei through an aneugenic mode of action. The available in vivo micronucleus studies were inconclusive and cannot be used to rule out potential aneugenicity of [FL‐no: 05.062] in vivo. Therefore, the Panel compared the lowest concentration resulting in aneugenicity in vitro with the use levels reported for this substance. Based on this comparison, the Panel concluded that the use of the flavouring substance [FL‐no: 05.062] at the reported use levels in several food categories would raise a concern for aneugenicity. Based on structural similarity, for the remaining four substances in this FGE [FL‐no: 05.099, 05.100, 05.175 and 05.222], an aneugenic potential may also be anticipated. For these four substances, individual data are needed to establish whether they have aneugenic potential. Accordingly, it is currently not appropriate to assess any of these five substances through the Procedure for the evaluation of flavouring substances.
Keywords: FGE.216; α,β‐unsaturated 2‐phenyl‐2‐alkenals; flavouring substances; safety evaluation; subgroup 3.3; FGE.19
1. Introduction
1.1. Background and terms of reference as provided by the requestor
The use of flavourings is regulated under Regulation (EC) No 1334/2008 1 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation an evaluation and approval are required for flavouring substances.
The Union List of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/2012 2 . The list contains flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/2000 3 .
On 4 July 2013 the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids adopted an opinion on Flavouring Group Evaluation 216 Revision 1 (FGE.216 Rev1): Consideration of genotoxicity data on α,β–unsaturated 2‐Phenyl‐2‐Alkenals from Subgroup 3.3 of FGE.19.
The Panel concluded that for representative substance 2‐phenylcrotonaldehyde [FL‐no: 05.062], the Panel's concern with respect to genotoxicity could not be ruled out and subsequently additional data are requested.
On 7 January 2014 the applicant submitted to the Commission and to EFSA (Ares(2014)96077) additional information on this group of substances. However, this new data does not completely address the request of data by EFSA in its opinion concerning the proof of sufficient systemic exposure to the test substance.
On 15 August 2014 the applicant submitted to the Commission and to EFSA (Ares(2015)886499) analytical data for plasma analysis of the representative substance 2‐phenylcrotonaldehyde [FL‐no: 05.062].
On 9 January 2015 Ares(2015)202297 the applicant has submitted the final report on the representative substance [FL‐no: 05.062]. The study concerns the development and limited validation of a method for the analysis of plasma samples which may contain 2‐phenylcrotonaldehyde.
1.1.1. Terms of Reference
The European Commission requests the European Food Safety Authority (EFSA) to evaluate this new information and, depending on the outcome, proceed to the full evaluation of this group of substances (FGE.19 subgroup 3.3) for which the substance [FL‐no: 05.062] is representative in accordance with Commission Regulation (EC) No 1565/2000.
2. Data and methodologies
2.1. History of the evaluation of FGE.19 substances
Flavouring Group Evaluation 19 (FGE.19) contains 360 flavouring substances from the EU Register being α,β‐unsaturated aldehydes or ketones and precursors which could give rise to such carbonyl substances via hydrolysis and/or oxidation (EFSA, 2008a).
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity. The Panel noted that there were limited genotoxicity data on these flavouring substances but that positive genotoxicity studies were identified for some substances in the group.
The α,β‐unsaturated carbonyls were subdivided into subgroups on the basis of structural similarity (EFSA, 2008a). In an attempt to decide which of the substances could go through the Procedure, a (quantitative) structure–activity relationship (Q)SAR prediction of the genotoxicity of these substances was undertaken considering a number of models (DEREKfW, TOPKAT, DTU‐NFI‐MultiCASE Models and ISS‐Local Models (Gry et al., 2007)).
The Panel noted that for most of these models, internal and external validation has been performed, but considered that the outcome of these validations was not always extensive enough to appreciate the validity of the predictions of these models for these α,β‐unsaturated carbonyls. Therefore, the Panel considered it inappropriate to totally rely on (Q)SAR predictions at this point in time and decided not to take substances through the Procedure based on negative (Q)SAR predictions only.
The Panel took note of the (Q)SAR predictions by using two ISS local models (Benigni and Netzeva, 2007) and four DTU‐NFI MultiCASE models (Gry et al., 2007; Nikolov et al., 2007) and the fact that there are available data on genotoxicity, in vitro and in vivo, as well as data on carcinogenicity for several substances.
Based on these data, the Panel decided that 15 subgroups (1.1.1, 1.2.1, 1.2.2, 1.2.3, 2.1, 2.2, 2.3, 2.5, 3.2, 4.3, 4.5, 4.6, 5.1, 5.2 and 5.3) (EFSA, 2008a,b) could not be evaluated through the Procedure due to concern with respect to genotoxicity. Corresponding to these subgroups, 15 flavouring group evaluations (FGEs) were established: FGE.200, 204, 205, 206, 207, 208, 209, 211, 215, 219, 221, 222, 223, 224 and 225.
For 11 subgroups, the Panel decided, based on the available genotoxicity data and (Q)SAR predictions, that a further scrutiny of the data should take place before requesting additional data on genotoxicity from the flavouring industry. These subgroups were evaluated in FGE.201, 202, 203, 210, 212, 213, 214, 216, 217, 218 and 220. For the substances in FGE.202, 214 and 218, it was concluded that a genotoxic potential could be ruled out, and accordingly, these substances have been evaluated using the Procedure. For all or some of the substances in the remaining FGEs, FGE.201, 203, 210, 212, 213, 216, 217 and 220, the genotoxic potential could not be ruled out.
To ease the data retrieval of the large number of structurally related α,β‐unsaturated substances in the different subgroups for which additional data are requested, EFSA has worked out a list of representative substances for each subgroup (EFSA, 2008c). Likewise, an EFSA genotoxicity expert group has worked out a test strategy to be followed in the data retrieval for these substances (EFSA, 2008b).
The flavouring industry has been requested to submit additional genotoxicity data according to the list of representative substances and test strategy for each subgroup.
The flavouring industry has submitted additional data and the present revision of FGE.216 (FGE.216Rev2) concerns the evaluation of these data requested on genotoxicity.
2.2. Presentation of the substances in flavouring group evaluation 216
The flavouring group evaluation 216 (FGE.216), corresponding to FGE.19 subgroup 3.3, concerns five α,β‐unsaturated 2‐phenyl substituted aldehydes, 2‐phenylcrotonaldehyde [FL‐no: 05.062], 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099], 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100], 2‐phenylpent‐2‐enal [FL‐no: 05.175] and 2‐phenyl‐4‐methyl‐2‐hexenal [FL‐no: 05.222], which are presented in Appendix A, Table A.1. In the EFSA Opinion, ‘List of α,β‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing’ (EFSA, 2008c), 2‐phenylcrotonaldehyde [FL‐no: 05.062] (Table 1) had been selected as representative flavouring substance for FGE.19, subgroup 3.3, corresponding to FGE.216.
Table 1.
Representative substance for subgroup 3.3 of FGE.19 (EFSA, 2008c)
| FL‐no JECFA‐no | Subgroup | EU Register name | Structural formula | Comments |
|---|---|---|---|---|
| 05.062 1474 | 3.3 | 2‐Phenylcrotonaldehyde |
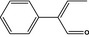
|
Data submitted in accordance to request and evaluated in FGE.216, FGE.216Rev1 and FGE.216Rev2 |
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity (EFSA, 2008a). Accordingly, the available data on genotoxic or carcinogenic activity for the five aldehydes [FL‐no: 05.062, 05.099, 05.100, 05.175 and 05.222] will be considered in this FGE.
2.3. History of the evaluation of the substances belonging to FGE.216
In the first scientific opinion on FGE.216 (EFSA, 2009), no data from genotoxicity or carcinogenicity studies with any of the substances in FGE.216 were available. The (Q)SAR predictions of these substances were limited to one endpoint (gene mutations in one strain of Salmonella), see Appendix C, Table C.1. 2‐Phenylcrotonaldehyde [FL‐no: 05.062], 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099] and 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100] were evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) as of no safety concern (JECFA, 2006), see Appendix B, Table B.1.
In FGE.216 (EFSA, 2009), the data available were insufficient to rule out the concern for genotoxicity; therefore, the CEF Panel requested to provide additional data on genotoxicity for the representative substance of this subgroup, according to the Genotoxicity Test Strategy for Substances Belonging to Subgroups of FGE.19 (EFSA, 2008b).
The flavouring industry submitted genotoxicity data on the representative substance 2‐phenylcrotonaldehyde [FL‐no: 05.062] that were evaluated in FGE.216Rev1 (EFSA CEF Panel, 2013): a bacterial reverse mutation assay, an in vitro and an in vivo micronucleus (MN) assay. The CEF Panel noted that 2‐phenylcrotonaldehyde induced micronuclei in vitro in the absence of metabolic activation. Equivocal results were obtained in the in vivo MN assay with no evidence of bone marrow exposure.
JECFA evaluated the same studies in 2014 (JECFA, 2015) together with a second in vivo MN assay, which is evaluated also in the present revision of FGE.216 (FGE.216Rev2). JECFA concluded that the Procedure cannot be applied until concerns regarding genotoxicity are addressed for [FL‐no: 05.062, 05.099, 05.100, 05.222].
In FGE.216Rev1 (EFSA CEF Panel, 2013), the CEF Panel requested to provide proof of sufficient systemic exposure of animals treated with 2‐phenylcrotonaldehyde [FL‐no: 05.062] and to provide an in vivo comet assay in the gastrointestinal system.
The flavouring industry has submitted data requested by the CEF Panel in FGE.216Rev1 that are evaluated in the present revision of FGE.216 (FGE.216Rev2).
Sections 2.4 and 2.5 report the same information that was presented in FGE.216 and FGE.216Rev1, respectively. Section 3 presents the evaluation of the new data submitted for 2‐phenylcrotonaldehyde [FL‐no: 05.062].
| FGE | Adopted by EFSA | Link | No. of substances |
|---|---|---|---|
| FGE.216 | 27 November 2008 | https://www.efsa.europa.eu/en/efsajournal/pub/881 | 5 |
| FGE.216Rev1 | 4 July 2013 | https://www.efsa.europa.eu/en/efsajournal/pub/3305 | 5 |
| FGE.216Rev2 | 29 June 2022 | https://www.efsa.europa.eu/it/efsajournal/pub/7420 | 5 |
2.4. Data evaluated in FGE.216 4
2.4.1. (Q)SAR predictions
In Appendix C, the outcomes of the (Q)SAR predictions for possible genotoxic activity in five in vitro (Q)SAR models (ISS Local Model‐Ames test, DTU‐NFI MultiCASE‐Ames test, ‐Chromosomal aberration test in Chinese hamster ovary cells (CHO), ‐Chromosomal aberration test in Chinese hamster lung cells (CHL), and ‐Mouse lymphoma test) are presented.
For all substances, a negative prediction was obtained in the ISS Local Model for mutagenicity in the Ames test with Salmonella typhimurium strain TA100 without activation. For the four DTU‐NFI MultiCASE models, these substances were invariably out of domain, meaning that these models did not provide indication on the presence or absence of a genotoxic potential (see Appendix C).
2.4.2. Carcinogenicity studies
No carcinogenicity studies are available for the substances in subgroup 3.3 of FGE.19.
2.4.3. Genotoxicity studies
No genotoxicity studies are available for the substances in subgroup 3.3 of FGE.19.
2.4.4. Conclusion on genotoxicity and carcinogenicity
No data from genotoxicity or carcinogenicity studies with any of the substances in FGE.216 are available. The available (Q)SAR predictions of these substances are limited to one endpoint (gene mutations in one strain of Salmonella). The data are insufficient to rule out the concern for genotoxicity.
2.4.5. Conclusions
The Panel concluded that a genotoxic potential of the five 2‐phenyl‐substituted aldehydes (i.e. 2‐phenyl‐2‐alkenals) in the present FGE.216 could not be ruled out, and therefore, these five substances cannot presently be evaluated through the Procedure. Additional data on genotoxicity for representative substances of this subgroup should be provided, according to the Genotoxicity Test Strategy for Substances Belonging to Subgroups of FGE.19 (EFSA, 2008b).
2.5. Additional genotoxicity data considered in FGE.216Rev1 5
The first revision of FGE.216, Revision 1 (FGE.216Rev1) concerned the evaluation of new genotoxicity data submitted by European Flavour and Fragrance Association (EFFA), in response to the request by EFSA in FGE.216, for the representative substance 2‐phenylcrotonaldehyde [FL‐no: 05.062], which is supposed to cover the genotoxicity evaluation of the four other substances in FGE.19, subgroup 3.3, 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099], 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100], 2‐phenylpent‐2‐enal [FL‐no: 05.175] and 2‐phenyl‐4‐methyl‐2‐hexenal [FL‐no: 05.222].
The new data submitted covered in vitro assays in bacteria and mammalian cell systems and in vivo data in the rat.
2.5.1. In vitro data
2.5.1.1. Bacterial reverse mutation assay
Ames assays were conducted in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 to assess the mutagenicity of 2‐phenylcrotonaldehyde [FL‐no: 05.062] (98.1% sum of isomers), both in the absence and in the presence of metabolic activation by an Aroclor 1,254‐induced rat liver post‐mitochondrial fraction (S9‐mix) in three separate assays using both standard plate incorporation and modified pre‐incubation treatments (Kilford, 2010). The protocol followed OECD Test Guideline (TG) 471 (OECD, 1997a) and the study was performed according to GLP.
In assay 1, no increases in revertant numbers were observed when S. typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 were incubated with 2‐phenylcrotonaldehyde [FL‐no: 05.062] up to 5,000 μg/plate in the absence and presence of S9‐mix using the standard plate incorporation method. A weak to moderate bacteriostatic activity was noted at concentrations of 1,000 μg/plate and above in strains TA98 and TA102 in the absence of S9‐mix and in strains TA1537 and TA102 in the presence of S9‐mix.
In assay 2, no increases in revertant numbers were observed when S. typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 were treated with 2‐phenylcrotonaldehyde [FL‐no: 05.062] up to 5,000 μg/plate in the absence of S9‐mix. In the presence of S9‐mix, the same concentrations were tested on strains TA98, TA100 and TA1535, whereas TA1537 and TA102 were treated up to 2000 μg/plate due to an excessive level of cytotoxicity in the first assay. A marked reduction in revertant numbers and/or slight thinning of the bacterial lawn was noted in all the high doses tested. No increase of revertants was observed except in the treatments of the TA100 strain in the absence of S9‐mix at a concentration of 2000 μg/plate and in the presence of S9‐mix at a concentration of 320 μg/plate. The increase in revertant mutations was statistically significant (p < 0.01), but these results were isolated and not reproducible in further assays.
To further explore the increase in mutations seen only in S. typhimurium strain TA100, assay 3 was performed in all tester strains in the presence of S9‐mix and in the absence of S9‐mix in strain TA100. No mutagenic effect was demonstrated.
Under these conditions, 2‐phenylcrotonaldehyde [FL‐no: 05.062] demonstrated no mutagenic activity in S. typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 both in the absence and in the presence of metabolic activation.
2.5.1.2. Micronucleus assays
2‐Phenylcrotonaldehyde [FL‐no: 05.062] was tested to determine its clastogenic or aneugenic potential in mammalian cells in vitro using the micronucleus test in cultured human peripheral blood lymphocytes with and without metabolic activation (Lloyd, 2012). The test was performed according to OECD TG 487 (OECD, 2010) (except that the assay with metabolic activation was not repeated) and performed according to GLP Guidelines.
The range of concentrations was determined in a preliminary range finding study.
In assay 1, 2‐phenylcrotonaldehyde [FL‐no: 05.062] was added for 3 h with a 21‐h recovery period in the absence of S9‐mix and examined at concentrations of 40, 60, 100 and 120 μg/mL. The frequency of micronucleated binucleate (MNBN) cells was statistically higher (p < 0.001) than vehicle controls at 100 and 120 μg/mL with 26 and 66% of cytotoxicity, respectively. The frequencies of MNBN cells exceeded the 95th percentile observed range only at 120 μg/mL (in both cultures), indicating a weak but significant induction of chromosomal damage. It was also added to cultures for 3 h with 21‐h recovery in the presence of S9‐mix at concentrations of 100, 130 and 140 μg/mL. The frequency of MNBN cells was significantly higher (p < 0.05) at the two highest concentrations analysed, 130 and 140 μg/mL, but fell clearly within normal ranges based on historical control data. Cultures were also treated for 24 + 0 h in the absence of S9‐mix at concentrations of 20, 23 and 26 μg/mL. The frequencies of MNBN cells were significantly higher (p < 0.05) than those observed in concurrent vehicle controls at all three concentrations (20, 23 and 26 μg/mL), but also fell within normal ranges based on historical control data. These data were considered difficult to interpret due to the steep concentration related cytotoxicity that was observed under all three treatment conditions as indicated by decreases in the replication index values of 13, 25 and 43%, respectively.
In assay 2, cultures were treated with 2‐phenylcrotonaldehyde [FL‐no: 05.062] at concentrations of 20, 60, 70 and 80 μg/mL for 3 h with 21‐h recovery in the absence of S9‐mix. The frequency of MNBN cells was significantly higher (p < 0.01) compared to those observed in concurrent vehicle controls at 20, 70 and 80 μg/mL, but not at 60 μg/mL. The MNBN cell frequencies in both cultures at 20 and 70 μg/mL and in one culture at 80 μg/mL exceeded the 95th percentile of the historical control range. These observations indicate the induction of micronuclei at concentrations at or below the limit of cytotoxicity. No second assay was performed with S9‐mix.
In conclusion, 2‐phenylcrotonaldehyde [FL‐no: 05.062] induced a significant increase of micronuclei in cultured human peripheral blood lymphocytes when tested for 3 + 21 h in the absence of rat liver metabolic activation (S9‐mix). In the same test system, 2‐phenylcrotonaldehyde did not induce micronuclei when tested up to toxic concentrations for 3 + 21 h in the presence of S9‐mix and for 24 + 0 h in the absence of S9‐mix.
A summary of the in vitro data is presented in Appendix D, Table D.1.
Table D.1.
Summary of in vitro genotoxicity data on 2‐phenylcrotonaldehyde [FL‐no: 05.062] of subgroup 3.3
|
Chemical name [FL‐no] |
Test system in vitro | Test object | Concentrations of substance and test conditions | Result | Reference | Comments |
|---|---|---|---|---|---|---|
|
2‐Phenylcrotonaldehyde [FL‐no: 05.062] |
Reverse Mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 and TA102 |
1.6, 8, 40, 200, 1,000 and 5,000 μg/plate (a) , (b) | Negative | Kilford (2010) | Toxicity was observed in all strains at 5,000 μg/plate in the absence and presence of S9‐mix, and at 1,000 μg/plate and above in strains TA98 and TA102 in the absence of S9‐mix and in strains TA1537 and TA102 in the presence of S9‐mix. All strains were negative. Study design complied with current recommendations. Acceptable top concentration was achieved. |
|
S. typhimurium TA98, TA100, TA1535 TA1537 and TA102 |
51.2, 128, 320, 800, 2,000 and 5,000 μg/plate (b) , (d) | Negative | Kilford (2010) | Toxicity was observed in strains TA1537 and TA102 at 2,000 μg/plate and above in the absence of S9‐mix and at 320 μg/plate in the presence of S9‐mix. Similar toxicity was also observed in strains TA98, TA100 and TA1535 at 5,000 μg/plate in the absence of S9 and at 800 μg/plate and above in the presence of S9‐mix. Statistically significant differences in mutation frequency were observed only in strain TA100 and only at levels of toxicity (in the absence of S9‐mix at a concentration of 2,000 μg/plate and in the presence of S9‐mix at 320 μg/plate). Study design complied with current recommendations. Acceptable top concentration was achieved. | ||
|
S. typhimurium TA98, TA100, TA1535 |
51.2, 128, 320, 800, 2,000 and 5,000 μg/plate (c) , (e) | Negative | ||||
| S. typhimurium TA1537 and TA102 | 20.48, 51.2, 128, 320, 800, 2,000 μg/plate (c) , (e) | Negative | ||||
|
S. typhimurium TA98, TA100, TA1535 |
31.25–1,000 μg/plate (c) , (e) | Negative | Kilford (2010) | Toxicity was observed at 3,500 μg/plate and above in strain TA100 in the absence of S9‐mix. In the presence of S9‐mix, toxicity was observed at 250 μg/plate and above in strains TA1537 and TA102 and at 1,000 μg/plate and above in strains TA100, TA98 and TA1535. | ||
|
S. typhimurium TA1537, TA102 |
15.625–500 μg/plate (c) , (e) | Negative | ||||
|
S. typhimurium TA100 |
320–5,000 μg/plate (b) , (d) | Negative | ||||
|
Micronucleus induction |
Human peripheral blood lymphocytes |
40, 60, 100, 120 |
Positive | Lloyd (2012) | The MNBN cell frequencies increases were statistically significant at the top two concentrations, but only slightly exceeded the 95% range of historical controls at the highest dose. All other treated cultures fell within the normal range. The study complies with OECD TG 487. | |
| 100, 130, 140 μg/mL (e) , (f) 20, 23, 26 μg/mL (d) , (g) |
Negative Negative |
Lloyd (2012) | The MNBN cell frequencies increases were statistically significant at the top two concentrations, but all treated cultures fell within the normal range. The study complies with OECD TG 487. | |||
| 20, 60, 70 and 80 μg/mL (d) , (f) | Positive | Lloyd (2012) | The MNBN cell frequencies in both cultures at 20 and 70 μg/mL and in one culture at 80 μg/mL exceeded the 95th percentile of the historical control range. The study complies with OECD TG 487. |
With and without S9 metabolic activation.
Plate incorporation method.
Pre‐incubation method.
Without S9 metabolic activation.
With S9 metabolic activation.
3‐h incubation with 21‐h recovery period.
24‐h incubation with no recovery period.
2.5.2. In vivo data
2.5.2.1. Bone Marrow Micronucleus Induction Assay in the rat
An in vivo micronucleus assay in rats was performed in compliance with OECD TG 474 (OECD, 1997b) (Henderson, 2013) to determine whether the results obtained in the initial in vitro micronucleus assay reflect the situation in vivo.
An initial range‐finding study was conducted in Han‐Wistar rats to estimate the maximum tolerated dose (MTD) of 2‐phenylcrotonaldehyde [FL‐no: 05.062] (purity 98%), administered by oral gavage. The dose of 700 mg/kg body weight (bw) per day was selected as the MTD based on displayed toxicity at the higher dose levels.
Groups of six male Han‐Wistar rats were treated via gavage with 2‐phenylcrotonaldehyde [FL‐no: 05.062] at doses of 0 (vehicle control), 70, 350 and 700 mg/kg bw per day. Animals were dosed at 0 and 24 h, followed by sacrifice and harvest of the femoral bone marrow at 24 h after the last treatment.
Rats treated with 2‐phenylcrotonaldehyde [FL‐no: 05.062] at all doses exhibited group mean % of polychromatic erythrocytes (PCE) that were similar to the vehicle control group. This parameter cannot be used to demonstrate systemic exposure of animals.
In rats treated with 2‐phenylcrotonaldehyde [FL‐no: 05.062], there were no statistically significant increases in frequency of micronucleated polychromatic erythrocytes (MNPCE) for any of the groups receiving the test article, compared to the concurrent vehicle control, with the exception of the intermediate dose group, which was nonetheless well within the historical control range and the difference was due to the very low concurrent control frequencies.
The authors of the report concluded that 2‐phenylcrotonaldehyde [FL‐no: 05.062] did not induce micronuclei in the polychromatic erythrocytes of the bone marrow of male rats treated at 70 and 700 mg/kg bw per day, but that it induced a small statistically significant increase in the frequency of MNPCEs observed at the intermediate dose (350 mg/kg bw per day), and they concluded that the small increase observed at 350 mg/kg bw per day is of questionable biological relevance. The intermediate dose produced a group mean MNPCEs that was twofold greater than and statistically (p < 0.05) higher than the vehicle control group. The mean value (2.83 MNPCEs/2000 PCE) was within the laboratory's historical range (0.74–4.46 MNPCEs/2000 PCE). However, individual results of the first reading demonstrated that percent values of MNPCE of 4 out of the 6 treated animals exceed the 95% confidence interval for mean of historical controls and all the individual values of the control animals were within the limit of historical controls.
The data generated from a second set of 2000 PCE gave a similar response across all test article groups with all individual values falling ‘normally’ within the historical distribution. However, the concurrent vehicle control frequencies were distributed at the low end of the historical data producing a low background level for comparison.
The percent values of MNPCE obtained in the first and second reading are shown in Table 2.
Table 2.
In vivo bone marrow micronucleus assay (Henderson, 2013), %MNPCE
| Treatment | Reading 1 | Reading 2 | Reading mean |
|---|---|---|---|
| Vehicle | 0.10 | 0.04 | 0.07 |
| 75 mg/kg | 0.09 | 0.12 | 0.10 |
| 350 mg/kg | 0.18 (a) | 0.10 | 0.14 (a) |
| 700 mg/kg | 0.12 | 0.12 | 0.12 |
p < 0.01.
The results demonstrate that after a second reading, the increase is still statistically significant when the data from the first and the second reading are pooled.
Plasma of animals of a satellite group was taken, but not analysed for 2‐phenylcrotonaldehyde [FL‐no: 05.062] content. Under these conditions, no clear proof of exposure was given.
Moreover, 2‐phenylcrotonaldehyde [FL‐no: 05.062] was found to be positive without metabolic activation in the in vitro micronucleus test. After oral absorption, the gastrointestinal tract (GIT) is the organ most exposed to high concentrations. These concentrations will not be achieved in the bone marrow. Therefore, it appears necessary to have information on genotoxicity potential of 2‐phenylcrotonaldehyde [FL‐no: 05.062] in the gastrointestinal mucosa by a Comet assay in the stomach or duodenum.
A summary of the in vivo data are presented in Appendix D, Table D.2.
Table D.2.
Summary of in vivo genotoxicity data on 2‐phenylcrotonaldehyde [FL‐no: 05.062] of subgroup 3.3
|
Chemical Name [FL‐no] |
Test system in vivo |
Test object Route |
Doses | Result | Reference | Comments |
|---|---|---|---|---|---|---|
|
2‐Phenylcrotonaldehyde [FL‐no: 05.062] |
Micronucleus assay in bone marrow |
Rat Gavage |
70, 350 and 700 mg/kg bw per day |
Negative | Henderson (2013) | The study complies with OECD TG 474. No clear evidence of bone marrow exposure, therefore the test is inconclusive. |
2.5.3. Conclusion on data considered in FGE.216Rev1
The FGE.216 concerned five α,β‐unsaturated 2‐phenyl substituted aldehydes, 2‐phenylcrotonaldehyde [FL‐no: 05.062], 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099], 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100], 2‐phenylpent‐2‐enal [FL‐no: 05.175] and 2‐phenyl‐4‐methyl‐2‐hexenal [FL‐no: 05.222], corresponding to subgroup 3.3 of FGE.19. The conclusion of the CEF Panel in FGE.216 was that the available data on genotoxicity were too limited to evaluate the five substances through the Procedure and additional genotoxicity data were requested.
The flavouring industry has submitted new data in reply to the above requested data for FGE.19 subgroup 3.3 (FGE.216) for the representative flavouring substance, 2‐phenylcrotonaldehyde [FL‐no: 05.062], covering the remaining four substances [FL‐no: 05.099, 05.100, 05.175 and 05.222].
2‐Phenylcrotonaldehyde [FL‐no: 05.062] did not demonstrate any mutagenic effect in a bacterial test with and without metabolic activation. However, it showed a genotoxic effect in the in vitro micronucleus test in cultured human lymphocytes in the absence of metabolic activation.
In order to verify that this genotoxic potential demonstrated in vitro was confirmed in vivo, a micronucleus test was conducted in the rat bone marrow by oral route which led to an ambiguous result, because only the intermediate dose induced a statistically significant increase of MNPCE, even after rereading the slides. No evidence of systemic exposure of animals was provided in this study, in particular, no change in the percentage of PCE in the bone marrow was noted and plasma of animals sampled in a satellite group have not been analysed.
Under these conditions, it appears necessary to provide proof of sufficient systemic exposure of animals treated with 2‐phenylcrotonaldehyde.
Moreover, since the substance was genotoxic only without metabolic activation, it appears necessary to prove the absence of genotoxic effect locally in the gastrointestinal system using the Comet assay.
3. Assessment
3.1. Additional data considered by the Panel in FGE.216Rev2
Data on genotoxicity
Following the request for additional data expressed by the CEF Panel in FGE.216Rev1, the industry has investigated the presence of 2‐phenylcrotonaldehyde [FL‐no: 05.062] in the plasma of a satellite group of animals (Covance, 2014) from two in vivo MN assays by Henderson (2013) and Covance (2013). The industry has repeated the in vivo MN assay using the same experimental conditions described in the study by Henderson, 2013 (Covance, 2013).
Upon request by EFSA (EFSA letter sent on 19 May 2015), an in vivo comet assay with scoring of duodenum cells has been submitted (Covance, 2016) on 1 July 2016. Since the available data did not allow to evaluate the aneugenic potential of 2‐phenylcrotonaldehyde [FL‐no: 05.062], the CEF Panel requested (EFSA letter sent on 9 February 2017) to test this substance in an in vitro MN assay with centromere staining. On 12 February 2018, the applicant provided the study requested (BioReliance, 2018a) and additional studies: a bacterial reverse mutation assay (BioReliance, 2016) and an in vivo gene mutation assay in transgenic mice (BioReliance, 2017, 2018b) that are evaluated in the present revision of FGE.216 (FGE.216Rev2). The new data evaluated in FGE.216Rev2 are listed in Table 3.
Table 3.
Data evaluated in FGE.216Rev2
| Chemical name [FL‐no] | Additional data submitted | Reference |
|---|---|---|
| 2‐Phenylcrotonaldehyde [FL‐no: 05.062] | In vitro bacterial reverse mutation assay | BioReliance (2016) |
| In vitro MN assay with CREST staining in TK6 cells | BioReliance (2018a) | |
| Development and limited validation of a method for the analysis of plasma samples which may contain 2‐phenylcrotonaldehyde | Covance (2014) | |
| In vivo MN assay in bone marrow | Covance (2013) | |
| In vivo comet assay in duodenum | Covance (2016) | |
| In vivo Oral Dose Range Finding Assay in C57BL/6 Mice | BioReliance (2017) | |
|
In vivo Mutation Assay at the cII Locus in Big Blue® Transgenic C57BL/6 Mice |
BioReliance (2018b) | |
| Use levels | Documentation provided to EFSA No. 10 | |
| 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099], 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100], 2‐phenylpent‐2‐enal [FL‐no: 05.175], 2‐phenyl‐4‐methyl‐2‐hexenal [FL‐no: 05.222] | Use levels | Documentation provided to EFSA No. 10 |
Data on uses and use levels and natural occurrence
New data on uses and use levels have been provided, documentation provided to EFSA No. 9 and No. 10, following a request by EFSA sent on 16 May 2018 and 8 November 2021, respectively. This latest submission (documentation provided to EFSA No. 10) replaces uses and use levels data provided in 2018 (documentation provided to EFSA No. 9). These data have been used in Appendix F to estimate exposure.
Based on information retrieved by EFSA from the Volatile Compounds in Food (VCF) database (VCF online database, 2022), the substances [FL‐no: 05.062, 05.099, 05.100, 05.175] are reported to be present in unprocessed and processed food. For substance [FL‐no: 05.222], no occurrence data are reported (Appendix F).
3.2. In vitro data
3.2.1. Bacterial reverse mutation assay
2‐Phenylcrotonaldehyde (purity: 97.6% sum of isomers, as per ‘post‐study’ final analytical report) was tested for mutagenicity in Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537 and Escherichia coli strain WP2 uvrA in the presence or absence of S9‐mix (prepared from Aroclor‐induced rats) (BioReliance, 2016). The study was performed according to GLP and OECD TG 471 (OECD, 1997a).
A preliminary toxicity‐mutation assay was performed to determine the range of concentrations to be used in the confirmatory assay. 2‐Phenylcrotonaldehyde was tested at 1.5, 5.0, 15, 50, 150, 500, 1,500 and 5,000 μg/plate in all strains both with and without S9‐mix (two plates per concentration). DMSO was used as vehicle control.
No precipitate was observed. Toxicity was observed at the two highest concentrations (1,500 or at 5000 μg/plate). An increase in the frequency of revertants was observed in E. coli WP2 uvrA in the presence and absence of S9‐mix. Increases were observed also in S. typhimurium strains TA98 and TA100 in the absence of S9‐mix and in strain TA1535 both in the presence and absence of S9‐mix. These increases in revertant counts were not clearly concentration‐related or consistently outside the historical control limits; therefore, in the confirmatory assay, the range of concentrations tested was adjusted in order to clarify the responses observed.
In the confirmatory assay, 2‐phenylcrotonaldehyde was tested at 15, 50, 150, 500, 1,000, 1,500 and 5,000 μg/plate with the plate incorporation method (in triplicate). No precipitate was observed. Toxicity was observed at 1,500 or at 5,000 μg/plate. Increases in revertant counts were observed in S. typhimurium strains TA98 and TA100 without metabolic activation and with tester strains TA100 in the presence of metabolic activation (1.5‐,1.9‐, 1.6‐fold, respectively). The authors of the study noted that some of the counts are outside of the historical control limits, but none of these increases were concentration‐related.
Increases in the frequency of revertants were confirmed in S. typhimurium strain TA1535 and in E. coli WP2 uvrA in the presence (2.8‐, 11.7‐fold, respectively) and absence of S9‐mix (6.3‐, 19.6‐fold respectively).
No increase was observed with tester strain TA98 in the presence of S9‐mix. Due to tester strain contamination, tester strain TA1537 was not evaluated for mutagenicity but was retested in a further experiment with the same concentrations of 2‐phenylcrotonaldehyde.
In this experiment, no increases in the frequency of revertants were observed in S. typhimurium strain TA1537 in either the presence or absence of S9‐mix. No precipitate was observed. Toxicity was observed at the highest concentrations tested (1,500 or at 5,000 μg/plate).
The authors of this study considered as equivocal the increase of revertants observed in strain TA100 in the absence of S9‐mix and in strain TA1535 in the presence of S9‐mix. The increase in revertants observed in strain TA98, in the absence of metabolic activation, was not concentration‐related and was inside the range of historical controls. The authors considered the results observed in TA98 as negative. They concluded that 2‐phenylcrotonaldehyde is positive in S. typhimurium strain TA1535, without S9‐mix, and in E. coli WP2 uvrA in the presence and absence of S9‐mix.
The Panel concluded that 2‐phenylcrotonaldehyde is positive in S. typhimurium strain TA1535, without S9‐mix, and in E. coli WP2 uvrA in the presence and absence of S9‐mix and equivocal in strain TA100 in the absence of S9‐mix and in strain TA1535 in the presence of S9‐mix.
3.2.2. In vitro micronucleus assay with kinetochores staining
2‐Phenylcrotonaldehyde (purity 99%) was tested in an in vitro micronucleus assay in the human lymphoblastoid cell line TK6 cells (BioReliance, 2018a), with the purpose of evaluating the aneugenic and clastogenic potential of the tested substance. DMSO was used as the vehicle. The study was performed according to GLP and OECD TG 487 (OECD, 2014a).
TK6 cells were treated for 4 h with 23 h of recovery period (4 + 23 h) in the absence or presence of S9‐mix (from aroclor‐induced rats) or for 27 h in the absence of S9‐mix. Positive controls were: mytomycin C (MMC), cyclophosphamide (CPA), vinblastine (VB).
In the preliminary toxicity test, concentrations between 0.146 and 1,460 μg/mL were tested. Precipitate was observed at concentrations above 438 μg/mL for all the three treatment conditions.
Cytotoxicity (more than 50% decrease in relative population doubling (RPD) compared to the vehicle control) was observed at concentrations above 146 μg/mL for the 4 h treatment both in the absence and in the presence of S9‐mix, and at concentrations above 43.8 μg/mL for the 27‐h treatment without S9‐mix.
Based on the results of the preliminary toxicity test, the following concentrations were tested:
-
–
For the treatment 4 + 23 h in the absence of metabolic activation 5, 10, 20, 40, 45, 50 μg/mL;
-
–
For the treatment 4 + 23 h in the presence of metabolic activation 20, 40, 60, 80, 100, 120, 140 μg/mL;
-
–
For the treatment at 27 h in the absence of metabolic activation 5, 15, 20, 25, 30, 35, 40 μg/mL;
Each concentration was tested in duplicate cultures; for each culture, 1,000 mononucleated cells were analysed for MN (a total of 2,000 cells per concentration). Due to cytotoxicity of approximatively 50%, the highest concentrations evaluated for MN induction were 45 μg/mL (for the 4 + 23 h treatment without S9‐mix), 20 μg/mL (for the 27 h treatment without S9‐mix) and 80 μg/mL (for the 4 + 23 h treatment with S9‐mix).
After 4 h treatment +23 h recovery period, in the absence of S9‐mix, at 20, 40 and 45 μg/mL and in the presence of S9‐mix, at 40, 60 and 80 μg/mL, no increase in micronucleated cell frequency was observed.
After 27 h treatment without recovery period, in the absence of S9‐mix, a statistically significant and concentration‐dependent increase in micronucleated cell frequency was observed at 20 μg/mL.
In order to confirm this positive result, the micronucleus assay was repeated with a narrower range of concentrations: 5, 16, 18, 20, 22, 24, 26 μg/mL. The cytotoxicity observed at the highest concentration tested was 41%; therefore, the experiment was repeated again at the following concentrations: 5, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, 45 μg/mL. In this third experiment, a cytotoxicity of 57% was observed at the concentration tested of 20 μg/mL, that was the highest concentration analysed for MN induction. No significant increase in micronucleated cell frequency was observed at 16 and 18 μg/mL, but a statistically significant increase in MN induction (1.25%) was observed at 20 μg/mL (p ≤ 0.01) versus 0.55% in the negative control. However, the effect was not concentration‐related (the Cochran–Armitage test was statistically not significant for a concentration‐dependent response (p > 0.05)).
The micronuclei induced by 2‐phenylcrotonaldehyde 20 μg/mL (after 27 h of treatment) were analysed through CREST staining (antikinetochore antibody staining) in order to determine the mechanism of action (aneugenicity or clastogenicity). The same was applied to vehicle and positive controls.
The results of the CREST analysis indicate that 77% of MN induced by 2‐phenylcrotonaldehyde were positive for kinetochore staining. The clastogen‐positive control MMC showed 30% of MN positive for kinetochore staining. The negative control showed 60% of MN positive for kinetochore staining. The aneugen‐positive control VB had 87% of MN positive for kinetochore staining. Therefore, the authors of this study considered that 2‐phenylcrotonaldehyde induces micronuclei in TK6 cells after 27 h of treatment, without metabolic activation, via an aneugenic mechanism.
The Panel agrees with this conclusion.
Results of in vitro studies are summarised in Appendix E, Table E.1.
Table E.1.
Summary of additional in vitro genotoxicity data on 2‐phenylcrotonaldehyde [FL‐no: 05.062] of subgroup 3.3
|
Chemical name [FL‐no] |
Test system in vitro | Test object | Concentrations of substance and test conditions | Result | Reference | Comments |
|---|---|---|---|---|---|---|
|
2‐Phenylcrotonaldehyde [FL‐no: 05.062] |
Reverse Mutation test |
S. typhimurium TA98, TA100, TA1535, TA1537 E. coli WP2 uvrA |
1.5, 5.0, 15, 50, 150, 500, 1,500 and 5,000 μg/plate (a) , (b) , (c) |
Positive | BioReliance (2016) | Reliable without restrictions. Study performed according to OECD TG 471 and in compliance with GLP. |
| Micronucleus assay with CREST staining | Human TK6 cells |
5, 15, 20 μg/mL (assay 1) |
Negative Negative Positive |
BioReliance (2018a) |
Reliable without restrictions. Study performed according to OECD TG 487 and in compliance with GLP. The given concentrations are those for the cultures that were scored for micronuclei. CREST analysis indicates that 2‐phenylcrotonaldehyde induced MN by an anuegenic mechanism. |
With and without S9 metabolic activation.
Plate incorporation method.
Preliminary toxicity and mutagenicity test.
Without S9 metabolic activation.
With S9 metabolic activation.
4‐h incubation with 23‐h recovery period.
27‐h incubation with no recovery period.
3.3. In vivo data
3.3.1. Plasma bioanalysis
In order to demonstrate the bone marrow exposure of animals treated with 2‐phenylcrotonaldehyde [FL‐no: 05.062] in the micronucleus assay by Henderson, 2013 (see Section 2.5.2.1) and by Covance, 2013 (see Section 3.3.2), a plasma analysis of a satellite group of animals was provided (Covance, 2014). Six male Han Wistar rats were dosed, by oral gavage, with 700 mg/kg bw per day (an estimate of the MTD) of 2‐phenylcrotonaldehyde. A method has been developed for the analysis of 2‐phenylcrotonaldehyde using gas chromatography with mass selective detection (GC–MSD). The method was developed from a literature method using a gas chromatography methodology with the mass selective detector in scan mode, and suitable ions were selected for use in single ion monitoring mode. A procedure was also developed to extract 2‐phenylcrotonaldehyde from the rat plasma samples.
According to the study authors, satisfactory linearity, recovery and repeatability were found for 2‐phenylcrotonaldehyde when the substance was spiked and analysed in rat plasma samples.
The Panel, however, noted that linearity in plasma extracts was in the range of 10–400 μg/mL, but the highest concentration reported for 2‐phenylcrotonaldehyde in rat plasma samples was below this range. Moreover, the recovery and accuracy of the method were only determined from 50 μg/mL and above.
No animals in the vehicle control group showed any detectable levels of 2‐phenylcrotonaldehyde in plasma, while less than 10 μg/mL 2‐phenylcrotonaldehyde were detected in the plasma of animals treated with 700 mg/kg bw per day, half an hour after dosage. The Panel, however, noted that there is some variability among treated animals. Although these results indicate that a very low amount of 2‐phenylcrotonaldehyde might be present in plasma shortly after dosing, the Panel concluded that the validation package provided by the study is not robust enough on its own to support this conclusion.
3.3.2. In vivo micronucleus assay
2‐Phenylcrotonaldehyde [FL‐no: 05.062] (purity 99.66%) was tested in a second bone marrow MN assay in rats which was performed in compliance with GLP and according to OECD TG 474 (OECD, 1997b) (Covance, 2013) as follow‐up of the in vivo MN assay by Henderson (2013). Based on the dose range finding study already performed by Henderson (2013), the same doses of 2‐phenylcrotonaldehyde [FL‐no: 05.062] were used in the new study by Covance (2013).
Groups of six Han‐Wistar rats were treated via gavage with 2‐phenylcrotonaldehyde [FL‐no: 05.062] at doses of 0 (vehicle control, aqueous methylcellulose), 70, 350 and 700 mg/kg bw per day. Cyclophosphamide at the concentration of 20 mg/kg bw per day was used as positive control. Animals were dosed at 0 and 24 h, except the positive control group that was dosed only at 24 h. All animals were sacrificed at 48 h and femoral bone marrow was harvested and prepared for the MN analysis.
The vehicle control data were comparable with the laboratory's historical vehicle control data. Positive controls resulted in a statistically significant increase in MNPCE (over the concurrent vehicle control), which was comparable with the laboratory's historical positive control data.
In rats treated with 2‐phenylcrotonaldehyde [FL‐no: 05.062], in all the three dose groups, there were no statistically significant increases in MNPCE frequency compared to the vehicle controls. Individual frequencies of MNPCE for all treated animals were consistent with historical vehicle control data.
2‐Phenylcrotonaldehyde [FL‐no: 05.062] did not induce micronuclei in PCE of male rats treated up to 700 mg/kg bw per day (an estimate of the MTD for this study). The PCE/NCE ratio was not affected by the treatment with 2‐phenylcrotonaldehyde [FL‐no: 05.062], indicating that the treatment did not induce bone marrow toxicity. Since signs of toxicity were not observed up to the highest dose tested (700 mg/kg bw per day), there is no evidence to presume systemic exposure to 2‐phenylcrotonaldehyde [FL‐no: 05.062]. Also taking into account the plasma analysis data (see above), the Panel considered that no clear evidence of bone marrow exposure is available. Therefore, the Panel evaluated the results of this in vivo MN assay as inconclusive.
3.3.3. In vivo comet assay
2‐Phenylcrotonaldehyde (purity 98.42%) was tested for its potential to induce DNA damage in the duodenum of treated rats (Covance, 2016). The study was performed according to GLP and OECD TG 489 (OECD, 2014b).
Six male Han Wistar rats were dosed twice with 2‐phenylcrotonaldehyde at 0 (Day 1) and 21 h (Day 2) by gavage. Dose levels were 175, 350 or 700 mg/kg bw per day. The maximum dose (700 mg/kg bw per day) was an estimate of the MTD based on toxicity data from a previous rat MN study by Henderson (2013). The vehicle was 0.5% (w/v) aqueous methylcellulose and the positive control was ethyl methanesulfonate (150 mg/kg bw per day, single oral administration at 21 h (Day 2)). No signs of clinical toxicity were observed.
Liver and duodenum were sampled on Day 2, 24 h after the first dosing. Only duodenum was analysed for comet assay.
There were no clinical chemistry findings considered to be related to administration of 2‐phenylcrotonaldehyde. Histopathology did not reveal any macroscopic or microscopic findings associated with the administration of 2‐phenylcrotonaldehyde.
The vehicle control data were comparable with the laboratory's historical data. The positive control resulted in a statistically significant increase in tail intensity (over the concurrent vehicle control) that was comparable with the laboratory's historical positive control data.
There was no dose‐related increase in % hedgehogs in duodenum following treatment with 2‐phenylcrotonaldehydethus demonstrating that the treatment did not cause excessive DNA damage, which could interfere with comet analysis.
No statistically significant increases in tail intensity at any dose levels of 2‐phenylcrotonaldehyde was observed compared to the vehicle control. All individual animal data at all dose levels were consistent with the data from vehicle control animals and fell within the laboratory's historical control data. The Panel concluded that 2‐phenylcrotonaldehyde did not induce DNA damage in the duodenum of rats treated up to 700 mg/kg bw per day (estimated MTD).
3.3.4. In vivo gene mutation assay in Big Blue® transgenic mice
2‐Phenylcrotonaldehyde was tested in a 5‐day dose range finding assay in C57BL/6 mice (BioReliance, 2017), in order to determine the MTD. The study was performed in compliance with GLP. Results from this study were used for selecting the dose levels tested in the in vivo gene mutation assay in Big Blue® Transgenic C57BL/6 mice (BioReliance, 2018b).
2‐Phenylcrotonaldehyde (purity 99%) was administered via oral gavage (0.5% methylcellulose used as vehicle) to five groups of wild‐type C57BL/6 male mice (three animals per group) corresponding to different dose levels: 50, 100, 250, 500, 1,000 mg/kg bw per day. An additional group (Group 6) of three Big Blue® C57BL/6 homozygous transgenic male mice was dosed at 750 mg/kg bw per day in order to verify whether the top dose selected for the 28‐day dosing period was the MTD.
All animals were observed twice daily, at least 6 h apart, for moribundity and mortality. Clinical observations were performed pre‐dose on Day 1 (Groups 1–6) and prior to sacrifice on Day 5 (Groups 1–5 only).
The three animals dosed at 1,000 mg/kg bw per day were found death or sacrificed moribund on day 1, 3 and 4. Prostration, laboured breathing, cold to touch and\or decreased motor activity was documented in all three animals treated with 1,000 mg/kg bw per day. One of the three transgenic mice dosed with 750 mg/kg bw was sacrificed in moribund condition a few hours after the first dose due to prostration and decreased motor activity.
No body weight changes associated with 2‐phenylcrotonaldehyde treatment were observed at any dose level up to 5 days. Based on these results, the authors of this study recommended for the 28‐day study in transgenic mice the doses of 100, 300 and 670 mg/kg bw per day (BioReliance, 2017). Upon further review of the available toxicity data, the dose of 670 mg/kg bw per day was considered to potentially exceed the MTD over the 28‐day treatment period. Therefore, for the in vivo gene mutation assay, the dose of 500 mg/kg bw per day was chosen as the highest dose because no mortality or toxicity were observed. The lower doses selected were 75 and 250 mg/kg bw per day.
In the in vivo gene mutation assay (BioReliance, 2018b), 2‐phenylcrotonaldehyde (purity 99%) was administered via oral gavage (0.5% methylcellulose used as vehicle) to four groups of male transgenic Big Blue® C57BL/6 mice (6 animals per group) at the dose levels of 0, 75, 250 and 500 mg/kg bw per day. This study was performed according to GLP and OECD TG 488 (OECD, 2013).
Dose formulations were prepared fresh each day just prior to dosing.
One animal treated at 500 mg/kg bw per day was terminated in moribund condition on Day 12. Ataxia, decreased motor activity, prostration, laboured breathing, white discharge from the eyes and squinting were noted at 500 mg/kg bw per day in all animals, mainly during the first week of treatment. Decreased motor activity was also noted in one animal at 250 mg/kg bw per day on Day 9.
Total absolute body weight gains were lower than control for all test article treated groups, reaching statistical significance at the 75 and 500 mg/kg bw per day. No differences in food consumption were observed.
Liver, duodenum and bone marrow samples from at least five animals per group were processed for DNA isolation. In addition, frozen tissue samples for liver, duodenum and bone marrow were processed for DNA isolation from five positive control animals, previously exposed to ENU (N‐ethyl‐N‐nitrosurea, via oral gavage, 40 mg/kg bw per day on Days 1, 2 and 3) in another study of the same laboratory.
Sufficient quantity and quality of DNA was obtained to permit 2–6 packagings of each DNA sample, yielding more than the OECD‐specified minimum of 125,000 plaques per tissue per animal.
Treatment with 2‐phenylcrotonaldehyde did not increase the mutation frequency at the cII gene in liver, bone marrow and duodenum of Big Blue® mice.
The authors concluded that in this in vivo gene mutation assay in transgenic mice, 2‐phenylcrotonaldehyde induced dose‐related toxicity based on: one early death, signs of toxicity at 500 mg/kg bw per day and a trend towards lower weekly body weights and total weight gain in all three treatment groups compared to the vehicle control. 2‐Phenylcrotonaldehyde did not induce a statistically significant increase in mutation frequency in duodenum, liver and bone marrow.
The Panel agrees with these conclusions.
Results of in vivo studies are summarised in Appendix E, Table E.2.
Table E.2.
Summary of additional in vivo genotoxicity data on 2‐phenylcrotonaldehyde [FL‐no: 05.062] of subgroup 3.3
| Chemical name [FL‐no] | Test system in vivo |
Test Object Route |
Doses | Result | Reference | Comments |
|---|---|---|---|---|---|---|
|
2‐Phenylcrotonaldehyde [FL‐no: 05.062] |
Micronucleus assay in bone marrow |
Rat gavage |
70, 350 and 700 mg/kg bw per day |
Inconclusive |
Covance (2013) | Reliable with restrictions (no clear evidence of bone marrow exposure). Study performed in compliance with GLP and according to OECD TG 474. The highest dose tested (700 mg/kg bw per day) was an estimate of the MTD according to the micronucleus study by Henderson (2013). |
| Plasma bioanalysis (Micronucleus assay) (a) |
Rat gavage |
700 mg/kg bw per day |
Inconclusive | Covance (2014) | CG‐MSD method validated (recovery, accuracy and precision). Linearity and working range were assessed. The concentration of 2‐phenylcrotonaldehyde detected was below the linearity range. Not a GLP study. | |
| Comet assay in duodenum |
Rat gavage |
175, 350, 700 mg/kg bw per day |
Negative | Covance (2016) | Reliable without restrictions. Study performed in compliance with GLP and according to OECD TG 489. The dose of 700 mg/kg bw per day was an estimate of the MTD according to the micronucleus study by Henderson (2013). | |
| Gene mutation assay in duodenum, liver and bone marrow |
Big Blue® C57BL/6 transgenic mice gavage |
75, 250 and 500 mg/kg bw per day | Negative | BioReliance (2018b) | Reliable without restrictions. Study performed in compliance with GLP and according to OECD TG 488. |
Plasma obtained from satellite group of animals in the study by Henderson (2013) and from satellite group of animals in the study by Covance (2013).
4. Discussion
2‐Phenylcrotonaldehyde [FL‐no: 05.062] was negative in a bacterial gene mutation assay (Kilford, 2010) with and without metabolic activation. In a second study by BioReliance (2016), a statistically significant increase in revertants was observed in S. typhimurium strain TA1535, without S9‐mix, and in E. coli WP2 uvrA in the presence and absence of S9‐mix.
In an in vivo follow‐up study, 2‐phenylcrotonaldehyde was tested for gene mutations in Big Blue® transgenic mice in which duodenum, liver and bone marrow were analysed. No statistically significant increase in mutation frequency was observed in the tissues analysed. The Panel considered that 2‐phenylcrotonaldehyde did not induce gene mutations in this in vivo assay.
Positive results were observed in two in vitro MN assays, in human peripheral blood lymphocytes and in TK6 cells in the absence of metabolic activation.
In the in vitro MN assay in TK6 cells, a statistically significant increase in micronucleated cell frequency was observed in the long‐term treatment, at a concentration of 20 μg/mL, in the absence of metabolic activation. The CREST analysis showed that 2‐phenylcrotonaldehyde [FL‐no: 05.062] induced MN mainly through an aneugenic mechanism.
As in vivo follow‐up study, a first rat bone marrow MN assay was performed by gavage (Henderson, 2013). The CEF Panel considered the results of this study as inconclusive because only the intermediate dose induced a statistically significant increase of MNPCE and there was no evidence of systemic exposure. In a second rat bone marrow micronucleus assay, performed under the same conditions, no increase in MNPCE was observed (Covance, 2013), but also in this study, there was no evidence of bone marrow exposure. Moreover, the results of a separate study on plasma bioanalysis (Covance, 2014) in rats treated with 2‐phenylcrotonaldehyde (700 mg/kg bw per day) did not provide a clear evidence of bone marrow exposure, either.
Since signs of toxicity were not observed up to the highest dose tested (700 mg/kg bw per day), there is no evidence of systemic exposure to 2‐phenylcrotonaldehyde [FL‐no: 05.062]. Therefore, the Panel concluded that results from the two in vivo MN studies are inconclusive.
Since 2‐phenylcrotonaldehyde was found to be positive without metabolic activation in the in vitro micronucleus test, the Panel agreed that in vivo the GIT would be the organ most exposed to high concentrations of the substance after oral exposure.
The negative results observed in the in vivo comet assay (Covance, 2016) in duodenum can overrule the potential clastogenicity at the site of contact, but not a possible aneugenic effect.
The Panel noted that at present, there are no validated in vivo methods to investigate aneugenicity in the GIT.
Following the recommendations for risk assessment in the EFSA Scientific Committee guidance on aneugenicity (EFSA Scientific Committee, 2021), the Panel compared the concentration resulting in aneugenicity in vitro (20 μg/mL) with the estimated concentration of the substance in the GIT following ingestion of food or beverage. Since the dilution in the upper parts of the GIT can be considered to be small, the estimated concentration of a substance in this part of the GIT would be in the same order of magnitude as its concentration in food and beverages.
The Panel noted that for 2‐phenylcrotonaldehyde [FL‐no: 05.062], for some food categories, the normal use levels and/or the maximum use levels (see Appendix F) are less than one order of magnitude below the concentration for which an aneugenic effect of this flavouring substance was observed in the in vitro MN assay (i.e. 20 μg/mL). Therefore, for the application in the respective food categories, the use of the flavouring substance [FL‐no: 05.062] at the reported use levels would raise a concern for aneugenicity.
Based on structural similarity, for the remaining four substances in this FGE [FL‐no: 05.099, 05.100, 05.175 and 05.222], an aneugenic potential may also be anticipated. However, the lowest concentration resulting in aneugenicity in vitro for [FL‐no: 05.062] cannot be used for the safety assessment for these four substances, because quantitative extrapolation of this concentration to estimate the aneugenic potency of these four substances would be connected to a high level of uncertainty. Therefore, for these four substances, individual data are needed to establish whether they have aneugenic potential. These four substances should be tested in an in vitro MN test with centromeres analysis. In case of increase in micronucleated cells frequency, an appropriate follow‐up test should be done, according to the EFSA guidance on genotoxicity testing strategy (EFSA Scientific Committee, 2011) and the EFSA guidance on aneugenicity (EFSA Scientific Committee, 2021).
5. Conclusions
The Panel concluded that, for all the substances in this FGE, concern for gene mutation and clastogenicity is ruled out by the negative results observed in the in vivo gene mutation assay and in the in vivo comet assay, respectively.
2‐Phenylcrotonaldehyde [FL‐no: 05.062] induced MN in vitro through an aneugenic mode of action. The available in vivo MN studies were inconclusive and cannot be used to rule out the potential aneugenicity of [FL‐no: 05.062] in vivo. Therefore, the Panel compared the lowest concentration resulting in aneugenicity in vitro with the use levels reported for this substance. Based on this comparison, the Panel concluded that the use of the flavouring substance [FL‐no: 05.062] at the reported use levels in several food categories would raise a concern for aneugenicity.
Based on structural similarity, for the remaining four substances in this FGE [FL‐no: 05.099, 05.100, 05.175 and 05.222], an aneugenic potential may also be anticipated. However, the lowest concentration resulting in aneugenicity in vitro for [FL‐no: 05.062] cannot be used for the safety assessment of these four substances, because quantitative extrapolation of this concentration to estimate the aneugenic potency of these four substances would be connected to a high level of uncertainty. Therefore, for these four substances, individual data are needed to establish whether they have aneugenic potential.
Accordingly, it is currently not appropriate to assess any of these five substances through the Procedure for the evaluation of flavouring substances.
6. Documentation as provided to EFSA
-
1
Benigni R and Netzeva T, 2007. Report on a QSAR model for prediction of genotoxicity of α,β‐unsaturated aldehydes in S. typhimurium TA100 and its application for predictions on α,β‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
-
2
BioReliance, 2016. Bacterial reverse mutation assay, 2‐phenyl‐2‐butenal. BioReliance Corporation, Rockville. Study number AD79XC.503.BTL. May 2016. Unpublished study report submitted by EFFA and IOFI.
-
3
BioReliance, 2017. In Vivo Oral Dose Range Finding Assay in C57BL/6 Mice, 2‐phenyl‐2‐butenal. BioReliance study number AD79XC.2G32NGLP.BTL. January 2017. Unpublished study report submitted by EFFA and IOFI.
-
4
BioReliance, 2018a. In Vitro Mammalian Cell Micronucleus Assay in TK6 Cells, 2‐Phenylcrotonaldehyde. BioReliance Corporation, Rockville. Study number AF04GF.361.BTL. February 2018. Unpublished study report submitted by EFFA and IOFI.
-
5
BioReliance, 2018b. In Vivo Mutation Assay at the cII Locus in Big Blue® Transgenic C57BL/6 Mice, 2‐phenyl‐2‐butenal. BioReliance Corporation, Rockville. Study number AD79XC.170.BTL. January 2018. Unpublished study report submitted by EFFA and IOFI.
-
6
Covance, 2013. Induction of micronuclei in the bone marrow of treated rats. 2‐Phenyl‐2‐butenal. Study Number: 8280126. August 2013. Covance Laboratories Ltd., North Yorkshire, England. Unpublished report submitted by EFFA.
-
7
Covance, 2014. Development and limited validation of a method for the analysis of plasma samples which may contain 2‐phenylcrotonaldehyde. Study no. 8302–748. September 2014. Covance Laboratories Ltd., North Yorkshire, England. Unpublished report submitted by EFFA and IOFI.
-
8
Covance, 2016. 2‐Phenylcrotonaldehyde: Rat Alkaline Comet assay. Study No. 8331882. June 2016. Covance Laboratories Ltd., North Yorkshire, England. Unpublished report submitted by EFFA and IOFI.
-
9
EFFA (European Flavour Association), 2018. Submission of EFFA FGE.19 data: use level information for 3 substances of Subgroup 3.3 (FGE.216 Rev1), 29 May 2018.
-
10
EFFA (European Flavour Association), 2022. Revised submission of EFFA data: use level information for five substances of FGE.19 subgroup 3.3 (FGE.216 Rev1), 25 February 2022.
-
11
Gry J, Beltoft V, Benigni R, Binderup M‐L, Carere A, Engel K‐H, Gürtler R, Jensen GE, Hulzebos E, Larsen JC, Mennes W, Netzeva T, Niemelä J, Nikolov N, Nørby KK and Wedebye EB, 2007. Description and validation of QSAR genotoxicity models for use in evaluation of flavouring substances in Flavouring Group Evaluation 19 (FGE.19) on 360 α,β‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
-
12
Henderson D, 2013. Induction of micronuclei in the bone marrow of treated rats. 2‐Phenyl‐2‐butenal. Covance Laboratories Ltd. Study no. 8262422. June 2012. Audited Draft Report. Unpublished report submitted by EFFA to FLAVIS Secretariat.
-
13
Kilford J, 2010. Reverse mutation in one histidine‐requiring strain of Salmonella typhimurium. 2‐Phenyl‐2‐butenal. Covance Laboratories Ltd, England. Study no. 8225706. August 2010. Unpublished report submitted by EFFA to FLAVIS Secretariat.
-
14
Lloyd M, 2012. Induction of micronuclei in cultured human peripheral blood lymphocytes. 2‐Phenyl‐2‐butenal. Covance Laboratories LTD. Study no. 8225707. May 2012. Unpublished report submitted by EFFA to FLAVIS Secretariat.
-
15
Nikolov N, Jensen GE, Wedebye EB and Niemelä J, 2007. Report on QSAR predictions of 222 α,β‐unsaturated aldehydes and ketones from Flavouring Group Evaluation 19 (FGE.19) on 360 α,β‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Abbreviations
- BW
Body Weight
- CAS
Chemical Abstract Service
- CEF
Scientific Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CHO
Chinese hamster ovary (cells)
- CHL
Chinese hamster lung (cells)
- CoE
Council of Europe
- FAO
Food and Agriculture Organisation of the United Nations
- FEMA
Flavour and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS
Flavour Information System database
- GLP
Good Laboratory Practice
- ID
Identity
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MN
Micronucleus
- MNBN
MicroNucleated BiNucleate cells
- MNPCE
MicroNucleated PolyChromatic Erythrocytes
- MS
Mass Spectrometry
- MTD
Maximum Tolerated Dose
- NMR
Nuclear Magnetic Resonance
- No
Number
- OECD
Organisation for Economic Co‐operation and Development
- PCE
PolyChromatic Erythrocytes
- (Q)SAR
(Quantitative) Structure–Activity Relationship
- WHO
World Health Organisation
Appendix A – Specification Summary of the Substances in the Flavouring Group Evaluation 216Rev2
Table A.1.
Specification Summary of the Substances in the Flavouring Group Evaluation 216Rev2 (JECFA, 2006)
| FL‐no JECFA‐no | Chemical name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubility (a) Solubility in ethanol (b) | Boiling point, °C (c) Melting point, °C ID test Assay minimum | Refrac. Index (d) Spec. gravity (e) |
|---|---|---|---|---|---|---|---|
| 05.062 1474 | 2‐Phenylcrotonaldehyde |
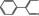
|
3224 670 4411‐89‐6 |
Liquid C10H10O 146.19 |
Insoluble Soluble |
177 (20 hPa) NMR 97% |
1.558–1.564 1.031–1.037 |
|
05.099 1472 |
5‐Methyl‐2‐phenylhex‐2‐enal |
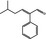
|
3199 10365 21834–92‐4 |
Liquid C13H16O 188.27 |
Insoluble Soluble |
96–100 (0.9 hPa) NMR 96% |
1.531–1.536 0.970–0.976 |
|
05.100 1473 |
4‐Methyl‐2‐phenylpent‐2‐enal |
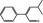
|
3200 10366 26643–91‐4 |
Liquid C12H14O 174.24 |
Insoluble Soluble |
96 (0.9 hPa) NMR 95% |
1.533–1.539 0.980–0.986 |
| 05.175 | 2‐Phenylpent‐2‐enal |
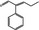
|
3491‐63‐2 |
Liquid C11H12O 160.22 |
Practically insoluble or insoluble Freely soluble |
126 (15 hPa) MS 95% |
1.545–1.553 1.005–1.015 |
|
05.222 2069 |
2‐Phenyl‐4‐methyl‐2‐hexenal |
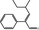
|
4194 26643–92‐5 |
Liquid C13H16O 188.27 |
Insoluble Soluble |
97 (0.6 hPa) 95% |
1.522–1.530 0.965–0.975 |
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
Appendix B – Summary of Safety Evaluation
Table B.1.
Summary of Safety Evaluation by JECFA of the substances in FGE.19 subgroup 3.3, applying the Procedure (based on intakes calculated by the MSDI approach) (JECFA, 2006, 2015)
|
FL‐no JECFA‐no CAS no |
EU Register name | Structural formula | MSDI (a) (μg/capita per day) |
Class (b) Evaluation procedure path (c) (JECFA) |
Outcome on the named compound (JECFA, 2006) | Outcome on the named compound (JECFA, 2015) | EFSA conclusion on the named compound (genotoxicity) |
|---|---|---|---|---|---|---|---|
|
05.062 1474 4411‐89‐6 |
2‐Phenylcrotonaldehyde |
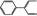
|
1.7 |
Class I A3: Intake below threshold |
(d) | (e) | Concern for aneugenicity cannot be ruled out at the reported use levels. |
|
05.099 1472 21834–92‐4 |
5‐Methyl‐2‐phenylhex‐2‐enal |
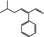
|
15 |
Class II A3: Intake below threshold |
(d) | (e) | Concern for aneugenicity cannot be ruled out. Additional genotoxicity data required. |
|
05.100 1473 26643–91‐4 |
4‐Methyl‐2‐phenylpent‐2‐enal |
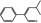
|
0.34 |
Class II A3: Intake below threshold |
(d) | (e) | Concern for aneugenicity cannot be ruled out. Additional genotoxicity data required. |
|
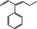
05.175 – 3491‐63‐2 |
2‐Phenylpent‐2‐enal |
|
0.011 | No evaluation | Not evaluated by JECFA | Not evaluated by JECFA | Concern for aneugenicity cannot be ruled out. Additional genotoxicity data required. |
|
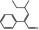
05.222 2069 26643–92‐5 |
2‐Phenyl‐4‐methyl‐2‐hexenal |
|
3.0 | No evaluation | Not evaluated by JECFA | (e) | Concern for aneugenicity cannot be ruled out. Additional genotoxicity data required. |
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day, calculated by EFSA based on the most recent data submitted.
Thresholds of toxicological concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
The Procedure cannot be applied until concerns regarding genotoxicity of 2‐phenyl‐2‐butenal (JECFA No. 1474) are resolved and the evaluation should be reconsidered at a future meeting.
Appendix C – (Q)SAR Predictions on Mutagenicity in Five Models for Five Aldehydes from Subgroup 3.3
Table C.1.
(Q)SAR Predictions on Mutagenicity for Five Aldehydes from Subgroup 3.3 considered in FGE.216 (EFSA, 2009)
|
FL‐no JECFA‐no |
Chemical name | Structural formula (a) |
FEMA no CoE no CAS no |
ISS Local Model Ames Test TA100 (b) |
MultiCASE Ames test (c) |
MultiCASE Mouse lymphoma test (d) |
MultiCASE Chromosomal aberration test in CHO (e) |
MultiCASE Chromosomal aberration test in CHL (f) |
|---|---|---|---|---|---|---|---|---|
|
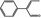
05.062 1474 |
2‐Phenylcrotonaldehyde |
|
3224 670 4411‐89‐6 |
NEG | OD | OD | OD | OD |
|
05.099 1472 |
5‐Methyl‐2‐phenylhex‐2‐enal |
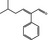
|
3199 10365 21834–92‐4 |
NEG | OD | OD | OD | OD |
|
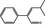
05.100 1473 |
4‐Methyl‐2‐phenylpent‐2‐enal |
|
3200 10366 26643–91‐4 |
NEG | OD | OD | OD | OD |
| 05.175 | 2‐Phenylpent‐2‐enal |
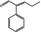
|
– – 3491‐63‐2 |
NEG | OD | OD | OD | OD |
|
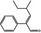
05.222 2069 |
2‐Phenyl‐4‐methyl‐2‐hexenal |
|
– – 26643–92‐5 |
NEG | OD | OD | OD | OD |
(Q)SAR: (Quantitative) Structure–Activity Relationship; FL‐no: FLAVIS number; FLAVIS: Flavour Information System (database); JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavour and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; CHO: Chinese hamster ovary (cells); CHL: Chinese hamster lung (cells); NEG: Negative; OD: Out of applicability domain (not matching the range of conditions where a reliable prediction can be obtained in this model. These conditions may be physico‐chemical, structural, biological, etc.)
Structure group 3.3: α, β‐unsaturated 2‐phenyl substituted aldehydes.
Local model on aldehydes and ketones, Ames TA100.
MultiCase Ames test.
MultiCase Mouse Lymphoma test.
MultiCase Chromosomal aberration in CHO.
MultiCase Chromosomal aberration in CHL.
Appendix D – Genotoxicity Data on 2‐phenylcrotonaldehyde [FL‐no: 05.062] evaluated in FGE.216Rev1
Appendix E – Genotoxicity Data on 2‐phenylcrotonaldehyde [FL‐no: 05.062] evaluated in FGE.216Rev2
Appendix F – Exposure
F.1. Presence in food
According to the Volatile Compounds in Food (VCF) database, 2‐phenylcrotonaldehyde [FL‐no: 05.062], 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099], 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100], 2‐phenylpent‐2‐enal [FL‐no: 05.175] are reported to be present in natural food sources and processed food as listed in Tables F.1, F.2, F.3 and F.4. These tables report a non‐exhaustive list of foodstuff containing [FL‐no: 05.062, 05.099, 05.100, 05.175]. 2‐Phenyl‐4‐methyl‐2‐hexenal [FL‐no: 05.222] is not reported to be present in food (VCF online database, 2022).
Table F.1.
Examples of 2‐phenylcrotonaldehyde [FL‐no: 05.062] occurrence in foodstuff
| Food item | Process | Qualitative | Quantitative (ppm) | Reference |
|---|---|---|---|---|
| Asparagus (Asparagus officinalis L.) | Cooked | 0.01 | Tressl et al. (1977) | |
| Raw | Yes | |||
| Black tea | Raw | Yes | Sato et al. (1970) | |
| Information not available | Yes | Bricout et al. (1967) | ||
| Microbial fermentation | Yes | Kawakami et al. (1987) | ||
| Fermentation/Microbial fermentation | Yes | Kobayashi and Kawakami (1991) | ||
| Brewing | 0.1–0.15 | Schreier and Mick (1984) | ||
| Buckwheat flour | Boiled | Yes | Yajima et al. (1983) | |
| Cocoa butter | From roasted and unroasted beans | Yes | Carlin et al. (1982) | |
| Cocoa liquor | Beans are roasted, shelled and finely ground into liquor | Yes | Counet et al. (2004) | |
| Baigrie and Rumbelow (1987) | ||||
| Muggler‐Chavan and Reymond (1967) | ||||
|
Coffee |
Roasting | 0.1–0.2 | Silwar et al. (1987) | |
| Information not available | 0.6 | Silwar (1982) | ||
| Dark chocolate | Produced in house | Yes | Afoakwa et al. (2009) | |
| Produced by roasted beans and conching | Owusu et al. (2012) | |||
| Milk chocolate | Storage | Yes | Ziegleder and Stojacic (1988) | |
| Cocoa beans | Roasting | 2.5–3 | Silwar (1988) | |
| Roasting | Yes | Van Praag et al. (1968) | ||
| Raw/Roasting | Yes | Ziegleder (1991) | ||
| Filbert hazelnut (Corylus avellana) | Roasting | Yes | Kinlin et al. (1972) | |
| Katsuobushi (Dried bonito – variety of fish) | Drying – soup stock | Yes | Washino et al. (1989) | |
| Macadama nut (Macadamia integrifolia) | Roasting and grinding | Yes | Crain and Tang (1975) | |
| Barley | Malting |
|
Kossa et al. (1979) | |
| Raw | Yes | Farley and Nursten (1980) | ||
| Fish meat paste, soy and rice miso | Fermentation | Yes | Giri et al. (2010) | |
| Mushrooms | Raw | 0.1 | Pyysalo (1975) | |
| Raw and pressed into juices | Yes | Pyysalo (1976) | ||
| Raw | Yes | Pyysalo and Honkanen (1976) | ||
| Okra (Hibiscus esculentus L.) | Raw | Yes | Ames and MacLeod (1990) | |
| Peanut oil | Cold‐pressed from raw and roasted peanuts | 0.1 | Brown et al. (1973) | |
| Roasted peanuts | Yes | Johnson et al. (1971) | ||
| Pork (liver) | Pressure Cooked | Yes | Mussinan and Walradt (1974) | |
| Potato chips (American) | Frying | Yes | Buttery (1973) | |
| Rice bran | Raw | Yes | Tsugita et al. (1978) | |
| Sesame seed | Roasting | Yes | Manley et al. (1974) | |
| Soybean | Extrusion from defatted flour | Yes | Ames and Macleod (1984) | |
| Yeast extract (Saccharomyces cerevisiae) | Processed in different ways and supplied as powder | Yes | Ames and Elmore (1992) | |
| Processed in different ways and supplied as paste | Yes | Mahadevan and Farmer (2006) |
Table F.2.
Examples of 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099] occurrence in foodstuff
| Food item | Process | Qualitative | Quantitative (ppm) | Reference |
|---|---|---|---|---|
| Black tea leaves | Brewing | Yes | Renold et al. (1974) | |
| Cocoa butter | Expeller pressing of heated cocoa beans | Yes | Carlin et al. (1982) | |
| Cocoa liquor | Beans are roasted, shelled and finely ground into liquor | Yes | Magi et al. (2012) | |
| Yes | Baigrie and Rumbelow (1987) | |||
| Cocoa powder | Roasting | 0.52–1.94 | Bonvehi (2005) | |
| Dark chocolate | Produced in house | Yes | Afoawka et al. (2009) | |
| Produced by roasted beans and conching | Yes | Owusu et al. (2012) | ||
| Before and after Conching | 0.496–0.546 | Counet et al. (2002) | ||
| Milk chocolate | Storage | Yes | Ziegleder and Stojacic (1988) | |
| Cocoa beans | Roasting | 0.3–0.5 | Silwar (1988) | |
| Raw/Fermentation/Roasting | 1 | Gill et al. (1984) | ||
| Roasting | Yes | Van Praag et al. (1968) | ||
| Roasting | Yes | Stofberg and Stoffelsma (1981) | ||
| Raw/Roasting | Yes | Ziegleder (1991) | ||
| Filbert hazelnut (Corylus avellana) | Roasting | Yes | Kinlin et al. (1972) | |
| Barley | Malting | < 0.01–0.065 | Kossa et al. (1979) | |
| Yes | Farley and Nursten (1980) | |||
| Peppermint oil (Mentha piperita L.) | Raw | Yes | Takahashi et al. (1980) | |
| Peanut Oil | Roasted peanuts | Yes | Johnson et al. (1971) | |
| Pork (liver) | Pressure cooked | Yes | Mussinan and Walradt (1974) | |
| Potato (Solanum tuberosum L.) | Frying | Yes | Carlin (1983) | |
| Sesame seed | Roasting | Yes | Manley et al. (1974) | |
| Wort | Boiling | Yes | Buckee et al. (1982) |
Table F.3.
Examples of 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100] occurrence in foodstuff
| Food item | Process | Qualitative | Quantitative (ppm) | Reference |
|---|---|---|---|---|
| Black tea leaves | Brewing | Yes | Renold et al. (1974) | |
| Cocoa butter | From roasted and unroasted beans | Yes | Carlin et al. (1982) | |
| Cocoa powder | Roasting | 0.025–0.31 | Bonvehi (2005) | |
| Milk chocolate | Storage | Yes | Ziegleder and Stojacic (1988) | |
| Cocoa beans | Roasting | 0.05–0.1 | Silwar (1988) | |
| Roasting | Yes | Van Praag et al. (1968) | ||
| Raw/Roasting | Yes | Ziegleder (1991) | ||
| Filbert hazelnut (Corylus avellana) | Roasting | Yes | Kinlin et al. (1972) | |
| Barley | Malting | < 0.01–0.02 | Kossa et al. (1979) | |
| Peppermint oil (Mentha piperita L.) | Raw | 6 | Takahashi et al. (1980) | |
| Potato (Solanum tuberosum L.) | Baking with skin | Yes | Coleman et al. (1981) | |
| Frying | Yes | Carlin (1983) | ||
| Potato chips (American) | Frying | Yes | Buttery (1973) | |
| Sesame seed | Roasting | Yes | Manley et al. (1974) | |
| Yeast extract (Saccharomyces cerevisiae) | Processed in different ways and supplied as powder | Yes | Ames and Elmore, (1992) |
Table F.4.
Examples of 2‐phenylpent‐2‐enal [FL‐no: 05.175] occurrence in foodstuff
F.2. Uses and use levels as provided by the Flavour Industry
For each substance, normal and maximum use levels have been provided by industry (documentation provided to EFSA No. 10) according to the different food categories reported in Annex I of Regulation (EC) 1565/2000 3 or according to the EFSA Guidance on the data required for the risk assessment of flavourings to be used in or on foods (EFSA CEF Panel, 2010). For substances 2‐phenylpent‐2‐enal [FL‐no: 05.175] and 2‐phenyl‐4‐methyl‐2‐hexenal [FL‐no: 05.222] use levels are reported only for the food categories according to of Regulation (EC) 1565/2000. Use levels data (Table F.5) have been used to calculate the mTAMDI. Since the calculation of mTAMDI is based on food categories as reported in Annex I of Regulation (EC) 1565/2000 (Table F.6), for the substances [FL‐no: 05.062, 05.099, 05.100] the highest values of the normal use levels among the subcategories have been selected.
Table F.5.
Use levels reported by industry for the flavouring substances in FGE.19 subgroup 3.3
| CODEX code | Food categories (a) , (d) | Standard portions (b) (g) | Occurrence level as added flavouring substance (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Max | Normal | Max | Normal | Max | Normal | Max | Normal | Max | |||
| Flavouring substances FL‐no: | 05.062 | 05.099 | 05.100 | 05.175 (c) | 05.222 (c) | |||||||
| 01.0 | Dairy products and analogues, excluding products of category 02.0 | 0.41 | 1.4 | 2.12 | 8.65 | 0.41 | 22.4 | 3 | 15 | 0.0002 | 0.0075 | |
| 01.1 | Milk and dairy‐based drinks | 200 | 0.25 | 0.56 | 1.56 | 5.43 | 0.19 | 0.53 | ||||
| 01.6 | Cheese and analogues | 40 | 0.37 | 1.4 | 1.64 | 3.21 | 0.41 | 22.4 | ||||
| 01.7 | Dairy‐based desserts (e.g. pudding, fruit or flavoured yoghurt) | 125 | 0.41 | 1.3 | 2.12 | 8.65 | 0.0022 | 0.5 | ||||
| 02.0 | Fats and oils and fat and oil emulsions (type water‐in‐oil) | 0.48 | 2 | 10 | 0.0002 | 0.0075 | ||||||
| 02.1 | Fats and oils essentially free from water | 15 | 0.16 | 1.56 | 5.68 | 17.38 | 0.11 | 5.61 | ||||
| 02.2 | Fat emulsions mainly of type water‐in‐oil | 15 | 0.16 | 1.56 | 5.68 | 17.38 | 0.11 | 5.61 | ||||
| 02.3 | Fat emulsions mainly of type water‐in‐oil, including mixed and/or flavoured products based on fat emulsions | 15 | 0.16 | 1.56 | 5.68 | 17.38 | 0.11 | 5.61 | ||||
| 02.4 | Fat‐based desserts excluding dairy‐based dessert products of category 1.7 | 50 | 0.48 | 1.23 | 0.85 | 9 | 0.03 | 0.79 | ||||
| 03.0 | Edible ices, including sherbet and sorbet | 50 | 0.44 | 1.15 | 3.89 | 13.15 | 0.03 | 0.55 | 3 | 15 | 0.002 | 0.005 |
| 04.1.2 | Processed fruit | 125 | 0.03 | 0.05 | 0.5 | 1.01 | 0.0021 | 0.09 | 2 | 10 | 0.0002 | 0.0075 |
| 04.1.2.5 | Jams, jellies, marmalades | 30 | 0.26 | 0.26 | 0.78 | 2.69 | 0.0032 | 0.16 | ||||
| 04.2.2 | Processed vegetables (including mushrooms and fungi, roots and tubers, pulses and legumes and aloe vera), seaweed and nut and seed purees and spreads (e.g. peanut butter) and nuts and seeds | 200 | 0.03 | 0.13 | 0.05 | 0.46 | 0.12 | 1.5 | ||||
| 05.0 | Confectionery | 2.52 | 4 | 20 | 0.02 | 0.75 | ||||||
| 05.1 | Cocoa products and chocolate products, including imitations and chocolate substitutes | 40 | 0.97 | 2.64 | 4.18 | 21.24 | 0.23 | 11.25 | ||||
| 05.2 | Confectionery, including hard and soft candy, nougats, etc., other than 05.1, 05.3 and 05.4 | 30 | 0.54 | 3.5 | 3.82 | 19.84 | 0.34 | 6.2 | ||||
| 05.3 | Chewing gum | 3 | 2.52 | 5.13 | 4.66 | 11.52 | 2.03 | 112 | ||||
| 05.4 | Decorations (e.g. for fine bakery wares), toppings (non‐fruit) and sweet sauces | 35 | 0.11 | 1.05 | 1.65 | 10.41 | 0.2 | 11.2 | ||||
| 06.0 | Cereals and cereal products, incl. Flours & starches from roots & tubers, pulses & legumes, excluding bakery | 0.45 | 2 | 10 | ||||||||
| 06.3 | Breakfast cereals, including rolled oats | 30 | 0.45 | 2 | 0.81 | 2.1 | 0.61 | 33.6 | ||||
| 06.4 | Pastas and noodles and like products (e.g. rice paper, rice vermicelli, soya bean pastas and noodles) | 200 | 0.01 | 0.04 | 0.03 | 0.26 | 0.12 | 1.5 | ||||
| 06.5 | Cereal and starch based desserts (e.g. rice pudding, tapioca pudding) | 200 | 0.19 | 1.33 | 1.04 | 1.58 | 0.41 | 22.4 | ||||
| 06.6 | Batters (e.g. for breading or batters for fish or poultry) | 30 | 0.04 | 0.3 | 1.08 | 3.08 | 0.23 | 3 | ||||
| 06.7 | Pre‐cooked or processed rice products, including rice cakes (Oriental type only) | 200 | 0.3 | 0.6 | 0.02 | 0.06 | 0.1 | 0.2 | ||||
| 06.8 | Soya bean products (excluding soya bean products of food category 12.9 and fermented soya bean products of food category 12.10) | 100 | 0.05 | 0.32 | 0.44 | 1.62 | 0.15 | 3.76 | ||||
| 07.0 | Bakery wares | 1.21 | 5 | 25 | 0.002 | 0.0075 | ||||||
| 07.1 | Bread and ordinary bakery wares | 50 | 0.51 | 2.14 | 1.7 | 6.71 | 1.42 | 9.51 | ||||
| 07.2 | Fine bakery wares (sweet, salty, savoury) and mixes | 80 | 1.21 | 3.3 | 6.81 | 22.32 | 1.84 | 11.14 | ||||
| 08.0 | Meat and meat products, including poultry and game | 0.02 | 1 | 5 | 0.0002 | 0.0075 | ||||||
| 08.2 | Processed meat, poultry and game products in whole pieces or cuts | 100 | 0.7 | 0.83 | 0.08 | 0.11 | 0.12 | 1.5 | ||||
| 08.3 | Processed comminute meat, poultry and game products | 100 | 0.7 | 0.83 | 0.08 | 0.11 | 0.12 | 1.5 | ||||
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | 0.42 | 1 | 5 | 0.0002 | 0.0075 | ||||||
| 09.2 | Processed fish and fish products, including molluscs, crustaceans and echinoderms | 100 | 0.42 | 0.47 | 4.13 | 12.88 | 0.12 | 1.5 | ||||
| 09.3 | Semi‐preserved fish and fish products, including molluscs, crustaceans and echinoderms | 100 | 0.42 | 0.47 | 4.13 | 12.88 | 0.12 | 1.5 | ||||
| 09.4 | Fully preserved, including canned or fermented, fish and fish products, including molluscs, crustaceans and echinoderms | 100 | 0.42 | 0.47 | 4.13 | 12.88 | 0.12 | 1.5 | ||||
| 10.0 | Eggs and egg products | 0.8 | ||||||||||
| 10.2 | Egg products | 100 | 0.02 | 0.15 | 0.05 | 0.51 | 0.12 | 1.5 | ||||
| 10.3 | Preserved eggs, including alkaline, salted and canned eggs | 100 | 0.02 | 0.15 | 0.05 | 0.51 | 0.12 | 1.5 | ||||
| 10.4 | Egg‐based desserts (e.g. custard) | 125 | 0.8 | 4 | 6.34 | 21.69 | 0.41 | 22.4 | ||||
| 11.0 | Sweeteners, including honey | 0.45 | ||||||||||
| 11.1 | Refined and raw sugar | 10 | 0.96 | 2.38 | ||||||||
| 11.2 | Brown sugar excluding products of food category 11.1 | 10 | 0.69 | 3.39 | ||||||||
| 11.3 | Sugar solutions and syrups, and (partially) inverted sugars, including molasses and treacle, excluding products of food category 11.1 | 30 | 0.01 | 0.04 | 0.26 | 0.85 | 0.0016 | 0.08 | ||||
| 11.4 | Other sugars and syrups (e.g. xylose, maple syrup, sugar toppings) | 30 | 0.45 | 1.45 | 1.29 | 5.5 | 0.0016 | 0.08 | ||||
| 11.5 | Honey | 15 | 0.5 | 3.01 | ||||||||
| 11.6 | Table‐top sweeteners, including those containing high‐intensity sweeteners | 1 | 0.004 | 0.02 | ||||||||
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. | 2.0 | 2 | 10 | 0.0002 | 0.0075 | ||||||
| 12.2 | Herbs, spices, seasonings and condiments (e.g. seasoning for instant noodles) | 1 | 0.41 | 0.56 | 0.22 | 9.18 | 0.09 | 0.81 | ||||
| 12.3 | Vinegars | 15 | 0.38 | 1.75 | 1 | 3.01 | 0.12 | 1.5 | ||||
| 12.4 | Mustards | 15 | 0.15 | 0.15 | 2.09 | 5.84 | 0.12 | 1.5 | ||||
| 12.5 | Soups and broths | 200 | 0.27 | 0.31 | 0.07 | 0.11 | 0.06 | 0.76 | ||||
| 12.6 | Sauces and like products | 30 | 0.38 | 1.88 | 0.42 | 1.63 | 0.12 | 1.5 | ||||
| 12.7.1 | Salads 120 g (e.g. macaroni salad, potato salad) excluding cocoa‐ and nut‐based spreads of food categories | 120 | 0.02 | 0.15 | 0.05 | 0.51 | 0.12 | 1.5 | ||||
| 12.7.2 | Sandwich spreads (20 g), excluding cocoa‐ and nut‐based spreads of food categories | 20 | 0.02 | 0.15 | 2.32 | 7.58 | 0.12 | 1.5 | ||||
| 12.9 | Protein products | 15 | 2 | 4 | 6.09 | 75.17 | 0.58 | 7.5 | ||||
| 12.10 | Fermented soya bean products | 40 | 1 | 1 | 7.25 | 25.01 | 0.12 | 1.5 | ||||
| 13.0 | Foodstuffs intended for particular nutritional uses | 0.3 | 3 | 15 | ||||||||
| 13.3 | Dietetic foods intended for special medical purposes (excluding food products of category 13.1) | 200 | 0.3 | 2.05 | 0.58 | 1.09 | 0.16 | 11.21 | ||||
| 13.4 | Dietetic formulae for slimming purposes and weight reduction | 200 | 0.3 | 2.05 | 0.58 | 1.09 | 0.16 | 11.21 | ||||
| 13.5 | Dietetic foods (e.g. supplementary foods for dietary use), excluding products of food categories 13.1–13.4 and 13.6 | 200 | 0.3 | 2.05 | 0.58 | 1.09 | 0.16 | 11.21 | ||||
| 13.6 | Food supplements | 5 | 1.14 | 6.6 | 0.07 | 0.57 | ||||||
| 14.1 | Non‐alcoholic (‘soft’) beverages | 300 | 0.55 | 2.29 | 1.54 | 9.87 | 2 | 10 | 0.02 | 0.75 | ||
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | 0.5 | 0.11 | 0.11 | 4 | 20 | 0.02 | 0.75 | ||||
| 14.2.1 | Beer and malt beverages | 300 | 0.13 | 0.13 | 0.47 | 1.29 | 0.0016 | 0.08 | ||||
| 14.2.2 | Grape wines | 150 | 0.23 | 0.23 | 2.47 | 8.39 | 0.0016 | 0.08 | ||||
| 14.2.3 | Mead | 150 | 0.5 | 0.5 | 4.84 | 16.71 | 0.0016 | 0.08 | ||||
| 14.2.4 | Spirituous beverages | 30 | 0.4 | 1.44 | 0.52 | 9.45 | 0 | 0.08 | ||||
| 15.0 | Ready‐to‐eat savouries | 30 | 2.53 (e) | 2.29 (e) | 3.64 | 10.04 | 0.78 | 1.79 | 5 | 25 | 0.0002 | 0.0075 |
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 01.0–15.0 | 300 | 0.08 | 0.34 | 0.55 | 2.25 | 2 | 10 | 0.0002 | 0.0075 | ||
Most of the categories reported are the subcategories of Codex GSFA (General Standard for Food Additives, available at http://www.codexalimentarius.net/gsfaonline/CXS_192e.pdf) used by the JECFA in the SPET technique (FAO/WHO, 2008).
For Adults. In case of foods marketed as powder or as concentrates, occurrence levels must be reported for the reconstituted product, considering the instructions reported on the product label or one of the standard dilution factors established by the JECFA (FAO/WHO, 2008):
– 1/25 for powder used to prepare water‐based drinks such as coffee, containing no additional ingredients,
– 1/10 for powder used to prepare water‐based drinks containing additional ingredients such as sugars (ice tea, squashes, etc.),
– 1/7 for powder used to prepare milk, soups and puddings,
– 1/3 for condensed milk.
Only use levels for ‘main’ food categories have been provided (see Table F.6).
Only food categories for which use has been reported are included in the table.
The Panel noted that the normal and maximum use levels for this food category for [FL‐no: 05.062] are not plausible. However, use level of 2.29 mg/kg was provisionally used for the calculation of mTAMDI.
Table F.6.
Distribution of the 18 food categories listed in Commission Regulation (EC) No 1565/2000 3 into the seven SCF food categories used for TAMDI calculation (SCF, 1995)
| Food categories according to Commission Regulation 1565/2000 | Distribution of the seven SCF food categories | |||
|---|---|---|---|---|
| Key | Food category | Foods | Beverages | Exceptions |
| 01.0 | Dairy products, excluding products of category 02.0 | Foods | ||
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | Foods | ||
| 03.0 | Edible ices, including sherbet and sorbet | Foods | ||
| 04.1 | Processed fruit | Foods | ||
| 04.2 | Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds | Foods | ||
| 05.0 | Confectionery | Exception a | ||
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery | Foods | ||
| 07.0 | Bakery wares | Foods | ||
| 08.0 | Meat and meat products, including poultry and game | Foods | ||
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | Foods | ||
| 10.0 | Eggs and egg products | Foods | ||
| 11.0 | Sweeteners, including honey | Exception a | ||
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. | Exception d | ||
| 13.0 | Foodstuffs intended for particular nutritional uses | Foods | ||
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | Beverages | ||
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | Exception c | ||
| 15.0 | Ready‐to‐eat savouries | Exception b | ||
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) ‐ foods that could not be placed in categories 01.0–15.0 | Foods | ||
F.3. Intake data from intended use
Annual production volumes of the flavouring substances as surveyed by industry are used to calculate the ‘Maximised Survey‐derived Daily Intake’ (MSDI) assuming that the production figure only represents 60% of the use in food, due to underreporting and that 10% of the total EU population are consumers (SCF, 1999).
Use levels for 2‐phenylcrotonaldehyde [FL‐no: 05.062], 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099], 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100], 2‐phenylpent‐2‐enal [FL‐no: 05.175] and 2‐phenyl‐4‐methyl‐2‐hexenal [FL‐no: 05.222] provided by industry (documentation provided to EFSA No. 10) and listed in Table F.5 have been used to calculate the ‘modified Theoretical Added Maximum Daily Intake’ (mTAMDI). 6 When use levels for subcategories are reported according to the EFSA Guidance on the data required for the risk assessment of flavourings (EFSA CEF Panel, 2010), the highest value of the normal use levels among the subcategories has been selected as the value for each main category that is used for the calculation of mTAMDI.
The MSDI and mTAMDI exposure estimates are given in Table F.7.
Table F.7.
Estimated exposure to 2‐phenylcrotonaldehyde [FL‐no: 05.062], 5‐methyl‐2‐phenylhex‐2‐enal [FL‐no: 05.099], 4‐methyl‐2‐phenylpent‐2‐enal [FL‐no: 05.100], 2‐phenylpent‐2‐enal [FL‐no: 05.175] and 2‐phenyl‐4‐methyl‐2‐hexenal [FL‐no: 05.222]
| FL‐no | Chemical name | EU MSDI μg/capita per day | mTAMDI μg/person per day |
|---|---|---|---|
| 05.062 | 2‐phenylcrotonaldehyde | 1.7 | 466.6 |
| 05.099 | 5‐methyl‐2‐phenylhex‐2‐enal | 15 | 1749.6 |
| 05.100 | 4‐methyl‐2‐phenylpent‐2‐enal | 0.34 | 285.9 |
| 05.175 | 2‐phenylpent‐2‐enal | 0.011 | 1,643 |
| 05.222 | 2‐phenyl‐4‐methyl‐2‐hexenal | 3 | 7.5 |
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Degen G, Engel KH, Fowler PJ, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Manco M, Moldeus P, Passamonti S, Shah R, Waalkens‐Berendsen I, Wright M, Benigni R, Bolognesi C, Chipman K, Cordelli E, Nørby K, Svendsen C, Carfì M, Martino C and Mennes W, 2022. Scientific opinion on flavouring group evaluation 216 revision 2 (FGE.216Rev2): consideration of the genotoxicity potential of α,β‐unsaturated 2‐phenyl‐2‐alkenals from subgroup 3.3 of FGE.19. EFSA Journal 2022;20(8):7420, 38 pp. 10.2903/j.efsa.2022.7420
Requestor European Commission
Question numbers EFSA‐Q‐2015‐00199, EFSA‐Q‐2015‐00201, EFSA‐Q‐2015‐00202, EFSA‐Q‐2015‐00203, EFSA‐Q‐2015‐00204
Panel members Maged Younes, Gabriele Aquilina, Laurence Castle, Gisela Degen, Karl‐Heinz Engel, Paul J Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Ursula Gundert‐Remy, Rainer Gürtler, Trine Husøy, Melania Manco, Wim Mennes, Peter Moldeus, Sabina Passamonti, Romina Shah, Ine Waalkens‐Berendsen and Matthew Wright.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The Panel wishes to thank the following for the support provided to this scientific output: Daniel Marzin (member of the Flavouring WG until September 2021) and EFSA staff Alkiviadis Stagkos‐Georgiadis and Giorgia Vianello.
Adopted: 29 June 2022
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
Data presented in Section 2.4 are cited from the first opinion on FGE.216 (EFSA, 2009).
Data presented in Section 2.5 are cited from the first revision of FGE.216 (FGE.216Rev1; EFSA CEF Panel, 2013).
mTAMDI estimation is based on an approach used by the SCF up to 1995 (SCF, 1995) and is calculated on the basis of standard portions and normal use levels for flavoured beverages and foods in general, with exceptional levels for particular foods.
References
- Afoakwa EO, Paterson A, Fowler M and Ryan A, 2009. Matrix effects on flavour volatiles release in dark chocolates varying in particle size distribution and fat content using GC–mass spectrometry and GC–olfactometry. Food Chemistry, 113(1), 208–215. 10.1016/j.foodchem.2008.07.088 [DOI] [Google Scholar]
- Ames JM and Macleod G, 1984. Volatile components of an unflavored textured soy protein. Journal of Food Science, 49, 1552–1565. 10.1111/j.1365-2621.1984.tb12842.x [DOI] [Google Scholar]
- Ames JM and Macleod G, 1990. Volatile components of okra. Phytochemistry, 29, 1201–1207. 10.1016/0031-9422(90)85429-J [DOI] [Google Scholar]
- Ames JM and Elmore JS, 1992. Aroma components of yeast extracts. Flavour and Fragrance Journal, 7, 89–103. [Google Scholar]
- Baigrie BD and Rumbelow SJ, 1987. Investigation of flavour defects in Asian cocoa liquors. Journal of the Science of Food and Agriculture, 39, 357–368. 10.1002/jsfa.2740390411 [DOI] [Google Scholar]
- Bonvehí JS, 2005. Investigation of aromatic compounds in roasted cocoa powder. European Food Research Technology, 221, 19–29. 10.1007/s00217-005-1147-y [DOI] [Google Scholar]
- Bricout J, Viani R, Mueggler‐Chavan F, Marion JP, Reymond D and Egli R, 1967. Sur la composition de l'arôme de thé noir II. Helvetica Chimica Acta, 50, 1517–1522. 10.1002/hlca.19670500610 [DOI] [Google Scholar]
- Brown DF, Senn VJ, Dollear FG and Goldblatt LA, 1973. Concentrations of some aliphatic aldehydes and ketones found in raw and roasted spanish and runner peanuts. Journal of the American Oil Chemists Society, 50, 16–20. 10.1007/BF02628733 [DOI] [Google Scholar]
- Buckee GK, Malcolm PT and Peppard TL, 1982. Evolution of volatile compounds during wort‐boiling. Journal of the Institute of Brewing, 88, 175–181. [Google Scholar]
- Buttery RG, 1973. Unusual volatile carbonyl components of potato chips. Journal of Agricultural and Food Chemistry, 21, 31–33. 10.1021/jf60185a017 [DOI] [Google Scholar]
- Carlin JT, Lee KN, Hsieh OAL, Hwang LS, Ho CT and Chang SS, 1982. Cocoa butter flavors. Manufacturing confectioner, 64–73. [Google Scholar]
- Carlin JT, 1983. Isolation and identification of the volatile flavor compounds from French fried potatoes. Rutgers The State University of New Jersey ‐ New Brunswick ProQuest Dissertations Publishing. (PhD thesis).
- Coleman EC, Ho CT and Chang SS, 1981. Isolation and identification of volatile compounds from baked potatoes. Journal of Agricultural and Food Chemistry, 29, 42–48. [DOI] [PubMed] [Google Scholar]
- Counet C, Callemien D, Ouwerx C and Collin S, 2002. Use of gas chromatography−olfactometry to identify key odorant compounds in dark chocolate. comparison of samples before and after conching. Journal of Agricultural and Food Chemistry, 50, 2385–2391. [DOI] [PubMed] [Google Scholar]
- Counet C, Ouwerx C, Rosoux D and Collin S, 2004. Relationship between procyanidin and flavor contents of cocoa liquors from different origins. Journal of Agricultural and Food Chemistry, 52, 6243–6249. 10.1021/jf040105b [DOI] [PubMed] [Google Scholar]
- Crain WO Jr and Tang CS, 1975. Volatile components of roasted macadamia nuts. Journal of Food Science (USA), 40, 207–208. [Google Scholar]
- EFSA (European Food Safety Authority) , 2008a. Minutes of the 26th Plenary meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Parma on 27–29 November 2007. Parma, 7 January 2008. Available online: http://www.efsa.europa.eu/sites/default/files/event/afc071127-m.pdf [Google Scholar]
- EFSA (European Food Safety Authority) , 2008b. Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19 ‐ Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA Journal 2008;854, 5 pp. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/854 [Google Scholar]
- EFSA (European Food Safety Authority) , 2008c. Scientific opinion. List of α,β‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing. Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA Journal 2008;910, 7 pp. 10.2903/j.efsa.2008.910 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Scientific Opinion of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids. Flavouring Group Evaluation 216: α,β‐unsaturated aldehydes from chemical subgroup 3.3 of FGE.19: 2‐Phenyl‐2‐alkenals. EFSA Journal 2009;ON‐881, 13 pp. 10.2903/j.efsa.2009.881 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2010. Scientific Opinion on guidance on the data required for the risk assessment of flavourings to be used in or on foods. EFSA Journal 2010;8(6):1623, 38 pp. 10.2093/j.efsa.2010.1623 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2013. Scientific Opinion on Flavouring Group Evaluation 216, Revision 1 (FGE.216Rev1). Consideration of genotoxic potential for α,β‐unsaturated 2‐Phenyl −2‐Alkenals from Subgroup 3.3 of FGE.19. EFSA Journal 2013;11(7):3305, 45 pp. 10.2903/j.efsa.2013.3305 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific opinion on Genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9 (9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2021. Scientific Opinion on the guidance on aneugenicity assessment. EFSA Journal 2021;19 (8):6770, 27 pp. 10.2903/j.efsa.2021.6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO , 2008. Joint FAO/WHO Expert Committee on Food Additives. Sixty‐ninth meeting, Rome, Italy, 17–26 June 2008. Summary and conclusions. Issued 4 July 2008. Available at: https://www.fao.org/documents/card/en/c/c1dfe308-c04e-444d-9885-e2b20ef6bb07/
- Farley DR and Nursten HE, 1980. Volatile flavour components of malt extract. Journal of the Science of Food and Agriculture, 31, 386–396. 10.1002/jsfa.2740310409 [DOI] [Google Scholar]
- Gill MS, Macleod AJ and Moreau M, 1984. Volatile components of cocoa with particular reference to glucosinolate products. Phytochemistry, 23, 1937–1942. 10.1016/S0031-9422(00)84945-6 [DOI] [Google Scholar]
- Giri A, Osako K and Ohshima T, 2010. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chemistry, 120, 621–631. 10.1016/j.foodchem.2009.10.036 [DOI] [Google Scholar]
- JECFA , 2006. Safety evaluation of certain food additives and contaminants. Sixty‐third Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 54. IPCS, WHO, Geneva. [Google Scholar]
- JECFA , 2015. Safety evaluation of certain food additives. Seventy‐nine Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 70. WHO, Geneva. [Google Scholar]
- Johnson BR, Waller GR and Foltz RL, 1971. Volatile components of roasted peanuts: neutral Fraction. Journal of Agricultural and Food Chemistry, 19, 1025–1027. [Google Scholar]
- Kawakami M, Kobayashi A, Yamanishi T and Shoujaku S, 1987. Flavor constituents of microbial‐fermented teas Chinese‐Zhuan‐cha and Koku‐cha. Journal of the Agricultural Chemical Society of Japan, 61, 457–465. [Google Scholar]
- Kinlin TE, Muralidhara R, Pittet AO, Sanderson A and Walradt JP, 1972. Volatile components of roasted filberts. Journal of Agricultural and Food Chemistry, 20, 1021–1028. 10.1021/jf60183a013 [DOI] [Google Scholar]
- Kobayashi A and Kawakami M, 1991. Analysis of essential oils of tea. Modern Methods of Plant Analysis, 12, 21–40. 10.1007/978-3-642-84023-4_2 [DOI] [Google Scholar]
- Kossa T, Bahri D and Tressl R, 1979. Aromastoffe des Malzes und deren Beitrag zum Bieraroma. Monatsschrift für Brauerei, 32, 249–254. [Google Scholar]
- Magi E, Bono L and Di Carro M, 2012. Characterization of cocoa liquors by GC–MS and LC–MS/MS: focus on alkylpyrazines and flavanols. Journal of Mass Spectrometry, 47, 1191–1197. 10.1002/jms.3034 [DOI] [PubMed] [Google Scholar]
- Mahadevan K and Farmer L, 2006. Key odor impact compounds in three yeast extract pastes. Journal of Agricultural and Food Chemistry, 54, 7242–7250. 10.1021/jf061102x [DOI] [PubMed] [Google Scholar]
- Manley CH, Vallon PP and Erickson RE, 1974. Some aroma components of roasted sesame seed (Sesamum indicum L.). Journal of Food Science, 39, 73–76. 10.1111/j.1365-2621.1974.tb00991.x [DOI] [Google Scholar]
- Müggler‐Chavan F and Reymond D, 1967. Constituants aromatiques de cacaos de diverses provenances. Mitt. Geb. Lebensmittelunters. Hygiene, 58, 466–473. [Google Scholar]
- Mussinan CJ and Walradt JP, 1974. Volatile constituents of pressure cooked pork liver. Journal of Agricultural and Food Chemistry, 22, 827–831. 10.1021/jf60195a002 [DOI] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 1997a. OECD Guideline for testing of chemicals No. 471: Bacterial Reverse Mutation Test.
- OECD (Organisation for Economic Co‐operation and Development) , 1997b. OECD Guideline for testing of chemicals No. 474: Mammalian Erythrocyte Micronucleus Test.
- OECD (Organisation for Economic Co‐operation and Development) , 2010. OECD Guideline for testing of chemicals No. 487: In Vitro Mammalian Cell Micronucleus Test.
- OECD (Organisation for Economic Co‐operation and Development) , 2013. OECD Guideline for testing of chemicals No. 488: Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2014a. OECD Guideline for testing of chemicals No. 487: In Vitro Mammalian Cell Micronucleus Test.
- OECD (Organisation for Economic Co‐operation and Development) , 2014b. OECD Guideline for testing of chemicals No. 489: In vivo Mammalian Alkaline Comet Assay. [Google Scholar]
- Owusu M, Petersen MA and Heimdal H, 2012. Effect of fermentation method, roasting and conching conditions on the aroma volatiles of dark chocolate. Journal of Food Processing and Preservation, 36, 446–456. 10.1111/j.1745-4549.2011.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyysalo H, 1975. Studies on the volatile compounds in mushrooms. Journal of Materials Processing Technology, 13, 1–139. [Google Scholar]
- Pyysalo H, 1976. Identification of volatile compounds in seven edible fresh mushrooms. Acta Chemica Scandinavica, 30, 235–244. [Google Scholar]
- Pyysalo H and Honkanen E, 1976. The aroma of fresh mushrooms. Analysis of Sensory Properties in Foods, 159–162. [Google Scholar]
- Renold W, Näf‐Müller R, Keller U, Willhalm B and Ohloff G, 1974. An investigation of the tea aroma ‐ Part I. New volatile black tea constituents. Helvetica Chimica Acta, 57, 1301–1308. 10.1002/hlca.19740570506 [DOI] [Google Scholar]
- Sato S, Sasakura S, Kobayashi A, Nakatani Y and Yamanishi T, 1970. Flavor of black tea: Part VI. Intermediate and high boiling components of the neutral fraction. Agricultural and Biological Chemistry, 34, 1355–1367. 10.1080/00021369.1970.10859779 [DOI] [Google Scholar]
- SCF (Scientific Committee for Food) , 1995. First annual report on chemically defined flavouring substances. May 1995, 2nd draft prepared by the SCF Working Group on Flavouring Substances (Submitted by the SCF Secretariat, 17 May 1995). CS/FLAV/FL/140‐Rev.2. Annex 6 to Document III/5611/95, European Commission, Directorate‐General III, Industry.
- SCF (Scientific Committee on Food) , 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). Scientific Committee on Food. SCF/CS/FLAV/TASK/11 Final 12 June 1999. Annex I the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Schreier P and Mick W, 1984. Analytische Differenzierung von zwei Qualitäten schwarzen Tees mittels Kapillargaschromatographie‐Massenspektrometrie. Chemie Mikrobiologie Technologie der Lebensmittel, 8, 97–104. [Google Scholar]
- Silwar R, 1982. GC‐MS Untersuchungen schwefelhaltiger Verbindungen in Röstkaffee und Cystein/Methionin‐Modellsystemen. Thesis, Berlin. [Google Scholar]
- Silwar R, Kamperschroer H and Tressl R, 1987. Gaschromatographlsch‐massenspektrometrlsche Untersuchungen des Rost‐kaffeearomas ‐Quantitative Bestlmmung wasserdampffluchtlger Aroma‐stoffe. Chem. Mlkrobiol. Technol. Lebensm., 10, 176–187. [Google Scholar]
- Silwar R, 1988. Gaschromatographic‐mass spectrometric investigation of cocoa aroma. Café Cacao Thé (Paris), 32, 243–250. [Google Scholar]
- Stofberg J and Stoffelsma J, 1981. Consumption of flavoring materials as food ingredients and food additives. Perfum. Flavor., 5, 19–35. [Google Scholar]
- Takahashi K, Someya T, Muraki S and Yoshida T, 1980. A new keto‐alcohol, (−)‐mintlactone, (+)‐isomintlactone and minor components in peppermint oil. Agricultural and Biological Chemistry, 44, 1535–1543. 10.1080/00021369.1980.10864154 [DOI] [Google Scholar]
- Tressl R, Bahri D, Holzer M and Kossa T, 1977. Formation of flavor components in asparagus. 2. Formation of flavor components in cooked asparagus. Journal of Agricultural and Food Chemistry, 25, 459–463. 10.1021/jf60211a048 [DOI] [Google Scholar]
- Tsugita T, Kurata T and Fujumaki M, 1978. Volatile components in the steam distillate of rice bran: identification of neutral and basic compounds. Agricultural and Biological Chemistry, 42, 643–651. 10.1080/00021369.1978.10863031 [DOI] [Google Scholar]
- Van Praag M, Stein HS and Tibbetts MS, 1968. Steam volatile aroma constituents of roasted cocoa beans. Journal of Agricultural and Food Chemistry, 16, 1005–1008. 10.1021/jf60160a011 [DOI] [Google Scholar]
- VCF (Volatile Compounds in Food) online database , 2022. Version 16.9, BeWiDo BV 1992–2022. Available online: https://www.vcf-online.nl/VcfHome.cfm
- Walradt JP, Pittet AO, Kinlin TE, Muralidhara R and Sanderson A, 1971. Volatile components of roasted peanuts. Journal of Agricultural and Food Chemistry, 19, 972–979. 10.1021/jf60177a017 [DOI] [Google Scholar]
- Washino Y, Kubota K and Kobayashi A, 1989. Aroma components of dried bonito soup stock ‐ Isolation of aroma component by using porous polymer resin. Nippon Kasei Gakkaishi, 40, 265–270. [Google Scholar]
- Yajima I, Yanai T, Nakamura M, Sakakibara H, Uchida H and Hayashi K, 1983. Volatile flavor compounds of boiled buckwheat flour. Agricultural and Biological Chemistry, 47, 729–738. 10.1080/00021369.1983.10865724 [DOI] [Google Scholar]
- Ziegleder G and Stojacic E, 1988. Lagerungsbedingte Veränderungen im Aroma von Milchschokoladen. Zeitschrift für Lebensmittel Untersuchung und Forschung, 186, 134–138. [Google Scholar]
- Ziegleder G, 1991. Composition of flavor extracts of raw and roasted cocoas. International Journal of Food Research and Technology, 192, 521–525. 10.1007/BF01202506 [DOI] [Google Scholar]


