Abstract
Adipose tissue (AT) is a complex organ, with multiple functions that are essential for maintaining metabolic health. A feature of AT is its capability to expand in response to physiological challenges, such as pregnancy and aging, and during chronic states of positive energy balance occurring throughout life. AT grows through adipogenesis and/or an increase in the size of existing adipocytes. One process that is required for healthy AT growth is the remodeling of the extracellular matrix (ECM), which is a necessary step to restore mechanical homeostasis and maintain tissue integrity and functionality. While the relationship between mechanobiology and adipogenesis is now well recognized, less is known about the role of adipocyte mechanosignaling pathways in AT growth. In this review article, we first summarize evidence linking ECM remodelling to AT expansion and how its perturbation is associated to a metabolically unhealthy phenotype. Subsequently, we highlight findings suggesting that molecules involved in the dynamic, bidirectional process (mechanoreciprocity) enabling adipocytes to sense changes in the mechanical properties of the ECM are interconnected to pathways regulating lipid metabolism. Finally, we discuss processes through which aging may influence the ability of adipocytes to appropriately respond to alterations in ECM composition.
Keywords: Adipocyte dysfunction, extracellular matrix stiffness, mechanical forces, mechanotransduction, adipose progenitor cells
1. Introduction
Adipose tissue (AT) is a metabolically active endocrine and immune organ that is involved in the regulation of whole-body energy homeostasis and metabolism (Choe et al., 2016; Kershaw and Flier, 2004), enables adaptive thermoregulation (Gregory, 1989), and serves as a physical barrier to infection (Alexander et al., 2015; Zwick et al., 2018). Studies in both humans and animal models also suggest that AT is at the center of mechanisms and pathways involved in health span and longevity, with most anti-aging interventions, such as caloric restriction, impacting AT (Huffman and Barzilai, 2010; Palmer and Kirkland, 2016). Because of its multifunctional nature, a remarkable feature of AT is its ability to undergo dynamic remodeling in response to physiological challenges (e.g. embryonic development, puberty, pregnancy, and aging) and changes in nutrient deprivation and excess that occur throughout life (Choe et al., 2016). The remodeling of AT is the result of a highly complex and well-orchestrated set of events that entail quantitative and qualitative alterations in AT-resident cells, including adipocytes, adipose stem cells, and macrophages, along with balanced angiogenic and proinflammatory responses and an appropriate renovation of the extracellular matrix (ECM) (Sun et al., 2011). In individuals with excessive adiposity perturbations in processes related to AT remodeling can promote a “metabolically unhealthy phenotype” characterized by impaired AT expandability, increased adipocyte size, altered lipid metabolism, and local inflammation (Goossens, 2017). This phenotype promotes the development of systemic insulin resistance that is one of the most critical risk factors for chronic cardiometabolic diseases (Smith et al., 2019; Vishvanath and Gupta, 2019). A deep understanding of the biological processes and molecular mechanisms that are involved in AT remodeling is thus instrumental for the development of efficient interventions aimed to prevent the onset of metabolic complications associated with impaired AT expansion.
Mechanical forces have long been implicated in multiple biological processes at the molecular, cellular, and tissue level (Gooch, 2012). In physiological conditions, growth and remodeling occur in native tissues to maintain a preferred level of the mechanical state, called “mechanical homeostasis”, which requires, in part, assessment of ECM mechanics by the cells (reviewed by (Ambrosi et al., 2019; Humphrey et al., 2014). Aging, however, perturbs the ability of the cell to reestablish the homeostasis of the mechanical microenvironment in several physiological systems, including the musculoskeletal, cardiovascular, neuronal, and respiratory systems, thereby increasing the risk for many common chronic diseases (Epro et al., 2017; Levy Nogueira et al., 2016; Lieber et al., 2004; Sicard et al., 2018). The progressive changes in the cell’s biophysical properties occurring with aging have such a profound impact on the cellular function that they can predict the cellular biological age with more accuracy than classical biomarkers of aging, such as DNA repair, cellular metabolism, and cellular secretions (Phillip et al., 2017). In recent years, it has become increasingly evident that mechanical signals, such as those resulting from cell-matrix adhesions, play a crucial role also in AT biology (Alkhouli et al., 2013; Ruiz-Ojeda et al., 2019; Yuan et al., 2015). The mechanical behavior and properties of the AT as well as the effect of mechanical stress on adipose cells’ behaviors have been previously summarized (Oomens and Peters, 2014; Pope et al., 2016; Shoham and Gefen, 2012; Yuan et al., 2015). In this review, we will focus on key mechanosensory transduction pathways in mature adipocytes and how they might be impacted by changes occurring with aging.
2. AT composition and anatomical distribution
AT is composed of lipid-laden mature adipocytes (~50% of cells) that are surrounded by connective tissue matrix, blood capillaries, nerve tissue, and stromal-vascular fraction (SVF) cells, including adipose progenitor cells (APCs), pericytes, lymphocytes, endothelial cells, and fibroblasts (Hausman and Dodson, 2013; Kershaw and Flier, 2004). Mature adipocytes are classified as white, brown, or beige/brite according to unique morphology, metabolic functions, biochemical features, and gene expression patterns (Park et al., 2014). White adipocytes compose the main parenchymal cells of white adipose tissue (WAT), which represents more than 95% of AT mass in adult humans. The primary role of white cells is to store neutral lipids (e.g. triglycerides and steryl esters) packed into a single large organelle called lipid droplet (LD) that occupies most of the cytoplasm (Konige et al., 2014). However, they also secrete pro- and anti-inflammatory adipokines, such as leptin, resistin, adiponectin, and omentin, which have crucial roles in satiety regulation and whole-body insulin sensitivity (Fasshauer and Bluher, 2015; Rabe et al., 2008). Anatomically, WAT is divided into subcutaneous WAT (sWAT) that is situated between muscle and skin, visceral WAT (vWAT) that is associated with internal visceral organs, and dermal WAT (dWAT) that underlies the reticular dermis (Driskell et al., 2014; Zwick et al., 2018). The distribution of sWAT and vWAT varies substantially among individuals and depends on several factors, such as nutrition, genetics, and sex (Kuk et al., 2009; Lee et al., 2013).
Unlike white fat cells, brown adipocytes are characterized by multilocular LDs, are richly vascularized, have abundant mitochondria (Park et al., 2014), and function prominently in thermoregulation by generating heat through the oxidation of available substrates (Harms and Seale, 2013). Moreover, brown adipocytes have a discrete localization, residing mainly in cervical-supraclavicular, perirenal/adrenal, and paravertebral regions around the major vessels in humans (Ravussin and Galgani, 2011), and share a common early gene expression profile with skeletal muscle cells (Timmons et al., 2007). Beige adipocytes, which are also called brite (brown-in-white), are phenotypically similar to brown adipocytes, but derive from WAT resident precursors or through reprogramming of mature white adipocytes in response to various activators, such as cold exposure or adrenergic stimulation (Harms and Seale, 2013; Shao et al., 2019).
The heat-generating feature of brown and beige adipocytes appears to have beneficial effects on both the reduction of body weight and insulin sensitivity (Harms and Seale, 2013; Peng et al., 2015). Moreover, more recent work demonstrated that perivascular AT (PVAT) displays characteristics of beige AT in humans (Efremova et al., 2020). PVAT is the fat located in close contact with the adventitial layer of most blood vessel walls, where it plays a crucial role in the regulation of vascular tone and remodeling by secreting several paracrine and endocrine mediators, such as adipokines and growth factors (Hu et al., 2020). There is growing evidence in animal studies that, during the development of obesity and with advancing age, beige-like adipocytes in PVAT can transition into white-like adipocytes that secrete factors causing constriction of blood vessels thereby leading to an increase in blood pressure (Chang et al., 2020). Thus, the activation or maintenance of brown and beige adipocytes through pharmacological and lifestyle interventions can be an efficient therapeutic approach for the prevention of Type-2 diabetes (T2D) and hypertension (Chang et al., 2020; Harms and Seale, 2013; Mulya and Kirwan, 2016).
3. The role of ECM in AT growth
3.1. Dynamics of AT growth
In humans, AT first appears during the second trimester of pregnancy (Poissonnet et al., 1984) and progressively develops through multiple cell lineages that are heterogeneously and dynamically distributed (Sanchez-Gurmaches and Guertin, 2014). After birth, the most abundant fat is brown AT (BAT), which protects the newborn from the cold extrauterine environment (Lidell, 2018), but its prevalence and functionality decline with increasing age (Florez-Duquet and McDonald, 1998; Zoico et al., 2019). On the other hand, most of WAT growth occurs during childhood and adolescence through the generation of new adipocytes (adipocyte hyperplasia through adipogenesis) or an increase in the size of existing adipocytes (adipocyte hypertrophy) (Hager et al., 1977; Hirsch and Knittle, 1970; Knittle et al., 1979; Vishvanath and Gupta, 2019). Adipose lineage-committed preadipocytes and SVF-resident adipose-derived stem cells constitute the pool of regenerative cells from which new adipocytes are produced (Raajendiran et al., 2019). Although the number of total adipocytes per depot appears to be mostly stable throughout adulthood in weight stable individuals (Spalding et al., 2008; Strawford et al., 2004), recent lineage-tracing models in rodents have demonstrated that adult WAT expansion may result from both hypertrophy and hyperplasia during chronic states of positive energy balance (e.g. when energy intake exceeds energy expenditure) (exhaustively reviewed in (Ghaben and Scherer, 2019). Additionally, substantial evidence indicates that healthy WAT expansion, as seen in “metabolically healthy obese” people, correlates with increased adipogenic capacity and smaller adipocyte size (Smith et al., 2019; Vishvanath and Gupta, 2019). The mechanism of AT growth, however, varies according to sex and specific depots (Lee et al., 2013), with the expansion of vWAT in males occurring through adipocyte hyperplasia in both mice (Jeffery et al., 2015) and humans (Tchkonia et al., 2013a; Tchoukalova et al., 2010).
3.2. ECM remodeling regulates AT growth
A crucial step in AT expansion, as well as in its contraction after weight loss, is the controlled remodeling of the ECM that surrounds adipocytes (Liu et al., 2016; Ruiz-Ojeda et al., 2019; van Baak and Mariman, 2019). As comprehensively reviewed by (Ruiz-Ojeda et al., 2019), the ECM of the AT is composed of two main classes of macromolecules: soluble proteoglycans and fibrous structural proteins, including collagens, fibronectins, laminins, and elastins. Collagens comprise the most abundant proteins in ECM, with 20 subunits of 12 different types identified in rodent adipocyte ECM using proteomics techniques (Mariman and Wang, 2010). Of these, type VI collagen (COL6) is specific to AT (Khan et al., 2009), is more expressed in vWAT than sWAT (Mori et al., 2014), and is involved in AT inflammation (Gesta et al., 2016). However, the composition and supramolecular organization of the ECM differ not only in specific AT depots but also in developmental periods and the pathophysiological events taking place in the setting of a long-term positive caloric imbalance (Liu et al., 2017; Mariman and Wang, 2010). For instance, it is well established that extensive ECM alterations occur during WAT growth through hyperplasia (Mariman and Wang, 2010). The formation of new adipocytes or adipogenesis is a tightly regulated process characterized by two essential phases (Ghaben and Scherer, 2019). The first phase consists of the proliferation and commitment of AT stem cells residing within the SVF in preadipocytes. This is followed by the differentiation phase during which preadipocytes accumulate a large amount of fat and become functional, insulin-responsive mature adipocytes (Ghaben and Scherer, 2019). As the fibroblast-like preadipocytes differentiate and assume the rounded form of the LD-rich adipocyte, cell shape is drastically altered (Mariman and Wang, 2010). To facilitate the change in adipocyte morphology, the ECM needs to transition from a fibronectin-rich stromal matrix to a laminin-rich basement membrane (Smas and Sul, 1995). This shift, which is regulated by insulin, energy metabolism, and mechanical forces (Mariman and Wang, 2010), is characterized by the replacement of the predominantly fibronectin-bound α5 integrin in preadipocytes to the laminin-bound α6 integrin in mature adipocytes (Liu et al., 2005). Furthermore, it requires the production and secretion of ECM components by local cells (e.g. adipocytes, fibroblast, adipocyte progenitors, and myofibroblast) and their degradation by enzymes belonging to either the fibrinolytic system or the matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of metalloproteinases [TIMPs]) (Datta et al., 2018; Ruiz-Ojeda et al., 2019). In this regard, it has been reported that, whereas the activity of MMP-2 and MMP-9 increases during adipocyte differentiation (Bouloumie et al., 2001), downregulation of TIMP-3 is necessary for the successful implementation of the adipocyte differentiation program (Bernot et al., 2010). It is also important to mention the critical role played by the membrane-anchored collagenase MMP-14 in ECM remodeling during adipogenesis. Unlike other MMPs, MMP-14 regulates not only the adipogenic collagenolytic turnover, but also the gene expression profile required for adipocyte differentiation by releasing the epigenetic constrains imposed by fibrillar type I collagen (Sato-Kusubata et al., 2011). Of note, more recent studies revealed that MMP-14 also digests COL6α3, one of the subunits of COL6, to generate endotrophin, a carboxy-terminally cleaved peptide of COL6α3 that enhances WAT fibrosis and inflammation during obesity development (Li et al., 2020). This work showed that, due to its multiple functions, overexpression of MMP-14 promotes healthy WAT expansion at early stages of obesity when collegenolysis is a necessary step to facilitate the change in adipocyte morphology. On the other hand, maintaining the expression levels of MMP-14 high at the late stages of obesity leads to massive WAT fibrosis and inflammation through the production of endotrophin (Li et al., 2020). Together, these findings highlight the complexity of the processes governing AT expansion and the importance of proper ECM remodeling.
3.3. Depot-specific perturbation of ECM remodeling during WAT expansion promotes a “metabolically unhealthy phenotype”
Given its pivotal role in AT biology, if ECM remodeling is perturbed because, for instance, an imbalance between degradation and replacement of structural components, WAT growth and plasticity can be compromised. This is supported by clinical observations of obese subjects, in whom adipocyte hypertrophy has been found associated with excess deposition of collagen fibers surrounding adipocytes, or pericellular fibrosis, in WAT (Dankel et al., 2014; Divoux et al., 2010). Moreover, studies in humans have revealed that increases in WAT collagen gene transcripts and their content, mainly COL6, are associated with obesity-induced inflammation and insulin resistance (Michaud et al., 2016; Pasarica et al., 2009). On the other hand, COL6-deficient mice fed a high-fat diet (HFD) display reduced fibrosis in WAT and are metabolically healthier than control mice (Khan et al., 2009). Because of these findings, it has been proposed that the fibrous (or rigid) ECM may prevent the expansion of mature adipocytes by exerting mechanical forces onto the adipocyte plasma membrane that counteract the pressure exerted by enlarged LDs within the cell. This would limit the capacity for cell size storage and ultimately lead to adipocyte death and inflammation (reviewed in (Datta et al., 2018). This idea, however, has been challenged by work from Divoux and colleagues (Divoux et al., 2010) that found a negative correlation of vWAT fibrosis with circulating triglycerides in morbidly obese subjects, suggesting that vWAT fibrosis may instead play a beneficial role in metabolic complications of obesity by limiting adipocyte hypertrophy and promoting adipocyte hyperplasia (Divoux et al., 2010). Interestingly, this relationship was not observed in sWAT, arguing for differential consequences of the presence of fibrosis depending on fat depot (Divoux et al., 2010). Along the same lines, it has been reported that increased tensile strength exerted by excess collagen accumulation in vWAT promotes metabolically healthy obesity in women (Lackey et al., 2014). Furthermore, a study from Muir and collaborators (Muir et al., 2016) demonstrated that obese people without T2D had increased vWAT fibrosis, higher preadipocyte frequency (e.g. enhanced adipogenic capacity), and smaller mature adipocytes than obese diabetic patients. The contrasting findings highlighted here strongly suggest that the underlying processes linking WAT fibrosis and obesity-associated metabolic complications are considerably more complex than predicted. Nonetheless, the evidence collected so far provides solid ground to consider WAT fibrosis as a potential target for manipulation of regional adiposity and systemic metabolism.
3.4. ECM, mechanotransduction, and adipocyte fate and function
3.4.1. Cellular mechanotransduction – an overview
Proper cell behaviors, such as proliferation, differentiation, and survival, are influenced by the properties and composition of the ECM (Vogel and Sheetz, 2006). This is because cells can sense the mechanical properties of the ECM through mechanosensitive receptors or structures (mechanosensors) that, in turn, activate various mechanotransduction pathways (reviewed by (Gasparski and Beningo, 2015; Jansen et al., 2015; Lim et al., 2018; Paluch et al., 2015). Once activated, the mechanochemical pathways convey a signal from the cell membrane to the nucleus through the actomyosin-based cytoskeletal components, or stress fibers, which are directly connected to the nuclear lamina and can pass on stress and strain and thereby deform the nucleus (Buxboim et al., 2014; Swift et al., 2013). This ‘outside-in’ mechanotransduction signal leads to the reorganization of chromatin structures and changes in the expression of genes that ultimately promote the remodeling of the ECM (Mohammed et al., 2019). Reciprocally, cells can alter the composition and mechanical properties of the matrix by altering architectural aspects of the cytoskeleton, modulating cellular elasticity, or generating a concomitant actomyosin-mediated contractile response to applied forces (‘inside-out’ mechanotransduction) (Martino et al., 2018; Mohammed et al., 2019). Thus, a “dynamic reciprocity” exists between the cell and the ECM and it is through this relationship that the cellular information can markedly impact the tissue microenvironment and the other way around (Xu et al., 2009). This close relationship enables cells to sense exogenous, physiological forces imparted upon them and restore mechanical homeostasis to maintain tissue integrity and functionality (Humphrey et al., 2014).
Several mechanosensors, such as integrins, G-protein coupled receptors, the glycocalyx, ion channels, and lipid rafts, have been identified so far (reviewed by (Gasparski and Beningo, 2015). Among them, there are the proteins of the focal adhesions (FAs), which are integrin-based transmembrane structures mediating the attachments of cells to the ECM (Kuo, 2014). FAs are very dynamic assemblies that consist of approx. 150 different structural and signaling proteins, including the mechanosensors talin, vinculin, and focal adhesion kinase (FAK), and serve as pivotal sites for both outside-in and inside-out mechanotransduction signals (Kuo, 2014). FA proteins are involved in activating and gathering integrins and linking integrins to F-actin filaments in response to force-induced conformational changes that expose cryptic peptide sequences that are otherwise hidden in the folded proteins (Lim et al., 2018). It is through FA proteins that cells can also exert F-actin cytoskeleton-mediated traction forces to sense their physical surrounding, such as matrix “stiffness” (e.g. the mechanical resistivity of the ECM), and respond to it (Paszek et al., 2005). Forces generated within the cytoskeleton produce active tension that is then applied to cell-ECM adhesions. As such, a tight relationship exists among FA assembly and growth, traction force transmission, and mechanosensing of ECM stiffness, such that perturbing one affects the other two (Wu et al., 2017).
It is well-recognized that alterations of matrix stiffness within or across a tissue have a profound effect on multiple aspects of cell behavior, including the lineage commitment of mesenchymal stem cells (MSCs) that are present in many adult tissues (Hadden and Choi, 2016). In this regard, remarkable are the experiments performed by Dupont and co-workers (Dupont et al., 2011) who demonstrated that changes in ECM stiffness regulate MSC differentiation commitment into either the adipogenic or osteogenic lineage through the transcriptional factors Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ). They also reported that mechanosensing of ECM stiffness via YAP/TAZ is independent of the Hippo kinase cascade and that nuclear localization and activity of YAP/TAZ requires the activation of the Ras homologous (Rho)/Rho-associated kinase (ROCK) signaling pathway and tension of the actomyosin cytoskeleton (Dupont et al., 2011). Later work also showed that YAP regulates cell mechanics in response to ECM cues by controlling FA assembly (Nardone et al., 2017) and that the FA protein vinculin is necessary for the nuclear localization of TAZ and its inhibition of the adipocyte differentiation on rigid ECM (Kuroda et al., 2017).
Other crucial downstream intracellular mediators of mechanotransduction are members of the small guanosine triphosphatase (GTPases) of the Rho-family (Rho-family GTPases), which include Rac1, Cdc42, and Rho family member A (RhoA). The latter is a key signaling component regulating the assembly of ECM receptors, FA proteins, and the actomyosin cytoskeleton in response to mechanical forces (Chrzanowska-Wodnicka and Burridge, 1996). Like other members of the Rho-family GTPases, RhoA acts as a molecular switch, cycling between an active GTP-bound state and an inactive GDP-bound state. In the active state, RhoA activates the kinase ROCK, which promotes actomyosin contractility by activating myosin via phosphorylation of the regulatory myosin light chain (MLC), and the formin protein mammalian Diaphanous, which stimulates actin polymerization (Chrzanowska-Wodnicka and Burridge, 1996). Apart from RhoA, Rac1 is also activated by ECM stiffness via a FAK- Cas-Rac1-lamellopidian signaling module that converts the external information encoded by ECM stiffness into stable intracellular stiffness and mechanosensitive cell cycling (Bae et al., 2014). Thus, changing the activities of Rho GTPases and/or their downstream effectors can severely alter cellular responses to substrate stiffness.
In recent years, it has become clear that members of the syndecan (SDCs) family, SDC1 and SDC4, are also involved in mechanosensing and mechanotransduction (Baeyens et al., 2014; Bellin et al., 2009; Chronopoulos et al., 2020; Huang et al., 2015; Jiang et al., 2020; Julien et al., 2007; Le et al., 2018) as well as obesity (De Luca et al., 2019; Kasza et al., 2014; Reizes et al., 2008). SDCs are type-I transmembrane glycoproteins belonging to the family of heparan sulfate proteoglycans that also include cell-surface glycosylphosphatidylinositol-anchored proteins (glypicans) and secreted proteoglycans found in basement membranes (agrin, collagen XVIII, and perlecan) (Sarrazin et al., 2011). Four SDC proteins, SDC1 to 4, are present in vertebrates, with SDC4 being distributed ubiquitously and the others having a tissue-specific expression pattern (Chakravarti and Adams, 2006). SDC4 is also the only mammalian syndecan that promotes focal adhesion assembly around pre-existing integrin clusters on fibronectin (Echtermeyer et al., 1999; Woods and Couchman, 2001). All SDCs are characterized by an extracellular domain (ectodomain) with attachment sites for glycosaminoglycan (GAG) chains (e.g. heparan sulfate and chondroitin sulfate) that mediate interactions with a wide array of ECM components, including fibronectin, laminins, and fibrillar collagens (Sarrazin et al., 2011). The ectodomain of all SDCs is constitutively released from the cell surface by proteolytic cleavage in a process known as ectodomain shedding that is mediated by matrix metalloproteinases (Manon-Jensen et al., 2013). Once released from the cell surface, the ectodomains may act as paracrine or autocrine effectors, or compete with cell surface receptors for the same ligand (Manon-Jensen et al., 2010). SDCs also contain a highly conserved transmembrane domain and a short cytoplasmic tail (Sarrazin et al., 2011). It is through their cytoplasmic domain, which includes binding sites for cytoskeletal proteins and protein kinases, that members of the SDC family can control cell behavior in synergy with the integrin-mediated signaling and/or independently of integrins (Morgan et al., 2007; Xian et al., 2010). Chaikof and collaborators were the first to report that mechanical stress promotes upregulation and shedding of SDC4 from vascular smooth muscle cells through differential activation of mitogen-activated protein kinase (MAPK) cascades (Julien et al., 2007; Li and Chaikof, 2002). A role for SDC4 as an initiator of cellular mechanotransduction without the direct extracellular binding of integrins was later revealed by Bellin and coworkers using NIH 3T3 fibroblasts (Bellin et al., 2009). Their study demonstrated not only that SDC4 can recruit FA proteins to sites of syndecan-specific cellular attachments, but also that mechanically induced SDC4 activation increases the phosphorylation of extracellular signal-regulated kinase (ERK) through the actin cytoskeleton (Bellin et al., 2009). More recently, an underlying mechanism for the role of SDC4 in cell mechanics, which, however, requires synergistic integrin activation, has been reported by (Chronopoulos et al., 2020) who integrated biophysical, cell biology, and computational techniques for their study. Based on their findings, it appears that SDC4 responds to mechanical tension by inducing Rho-dependent adaptive cell stiffening via engagement of a phosphoinositide 3-kinase (PI3K)/kindlin-2/β1integrin axis as well as modulating YAP activation (Chronopoulos et al., 2020). Overall, these data imply a major function for SDC4 in mechanotranduction at the cell-matrix interface.
3.4.2. Mechanotransduction signals in adipocytes
Adipocytes may experience various physical forces in vivo, including shear stress and tensile and compressive forces (Lee and Kuo, 2013). These forces can lead to intracellular metabolic changes and the activation of mechanotransduction pathways that promote the formation of new adipocytes and the secretion of immune cell recruitment factors (Lee and Kuo, 2013). As previously reviewed and discussed by (Shoham and Gefen, 2012), studies in cell culture, animal models, and humans consistently suggest that, while static stretching enhances adipogenesis, dynamic (cyclic stretching and vibration) mechanical stimuli inhibit adipogenesis and reduces body fat. Despite this progress, the mechanotransduction pathways activated by the mechanical stimuli have not been fully characterized yet. As mentioned above (see ECM remodeling regulates AT growth sections), a change in cell shape marks the conversation of the fibroblast-like preadipocytes into the rounded form of the LD-rich adipocyte during the process of adipogenesis. Elegant work performed by Shoham and collaborators (Shoham et al., 2014), who used atomic force microscopy, interferometric phase microscopy, and finite element simulations to verify the experimental findings, demonstrated that the change in cell shape occurring during differentiation is accompanied by an increase in adipocyte stiffness that is triggered by the production and accumulation of lipids in LD. This, in turn, promotes changes in intracellular strain/stress distribution and in cytoskeletal rearrangements that, ultimately, lead to alterations in ECM composition (Shoham et al., 2014). In this regard, several studies have shown that activation of RhoA/ROCK signaling and the resulting less organized cytoskeleton and low tension is necessary for the induction of adipogenic differentiation in 3T3- L1 preadipocytes (reviewed by (Lee and Kuo, 2013). However, the role of RhoA/ROCK in AT is not only limited to adipogenesis but extends also to mature adipocytes. Long-lasting (72 hours) stretching of 3T3-L1 mature adipocytes grown on a collagen-coated silicon substratum up to 120% of initial diameter has been reported to promote Rho-kinase activation and stress fibers (Hara et al., 2011). Notably, the degree of mechanical stretching applied on the 3T3-L1 mature adipocytes corresponded to an ~ 20% increase in diameter of adipocytes (hypertrophic adipocytes) isolated from mice that were fed a HFD compared to mice fed a low-fat diet (LFD) (Hara et al., 2011). This increase in diameter of the adipocytes provided sufficient mechanical stress to elicit activation of the RhoA/ROCK signaling pathway, which, in turn, induced the expression of cytokines and chemokines, including monocyte chemoattractant protein-1 and tumor necrosis factor-α (TNFα) (Hara et al., 2011). On the other hand, inhibition of the RhoA/ROCK signaling pathway through pharmacological and genetic approaches led to mice with decreased adipocyte hypertrophy, reduced macrophage recruitment to AT, and lower gene expression of several adipocytokines despite being fed an HFD (Hara et al., 2011). Thus, lipid accumulation in adipocytes activates the Rho-Rho kinase signaling, partly, through the mechanical stretch. The critical role of ROCK signaling pathway in adipocyte function has been further corroborated by studies using HFP-fed adipocyte specific Rock1 mice, which revealed that ROCK1 regulates insulin signaling in the adipocyte (Lee et al., 2014).
Other candidates likely involved in mediating mechanotransduction signals in adipocytes are YAP and TAZ. As discussed in the previous section, nuclear localization of the transcriptional co-activators YAP and TAZ inhibits ECM stiffness-dependent differentiation of MSC into adipocytes (Dupont et al., 2011; Kuroda et al., 2017). On the other hand, adipogenesis is promoted when YAP and TAZ are re-localized to the cytoplasm on soft substrates (Dupont et al., 2011). However, recent research has also revealed that YAP/TAZ are activated by the proinflammatory adipokines TNFα and IL-1β in both human and mouse mature white adipocytes to promote adipocyte survival during WAT expansion (Wang et al., 2020). As argued by Wang and collaborators (Wang et al., 2020), activation of the YAP/TAZ signaling pathway in the adipocyte is likely induced to regulate the balance between adipocyte death and the formation of new adipocytes during obesity. In this regard, it is important to mention that computational modeling studies have revealed that large tensile strains generated by the production of LDs in mature adipocytes can be projected onto neighboring immature cells through the deformation of their plasma membrane (Ben-Or Frank et al., 2015). This finding is notable because it implies that adipocyte hypertrophy may influence the biomechanical microenvironment of neighboring cells in early-stage differentiation and thereby their fate (Ben-Or Frank et al., 2015).
The discovery that YAP/TAZ activation is induced by pro-inflammatory cytokines is also in line with previous evidence that an appropriate pro-inflammatory response at the level of the adipocyte, particularly activation of TNFα, is required for healthy WAT expansion and remodeling (Wernstedt Asterholm et al., 2014). The subtle balance between lipogenesis and lipolysis in adipocytes is critical for maintaining systemic energy homeostasis and insulin sensitivity and, therefore, it is highly regulated by numerous extracellular and intracellular stimuli (reviewed by (Luo and Liu, 2016). Elevation of TNFα levels, induced by β-adrenoreceptors (β-AR) stimulation (Orban et al., 1999), is a key step involved in the control of adipocyte lipid metabolism and lipolysis (Cawthorn and Sethi, 2008). TNFα can impair glucose uptake into adipocytes and inhibit the uptake of free fatty acids (Cawthorn and Sethi, 2008). Moreover, studies in human adipocytes revealed that TNFα promotes lipolysis through activation of ERK and elevation of the intracellular cyclic AMP (cAMP)-protein kinase A (PKA) pathway, which, in turn, leads to phosphorylation of perilipin-1 (PLIN1) (a protective coat protein until phosphorylated) and hormone-sensitive lipase (HSL), with consequent translocation of phosphorylated HSL from the cytosol to the LD (Zhang et al., 2002). TNFα can also destabilize the interaction between the insulin receptor and caveolin-1 (Cav-1) in mature adipocytes (Kabayama et al., 2007). Cav-1 is a component of caveolae, which are mechanosensitive membrane invaginations linking mechanotransduction pathways to actin-controlled changes in tension (Echarri and Del Pozo, 2015), and is found in high abundance in AT where it plays a role in insulin signaling through direct binding to the insulin receptor (Nystrom et al., 1999) and the modulation of lipolysis and LD formation (Cohen et al., 2004). Together, these observations strongly suggest that mechanical signals and molecular pathways regulating the balance between lipogenesis and lipolysis in adipocytes are highly interconnected. This idea is corroborated by evidence from studies performed by Pellegrinelli and collaborators (Pellegrinelli et al., 2014) who used decellularized material of AT (dMAT) that were embedded in a peptide hydrogel to assess the impact of increased fibrosis on adipocytes. They reported that, when cultured with dMAT from obese individuals or subjected to compressive forces, adipocytes displayed reduced lipolysis and increased production of pro-inflammatory cytokines and fibrotic mediators. This phenotypic profile was induced by the activation of mechanosensitive molecules, such as integrins (Pellegrinelli et al., 2014). Along these lines, it has been further shown that β1 and β3 integrins interact with the insulin receptor in subcutaneous white adipocytes, and blunting their activity in the mature adipocyte through genetic inactivation of the Kindlin-2 protein, a regulator of integrin activation, results in lipodystrophy due to reduced insulin signaling (Gao et al., 2019; Ruiz-Ojeda et al., 2021).
A role in promoting adipocyte survival and maintaining insulin sensitivity during healthy AT expansion is also played by the mechanosensor molecule FAK. Studies in mice have revealed that knockout (KO) of Ptk2 (encoding FAK1) specifically in adipocytes leads to animals with increased adipocyte cell death and inflammation as well as insulin resistance as a result of caloric excess (Luk et al., 2017). The mechanism behind these findings, which were replicated in the offspring of adipocyte-specific Ptk2 KO mice crossed over genetically induced obese mice, involves increased activation of the p53 pro-apoptotic activity and decreased phosphorylation of ERK (Luk et al., 2017). It is well-established that death of adipocytes, resulting from apoptosis or necrosis, promotes macrophage infiltration and the formation of crown-like structures (CLS), which are composed of macrophages surrounding dead or dying adipocytes (Cinti et al., 2005) and are associated with AT inflammation and insulin resistance in obesity (Strissel et al., 2007). In this regard, we recently reported that deletion of the Sdc4 gene in mice promoted sex-specific metabolic derangements associated with diet-induced obesity (De Luca et al., 2019). Specifically, HFD-fed Sdc4 KO female mice, but not males, displayed increased levels of plasma total cholesterol, triglyceride, and glucose, as well as reduced whole-body insulin sensitivity. Additionally, they had increased adipocyte size, macrophage infiltration, and CLS in the vWAT (De Luca et al., 2019). The deletion of Sdc4 was not specific to the adipocytes and it is, therefore, not possible to conclude that the effects on adipocyte size and macrophage infiltration are due to an adipocyte-mediated molecular mechanism. However, the results in mice echo our previous findings in Drosophila melanogaster showing that flies with decreased expression of the Syndecan gene in the fat body (the fly equivalent of mammalian adipose tissue and liver) had increased fat levels and reduced phosphorylation levels of prosurvival mediators, Akt and ERK (Eveland et al., 2016). Furthermore, cell culture studies have demonstrated that the SDC4 protein is expressed in mature white adipocytes and its synthesis and ectodomain shedding is regulated by insulin (Reizes et al., 2006). These studies also indicate that shed adipocyte syndecans associate with the lipoprotein lipase and stabilize its activity (Reizes et al., 2006). Together with the solid evidence that SDC4 acts as a cellular mechanosensory and mechanotransmitter (Chronopoulos et al., 2020), these observations suggest that SDC4 is involved in mechanotransduction and lipid metabolism in adipocytes. The reason behind the sex-specific effects of the Sdc4 deletion in mice is still unknown, but it is important to point out that WAT displays a large number of significant differences in gene expression between the sexes in both mice (Yang et al., 2006) and humans (Gershoni and Pietrokovski, 2017). Among the sexually dimorphic genes identified in the mouse WAT, there are those encoding actinins, cadherins, and calcium channel subunits (Yang et al., 2006), which are molecules involved in cell–matrix and cell–cell adhesions and, therefore, likely to functionally interact with SDC4 (Gopal et al., 2017). Moreover, elevated levels of SDC4 gene transcription are often observed in estrogen receptor-positive breast cancer cells, suggesting that sex hormones may play a role in its regulation (Lendorf et al., 2011).
As depicted in Figure 1, the studies reviewed in this section suggest a critical role of adipocyte mechanotransduction signaling pathways in healthy WAT growth in normal physiological conditions. However, when the “mechanoreciprocity” that maintain tensional homeostasis is lost due to factors that perturb ECM remodeling and induce abnormal ECM deposition and stiffness, like obesity, genetics, and aging (see Aging, mechanotransduction, and adipocyte sections and Figure 2), then adipocyte cell death is promoted (Takao et al., 2019). This induces monocyte infiltration to manage tissue remodeling. If the inflammatory response is sustained, the chronic inflammation leads to massive fibrosis through excess collagen production mainly by the fibro-inflammatory stromal cells, with consequent unhealthy WAT expansion that, in turn, is associated with systemic insulin resistance (Smith et al., 2019).
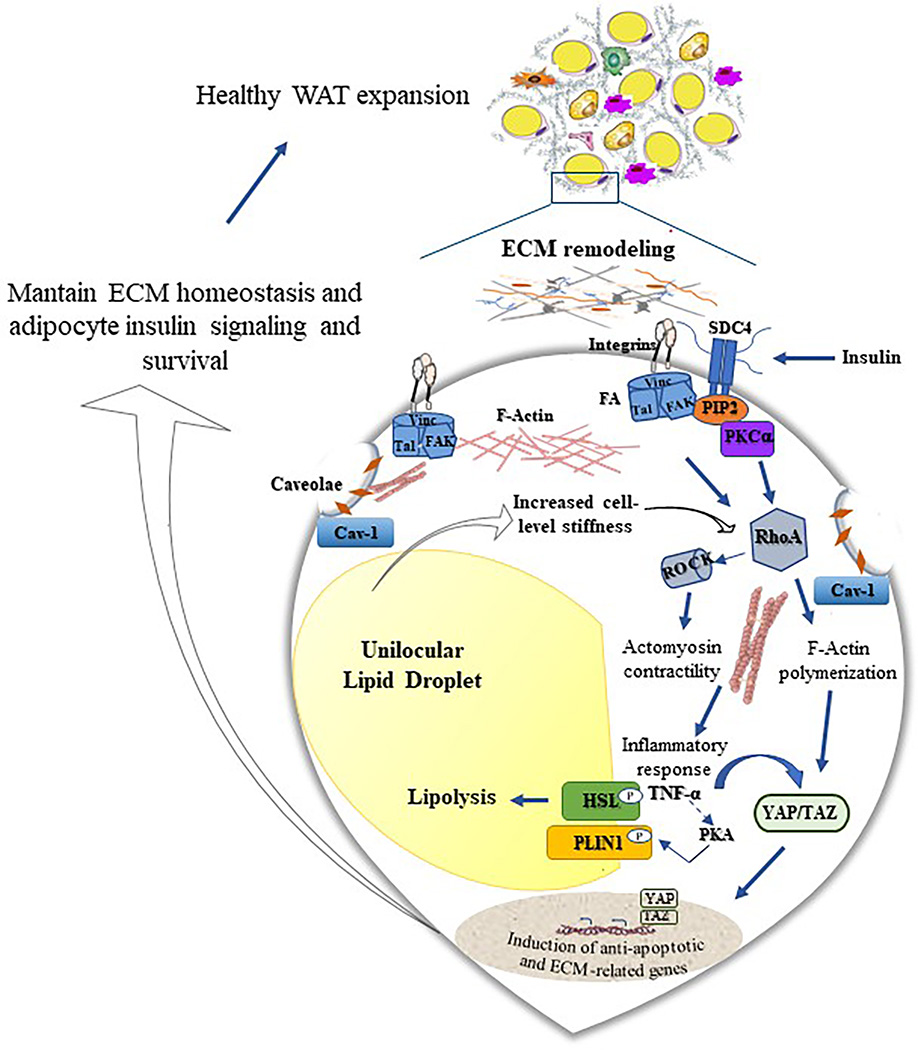
Figure 1. Schematic representation of the mechanosensing machinery in the adipocyte and its impact on white adipose tissue (WAT) expansion.
Mechanical properties of the ECM are sensed by the adipocyte via a range of membrane-bound mechanosensors, including integrins, syndecan 4 (SDC4), focal adhesion (FA) complex proteins, and Caveolin-1 (Cav-1), the principal component of caveolae in adipocytes. These mechanosensors dynamically regulate the transfer of extracellular/intracellular mechanical signals inside/outside the cell. ECM-mediated activation of the FA protein vinculin (Vinc), talin (Tal), and focal adhesion kinase (FAK) produces downstream signaling events regulating the activity of Rho family member A (RhoA). RhoA activation, which is also mediated by increased cell-level stiffness and SDC4-induced phosphorylation of extracellular signal-regulated kinase, leads to activation of the Ras homologous (Rho)/Rho-associated kinase (ROCK) signaling pathway and a consequent increase in tension of the actomyosin cytoskeleton. This promotes the nuclear translocation of the transcriptional factors Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) resulting in transcriptional control of genes that sustain ECM homeostasis and adipocyte survival. The increased cellular tension also induces the expression of tumor necrosis factor-α (TNFα), which in turn, stimulates lipolysis through activation of cAMP-dependent protein kinase A (PKA) and its phosphorylation of perilipin-1 (PLIN1) and hormone-sensitive lipase (HSL). The interaction between phosphorylated PLIN1 and HSL allows HSL to translocate from the cytosol to the lipid droplet where it promotes lipolysis. Activation of lipolysis may, in turn, counterbalance the adipocyte hypertrophy-induced cellular tension.
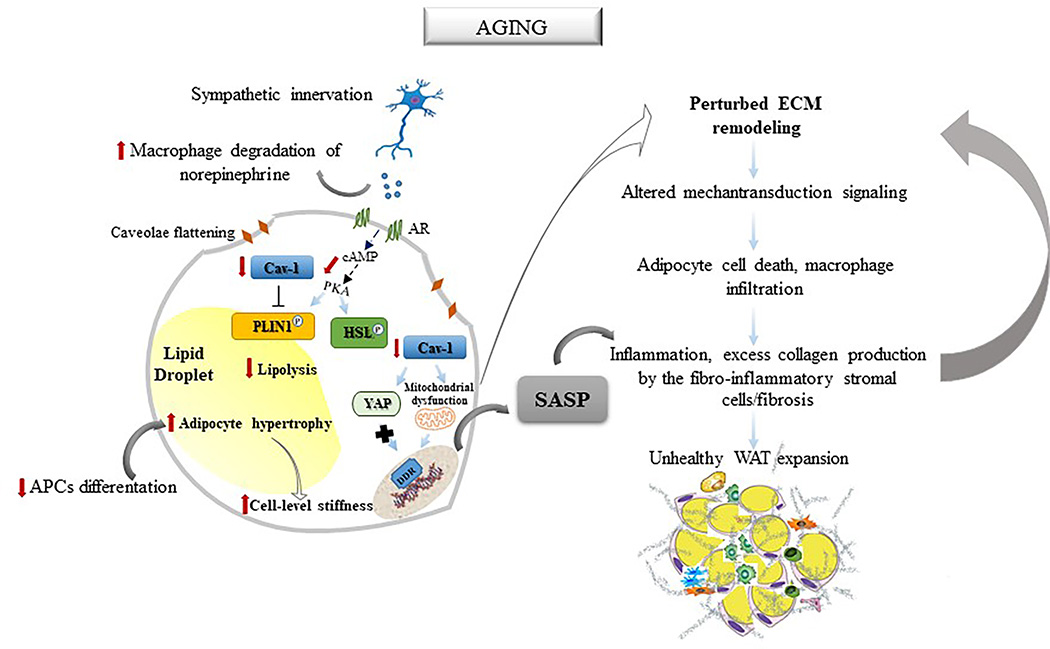
Figure 2. Schematic illustration of the hypothetical model through which aging may affect the mechanoreciprocity process in the white adipocyte cell.
Aging is characterized by reduced catecholamine-stimulated lipolysis caused by lowered bioavailability of norepinephrine by adipose tissue macrophages and decreased activation of the hormone-sensitive lipase (HSL)/perilipin-1 (PLIN1) complex. Age-related reduction of caveolin-1 (Cav-1) prevents caveolae formations in adipocytes and leads to an increase in net collagen synthesis. Moreover, reduced Cav-1 levels reduce actin-dependent mechanoregulation of Yes-associated protein (YAP) activity and induce mitochondrial dysfunction-associated senescence, which, in turn, promotes acquisition of a senescence-associated secretory phenotype (SASP). Together with the age-related decline in adipose progenitor cells (APCs) ability to differentiate into adipocytes and increased adipocyte hypertrophy and stiffness, these events disrupt the feedback loops that regulate extracellular matrix (ECM) composition and mechanical properties. This alters the adipocyte mechanotransduction signaling resulting in a vicious cycle that ultimately leads to adipocyte death, macrophage infiltration, and excess collagen production by the fibro-inflammatory stromal cells, an additional source of ECM perturbation. The senescence-associated secretory phenotype (SASP) produced by the senescent adipocyte contributes to the chronic inflammation and fibrosis that are responsible for the unhealthy expansion of the white adipose tissue (WAT).
4. Aging, mechanotransduction, and adipocyte
Aging is characterized by significant changes in the regional distribution of WAT that consist of a relative loss of sWAT, particularly from the limbs, and an accumulation of fat in abdominal sWAT and vWAT (Kuk et al., 2009). These fat distribution shifts are accompanied by an inadequate capacity to store lipids by hypertropic adipocytes and a subsequent conspicuous deposition of lipids in nonadipose tissues, which causes systemic lipotoxicity, a process that contributes to adverse metabolic and cardiovascular outcomes (reviewed by (De Carvalho et al., 2019; Palmer and Kirkland, 2016). The age-related loss of sWAT appears to occur through a decline in the function of APCs and their potential to differentiate into adipocytes as well as via a considerable accumulation of senescent cells (Palmer and Kirkland, 2016). Senescent cells are metabolically active cells that, however, are resistant to mitogenic stimulation (Shelton et al., 1999). While the growth arrest is achieved via either p16INK4A or the p53/p21CIP1 pathway as key executers of cell-cycle arrest, the senescent phenotype is triggered by increased production of reactive oxidative species (ROS) followed by a chronically active DNA damage response (DDR), which promotes an irreversible cell cycle arrest by activating the p53/p21CIP1 pathway (Tchkonia et al., 2013b). Senescent cells are also characterized by widespread changes in chromatin organization and gene expression that ultimately lead to the secretion of numerous pro-inflammatory cytokines, chemokines, and growth factors (a phenotype termed the “senescence-associated secretory phenotype” or SASP) (Tchkonia et al., 2013b). The number of senescent cells increases exponentially with age (Tchkonia et al., 2010) and signals from the ECM can induce cellular senescence (Choi et al., 2011). In addition, senescent cells participate in tissue remodeling through the release, accumulation, and modification of ECM components, including the production and secretion of multiple MMP proteins and fibronectin, and the recruitment of macrophages (Coppe et al., 2010; De Luca, 2019; Elder and Emmerson, 2020; Mavrogonatou et al., 2019). Although little is still known regarding the role of adipose tissue senescence in ECM quality/composition/stiffness, the enhanced senescence-induced secretion of MMPs and fibronectin could produce ECM reorganization and changes in behavior of surrounding cells. For instance, adipogenesis in obesity requires an interplay between differentiating adipocytes, stromal cells, and blood vessels (Nishimura et al., 2007). The ECM plays an essential role in the crosstalk between APCs and endothelial cells in AT, with ECM components released by microvascular endothelial cells promoting preadipocyte differentiation (Varzaneh et al., 1994). This is particularly significant considering that the maintenance of the mechanical and mechanotransduction integrity of tissues rely on the interdependence between the cell-ECM traction force and the tension that it exerts at the cell-cell interface (Maruthamuthu et al., 2011). Thus, changes in ECM mechanical properties can directly impact the force levels at cell-cell adhesions and therefore modulate the strength of adhesion and influence intercellular dynamics (Tang, 2018).
As the APCs present in adipose SVF, mature adipocytes undergo senescence (Smith et al., 2021). Evidence suggests that adipocyte senescence results from a DNA damage response to elevated oxidative stress (Chen et al., 2015). Additionally, it has been reported that senescent endothelial cells in adipose SVF can induce senescence features in mature adipocytes through SASP (Barinda et al., 2020). Yet, the senescent characteristics of adipocytes and molecular mechanisms responsible for triggering the senescence program remain poorly understood. Recent data collected from an in vitro model of adipocyte aging revealed that, compared to young mature adipocytes, older adipocytes were characterized by not only an increase in senescence but also a higher expression level of profibrotic genes (Zoico et al., 2020). Notably, the ECM alterations were also accompanied by a significant decrease in expression of the CAV1 gene (Zoico et al., 2020), suggesting that reduced levels of Cav-1 could contribute to adipocyte dysfunction with age.
As mentioned above, Cav-1 is a small integral membrane protein that is essential for caveolae formation in adipocytes (Pilch et al., 2011). Earlier studies in mice revealed that genetic deficiency of Cav1 leads to an imbalance between degradation and replacement of collagen in WAT as a result of an increase in net collagen synthesis (Martin et al., 2012). Interestingly, this perturbation in ECM remodeling correlated with reduced lipolysis in adipocytes due to a decrease in the levels of the LD protein PLIN1 and increased susceptibility to cell death (Martin et al., 2012). Catecholamine-stimulated lipolysis is significantly reduced (50%) in healthy, nonobese elderly subjects compared to young subjects because of a declined function of the HSL complex (Lonnqvist et al., 1990). Given that translocation of HSL to the LD requires phosphorylation of PLIN1 (Sztalryd et al., 2003), which, in turn, is controlled by Cav-1 (Cohen et al., 2004), it is possible that the age-associated impairment in catecholamine-stimulated lipolysis is, in part, caused by reduced Cav-1 levels. Of note, studies in mice revealed that AT macrophages are also involved in the age-related reduction in adipocyte lipolysis by decreasing the bioavailability of norepinephrine and thereby its available concentration in adipocytes (Camell et al., 2017). Thus, this could further exacerbate the situation (Figure 2).
Evidence from both in vitro and in vivo studies has demonstrated that Cav-1 regulates mechanotransduction response to ECM stiffness through actin-dependent control of YAP (Moreno-Vicente et al., 2018). Loss of Cav-1 can also prevent caveolae formation in adipocytes (Razani et al., 2002) and the flattening and rapid disassembly of caveolae is a process that enables cells to respond to surges in membrane tension occurring during mechanical stress (Sinha et al., 2011). Furthermore, it has been reported that Cav-1 deficiency promotes senescence in human fibroblasts via mitochondrial dysfunction and inactivation of SIRT1, which, in turn, leads to the activation of the p53/p21 pathway (Yu et al., 2017). Adipocytes respond to HFD by adopting a fibroblast-like phenotype characterized by enhanced expression of ECM, FA, and cytoskeletal-related genes, and reduced expression of genes involved in mitochondrial function (Jones et al., 2020). Thus, as illustrated in Figure 2, we propose that an age-related reduction of Cav-1 levels may not only lead to caveolae flattening but also to mitochondrial dysfunction-associated senescence, as seen in fibroblasts. At the same time, the age-related decline in APCs capacity to differentiate into adipocytes in sWAT promotes an increase in adipocyte hypertrophy (Palmer and Kirkland, 2016), with consequent cell stiffness augmentation. However, because of perturbation of ECM remodeling resulting from reduced Cav-1 levels and the adipocyte secretion of SASP factors, adipocytes lose their ability to restore mechanical homeostasis. The disrupted mechanoreciprocity response in the adipocyte eventually leads to cell death and macrophage infiltration. The situation is further exacerbated by the contribution of SASPs to chronic inflammation and profibrotic changes of fibroblast and other SVF cells that promote unhealthy expansion of sWAT (Smith et al., 2021) (Figure 2).
In addition to changes in WAT, a loss of brown and beige adipocytes also happens with advancing age (Zoico et. al, 2019). Activation of β-adrenergic receptors in response, for instance, to cold stress stimulates BAT thermogenesis in humans (Blondin et al., 2020; Gavrila et al., 2017). Moreover, it has been reported that actomyosin stiffness induced by β-adrenergic stimulation is required for the induction of thermogenic capacity in brown adipocytes through YAP/TAZ activation and translocation into the nucleus (Tharp et al., 2018). Thus, age-related changes resulting in altered mechanosensitive signals may also be partly responsible for the impairment of brown adipocyte regenerative capacity occurring with age.
5. Concluding Remarks
In conclusion, there is a large body of literature showing that the composition and mechanical properties of the ECM have an essential role in AT remodeling and healthy expansion. Most research so far has focused on the influence of matrix stiffness on adipogenesis, and we refer the interested readers to other excellent reviews on this topic (Mor-Yossef Moldovan et al., 2019; Pope et al., 2016; Yuan et al., 2015). In this review article, we sought to draw attention to the molecules and mechanisms of mechanotransduction involved in mature adipocyte function and fate. Although the experimental data is still very limited, current evidence suggests that the cellular pathways involved in the adaptative mechanoreciprocity process and lipid metabolism of adipocytes are highly interconnected and that their coordinated regulation is critical to preserve adipocyte function and survival under physiological conditions. Because of this, loss of mechanoreciprocity may result in adipocyte dysfunction and impaired AT expansion, which is intimately linked to the development of systemic insulin resistance, T2D, and cardiovascular disease (Smith et al., 2019; Vishvanath and Gupta, 2019). Most of our basic understanding of the effects of disruption of the mechanoreciprocity process in adipocytes at the organismal level comes from in vivo studies using adipocyte-specific transgenic and KO mice models of genes related to mechanotransduction pathways (see Table 1). Molecular genetic studies have also reported significant associations of polymorphisms in ECM-related genes with human obesity and diabetes traits (Chun et al., 2010; Saravani et al., 2017), highlighting its clinical relevance. As such, future success in the prevention and treatment of these chronic diseases is, in part, dependent on a deeper understanding of the molecular mechanisms that regulate the mechanoreciprocity process in adipocytes. This includes our knowledge of the effects of aging on adipocyte ECM stiffness, which remains largely unknown due to the limited availability of techniques allowing the measurement of mechanical forces generated by adipocyte activities in vivo. The application of novel and more advanced tools, such as biocompatible and implantable optical fibers and waveguides (Choi et al., 2013; Nazempour et al., 2018), will likely be valuable in moving the field forward.
Table 1.
Summary of mouse studies in which genes related to cellular mechanotransduction are altered in mature adipocytes
| Target gene/protein | Adipocyte-Specific Mouse Model | Phenotypes of genetically altered mice vs control mice | References |
|---|---|---|---|
| Rhoa/Transforiming protein RhoA | HFD-induced obese transgenic mice overexpressing dominant-negative allele | Attenuated weight gain, adipocyte hypertrophy, and macrophage infiltration Lower serum free fatty acids Improved systemic insulin sensitivity Higher fasting glucose and insulin levels |
Hara et al., 2011 |
| Rockl/Rho-associated protein kinase 1 | HFD-induced obese KO mice | No differences in weight gain, body composition, or biochemical parameters Improved systemic insulin sensitivity Enhanced adipocyte insulin signaling |
Lee et al., 2014 |
| Yapl and Wwtrl/Transcriptional coactivator YAP1 and TAZ | HFD-induced obese double KO mice | Attenuated weight gain No differences in fasting glucose levels Improved glucose tolerance Lipodystrophy Increased adipocyte death and macrophage infiltration Increased adipogenesis |
Wang et al., 2020 |
| Ptk2/Focal Adhesion Kinase 1 | NCD-fed KO mice HFD-induced obese KO mice |
No differences in total body weight Increased adipocyte death and macrophage infiltration Elevated fasting glucose levels and insulin resistance Hyperlipidemia Attenuated weight gain Enhanced adipocyte death, macrophage infiltration, and fibrosis Higher fasting glucose levels and insulin resistance |
Luk et al., 2017 |
| Fermt2/Kindlin-2 | NCD-fed KO mice HFD-induced obese KO mice |
Severe lipodystrophy, AT fibrosis and inflammation, adipocyte death Attenuated weight gain, smaller size, and reduced fat mass Adipocyte death, AT fibrosis and inflammation Higher fasting glucose levels, glucose intolerance, peripheral insulin resistance Hyperlipidemia and massive fatty liver |
Gao et al., 2019 Ruiz-Ojeda et al., 2021 |
| Itgbl/Integrin beta-1 | HFD-induced obese KO mice | Reduced weight gain and fat mass No differences in fasting glucose and insulin levels and glucose tolerance |
Ruiz-Ojeda et al., 2021 |
Abbreviations: HFD, High-fat diet; NCD, Normal chow diet; KO, knockout; AT, Adipose tissue
Acknowledgments
We acknowledge the financial support of U.S. National Institutes of Health grant R21AG063197 to M.D.
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander CM, Kasza I, Yen CL, Reeder SB, Hernando D, Gallo RL, Jahoda CA, Horsley V and MacDougald OA, 2015. Dermal white adipose tissue: a new component of the thermogenic response. J Lipid Res. 56, 2061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N, Tham JC, Welbourn R, Shore AC, Kos K and Winlove CP, 2013. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab. 305, E1427–35. [DOI] [PubMed] [Google Scholar]
- Ambrosi D, Ben Amar M, Cyron CJ, DeSimone A, Goriely A, Humphrey JD and Kuhl E, 2019. Growth and remodelling of living tissues: perspectives, challenges and opportunities. J R Soc Interface. 16, 20190233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YH, Mui KL, Hsu BY, Liu SL, Cretu A, Razinia Z, Xu T, Pure E and Assoian RK, 2014. A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci Signal. 7, ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J and Schwartz MA, 2014. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci U S A. 111, 17308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinda AJ, Ikeda K, Nugroho DB, Wardhana DA, Sasaki N, Honda S, Urata R, Matoba S, Hirata KI and Emoto N, 2020. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat Commun. 11, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin RM, Kubicek JD, Frigault MJ, Kamien AJ, Steward RL Jr., Barnes HM, Digiacomo MB, Duncan LJ, Edgerly CK, Morse EM, Park CY, Fredberg JJ, Cheng CM and LeDuc PR, 2009. Defining the role of syndecan-4 in mechanotransduction using surface-modification approaches. Proc Natl Acad Sci U S A. 106, 22102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Or Frank M, Shoham N, Benayahu D and Gefen A, 2015. Effects of accumulation of lipid droplets on load transfer between and within adipocytes. Biomech Model Mechanobiol. 14, 15–28. [DOI] [PubMed] [Google Scholar]
- Bernot D, Barruet E, Poggi M, Bonardo B, Alessi MC and Peiretti F, 2010. Downregulation of tissue inhibitor of metalloproteinase-3 (TIMP-3) expression is necessary for adipocyte differentiation. J Biol Chem. 285, 6508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, Miard S, Jespersen NZ, Kooijman S, Boon MR, Fortin M, Phoenix S, Frisch F, Guerin B, Turcotte EE, Haman F, Richard D, Picard F, Rensen PCN, Scheele C and Carpentier AC, 2020. Human Brown Adipocyte Thermogenesis Is Driven by beta2-AR Stimulation. Cell Metab. 32, 287–300 e7. [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Sengenes C, Portolan G, Galitzky J and Lafontan M, 2001. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 50, 2080–6. [DOI] [PubMed] [Google Scholar]
- Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A, Kao YR, Cho S, Harada T, Shin JW and Discher DE, 2014. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol. 24, 1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, Goldberg EL, Youm YH, Brown CW, Elsworth J, Rodeheffer MS, Schultze JL and Dixit VD, 2017. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature. 550, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn WP and Sethi JK, 2008. TNF-alpha and adipocyte biology. FEBS Lett. 582, 117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti R and Adams JC, 2006. Comparative genomics of the syndecans defines an ancestral genomic context associated with matrilins in vertebrates. BMC Genomics. 7, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Garcia-Barrio MT and Chen YE, 2020. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 40, 1094–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Harris RA, Hatahet Z and Chou KM, 2015. Ablation of XP-V gene causes adipose tissue senescence and metabolic abnormalities. Proc Natl Acad Sci U S A. 112, E4556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe SS, Huh JY, Hwang IJ, Kim JI and Kim JB, 2016. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne). 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HR, Cho KA, Kang HT, Lee JB, Kaeberlein M, Suh Y, Chung IK and Park SC, 2011. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging Cell. 10, 148–57. [DOI] [PubMed] [Google Scholar]
- Choi M, Choi JW, Kim S, Nizamoglu S, Hahn SK and Yun SH, 2013. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat Photonics. 7, 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulos A, Thorpe SD, Cortes E, Lachowski D, Rice AJ, Mykuliak VV, Rog T, Lee DA, Hytonen VP and Del Rio Hernandez AE, 2020. Syndecan-4 tunes cell mechanics by activating the kindlin-integrin-RhoA pathway. Nat Mater. 19, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M and Burridge K, 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 133, 1403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Inoue M, Morisaki H, Yamanaka I, Miyamoto Y, Okamura T, Sato-Kusubata K and Weiss SJ, 2010. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 59, 2484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS and Obin MS, 2005. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 46, 2347–55. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE and Lisanti MP, 2004. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 53, 1261–70. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A and Campisi J, 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankel SN, Svard J, Mattha S, Claussnitzer M, Kloting N, Glunk V, Fandalyuk Z, Grytten E, Solsvik MH, Nielsen HJ, Busch C, Hauner H, Bluher M, Skurk T, Sagen JV and Mellgren G, 2014. COL6A3 expression in adipocytes associates with insulin resistance and depends on PPARgamma and adipocyte size. Obesity (Silver Spring). 22, 1807–13. [DOI] [PubMed] [Google Scholar]
- Datta R, Podolsky MJ and Atabai K, 2018. Fat fibrosis: friend or foe? JCI Insight. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho FG, Justice JN, Freitas EC, Kershaw EE and Sparks LM, 2019. Adipose Tissue Quality in Aging: How Structural and Functional Aspects of Adipose Tissue Impact Skeletal Muscle Quality. Nutrients. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, 2019. The role of the cell-matrix interface in aging and its interaction with the renin-angiotensin system in the aged vasculature. Mech Ageing Dev. 177, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Vecchie D, Athmanathan B, Gopalkrishna S, Valcin JA, Swain TM, Sertie R, Wekesa K, Rowe GC, Bailey SM and Nagareddy PR, 2019. Genetic Deletion of Syndecan-4 Alters Body Composition, Metabolic Phenotypes, and the Function of Metabolic Tissues in Female Mice Fed A High-Fat Diet. Nutrients. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P and Clement K, 2010. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 59, 2817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Jahoda CA, Chuong CM, Watt FM and Horsley V, 2014. Defining dermal adipose tissue. Exp Dermatol. 23, 629–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N and Piccolo S, 2011. Role of YAP/TAZ in mechanotransduction. Nature. 474, 179–83. [DOI] [PubMed] [Google Scholar]
- Echarri A and Del Pozo MA, 2015. Caveolae - mechanosensitive membrane invaginations linked to actin filaments. J Cell Sci. 128, 2747–58. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Baciu PC, Saoncella S, Ge Y and Goetinck PF, 1999. Syndecan-4 core protein is sufficient for the assembly of focal adhesions and actin stress fibers. J Cell Sci. 112 ( Pt 20), 3433–41. [DOI] [PubMed] [Google Scholar]
- Efremova A, Senzacqua M, Venema W, Isakov E, Di Vincenzo A, Zingaretti MC, Protasoni M, Thomski M, Giordano A and Cinti S, 2020. A large proportion of mediastinal and perirenal visceral fat of Siberian adult people is formed by UCP1 immunoreactive multilocular and paucilocular adipocytes. J Physiol Biochem. 76, 185–192. [DOI] [PubMed] [Google Scholar]
- Elder SS and Emmerson E, 2020. Senescent cells and macrophages: key players for regeneration? Open Biol. 10, 200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epro G, Mierau A, Doerner J, Luetkens JA, Scheef L, Kukuk GM, Boecker H, Maganaris CN, Bruggemann GP and Karamanidis K, 2017. The Achilles tendon is mechanosensitive in older adults: adaptations following 14 weeks versus 1.5 years of cyclic strain exercise. J Exp Biol. 220, 1008–1018. [DOI] [PubMed] [Google Scholar]
- Eveland M, Brokamp GA, Lue CH, Harbison ST, Leips J and De Luca M, 2016. Knockdown expression of Syndecan in the fat body impacts nutrient metabolism and the organismal response to environmental stresses in Drosophila melanogaster. Biochem Biophys Res Commun. 477, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer M and Bluher M, 2015. Adipokines in health and disease. Trends Pharmacol Sci. 36, 461–70. [DOI] [PubMed] [Google Scholar]
- Florez-Duquet M and McDonald RB, 1998. Cold-induced thermoregulation and biological aging. Physiol Rev. 78, 339–58. [DOI] [PubMed] [Google Scholar]
- Gao H, Guo Y, Yan Q, Yang W, Li R, Lin S, Bai X, Liu C, Chen D, Cao H and Xiao G, 2019. Lipoatrophy and metabolic disturbance in mice with adipose-specific deletion of kindlin-2. JCI Insight. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparski AN and Beningo KA, 2015. Mechanoreception at the cell membrane: More than the integrins. Arch Biochem Biophys. 586, 20–6. [DOI] [PubMed] [Google Scholar]
- Gavrila A, Hasselgren PO, Glasgow A, Doyle AN, Lee AJ, Fox P, Gautam S, Hennessey JV, Kolodny GM and Cypess AM, 2017. Variable Cold-Induced Brown Adipose Tissue Response to Thyroid Hormone Status. Thyroid. 27, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni M and Pietrokovski S, 2017. The landscape of sex-differential transcriptome and its consequent selection in human adults. BMC Biol. 15, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Guntur K, Majumdar ID, Akella S, Vishnudas VK, Sarangarajan R and Narain NR, 2016. Reduced expression of collagen VI alpha 3 (COL6A3) confers resistance to inflammation-induced MCP1 expression in adipocytes. Obesity (Silver Spring). 24, 1695–703. [DOI] [PubMed] [Google Scholar]
- Ghaben AL and Scherer PE, 2019. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 20, 242–258. [DOI] [PubMed] [Google Scholar]
- Gooch KJT, C.J., 2012. Mechanical Forces: Their Effects on Cells and Tissues, Springer-Verlag Berlin and Heidelberg GmbH & Co. KG, Berlin, Germany. [Google Scholar]
- Goossens GH, 2017. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts. 10, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Multhaupt HAB, Pocock R and Couchman JR, 2017. Cell-extracellular matrix and cell-cell adhesion are linked by syndecan-4. Matrix Biol. 60–61, 57–69. [DOI] [PubMed] [Google Scholar]
- Gregory EL, 1989. Thermoregulatory aspects of adipose tissue. Clin Dermatol. 7, 78–92. [DOI] [PubMed] [Google Scholar]
- Hadden WJ and Choi YS, 2016. The extracellular microscape governs mesenchymal stem cell fate. J Biol Eng. 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Sjostrm L, Arvidsson B, Bjorntorp P and Smith U, 1977. Body fat and adipose tissue cellularity in infants: a longitudinal study. Metabolism. 26, 607–14. [DOI] [PubMed] [Google Scholar]
- Hara Y, Wakino S, Tanabe Y, Saito M, Tokuyama H, Washida N, Tatematsu S, Yoshioka K, Homma K, Hasegawa K, Minakuchi H, Fujimura K, Hosoya K, Hayashi K, Nakayama K and Itoh H, 2011. Rho and Rho-kinase activity in adipocytes contributes to a vicious cycle in obesity that may involve mechanical stretch. Sci Signal. 4, ra3. [DOI] [PubMed] [Google Scholar]
- Harms M and Seale P, 2013. Brown and beige fat: development, function and therapeutic potential. Nat Med. 19, 1252–63. [DOI] [PubMed] [Google Scholar]
- Hausman GJ and Dodson MV, 2013. Stromal Vascular Cells and Adipogenesis: Cells within Adipose Depots Regulate Adipogenesis. J Genomics. 1, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J and Knittle JL, 1970. Cellularity of obese and nonobese human adipose tissue. Fed Proc. 29, 1516–21. [PubMed] [Google Scholar]
- Hu H, Garcia-Barrio M, Jiang ZS, Chen YE and Chang L, 2020. Roles of Perivascular Adipose Tissue in Hypertension and Atherosclerosis. Antioxid Redox Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CP, Cheng CM, Su HL and Lin YW, 2015. Syndecan-4 Promotes Epithelial Tumor Cells Spreading and Regulates the Turnover of PKCalpha Activity under Mechanical Stimulation on the Elastomeric Substrates. Cell Physiol Biochem. 36, 1291–304. [DOI] [PubMed] [Google Scholar]
- Huffman DM and Barzilai N, 2010. Contribution of adipose tissue to health span and longevity. Interdiscip Top Gerontol. 37, 1–19. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Dufresne ER and Schwartz MA, 2014. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 15, 802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KA, Donato DM, Balcioglu HE, Schmidt T, Danen EH and Koenderink GH, 2015. A guide to mechanobiology: Where biology and physics meet. Biochim Biophys Acta. 1853, 3043–52. [DOI] [PubMed] [Google Scholar]
- Jeffery E, Church CD, Holtrup B, Colman L and Rodeheffer MS, 2015. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 17, 376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XZ, Luo KH and Ventikos Y, 2020. Principal mode of Syndecan-4 mechanotransduction for the endothelial glycocalyx is a scissor-like dimer motion. Acta Physiol (Oxf). 228, e13376. [DOI] [PubMed] [Google Scholar]
- Jones JEC, Rabhi N, Orofino J, Gamini R, Perissi V, Vernochet C and Farmer SR, 2020. The Adipocyte Acquires a Fibroblast-Like Transcriptional Signature in Response to a High Fat Diet. Sci Rep. 10, 2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien MA, Wang P, Haller CA, Wen J and Chaikof EL, 2007. Mechanical strain regulates syndecan-4 expression and shedding in smooth muscle cells through differential activation of MAP kinase signaling pathways. Am J Physiol Cell Physiol. 292, C517–25. [DOI] [PubMed] [Google Scholar]
- Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y and Inokuchi J, 2007. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci U S A. 104, 13678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, MacDougald OA, Shedd TA, Nelson DW, Yen MI, Yen CL and Alexander CM, 2014. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet. 10, e1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw EE and Flier JS, 2004. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 89, 2548–56. [DOI] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S and Scherer PE, 2009. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 29, 1575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE and Katz DP, 1979. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 63, 239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konige M, Wang H and Sztalryd C, 2014. Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochim Biophys Acta. 1842, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk JL, Saunders TJ, Davidson LE and Ross R, 2009. Age-related changes in total and regional fat distribution. Ageing Res Rev. 8, 339–48. [DOI] [PubMed] [Google Scholar]
- Kuo JC, 2014. Focal adhesions function as a mechanosensor. Prog Mol Biol Transl Sci. 126, 55–73. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Wada H, Kimura Y, Ueda K and Kioka N, 2017. Vinculin promotes nuclear localization of TAZ to inhibit ECM stiffness-dependent differentiation into adipocytes. J Cell Sci. 130, 989–1002. [DOI] [PubMed] [Google Scholar]
- Lackey DE, Burk DH, Ali MR, Mostaedi R, Smith WH, Park J, Scherer PE, Seay SA, McCoin CS, Bonaldo P and Adams SH, 2014. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab. 306, E233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le V, Lee J, Chaterji S, Spencer A, Liu YL, Kim P, Yeh HC, Kim DH and Baker AB, 2018. Syndecan-1 in mechanosensing of nanotopological cues in engineered materials. Biomaterials. 155, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K and Kuo CK, 2013. Extracellular Matrix Remodeling and Mechanical Stresses as Modulators of Adipose Tissue Metabolism and Inflammation, in: Gefen A, Benayahu D (Ed.), The Mechanobiology of Obesity and Related Diseases. Studies in Mechanobiology, Tissue Engineering and Biomaterials. Springer, Cham, pp. 105–122. [Google Scholar]
- Lee MJ, Wu Y and Fried SK, 2013. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 34, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Huang H, Choi K, Lee DH, Shi J, Liu T, Chun KH, Seo JA, Lima IS, Zabolotny JM, Wei L and Kim YB, 2014. ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 306, E332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendorf ME, Manon-Jensen T, Kronqvist P, Multhaupt HA and Couchman JR, 2011. Syndecan-1 and syndecan-4 are independent indicators in breast carcinoma. J Histochem Cytochem. 59, 615–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Nogueira M, Lafitte O, Steyaert JM, Bakardjian H, Dubois B, Hampel H and Schwartz L, 2016. Mechanical stress related to brain atrophy in Alzheimer’s disease. Alzheimers Dement. 12, 11–20. [DOI] [PubMed] [Google Scholar]
- Li L and Chaikof EL, 2002. Mechanical stress regulates syndecan-4 expression and redistribution in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 22, 61–8. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao Y, Chen C, Yang L, Lee HH, Wang Z, Zhang N, Kolonin MG, An Z, Ge X, Scherer PE and Sun K, 2020. Critical Role of Matrix Metalloproteinase 14 in Adipose Tissue Remodeling during Obesity. Mol Cell Biol. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, 2018. Brown Adipose Tissue in Human Infants., in: Pfeifer A, K.M., Herzig S (Ed.), Brown Adipose Tissue. Handbook of Experimental Pharmacology. Springer, Cham. [DOI] [PubMed] [Google Scholar]
- Lieber SC, Aubry N, Pain J, Diaz G, Kim SJ and Vatner SF, 2004. Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol. 287, H645–51. [DOI] [PubMed] [Google Scholar]
- Lim CG, Jang J and Kim C, 2018. Cellular machinery for sensing mechanical force. BMB Rep. 51, 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Huang K, Li G, Wang P, Liu C, Guo C, Sun Z and Pan J, 2017. Ascorbic acid promotes 3T3-L1 cells adipogenesis by attenuating ERK signaling to upregulate the collagen VI. Nutr Metab (Lond). 14, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, DeYoung SM, Zhang M, Zhang M, Cheng A and Saltiel AR, 2005. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2, 165–77. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aron-Wisnewsky J, Marcelin G, Genser L, Le Naour G, Torcivia A, Bauvois B, Bouchet S, Pelloux V, Sasso M, Miette V, Tordjman J and Clement K, 2016. Accumulation and Changes in Composition of Collagens in Subcutaneous Adipose Tissue After Bariatric Surgery. J Clin Endocrinol Metab. 101, 293–304. [DOI] [PubMed] [Google Scholar]
- Lonnqvist F, Nyberg B, Wahrenberg H and Arner P, 1990. Catecholamine-induced lipolysis in adipose tissue of the elderly. J Clin Invest. 85, 1614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk CT, Shi SY, Cai EP, Sivasubramaniyam T, Krishnamurthy M, Brunt JJ, Schroer SA, Winer DA and Woo M, 2017. FAK signalling controls insulin sensitivity through regulation of adipocyte survival. Nat Commun. 8, 14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L and Liu M, 2016. Adipose tissue in control of metabolism. J Endocrinol. 231, R77–R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manon-Jensen T, Itoh Y and Couchman JR, 2010. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 277, 3876–89. [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Multhaupt HA and Couchman JR, 2013. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 280, 2320–31. [DOI] [PubMed] [Google Scholar]
- Mariman EC and Wang P, 2010. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 67, 1277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Fernandez-Rojo MA, Stanley AC, Bastiani M, Okano S, Nixon SJ, Thomas G, Stow JL and Parton RG, 2012. Caveolin-1 deficiency leads to increased susceptibility to cell death and fibrosis in white adipose tissue: characterization of a lipodystrophic model. PLoS One. 7, e46242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino F, Perestrelo AR, Vinarsky V, Pagliari S and Forte G, 2018. Cellular Mechanotransduction: From Tension to Function. Front Physiol. 9, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthamuthu V, Sabass B, Schwarz US and Gardel ML, 2011. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A. 108, 4708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogonatou E, Pratsinis H, Papadopoulou A, Karamanos NK and Kletsas D, 2019. Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol. 75–76, 27–42. [DOI] [PubMed] [Google Scholar]
- Michaud A, Tordjman J, Pelletier M, Liu Y, Laforest S, Noel S, Le Naour G, Bouchard C, Clement K and Tchernof A, 2016. Relevance of omental pericellular adipose tissue collagen in the pathophysiology of human abdominal obesity and related cardiometabolic risk. Int J Obes (Lond). 40, 1823–1831. [DOI] [PubMed] [Google Scholar]
- Mohammed D, Versaevel M, Bruyere C, Alaimo L, Luciano M, Vercruysse E, Proces A and Gabriele S, 2019. Innovative Tools for Mechanobiology: Unraveling Outside-In and Inside-Out Mechanotransduction. Front Bioeng Biotechnol. 7, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor-Yossef Moldovan L, Lustig M, Naftaly A, Mardamshina M, Geiger T, Gefen A and Benayahu D, 2019. Cell shape alteration during adipogenesis is associated with coordinated matrix cues. J Cell Physiol. 234, 3850–3863. [DOI] [PubMed] [Google Scholar]
- Moreno-Vicente R, Pavon DM, Martin-Padura I, Catala-Montoro M, Diez-Sanchez A, Quilez-Alvarez A, Lopez JA, Sanchez-Alvarez M, Vazquez J, Strippoli R and Del Pozo MA, 2018. Caveolin-1 Modulates Mechanotransduction Responses to Substrate Stiffness through Actin-Dependent Control of YAP. Cell Rep. 25, 1622–1635 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ and Bass MD, 2007. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 8, 957–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Kiuchi S, Ouchi A, Hase T and Murase T, 2014. Characteristic expression of extracellular matrix in subcutaneous adipose tissue development and adipogenesis; comparison with visceral adipose tissue. Int J Biol Sci. 10, 825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG, Chang JS, DelProposto JB, Geletka L, Martinez-Santibanez G, Kaciroti N, Lumeng CN and O’Rourke RW, 2016. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity (Silver Spring). 24, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulya A and Kirwan JP, 2016. Brown and Beige Adipose Tissue: Therapy for Obesity and Its Comorbidities? Endocrinol Metab Clin North Am. 45, 605–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, Pribyl J, Skladal P, Pesl M, Caluori G, Pagliari S, Martino F, Maceckova Z, Hajduch M, Sanz-Garcia A, Pugno NM, Stokin GB and Forte G, 2017. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. 8, 15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazempour R, Zhang Q, Fu R and Sheng X, 2018. Biocompatible and Implantable Optical Fibers and Waveguides for Biomedicine. Materials (Basel). 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R and Sugiura S, 2007. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 56, 1517–26. [DOI] [PubMed] [Google Scholar]
- Nystrom FH, Chen H, Cong LN, Li Y and Quon MJ, 1999. Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected Cos-7 cells and rat adipose cells. Mol Endocrinol. 13, 2013–24. [DOI] [PubMed] [Google Scholar]
- Oomens C and Peters G, 2014. Mechanical Behavior and Properties of Adipose Tissue, in: Gefen A and Benayahu D Eds.), The mechanobiology of obesity and realted diseases. Studies in Mechanobiology, Tissue Engineering and Biomaterials, vol 16. Springer, Cham, pp. 3–9. [Google Scholar]
- Orban Z, Remaley AT, Sampson M, Trajanoski Z and Chrousos GP, 1999. The differential effect of food intake and beta-adrenergic stimulation on adipose-derived hormones and cytokines in man. J Clin Endocrinol Metab. 84, 2126–33. [DOI] [PubMed] [Google Scholar]
- Palmer AK and Kirkland JL, 2016. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol. 86, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluch EK, Nelson CM, Biais N, Fabry B, Moeller J, Pruitt BL, Wollnik C, Kudryasheva G, Rehfeldt F and Federle W, 2015. Mechanotransduction: use the force(s). BMC Biol. 13, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Kim WK and Bae KH, 2014. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J Stem Cells. 6, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA and Smith SR, 2009. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 94, 5155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA and Weaver VM, 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8, 241–54. [DOI] [PubMed] [Google Scholar]
- Pellegrinelli V, Heuvingh J, du Roure O, Rouault C, Devulder A, Klein C, Lacasa M, Clement E, Lacasa D and Clement K, 2014. Human adipocyte function is impacted by mechanical cues. J Pathol. 233, 183–95. [DOI] [PubMed] [Google Scholar]
- Peng XR, Gennemark P, O’Mahony G and Bartesaghi S, 2015. Unlock the Thermogenic Potential of Adipose Tissue: Pharmacological Modulation and Implications for Treatment of Diabetes and Obesity. Front Endocrinol (Lausanne). 6, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillip JM, Wu PH, Gilkes DM, Williams W, McGovern S, Daya J, Chen J, Aifuwa I, Lee JSH, Fan R, Walston J and Wirtz D, 2017. Biophysical and biomolecular determination of cellular age in humans. Nat Biomed Eng. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch PF, Meshulam T, Ding S and Liu L, 2011. Caveolae and lipid trafficking in adipocytes. Clin Lipidol. 6, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissonnet CM, Burdi AR and Garn SM, 1984. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 10, 1–11. [DOI] [PubMed] [Google Scholar]
- Pope BD, Warren CR, Parker KK and Cowan CA, 2016. Microenvironmental Control of Adipocyte Fate and Function. Trends Cell Biol. 26, 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raajendiran A, Ooi G, Bayliss J, O’Brien PE, Schittenhelm RB, Clark AK, Taylor RA, Rodeheffer MS, Burton PR and Watt MJ, 2019. Identification of Metabolically Distinct Adipocyte Progenitor Cells in Human Adipose Tissues. Cell Rep. 27, 1528–1540 e7. [DOI] [PubMed] [Google Scholar]
- Rabe K, Lehrke M, Parhofer KG and Broedl UC, 2008. Adipokines and insulin resistance. Mol Med. 14, 741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E and Galgani JE, 2011. The implication of brown adipose tissue for humans. Annu Rev Nutr. 31, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE and Lisanti MP, 2002. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 277, 8635–47. [DOI] [PubMed] [Google Scholar]
- Reizes O, Benoit SC and Clegg DJ, 2008. The role of syndecans in the regulation of body weight and synaptic plasticity. Int J Biochem Cell Biol. 40, 28–45. [DOI] [PubMed] [Google Scholar]
- Reizes O, Goldberger O, Smith AC, Xu Z, Bernfield M and Bickel PE, 2006. Insulin promotes shedding of syndecan ectodomains from 3T3-L1 adipocytes: a proposed mechanism for stabilization of extracellular lipoprotein lipase. Biochemistry. 45, 5703–11. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ojeda FJ, Mendez-Gutierrez A, Aguilera CM and Plaza-Diaz J, 2019. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int J Mol Sci. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ojeda FJ, Wang J, Backer T, Krueger M, Zamani S, Rosowski S, Gruber T, Onogi Y, Feuchtinger A, Schulz TJ, Fassler R, Muller TD, Garcia-Caceres C, Meier M, Bluher M and Ussar S, 2021. Active integrins regulate white adipose tissue insulin sensitivity and brown fat thermogenesis. Mol Metab. 45, 101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J and Guertin DA, 2014. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 5, 4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravani S, Yari D, Saravani R and Azadi Ahmadabadi C, 2017. Association of COL4A3 (rs55703767), MMP-9 (rs17576)and TIMP-1 (rs6609533) gene polymorphisms with susceptibility to type 2 diabetes. Biomed Rep. 6, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Lamanna WC and Esko JD, 2011. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato-Kusubata K, Jiang Y, Ueno Y and Chun TH, 2011. Adipogenic histone mark regulation by matrix metalloproteinase 14 in collagen-rich microenvironments. Mol Endocrinol. 25, 745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Wang QA, Song A, Vishvanath L, Busbuso NC, Scherer PE and Gupta RK, 2019. Cellular Origins of Beige Fat Cells Revisited. Diabetes. 68, 1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton DN, Chang E, Whittier PS, Choi D and Funk WD, 1999. Microarray analysis of replicative senescence. Curr Biol. 9, 939–45. [DOI] [PubMed] [Google Scholar]
- Shoham N and Gefen A, 2012. Mechanotransduction in adipocytes. J Biomech. 45, 1–8. [DOI] [PubMed] [Google Scholar]
- Shoham N, Girshovitz P, Katzengold R, Shaked NT, Benayahu D and Gefen A, 2014. Adipocyte stiffness increases with accumulation of lipid droplets. Biophys J. 106, 1421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard D, Haak AJ, Choi KM, Craig AR, Fredenburgh LE and Tschumperlin DJ, 2018. Aging and anatomical variations in lung tissue stiffness. Am J Physiol Lung Cell Mol Physiol. 314, L946–L955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C and Nassoy P, 2011. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 144, 402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smas CM and Sul HS, 1995. Control of adipocyte differentiation. Biochem J. 309 ( Pt 3), 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Mittendorfer B and Klein S, 2019. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 129, 3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U, Li Q, Ryden M and Spalding KL, 2021. Cellular senescence and its role in white adipose tissue. Int J Obes (Lond) [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J and Arner P, 2008. Dynamics of fat cell turnover in humans. Nature. 453, 783–7. [DOI] [PubMed] [Google Scholar]
- Strawford A, Antelo F, Christiansen M and Hellerstein MK, 2004. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab. 286, E577–88. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS and Obin MS, 2007. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 56, 2910–8. [DOI] [PubMed] [Google Scholar]
- Sun K, Kusminski CM and Scherer PE, 2011. Adipose tissue remodeling and obesity. J Clin Invest. 121, 2094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW and Discher DE, 2013. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 341, 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR and Londos C, 2003. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 161, 1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao S, Taya M and Chiew C, 2019. Mechanical stress-induced cell death in breast cancer cells. Biol Open. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang VW, 2018. Cell-cell adhesion interface: orthogonal and parallel forces from contraction, protrusion, and retraction. F1000Res. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD and Kirkland JL, 2010. Fat tissue, aging, and cellular senescence. Aging Cell. 9, 667–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD and Kirkland JL, 2013a. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 17, 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J and Kirkland JL, 2013b. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 123, 966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL and Jensen MD, 2010. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 107, 18226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharp KM, Kang MS, Timblin GA, Dempersmier J, Dempsey GE, Zushin PH, Benavides J, Choi C, Li CX, Jha AK, Kajimura S, Healy KE, Sul HS, Saijo K, Kumar S and Stahl A, 2018. Actomyosin-Mediated Tension Orchestrates Uncoupled Respiration in Adipose Tissues. Cell Metab. 27, 602–615 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J and Cannon B, 2007. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 104, 4401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baak MA and Mariman ECM, 2019. Mechanisms of weight regain after weight loss - the role of adipose tissue. Nat Rev Endocrinol. 15, 274–287. [DOI] [PubMed] [Google Scholar]
- Varzaneh FE, Shillabeer G, Wong KL and Lau DC, 1994. Extracellular matrix components secreted by microvascular endothelial cells stimulate preadipocyte differentiation in vitro. Metabolism. 43, 906–12. [DOI] [PubMed] [Google Scholar]
- Vishvanath L and Gupta RK, 2019. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest. 129, 4022–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V and Sheetz M, 2006. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 7, 265–75. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang S, Shi Y, Li R, Gunther S, Ong YT, Potente M, Yuan Z, Liu E and Offermanns S, 2020. YAP and TAZ protect against white adipocyte cell death during obesity. Nat Commun. 11, 5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV and Scherer PE, 2014. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20, 103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A and Couchman JR, 2001. Syndecan-4 and focal adhesion function. Curr Opin Cell Biol. 13, 578–83. [DOI] [PubMed] [Google Scholar]
- Wu Z, Plotnikov SV, Moalim AY, Waterman CM and Liu J, 2017. Two Distinct Actin Networks Mediate Traction Oscillations to Confer Focal Adhesion Mechanosensing. Biophys J. 112, 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian X, Gopal S and Couchman JR, 2010. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 339, 31–46. [DOI] [PubMed] [Google Scholar]
- Xu R, Boudreau A and Bissell MJ, 2009. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 28, 167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA and Lusis AJ, 2006. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DM, Jung SH, An HT, Lee S, Hong J, Park JS, Lee H, Lee H, Bahn MS, Lee HC, Han NK, Ko J, Lee JS and Ko YG, 2017. Caveolin-1 deficiency induces premature senescence with mitochondrial dysfunction. Aging Cell. 16, 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Gao J and Ogawa R, 2015. Mechanobiology and Mechanotherapy of Adipose Tissue-Effect of Mechanical Force on Fat Tissue Engineering. Plast Reconstr Surg Glob Open. 3, e578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Halbleib M, Ahmad F, Manganiello VC and Greenberg AS, 2002. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 51, 2929–35. [DOI] [PubMed] [Google Scholar]
- Zoico E, Policastro G, Rizzatti V, Nori N, Darra E, Rossi AP, Fantin F and Zamboni M, 2020. Mechanisms of adipose tissue extracellular matrix alterations in an in vitro model of adipocytes hypoxia and aging. Mech Ageing Dev. 192, 111374. [DOI] [PubMed] [Google Scholar]
- Zoico E, Rubele S, De Caro A, Nori N, Mazzali G, Fantin F, Rossi A and Zamboni M, 2019. Brown and Beige Adipose Tissue and Aging. Front Endocrinol (Lausanne). 10, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick RK, Guerrero-Juarez CF, Horsley V and Plikus MV, 2018. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 27, 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]