Preface.
Alzheimer disease (AD) is a major cause of age-related dementia. We do not fully understand AD etiology and pathogenesis, but oxidative damage is a key component. Brain mostly uses glucose for energy, but in AD and amnestic mild cognitive impairment (aMCI) glucose metabolism is dramatically decreased, probably due, at least in part, to oxidative damage to enzymes involved in glycolysis, the tricarboxylic acid (TCA) cycle, and ATP biosynthesis. Consequently, ATP-requiring processes for cognitive function are impaired, and synaptic dysfunction and neuronal death result, with ensuing thinning of key brain areas. We summarize current research on the interplay and sequence of these processes and suggest potential pharmacological interventions to retard AD progression.
INTRODUCTION
AD is characterized by an accumulation of senile plaques (SP) [composed mostly of fibrillary amyloid beta-peptide (Aβ) and dystrophic neurites] and neurofibrillary tangles (NFT) [composed of hyperphosphorylated tau protein] in the brain, leading to dysfunction and loss of synapses and eventual neuronal death1,2. Clinically, AD is characterized by several features, notably a progressive cognitive decline involving loss of memory and higher executive functioning1. Arguably the earliest stage of AD is preclinical AD (PCAD) in which persons have normal cognitive status, but upon death and autopsy their brains display evidence of substantial AD neuropathology. Amnestic mild cognitive impairment (aMCI) is a progressive condition in which there is some degree of memory loss and is widely thought to be a prodromal early stage of AD in which AD neuropathology is present, albeit to a lesser degree. In contrast to AD patients, however, aMCI individuals can perform the activities of daily living. It has been estimated that approximately 15 percent of people with aMCI progress to AD annually3.
AD pathology occurs well before (up to two decades) the onset of clinical symptoms2–4. It follows that a) therapy begun when symptoms appear may be too late to be effective; and b) understanding key molecular processes in the progression of AD is needed to facilitate earlier diagnosis and to develop new interventions to slow or stop its progression.
One important process that becomes dysfunctional in AD and aMCI is metabolism of glucose5,6. Glucose is normally the major energy source for the brain and is metabolized to ATP via glycolysis, the tricarboxylic acid (TCA) cycle, and the electron transport chain (ETC), as shown in Figure 1. Glucose enters the brain from the vasculature through highly efficient glucose transporters and requires insulin for optimal cellular utilization7. In AD and aMCI, however, brain insulin resistance is present6,7. Indeed, type 2 diabetes (T2DM), a key component of which is insulin resistance, is a significant risk factor for developing AD7. Given the huge increase in T2DM development worldwide, combined with ageing populations, AD is a major and growing problem.
Figure 1. Schematic diagrams of the biochemistry of glucose catabolism and ATP synthesis and their oxidative dysfunction in AD and aMCI brains.
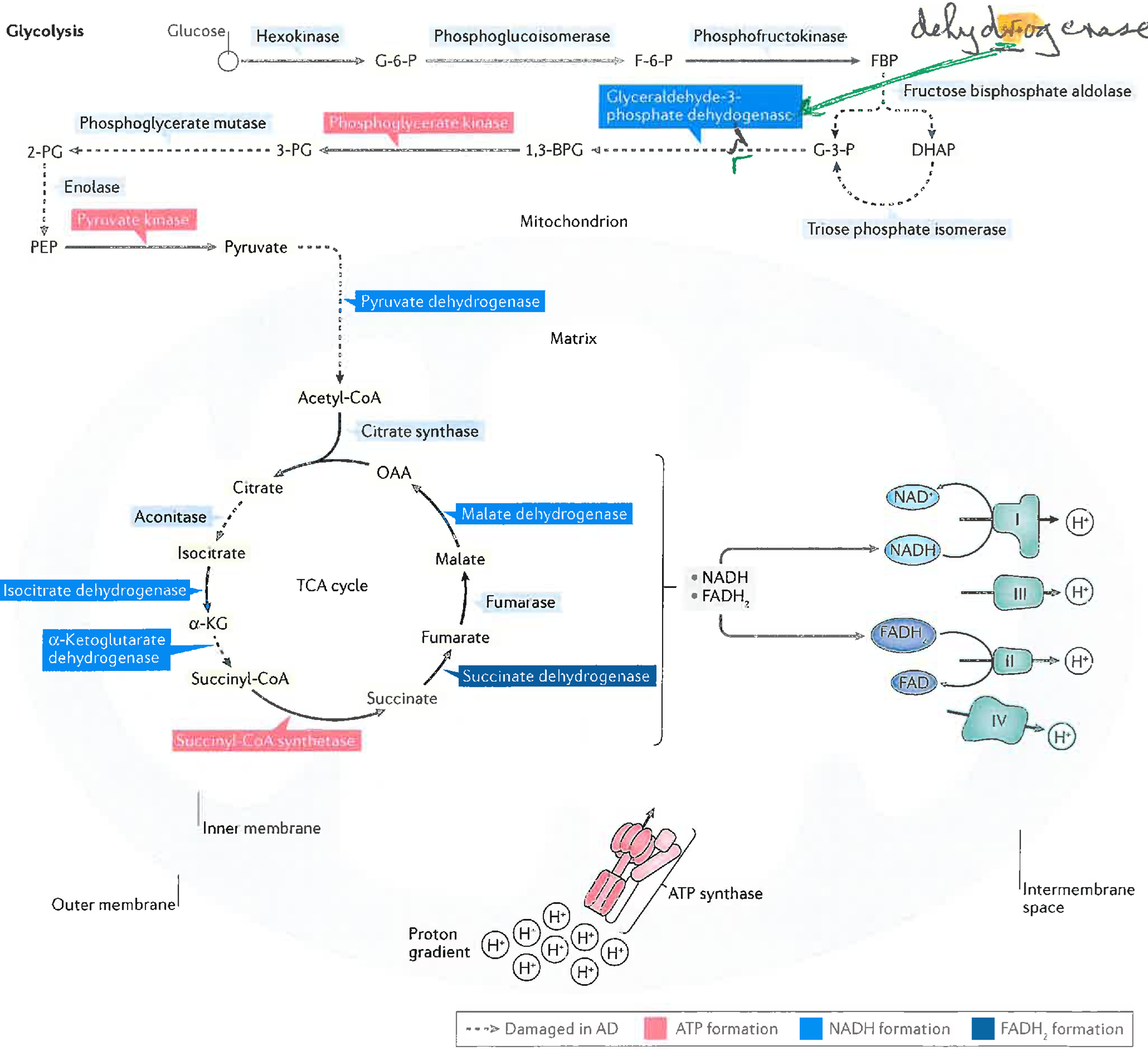
Glycolysis, the tricarboxylic acid (TCA) cycle, and the electron transport chain (ETC), the latter localized on the inner mitochondrial membrane, work together to catabolize glucose and drive ATP synthesis via the ATP synthase complex. Complexes I to IV of the ETC are shown. Also shown is ATP Synthase, whose α-chain is oxidatively modified in brains of subjects with AD. Briefly, this figure shows that glucose is converted to pyruvate in glycolysis. Pyruvate is converted to acetyl coenzyme A, which enters the TCA cycle, and resulting reducing equivalents (NADH and FADH2) from glycolysis and the TCA cycle enter the mitochondrial ETC. (The inner mitochondrial membrane is impermeable to NADH, so the Malate-Aspartate shuttle leads to NADH synthesis in the matrix via NADH in the cytosol) to reduce oxygen to water, leading to production of a mitochondrial proton gradient in the Intermembrane Space that drives ATP synthesis. Reactions catalyzed by specific enzymes or enzyme complexes identified by redox proteomics or other techniques to be oxidatively damaged (and likely thereby dysfunctional) in AD brain (and most also in aMCI brains)12,22,24–26,34,35,115 are indicated as dashed lines in the Figure.
Abbreviations:
G-6-P, glucose-6-phosphate; F-6-P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; G-3-P, glyceraldehyde-3-phosphate; 1,3-BPG, 1,3-bisphosphoglycerate; 3-PG, 3-phosphoglycerate; 2-PG, 2-phosphoglycerate; PEP, phosphoenolpyruvate; acetyl-CoA, acetyl coenzyme A; α-KG, α-ketoglutarate; succinyl-CoA, succinyl-coenzyme A; OAA, oxaloacetate.
The present article summarizes the role of oxidative damage in aMCI and AD, how it affects glucose metabolism, and how it is a key mechanism behind insulin resistance. We review the reasons for the failures of certain therapeutic approaches in AD, and suggests possible new approaches.
Oxidative Damage is relevant to AD
Oxidative Damage is the damage that is done to biomolecules during oxidative stress (for a detailed review and discussion see8). Oxidative stress is a serious imbalance between the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and antioxidant defences8, and has been shown in a wide range of studies to contribute significantly to the pathogenesis and progression of AD2,8–19. Diabetes also leads to oxidative stress (ref8, also see later discussion), which may make a contribution to its propensity to favour AD development9. The term “reactive” is variable: some ROS and RNS are highly reactive (e.g., OH•), whereas other are much more selective in their reactions (e.g., H2O2, NO•, ). Box 1 lists several biologically-important ROS and RNS.
When certain ROS or RNS react with biomolecules, oxidative or nitrosative damage occurs, which can be detected by measuring specific products that result from such damage (“biomarkers of oxidative or nitrosative damage”)8. Some of the most commonly used biomarkers of oxidative damage to lipids, proteins and nucleic acids are listed in Table 1.
Table 1.
Some of the major biomarkers of oxidative damage in brain.
| Target | Products measured | Comments | Refs. |
|---|---|---|---|
| PROTEINS | 3-Nitrotyrosine | Mostly produced when superoxide radical () reacts with nitric oxide (NO•) to give peroxynitrite (ONOO−), which leads to protein nitration on tyrosine (and other residues) by complex mechanisms. | 8,18 |
| Protein carbonyls Methionine sulphoxide - product of attack by ROS/RNS on methionine residues Highly reactive aldehydes, e.g. 4-hydroxy-2-nonenal (HNE), hexenal (HHE); and 2-propene-1-al (acrolein). These latter molecules can form covalent adducts by Michael addition reactions with Cys, Lys, and His residues on proteins. Such Michael adducts alter the conformation and function of proteins and can be detected by immunochemical or mass spectrometric methods. |
There are four principal mechanisms that generate PC.
|
8,14,15,118 | |
| LIPIDS | Lipid peroxides Cyclic peroxides |
Lipid peroxidation usually results from abstraction of labile allylic H-atoms from | 8,11,13–16,119 |
| F2-isoprostanes F3-isoprostanes F4-isoprostanes |
unsaturated fatty acid side chains of phospholipids by ROS and/or RNS. This generates carbon-centered radicals (C•). Subsequent chain reaction steps involve:
|
||
| Isoprostanes are a family of lipid peroxides mostly derived from arachidonic (F2 −), eicosapentaenoic (F3 −) or docosahexaenoic (F4 −) acids. They are often regarded as the most reliable “gold standard” biomarkers of lipid peroxidation (especially F2 −, F4 −). | 119 | ||
| DNA | 8-hydroxydeoxyguanosine (8OHdG) A range of other base oxidation products |
8OHdG is a mutagenic product and the most commonly measured biomarker of oxidative DNA damage. It is generated by attack of several ROS/RNS on guanine residues in DNA. However, some highly-reactive ROS/RNS such as OH• and ONOOH generate a range of other products in addition. | 8,120 |
| RNA | 8-hydroxyguanine (8OHG) | 8OHG is the most commonly measured biomarker of oxidative RNA damage and is generated by attack of several ROS/RNS on guanine residues in RNA. RNA oxidation has a range of deleterious consequences. | 8,121–123 |
Increased levels of biomarkers of oxidative and nitrosative damage can be due to increased ROS/RNS production, decreased clearance of oxidatively damaged molecules (or most likely both). Each of these processes plays a role in AD and aMCI.
In the brains of subjects with PCAD and AD, levels of oxidative damage to a wide range of molecules are increased8–19. For example, levels of protein carbonyls (PC) are elevated in AD in brain regions that are rich in amyloid beta-peptide (Aβ)-containing senile plaques (Table 1), but at normal levels from brain regions devoid of Aβ-rich plaques19. Even in aMCI patients, oxidative damage is already significantly increased: PC are significantly elevated in aMCI brain or cerebrospinal fluid (CSF)8,14,20. Increased lipid peroxidation (a term explained in Table 1) in AD and aMCI brains or CSF and in PCAD hippocampus is further evidenced by rises in the levels of protein-conjugated HNE, F2-isoprostanes, and F4-isoprostanes8,11,12,13,15,16,19–23. Elevated levels of 3-nitrotyrosine (3-NT), suggestive of damage by peroxynitrite (Table 1) are also observed in AD18,24 and aMCI25. 8-Hydroxy-deoxyguanosine (8-OHdG), a biomarker of oxidative damage to DNA (Table 1), is also elevated in AD (in both nuclear and mitochondrial DNA)26–28, as is oxidative damage to RNA29,30. For example, neuritic plaques (rich in fibrillar Aβ42 and Aβ40) and neurofibrillary tangles contain oxidized, glycated, and nitrated proteins. Consequences of this increased oxidative and nitrosative damage are likely to include glucose dysmetabolism (see section on oxidative damage below) and loss of ion gradients with resulting impaired action potentials and Ca2+ dyshomeostasis, because oxidative stress is well known to raise intracellular free Ca2+ levels, from which several deleterious consequences can follow8. Moreover, oxidative DNA damage can interfere with gene transcription and affect promoter function, which can lead to impaired transcription of essential genes and to mutations. Oxidative RNA damage can impair protein translation and the damaged RNA can be prematurely degraded, further impairing synthesis of essential proteins (Table 1).
Learning and memory deficits, decreased higher executive function, and diminished reasoning ability characterize AD patients, whereas memory deficits are a hallmark of aMCI. In both conditions, these altered functions largely originate from synaptic dysfunction relating to altered synaptic proteomes31,32. Aβ42 oligomers contribute to this synaptic dysfunction, impairing learning and memory31. Aβ42 oligomers also cause oxidative damage to synaptic membranes12,16, and there seems to be an intimate relationship between this oligomer-induced oxidative damage and synaptic dysfunction. Indeed, the first pathological insults to neurons in AD and aMCI occur at pre- and post-synaptic membranes1,3. Interestingly, large oligomers of Aβ42, which would have difficulty solubilizing in the neuronal lipid bilayer, seem relatively non-toxic, whereas small oligomers of Aβ42 (for example, dimers or trimers that easily enter lipid bilayers) appear highly toxic to synapses33. These considerations support the notion that lipid peroxidation, and perhaps other forms of oxidative damage in synaptic membranes, accounts for the loss of long-term potentiation (LTP) and other synaptic functions involved in learning and memory12,15,16,20–22,34,35.
Dysfunctional glucose metabolism in AD
The brain is an energy-demanding organ and relies heavily on efficient ATP production via glycolysis, the TCA cycle and oxidative phosphorylation (Fig. 1)7. Yet glucose metabolism in AD and aMCI brains is significantly impaired5–7,9,38. What causes this loss of glucose utilization?
Contribution of oxidative damage
Research from our laboratories11,12,34 and many others2,4,8,10 has shown that inefficient glucose utilization (and thus impaired ATP production) and oxidative damage are intimately related. A major contributor to inefficient glucose utilization may well be oxidative modification, which almost always leads to decreased activity of enzymes involved in glucose metabolism (Figure 1).
The techniques of redox proteomics34 allowed specific oxidatively or nitrosatively modified proteins (that is, PC-, protein bound HNE-, and/or 3-NT-modified proteins) to be identified in brain from subjects with late-stage AD, aMCI and PCAD20,24,34,35. For example, redox proteomics of AD brain tissue revealed that in affected brain areas, oxidative modification of the glycolytic enzymes aldolase, triosephosphate isomerase (TPI), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate mutase (PGM1), and α-enolase occurs12,34. In addition, oxidative modifications to aconitase, a key iron-sulfur containing enzyme in the TCA cycle, creatine kinase (an enzyme which helps neurons to maintain ATP levels) and ATP synthase in brain mitochondria, help to explain decreased glucose metabolism and consequent decreased ATP production in the brains of people with aMCI and AD12,25,34. Oxidative damage to mitochondrial DNA27,28 might also contribute to impaired energy production, and there has been a suggestion that defects in sirtuin 3 (SIRT 3) contribute to oxidative damage in AD mitochondria37. Indeed, mitochondrial dysfunction and insulin resistance are intimately related7,38.
The consequences of this decreased ATP production in AD and aMCI are profound. For example, decreased ATP will diminish the neuron’s ability to maintain ionic gradients, hindering production and propagation of action potentials and therefore neurotransmission. Moreover, loss of ion gradients can allow extracellular Ca2+ to enter, which can further raise intracellular free Ca2+ levels, stimulating Ca2+-dependent endonuclease, phospholipase, and proteinase activities8, contributing to synaptic dysfunction and eventual neuronal death. Excess Ca2+ can saturate the ability of endoplasmic reticulum (ER) and mitochondria to buffer and cycle Ca2+, causing swelling of the latter with consequent opening of the mitochondrial permeability transition pore, leading to release of cytochrome c and apoptosis inducing factor-1, provoking neuronal apoptotic death39. Excess intra-neuronal free Ca2+ can also cause loss of fidelity of microtubule assembly40, with consequent decreased anterograde and retrograde transport of mitochondria and neurotransmitter vesicles, starving presynaptic terminals of energy and decreasing neurotransmission. This in turn leads to synaptic dysfunction, neuronal death, and ultimately to cognitive dysfunction.
mTOR Activation and AD
Brain insulin resistance is common in AD7,38,41. One of the mechanisms by which insulin resistance can develop is by activation of the mechanistic target of rapamycin (sometimes called the mammalian target of rapamycin), usually abbreviated to mTOR. mTOR is a highly integrated complex of many proteins41,42, and exists in two functionally distinct forms, mTORC1 and mTORC2. Activated mTORC1 is intimately involved in regulation of protein synthesis, autophagy, mitochondrial function, lipogenesis, ketogenesis, and insulin signaling, and is crucially linked to glucose metabolism, where it becomes activated by growth factors, amino acids, and high cellular energy status41,42 (Fig 2).
Figure 2. Schematic representation of biochemical events associated with insulin binding to its receptor, leading to activation of mTORC1 with subsequent inhibition of autophagy and development of insulin resistance.
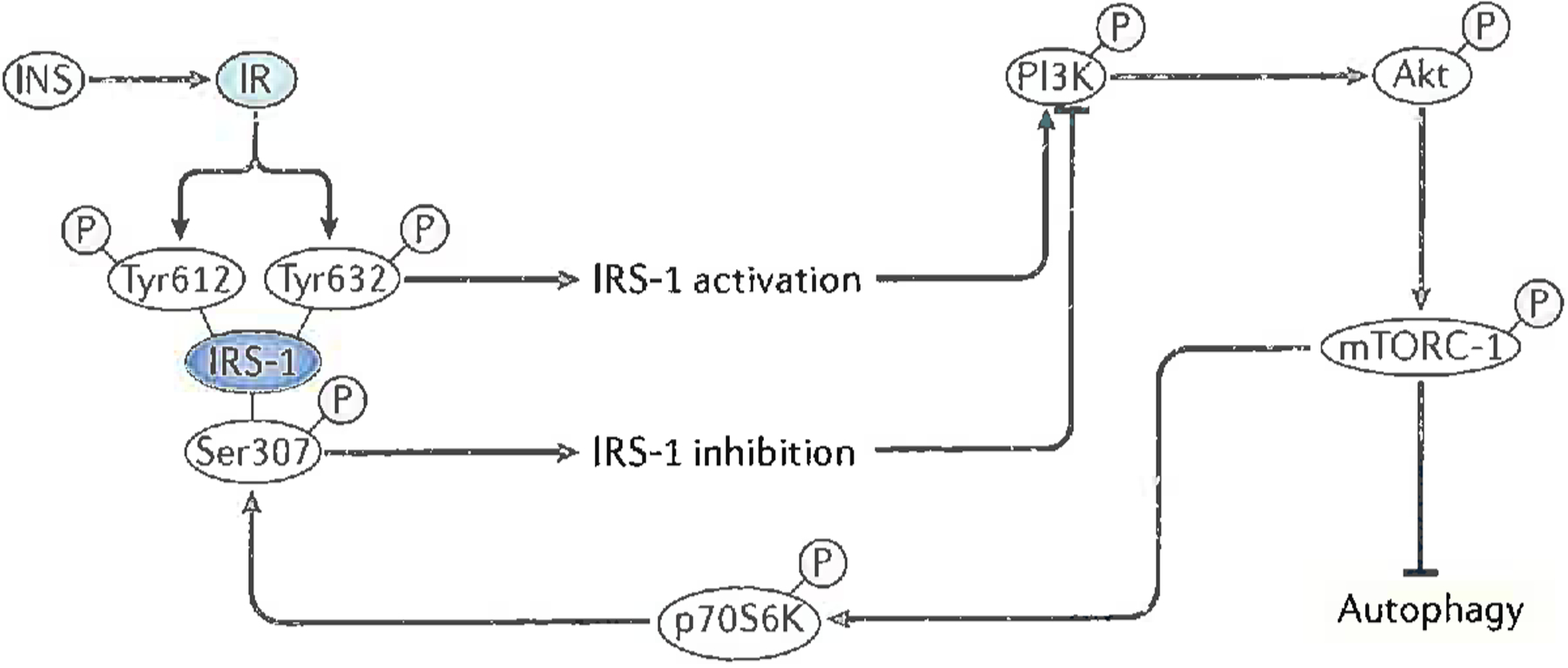
After insulin (INS) binds to the insulin receptor (IR) on neuronal membranes, the IR dimerizes and auto-phosphorylation on tyrosine residues occurs. The insulin receptor substrate-1 (IRS-1) recognizes IR phosphotyrosine residues and binds to the IR, which in turn leads to phosphorylation of IRS-1 on tyrosine residues 612 and 632 with resultant activation of IRS-1. Activated IRS-1 leads to phosphorylation and activation of two pathways for the insulin signaling cascade, one of which is the PI3/Akt pathway. Phosphorylated PI3K leads to phosphorylation and activation of Akt, which leads to phosphorylation of Ser-2448 of the mechanistic target of rapamycin complex 1 (mTORC1), the latter becoming activated as a kinase7,41,42,62. Activated mTORC1 kinase has several key downstream effects (two of which are shown in the figure) that impair neuronal survival (and are thus relevant to AD). There are (a) inhibition of autophagy; and (b) phosphorylation of the protein, p70S6K, which then becomes a kinase, one of whose substrates is Ser-307 of IRS-1. Once phosphorylated on Ser-307, IRS-1 function ceases, leading to and becoming a marker for insulin resistance34,41,62.
Modified with permission from Ref. 62.
Inhibition of autophagy following activation of mTOR in AD (Fig 2) causes accumulation of aggregated, misfolded proteins and damaged organelles, particularly mitochondria, which can lead to inhibition of normal cellular processes. This significant mediator of neuronal death is present in the early stages of AD, and evidence of impaired autophagy is also found in aMCI brain and PCAD brains41–43. Insulin resistance is another detrimental consequence of mTOR activation in aMCI and AD brain41 (Fig 2). These mTOR-mediated events may help explain the observation that type 2 diabetes (T2DM) is a significant risk factor for development of AD7,41. In addition to mTOR activation, insulin resistance can also result in neuronal glucose deficiency, with consequent decreased glucose metabolism, impaired ATP production, and dysfunction and/or death7,42
Glycation and brain oxidative damage
When reducing sugars react with the side chain amino groups of lysine residues on proteins, several processes occur that eventually generate advanced glycation end products (AGEs). AGE formation involves not only direct reaction of these residues with the sugars but also oxidative damage to the proteins: the combination of these reactions is often referred to as glycoxidation (reviewed in8,44). AGEs are ligands for receptors for AGEs (RAGE)44–47. Glycoxidation is highly relevant to AD, in part because Aβ extracellular fibrillar aggregates have characteristics of AGEs and bind to RAGE in neurons and brain endothelial cells. AGE and Aβ binding to RAGE leads to further oxidative stress that contributes to neuronal death and vascular dementia in AD45–48. Glycation may slow the conversion of Aβ to fibrils, keeping them longer in the toxic oligomeric forms48. Vascular dysfunction worsens cognitive defects in AD49.
Proteostasis Abnormalities in AD
The proteostasis network consists of autophagy (all types), the ubiquitin-proteasome system (UPS) and the unfolded-protein response (UPR) in the ER43,50–54 (Fig 3). One might predict that as a consequence of decreased autophagy in AD (see above), the UPS would become hyperactivated to compensate for decreased removal of damaged proteins. In fact, proteasome activity seems to be decreased in AD50–52,. In addition, a crucial part of the UPS is oxidatively damaged and dysfunctional in both aMCI and AD brain, namely, ubiquitin carboxyl-terminal hydrolase L-1 (UCH L1)34,55–57. UCHL1 removes (one residue at a time from the carboxyl terminal) the polymeric ubiquitin chains that have been added to the damaged proteins by ubiquitin ligases, so that the protein can enter the 19S cap of the 26S proteasome. In aMCI and AD brains, the failure of oxidatively damaged UCH L1 to perform its function in aMCI and AD brains leads to accumulation of ubiquitinylated proteins, decreased proteasome function, and subsequent accumulation of damaged, aggregated proteins55. This UCH L1 dysfunction synergizes with dysfunctional autophagy, ER stress (described below), and other impairments of the proteostasis network (Fig 3) to contribute to the cellular detritus in neurons, hastening their demise. In the presence of excessive oxidative stress, the two 19S caps can be separated from the 26S proteasome, leaving the 20S proteasome capable of degradation of certain oxidatively damaged proteins without the involvement of ubiquitin50. One implication of the ability of the 20S proteasome to degrade oxidatively modified proteins independent of the 19S components of the 26S proteasome is that the involvement of UCH L1 is not needed, and, as noted above, this critical enzyme is oxidatively dysfunctional in AD. This could be regarded as an attempt to rescue dysfunctional proteostasis. Unfortunately, degradation of oxidatively modified proteins is still compromised in AD brain as the 20S proteasome enzymatic activities are dysfunctional51,52 (including inhibition by Aβ), resulting in accumulation of oxidatively modified, often misfolded and dysfunctional proteins.
Figure 3. Schematic drawings of the three components of the proteostasis network in brain cells.
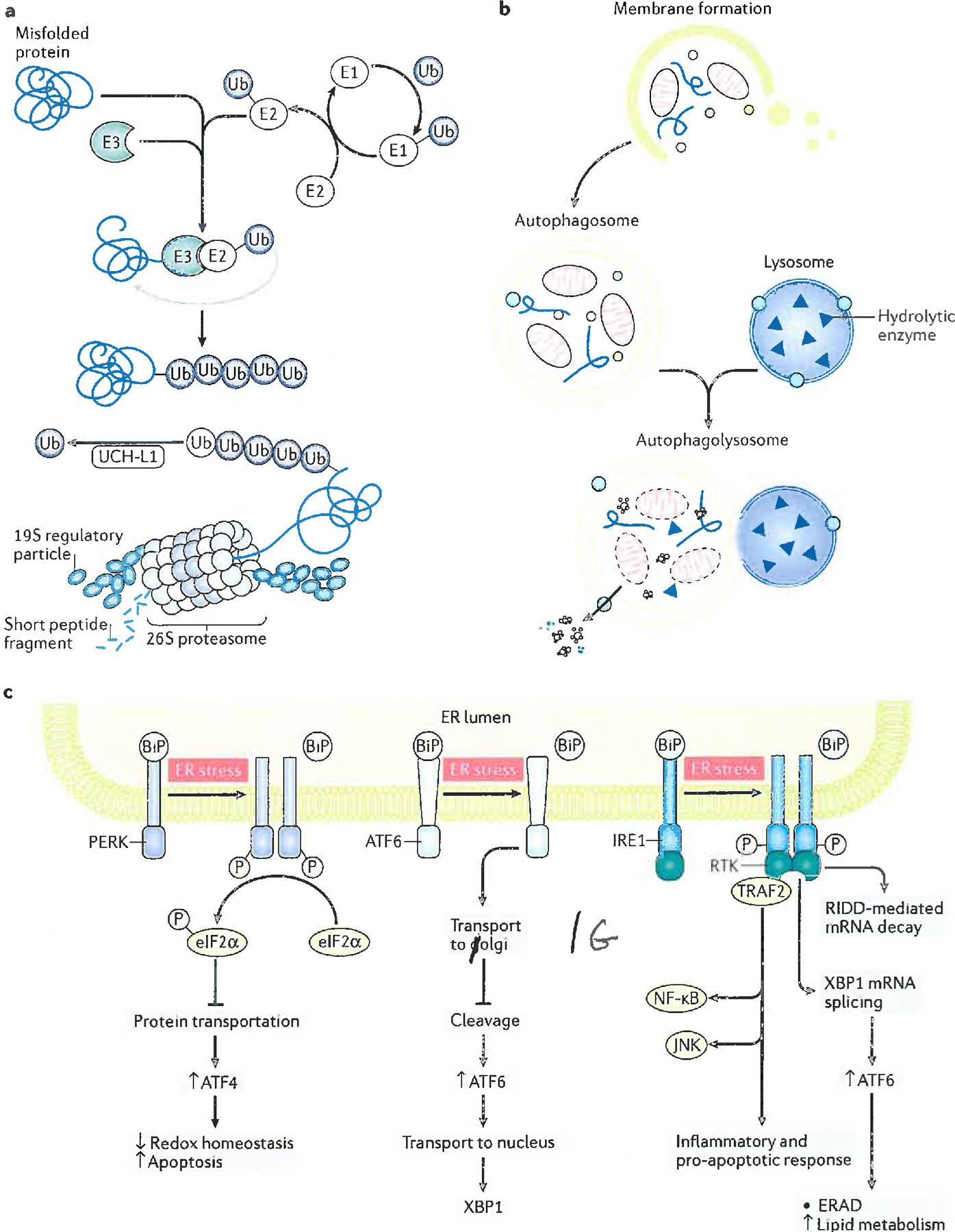
A: The Ubiquitin Proteasomal System (UPS). Damaged proteins are polyubiquitinylated by the Ubiquitin Ligase Enzymes, E1, E2, E3. E1 requires ATP for its function. An initial ubiquitin (Ub) molecule is bound to the damaged protein or organelle by this process and is repeated to form a polyubiquitinylated chain. Polyubiquitinylated damaged proteins are destined for degradation by the 26S proteasome, but prior to entering the 19S cap, these proteins must be de-ubiquitinylated, one ubiquitin residue at a time by the enzyme, ubiquitin carboxyl-terminal hydrolase L-1 (UCH L1). The de-ubiquitinylated, damaged protein is degraded by the proteinases in the 20S portion of the 26S proteasome, and small peptides are ejected by the bottom 19S portion of the 26S proteasome to become degraded by soluble peptidases to individual amino acids for reuse55–57.
B: Autophagic Degradation of Aggregated Proteins or Organelles. The process starts with formation of a double membrane enveloping the aggregated, damaged protein or organelle, to form an autophagosome. This is transported to the lysosome, where membrane fusion leads to formation of the autophagolysosome. Endocytosis of the contents of the autophagosome into the acidic interior of the lysosome leads to their proteolytic degradation, with peptides, amino acids, and other biomolecules being ejected from the autophagolysosome for reuse43.
C: The Unfolded Protein Response (UPR) Associated with ER Stress. Following an elevation in the levels of misfolded proteins and/or Ca2+ in the ER lumen, the UPR is usually engaged. This consists of activation of one or more of three stress transducers: protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1alpha (IRE1), and activating transcription factor-6 alpha and beta (ATF6). Activation of each stress sensor is accompanied by removal from the stress sensor of binding immunoglobulin protein (BiP, also known as glucose regulated protein 78, Grp78), which when bound inactivates each of the three stress transducers. Each activated stress sensor induces one or more down-stream response mechanisms. In the figure, up arrows denote increased process or level, whereas down arrows denote decreased process or level. For PERK, receptor Tyr-kinase activity phosphorylates eIF2α that leads to decreased protein translation, which causes elevated rates of translation of normally poorly translated mRNAs, among which is ATF4. This, in turn, leads to decreased redox homeostasis and elevated apoptosis.
ATF6 transduces ER stress by inducing transport of the ATF6 precursor protein to the Golgi apparatus, where the shorter ATF6 is produced. The latter translocates to the nucleus leading to expression of X-box binding protein (XBP1).
In the case of IRE1, transduced ER stress results in dimerization and formation of a transmembrane kinase – Receptor Tyr Kinase (RTK), which in turn results in: a) RNA degradation by regulated IRE1-dependent decay (RIDD); b) XBP1-mediated alternative mRNA splicing that leads to ER-associated degradation (ERAD) and increased lipid metabolism (from which elevated levels of the lipid peroxidation product, 4-hydroxynonenal (HNE) can arise); and c) tumor necrosis factor receptor-associated factor 2 (TRAF2)-mediated inflammatory or pro-apoptotic gene induction, particularly those of nuclear factor kappa-light chain enhancer of activated B cells (NF-κB) and c-Jun N-terminal kinase (JNK). The endoplasmic reticulum (ER)-resident component of the proteostasis network, particularly the UPR, is impaired in aMCI and AD brain, due to accumulation of abnormal proteins and alterations in Ca2+ homeostasis53–55,58. In AD brain, markers of ER stress are elevated and correlate with progression of the disease53–55,58.
Modified with permission from Ref. 58.
In response to abnormal elevated levels of misfolded proteins and/or free Ca2+ in the ER lumen, the unfolded protein response (UPR) is engaged53,54,58. This consists of activation of one or more of three stress transducers that lead to rapid responses designed to repair the defective mechanisms that caused the cellular dysfunction: protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1alpha (IRE1), and activating transcription factor-6 alpha and beta (ATF6). Each stress sensor induces one or more down-stream response mechanisms that are designed to lower ER stress (Fig. 3). Normally, these three stress sensors are inactive due to interaction with binding immunoglobulin protein (BiP, also called glucose regulated protein 78 (Grp78)), which, when removed, initiates activation of the stress response. Addition of oligomeric Aβ to neurons leads to large elevations in Ca2+ with consequent oxidative stress and ER stress-dependent neuronal death.
Inhibition of autophagy, oxidative stress, and mitochondrial function loss also activate the UPR, and as noted above, all of these alterations are observed in AD34,41–43,53,54,58 (Fig 3). For PERK, receptor Tyr-kinase activity phosphorylates eIF2α, which leads to decreased protein translation and causes elevated rates of translation of normally poorly translated mRNAs, among which is ATF4. This, in turn, leads to decreased redox homeostasis and elevated apoptosis. ATF6-transduced ER stress response involves inducing transport of the ATF6 precursor protein to the Golgi apparatus, where a shorter form of ATF6 is produced. This then translocates to the nucleus where expression of X-box binding protein (XBP1) occurs. In the case of IRE1, transduced ER stress results in dimerization and formation of a transmembrane kinase – Receptor Tyr Kinase (RTK), which in turn results in: a) RNA degradation by regulated IRE1-dependent decay (RIDD); b) XBP1-mediated alternative mRNA splicing that leads to ER-associated degradation (ERAD) and increased lipid metabolism, which can lead to elevated levels of the lipid peroxidation product, 4-hydroxynonenal (HNE); and c) tumor necrosis factor receptor-associated factor 2 (TRAF2)-mediated inflammatory or pro-apoptotic gene induction, including nuclear factor kappa-light chain enhancer of activated B cells (NF-κB) and c-Jun N-terminal kinase (JNK). In AD brain, markers of ER stress are elevated and correlate with progression of this disorder53,54,58.
Thus, three key components of the proteostasis network are dysfunctional in aMCI and AD brains. This dysfunction contributes to neuronal damage and death by several mechanisms, including ER stress and the accumulation of oxidatively damaged proteins, which in the normal brain are usually degraded by the UPS and autophagy to keep their levels low8,50,53,55.
In summary, oxidative and/or nitrosative damage to multiple proteins, including synaptic proteins is very likely to contribute to the memory problems and other cognitive deficits, reduced ATP availability in neurons, and decreased clearance of abnormal proteins34, creating a “perfect storm” (or sTORm perhaps!) of detrimental cognitive effects in AD9,34,35,41,42.
Down Syndrome and AD
Further evidence for a key role of oxidative damage in AD comes from studies of Down syndrome (DS). The varied phenotypes of DS result from full or partial triplication of chromosome 21, the most common human chromosomal disorder59. There are several genes associated with AD and oxidative stress on chromosome 21, including amyloid precursor protein (APP), from which Aβ arises, and beta-secretase-2, one of the proteinases that act on APP to form Aβ. One striking characteristic of people with DS is that at approximately 40–50 years of age, AD-like dementia often appears59,60. It is likely that both a dose effect of triplication of APP and gene-environment interactions play a role59,60. Other proteins encoded on chromosome 21 may be involved59, such as Cu/Zn-superoxide dismutase (which is an important antioxidant enzyme normally, but can be deleterious if excess is present8) and Bach1 (transcriptional inhibitor of heme oxygenase-1, HO-1). HO-1 is an important cellular antioxidant system that degrades free heme (a pro-oxidant)8 and interference with its action can contribute to oxidative stress8,61,62.
Increased oxidative damage occurs early in DS development, as evidenced by the fact that neurons obtained from aborted fetuses with DS show evidence of increased oxidative damage63 as does amniotic fluid from mothers carrying fetuses with DS64. These observations are consistent with the notion that oxidative damage contributes significantly to pathogenesis and progression of DS42,64. Redox proteomics has identified several oxidatively damaged proteins (including ceruloplasmin, transferrin, retinol-binding protein 4, apolipoprotein Al, complement C9, and collagen α−1Vchain) in amniotic fluid that correlate with the various phenotypes presented in individuals with DS, suggesting that oxidative stress contributes to these phenotypes64. Other biomarkers of increased oxidative damage in the brains of persons with DS and persons with DS with AD neuropathology (DS with AD) include elevated levels of HNE and PC15,42; the increases are greater in people with DS with AD62,65,66. Moreover, redox proteomics identified brain proteins oxidatively modified by PC and HNE that are similar to those in AD42,65. These oxidatively modified, and likely dysfunctional, proteins include ones associated with altered glucose metabolism, mTOR signaling (including inhibited autophagy and increased insulin resistance), and the proteostasis network42,61,62,65,66. All of these events will impair brain function and development in DS.
Proteins encoded on chromosome 21, excess levels of Aβ and of hyperphosphorylated tau protein42 constitute a vicious cycle for neuronal damage in DS. Elevated oxidative stress and mTOR activation with consequent inhibited autophagy that results in elevated levels of neuronal detritus are observed, and AD-like senile plaques form. Among the non-degraded moieties is Aβ itself, whose accumulation leads to more oxidative damage, RAGE activation, and activation of mTOR, continuing the vicious cycle. Hyperphosphorylated tau leads to destabilization of microtubules, affecting mitochondrial trafficking thus leading to presynaptic energy deficits. Consequent loss of ion gradients in presynaptic membranes that is secondary to loss of mitochondrial anterograde transport down axons, with consequent damaging Ca2+-related changes (discussed earlier), occurs12,24,34,42. Tau hyperphosphorylation also facilitates deposition of neurofibrillary tangles similar to those in AD brain. Furthermore, activated mTOR-mediated phosphorylation of IRS-1 (via phosphorylated p70S6K; Fig 2) leads to insulin resistance in DS, contributing to glucose hypometabolism. It is conceivable that mTOR-mediated alteration of insulin signaling due to IRS-1 inhibition, coupled to glucose hypometabolism, could lead to elevated tau phosphorylation, causing NFT formation and neuronal death7,36,42.
To summarize, the genetic abnormalities underlying DS drive mechanisms similar to those in AD, providing insights not only into DS but also into aMCI and AD and also suggesting novel therapeutic targets and approaches67. Indeed, ongoing investigations of the temporal changes in damaged brain proteins associated with glucose metabolism, the proteostasis network, and glutamate metabolism (the latter changes related to the functioning of the TCA cycle) are likely to provide insights into the molecular bases for conversion of DS into DS with AD.
Early detection is important
As mentioned earlier, brain pathology in patients who will eventually develop AD probably begins at least two decades before clinical symptoms appear2,4. Therefore, identifying who is at risk of developing AD and early detection of the disease are essential to allow potential disease-modifying treatments to be administered before substantial neuronal dysfunction and death have occurred.
Simple blood tests to detect early development of AD pathology would be the ‘Holy Grail’ and there has been some progress towards this goal. A recent study68 measured plasma amyloid-β biomarkers by immunoprecipitation coupled with mass spectrometric analysis. Plasma from hundreds of well-characterized controls, aMCI, and AD patients from Japan and Australia was examined. The results demonstrated, with very high confidence (over 94% of samples tested), that amyloid β-biomarkers in plasma can correctly discriminate among control, aMCI, and AD subjects. This study raises hopes that this far less invasive approach (compared to lumbar puncture to obtain CSF) will allow physicians and scientists to both treat patients earlier in the progression of this disorder than is currently possible and gain insights into molecular processes involved in the etiology and progression of AD. Exosomal biomarkers might be another promising approach in DS with AD69.
CSF biomarkers have also been proposed but not yet validated, but these are less desirable than plasma biomarkers because extracting CSF is more invasive2,70; using biomarkers detected through retinal imaging has also been proposed2,71,72. Another approach underway to address this need for predictive biomarkers in AD involves the Dominantly Inherited Alzheimer Network (DIAN)73: persons carrying one of several dominantly-inherited genes that cause familial AD agreed to provide fluids and tissue, and undergo neuroimaging to detect AD pathology. The combined data allowed researchers to gain insights into factors and biomarkers that might predict onset of AD pathology and clinical symptoms. The results of studies using DIAN should be extremely helpful in AD treatment and AD research. Indeed, a recent neuroimaging study using DIAN shows that preferential degradation of cognitive networks differentiates AD from normal aging74. Other valuable networks contributing to our understanding of the above events have been AIBL (the Australian Imaging Biomarkers and Lifestyle study)2 and ADNI (the Alzheimer Disease Neuroimaging Initiative)75, as well as cohorts of people with DS67,69.
Using a different approach, oxidative stress was found to be elevated in mitochondria isolated from peripheral lymphocytes of people with aMCI and AD76, results that potentially could be part of a putative panel of biomarkers to identify development of aMCI or AD prior to appearance of symptoms. Given the involvement of oxidative stress in the progression and pathogenesis of AD, it is possible that unless AD therapies (such as antioxidants) are used much earlier in AD than the onset of symptoms, they will have limited effectiveness and may be totally unsuccessful. So let us move on to consider the value of antioxidants.
Antioxidant Interventions in AD
Vitamin E
The key role of oxidative damage in the pathology of aMCI, PCAD and AD suggests that inhibiting it should have therapeutic benefit8,11. Consistent with this view, many preclinical models of aMCI and AD, such as neuronal cell cultures or transgenic mice, have shown significant protective effects of antioxidant treatment (e.g. refs 2, 8, 77–79). It is therefore surprising that the results of clinical trials in aMCI and AD involving antioxidant therapies (such as vitamin E) have been largely disappointing2,8,70,78,80.
The trials that have been conducted using vitamin E are worth examining, since they may have lessons relevant to the use of other antioxidants. Following a report of success of high-dose vitamin E in keeping late-stage AD patients out of long-term care facilities longer than patients on a placebo81, other studies with vitamin E have been less impressive70,78,80. So, what went wrong? Although vitamin E administration has been observed to decrease oxidative damage levels in brain in vivo in some studies82, administering extra vitamin E has not proved particularly effective as an antioxidant in humans in vivo8. If administered vitamin E does not decrease oxidative damage in the brain significantly, then it will not be effective against MCI or AD. One reason is that transport of extra vitamin E into brain is limited83.
The most important biological form of vitamin E is RRR-alpha(α)-tocopherol, the major form found in the brain. However, three other tocopherols (β, γ, ξ) are known, with frequent suggestions (although limited evidence) that they may be important antioxidants in vivo8. Although α-tocopherol is efficient in trapping lipid peroxyl radicals, gamma-tocopherol appears more efficient at scavenging RNS species84. Because both elevated oxidative and nitrosative stress occur in AD (see above), clinical trials of vitamin E in AD should perhaps have included both of these forms of tocopherol.
Polyphenols
Some so-called antioxidant molecules (for example, polyphenols such as resveratrol or quercetin) are able to enter the brain to a limited extent85. In preclinical models, such molecules have shown promise in treatment of AD77. One mechanism by which polyphenols could exert protective properties is by generation of a hormetic response to their use85–88. In other words, they generate a mild oxidative stress that the body tries to mitigate by upregulating protective genes. This often leads to rises in the levels of antioxidants such as GSH and HO-1, mediated by activation of the transcription factor Nrf-2, (nuclear factor erythroid 2-related factor 2)8,89.
How does this system work? Nrf2 is widely expressed in animal tissues but most of it is kept in an inactive form, largely in the cytosol, by binding to the Keap1 (Kelch-like erythroid cell-derived protein with CNC-homology associated protein 1) protein, which also promotes its rapid degradation by the proteasome8,89. Many xenobiotics induce oxidative stress and are, or can form, electrophiles, agents that react with sites of high electron density on proteins, DNA, or lipids (an example being unsaturated aldehydes such as HNE formed during lipid peroxidation; Table 1). An increase in oxidative/electrophilic stress can activate protein kinases that phosphorylate cytoplasmic Nrf2, causing it to fall away from Keap1. The Nrf2 then migrates into the nucleus and binds to an antioxidant response element to initiate gene transcription. In addition, Keap1 is rich in cysteine residues and its direct modification by reactive oxygen / nitrogen species and electrophiles, including α,β-unsaturated aldehydes, and dietary xenobiotics such as sulphoraphane or curcumin can also stop the proteasomal degradation of Nrf2 and cause it to accumulate89. Indeed, impairment of Nrf2 function worsened cognitive defects in a mouse model of AD90, suggesting the importance of the Nrf2 system in AD. In vivo use of polyphenols (especially curcumin) in some mouse models of AD has been successful77,87, but this has not (yet) been translated to usefulness in human AD2,70,87,88.
However, one must be prudent when using polyphenols as “antioxidant” therapeutic agents. If their beneficial effects actually rely on a mild pro-oxidant action that triggers hormesis, too many of these molecules in the brain would likely aggravate oxidative stress rather than ameliorate it. There can be other problems. For example, the polyphenol curcumin, found in turmeric, has been widely used in preclinical studies, several of which suggest that its antioxidant properties, ability to promote Aβ clearance, and other actions may be a potential treatment for AD2,87,88. But, curcumin is actually a poor antioxidant in vitro8 and readily breaks down, for example in cell culture91, to a range of biologically active products. Curcumin can bind to many proteins and membranes, sometimes disguising in in vitro assays the effects of potential new drugs that might eventually prove useful for AD or other disorders89. The true value of curcumin in AD therapy remains unclear70. Brain penetrant antioxidant and cytoprotective agents without pro-oxidant effects, such as ergothioneine, may be a promising approach92,93. Other approaches include the use of different agents, such as triterpenoids, that can activate Nrf294, although they (and other agents that act via Nrf2) should be used with caution because too much Nrf2 activation can be deleterious89,95. Mitochondrially-targeted antioxidants, to reduce mitochondrial oxidative damage in AD94,96, might also prove useful.
Deuterated lipids
Lipid peroxidation involves abstraction of labile hydrogen atoms from unsaturated fatty acid side chains (Table 1). Chains in which labile hydrogen atoms are replaced by deuterium atoms are more resistant to peroxidation. This is because abstraction of deuterium by ROS is harder, since the C-D bond is much stronger than the C-H bond. Deuterated lipids have been claimed to be effective in some preclinical models of AD97, but more research is needed to establish their true value.
Other interventions
Intranasal insulin
Craft and co-workers investigated insulin resistance in AD7,38 and reported improved cognition in early AD patients and decreased levels of neurotoxic Aβ42 following a short-term treatment with intranasal insulin98. The results differed according to gender and apoE genotype98. This potentially important intervention to slow or halt the progress of AD requires an understanding of the molecular basis of the improvement. Recently, intranasal treatment of a 3 X Tg mice model of AD with insulin showed a significant reduction of brain nitrosative stress, tau phosphorylation, and Aβ oligomers coupled to improved cognition, and these effects were dependent on the activity of biliverdin reductase-A (BVR-A)99,100. BVR-A, in addition to being a reductase, is a kinase that can phosphorylate insulin receptor substrate-1100. BVR-A is nitrosatively modified and dysfunctional in AD and MCI brain100, and its impairment promotes insulin resistance101. Further studies of the molecular processes involved in cognitive improvement in AD and MCI by intranasal insulin treatment likely will be driven by the results of an ongoing multicenter clinical trial using intranasal insulin.
Alternative energy sources?
In certain circumstances the brain can use ketone bodies (acetoacetate and β-hydroxybutyrate) as metabolic fuel102. Thus it has been suggested that a ketogenic diet might have some beneficial effect in aMCI and mild-to-moderate AD, because brain ketone body metabolism seems unchanged in AD102. Its value is uncertain as yet70. Studies on human ApoE gene-targeted female replacement mice show that apoE status appears to influence brain glucose and ketone metabolism, with the apoE4 allele being the most deleterious for the former103.
Diet and Lifestyle
An additional consideration when considering the effectiveness (or lack of it) of antioxidants is that, though aMCI and AD are strongly associated with oxidative damage, they are highly complex disorders, and processes other than oxidative damage are likely to contribute to their pathogenesis and progression. For example, APP is processed in several ways by proteinases, so in addition to Aβ42 oligomers, the soluble β-secretase fragment and parts of the C-terminal fragments of APP are reportedly neurotoxic104,105. Consequently, therapeutic approaches for aMCI and AD probably need to be multifactorial. Toward this end, studies involving aged beagle dogs, which accumulate Aβ42 and Aβ40 brain deposits with an amino acid sequence identical to that of humans, have proven illustrative. The study found that aged beagles (12 years old) placed on a high antioxidant diet, given environmental enrichment to produce more cognitive stimulation (resulting in more synapse formation), and provided exercise for three years (exercise leads to elevation of brain-derived nerve growth factor, among other benefits106), had an error rate on behavioral tests, brain Aβ42 levels, and brain oxidative damage similar to 4 year-old dogs107. If this promising result is translatable to humans, then at a certain age (for example age 40, to account for pathology of AD being present for at least two decades prior to appearance of clinical symptoms of aMCI or AD4), or ideally throughout a lifetime, persons should eat a diet high in antioxidants, develop new intellectual tasks (such as learning a new language, learning to play a new musical instrument, etc.), and undertake a reasonable degree of exercise. Several studies have indicated that these approaches can yield results2,108,109. A recent study is the Finnish Geriatic Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). This was a two year intervention consisting of diet, exercise, cognitive training and management of metabolic and vascular risk factors. Positive effects on cognition were reported108,110 even in subjects with the apoE4 allele110. By contrast, single interventions (for example, decreasing vascular risk, as in the preDIVA trial, or administering nutritional supplements) seem in general to be less effective70,109,111.
Indirect Antioxidants
A final consideration of this topic is potential treatment of aMCI and AD with indirect antioxidants8, agents that can decrease oxidative stress but not by direct antioxidant mechanisms. Polyphenols may be one example, acting as mild pro-oxidants (see above). Another example is the intersection of oxidative stress, glucose metabolism, and statins, such as atorvastatin, as promising agents to decrease damage in aMCI and AD brains112.
The enzyme heme oxygenase 1 (HO-1) oxidizes heme (a pro-oxidant)8 to produce biliverdin, which is reduced by a biliverdin reductase-A (BVR-A) enzyme to bilirubin (a scavenger of ROS/RNS8,101). Both HO-1 and BVR-A are oxidatively damaged in aMCI and AD brain100. Moreover, oxidative modification of BVR-A in brain promotes insulin resistance in a triple transgenic mouse model of this disorder101, providing a further connection between oxidative damage and glucose dysmetabolism in brain.
Many statins, including atorvastatin, do not penetrate the blood-brain barrier (BBB) significantly, yet atorvastatin use is associated with lowered risk of developing AD113,114. Atorvastatin given to aged beagle dogs protected against oxidation of BVR-A and this statin increased HO-1 levels, whereas oxidative damage levels in brain were lowered114. These studies suggest that following atorvastatin use, some moiety in the periphery is able to penetrate the BBB to target the HO-1–BVR-A system or other systems to protect brain. It also suggests that atorvastatin, although not a direct antioxidant and not able to cross the BBB, leads to antioxidant-like effects in brain100. There is also growing interest in agents (which may include polyphenols), that affect the gut microbiome to exert neuroprotection, but that is beyond the scope of this review.
Concluding remarks
The evidence for oxidative damage being a critical component of the pathology and progression of AD is compelling8,11,115. Glucose metabolism, a key source of energy for the brain, is defective in PCAD, aMCI and AD. This defect likely results in significant part from oxidative damage to key proteins in glycolysis, the TCA cycle and ATP synthase. Other proteins are also oxidatively damaged, such as BVR-A, UCHL-1, and the proteasome. Decreased ATP results in numerous changes in brain function, such as impaired maintenance of membrane potentials, and increased intracellular Ca2+ levels leading to detrimental downstream effects on cell function and survival.
Small oligomers of Aβ42 solubilize in the membrane to promote oxidative damage, such as lipid peroxidation. This produces reactive aldehydes (Table 1) that bind to critical brain proteins to change their conformation and to lower their activity in aMCI and AD brain. Presynaptic membranes are particularly vulnerable to such oxidative damage, leading to diminished learning and memory and ultimately dementia. Subsequent neuronal death also contributes to the clinical presentation and pathology observed in aMCI and AD. Clinical trials in both conditions using antioxidants have been disappointing, but better design of such trials combined with the use of appropriate biomarkers of disease progression and of oxidative damage8 (to ensure that the antioxidants are actually decreasing it) offers hope2,8,92. As AD neuropathology begins at least two decades before symptoms appear, increased efforts to discover biochemical (e.g., plasma) and/or imaging biomarkers for the presymptomatic presence of AD are essential. Given the expected large increase in AD cases as the world’s population of aged individuals grows and diabetes becomes more frequent, better therapeutic approaches to preserve and/or improve glucose utilization, decrease oxidative damage and protect the brain against neuropathological changes will be needed.
The overall hypothesis presented in this review for the pathogenesis and progression of AD is that Aβ42 oligomer-induced oxidative stress impairs glucose metabolism, leading to synaptic dysfunction and eventual neuronal death (demonstrated by thinning of key brain areas), ultimately causing aMCI, PCAD, and AD. A recent paper is highly consistent with sequence of events116. The authors employed various imaging modalities using subjects from the DIAN network (see above) who had been regularly scanned from as long as 22 years prior, to 3 years after, the onset of symptoms, using 11C-PiB (a PET ligand that detects Aβ aggregates), 18FDG (to determine glucose metabolism), and MRI (to determine thickness of various brain regions). The authors demonstrated that the first detectable pathological change in asymptomatic persons in the DIAN network is deposition of Aβ aggregates (which would imply membrane-resident oligomers that would lead to oxidative damage), followed by decreased glucose metabolism, and finally thinning of key brain areas116. Continued studies interrogating this sequence of changes should lead to greater understanding of the pathogenesis and progression of AD and better means to monitor therapeutic efficacy of promising new agents directed against production of Aβ, oxidative stress, impaired glucose metabolism, and neuronal death. Other cohorts such as AIBL and ADNI will also continue to contribute valuable insights in this context2,75,117. This is an era of exciting new developments in aMCI and AD research and potential therapeutic modalities. We both look forward to contributing to this future.
Box 1.
Some biologically important ROS and RNS.
For a detailed discussion see8, from which this box is adapted.
| ROS Free Radicals | ROS Non-radicals |
| Superoxide, | Hydrogen peroxide, H2O2 |
| Hydroxyl, OH• | Hypochlorous acid, HOCl |
| Peroxyl, RO2• | Singlet oxygen, O21 Δg |
| Alkoxyl, RO• | Organic peroxides, ROOH |
| Singlet O2 1∑g+ | Peroxynitrite, ONOO− |
| Peroxynitrous acid, ONOOH | |
| RNS Free Radicals | RNS Non-radicals |
| Nitric Oxide, NO• | Nitrous acid, HNO2 |
| Nitrogen dioxide, NO2• | Nitrosyl cation, NO+ |
| Nitrate, NO3• | Nitrosyl anion, NO− |
| Peroxynitrite, ONOO− | |
| Peroxynitrous acid, ONOOH |
Note that NO•, ONOO− and ONOOH are classified as both RNS and ROS
ACKNOWLEDGEMENTS
This work was supported in part by grants from the National Institutes of Health [1R01 AG060056–01] (DAB) and the National Medical Research Council and Tan Chin Tuan Centennial Foundation, Singapore (BH). We thank Ms. Xiaojia Ren for assistance with Figures 1–3 and the three reviewers for their very helpful suggestions.
Glossary:
- Reactive Oxygen Species (ROS)
Oxygen-containing species that contain unpaired electrons (which makes them free radicals) or from which free radicals are easily derived.
- Reactive nitrogen species (RNS)
Nitrogen-containing species that are free radicals or moieties from which free radicals are easily derived.
- Higher executive functioning
Cognitive processes that include planning, reasoning, and problem solving that in humans largely involve the prefrontal cortex, with connections to other brain areas.
- Redox proteomics
A method for identification of oxidatively modified proteins that most often involves protein separation and digestion, mass spectrometric utilization to sequence the amino acids of the resulting peptides, and protein identification and informatics.
- Proteostasis
Sometimes called protein quality control, is a term encompassing three different cellular processes (the ubiquitin proteasomal system, autophagy and the endoplasmic reticulum resident unfolded-protein response) used to degrade aggregated, damaged proteins, or sometimes cellular organelles.
- Autophagy
One of the components of the proteostasis network, and involves formation of a double membrane (autophagosome) that surrounds the aggregated, damaged protein or organelle, transport of the autophagosome to and fusion with a lysosome, exposing the contents of the autophagosome to proteolysis and degradation.
Footnotes
Competing interests
Competing interests policy There is NO Competing Interest.
REFERENCES
- 1.Nelson PT, Braak H & Markesbery WR Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropathol. Exp. Neurol 68,1–14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martins RN et al. Alzheimer’s disease: a journey from amyloid peptides and oxidative stress, to biomarker technologies and disease prevention strategies—gains from AIBL and DIAN cohort studies. J. Alzheimers Dis 62, 965–992 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; A detailed review of how cohort studies have contributed to our understanding of AD
- 3.Markesbery WR Neuropathologic alterations in mild cognitive impairment: a review. J. Alzheimers Dis 19, 221–228 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landau SM & Frosch MP Tracking the earliest pathological changes in Alzheimer disease. Neurology 82, 878–883 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Weise CM et al. Left lateralized cerebral glucose metabolism declines in amyloid-β positive persons with mild cognitive impairment. Neuroimage. Clin 20, 286–296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croteau E et al. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp Gerontol. 107, 18–26 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Arnold SE et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nature Rev. Neurol 14,168–181 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review of the links between AD, dysfunctional glucose metabolism and diabetes.
- 8.Halliwell B & Gutteridge JMC Free Radicals in Biology and Medicine, 5th Ed., (Oxford University Press, Oxford, 2015). [Google Scholar]; A thorough and detailed summary of the role of ROS and antioxidants in human health and disease, including neurodegenerative diseases.
- 9.Butterfield DA, Di Domenico F & Barone E Elevated Risk of Type 2 Diabetes for Development of Alzheimer Disease: a Key Role for Oxidative Stress in Brain. Biochim. Biophys. Acta 1842, 1693–1706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheignin C et al. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biology 14, 450–464 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliwell B Oxidative stress and neurodegeneration: where are we now? J. Neurochem 97, 1634–1658 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Butterfield DA & Boyd-Kimball D Oxidative stress, amyloid β-peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. J. Alzheimers Dis 62, 1345–1367 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper highlights the role of Aβ42 oligomers in oxidative stress in amnestic MCI and AD brains in in vitro and in vivo models, and how redox proteomics-mediated identification of oxidatively modified key brain proteins is correlated with glucose dysmetabolism, dysfunction of the proteostasis network, activation of mTORC1, and altered protein phosphorylation, all contributing to neuronal death.
- 13.Nourooz-Zadeh J, Liu EH, Yhlen B, Anggard EE & Halliwell B. F4-isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer’s disease. J. Neurochem 72, 734–740 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Di Domenico F et al. Oxidative signature of cerebrospinal fluid from mild cognitive impairment and Alzheimer disease. Free Radic. Biol. Med 91:1–9 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Di Domenico F, Tramutola A & Butterfield DA Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of Alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med 111, 253–261 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Lauderback CM et al. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: Role of Aβ1–42. J. Neurochem 78, 413–416 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Martins RN, Harper CG, Stokes GB & Masters CL Increased cerebral glucose-6-phosphate dehydrogenase activity in Alzheimer’s disease may reflect oxidative stress. J Neurochem. 46, 1042–5 (1986). [DOI] [PubMed] [Google Scholar]
- 18.Smith MA, Richey Harris PL, Sayre LM, Beckman JS & Perry G Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci 15, 2653–2657 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensley K et al. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem 65, 2146–2156 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Butterfield DA et al. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol. Dis 22, 223–232 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Butterfield DA et al. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci. Lett 397,170–173 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Sultana R, Perluigi M & Butterfield DA Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med 62,157–169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows mechanisms of lipid peroxidation in AD and MCI brain, with resulting HNE covalently modifying and causing dysfunction to brain proteins involved in glucose metabolism and in synaptic remodeling needed for effective neurotransmission, as revealed by redox proteomics.
- 23.Bradley MA, Markesbery WR & Lovell MA Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic. Biol. Med 48, 1570–1576 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed TT, Pierce WM, Turner DM, Markesbery WR & Butterfield DA Proteomic identification of nitrated brain proteins in early Alzheimer’s disease inferior parietal lobule. J. Cell. Mol. Med 13(8B), 2019–2029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sultana R et al. Proteomic identification of nitrated brain proteins in amnestic mild cognitive impairment. J Cell Mol Med 11, 839–851 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyras L, Cairns NJ, Jenner A, Jenner P & Halliwell B An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J. Neurochem 68, 2061–2069 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Santos RX et al. Nuclear and mitochondrial DNA oxidation in Alzheimer’s disease. Free Radic. Res 46, 565–576 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Abolhassani N et al. Molecular pathophysiology of impaired glucose metabolism, mitochondrial dysfunction, and oxidative DNA damage in Alzheimer’s disease brain. Mech Ageing Dev. 161, 95–104 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Shan X & Lin CL Quantification of oxidized RNAs in Alzheimer’s disease. Neurobiol Aging. 27, 657–62 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Nunomura A et al. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol.118, 151–166 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Li S et al. Soluble oligomers of amyloid β-protein facilitate hippocampal long term depression by disrupting glutamate uptake. Neuron 62, 788–801 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bereczki E et al. Synaptic markers of cognitive decline in neurodegenerative diseases: a proteomic approach. Brain 141, 582–595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang T, Li S, Xu H, Walsh DM & Selkoe DJ Large soluble oligomers of amyloid β-protein from Alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. J. Neurosci 37, 152–163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Domenico F, Barone E, Perluigi M & Butterfield DA The triangle of death in Alzheimer’s disease brain: the aberrant cross-talk among energy metabolism, mammalian target of rapamycin signaling, and protein homeostasis revealed by redox proteomics. Antioxidant Redox Signal 26, 364–387 (2017). [DOI] [PubMed] [Google Scholar]; This paper demonstrates the utility of redox proteomics to increase our understanding of molecular processes involved in neurodegeneration in the pathogenesis and progression of AD.
- 35.Reed T et al. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol. Dis 30,107–120 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Vlassenko AG et al. Aerobic glycolysis and tau deposition in preclinical Alzheimer’s disease. Neurobiol Aging. 67, 95–98 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell 17, e12679 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neth BJ & Craft S Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Frontiers in Aging Neurosci. 9, 1–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bezprozvanny I & Mattson MP Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 31, 454–463 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pirollet F, Margolis RL & Job D Ca(2+)-calmodulin regulated effectors of microtubule stability in neuronal tissues. Biochim Biophys Acta. 1160, 113–119 (1992). [DOI] [PubMed] [Google Scholar]
- 41.Tramutola A et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment, and late-stage AD. J Neurochem.133, 739–749 (2015). [DOI] [PubMed] [Google Scholar]; This paper contains the first report of mTORC1 activation and consequent markers of insulin resistance and inhibition of autophagy in brains of subjects with aMCI, consistent with the notion that mTORC1 activation occurs well before dementia in the progression of AD and reflects glucose dysmetabolism early in the disease.
- 42.Di Domenico F et al. mTOR in Down syndrome: Role in Aβ and Tau neuropathology and transition to redox Alzheimer disease-like dementia. Free Radic. Biol. Med 114, 94–101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nixon RA Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease. FASEB J. 31, 2729–2743 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabbani N, Xue M & Thornalley P Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj J. 33, 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan SD et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382, 685–691 (1996). [DOI] [PubMed] [Google Scholar]
- 46.Pugazhenthi S, Qin L & Reddy PH Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s Disease. Biochim. Biophys. Acta 1863, 1037–1045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piras S et al. Monomeric Aβ1–42 and RAGE: key players in neuronal differentiation. Neurobiology of Aging 35, 1301–1308 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Emendato A et al. Glycation affects fibril formation of Aβ peptides. J. Biol Chem 293, 13100–13111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sweeney MD, Kisler K, Montagne A, Toga AW & Zlokovic BV The role of brain vasculature in neurodegenerative disorders. Nature Neurosci. 21, 1318–1331 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonet-Costa V, Corrales-Diaz Pomatto L & Davies KJA The proteasome and oxidative stress in Alzheimer disease. Antioxidant Redox Signal 25, 886–901 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keller JN, Hanni KB, Markesbery WR Impaired proteasome function in Alzheimer’s disease. J Neurochem. 75, 438–439 (2000). [DOI] [PubMed] [Google Scholar]; An early paper identifying proteasomal dysfunction in AD.
- 52.Tseng BP, Green KN, Chan JL, Blurton-Jones M & LaFerla FM Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol. Aging 29, 1607–1618 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mota SI et al. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer’s disease. Biochim. Biophys. Acta 1852, 1428–1441 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Hashimoto S & Saido TC Critical review: involvement of endoplasmic reticulum stress in aetiology of Alzheimer’s disease. Open Biol. 8, 180024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Domenico F, Head E, Butterfield DA & Perluigi M Oxidative stress and proteostasis network: Culprit and Casualty of Alzheimer’s-like neurodegeneration. Adv. Geriatr 2014, 1–14 (2014). [Google Scholar]
- 56.Choi J et al. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem. 279, 13256–64 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Castegna A et al. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 33, 562–571 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Gerakis Y & Hetz C Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 285, 995–1011 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Wiseman FK et al. Trisomy of human chromosome 21 enhances amyloid-β deposition independently of an extra copy of APP. Brain 141, 2457–2474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lott IT Neurological phenotypes for Down syndrome across the life span. Prog. Brain Res 197, 101–121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Domenico F et al. Bach1 overexpression in Down syndrome correlates with the alteration of the HO-1/BVR-A system: Insights for transition to Alzheimer disease. J. Alzheimers Dis 44, 1107–1120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perluigi M, et al. Neuropathological role of PI3K/Akt/mTOR axis in Down syndrome. Biochim. Biophys. Acta 1842, 1144–1153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busciglio J & Yankner BA Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature 378, 776–779 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Perluigi M et al. Oxidative Stress Occurs Early in Down Syndrome Pregnancy A Redox Proteomics: Analysis of Amniotic Fluid. Proteomics—Clin. Appl 5, 167–178 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Barone E, Head E, Butterfield DA & Perluigi M HNE-modified proteins in Down syndrome: Involvement in development of Alzheimer disease neuropathology,” Free Radic. Biol. Med 111, 262–269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tramutola A et al. Polyubiquitinylation profile in Down syndrome brain before and after the development of Alzheimer neuropathology. Antioxidants Redox Signal. 26, 280–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caraci F et al. Searching for new pharmacological targets for the treatment of Alzheimer’s disease in Down syndrome. Eur J Pharmacol. 817, 7–19 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Nakamura A et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 554, 249–254 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Hamlett ED et al. Exosomal biomarkers in Down syndrome and Alzheimer’s disease. Free Radic Biol Med. 114, 110–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winblad B et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 15, 455–532 (2016). [DOI] [PubMed] [Google Scholar]; A comprehensive review of the current state of AD research and prospects for treatment.
- 71.Golzan SM et al. Retinal vascular and structural changes are associated with amyloid burden in the elderly: opthalmic biomarkers of preclinical Alzheimer’s disease. Alzheimers Res Ther. 9, 13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koronyo Y et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight, e93621 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang M et al. Dominantly Inherited Alzheimer Network (DIAN) Neurological manifestations of autosomal dominant familial Alzheimer disease: a comparison of the published literature with the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS). Lancet-Neurol. 15, 1317–1325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper extensively describes the dominantly inherited Alzheimer disease network (DIAN) and how neurological studies employing DIAN compare to results of the published literature on AD.
- 74.Chhatwal JP et al. Dominantly Inherited Alzheimer Network. Preferential degradation of cognitive networks differentiates Alzheimer’s disease from ageing. Brain 141, 1486–1500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casamitjana A et al. MRI-Based Screening of Preclinical Alzheimer’s Disease for Prevention Clinical Trials. J Alzheimers Dis. 64, 1099–1112 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Sultana R et al. Lymphocyte mitochondria: toward identification of peripheral biomarkers in progression of Alzheimer disease. Free Radic. Biol. Med 65, 595–606 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perluigi M et al. In vivo protective effects of ferulic acid ethyl ester against amyloid β-peptide (1–42)-induced oxidative stress. J. Neurosci. Res 84, 418–426 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Polidori MC & Nelles G Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease-challenges and perspectives. Curr. Pharm. Des 20, 3083–3092 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Butterfield DA, Koppal T, Subramaniam R & Yatin S Vitamin E as an antioxidant/free radical scavenger against amyloid beta-peptide-induced oxidative stress in neocortical synaptosomal membranes and hippocampal neurons in culture: insights into Alzheimer’s disease. Rev Neurosci. 10, 141–9 (1999). [DOI] [PubMed] [Google Scholar]
- 80.Petersen RC et al. Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med 352, 2379–2388 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Sano M et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med 336,1216–1222 (1996). [DOI] [PubMed] [Google Scholar]
- 82.Ulatowski L et al. Vitamin E is essential for Purkinje neuron integrity. Neuroscience 260, 120–129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spector R & Johanson CE Vitamin transport and homeostasis in mammalian brain: focus on vitamins B and E. J. Neurochem 103, 425–438 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Pei R, Mah E, Leonard SW, Traber MG & Bruno RS α-Tocopherol supplementation reduces 5-nitro-γ-tocopherol accumulation by decreasing γ-tocopherol in young adult smokers. Free Radic. Res 49, 1114–1121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schaffer S & Halliwell B Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 7, 99–109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mattson MP, Son TG & Camandola S Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Resp. 5,174–186 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Begum AN et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther 326,196–208 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson KM et al. The essential medicinal chemistry of curcumin. J Med Chem 60, 1620–1637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma Q & He X Molecular basis of electrophilic and oxidative defence: promises and perils of Nrf2. Pharmacol. Rev 62, 1055–1081 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Branca C et al. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Human Molecular Genetics 26, 4823–4835 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Long LH, Hoi A & Halliwell B Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch. Biochem. Biophys 501, 162–169 (2010). [DOI] [PubMed] [Google Scholar]
- 92.Halliwell B, Cheah IK & Tang RMY. Ergothioneine, a diet-derived antioxidant with therapeutic potential? FEBS Letters 592, 3357–3366 (2018) [DOI] [PubMed] [Google Scholar]
- 93.Cheah IK, Tang RM, Yew TS, Lim KH & Halliwell B Administration of pure ergothioneine to healthy human subjects: uptake, metabolism, and effects on biomarkers of oxidative damage and inflammation. Antioxid. Redox Signal 26, 193–206 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Valero T Mitochondrial Biogenesis: Pharmacological Approaches. Current Pharmaceutical Design 20, 5507–5509 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Huang Z et al. Novel derivative of bardoxolone methyl improves safety for the treatment of diabetic nephropathy. J. Med. Chem 60, 8847–8857 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Ng LF et al. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 71, 390–401 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Raefsky S et al. Deuterated polysaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid beta-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer disease. Neurobiol. Aging 66, 165–176 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Claxton A et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis. 35, 789–97 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barone E et al. Biliverdin reductase-A mediates the beneficial effects of intranasal insulin in Alzheimer disease. Mol Neurobiol. [EPub ahead of print] (2018) doi: 10.1007/s12035-018-1231-5. [DOI] [PubMed] [Google Scholar]; This paper improves our mechanistic understanding of the beneficial effects of intranasal insulin (INI) in cognitive improvement of AD and MCI patients by demonstrating in the 3XTg mouse model of AD that biliverdin reductase-A (BVR-A) was prevented from early impairment in adult mice, or rescued from damage in aged mice, following INI that was correlated with improved insulin signaling, decreased nitrosative stress, and improved cognition.
- 100.Barone E, Di Domenico F, Mancuso C & Butterfield DA The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: It’s time for reconciliation. Neurobiol. Dis 62,144–159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barone E et al. Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: a new paradigm. Free Radic. Biol. Med 91, 127–142 (2016). [DOI] [PubMed] [Google Scholar]
- 102.Cunnane SC et al. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann N Y Acad Sci. 1367, 12–20 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Wu L, Zhang X & Zhao L Human ApoE isoforms differentially modulate brain glucose and ketone body metabolism: Implications for Alzheimer’s disease risk reduction and early intervention. J. Neurosci 38, 6665–6681 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu DC et al. A second cytotoxic proteolytic peptide derived from amyloid β-protein precursor. Nat. Med 6, 397–404 (2000). [DOI] [PubMed] [Google Scholar]
- 105.Nikolaev A, McLaughlin T, O’Leary DD & Tessier-Lavigne M APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457, 981–989 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Intlekofer KA & Cotman CW Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol. Dis 57, 47–55 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Opii WO et al. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and program of behavioral enrichment: Relevance to Alzheimer’s disease. Neurobiol. Aging 29, 51–70 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Solomon A et al. Effect of the Apolipoprotein E Genotype on Cognitive Change During a Multidomain Lifestyle Intervention: A Subgroup Analysis of a Randomized Clinical Trial. JAMA Neurol. 75, 462–470 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scarmeas N, Anastasiou CA & Yannakoulia M Nutrition and prevention of cognitive impairment. Lancet Neurol. 17, 1006–1015 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Fyfe I APOE ε4 affects cognitive decline but does not block benefits of healthy lifestyle. Nature Reviews Neurology 14, 25 (2018). [DOI] [PubMed] [Google Scholar]
- 111.van Dalen JW et al. Effect of Long-Term Vascular Care on Progression of Cerebrovascular Lesions: Magnetic Resonance Imaging Substudy of the PreDIVA Trial (Prevention of Dementia by Intensive Vascular Care). Stroke. 48, 1842–1848 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Chu CS et al. Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci Rep. 8, 5804 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Butterfield DA, Barone E & Mancuso C Cholesterol-independent neuroprotective and neurotoxic activities of statins: Perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol. Res 64,180–186 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barone E, et al. Biliverdin reductase-A: a novel drug target for atorvastatin in a dog preclinical model of Alzheimer disease. J. Neurochem 120, 135–146 (2012). [DOI] [PubMed] [Google Scholar]
- 115.Butterfield DA & Boyd-Kimball D Redox proteomics and amyloid β-peptide: Insights into Alzheimer disease. J. Neurochem [Epub ahead of print] (2018) doi: 10.1111/jnc.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gordon BA et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol. 17, 241–250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports longitudinal PET and MRI imaging results on brains in persons with autosomal dominantly inherited AD obtained regularly 22 years before up to 3 years after the onset of AD symptoms, and demonstrates the temporal order of brain pathology observed prior to symptoms to be: deposition of Aβ; followed by glucose dysmetabolism (which means the decreased ATP produced would lead to loss of neuronal cell potential and consequent opening of voltage-gated Ca2+channels and subsequent excess Ca2+-mediated necrotic and apoptotic process to occur); and followed by thinning of frontal cortical and hippocampal structures (resulting from neuronal death in these vulnerable brain areas involved in learning and memory) that is consonant with progressive cognitive loss in MCI and AD.
- 117.Zhang H et al. Cerebrospinal fluid synaptosomal-associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther. 10, 80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davies MJ Protein oxidation and peroxidation. Biochem J. 473, 805–825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; A very good account of the mechanisms and consequences of oxidative protein damage.
- 119.Milne GL, Dai Q & Roberts LJ 2nd. The isoprostanes−−25 years later. Biochim Biophys Acta. 1851, 433–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review of the formation of isoprostanes in biological systems and their role as biomarkers of lipid peroxidation in disease.
- 120.Dizdaroglu M, Coskun E & Jaruga P Measurement of oxidatively induced DNA damage and its repair, by mass spectrometric techniques. Free Radic Res. 49, 525–48 (2015). [DOI] [PubMed] [Google Scholar]; A detailed and comprehensive review of mechanisms of oxidative DNA damage and how it can be measured in vivo.
- 121.Ishii T et al. Specific binding of PCBP1 to heavily oxidized RNA to induce cell death. Proc. Natl. Acad. Sci. USA 115, 6715–6720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J-X et al. Oxidative modification of miR-184 enables it to target Bcl-xL and Bcl-w. Molecular Cell 59, 50–61 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Dai D-P. et al. Transcriptional mutagenesis mediated by 8-oxoG induces translational errors in mammalian cells. Proc. Natl. Acad. Sci. USA 115, 4218–4222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


