ABSTRACT
The barrier-forming, self-renewing mammalian epidermis comprises keratinocytes, pigment-producing melanocytes and resident immune cells as first-line host defense. In murine tail skin, interfollicular epidermis patterns into pigmented ‘scale’ and hypopigmented ‘interscale’ epidermis. Why and how mature melanocytes accumulate in scale epidermis is unresolved. Here, we delineate a cellular hierarchy among epidermal cell types that determines skin patterning. Already during postnatal development, melanocytes co-segregate with newly forming scale compartments. Intriguingly, this process coincides with partitioning of both Langerhans cells and dendritic epidermal T cells to interscale epidermis, suggesting functional segregation of pigmentation and immune surveillance. Analysis of non-pigmented mice and of mice lacking melanocytes or resident immune cells revealed that immunocyte patterning is melanocyte and melanin independent and, vice versa, immune cells do not control melanocyte localization. Instead, genetically enforced progressive scale fusion upon Lrig1 deletion showed that melanocytes and immune cells dynamically follow epithelial scale:interscale patterns. Importantly, disrupting Wnt-Lef1 function in keratinocytes caused melanocyte mislocalization to interscale epidermis, implicating canonical Wnt signaling in organizing the pigmentation pattern. Together, this work uncovers cellular and molecular principles underlying the compartmentalization of tissue functions in skin.
KEY WORDS: Epidermis, Skin, Tissue patterning, Langerhans cell, Immune cell, Melanocyte, Wnt signaling, Intercellular communication, Mouse
Summary: Pigmentation and immune surveillance functions in murine tail skin are spatially segregated by Lrig1- and Wnt-Lef1-dependent keratinocyte lineages that control the partitioning of melanocytes and tissue-resident immune cells into distinct epidermal niches.
INTRODUCTION
The skin acts as dynamic interface between the organism and its environment. It protects from uncontrolled water loss and external threats such as pathogens, toxins, mechanical damage and temperature variation. Its outermost stratified epidermis is continuous with epidermal appendages like hair follicles and sweat glands (Chuong and Noveen, 1999). Proliferative, undifferentiated basal layer keratinocytes (KCs) attach to the underlying basement membrane and, following asymmetric cell division or delamination, progressively differentiate to constitute the spinous, granular and cornified layers (Blanpain and Fuchs, 2006; Dias Gomes and Iden, 2021; Gonzales and Fuchs, 2017; Ray and Lechler, 2011). The epidermis also hosts other resident cell types with crucial functions for the organism: neural-crest derived melanocytes (MCs) provide melanin for hair colorization and to protect KCs from ultraviolet (UV) damage (D'Orazio et al., 2013), whereas Langerhans cells (LCs) and dendritic epidermal T cells (DETCs) constitute a first line of defense against environmental pathogens and malignant transformation in murine skin (Deckers et al., 2018; MacLeod et al., 2013). Various UV-induced KC-derived soluble factors have been implicated in melanin induction in MCs (Upadhyay et al., 2021). Yet, how KC, LC, DETC and MC networks are spatiotemporally coordinated within the densely packed epidermis to simultaneously ensure skin barrier, pigmentation and host defense is largely unresolved.
The interfollicular epidermis (IFE) of murine ear and back-skin harbors evenly distributed immune cells (Bergstresser et al., 1980) and is largely devoid of MCs, which mostly reside in the dermis (Hirobe, 1984; Kunisada et al., 2000). In adult ear epidermis, LCs and DETCs actively maintain a non-random distribution that depends on KC density and engages LC-expressed Rac1 (Park et al., 2021), yet the molecular mechanisms through which KCs control resident immune cells remain unknown. Tail skin exhibits two distinct epidermal compartments: ‘scale’ IFE forms a spherical epidermal patch just above hair-follicle triplets and undergoes parakeratotic differentiation, characterized by lack of a granular layer and nucleated cornified layer KCs; ‘interscale’ IFE surrounds the scale IFE and, like ear and back-skin IFE, displays orthokeratotic differentiation with a granular layer and enucleated cornified layer KCs (Didierjean et al., 1982). Gomez et al. (2013) demonstrated that epidermal lineages of scale and interscale IFE express unique marker genes and are regulated by Wnt, Edaradd and Lrig1. Strikingly, these intraepidermal patterns in tail skin correlate with a strict compartmentalization of LCs, which populate the interscale IFE (Schweizer and Marks, 1977), and of pigmented MCs, which localize to the scale IFE where they persist throughout adulthood (Glover et al., 2015; Gomez et al., 2013). Further, a small population of amelanotic, quiescent MCs in interscale IFE has been reported (Glover et al., 2015; Köhler et al., 2017). How such segregation of epidermis-resident cells is achieved and maintained is poorly understood. Gomez et al. (2013) showed that MCs are dispensable for scale IFE formation; however, molecular and cellular signals orchestrating the mutually exclusive MC:immune cell distribution remain to be identified. It is currently not known whether scale-based MCs antagonize the localization of immune cells (or vice versa). Mouse scale IFE closely resembles the epidermal MC distribution of human skin and hence is used to study mechanisms of skin pigmentation (Fitch et al., 2003; Van Raamsdonk et al., 2004) and intraepidermal melanoma (Köhler et al., 2017). Gaining insight into heterologous communication between different epidermis-resident cell types will thus be beneficial to better understand human skin physiology and disease. In this study, we therefore set out to reveal potential interactions and interdependencies of KCs, MCs and immune cells regarding functional compartmentalization in murine tail skin.
RESULTS
DETCs, LCs and MCs reside in distinct compartments of the tail IFE
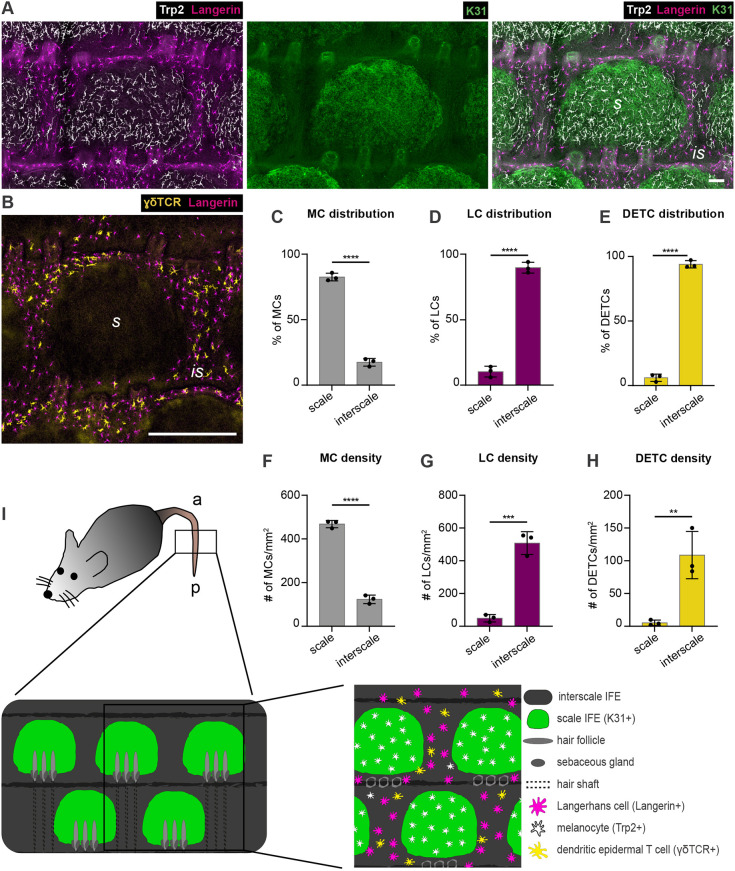
To first map the localization of DETCs, LCs and MCs with respect to IFE niches we performed immunohistochemistry on tail epidermis wholemounts of 6- to 15-week-old C57BL/6 mice. Co-immunostaining for keratin 31 (K31; also known as Krt31) marked parakeratotic differentiation and hence scale IFE in epidermal tail wholemounts (Gomez et al., 2013). In agreement with earlier reports, Trp2 (DCT)-positive MCs were highly enriched in scale IFE (Glover et al., 2015; Gomez et al., 2013) (Fig. 1A,C,F,I). In the adjacent K31-negative interscale IFE, only a few MCs were found (Fig. 1A,C,F), producing melanin and exhibiting a morphology comparable with scale MCs (Fig. S1A-D). Intriguingly, resident immune cells were largely excluded from scale IFE: langerin (CD207)-positive LCs were confined to interscale IFE (Schweizer and Marks, 1977) (Fig. 1A,D,G,I), and co-immunostaining of γδTCR (marking DETCs) and langerin revealed that DETCs and LCs co-distribute within the interscale IFE (Fig. 1B,E,H,I). Together, these data underpin a remarkable separation of epidermal pigmentary units and resident immune cells in tail IFE, opening the possibility that the scale IFE is an immune-privileged niche.
Fig. 1.
Mutually exclusive localization of MCs and epidermis-resident immunocytes to scale and interscale IFE compartments of murine tail epidermis. (A) Micrographs of tail epidermis wholemounts from 3-month-old wild-type C57BL/6 mice, immunostained for markers of LCs (langerin), MCs (Trp2), and scale IFE (K31). Representative for n=4. (B) Immunostaining of tail epidermis wholemounts from 3-month-old wild-type C57BL/6 mice for langerin and DETC marker γδTCR. Representative for n=3. (C) Quantification of A: MC distribution per scale:interscale unit (% of MCs) in tail epidermis. MC numbers in each compartment (scale, interscale) were normalized to the total MC number per scale:interscale unit. (D) Quantification of A: LC distribution per scale:interscale unit (% of LCs) in tail epidermis. LC numbers in each compartment (scale, interscale) were normalized to the total LC number per scale:interscale unit. (E) Quantification of B: DETC distribution per scale:interscale unit (% of DETCs) in tail epidermis. DETC numbers in each compartment (scale, interscale) were normalized to the total DETC number per scale:interscale unit. (F) Quantification of A: MC density (MC number/mm2) in tail epidermis. (G) Quantification of A: LC density (LC number/mm2) in tail epidermis. (H) Quantification of B: DETC density (DETC number/mm2) in tail epidermis. Data are mean±s.d.; n=3; ****P<0.0001, ***P=0.0004, **P=0.0079; unpaired Student's t-test. (I) Schematic of scale:interscale IFE patterns and associated structures and cell types in murine tail epidermis; a, anterior; is, interscale; p, posterior; s, scale. Scale bars: 75 µm (A); 300 µm (B).
IFE-resident cell types already segregate during postnatal tail skin development
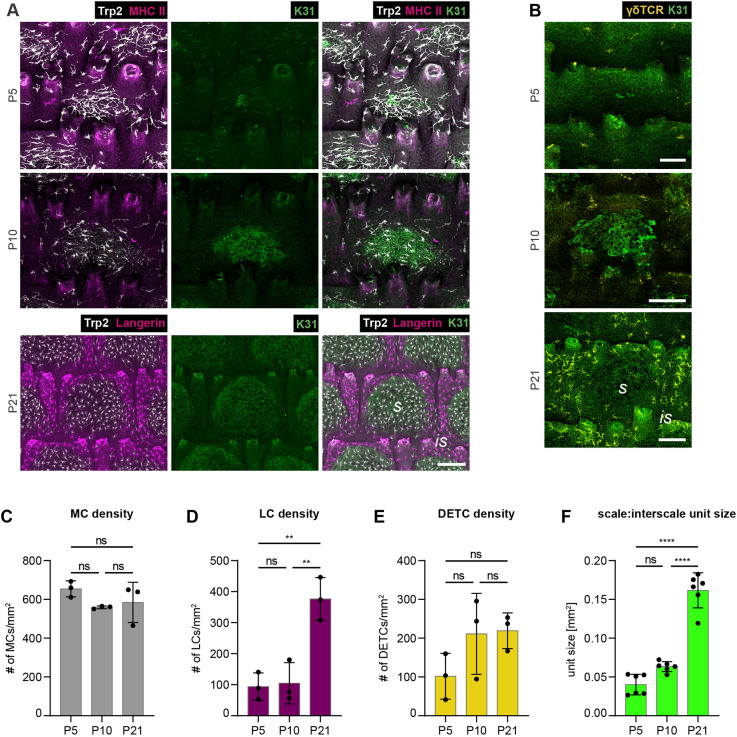
To shed light into the establishment of these MC:immune cell patterns, we next examined early postnatal tissues at the onset of scale formation (Gomez et al., 2013). At postnatal day (P) 5, MCs were already prominently enriched at sites of scale induction (Fig. 2A), a phenomenon that reinforced towards P10 (Fig. 2A). This indicated that MCs co-segregate with the parakeratotic lineage early during skin development. MHCII immunostaining served to visualize LCs at P5, as langerin is weakly expressed at these stages (Tripp et al., 2004). MHCII, similar to langerin, is solely found on LCs in unchallenged epidermis, enabling their identification (Tsuruta et al., 1999). Albeit low in numbers owing to the postnatal LC proliferation burst yet to occur (Chang-Rodriguez et al., 2005; Kobayashi et al., 1987), LCs were confined to interscale IFE in P5 tail epidermis (Fig. 2A,D). The differential distribution of MCs, LCs and DETCs became even more pronounced with further scale expansion (P10 to P21; Fig. 2A-F), culminating in the dense LC and DETC networks in interscale and MC networks in scale IFE observed in adult tail skin (Fig. 1A-I). These data thus demonstrate that the formation of IFE compartments coincides with the patterned distribution of MCs and epidermis-resident immune cells.
Fig. 2.
Progressive segregation of IFE-resident cell types during postnatal tail skin development. (A) Immunostaining of tail epidermis wholemounts from wild-type C57BL/6 mice for Trp2, K31 and LC markers MHCII (P5, P10) or langerin (P21). Representative for n=3. (B) Immunostaining of tail epidermis wholemounts from wild-type C57BL/6 mice for K31 and γδTCR. Representative for n=3. (C) Quantification of A: MC density (MC number/mm2) in tail epidermis. n=3; ns, P=0.2458 (P5 versus P10); ns, P=0.4338 (P5 versus P21); ns, P=0.8820 (P10 versus P21); mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (D) Quantification of A: LC density (LC number/mm2) in tail epidermis. n=3; ns, P=0.9750 (P5 versus P10); **P=0.0031 (P5 versus P21); **P=0.0038 (P10 versus P21); mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (E) Quantification of B: DETC density (DETC number/mm2) in tail epidermis. n=3; ns, P=0.2455 (P5 versus P10); ns, P=0.2082 (P5 versus P21); ns, P=0.9905 (P10 versus P21); mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (F) Quantification of A and B: scale:interscale unit size (mm2). n=6; ns, P=0.0523 (P5 versus P10); ****P<0.0001 (P5 versus P21); ****P<0.0001 (P10 versus P21); mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. is, interscale; ns, not significant; s, scale. Scale bars: 75 µm (A); 100 µm (B).
Distinct IFE localization of MCs and immune cells is not interdependent
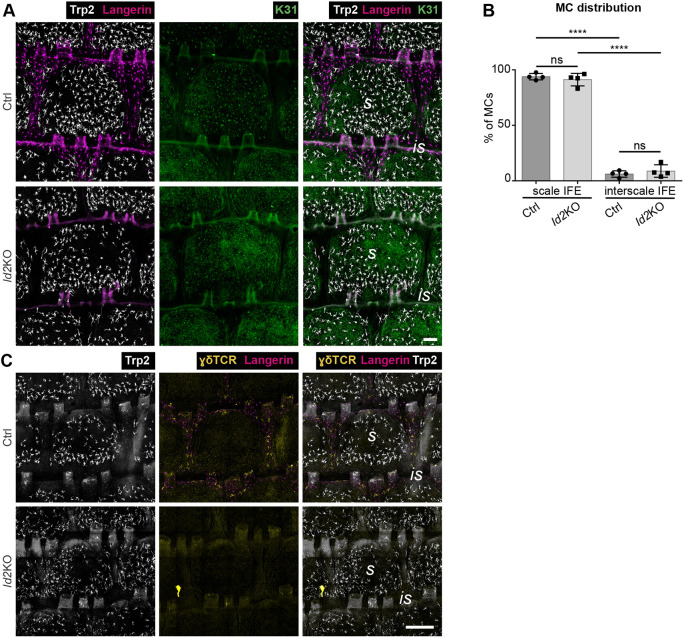
We next aimed to dissect a potential hierarchy underlying the positioning of MCs, LCs, DETCs and KC lineages. The role of resident immune cells in patterning of the other cell types was analyzed using mice deficient for the transcription factor Id2, which are characterized by complete LC and DETC loss by young adulthood (Hacker et al., 2003; Seré et al., 2012; Yokota et al., 1999). As expected, LCs and DETCs were undetectable in adult Id2 knockout (KO) tail epidermis (Fig. 3A,C). The scale:interscale IFE pattern of mutant mice, however, was comparable with control mice (Fig. S2A), and MCs were enriched within scale IFE at the age of 3 months (Fig. 3A,B) up to one year (S.C.B. and S.I., unpublished). Likewise, numbers of MCs were similar in control and mutant mice (Fig. S2B). This suggests that both MC distribution and KC lineage progression into scale and interscale identity are independent of resident immune cells. We cannot formally rule out that a transient presence of immune cells during ontogeny might contribute to initial MC and IFE patterning. Nevertheless, our data clearly demonstrate that LCs and DETCs are not required for scale confinement of MCs throughout adulthood.
Fig. 3.
MC localization to scale IFE is independent of epidermis-resident immune cells. (A) Langerin, Trp2 and K31 immunostaining of tail epidermis wholemounts from 3-month-old control and Id2 KO mice. Representative for n=4. (B) Quantification of A: MC distribution per scale:interscale unit (% of MCs). MC numbers in each compartment (scale, interscale) were normalized to the total MC number per scale:interscale unit. n=4; ns, P=0.8370; ****P<0.0001; mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (C) Langerin and γδTCR immunostaining of tail epidermis wholemounts from 3-month-old control and Id2 KO mice. Representative for n=3. Is, interscale; s, scale. Scale bars: 100 µm (A); 300 µm (C).
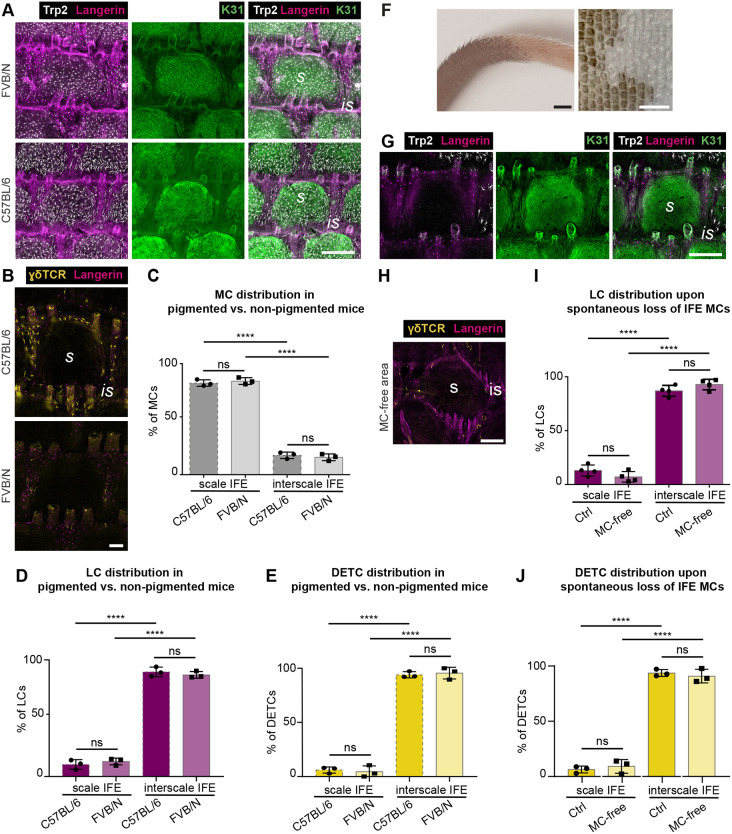
Next, we asked whether, vice versa, MCs antagonize the localization of IFE-resident immune cells. Insects use melanin as innate immune defense (Gillespie et al., 1997), and immunomodulatory roles have also been proposed for mammalian MCs and MC-derived melanin (Burkhart and Burkhart, 2005; Hong et al., 2015). LCs can take up melanin (Breathnach and Wyllie, 1965; Mishima, 1966; Tobin, 1998), which may cause LC emigration to draining lymph nodes (Hemmi et al., 2001). We therefore explored whether MCs, or melanin levels, counteract immune cell localization to scale IFE. Comparison of adult pigmented C57BL/6 mice and non-pigmented (albino) FVB/N mice revealed no difference in MC, LC and DETC distribution per scale:interscale unit (Fig. 4A-E). Similarly, localized spontaneous depletion of MCs and subsequent loss of pigmentation in tail epidermis of C57BL/6 mice (Fig. 4F) did not cause redistribution of LCs or DETCs into the MC-free scale IFE (Fig. 4G-J). Of note, despite stable immune cell distributions, DETC densities were reduced in FVB/N mice but not in mice with spontaneous MC loss (Fig. S3A-E), pointing to strain-dependent variation in numbers of DETCs. Together, epidermal MCs and resident immune cells do not affect each other's positioning in tail epidermis.
Fig. 4.
LCs and DETCs localize independently of MCs and melanin production to interscale IFE. (A) Langerin, Trp2 and K31 immunostaining of tail epidermis wholemounts of 3-month-old wild-type C57BL/6 and FVB/N mice. Representative for n=3. (B) γδTCR and langerin immunostaining of tail epidermis wholemounts of 3-month-old wild-type C57BL/6 and FVB/N mice. Representative for n=3. (C) Quantification of A: MC distribution to scale (pigmented) and interscale (hypopigmented) IFE (% of MCs). MC numbers in each compartment (scale, interscale) were normalized to the total MC number per scale:interscale unit. n=3; ns, P=0.8755; ****P<0.0001; mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. Data for C57BL/6 as shown in Fig. 1C. (D) Quantification of A: LC distribution per scale:interscale unit (% of LCs). LC numbers in each compartment (scale, interscale) were normalized to the total LC number per scale:interscale unit. n=3; ns, P=0.8186; ****P<0.0001; mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. Data for C57BL/6 as shown in Fig. 1D. (E) Quantification of B: DETC distribution per scale:interscale unit (% of DETCs). DETC numbers in each compartment (scale, interscale) were normalized to the total DETC number per scale:interscale unit. n=3; ns, P=0.9661; ****P<0.0001; mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. Data for C57BL/6 as shown in Fig. 1E. (F) Representative images of tail skin from 3-month-old wild-type C57BL/6 with partial spontaneous loss of MCs. Left: intact tail skin. Right: epidermis wholemount. Representative for n=4. (G) Langerin, Trp2 and K31 immunostaining of tail epidermis wholemounts of 3-month-old wild-type C57BL/6 mice with spontaneous loss of MCs (center) in posterior tail region. Representative for n=4. (H) Langerin and γδTCR immunostaining of tail epidermis wholemounts of 3-month-old wild-type C57BL/6 mice with spontaneous loss of MCs in posterior tail epidermis. Representative for n=3. (I) Quantification of G: LC distribution per scale:interscale unit (% of LCs) in MC-free and MC-containing tail epidermis. LC numbers in each compartment (scale, interscale) were normalized to the total LC number per scale:interscale unit. n=4; ns, P=0.3839; ****P<0.0001; mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (J) Quantification of H; DETC distribution per scale:interscale unit (% of DETCs) in MC-free and MC-containing tail epidermis. DETC numbers in each compartment (scale, interscale) were normalized to the total DETC number per scale:interscale unit. n=3; ns, P=0.8854; ****P<0.0001; mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. is, interscale; s, scale. Scale bars: 250 µm (A,G); 100 µm (B); 3000 µm (F, left); 1500 µm (F, right); 150 µm (H).
MC:immune cell distribution follows scale:interscale patterning
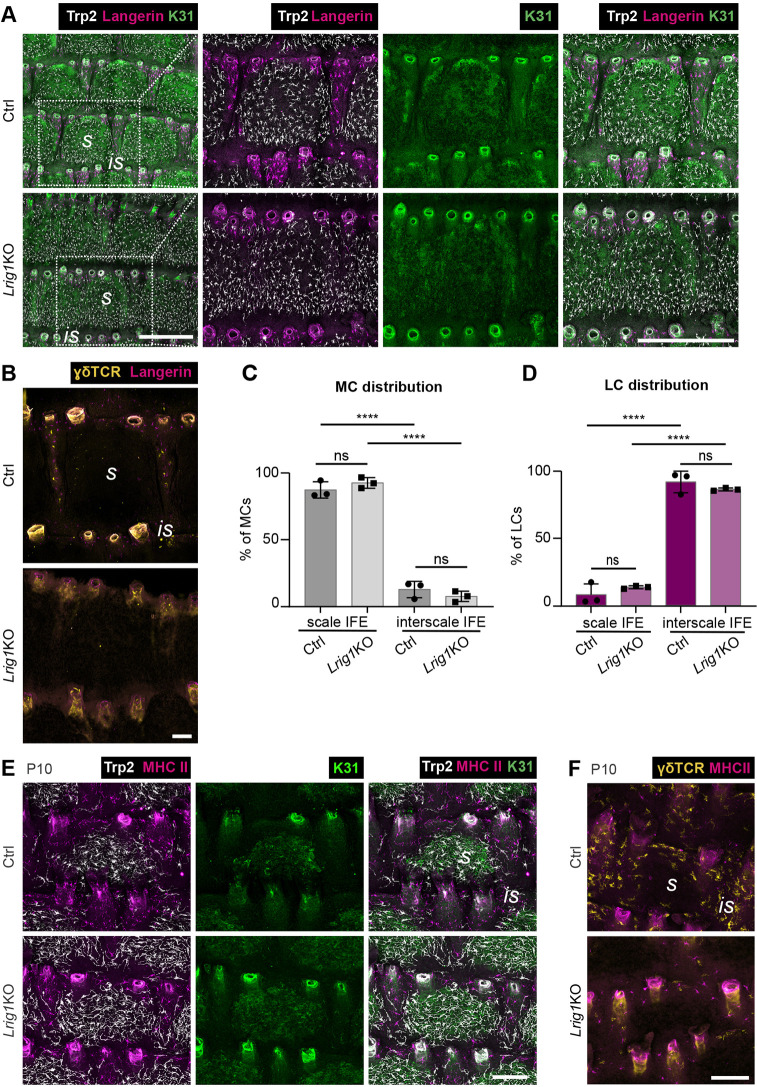
Our observations and the strikingly similar dynamics of scale formation and MC clustering during postnatal development (Fig. 2A) prompted us to investigate the role of parakeratotic scale and orthokeratotic interscale IFE compartments for immunocyte and MC patterning. Scale progenitors divide more rapidly than interscale progenitors (Gomez et al., 2013; Sada et al., 2016), with spatial control of scale progenitor proliferation likely contributing to scale:interscale patterning and boundary formation. Lrig1, a negative regulator of EGFR signaling, is expressed in the dermis underneath interscale IFE where it is thought to antagonize scale fate and size through dermal-epidermal signaling (Gomez et al., 2013). Upon constitutive Lrig1 deletion, initial scale induction is normal but scale IFE compartments progressively enlarge and eventually laterally fuse into wide dorsoventral bands in adult mice (Gomez et al., 2013) (Fig. 5A). Interestingly, analysis of Lrig1 KO mice at P10 and adult stages showed that, concomitant with lateral scale fusion, MCs assumed a band-like expansion corresponding to the K31-labeled scale band, whereas LCs and DETCs were restricted to the diminished interscale stripe (Fig. 5A-F). Notably, despite their different scale and interscale architectures, both MC and LC densities were comparable in control and Lrig1 KO mice (Fig. S4A,B). Likewise, LC densities were similar during and after scale fusion in Lrig1 KO mice (Fig. S4C). This opens the possibility that LCs are expelled or lost when scales fuse; however, we did not observe apoptotic LCs in Lrig1 KO tail skin (Fig. S4D). Together, the above data indicate that patterning of MCs and immune cells is primarily determined by compartmentalization of the epidermis into scale and interscale IFE.
Fig. 5.
MC:immunocyte distribution dynamically adapts to changes of epidermal scale:interscale patterning in Lrig1 KO mice. (A) Langerin, Trp2 and K31 immunostaining of tail epidermis wholemounts from 3-month-old control and Lrig1 KO mice. Representative for n=3. Right panels show magnification of boxed area in left panels. (B) γδTCR and langerin immunostaining of tail epidermis wholemounts from 3-month-old control and Lrig1 KO mice. Representative for n=3. (C) Quantification of A: MC distribution per scale:interscale unit (% of MCs). MC numbers in each compartment (scale, interscale) were normalized to the total MC number per scale:interscale unit. n=3; ns, P=0.6203; ****P<0.0001; mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (D) Quantification of A: LC distribution per scale:interscale unit (% of LCs). LC numbers in each compartment (scale, interscale) were normalized to the total LC number per scale:interscale unit. n=3; ns, P=0.6504; ****P<0.0001; mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (E) MHCII, Trp2 and K31 immunostaining of tail epidermis wholemounts from control and Lrig1 KO mice at P10. Representative for n=4. (F) MHCII and γδTCR immunostaining of tail epidermis wholemounts from control and Lrig1 KO mice at P10. Representative for n=4. is, interscale; s, scale. Scale bars: 500 µm (A); 100 µm (B,E,F).
Defective epidermal Wnt-Lef1 signaling causes MC redistribution to interscale IFE
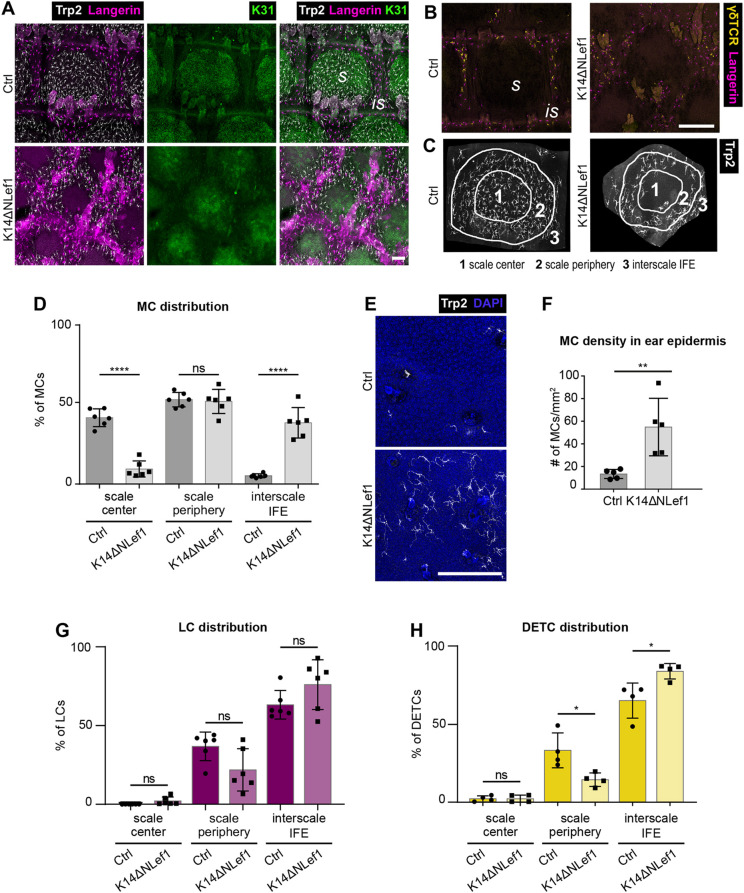
We next set out to identify intraepidermal factors mediating MC and immune cell patterning. Among candidate pathways, we hypothesized that canonical Wnt signaling could be involved, as it is known for steering epidermal lineage selection in postnatal epidermis (Watt and Collins, 2008) and because Lef1, a key transcriptional effector of canonical Wnt signaling, is predominantly expressed in scale IFE (Gomez et al., 2013; Niemann et al., 2002). Expression of N-terminally truncated Lef1 in epidermal KCs (K14ΔNLef1; Niemann et al., 2002), acting as dominant negative inhibitor of canonical Wnt signaling, results in impaired scale differentiation and size with irregular scale:interscale patterning (Gomez et al., 2013). Strikingly, analysis of skin-resident cell types in these mice revealed that MC distribution was largely inverted compared with control mice, with MCs localizing to interscale IFE and to the scale periphery, whereas the scale center was mostly devoid of MCs (Fig. 6A-D). Next to this repositioning, total numbers of MCs in K14ΔNLef1 tissues were reduced (Fig. S5A,B), and remaining scale-based MCs showed increased mean area, axis length and dendricity (Fig. S6A-F). Interscale MCs in mutant mice retained the ability to produce melanin (Fig. S6G) and did not show signs of increased apoptosis (Fig. S6H). Congruent with the redistribution of MCs in mutant tail skin, MC numbers were significantly increased in K14ΔNLef1 ear epidermis, whereas they were rarely detected in control ear epidermis (Fig. 6E,F). These combined findings in tail and ear epidermis strongly suggest that inhibition of canonical Wnt signaling renders orthokeratotic epidermis permissive for MC colonization. Notably, resident immune cells remained confined to the interscale IFE in K14ΔNLef1 mice (Fig. 6A,G,H), whereby densities of LCs in interscale regions and of DETCs in ear epidermis of mutant mice were reduced (Fig. S5C-H).
Fig. 6.
Repression of epidermal Wnt signaling causes ectopic localization of MCs to interscale IFE. (A) Immunostaining of tail epidermis wholemounts of 3-month-old control and K14ΔNLef1 mice for langerin, Trp2 and K31. Representative for n=6. (B) γδTCR and langerin immunostaining of tail epidermis wholemounts from 3-month-old control and K14ΔNLef1 mice. Representative for n=4. (C) Illustration of one scale:interscale unit with corresponding categorization into scale center, scale periphery and interscale IFE for analysis of MC distribution in K14ΔNLef1 mice as in D, G and H. (D) Quantification of A: MC distribution per scale:interscale unit (% of MCs) in control and K14ΔNLef1 mice. MC numbers per compartment were normalized to the total number of the scale:interscale unit. n=6; ****P<0.0001 (scale center, Ctrl versus K14ΔNLef1; interscale IFE, Ctrl versus K14ΔNLef1); ns, P=0.9995 (scale periphery, Ctrl versus K14ΔNLef1); mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (E) Trp2 immunostaining of ear epidermis wholemounts of 3-month-old control and K14ΔNLef1 mice. Nuclei were counterstained using DAPI. Representative for n=5. (F) Quantification of E: MC density (MC number/mm2) in ear epidermis. n=5; **P=0.0079; mean±s.d.; Mann–Whitney test. (G) Quantification of A: LC distribution per scale:interscale unit (% of LCs) in control and K14ΔNLef1 mice. LC numbers per compartment were normalized to the total number of the scale:interscale unit. n=6; ns, P=0.9991 (scale center, Ctrl versus K14ΔNLef1); ns, P=0.1341 (scale periphery, Ctrl versus K14ΔNLef1); ns, P=0.2596 (interscale IFE, Ctrl versus K14ΔNLef1); mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. (H) Quantification of B: DETC distribution per scale:interscale unit (% of DETCs) in control and K14ΔNLef1 mice. DETC numbers per compartment were normalized to the total number of the scale:interscale unit. n=6; ns, P>0.9999 (scale center, Ctrl versus K14ΔNLef1); *P=0.0163 (scale periphery, Ctrl versus K14ΔNLef1); *P=0.0166 (interscale IFE, Ctrl versus K14ΔNLef1); mean±s.d.; one-way ANOVA/Tukey's multiple comparisons test. is, interscale; s, scale. Scale bars: 100 µm (A); 250 µm (B,E).
To understand whether the observed striking MC mislocalization upon altered Wnt-Lef1 function is a progressive event, we next assessed the distribution of resident cell types in mutant tissues during postnatal development. K31 immunolabeling of P5 and P10 tail epidermis illustrated that scale formation in K14ΔNLef1 mice was delayed when compared with control mice (Fig. 7A) (Gomez et al., 2013). Importantly, already at this early stage MCs failed to cluster to scale IFE and instead overlapped with LCs in the K31-negative compartment in K14ΔNLef1 mice early-on (Fig. 7A,B). This indicates that canonical Wnt signaling in KCs orchestrates not only scale formation and maintenance but also the distinct patterning of epidermis-resident MCs and hence tail skin pigmentation.
Fig. 7.
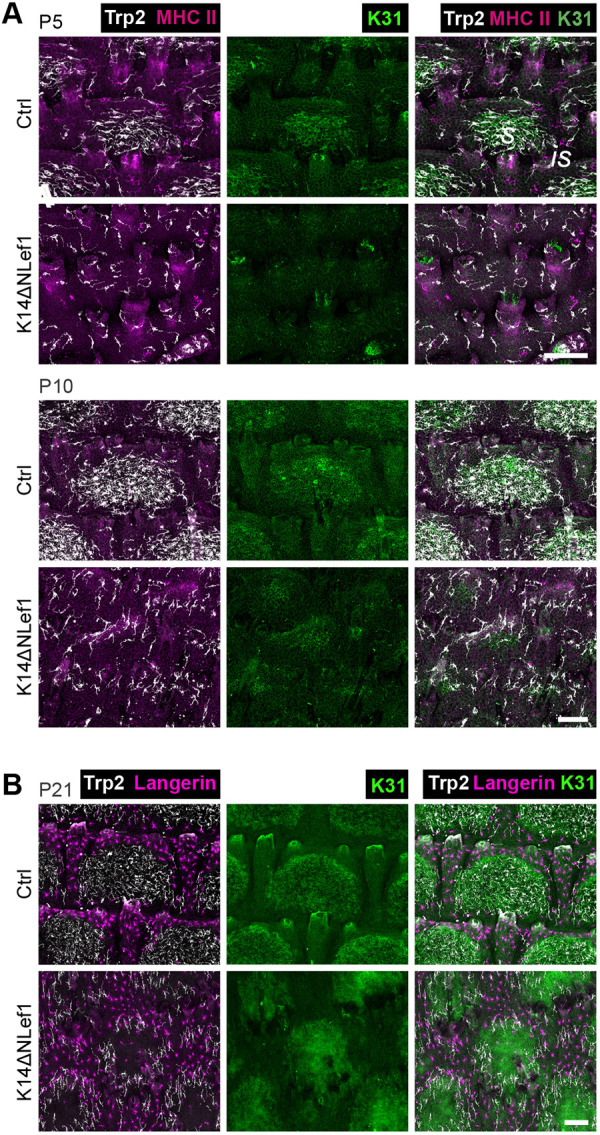
During postnatal tail skin development, MCs in K14ΔNLef1 mice fail to enrich in forming scale regions. (A) Trp2, K31 and MHCII immunostaining of tail epidermis wholemounts of control and K14ΔNLef1 mice at P5 and P10. Representative for n=3. (B) Trp2, K31 and langerin immunostaining of tail epidermis wholemounts from P21 control and K14ΔNLef1 mice. Representative for n=3. is, interscale; s, scale. Scale bars: 100 µm.
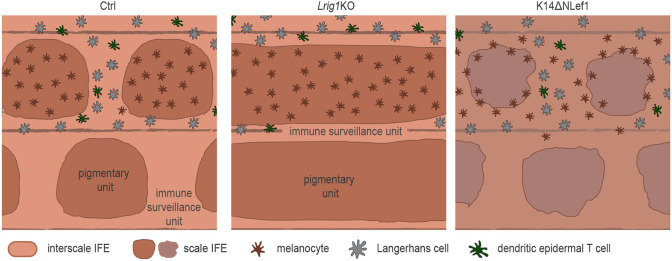
Collectively, these findings demonstrate that the mutually exclusive localization of MCs and epidermis-resident immune cells depends on epidermal patterns that are orchestrated by antagonistic signaling downstream of Lrig1 and Wnt-Lef1 (Fig. 8). Our work identified epidermal KCs at the top of a cellular hierarchy that guides the formation and maintenance of functionally distinct tail IFE compartments.
Fig. 8.
Schematic summary of Lrig1- and Wnt-dependent partitioning of MCs and resident immunocytes into distinct epidermal niches. Epidermal niches and their resident cell types in control mice (left), Lrig1 KO mice exhibiting scale IFE fusion (middle) and K14ΔNLef1 mice with MC localization inverted to interscale IFE, resulting in loss of functional segregation of pigmentary units and immune surveillance unit in mouse tail skin (right).
DISCUSSION
Evolutionarily, pigmentation of scale IFE may have prevailed to protect the underlying hair follicle stem cells from UV-mediated damage. Yet, it is intriguing that immune cells are strictly excluded from this compartment. Melanin synthesis involves generation of cytotoxic intermediates, increasing oxidative stress (Denat et al., 2014; Graham et al., 1978; Urabe et al., 1994); however, we show that the epidermal lineages, but not MCs or melanin, determine LC/DETC patterning. KC-derived paracrine factors (e.g. α-melanocyte stimulating hormone, endothelin, TGFβ) regulate follicular MC stem cell maintenance (Nishimura, 2011) and induction of melanin synthesis (Li et al., 2020; Upadhyay et al., 2021). In contrast, principles of KC:immune cell communication are much less understood. mRNA transfer from KCs to MCs through exosomes (Lo Cicero et al., 2015; Liu et al., 2019) and from LCs to KCs and DETCs has been reported, likely through tunneling nanotubes (Su and Igyártó, 2019), opening the possibility that heterologous cell types in the epidermis actively communicate via such structures. Yet, these studies used in vitro systems or non-patterned orthokeratotic epidermis, which leaves open the question of which molecules and signaling pathways mediate the partitioning of epidermis-resident cells to different epidermal niches. Our work identifies an antagonistic framework in which Lrig1-dependent IFE lineage commitment instructs the segregation of epidermal MCs, LC and DETCs, and epidermal Wnt-Lef1 signaling restricts mature MCs to scale IFE.
Our data derived from the Lrig1 KO model, characterized by progressive scale fusion, point to remarkably stable LC densities during interscale shrinkage and hence adaptation of the LC network to changes in scale:interscale IFE ratios. Though not directly comparable, this partly resembles the recently reported modulation of LC numbers upon alterations of KC densities in adult, non-patterned orthokeratotic ear epidermis (Park et al., 2021). These combined findings illustrate previously unrecognized dynamics of resident immune cells and their ability to adapt to changes in tissue epithelia. Regarding our results on Wnt-Lef1-mediated tissue patterning, further work is necessary to reveal Lef1 transcriptional targets that control MC scale localization. Albeit hypothetical at this point, both the strong decline and the associated morphological alterations of scale-restricted MCs in K14ΔNLef1 IFE shown here, combined with the reported high expression of endogenous Lef1 in wild-type scale IFE (Gomez et al., 2013; Niemann et al., 2002), suggests that canonical Wnt signaling elicits expression of MC-retention factors in the scale IFE. However, as transgene expression in the K14ΔNLef1 model is not restricted to scale IFE, an active recruitment of MCs to K14ΔNLef1 interscale IFE through yet unknown mechanisms is possible as well.
We were also intrigued by previous reports of differential transformation susceptibility of scale and interscale IFE cells: oncogenic hedgehog signaling causes basal cell carcinoma predominantly in interscale IFE (Sánchez-Danés et al., 2016), whereas oncogenic Braf-induced melanomas arise from pigmented MCs in scale IFE (Köhler et al., 2017). This poses the question whether such differential oncogenic outcome is, next to cell-intrinsic factors such as proliferative capacity, also linked to the functional separation of pigmentation and skin immunity. LC sample environmental antigens (Cho et al., 2010; de Jong et al., 2010; Leclercq and Plum, 1995), present self-antigens to naïve T cells to induce tolerance (Stoitzner et al., 2006), and detect altered self-antigens or neo-antigens following external stresses or during malignant transformation (Cao et al., 2007; Schreurs et al., 2000). The role of LCs in various skin cancers and wound healing, however, remains controversial, likely due to recently identified additional dermal langerin-positive non-LC populations that require reinterpretation of data from genetic models with langerin promoters (Deckers et al., 2018; Sheng et al., 2021; Li et al., 2021). DETCs have been clearly implicated in immune surveillance, shown to protect against skin tumors and to promote wound healing (Girardi et al., 2003; Havran and Jameson, 2010; Jameson et al., 2002; Kaminski et al., 1993; Schuhmachers et al., 1995; Xiang et al., 2020). Although it is unclear whether the above immune cell functions also apply to tail skin, this opens the possibility that immune surveillance in interscale IFE, and lack thereof in scale IFE, is decisive for tumorigenesis. Interestingly, MCs themselves may serve as non-professional antigen presenting cells (Le Poole et al., 1993a), perform phagocytosis (Le Poole et al., 1993b) and produce immune-regulatory cytokines and chemokines (Hong et al., 2015). Though requiring further investigation, considering that scale IFE lacks resident immune cells, it seems possible that MCs, in principle, compensate for certain immune cell functions in this compartment. Moreover, it will be interesting to learn whether the rare, pigmented interscale MCs described in this study serve different functions than their scale counterparts, even though they are phenotypically comparable (Fig. S1A-D). In addition, how this pool of MCs relates to the amelanotic interscale MCs reported by Glover et al. (2015) and Köhler et al. (2017) requires further investigation. Together, dissecting the cellular complexity of epidermal niches and communication of the different resident cell types is an important future task to advance our understanding of cell transformation and malignant progression in the skin.
MATERIALS AND METHODS
Mice
All animal breeding and tissue analyses were performed according to institutional guidelines and in compliance with the German federal law for animal protection under control of the North Rhine-Westphalian State Agency for Nature, Environment and Consumer Protection (LANUV, NRW, Germany; file references 81-02.04.2018.A384 and 81-02.04.2018.A401) and the Veterinary Office City of Cologne, Germany (file references UniKöln_Anzeige §4.17.018 and §4.18.020). Id2 KO (Id2tm1Yyk) (Yokota et al., 1999) and K14ΔNLef1 (Niemann et al., 2002) mice have been previously described. Lrig1 KO mice were generated by crossing Lrig1-CreERT2/EGFP mice (Page et al., 2013) (kindly provided by Prof. Kim B. Jensen, University of Copenhagen, Danstem, Denmark) on 129 background to homozygosity, resulting in Lrig1 deletion.
Immunohistochemistry of tail epidermal whole mounts
Preparation and staining of mouse tail wholemounts was performed as previously described (Braun et al., 2003). Briefly, mouse tail skin was peeled off the bone and incubated in 5 mM EDTA/PBS for 2 h at room temperature (RT) with gentle shaking. After replacing half of the EDTA/PBS volume, the tissue was incubated for another 2 h. Next, the epidermis was separated from the dermis, leaving hair follicles attached to the epidermal sheet. Depilation was performed to eliminate autofluorescence caused by hair. The epidermis was fixed in pre-cooled acetone for 30 min on ice. Samples stained with directly conjugated antibodies (γδTCR-FITC) were first incubated with the antibody diluted in PBS overnight at 4°C, followed by blocking the next day. For blocking, samples were incubated in PB buffer [0.5% skim milk powder, 0.25% fish skin gelatin (Sigma-Aldrich), 0.5% Triton X-100 in HBS (20 mM HEPES pH 7.2, 0.9% NaCl)] or 3% milk/PBS for 1 h at RT, followed by incubation with primary antibodies diluted in blocking buffer overnight at RT. The next day, samples were extensively washed for 6-8 h with 0.5% Triton X-100/PBS and multiple exchanges of washing buffer. Samples were then incubated with secondary antibodies (diluted in blocking buffer) over-night at RT. Finally, washing steps for 6-8 h were repeated at RT and samples were mounted in Mowiol with the basal side facing upwards. For details of antibodies used see Table S1.
Co-immunostaining of K31 and Trp2, and of γδTCR, langerin and Trp2 in tail epidermal wholemounts
Owing to limited choices of primary antibodies suited for tail skin wholemount preparations, co-detection of K31 and Trp2 required sequential immunostaining to avoid cross-reactivity of secondary antibodies (AF647 donkey-anti-goat and AF488 goat-anti-guinea pig, see Table S1). Samples were incubated in secondary antibodies except for AF488-conjugated anti-guinea pig overnight at RT, followed by 6-8 h of washing in 0.5% Triton X-100/PBS and subsequent incubation with AF488 goat anti-guinea pig antibodies overnight. Using this protocol, cross-reactivity with Trp2 primary antibodies could be strongly diminished, though not completely eliminated. In some samples, dim signals for MCs were noted when acquiring K31 signals. This was attributed to secondary antibodies, as we could validate that the anti-K31 antibody itself does not recognize MC antigens (S.C.B., unpublished). Importantly, K31 signals solely served to identify scale areas, but not to quantify signal intensities or cell morphologies, hence this weak MC signal was not relevant for the analyses presented in this study.
Similarly, co-detection of γδTCR, langerin and Trp2 required sequential immunostaining to avoid cross-reactivity of secondary antibodies (AF568 donkey-anti-goat and AF647 goat-anti-rat, see Table S1). Samples were incubated in secondary antibodies except for AF647 goat-anti-rat overnight at RT, followed by 6-8 h of washing in 0.5% Triton X-100/PBS and subsequent incubation with AF647 goat-anti-rat antibody overnight.
Immunohistochemistry of ear epidermal wholemounts
Preparation and staining of mouse ear epidermis wholemounts was performed as previously described (Ross et al., 1998). Briefly, mouse ears were depilated using Veet Hair Removal Cream and residual ventral cartilage was removed. Ears were split while floating on PBS, and for further use only the inner ear skin was kept. To separate dermis and epidermis, the skin was placed on 0.5 M ammonium thiocyanate for 25 min at 37°C with the dermal side down. After removing the dermis from the epidermis using forceps, the epidermis was spread on the bottom of a 12-well plate, covered with ice-cold acetone and fixed on ice for 20 min. Next, ear tissues were blocked in 3% milk/PBS for 2 h at RT and subsequently incubated with primary antibodies overnight at 4°C. The next day, ear epidermis was washed three times in PBS for 20 min at RT, followed by incubation with secondary antibodies (diluted in AB buffer, 0.4% bovine serum albumin, 0.5% Triton-X100 in PBS) for 2 h at 37°C. Finally, the ear tissues were washed three times in PBS at RT and then mounted in Mowiol, with the basal side facing upwards. For details of antibodies used see Table S1.
Melanin detection in tail skin using Fontana-Masson staining
Fontana-Masson silver staining served to visualize melanin in skin tissues, based on melanin-mediated reduction of silver nitrate to metallic silver. For this, the Fontana-Masson Staining Kit (Sigma Aldrich, HT200) was used. Tail epidermal wholemounts were fixed with 4% paraformaldehyde/PBS for 30 min at RT and incubated in freshly prepared ammoniacal silver solution at 60°C for 1 min. After rinsing in water, samples were quickly mounted in Mowiol and micrographs directly acquired.
Microscopy
Confocal images were acquired using a Leica SP8 confocal laser scanning microscope and LASX software using a PL Apo 10×/0.40 CS2 air objective or a Zeiss LSM880 confocal laser scanning microscope and ZEN 2.3 SP1 software using a PL Apo 40×/1.3 Oil DIC UV-IR M27 objective. For detection of Fontana-Masson staining, the 633 nm laser and T-PMT detector were used.
Analysis of MC, LC and DETC distributions in tail epidermal wholemounts
Immunostaining for the scale IFE marker K31 was used to distinguish scale from interscale IFE. Using the free image processing software FIJI, a mask was created based on K31 immunostaining only considering depilated but otherwise intact scale:interscale units for the analysis. Immunostainings for Trp2 (MCs) and langerin (LCs) were merged with the scale:interscale mask for further compartment-specific quantification of MC or LC numbers. For analysis of DETC distribution, a mask based on langerin immunostaining was created and merged with γδTCR immunostaining. For analysis of MC, LC or DETC distribution in Id2 Ctrl/KO, FVB/N, C57BL/6 WT and partially MC-free tail epidermis of C57BL/6 mice, percentages of the total MC, LC or DETC population per scale:interscale unit localizing to either scale or interscale IFE were calculated. For analysis of MC, LC or DETC distribution in control and K14ΔLef1 mice, the mask of the scale IFE [based on K31 immunostaining (MC, LC) or langerin immunostaining (DETC)] was either decreased to 60% of its size to mark the center region of the scale IFE, or expanded to 110% to span the scale IFE and its periphery. Remaining K31-negative IFE was considered as the third zone (interscale). Percentages of the total MC, LC or DETC populations per scale:interscale unit localizing to one of the three defined regions was calculated per scale:interscale unit. Analysis of distribution and densities of MCs, LCs and DETCs comprised 2-15 scale:interscale units per animal.
Analysis of apoptosis in tail epidermal wholemounts
For analysis of apoptotic MCs in K14ΔNLef1 mice and of apoptotic immune cells in Lrig1 KO mice, partly depilated and acetone-fixed tail epidermal wholemounts were blocked in PB buffer for 1 h at RT followed by incubation with primary antibodies diluted in PB buffer overnight at RT. The next day, samples were washed three times for 10 min each with 0.05% Triton X-100/PBS. Subsequently, samples were incubated with secondary antibodies (diluted in PB buffer) for 2 h at RT, followed by three washing steps (10 min each, RT) and mounting of samples in Mowiol, with the basal side facing upwards. Apoptotic cells in the remaining hair follicles served as internal positive control for cleaved caspase3 immunostaining, as apoptosis is a natural process occurring during catagen. For details of antibodies used see Table S1.
Quantitative analysis of MC, LC and DETC densities in tail epidermal wholemounts
For analysis of MC, LC or DETC densities in tail epidermis of Id2 Ctrl/KO, FVB/N, C57BL/6 WT and partially MC-free tail epidermis of C57BL/6 mice, respective cell numbers per scale or interscale compartment [determined by K31 (MC, LC) or langerin immunostaining (DETC)] were counted and cell numbers per mm2 were calculated. For analysis of MC, LC or DETC densities in tail epidermis of C57BL/6 mice at P5, P10 and P21, respective cell numbers per complete scale:interscale unit were counted and cell numbers per mm2 were calculated. For analysis of MC, LC, or DETC densities in tail epidermis of control and K14ΔLef1 mice, respective cell numbers per compartment [scale center, scale periphery or interscale IFE, determined by K31 (MC, LC) or langerin immunostaining (DETC) as previously described] were counted and cell numbers per mm2 were calculated.
Quantitative analysis of MC and immune cell densities in ear epidermal wholemounts
For quantification of MC, LC and DETC density in the ear epidermis, respective cell numbers were counted and numbers per mm2 were calculated. At least 0.825 mm2 per animal was analyzed.
Quantification of MC mean area and major axis length
A CellProfiler (Carpenter et al., 2006) pipeline was used to calculate MC mean area and major axis length. Briefly, Trp2 immunostaining was used for primary object identification. MC objects were assigned to tail IFE compartments [scale or interscale (C57BL/6), scale center or scale periphery or interscale IFE (K14ΔNLef1 mice)] and subsequent analysis was carried out automatically with supervision.
Quantification of numbers of MC dendrites
MCs were assigned to tail IFE compartments [scale:interscale (C57BL/6), scale center or scale periphery or interscale IFE (K14ΔNLef1 mice)] and subsequently numbers of dendrites of MCs per compartment were determined.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software (GraphPad, version 6.0). Significance was determined as indicated in the figure legends. n-values refer to biological replicates (=mice), are specified in the figure legends and correspond to the sample size used to derive statistics. All datasets were subjected to normality tests (D'Agostino–Pearson omnibus test, KS normality test or Shapiro–Wilk normality test) when applicable. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. For all experiments, measurements were taken from a minimum of three independent biological samples.
Software
For data analysis, the following software was used: GraphPad PRISM VI, Inkscape, ImageJ/Fiji (Rueden et al., 2017; Schindelin et al., 2012), Cell Profiler (Carpenter et al., 2006) and ZEN 3.0 (blue edition; Carl Zeiss Microscopy GmbH, 2019).
Supplementary Material
Acknowledgements
We thank the imaging facilities at CECAD and Saarland University (ALM-CF), and the imaging facility and University of Cologne animal facilities for important services, and Florian Kuester and CMMC Imaging facility for assisting with mouse analysis. We are grateful to Wendy Havran (deceased) and Deborah Witherden for advice on DETC immunostaining. We acknowledge Kim B. Jensen (University of Copenhagen, Danstem, Denmark) for sharing Lrig1-CreERT2/EGFP mice. We thank all members of the Iden laboratory for stimulating discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.C.B., A.G., S.I.; Methodology: S.C.B., A.G., S.I.; Validation: S.C.B., A.G., S.I.; Formal analysis: S.C.B., S.I.; Investigation: S.C.B., A.K.B., S.C., S.I.; Resources: K.S., M.Z., C.N., S.I.; Data curation: S.C.B.; Writing - original draft: S.C.B., S.I.; Writing - review & editing: S.C.B., S.I.; Visualization: S.C.B.; Supervision: S.I.; Project administration: S.I.; Funding acquisition: S.I.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (grants SPP1782-ID79/2-2 to S.I.; Projektnummer 73111208-SFB 829, A03 to C.N., A10 to S.I.; and Projektnummer 200049484-SFB 1027, A12 to S.I.) and the Center for Molecular Medicine Cologne, University of Cologne (CMMC; project grants to C.N. and to S.I.). Part of this work was funded by a donation of U. Lehmann to M.Z. Work in the Iden laboratory was further supported by the Universität des Saarlandes and Excellence Initiative of the German federal and state governments (CECAD Cologne). Open Access funding provided by the University of Cologne. Deposited in PMC for immediate release.
Peer review history
The peer review history is available online at https://journals.biologists.com/dev/article-lookup/doi/10.1242/dev.200154.
References
- Bergstresser, P. R., Toews, G. B. and Streilein, J. W. (1980). Natural and perturbed distributions of Langerhans cells: responses to ultraviolet light, heterotopic skin grafting, and dinitrofluorobenzene sensitization. J. Invest. Dermatol. 75, 73-77. 10.1111/1523-1747.ep12521261 [DOI] [PubMed] [Google Scholar]
- Blanpain, C. and Fuchs, E. (2006). Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 22, 339-373. 10.1146/annurev.cellbio.22.010305.104357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, K. M., Niemann, C., Jensen, U. B., Sundberg, J. P., Silva-Vargas, V. and Watt, F. M. (2003). Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development 130, 5241-5255. 10.1242/dev.00703 [DOI] [PubMed] [Google Scholar]
- Breathnach, A. S. and Wyllie, L. M.-A. (1965). Melanin in langerhans cells. J. Invest. Dermatol. 45, 401-403. 10.1038/jid.1965.151 [DOI] [PubMed] [Google Scholar]
- Burkhart, C. G. and Burkhart, C. N. (2005). The mole theory: primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection). Int. J. Dermatol. 44, 340-342. 10.1111/j.1365-4632.2004.02556.x [DOI] [PubMed] [Google Scholar]
- Cao, T., Ueno, H., Glaser, C., Fay, J. W., Palucka, A. K. and Banchereau, J. (2007). Both Langerhans cells and intestinal DC cross-present melanoma antigens and efficiently activate antigen-specific CTL. Eur. J. Immunol. 37, 2657-2667. 10.1002/eji.200636499 [DOI] [PubMed] [Google Scholar]
- Carpenter, A. E., Jones, T. R., Lamprecht, M. R., Clarke, C., Kang, I. H., Friman, O., Guertin, D. A., Chang, J. H., Lindquist, R. A., Moffat, J.et al. (2006). CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100. 10.1186/gb-2006-7-10-r100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Rodriguez, S., Hoetzenecker, W., Schwärzler, C., Biedermann, T., Saeland, S. and Elbe-Bürger, A. (2005). Fetal and neonatal murine skin harbors Langerhans cell precursors. J. Leukoc. Biol. 77, 352-360. 10.1189/jlb.1004584 [DOI] [PubMed] [Google Scholar]
- Cho, J. S., Modlin, R. L., Miller, L. S., Cho, J. S., Pietras, E. M., Garcia, N. C., Ramos, R. I., Farzam, D. M., Monroe, H. R., Magorien, J. E.et al. (2010). Staphylococcus Aureus infection in mice IL-17 is essential for host defense against cutaneous Staphylococcus Aureus infection in mice. J. Clin. Invest. 120, 1762-1773. 10.1172/JCI40891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong, C. M. and Noveen, A. (1999). Phenotypic determination of epithelial appendages: genes, developmental pathways, and evolution. J. Investig. Dermatol. Symp. Proc. 4, 307-311. 10.1038/sj.jidsp.5640235 [DOI] [PubMed] [Google Scholar]
- de Jong, M. A. W. P., de Witte, L., Taylor, M. E. and Geijtenbeek, T. B. H. (2010). Herpes simplex virus type 2 enhances HIV-1 susceptibility by affecting Langerhans cell function. J. Immunol. 185, 1633-1641. 10.4049/jimmunol.0904137 [DOI] [PubMed] [Google Scholar]
- Deckers, J., Hammad, H. and Hoste, E. (2018). Langerhans cells: sensing the environment in health and disease. Front. Immunol. 9, 93. 10.3389/fimmu.2018.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denat, L., Kadekaro, A. L., Marrot, L., Leachman, S. A. and Abdel-Malek, Z. A. (2014). Melanocytes as instigators and victims of oxidative stress. J. Invest. Dermatol. 134, 1512-1518. 10.1038/jid.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias Gomes, M. and Iden, S. (2021). Orchestration of tissue-scale mechanics and fate decisions by polarity signalling. EMBO J. 40, e106787. 10.15252/embj.2020106787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierjean, L., Wrench, R. and Saurat, J. H. (1982). Expression of cytoplasmic antigens linked to orthokeratosis during the development of parakeratosis in newborn mouse tail epidermis. Differentiation 23, 250-255. 10.1111/j.1432-0436.1982.tb01290.x [DOI] [PubMed] [Google Scholar]
- D'Orazio, J., Jarrett, S., Amaro-Ortiz, A. and Scott, T. (2013). UV radiation and the skin. Int. J. Mol. Sci. 14, 12222-12248. 10.3390/ijms140612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch, K. R., McGowan, K. A., Van Raamsdonk, C. D., Fuchs, H., Lee, D., Puech, A., Hérault, Y., Threadgill, D. W., De Angelis, M. H. and Barsh, G. S. (2003). Genetics of dark skin in mice. Genes Dev. 17, 214-228. 10.1101/gad.1023703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, J. P., Kanost, M. R. and Trenczek, T. (1997). Biological mediators of insect immunity. Annu. Rev. Entomol. 42, 611-643. 10.1146/annurev.ento.42.1.611 [DOI] [PubMed] [Google Scholar]
- Girardi, M., Glusac, E., Filler, R. B., Roberts, S. J., Propperova, I., Lewis, J., Tigelaar, R. E. and Hayday, A. C. (2003). The distinct contributions of murine T cell receptor (TCR)γδ+ and TCRαβ+ T cells to different stages of chemically induced skin cancer. J. Exp. Med. 198, 747-755. 10.1084/jem.20021282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, J. D., Knolle, S., Wells, K. L., Liu, D., Jackson, I. J., Mort, R. L. and Headon, D. J. (2015). Maintenance of distinct melanocyte populations in the interfollicular epidermis. Pigment Cell Melanoma Res. 28, 476-480. 10.1111/pcmr.12375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, C., Chua, W., Miremadi, A., Quist, S., Headon, D. J. and Watt, F. M. (2013). The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Rep. 1, 19-27. 10.1016/j.stemcr.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales, K. A. U. and Fuchs, E. (2017). Skin and its regenerative powers: an alliance between stem cells and their niche. Dev. Cell 43, 387-401. 10.1016/j.devcel.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, D. G., Tiffany, S. M. and Vogel, F. S. (1978). The toxicity of melanin precursors. J. Invest. Dermatol. 70, 113-116. 10.1111/1523-1747.ep12541249 [DOI] [PubMed] [Google Scholar]
- Hacker, C., Kirsch, R. D., Ju, X. S., Hieronymus, T., Gust, T. C., Kuhl, C., Jorgas, T., Kurz, S. M., Rose-John, S., Yokota, Y.et al. (2003). Transcriptional profiling identifies Id2 function in dendritic cell development. Nat. Immunol. 4, 380-386. 10.1038/ni903 [DOI] [PubMed] [Google Scholar]
- Havran, W. L. and Jameson, J. M. (2010). Epidermal T cells and wound healing. Bone 184, 5423-5428. 10.4049/jimmunol.0902733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi, H., Yoshino, M., Yamazaki, H., Naito, M., Iyoda, T., Omatsu, Y., Shimoyama, S., Letterio, J. J., Nakabayashi, T., Tagaya, H.et al. (2001). Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-β1-dependent cells. Int. Immunol. 13, 695-704. 10.1093/intimm/13.5.695 [DOI] [PubMed] [Google Scholar]
- Hirobe, T. (1984). Histochemical survey of the distribution of the epidermal melanoblasts and melanocytes in the mouse during fetal and postnatal periods. Anat. Rec. 208, 589-594. 10.1002/ar.1092080414 [DOI] [PubMed] [Google Scholar]
- Hong, Y., Song, B., Chen, H.-D. and Gao, X.-H. (2015). Melanocytes and skin immunity. J. Investig. Dermatol. Symp. Proc. 17, 37-39. 10.1038/jidsymp.2015.14 [DOI] [PubMed] [Google Scholar]
- Jameson, J., Ugarte, K., Chen, N., Yachi, P., Fuchs, E., Boismenu, R. and Havran, W. L. (2002). A role for skin γδ T cells in wound repair. Science 296, 747-749. 10.1126/science.1069639 [DOI] [PubMed] [Google Scholar]
- Kaminski, M. J., Cruz, P. D., Bergstresser, P. R. and Takashima, A. (1993). Killing of skin-derived tumor cells by mouse dendritic epidermal T-cells. Cancer Res. 53, 4014-4019. [PubMed] [Google Scholar]
- Kobayashi, M., Asano, H., Fujita, Y. and Hoshino, T. (1987). Development of ATPase-positive, immature Langerhans cells in the fetal mouse epidermis and their maturation during the early postnatal period. Cell Tissue Res. 248, 315-3322. 10.1007/BF00218198 [DOI] [PubMed] [Google Scholar]
- Köhler, C., Nittner, D., Rambow, F., Radaelli, E., Stanchi, F., Vandamme, N., Baggiolini, A., Sommer, L., Berx, G., van den Oord, J. J.et al. (2017). Mouse cutaneous melanoma induced by mutant BRaf arises from expansion and dedifferentiation of mature pigmented melanocytes. Cell Stem Cell 21, 679-693.e6. 10.1016/j.stem.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Kunisada, T., Yamazaki, H., Hirobe, T., Kamei, S., Omoteno, M., Tagaya, H., Hemmi, H., Koshimizu, U., Nakamura, T. and Hayashi, S. I. (2000). Keratinocyte expression of transgenic hepatocyte growth factor affects melanocyte development, leading to dermal melanocytosis. Mech. Dev. 94, 67-78. 10.1016/S0925-4773(00)00308-7 [DOI] [PubMed] [Google Scholar]
- Le Poole, I. C., Mutis, T., Wijngaard, V. d., Westerhof, R. M. J. G. J., Ottenhoff, W., De Vries, T. and Das, R. R. P. K. (1993a). A novel, antigen-presenting function of melanocytes and its possible relationship to hypopigmentary disorders. J. Immunol. 151, 7284-7292. [PubMed] [Google Scholar]
- Le Poole, I. C., Van Den Wijngaard, R. M. J. G. J., Westerhof, W., Verkruisen, R. P., Dutrieux, R. P., Dingemans, K. P. and Das, P. K. (1993b). Phagocytosis by normal human melanocytes in vitro. Exp. Cell Res. 205, 388-395. 10.1006/excr.1993.1102 [DOI] [PubMed] [Google Scholar]
- Leclercq, G. and Plum, J. (1995). Stimulation of TCR V gamma 3 cells by gram-negative bacteria. J. Immunol. 154, 5313-5319. [PubMed] [Google Scholar]
- Li, M., Knapp, S. K. and Iden, S. (2020). Mechanisms of melanocyte polarity and differentiation: what can we learn from other neuroectoderm-derived lineages? Curr. Opin. Cell Biol. 67, 99-108. 10.1016/j.ceb.2020.09.001 [DOI] [PubMed] [Google Scholar]
- Li, Z., Lamb, R., Coles, M. C., Bennett, C. L. and Ambler, C. A. (2021). Inducible ablation of CD11c+ cells to determine their role in skin wound repair. Immunology 163, 105-111. 10.1111/imm.13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Xue, L., Gao, H., Chang, L., Yu, X., Zhu, Z., He, X., Geng, J., Dong, Y., Li, H.et al. (2019). Exosomal miRNA derived from keratinocytes regulates pigmentation in melanocytes. J. Dermatol. Sci. 93, 159-167. 10.1016/j.jdermsci.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Lo Cicero, A., Delevoye, C., Gilles-Marsens, F., Loew, D., Dingli, F., Guéré, C., André, N., Vié, K., Van Niel, G. and Raposo, G. (2015). Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 6, 7506. 10.1038/ncomms8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod, A. S., Hemmers, S., Garijo, O., Chabod, M., Mowen, K., Witherden, D. A. and Havran, W. L. (2013). Dendritic epidermal T cells regulate skin antimicrobial barrier function. J. Clin. Invest. 123, 4364-4374. 10.1172/JCI70064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima, Y. (1966). Melanosomes in phagocytotic vacuoles in Langerhans cells. J. Cell Biol. 30, 417-423. 10.1083/jcb.30.2.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann, C., Owens, D. M., Hülsken, J., Birchmeier, W. and Watt, F. M. (2002). Expression of ΔNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129, 95-109. 10.1242/dev.129.1.95 [DOI] [PubMed] [Google Scholar]
- Nishimura, E. K. (2011). Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 24, 401-410. 10.1111/j.1755-148X.2011.00855.x [DOI] [PubMed] [Google Scholar]
- Page, M. E., Lombard, P., Ng, F., Göttgens, B. and Jensen, K. B. (2013). The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471-482. 10.1016/j.stem.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S., Matte-Martone, C., Gonzalez, D. G., Lathrop, E. A., May, D. P., Pineda, C. M., Moore, J. L., Boucher, J. D., Marsh, E., Schmitter-Sánchez, A.et al. (2021). Skin-resident immune cells actively coordinate their distribution with epidermal cells during homeostasis. Nat. Cell Biol. 23, 476-484. 10.1038/s41556-021-00670-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, S. and Lechler, T. (2011). Regulation of asymmetric cell division in the epidermis. Cell Div 6, 12. 10.1186/1747-1028-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, R., Gillitzer, C., Kleinz, R., Schwing, J., Kleinert, H., Förstermann, U. and Reske-Kunz, A. B. (1998). Involvement of NO in contact hypersensitivity. Int. Immunol 10, 61-69. 10.1093/intimm/10.1.61 [DOI] [PubMed] [Google Scholar]
- Rueden, C. T., Schindelin, J., Hiner, M. C., DeZonia, B. E., Walter, A. E., Arena, E. T. and Eliceiri, K. W. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 18, 529. 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada, A., Jacob, F., Leung, E., Wang, S., White, B. S., Shalloway, D. and Tumbar, T. (2016). Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nat. Cell Biol. 18, 619-631. 10.1038/ncb3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Danés, A., Hannezo, E., Larsimont, J.-C. C., Liagre, M., Youssef, K. K., Simons, B. D. and Blanpain, C. (2016). Defining the clonal dynamics leading to mouse skin tumour initiation. Nature 536, 298-303. 10.1038/nature19069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B.et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs, M. W. J., Eggert, A. A. O., De Boer, A. J., Vissers, J. L. M., Van Hall, T., Offringa, R., Figdor, C. G. and Adema, G. J. (2000). Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Res. 60, 6995-7001. [PubMed] [Google Scholar]
- Schuhmachers, G., Ariizumi, K., Mathew, P. A., Bennett, M., Kumar, V. and Takashima, A. (1995). 2B4, a new member of the immunoglobulin gene superfamily, is expressed on murine dendritic epidermal T cells and plays a functional role in their killing of skin tumors. J. Invest. Dermatol. 105, 592-596. 10.1111/1523-1747.ep12323533 [DOI] [PubMed] [Google Scholar]
- Schweizer, J. and Marks, F. (1977). Developmental study of the distribution and frequency of Langerhans cells in relation to the formation of patterning in mouse tail epidermis. J. Invest. Dermatol. 69, 198-204. 10.1111/1523-1747.ep12506298 [DOI] [PubMed] [Google Scholar]
- Seré, K., Baek, J. H., Ober-Blöbaum, J., Müller-Newen, G., Tacke, F., Yokota, Y., Zenke, M. and Hieronymus, T. (2012). Two distinct types of langerhans cells populate the skin during steady state and inflammation. Immunity 37, 905-916. 10.1016/j.immuni.2012.07.019 [DOI] [PubMed] [Google Scholar]
- Sheng, J., Chen, Q., Wu, X., Dong, Y. W., Mayer, J., Zhang, J., Wang, L., Bai, X., Liang, T., Sung, Y. H.et al. (2021). Fate mapping analysis reveals a novel murine dermal migratory Langerhans-like cell population. Elife 10, e65412. 10.7554/eLife.65412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitzner, P., Tripp, C. H., Eberhart, A., Price, K. M., Jung, J. Y., Bursch, L., Ronchese, F. and Romani, N. (2006). Langerhans cells cross-present antigen derived from skin. Proc. Natl. Acad. Sci. USA 103, 7783-7788. 10.1073/pnas.0509307103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, Q. and Igyártó, B. Z. (2019). Keratinocytes share gene expression fingerprint with epidermal langerhans cells via mRNA transfer. J. Invest. Dermatol. 139, 2313-2323.e8. 10.1016/j.jid.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Tobin, D. J. (1998). A possible role for Langerhans cells in the removal of melanin from early catagen hair follicles. Br. J. Dermatol. 138, 795-798. 10.1046/j.1365-2133.1998.02215.x [DOI] [PubMed] [Google Scholar]
- Tripp, C. H., Chang-rodriguez, S., Stoitzner, P., Holzmann, S., Stössel, H., Douillard, P., Saeland, S., Koch, F. and Elbe-bu, A. (2004). Ontogeny of Langerin/CD207 expression in the epidermis of mice. J. Invest. Dermatol. 122, 670-672. 10.1111/j.0022-202X.2004.22337.x [DOI] [PubMed] [Google Scholar]
- Tsuruta, D., Kaneda, K., Teramae, H. and Ishii, M. (1999). In vivo activation of Langerhans cells and dendritic epidermal T cells in the elicitation phase of murine contact hypersensitivity. Br. J. Dermatol. 140, 392-399. 10.1046/j.1365-2133.1999.02698.x [DOI] [PubMed] [Google Scholar]
- Upadhyay, P. R., Ho, T. and Abdel-Malek, Z. A. (2021). Participation of keratinocyte- and fibroblast-derived factors in melanocyte homeostasis, the response to UV, and pigmentary disorders. Pigment Cell Melanoma Res. 34, 762-776. 10.1111/pcmr.12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe, K., Aroca, P., Tsukamoto, K., Mascagna, D., Palumbo, A., Prota, G. and Hearing, V. J. (1994). The inherent cytotoxicity of melanin precursors: a revision. Biochim. Biophys. Acta 1221, 272-278. 10.1016/0167-4889(94)90250-x [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk, C. D., Fitch, K. R., Fuchs, H., De Angelis, M. H. and Barsh, G. S. (2004). Effects of G-protein mutations on skin color. Nat. Genet. 36, 961-968. 10.1038/ng1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt, F. M. and Collins, C. A. (2008). Role of β-catenin in epidermal stem cell expansion, lineage selection, and cancer. Cold Spring Harb. Symp. Quant. Biol. 73, 503-512. 10.1101/sqb.2008.73.011 [DOI] [PubMed] [Google Scholar]
- Xiang, J., Qiu, M. and Zhang, H. (2020). Role of dendritic epidermal T cells in cutaneous carcinoma. Front. Immunol. 11, 1266. 10.3389/fimmu.2020.01266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota, Y., Mansouri, A., Mori, S., Sugawara, S., Adachi, S., Nishikawa, S. and Gruss, P. (1999). Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702-706. 10.1038/17812 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.