Abstract
A neural architecture that preferentially processes immediate survival threats relative to other negatively and positively valenced stimuli presumably evolved to facilitate survival. The empirical literature on threat superiority, however, has suffered two problems: methodologically distinguishing threatening stimuli from negative stimuli and differentiating whether responses are sped and strengthened by threat superiority or delayed and diminished by conscious processing of nonthreatening stimuli. We addressed both problems in three within-subject studies that compared responses to empirically validated sets of threating, negative, positive, and neutral stimuli, and isolated threat superiority from the opposing effect of conscious attention by presenting stimuli outside conscious perception. Consistent with threat superiority, threatening stimuli elicited stronger skin-conductance (Study 1), startle-eyeblink (Study 2), and more negative downstream evaluative responses (Study 3) relative to the undifferentiated responses to negative, positive, and neutral stimuli.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42761-021-00090-6.
Keywords: Threat, Valence, Skin-conductance, Startle eyeblink, Subliminal
Rapid detection and avoidance of imminent danger is a crucial responsibility of the mind. Threatening stimuli, such as snakes and weapons, pose an immediate risk of physical harm and are functionally distinct from other negatively and positively valenced stimuli that do not require rapid detection to avoid harm (e.g., bugs, feces, kittens, flowers). Humans ostensibly inherited neural architecture that preferentially processes and responds to immediate survival threats (of phylogenetic or ontogenetic origin; Blanchette, 2006; LeDoux, 2012; Öhman & Mineka, 2001; Vuilleumier, 2005). A neural “high road” provides delayed yet more processed information to the amygdala via the visual and temporal cortices, and contrasts a “low road” that includes a subcortical pathway to the amygdala and periaqueductal gray via the superior colliculus and pulvinar nuclei of the thalamus to quickly detect threat and activate associated responses without recruiting explicit processes (Garrido et al., 2012; Garvert et al., 2014; Koller et al., 2019; LeDoux, 2012; Lischinsky & Lin, 2019; Rafal et al., 2015; Tamietto et al., 2012; cf. Pessoa & Adolphs, 2010).
Threat superiority manifests as earlier and stronger detection and responses to threatening than nonthreatening stimuli (March et al., 2018a, b). Research using consciously perceived stimuli suggests that threatening stimuli (e.g., angry faces, snakes, guns), relative to innocuous stimuli (e.g., happy faces, flowers, mushrooms), are detected faster (Blanchette, 2006; Eastwood et al., 2001; Öhman et al., 2001a, b), and evoke stronger skin-conductance (Knight et al., 2009; Morris et al., 1998; Vrana, 1995) and startle-eyeblinks (Knight et al., 2009; Lang et al., 1990) and earlier/stronger responses in the amygdala (Kveraga et al., 2015; Méndez-Bértolo et al., 2016; Whalen et al., 1998) and cortices (Costa et al., 2014).
However, two problems complicate attributing those effects to threat superiority. One problem, which has been addressed but remains relevant, is that threatening stimuli are also negative. With few exceptions (March et al., 2017; Kveraga et al., 2015), research did not methodologically distinguish threatening from negative stimuli, which precludes isolating sensitivity to threat per se. March et al., (2017; also see Kveraga et al., 2015) addressed this by comparing responses to four consciously perceived and empirically validated stimulus categories: threatening (e.g., snarling predators, gunmen), nonthreatening-negative (herein “negative,” e.g., excrement, injured animals), positive (e.g., puppies, babies), and neutral (e.g., doorknobs, mugs). Relative to the other stimuli, threatening stimuli were detected faster, more frequent targets of initial eye-gaze, and elicited stronger startle-eyeblinks.
The second, and more challenging, problem is that utilizing consciously perceived stimuli does not distinguish whether faster/stronger responses to threat are due to threat superiority or the opposing effects of attention and conscious processes evoked by nonthreatening stimuli. For example, attention can delay/diminish responses via slower disengagement and inhibited muscle movement. Faster detection of threat in visual-search tasks might reflect slower disengagement from nonthreatening stimuli (West et al., 2009). Stronger startle-eyeblinks to threat might reflect greater attentional capture, which inhibits blinking, of nonthreatening stimuli (Filion et al., 1998; March et al., 2017). This problem could be rectified by presenting stimuli outside conscious perception thereby eliminating the competing effects of conscious processing and isolating the hypothesized effect of threat superiority.
Studies that present stimuli outside conscious perception are consistent with threat superiority in terms of stronger skin-conductance (Esteves et al., 1994; Morris et al., 1998; Öhman & Soares, 1998; Reagh & Knight, 2013) and startle-eyeblink (Reagh & Knight, 2013; Ruiz-Padial & Vila, 2007). Unfortunately, none of those studies distinguished threatening from negative stimuli. Some assessed responses to threatening stimuli without assessing responses to negative stimuli (e.g., Dimberg et al., 2000; Esteves et al., 1994; Morris et al., 1998; Öhman & Soares, 1998; Ruiz-Padial & Vila, 2007). Others averaged responses to threatening and negative stimuli (e.g., Hermans et al., 2003; Lähteenmäki et al., 2015; Reagh & Knight, 2013). Consequently, those studies do not clarify whether the effects are unique to threat.
The current research addresses both problems to test whether the mind is uniquely sensitive to threatening than nonthreatening (i.e., negative, positive, and neutral) stimuli. Two pilot studies ensured that stimuli were presented outside conscious perception. Three within-subject studies presented threatening, negative, positive, and neutral stimuli (March et al., 2017) and assessed skin-conductance (Study 1), startle-eyeblink (Study 2), and evaluative inference (Study 3). If the mind is uniquely sensitive to threat as a functional adaptation for survival, reflexive reactions (such as skin-conductance and startle-eyeblink) should be stronger to threatening than nonthreatening stimuli and influence downstream inferences (e.g., Bechara & Damasio, 2005).
Pilot Studies
We designed the pilot studies to ensure that the stimulus presentations minimized conscious perception. Our concern is not whether participants are aware that a stimulus is presented, but whether they can actively attend to and think about the stimulus. We purposefully avoided detection and discrimination tasks, which compare rates at which participants detect the presence of a stimulus or distinguish it from foils. Neither task necessarily assesses conscious perception and are compromised by feature-matching (e.g., a dark edge in the stimulus that is not in the foils) and affective inference (e.g., Bechara & Damasio, 2005; Storbeck & Clore, 2008). If participants can affectively feel the stimulus (despite not knowing what it is), they can discern its presence and distinguish it from foils. We motivated participants with payment to actively attend and accurately describe the stimulus. If they could, it would imply that they have conscious perception of the stimulus (Lähteenmäki et al., 2015; Wiens, 2006).
Method
Undergraduates at a US public university participated (N = 43 and 50 for Pilots 1 and 2, respectively) for partial credit in an introductory psychology-course seated in individual cubicles ~ 75 cm from a 60-cm 144 Hz high-speed monitor. Instructions explained that every trial would quickly present an image and, to motivate attention and accuracy, participants would earn $1 for every image they described correctly. To rule-out laziness and quickly clicking to the end, we required participants to type a response on each trial (a brief description or “do not know”). The images were those of the main studies and were from March et al.’s (2017) validated stimulus categories: threatening, negative, positive, and neutral. Figure 1 provides examples, and the Supplemental Material provides all stimuli—as detailed in Supplemental Table1, the stimuli do not differ in luminance or red value, and the threatening stimuli are not more negative or arousing than the negative stimuli.
Fig. 1.
Examples of stimuli used in all studies
Pilot 1 mimicked the trial structure of Study 1. Participants experienced 64 trials that (1) began with a centrally located 4000-ms fixation-dot that was (2) replaced by a 25-ms 500 × 500 pixel image that was (3) backward masked by a 150-ms colorful mosaic that was (4) replaced by a 4000-ms blank screen, and (5) ended with a prompt to describe the image. We randomly presented across the 64 trials, sixteen images from each of the four stimulus categories. Pilot 2 mimicked the trial structure of Studies 2 and 3. Participants experienced 100 trials that each (1) began with a 2000-ms centrally located pre-mask of a mosaic that was (2) replaced by a 25-ms 500 × 500 pixel image, that was (3) backward masked by a 3000–5000 ms mosaic (rotated 90° clockwise) which was, (4) replaced with a 4000-ms blank screen, and (5) ended with a prompt to describe the image. We randomly presented across the 100 trials twenty-five images from each of the four stimulus categories.
Data preparation
Two assistants blind to the purpose of the study-coded responses for accuracy with instructions to use a liberal standard such that responses needed to be generally accurate but not exact. For example, it would be correct to respond “bug” to a cockroach, “bowl” or “cylinder” to a saucepan, or “burglar,” “man in a mask,” or “gunman” to a masked man holding a gun. The assistants agreed on 7745 of 7752 responses (99.91%) and the first author rectified the seven disagreements.
Results
Despite the monetary incentive to actively attend and accurately describe the stimuli, participants were remarkably unable to do so. In particular, they were unable to accurately describe an average of 94.26% and 99.32% of the stimuli in Pilots 1 and 2, respectively, with the modal response being “I don’t know.” These data suggest that the stimulus presentations effectively minimize conscious perception. See Supplement for further details.
Study 1
A skin-conductance response (SCR; Dawson et al., 2011) results from arousal of the sympathetic nervous system which activates sweat gland activity. Increased sweat enhances the skin’s electrical resistance and greater arousal registers as a stronger SCR.
Method
Existing skin-conductance studies that presented threatening stimuli outside conscious perception used a within-subject design with sample sizes ranging from 10 to 46 participants (Esteves et al., 1994; Lapate et al., 2014; Morris et al., 1999; Öhman & Soares, 1998; Reagh & Knight, 2013). We attempted to well exceed those sample sizes by collecting data from the beginning to end of a semester. Undergraduates at a US public university participated (N = 111) for partial credit in an introductory psychology-course seated in individual cubicles ~ 75 cm from a 60-cm 144 Hz high-speed monitor. Instructions indicated that the study examined whether participants could perceive quickly presented images with trials varying in the presence of an image (all trials actually presented an image). Participants experienced 64 trials that (1) began with a centrally located 4000-ms fixation-dot that was (2) replaced by a 14-ms 500 × 500 pixel image that was (3) backward masked by a 150-ms colorful mosaic that was (4) replaced by a 4000-ms blank screen, and (5) ended with a prompt of whether an image was presented (response to the prompt and demographics questions were lost when lab computers were replaced). The 4000-ms delay isolated SCR to the stimulus vs. prompt. We randomly presented sixteen images from each category (threatening, negative, positive, neutral) across trials. A Biopac MP36 amplifier with AcqKnowledge software recorded SCR via two 6-mm diameter Ag–AgCl electrodes attached to the medial phalanges of the ring and middle finger on the volar surface of the non-dominant hand. The MP36 passed a constant 0.5 V current through one electrode and measured conductivity as deviation from the constant at 2 kHz.
Data preparation
We exported the data into the PsPM package of MATLAB (Bach et al., 2010) which estimates SCRs through general linear convolution models that separate overlapping responses, such as those to the fixation-dot and image. Data were filtered through 1st-order low-pass (5 Hz) and high-pass (0.05 Hz) Butterworth filters, resampled to 10 Hz, and Z-transformed within-person to account for between-person variability (Bach et al., 2013). We constructed critical data-epochs containing the 4 s after image onset. To derive responses to each category, we averaged the data across all epochs of a given category for each participant and submitted them to a subject-specific GLM which yielded four mean SCRs for each participant. We submitted the four responses to a multivariate repeated measures analysis and tested threat superiority with two a-priori comparisons that are orthogonal to each other: (1) the mean response to threat versus the mean of the responses to the negative, neutral, and positive stimuli, and (2) whether there was systematic variability among responses to the latter three stimuli (see Supplement for further details).
Results
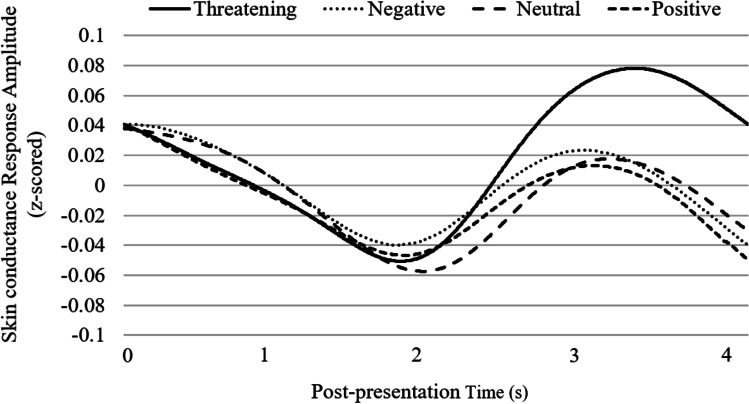
Consistent with threat superiority and as depicted in Fig. 2, threatening stimuli yielded a stronger SCR (M = 0.232) than the mean SCR to negative (M = 0.104), positive (M = 0.094), and neutral stimuli (M = 0.084), F(1, 110) = 4.63, p = 0.0336, d = 0.21, and there was no systematic variability among the latter three SCRs, F(2, 109) = 0.03, p = 0.9687.
Fig. 2.
Mean Pre-PpSM processed skin-conductance response to each stimulus type
Discussion
We isolated threat superiority by eliminating conscious attention to the stimuli and observed stronger SCR to threatening stimuli relative to the undifferentiated SCRs to negative, positive, and neutral stimuli. Study 2 provides a conceptual replication with a different physiological response.
Study 2
Startle-eyeblink is a reflexive response modulated by at least two influences (Filion et al., 1998). Affective arousal facilitates the response and attention inhibits the response, and both can co-occur when stimuli are presented with conscious perception.
Method
Existing startle-eyeblink studies that presented threatening stimuli outside conscious perception used a within-subject design with sample sizes ranging from 19 to 48 participants (Reagh & Knight, 2013; Ruiz-Padial & Vila, 2007). We attempted to well exceed those sample sizes by collecting data from the beginning to end of a semester. Undergraduates at a US public university participated (N = 142; 103 females, 39 males) for partial credit in an introductory psychology-course seated in individual cubicles ~ 75 cm from a 60-cm 144 Hz high-speed monitor with headphones.
Instructions explained that the study examined sound perception with trials randomly varying in the presence of a noise, and the participant’s task was to judge the loudness of the noise. Participants sampled the noise (i.e., the startle probe—a 50-ms binaural burst of 1000 Hz, 100 dB white noise; calibrated daily) and completed 144 trials that each (1) began with a 2000 ms centrally located pre-mask of a mosaic that was (2) replaced by a 21-ms image, that was (3) backward masked by a 3000–5000-ms mosaic (rotated 90° clockwise) and, (4) ended with either a 4000-ms blank screen, or, for trials including a startle-probe, a text-box prompting the perceived loudness (85–115 dB) of the noise (this rating did not vary as a function of the stimulus image), and a 4000-ms blank screen. On 48 critical trials, the startle-probe sounded 2000–4000 ms after stimulus-image onset (12 trials for each of the four categories). The 96 non-critical trials were also equally composed of the four categories (24 trials of each). We randomly ordered all trials and images. We measured startle-eyeblink with facial electromyography (fEMG) via 4 mm Ag–AgCl electrodes placed ~ 20 mm apart over the orbicularis oculi muscle under the left eye, with a forehead ground.
Data preparation
We recorded and cleaned data via a Biopac MP36 amplifier and AcqKnowledge 4.1 software at a rate of 2000 Hz, amplified with a gain of 5000, notch (60 Hz) and band-pass filtered (HP = 10 Hz, LP = 500 Hz) online, stop (57-63 Hz) and band-pass (HP = 28 Hz, LP = 500 Hz) filtered offline, and rectified, fully integrated, and averaged over 20 samples with the root mean square (Blumenthal et al., 2005).
Thirty-six participants provided unusable data: 33 were non-responders (i.e., rarely blinked to the noise-probe, resulting in more than 50% data loss prior to removing outliers), 1 cringed excessively, thereby impeding assessment of eyeblink amplitude, and 2 dislodged an fEMG electrode, which yielded a sample of 106 participants (75 females, 31 males) and 5088 critical trials. We calculated startle-eyeblink amplitude for each critical trial by subtracting the mean fEMG amplitude across the 50 ms baseline preceding the probe from the maximum amplitude during the 200 ms following the probe. We could not compute a startle-eyeblink for 855 trials (16.8% of all trials) due to the absence of a blink (n = 612), blink during baseline (n = 115), or excessive orbicularis oculi movement during the trial (n = 130); unusableness was unrelated to stimulus type, Fmulti-level logistic regression(3, 4979) = 0.90, p = 0.4388. We standardized startle-eyeblink amplitudes within-person to control between-person variation in baseline and blink fEMG (Blumenthal et al., 2005). We excluded amplitudes beyond 2.5SDs of the person-mean (n = 97; exclusion was unrelated to stimulus type, Fmulti-level logistic regression (3, 4229) = 0.56, p = 0.6389) and 5 participants with 50% or fewer critical trials remaining (conclusions based on p values and direction of effects are the same with those participants included). We computed a mean startle-eyeblink to each of the four stimulus types for each of the remaining 101 participants. We submitted the four responses to a multivariate repeated measures analysis and tested threat superiority with two a-priori comparisons: (1) the mean response to threat versus the mean of the responses to the negative, neutral, and positive stimuli, and (2) whether there was systematic variability among responses to the latter three stimuli.
Results
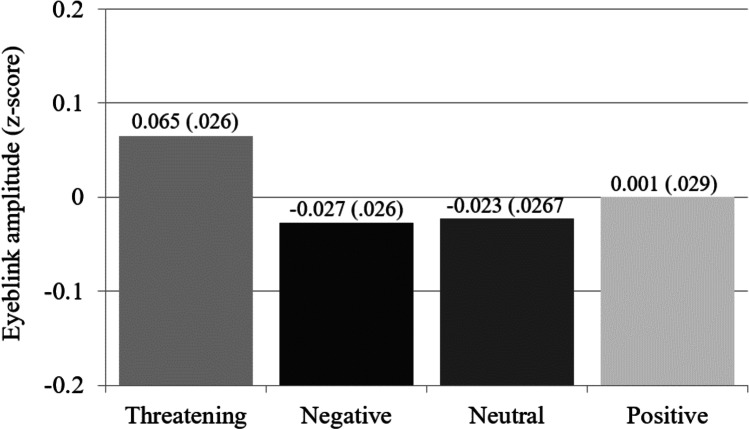
Consistent with threat superiority and as depicted in Fig. 3, threatening stimuli elicited a larger startle-eyeblink (M = 0.065) than the mean startle-eyeblink to negative (M = − 0.027), neutral (M = − 0.023), and positive stimuli (M = 0.005), F(1, 100) = 5.36, p = 0.0226, d = 0.23, and there was no systematic variability among the latter three startle-eyeblinks, F(2, 99) = 0.30, p = 0.7442.
Fig. 3.
Mean standardized eye-blink amplitude (and SEM calculated within-participants; O’Brien & Cousineau, 2014) as a function of stimulus type
Discussion
Assessing startle-eyeblink to stimuli presented outside conscious perception, we conceptually replicated the Study 1 pattern consistent with threat superiority. As with SCR, we observed a stronger startle-eyeblink in response to threatening stimuli relative to the undifferentiated startle-eyeblink to negative, positive, and neutral stimuli.
Study 3
Given that threat superiority is functional for survival, autonomic responses to threatening stimuli should influence downstream judgement (e.g., Bechara & Damasio, 2005). To test this, we used the procedure of Study 2 and had participants rate the valence of each stimulus.
Method
The procedure was identical to Study 2 with two exceptions: (1) Participants (N = 83; 46 females, 35 males, 2 unreported) were informed that every trial would present an image too quickly to see, but they might feel whether it is bad or good; (2) Rather than rating the loudness of the startle-probe, they rated after each trial the valence (“1 = Very Negative” to “5 = Very Positive”) of the unseen image. Two participants exited the study early (after 13 and 16 trials), which yielded useable data from 81 participants. Unfortunately, the valence-rating task rendered useless the startle-eyeblink measure. Participants squinted to try to see the images, which inhibited blinking and prevented assessment of startle-eyeblink (over 60% of trials were unusable). We limit discussion to the valence ratings.
Data preparation
We computed for each participant a mean valence-rating of the four stimulus types for probe and no-probe trials, respectively. We submitted the eight ratings to a 2(probe: yes, no) × 4(stimulus: threatening, negative, neutral, positive) multivariate repeated measures analysis and examined stimulus effects with two a-priori comparisons to test threat superiority: (1) the mean response to threat versus the mean of the responses to the negative, neutral, and positive stimuli, and (2) whether there was systematic variability among responses to the latter three stimuli.
Results
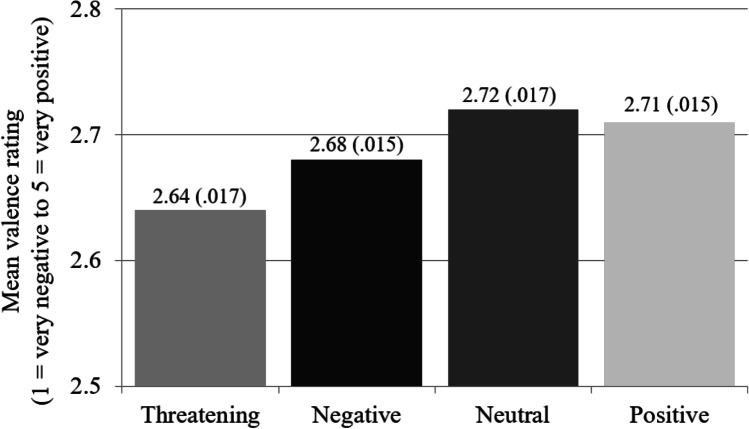
Consistent with threat superiority and as depicted in Fig. 4, participants rated less positively unseen threatening stimuli (M = 2.64) than the mean valence of negative (M = 2.68), neutral (M = 2.72), and positive stimuli (M = 2.71), F(1, 80) = 12.17, p = 0.0008, d = 0.39, and there was no systematic variability among the latter three ratings, F(2, 79) = 1.88, p = 0.1588. Neither comparison was moderated by probe, F(1, 80) = 0.63, p = 0.4304, and F(2, 79) = 1.51, p = 0.2283, nor was there a stimulus × probe interaction, F(3, 78) = 1.30, p = 0.2809.
Fig. 4.
Mean valence ratings (and SEM, calculated with-in participants; O’Brien & Cousineau, 2014) as a function of stimulus
Discussion
Consistent with the possibility that threat presented outside conscious perception uniquely influences downstream judgement, participants more negatively evaluated threatening stimuli relative to their undifferentiated evaluation of negative, positive, and neutral stimuli. Ostensibly, heightened autonomic arousal to threat, which registered in Studies 1 and 2 as stronger SCR and startle-eyeblink, influenced explicit judgements and manifested in self-reported valence ratings. This is consistent with the proposition that persons can utilize autonomic arousal to inform judgment absent conscious perception of the eliciting stimulus (Bechara & Damasio, 2005). As a limitation, the absence of a reliably assessed physiological response precluded testing its presumed role as a mediator of inferred evaluation.
General Discussion
Neural architecture that preferentially processes and responds to immediate danger relative to other positively and negatively valenced stimuli ostensibly evolved to facilitate survival. Empirical work on threat superiority, however, suffered two problems: methodologically distinguishing threatening from negative stimuli and differentiating whether responses are sped/strengthened by threat or delayed/diminished by conscious perception of nonthreatening stimuli. We addressed both problems by exposing participants to threating, negative, positive, and neutral stimuli, and isolating threat superiority from the opposing effect of conscious attention by presenting stimuli outside conscious perception. Threatening stimuli elicited stronger skin-conductance, startle-eyeblink, and more negative evaluative responses relative to the undifferentiated responses to negative, positive, and neutral stimuli. These findings make clear that threat superiority is not a byproduct of conscious attention to nonthreatening stimuli and, instead, arises from the mind’s sensitivity to survival threats. We conclude with consideration of four issues.
Active and Passive Threat
Rapid detection of and response to immediate danger (e.g., gunman, snake) is functional and promotes survival. Here the key is the imminent and active nature of the threat. Other stimuli (e.g., feces, diseased person) can also pose a potential threat. However, harm from such stimuli is neither certain nor sudden. Such stimuli pose a passive threat that manifests only if they are approached or engaged. Rather than evoking rapid avoidance, those stimuli may evoke a delayed response as a means of obtaining additional information about the potential nature of the threat (e.g., morbid fascination; March et al., 2017; Oosterwijk et al., 2016). We note this distinction to emphasize that a rapid response is not always a functional response and that immediate threats are not the only stimuli that evoke a functional response.
Threatening Versus Negative Stimuli
Threat superiority is the preferential processing of threatening stimuli relative to other negatively and positively valenced stimuli. For readers curious about a direct comparison of threatening and negative stimuli, responses were directionally stronger to threatening than negative stimuli in all studies with the test being significant in Study 2, F(1, 100) = 4.98, p = 0.0279, d = 0.22, and Study 3, F(1, 80) = 4.37, p = 0.0397, d = 0.24, but not in Study 1, F(1, 110) = 2.34, p = 0.1290, d = 0.14. To better estimate the effect, we performed a random effects meta-analysis of our studies using R’s Metaphor package (Viechtbauer, 2010; Goh et al., 2016). As Fig. 5 depicts, the average effect was significant across all three studies, d = 0.20, Z = 3.35, p = 0.0008, and when limited to the physiological data of Studies 1 and 2, d = 0.18, Z = 2.61, p = 0.0090.
Fig. 5.
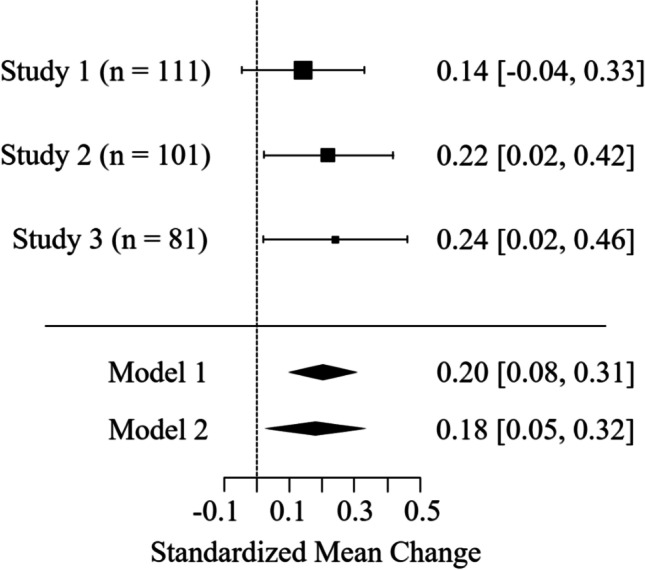
Forest plot of meta-analyzed effect sizes with average effect estimated from all three studies (Model 1) or only Studies 1 and 2 (Model 2)
Is It Really Threat Superiority?
Readers eager for a physiological measure with perfect correspondence to a singular psychological process might be skeptical of our conclusion for threat superiority. Unfortunately, we know of no such measure (Cacioppo & Tassinary, 1990). We measured skin conductance and startle eye-blink because they are prominent in the threat superiority literature. Skin conductance is certainly sensitive to processes beyond threat, such as attention, effort, and sexual interest. Startle eye-blink might be closer to the mark of a “threat” measure in that it is believed to be a defensive response (Bradley et al., 2001; Lang et al., 1990)—but it too can be affected by attention (Filion et al., 1998). In the absence of a pure measure, we relied on a different tactic to garner confidence that the data might reflect threat superiority. We systematically varied the stimuli to assess whether the physiological system responded selectively to a particular type of stimulus (when attention and active cognition were minimized). And it did: only threatening stimuli elicited elevated responses. We could have employed other measures. Facial EMG of corrugator and zygomaticus activity could be used to assess negative vs. positive affect in response to the stimuli (Brown & Schwartz, 1980), which would be conceptually similar to Study 3’s measure of inferred valence as would be the use of an approach-avoidance measure (e.g., push–pull on a joystick). Facial EMG could also assess muscular activation of a fear face (Van Den Broek et al., 2006). This probably would be more fruitful in response to consciously perceived stimuli because we doubt the current paradigm elicits reactions of sufficient strength to manifest an emotional response.
A related issue is whether threat is really superior? Do other motivational states rival threat? Among the basic (i.e., evolutionarily necessary) needs (i.e., avoid harm, eat, sleep, procreate), we suspect that harm avoidance is primary given that other needs cannot be met (nor matter) when dead. This is not to suggest that other needs elicit weak motivations and reactions. But when paired in competition, it is unlikely that any other need would elicit a stronger or earlier response than that of threat. It is difficult to imagine a delicious meal, alluring partner, or comfy bed being prioritized in the presence of a snarling wolf. Of course, this is an empirical issue and, unfortunately, none of the positive stimuli were appropriate for comparing needs of sustenance, sleep, and procreation to that of survival.
Facial and Non-Facial Stimuli
Study 3 found that threatening stimuli presented outside conscious perception yielded a stronger valence inference than the undifferentiated inference of positive, negative, and neutral stimuli. This is discrepant with Murphy and Zajonc (1993) who found both positive and threatening (relative to neutral) primes yielded stronger affective misattribution to a subsequent stimulus (Chinese ideograph). Although the inference tasks differed—i.e., inferring the valence of the stimulus vs. attributing the valence of the stimulus to a subsequent stimulus—we suspect stimulus differences had more to do with the discrepant results. Murphy and Zajonc used happy and angry faces. When presented outside conscious perception, happy-faces are detected better than angry faces (Sweeny et al., 2013), which is also discrepant with threat superiority. One possibility for the discrepancy could be the greater difficulty of masking happy faces (Maxwell & Davidson, 2004).
A more interesting possibility is that faces are processed differently than other stimuli. Humans have an anatomically discrete cortical area (McCarthy et al., 1997) facilitating face processing, and decoding different facial expressions involves partially dissociable neural circuits (Adolphs et al., 1996). Such anatomy likely arose through the importance of interpersonal and intragroup relations in human evolution (Caporael, 1997). Considering the proximity required to discern facial expressions, trust is likely necessary. Perhaps facial expressions of happiness are more critical for consensual acts of reproduction and resource sharing than are expressions of anger. Indeed, facial changes to happiness are better detected on ingroup than outgroup faces (Hugenberg, 2005). Human faces might activate a variety of processes and not clearly conform to patterns of threat superiority. Future work should examine whether human facial-expressions of anger and happiness are processed differently than other forms of threat and positivity.
Conclusion
By comparing responses to threating, negative, positive, and neutral stimuli, and isolating threat superiority from the opposing effect of conscious attention, the current work demonstrates the unique sensitivity of the mind to survival threats. Preferentially processing and responding to immediate danger is functional for survival.
Supplementary Information
(PDF 3022 KB)
Acknowledgements
We thank Dominic Bach for his help with the PSPM treatment of the Study 1 SCR data.
Additional Information
Funding Information
Not Applicable.
Data Availability
Data and materials are available at https://osf.io/2sx4h/.
Ethical Approval
The methodology for these studies were approved by the Institutional Review Board of the University of Tennessee (IRB# 15–02,107-FB).
Conflicts of Interest
The authors have no conflicts of interest to declare.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Author Contributions
All authors contributed to study design, data analysis, and manuscript development.
Footnotes
The original online version of this article was revised due to the incorrect data in the Method of Study 2, the data should be "21-ms image".
Change history
2/21/2022
A Correction to this paper has been published: 10.1007/s42761-022-00106-9
References
- Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. Journal of Neuroscience. 1996;16:7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Flandin G, Friston KJ, Dolan RJ. Modelling event-related skin conductance responses. International Journal of Psychophysiology. 2010;75:349–356. doi: 10.1016/j.ijpsycho.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Friston KJ, Dolan RJ. An improved algorithm for model-based analysis of evoked skin conductance responses. Biological Psychology. 2013;94:490–497. doi: 10.1016/j.biopsycho.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio A. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Blanchette I. Snakes, spiders, guns, and syringes: How specific are evolutionary constraints on the detection of threatening stimuli? The Quarterly Journal of Experimental Psychology. 2006;59:1484–1504. doi: 10.1080/02724980543000204. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Brown SL, Schwartz GE. Relationships between facial electromyography and subjective experience during affective imagery. Biological Psychology. 1980;11:49–62. doi: 10.1016/0301-0511(80)90026-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG. Inferring psychological significance from physiological signals. American Psychologist. 1990;45:16–28. doi: 10.1037//0003-066x.45.1.16. [DOI] [PubMed] [Google Scholar]
- Caporael LR. The evolution of truly social cognition: The core configurations model. Personality and Social Psychology Review. 1997;1:276–298. doi: 10.1207/s15327957pspr0104_1. [DOI] [PubMed] [Google Scholar]
- Costa T, Cauda F, Crini M, Tatu MK, Celeghin A, de Gelder B, Tamietto M. Temporal and spatial neural dynamics in the perception of basic emotions from complex scenes. Social Cognitive and Affective Neuroscience. 2014;9:1690–1703. doi: 10.1093/scan/nst164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Courtney CG. The skin conductance response, anticipation, and decision-making. Journal of Neuroscience, Psychology, and Economics. 2011;4:111. [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Eastwood JD, Smilek D, Merikle PM. Differential attentional guidance by unattended faces expressing positive and negative emotion. Perception & Psychophysics. 2001;63:1004–1013. doi: 10.3758/bf03194519. [DOI] [PubMed] [Google Scholar]
- Esteves F, Dimberg U, Öhman A. Automatically elicited fear: Conditioned skin conductance responses to masked facial expressions. Cognition & Emotion. 1994;8:393–413. [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: A review. Biological Psychology. 1998;47:1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Garrido MI, Barnes GR, Sahani M, Dolan RJ. Functional evidence for a dual route to amygdala. Current Biology. 2012;22:129–134. doi: 10.1016/j.cub.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvert MM, Friston KJ, Dolan RJ, Garrido MI. Subcortical amygdala pathways enable rapid face processing. NeuroImage. 2014;102:309–316. doi: 10.1016/j.neuroimage.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh, J. X., Hall, J. A., & Rosenthal, R. (2016). Mini meta‐analysis of your own studies: Some arguments on why and a primer on how. Social and Personality Psychology Compass, 10(10), 535–549.
- Hermans D, Spruyt A, De Houwer J, Elen P. Affective priming with subliminally presented pictures. Canadian Journal of Experimental Psychology. 2003;57:97–114. doi: 10.1037/h0087416. [DOI] [PubMed] [Google Scholar]
- Hugenberg K. Social categorization and the perception of facial affect: Target race moderates the response latency advantage for happy faces. Emotion. 2005;5:267–276. doi: 10.1037/1528-3542.5.3.267. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters N, Bandettini PA. Neural substrates of explicit and implicit fear memory. NeuroImage. 2009;45:208–214. doi: 10.1016/j.neuroimage.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K, Rafal RD, Platt A, Mitchell ND. Orienting toward threat: Contributions of a subcortical pathway transmitting retinal afferents to the amygdala via the superior colliculus and pulvinar. Neuropsychologia. 2019;128:78–86. doi: 10.1016/j.neuropsychologia.2018.01.027. [DOI] [PubMed] [Google Scholar]
- Kveraga, K., Boshyan, J., Adams, R. B., Mote, J., Betz, N., Ward, N., ... & Barrett, L. F. (2015). If it bleeds, it leads: separating threat from mere negativity. Social Cognitive and Affective Neuroscience, 10, 28–35. [DOI] [PMC free article] [PubMed]
- Lähteenmäki M, Hyönä J, Koivisto M, Nummenmaa L. Affective processing requires awareness. Journal of Experimental Psychology General. 2015;144:339–365. doi: 10.1037/xge0000040. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377. [PubMed] [Google Scholar]
- Lapate, R. C., Rokers, B., Li, T., & Davidson, R. J. (2014). Nonconscious emotional activation colors first impressions: A regulatory role for conscious awareness. Psychological Science, 25(2), 349–357. [DOI] [PMC free article] [PubMed]
- LeDoux JE. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischinsky JE, Lin D. Looming Danger: Unraveling the Circuitry for Predator Threats. Trends in Neurosciences. 2019;42:841–842. doi: 10.1016/j.tins.2019.10.004. [DOI] [PubMed] [Google Scholar]
- March DS, Gaertner L, Olson MA. In harm’s way: On preferential response to threatening stimuli. Personality and Social Psychology Bulletin. 2017;43:1519–1529. doi: 10.1177/0146167217722558. [DOI] [PubMed] [Google Scholar]
- March DS, Gaertner L, Olson MA. On the prioritized processing of threat in a Dual Implicit Process Model of evaluation. Psychological Inquiry. 2018;19:1–13. [Google Scholar]
- March, D. S., Gaertner, L., & Olson, M. A. (2018b). Clarifying the explanatory scope of the dual implicit process model. Psychological Inquiry, 29(1), 38–43.
- Maxwell JS, Davidson RJ. Unequally masked: Indexing differences in the perceptual salience of “unseen” facial expressions. Cognition and Emotion. 2004;18:1009–1026. [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- Méndez-Bértolo, C., Moratti, S., Toledano, R., Lopez-Sosa, F., Martínez-Alvarez, R., Mah, Y. H., ... & Strange, B. A. (2016). A fast pathway for fear in human amygdala. Nature neuroscience, 19(8), 1041. [DOI] [PubMed]
- Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. S., Öhman, A., & Dolan, R. J. (1998). Conscious and unconscious emotional learning in the human amygdala. Nature, 393(6684), 467–470. [DOI] [PubMed]
- Murphy ST, Zajonc RB. Affect, cognition, and awareness: Affective priming with optimal and suboptimal stimulus exposures. Journal of Personality and Social Psychology. 1993;64:723–739. doi: 10.1037//0022-3514.64.5.723. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares JJF. Emotional conditioning to masked stimuli: Expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. Journal of Experimental Psychology General. 1998;127:69–82. doi: 10.1037//0096-3445.127.1.69. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;3:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: A threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;3:381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Oosterwijk S, Lindquist KA, Adebayo M, Barrett LF. The neural representation of typical and atypical experiences of negative images: Comparing fear, disgust and morbid fascination. Social Cognitive and Affective Neuroscience. 2016;11:11–22. doi: 10.1093/scan/nsv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal RD, Koller K, Bultitude JH, Mullins P, Ward R, Mitchell AS, Bell AH. Connectivity between the superior colliculus and the amygdala in humans and macaque monkeys: Virtual dissection with probabilistic DTI tractography. Journal of Neurophysiology. 2015;114:1947–1962. doi: 10.1152/jn.01016.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh Z, Knight D. Negative, but not positive emotional images modulate the startle response independent of conscious awareness. Emotion. 2013;4:782–791. doi: 10.1037/a0032286. [DOI] [PubMed] [Google Scholar]
- Ruiz-Padial E, Vila J. Fearful and sexual pictures not consciously seen modulate the startle reflex in human beings. Biological Psychiatry. 2007;61:996–1001. doi: 10.1016/j.biopsych.2006.08.046. [DOI] [PubMed] [Google Scholar]
- Storbeck J, Clore GL. Affective arousal as information: How affective arousal influences judgements, learning, and memory. Social and Personality Psychology Compass. 2008;2:1824–1843. doi: 10.1111/j.1751-9004.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny TD, Suzuki S, Grabowecky M, Paller KA. Detecting and categorizing fleeting emotions in faces. Emotion. 2013;13:76–91. doi: 10.1037/a0029193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M, Pullens P, de Gelder B, Weiskrantz L, Goebel R. Subcortical connections to human amygdala and changes following destruction of the visual cortex. Current Biology. 2012;22:1449–1455. doi: 10.1016/j.cub.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Van Den Broek, E. L., Schut, M. H., Westerink, J. H., van Herk, J., & Tuinenbreijer, K. (2006, May). Computing emotion awareness through facial electromyography. In European Conference on Computer Vision (pp. 52–63). Springer, Berlin, Heidelberg.
- Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48.
- Vrana SR. Emotional modulation of skin conductance and eyeblink responses to a startle probe. Psychophysiology. 1995;32:351–357. doi: 10.1111/j.1469-8986.1995.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- West GL, Anderson AA, Pratt J. Motivationally significant stimuli show visual prior entry: Evidence for attentional capture. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1032–1042. doi: 10.1037/a0014493. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens, S. (2006). Current concerns in visual masking. Emotion, 6(4), 675–680. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 3022 KB)
Data Availability Statement
Data and materials are available at https://osf.io/2sx4h/.