Abstract
We report the first functional neuroimaging meta-analysis on age-related differences in adult neural activity during affect. We identified and coded experimental contrasts from 27 studies (published 1997–2018) with 490 older adults (55–87 years, Mage = 69 years) and 470 younger adults (18–39 years, Mage = 24 years). Using multilevel kernel density analysis, we assessed functional brain activation contrasts for older vs. younger adult affect across in-scanner tasks (i.e., affect induction and perception). Relative to older adults, younger adults showed more reliable activation in subcortical structures (e.g., amygdala, thalamus, caudate) and in relatively more posterior aspects of specific brain structures (e.g., posterior insula, mid- and posterior cingulate). In contrast, older adults exhibited more reliable activation in the prefrontal cortex and more anterior aspects of specific brain structures (e.g., anterior insula, anterior cingulate). Meta-analytic coactivation network analyses further revealed that in younger adults, the amygdala and mid-cingulate were more central, locally efficient network nodes, whereas in older adults, regions in the superior and medial prefrontal cortex were more central, locally efficient network nodes. Collectively, these findings help characterize age differences in the brain basis of affect and provide insights for future investigations into the neural mechanisms underlying affective aging.
Electronic supplementary material
The online version of this article (10.1007/s42761-020-00016-8) contains supplementary material, which is available to authorized users.
Keywords: Aging, Affective neuroscience, Brain, Emotion, Meta-analysis, Functional neuroimaging
Affect is the mental representation of ongoing bodily states (e.g., autonomic, immune, metabolic, and temperature shifts) and predictions about how objects and events (e.g., a rabid dog, meeting a stranger) will impact those states; as such, affect is thought to form the basis of emotion, motivated behavior, and even consciousness (Barrett & Bar, 2009; Cabanac, 2002; Craig, 2009; Damasio, 1999; Duncan & Barrett, 2007; LeDoux & Brown, 2017; MacCormack & Lindquist, 2017; Northoff, 2012; Schwarz & Clore, 1983; Seth, 2018). Affect is most frequently characterized as having two subjective qualities: valence, or subjective pleasantness vs. unpleasantness (“positive” vs. “negative”), and arousal, or subjective activation vs. relaxation (“high arousal” vs. “low arousal”) (Bradley & Lang, 1994; Posner, Russell, & Peterson, 2005; Satpute, Kragel, Barrett, Wager, & Bianciardi, 2019). Although it is often assumed that the neurobiology underlying affect remains stable after reaching adulthood (Davidson, 2003), behavioral findings show age-related shifts in affect from young adulthood (i.e., 18 years old) into late life (i.e., > 60 years old).
For example, cross-sectional and longitudinal studies suggest that older adults tend to experience greater positive and less negative affect, greater low arousal and less high arousal affect, greater affective stability, and less affective reactivity than younger adults (Birditt, Fingerman, & Almeida, 2005; Brose, Scheibe, & Schmiedek, 2013; Bruine de Bruin, van Putten, van Emden, & Strough, 2018; Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Charles & Piazza, 2009; Charles, Reynolds, & Gatz, 2001; Cheng, 2004; Coats & Blanchard-Fields, 2008; English & Carstensen, 2014; Gross et al., 1997; Kan, Garrison, Drummey, Emmert, & Rogers, 2018; Kessler & Staudinger, 2009; MacCormack, Henry, Davis, Oosterwijk, & Lindquist, 2019; Mather & Carstensen, 2005; Mikkelsen, O’Toole, Lyby, Wallot, & Mehlsen, 2019; Mogilner, Kamvar, & Aaker, 2011; Neupert, Almeida, & Charles, 2007; Shallcross, Ford, Floerke, & Mauss, 2013). Similar patterns emerge when perceiving affect in nonverbal expressions such as on faces. In some studies, older adults performed well at identifying positive expressions (e.g., happiness) but were less able to infer the meaning of posed facial expressions conveying negative affect (e.g., sadness; Calder et al., 2003; McDowell, Harrison, & Demaree, 1994; Moreno, Borod, Welkowitz, & Alpert, 1993; but see differing meta-analytic evidence in Ruffman, Henry, Livingstone, & Phillips, 2008 and see Mather & Knight, 2006 for work on threat perception preservation across adulthood). Other studies find that older adults perceive posed angry and happy faces to be less highly arousing than do younger adults (Svärd, Fischer, & Lundqvist, 2014).
Although there is clear behavioral evidence for age-related shifts in affect, we still know relatively little about how neural activity during affect might differ across adulthood. Since the late 1990s, more than two dozen functional magnetic resonance imaging (fMRI) studies have examined differences in functional brain activity during healthy older vs. younger adult affect. Herein, we applied quantitative meta-analysis to statistically summarize this growing literature, identifying which brain regions show the most reliable age-related differences in functional activation during affect across studies of older versus younger adults. Furthermore, we used network-based meta-analytic coactivation analyses to pinpoint the groups of brain regions that most reliably co-activate during affective states for younger and older adults and then identify which of these regions serve as influential hubs. Ultimately, this research has the potential to (i) identify neural mechanisms that may be associated with observed affective differences across adulthood and more generally (ii) underscore how aging nervous systems produce aging minds.
The Brain Basis of Affect
Over a hundred years of research have examined the peripheral and, more recently, central nervous system representations of affect. For instance, since the late nineteenth century, it was recognized that affect is related to and represented in peripheral nervous system changes (e.g., heart rate and skin conductance; Fere, 1888; James, 1890; Tarchanoff, 1890). In recent decades, human functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) have investigated functional brain differences during affect. Meta-analytic summaries of this work in young adults reveal that pleasant and unpleasant states are represented by brain regions spanning subcortical, limbic, and cortical regions (Fusar-Poli et al., 2009; Kober et al., 2008; Lindquist, Satpute, Wager, Weber, & Barrett, 2016; Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012; Murphy, Nimmo-Smith, & Lawrence, 2003; Phan, Wager, Taylor, & Liberzon, 2004; Vytal & Hamann, 2010; Wager, Phan, Liberzon, & Taylor, 2003). More specifically, affective states are associated with increased activity across the brain within regions such as the brainstem; cerebellum; amygdala; basal ganglia; anterior, mid, and posterior insula; ventromedial prefrontal cortex (vmPFC); dorsomedial prefrontal cortex (dmPFC); motor and premotor cortex; ventrolateral and dorsolateral prefrontal cortex; anterior, mid, and posterior regions of the cingulate cortex (ACC, MCC, PCC); temporoparietal cortex; lateral temporal cortex; and visual cortex (e.g., Kober et al., 2008; Lindquist et al., 2012, 2016). These regions interact as sets of broadly distributed functional networks that are thought to perform domain-general functions (i.e., functions not just specific to affect) and are undergirded by the brain’s structural architecture (Barrett & Satpute, 2019; Bressler & Menon, 2010; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008; Park & Friston, 2013; Petersen & Sporns, 2015; Power et al., 2011; Yeo et al., 2011).
Candidate functional networks that likely contribute to affect include the central autonomic, salience, default mode, dorsal attention, and frontoparietal networks. The central autonomic network (i.e., insular cortex, ACC, amygdala, hypothalamus, periaqueductal gray, and parts of the ventrolateral medulla) helps regulate preganglionic sympathetic and parasympathetic neurons and is thought to support the management and integration of visceral signals and functions, including during affect (Benarroch, 1993; Ding et al., 2020; Kleckner et al., 2017). The salience network (i.e., anterior insula, ACC, middle frontal gyrus, MCC, amygdala, ventral striatum, and substantia nigra/ventral tegmental area) helps direct attention and behavior to motivationally relevant stimuli (e.g., noticing a threat, being distracted by cake) (Kleckner et al., 2017; Lindquist & Barrett, 2012; Menon, 2015; Menon & Uddin, 2010; Seeley et al., 2007; Touroutoglou, Andreano, Adebayo, Lyons, & Barrett, 2019; C. Xia, Touroutoglou, Quigley, Barrett, & Dickerson, 2017). Finally, the default mode network (i.e., vmPFC, dmPFC, PCC, lateral prefrontal cortex, and lateral temporoparietal and temporal cortex) supports mentalizing, autobiographical memory, and self-referential, introspective processes, whereas the dorsal attention network (i.e., the intraparietal sulcus, frontal eye fields, superior parietal lobule, and ventral premotor cortex) and the frontoparietal control network (i.e., the dorsolateral and lateral parietal cortex) support perceptual attention, executive function, and some aspects of cognitive control (Buckner, Andrews-Hanna, & Schacter, 2008; Cole, Repovš, & Anticevic, 2014; Denny, Kober, Wager, & Ochsner, 2012; Dixon et al., 2018; Dixon, Thiruchselvam, Todd, & Christoff, 2017; Spreng, Mar, & Kim, 2009; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013; Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010). In the context of affect, the default mode network in particular may support the concepts, autobiographical narratives, and mentalization that help the brain predict and categorize experiences and stimuli as affective in nature (Barrett, 2017; Satpute & Lindquist, 2019). Ultimately, these functional networks show evidence of functional connectivity within and between nodes during affective states or affect perception (see reviews in Barrett & Satpute, 2013; Lindquist & Barrett, 2012; Satpute & Lindquist, 2019; Touroutoglou, Lindquist, Dickerson, & Barrett, 2015). What remains in question is whether and how these brain regions and networks associated with affect differ in their functional profiles across age.
The Neuroscience of Aging
Although much research has examined how brain regions and networks activate during affective states in young adults, less work examines how such functional activation differs and changes across adulthood. Yet there are multiple reasons why one might predict age-related shifts in functional brain activity during affect. First, healthy aging is generally accompanied by structural and functional brain changes (although the severity and types of change can vary across individuals, e.g., “super-agers”: Rogalski et al., 2013; J. Zhang, Andreano, Dickerson, Touroutoglou, & Barrett, 2020). For example, older adult brains tend to exhibit increased gray matter atrophy (especially in frontal regions) and decreases in overall white matter volume when compared with younger adult brains (J. S. Allen, Bruss, Brown, & Damasio, 2005; Bagarinao et al., 2018; Fjell & Walhovd, 2010; Fjell et al., 2013; Good et al., 2001; Smith, Chebrolu, Wekstein, Schmitt, & Markesbery, 2007). These structural differences may impact functional activation in degree and/or kind. During cognitive tasks, older adult brains are typically characterized by increased functional activation in prefrontal regions and greater recruitment across both brain hemispheres relative to younger adults (e.g., Cabeza, Anderson, Locantore, & McIntosh, 2002; Park & Reuter-Lorenz, 2009; Turner & Spreng, 2012). A meta-analysis of functional neuroimaging studies on cognitive aging revealed that older adults exhibit more reliable activation in the prefrontal cortex during cognitive tasks relative to younger adults, whereas younger adults exhibit more reliable activation in exteroceptive sensory regions such as the occipital lobe (Spreng, Wojtowicz, & Grady, 2010). This pattern has been called the posterior-anterior shift in aging (PASA) and describes the increasing functional involvement of prefrontal regions over sensory processing regions as age increases (e.g., Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; McCarthy, Benuskova, & Franz, 2014).
The healthy aging brain also demonstrates reorganization of functional brain networks that may influence the neural basis of affect (see review in Sala-Llonch, Bartrés-Faz, & Junqué, 2015). The default mode and dorsal attention networks appear especially sensitive to age-related disruptions, with relative maintenance of somatosensory and subcortical networks (Tomasi & Volkow, 2012). Indeed, some of the most consistent findings are that late life is accompanied by reductions in default mode and frontoparietal network connectivity both at rest and during cognitive tasks (Andrews-Hanna et al., 2007; Betzel et al., 2014; Campbell, Grady, Ng, & Hasher, 2012; Esposito et al., 2008; He et al., 2014; Nashiro, Sakaki, Braskie, & Mather, 2017; Onoda, Ishihara, & Yamaguchi, 2012; Shaw, Schultz, Sperling, & Hedden, 2015; Liang Wang et al., 2010; Wang, Lubin, Su, Shen, & Hu, 2012; Ward et al., 2015; L. Zhang et al., 2020). More recent work suggests that older adults’ brains are characterized by changes in network integration, with greater between-network connectivity and lower within-network connectivity in visual, sensorimotor, and frontoparietal control networks during cognitive control and attention tasks (Bagarinao et al., 2019). Collectively, these findings could be interpreted as evidence that older adult brains shift function to prefrontal regions to compensate for structural or functional changes elsewhere (Cabeza et al., 2018; Grady et al., 1994) or alternatively that prefrontal regions are increasingly recruited because neural activity in frontal regions becomes less efficient with age due to structural declines predominating in these prefrontal regions (Morcom & Henson, 2018).
Hypotheses about Age-Related Nervous System Shifts During Affect
In addition to structural and functional brain changes associated with cognitive aging more generally, there are known structural and functional changes in the brain and peripheral nervous system that may specifically contribute to age-related differences in affect. First, there are peripheral nervous system changes that may alter affect in later life. The theory of maturational dualism suggests that these structural changes may result in a disconnect between peripheral signals and mental processes such as affect and cognition (MacCormack et al., 2019; Mendes, 2010; Mikkelsen et al., 2019). Although it is debated whether peripheral signals are necessary for affective experience (Barrett, 2017; Barrett & Bliss-Moreau, 2009; Berntson, Gianaros, & Tsakiris, 2018; Cannon, 1927; Ekman & Cordaro, 2011; Feinstein et al., 2016; Friedman, 2010; Garfinkel & Critchley, 2013; Harrison, Gray, Gianaros, & Critchley, 2010; MacCormack & Lindquist, 2017), there is increasing experimental evidence that ongoing efferent signals to and afferent signals from the body can indeed contribute to the quality of affective experiences and perceptions (Durso, Luttrell, & Way, 2015; Eisenberger, Moieni, Inagaki, Muscatell, & Irwin, 2017; Garfinkel et al., 2014; Gray et al., 2012; MacCormack et al., 2020; MacCormack & Muscatell, 2019; Muscatell et al., 2016).
The theory of maturational dualism suggests that peripheral nerve demyelination and neuropathy (i.e., cell death) that occur from mid-to-late life may be a source of age-related changes in affect (Melcangi, Magnaghi, & Martini, 2000; Mendes, 2010; Sato, Sato, & Suzuki, 1985; Verdú, Ceballos, Vilches, & Navarro, 2000). For instance, older adults tend to show less autonomic nervous system reactivity during high arousal affect inductions such as stress, conflict, and amusement (Levenson, Carstensen, Friesen, & Ekman, 1991; Levenson, Carstensen, & Gottman, 1994; Neiss, Leigland, Carlson, & Janowsky, 2009; Tsai, Levenson, & Carstensen, 2000; Uchino, Birmingham, & Berg, 2010). One the one hand, older adults’ reduced autonomic reactivity could be due to them finding certain affective stimuli or situations less aversive or arousing to begin with or due to improved affect regulation, but it is also possible that structural and functional peripheral aging could be contributing to their autonomic blunting. Consistent with the latter explanation, older adults show reduced sensitivity to internal bodily signals (i.e., “interoception”) than younger adults (Khalsa, Rudrauf, & Tranel, 2009; J. Murphy, Geary, Millgate, Catmur, & Bird, 2018), perhaps reflecting changes to both afferent pathways and how the brain represents those afferent signals.
Although the theory of maturational dualism has focused primarily on the peripheral nervous system (Mendes, 2010), it follows that changes to peripheral nerve involvement in emotion might be reflected in central nervous processing of this information. Specifically, relative to older adults, younger adult brains might show greater activation and coactivation of regions and networks involved in autonomic regulation and interoception such as the central autonomic and salience networks. To our knowledge, little research has examined shifts in the autonomic network across adulthood. There are, however, known structural and functional late-life shifts in the salience network. For example, older adult brains show decreased gray matter volume within hubs of the salience network (e.g., insula, dorsal anterior cingulate cortex or dACC) relative to younger adults (He et al., 2014; Sun et al., 2016) as well as altered functional connectivity between hubs within the salience network and between the salience network and other functional networks. Yet some findings are inconsistent: some studies show either preservation or increased age-related salience network connectivity (Cao et al., 2014; Xiao et al., 2018) whereas others show age-related declines in connectivity (E. A. Allen et al., 2011; He et al., 2013, 2014; Onoda et al., 2012; Roski et al., 2013). Still, other work finds divergent patterns of aging depending on which salience subnetwork is examined (Touroutoglou, Zhang, Andreano, Dickerson, & Barrett, 2018). For example, as age increased, there were decreases in coactivation within a dorsal salience subnetwork (e.g., between the dorsal anterior insula and MCC) thought to support attention to affect and cognitive control. On the other hand, as age increased, there was increased coactivation within the ventral subsystem (e.g., between the ventral anterior insula and amygdala) thought to support visceromotor processes and felt arousal.
Beyond the aging of systems supporting visceromotor control and interoception, there are known age-related differences in other functional networks that point to other potential mechanisms for affective aging. For instance, there are age-related shifts in default mode network and frontoparietal network function during affect (see discussions in Martins & Mather, 2016; Mather, 2016). The role of these networks in self-reflective processes and cognitive control has been taken as evidence for socioemotional selectivity theory (SST), which proposes that as older adults approach the end of their lives, they are more likely to prioritize socioemotional goals (e.g., close relationships), including being more motivated to attend to pleasant stimuli and select situations or regulate feelings that promote greater positivity and well-being (Carstensen & DeLiema, 2018; Carstensen, Isaacowitz, & Charles, 1999; Carstensen & Mikels, 2005; Scheibe, English, Tsai, & Carstensen, 2013). These goals may lead older adults to implicitly regulate their affective states in different ways than younger adults. Indeed, it has been suggested that, after building a lifetime of affective “expertise,” older adults may rely more on self-referential processes supported by the default mode network than do younger adults during affect (Martins & Mather, 2016). As such, SST might predict that older adult brains are characterized by greater activity in prefrontal regions within the default mode and frontoparietal networks during affect—as well as altered functional connectivity between frontal and limbic regions in order to facilitate the pursuit of positive and avoidance of negative stimuli and experiences (Martins & Mather, 2016; Mather, 2012; Mather & Carstensen, 2005; Mather & Knight, 2005; Samanez-Larkin & Carstensen, 2011). Furthermore, older adults might show increased or maintained activity within the salience network during the experience or perception of positive affective stimuli (i.e., the “positivity” effect) relative to younger adults, but conversely, younger adults might show greater activity herein during negative affect.
Of course, the predictions outlined by affective aging theories such as maturational dualism and SST may not be mutually exclusive. A first step is to examine which brain regions are most reliably activated during affect in older and younger adults across the literature. The present meta-analysis examined (i) age differences in which brain regions are reliably activated during affect as well as (ii) age differences in which brain regions show reliable functional coactivation, in order to begin clarifying possible neural mechanisms underlying age-related differences in affect.
The Present Meta-Analysis
Roughly two decades of neuroimaging studies have directly compared differences in functional brain activity when younger vs. older adults experience affective states and perceive portrayals of affective behaviors (e.g., affective facial, bodily, and vocal expressions). The primary goal of this meta-analysis was to quantitatively summarize this literature to reveal which regions and sets of coactivated regions most reliably co-occur with affect in older relative to younger adults and vice versa. As a secondary goal, we sought to examine age-related neural differences in valence and arousal, with the hopes of shedding light on theories of affective aging outlined above.
Although individual neuroimaging studies are valuable in their own right, meta-analysis overcomes the limitations associated with sample size, power, and limited experimental designs inherent in individual studies (Cremers, Wager, & Yarkoni, 2017; Turner, Paul, Miller, & Barbey, 2018). Meta-analysis can also reveal the functional neuroanatomy or “neural reference space” (see Lindquist et al., 2012) consistently related to a process of interest and begin weighing in on questions about how those processes might be instantiated in the brain. Our primary aim was to identify the neural reference spaces for younger and older adult brains during affect in general and with respect to valence and arousal. Our second goal was to examine which brain regions were most reliably coactivated for older adults than younger adults and vice versa. We used network-based statistics to reveal which brain regions were most central (i.e., influential) within networks and which were most locally efficient (i.e., tightly interconnected).
Methods
Literature Search and Study Selection
We conducted a review of all functional neuroimaging studies that assessed affective processing in older versus younger adults. Using PRISMA standards (Liberati et al., 2009), we identified and coded individual study-level experimental contrasts from 27 studies containing a total N = 960 healthy participants, with 490 older adults (58% female; Mage = 69.04 years, 55–87 years) and 470 younger adults (53% female; Mage = 24.22 years, 18–39 years). See Table 1 for study-specific demographics and design details. We only included studies that had contrasts for healthy samples of adults. For example, we did not include special population samples such as individuals with Alzheimer’s disease, diabetes, depression, etc. We also strove to only include samples where the older adults were cognitively healthy (i.e., no evidence of clinically significant cognitive declines or impairments). See the Supplementary Materials (SMs) Table S1 for study-specific information on older adult cognitive assessments.
Table 1.
Summary of included studies in the final meta-analytic database
| Study | Older adult N (no. of female), Mage |
Younger adult N (no. of female), Mage | In-scanner task description | Affective categories |
|---|---|---|---|---|
| Allard and Kensinger (2014) | 30 (20), 68.47 years | 34 (16), 23.79 years | Passive affect induction and perception task using video clips | Positive, negative, neutral |
| Brassen, Gamer, and Büchel (2011) | 21 (14), 65.80 years | 22 (8), 25.20 years | Spatial cueing paradigm with affect perception of faces | Happy, fearful, sad, neutral |
| Cassidy, Leshikar, Shih, Aizenman, and Gutchess (2013) | 18 (12), 75.56 years | 19 (11), 24.32 years | Passively viewed facial expressions | Positive, negative, neutral |
| Dolcos, Katsumi, and Dixon (2014) | 16 (11), 68.56 years | 18 (10), 23.61 years | Affect induction with pictures | High arousal negative, medium arousal negative, low arousal negative, neutral |
| Ebner, Johnson, and Fischer (2012) | 32 (18), 68.2 years | 30 (16), 25.1 years | Judged facial expressions | Happy, angry, neutral |
| Everaerd, Klumpers, Oude Voshaar, Fernández, and Tendolkar (2017) | 25 (0), 66.7 years | 25 (0), 21.5 years | Movie clip stress induction followed by in-scanner passive viewing facial expressions | Happy, fearful |
| Fischer et al. (2005) | 22 (11), 74.1 years | 24 (12), 24.7 years | Passively viewed facial expressions | Angry, neutral |
| Fischer, Nyberg, and Bäckman (2010) | 18 (9), 74.3 years | 24 (12), 24.7 years | Judged facial expressions | Fearful, neutral |
| Gunning-Dixon et al. (2003) | 8 (4), 72.3 years | 8 (4), 25.8 years | Judged facial expressions | Happy, sad, angry, fearful, disgusted, neutral |
| Iidaka et al. (2002) | 12 (6), 65.2 years | 12 (6), 25. 1 years | Judged facial expressions | Positive, negative, neutral |
| Kehoe, Toomey, Balsters, and Bokde (2013) | 23 (23), 61.0 years | 23 (23), 23.0 years | Affect induction with pictures; subjects categorized whether things in the pictures were living or non-living | High arousal positive, high arousal neutral, low arousal positive, low arousal neutral |
| Keightley, Chiew, Winocur, and Grady (2007) | 11 (6), 69.6 years | 10 (5), 27.2 years | Judged facial expressions | Angry, contempt, disgusted, fearful, happy, sad, surprised, neutral |
| Kensinger and Schacter (2008) | 17 (11), 73.3 years | 17 (12), 21.6 years | Affect induction with pictures; subjects judged the size of objects in pictures | High arousal negative, high arousal positive, neutral |
| Leclerc and Kensinger (2008) | 20 (13), n/a | 17 (12), n/a | Affect induction with pictures; subjects judged the size of objects in pictures | High arousal negative, high arousal positive, neutral |
| Leclerc and Kensinger (2011): Study 1 | 18 (10), 72.2 years | 18 (10), 21.5 years | Affect induction with pictures using a visual search task | High arousal negative, high arousal positive, neutral |
| Leclerc and Kensinger (2010): Study 2 | 24 (14), 73.7 years | 24 (9), 23.9 years | Affect induction with pictures; subjects judged whether objects were animate or common objects | High arousal negative, high arousal positive, neutral |
| Leclerc and Kensinger (2011) | 19 (11), 71.7 years | 20 (10), 23.4 years | Affect induction with pictures and words while judging whether objects were animate or common objects | High arousal negative, high arousal positive, neutral |
| Murty et al. (2009) | 30 (14), 61.2 years | 30 (14), 25.6 years | Affect induction with pictures while judging whether picture was indoors or outdoors | “Aversive” (high arousal negative), neutral |
| Paradiso et al. (1997) | 8 (6), 62.6 years | Affect induction with film clips | Happy, fearful, “fear-disgust,” neutral | |
| Paradiso, Robinson, Boles Ponto, Watkins, and Hichwa (2003) | 17 (8), 65.0 years | Affect induction and perception with pictures and facial expressions | Happy, sad, neutral | |
| Ritchey, Bessette-Symons, Hayes, and Cabeza (2011) | 15 (7), 66.7 years | 19 (10), 23.2 years | Affect induction with pictures | High arousal positive, high arousal negative, low arousal neutral |
| Roalf, Pruis, Stevens, and Janowsky (2011) | 22 (13), 72.5 years | 14 (7), 25.2 years | Affect induction with passive viewing of pictures | High arousal negative, high arousal positive, low arousal neutral |
| St. Jacques, Dolcos, and Cabeza (2009)* | 15 (15), 70.2 years | 15 (15), 24.8 years | Affect induction with pictures | Positive, negative, neutral |
| St. Jacques, Dolcos, and Cabeza (2010)* | 15 (15), 70.2 years | 15 (15), 24.8 years | Affect induction with pictures | Positive, negative, neutral |
| Tessitore et al. (2005) | 14 (7), 67.0 years | 12 (6), 25.0 years | Judged facial expressions | Fearful, angry, control (a shape) |
| Wright, Wedig, Williams, Rauch, and Albert (2006) | 18 (12), 71.6 years | 18 (12), 24.0 years | Passively viewed facial expressions | Fearful, neutral |
| Zsoldos, Cousin, Klein-Koerkamp, Pichat, and Hot (2016) | 17 (8), 68.6 years | 17 (11), 24.9 years | Judged facial expressions | Fearful, neutral |
*Note that St. Jacques et al. (2009) and St. Jacques et al. (2010) are likely from the same study and sample but report different analyses and contrasts, and thus are both retained in the database. However, we only included the St. Jacques papers once in sample size and demographic counts so as to avoid overestimating the number of unique individuals in the meta-analytic database
Inclusion and Exclusion Criteria
Prior to performing the literature search, we established inclusion and exclusion criteria on the basis of our prior published neuroimaging meta-analyses (Brooks et al., 2017; Kober et al., 2008; Lindquist et al., 2016, 2012; Satpute et al., 2015). Studies could either use fMRI or PET methods, must have included a sample of healthy (non-clinical) older and younger adults (as defined in the given study), and must have used an affect induction or any task where affective stimuli were passively viewed, categorized, or rated in the scanner. Per our prior meta-analytic work, we specifically focused on studies designed to manipulate affective experiences (e.g., feelings of emotion) or affective perceptions (e.g., seeing or hearing affect or emotion in others’ facial, bodily, or vocal behaviors) and excluded studies designed to explicitly measure the neural basis of learning, memory, priming, or pain given that these types of tasks are likely to involve additional psychological and hence neural processes (e.g., studies assessing brain activity during affective learning reveal neural processes linked to learning in addition to affect). Furthermore, although there are hypotheses that older adults might be better at engaging explicit emotion regulation (Birditt & Fingerman, 2005; Sands, Garbacz, & Isaacowitz, 2016; Scheibe & Blanchard-Fields, 2009; although see Livingstone & Isaacowitz, 2019), we excluded these kinds of studies, as explicit emotion regulation involves effortful cognitive control processes that may be distinct from affective experiences or perceptions. This exclusion criterion is consistent with our prior meta-analytic work which also excludes emotion regulation studies (Brooks et al., 2017; Lindquist et al., 2016, 2012; Satpute et al., 2015).
To be included in our database, papers had to (i) conduct functional neuroimaging analyses, (ii) report peak coordinates from an experimental contrast, and (iii) report a contrast that was relevant to the research question (e.g., a contrast examining older adult affect on a drug vs. placebo would not be eligible because the contrast was confounded with drug effects rather than age effects on affect). We considered any peer-reviewed publications in press or published up until January 2019 that met our search parameters. Literature searches were conducted using UNC Libraries (Web of Science, Elsevier, ScienceDirect) and Google Scholar, with Boolean that included combinations of the following words: [Emotion OR Arousal OR Valence OR Affect] AND [Aging OR Old Age OR Older Adult OR Late Adulthood] AND [Brain OR fMRI OR Positron Emission Tomography OR Neural]. Theses, dissertations, and book chapters were excluded. We found 64,724 papers in UNC Libraries using this Boolean, sorted by relevance. In Google Scholar, using the same Boolean, we found roughly 49,700 results, sorted by relevance.
Our literature search team included two trained research assistants plus the first and second authors. The process was overseen by the senior author. The research assistants and second author jointly looked through the combined results for relevant papers. The first author also examined the first 5000 hits on each search to confirm that all relevant papers had been found by the first team. Many items were duplicates, significantly inflating the number of papers. Additionally, although many papers discussed older adult affect or the brain, they did not actually study the brain. Through these concerted efforts, we identified 82 possible papers.
We excluded 14 papers that used EEG or structural/functional connectivity approaches, unless those papers also provided functional activation peak coordinates from relevant contrasts, given that the MKDA method we used does not analyze functional connectivity data. Eight papers were review articles that were examined for other relevant studies but did not contain original data. A further six papers were excluded because they only reported continuous age analyses, but results derived from such analyses are not compatible with MKDA. Another 13 papers contained only contrasts that did not fit our inclusion criteria (e.g., contrasts only compared healthy vs. unhealthy groups; compared older adults on drug vs. placebo). Finally, 15 papers did not actually investigate affective experience or perception, but instead assessed constructs such as empathy, theory of mind, prejudice, or social cognition which we a priori excluded on the basis that these constructs are related to, but distinct from, affective experience or perception. Importantly, we cross-checked papers by the same authors to ensure that all included studies were independent and did not represent different publications from the same sample or analyses.
Thus, the search concluded with a final set of 26 papers spanning 27 studies, of which 26 studies used fMRI and 1 study used PET. (See Fig. S1 in SMs for PRISMA flowchart.) We included the single PET study given that PET and fMRI both measure blood flow and use the same coordinate system; PET inclusion is also common practice in other neuroimaging meta-analyses (e.g., Kraynak, Marsland, Wager, & Gianaros, 2018; Lindquist et al., 2016, 2012; Phan, Wager, Taylor, & Liberzon, 2002; Thayer et al., 2012; Wager, Jonides, & Reading, 2004). Note that the single PET study included in the database only contains within-age contrasts and no between-age contrasts. Given our meta-analytic focus on between-group age differences, contrasts from the single PET study only appear in supplementary analyses that include within-age contrasts.
Coding
After all relevant papers were identified, the first and second authors read all papers, independently coded them, and extracted peak coordinates for the database. Coders met every 5 papers to review coding and ensure that codes were applied correctly and consistently between coders. Coded variables included sample characteristics (e.g., sample size, age, sex), task characteristics (e.g., passive viewing of affective stimuli, judging or categorizing stimuli into emotion categories, etc.), and contrast characteristics—specifically, the age groups (older adults or OAs, younger adults or YAs), valence (negative, neutral, positive), and arousal (high, medium, low) of each target and comparison within the contrast. Of note, all study stimuli were either normed for discrete emotions that differed in valence and arousal (e.g., in face perception tasks) or normed for valence and arousal (e.g., International Affective Picture System; IAPS). Although discrete emotion categories included happy, sad, angry, fear, disgust, contempt, and surprise, the most prevalent category studied was fear. In studies that used affective images such as IAPS, studies generally compared negative, positive, and neutral images that were normed at mid-to-high arousal.
In total, coding produced 72 contrasts that directly contrasted older vs. younger adults, with 41 contrasts where younger adults were the target (YA > OA) and 31 contrasts where older adults were the target age group (OA > YA). See Table 2. There were an additional 92 contrasts that contrasted within-age group effects (e.g., OA negative affect > OA neutral affect) which are relevant for additional results presented in the SMs.
Table 2.
Study and between-age contrast characteristics within the meta-analytic database
| Characteristic | No. of studies | OA > YA no. contrasts | YA > OA no. contrasts |
|---|---|---|---|
| Affective process | |||
| Experience | 15 | 23 | 29 |
| Perception | 10 | 7 | 11 |
| Both | 2 | 1 | 1 |
| Affective stimuli | |||
| Facial expressions | 12 | 9 | 12 |
| Affective images | 13 | 21 | 27 |
| Film clips | 1 | 1 | 0 |
| Words | 1 | 0 | 2 |
| Affective task | |||
| Emotion judgment | 15 | 16 | 23 |
| Passive viewing | 6 | 10 | 9 |
| Likert rating | 6 | 5 | 9 |
Meta-Analytic Approach
Functional Activations
To assess age differences in functional activations during affect, we used multilevel kernel density analysis (MKDA; Kober et al., 2008; Kober & Wager, 2010; Salimi-Khorshidi, Smith, Keltner, Wager, & Nichols, 2009; Wager, Lindquist, & Kaplan, 2007; Wager, Lindquist, Nichols, Kober, & Van Snellenberg, 2009), which has been extensively validated and used in functional neuroimaging meta-analyses of human affect, pain, and cognition over the past decade. MKDA is used to compute meta-analytic summary contrasts of brain regions that are more reliably active than would be expected by chance during one condition vs. another (e.g., OAs > YAs) across the literature. Using the Matlab toolbox NeuroElf (http://neuroelf.net/), the MKDA uses voxel-wise peak activations within study contrasts to generate a meta-analytic map of neural activity.
As per standard MKDA and neuroimaging meta-analytic procedures (Kober et al., 2008; Müller et al., 2018; Wager et al., 2007), contrast coordinates in Talairach space were first converted to MNI space and then convolved using a smoothing kernel of 12 mm to produce binary indicator contrast maps. In most neuroimaging meta-analyses, coordinates are convolved with spheres between 10 and 15 mm, and we picked 12 mm, building off prior data-driven approaches and other similar studies using MKDA (Brooks et al., 2017; Lindquist et al., 2016, 2012; Salimi-Khorshidi et al., 2009). MKDA weights study contrasts by sample size and down-weights study contrasts that do not model random effects in their analyses, with the goal of ensuring that adequately powered, more generalizable studies contribute more strongly to the meta-analytic findings. Thus, contrast maps were weighted by the square root of the sample size. Studies that used only fixed-effects analysis techniques were then down weighted by 0.75. These weighted averages of the kernels across individual study contrasts were then used to derive a map of proportional brain activation from N contrasts. Ultimately, MKDA treats contrasts as independent and nest coordinates within contrasts to control for dependencies therein. We carried out Monte Carlo tests to assess the significance of each voxel in the MKDA maps. In particular, we simulated 5000 samples to create a null distribution of the expected maximum proportion of voxels with significant activation that is greater than expected by chance, according to which all MKDA maps were thresholded. For all analyses, we set this a priori threshold to a stringent height-based threshold of p < .01 (using family-wise error or FWE-correction for multiple comparisons) to determine whether voxels were significant. However, given the small size of our database and given that this is the first meta-analysis to examine age-related differences in functional brain activity during affect, we also report exploratory findings for regions that showed significant differences at the less stringent FWE-correction thresholds of p < .02 and p < .05. As part of quality control, we confirmed that coordinates for all meta-analytic maps were located in gray matter and not ventricles.
We performed two different rounds of MKDAs. Of primary interest, we first examined brain regions that were reliably active across affective contrasts comparing older and younger adults. Specifically, we compared YA > OA and OA > YA study contrasts across all tasks and dimensions of affect (e.g., negative affect > neutral affect and positive affect > neutral affect). The goal with these analyses was to produce a “neural reference space” representing functional brain activations that are reliably more activated across studies for younger adults compared with older adults or that are reliably more activated for older adults compared with younger adults during any affective experience or perception. These maps are akin to assessing the reliable brain activity for younger and older adults across the literature. (For full neural reference space across affect and age groups, see SMs Table S2). After determining age differences across all affective contrasts, we next examined age differences in functional activation for specific dimensions of affect, broken down by valence (e.g., negative affect > neutral affect, positive affect > neutral affect) and arousal (e.g., high arousal > low and medium arousal, medium arousal > high and low arousal, low arousal > high and medium arousal). These maps are akin to assessing the average reliable brain activity for each condition of interest: i.e., brain activation in younger adults (YA > OA) or older adults (OA > YA) for a specific aspect of valence or arousal. These maps also are the most specific analyses, allowing us to pinpoint age differences in affect.
In addition to the primary analyses examining between-age group contrasts (e.g., OA > YA or YA > OA) across affect, valence, and arousal, we also conducted secondary analyses that contrasted both sets of age contrasts against each other, i.e., [(YA > OA) > (OA > YA)] and [(OA > YA) > (YA > OA)]. The motivation for these additional analyses was to determine the specificity of contrast effects observed by subtracting out anything due to chance within each age group. Although less straightforward to interpret than the simpler YA > OA and OA > YA contrasts, these secondary analyses allowed us to combine all YA > OA and OA > YA contrasts and thus provided more power to provisionally examine contrast effects. These secondary results are reported in the SMs (Tables S3-S4). Finally, we were most interested in age differences across affective processes overall and by valence vs. arousal. However, we did conduct supplementary analyses to assess potential age differences by affective experience vs. perception. Interestingly, there were only some age differences by type of affective process. These findings are presented in the SMs (Table S5-S6).
Functional Coactivation
To assess age differences in functional coactivation during affect, we followed a method similar to Robinson, Laird, Glahn, Lovallo, and Fox’s (2009) approach to meta-analytic coactivation modeling. First, we extracted separate functional activation coordinates from the neural reference spaces for YA > OA and OA > YA that were identified by MKDA as being the most reliably activated across studies. Using these MKDA-thresholded coordinates, we extracted study-level activation frequencies for each coordinate at the level of the MKDA contrast, coded as 1 = coordinate activation was observed, 0 = coordinate activation was not observed. From these binary variables, we then computed phi-coefficients (which are computationally equivalent to Pearson correlations) between coordinates to determine coactivation of regions at the meta-analytic level. To test the significance of these phi-coefficients, we used the χ2 test of independence for binary variables. To correct for multiple comparisons and minimize false positives, we used the Benjamani-Hochberg correction to control for false discovery rate at the nominal level of 0.05 (Benjamini & Hochberg, 1995). All coactivation analyses were conducted in R. Coactivation visualizations of the significant nodes that survived multiple comparisons correction were created using BrainNet Viewer (M. Xia, Wang, & He, 2013) (https://www.nitrc.org/projects/bnv/).
To characterize coactivation patterns, we computed network statistics on the coactivation patterns between functional coordinates (“nodes”) that survived multiple comparisons correction. Specifically, we computed three network statistics for the YA > OA and OA > YA coactivation networks: (1) eigenvector centrality (Borgatti, 2005), (2) betweenness centrality (Freeman, 1977), and (3) local efficiency (Latora & Marchiori, 2001). Eigenvector centrality indicates how central a node is to the network overall (e.g., how many edges a node shares with other nodes, taking into account whether related nodes are also central). Nodes with high eigenvector centrality indicate that a given node shares many edges with other central nodes; nodes with high eigenvector centrality are thus particularly influential in relation to other nodes. Betweenness centrality indicates whether a node is more likely to serve as a “bridge” or “bottleneck” when connecting other sets of nodes. Nodes with higher betweenness help connect other nodes with each other and thus may help “gate” the flow of information across a network. Finally, local efficiency is a measure of how closely connected a node’s immediate neighbors are. A node with high local efficiency has nodal neighbors that are tightly interconnected, making it more efficient for information to flow between nearby nodes.
We focused on these three network statistics because they provide a broad descriptive view of network topology. Given that the YA > OA and OA > YA networks have different node sets and edge densities, it is important to note that the value of network statistics is not directly comparable across YA > OA and OA > YA networks (van Wijk, Stam, & Daffertshofer, 2010). Rather, these statistics characterize key nodes within each network. Moreover, network statistic values are not standardized scales so values cannot be compared between metrics (e.g., between eigenvector centrality and betweenness centrality). Values can, however, be interpreted relative to other node values within the same metric (e.g., within local efficiency). It is also possible to compare the relative rankings of nodes between networks (e.g., if the insula were to have the highest eigenvector centrality in both networks) for descriptive purposes. Network properties were analyzed and visualized with the igraph (http://igraph.org) and brainGraph (https://github.com/cwatson/brainGraph) packages in R (Csardi & Nepusz, 2006; Watson, 2018). Layouts for network metrics were computed using the Fruchterman-Reingold algorithm (Fruchterman & Reingold, 1991).
Results
Age Differences in Functional Activation during Affect
Age Differences in Affect
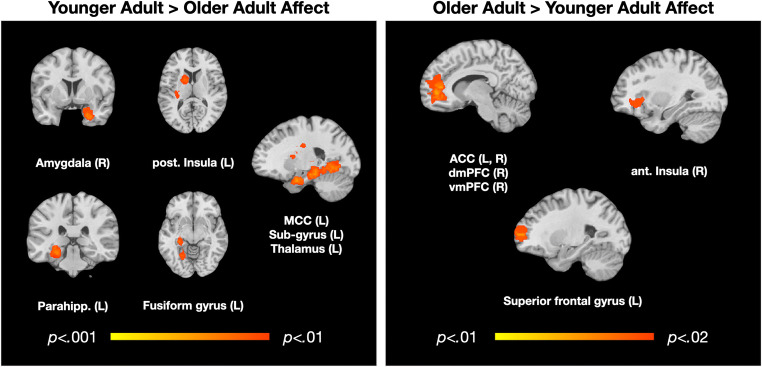
We first examined brain regions that were more frequently activated in YA > OA across affect (375/553 points; 40/72 contrasts). We observed three clusters centered on the right amygdala (27, 3, − 36; k = 420, p < .01), left rostral parahippocampal gyrus (− 24, − 33, − 15; k = 745, p < .01), and the mid-cingulate cortex (MCC; 6, 6, 24; k = 386, p < .01). The right amygdala cluster extended into the hippocampus, the entorhinal and perirhinal cortex, the inferior frontal gyrus including some anterior (more ventral) insula, and other parts of the parahippocampal gyrus. The left rostral parahippocampal cluster extended into the left amygdala, hippocampus, fusiform gyrus, superior temporal gyrus, posterior insula extending into the somatosensory cortex, the agranular retrolimbic area extending into the lingual gyrus, and the lingual gyrus and thalamus. The MCC cluster included the anterior MCC and extended into the left caudate body. See Table 3 and Fig. 1.
Table 3.
Coordinates for younger > older adult affect, k-threshold of p < .01, FWE-corrected for multiple comparisons
| Region | BA | x | y | z | k | Max | Mean |
|---|---|---|---|---|---|---|---|
| R amygdala (cluster) | 29 | 0 | − 25 | 420 | 0.21 | 0.13 | |
| R amygdala | 29 | 0 | − 25 | 0.21 | 0.15 | ||
| R hippocampus | 34 | − 8 | − 19 | 0.18 | 0.14 | ||
| R parahippocampal gyrus | 34 | 22 | 0 | − 11 | 0.18 | 0.14 | |
| R parahippocampal gyrus | 36 | 23 | − 30 | − 12 | 0.15 | 0.12 | |
| R inferior frontal gyrus | 13 | 35 | 6 | − 13 | 0.15 | 0.13 | |
| R culmen | 24 | − 44 | − 11 | 0.14 | 0.11 | ||
| R parahippocampal gyrus | 35 | 20 | − 30 | − 7 | 0.14 | 0.11 | |
| R hippocampus | 33 | − 20 | − 11 | 0.14 | 0.12 | ||
| R inferior frontal gyrus | 47 | 30 | 14 | − 20 | 0.13 | 0.12 | |
| R parahippocampal gyrus | 36 | 33 | − 33 | − 13 | 0.11 | 0.11 | |
| R parahippocampal gyrus | 19 | 21 | − 48 | − 4 | 0.11 | 0.11 | |
| L parahippocampal gyrus (cluster) | 27 | − 23 | − 31 | − 8 | 745 | 0.21 | 0.13 |
| L parahippocampal gyrus | 27 | − 23 | − 31 | − 8 | 0.21 | 0.14 | |
| L amygdala | − 27 | − 4 | − 20 | 0.20 | 0.14 | ||
| L parahippocampal gyrus | 28 | − 20 | − 23 | − 9 | 0.19 | 0.14 | |
| L parahippocampal gyrus | 18 | − 25 | − 57 | 3 | 0.18 | 0.14 | |
| L fusiform gyrus | 19 | − 22 | − 66 | − 4 | 0.18 | 0.13 | |
| L superior temporal gyrus | 38 | − 37 | 5 | − 18 | 0.15 | 0.12 | |
| L hippocampus | − 33 | − 12 | − 17 | 0.15 | 0.12 | ||
| L superior temporal gyrus | 38 | − 46 | 4 | − 10 | 0.15 | 0.13 | |
| L posterior insula | 13 | − 34 | − 19 | 12 | 0.14 | 0.11 | |
| L agranular retrolimbic area | 30 | − 23 | − 70 | 6 | 0.13 | 0.11 | |
| L hippocampus | − 27 | − 41 | 1 | 0.13 | 0.12 | ||
| L lingual gyrus | 18 | − 12 | − 57 | 6 | 0.11 | 0.11 | |
| L thalamus | − 27 | − 24 | 4 | 0.11 | 0.11 | ||
| R mid-cingulate (cluster) | 24 | 7 | 5 | 23 | 396 | 0.19 | 0.12 |
| R mid-cingulate | 24 | 7 | 5 | 23 | 0.19 | 0.13 | |
| L caudate body | − 16 | 9 | 23 | 0.18 | 0.12 | ||
| L caudate body | − 16 | 8 | 17 | 0.16 | 0.12 | ||
| L anterior mid-cingulate | 24 | − 4 | 15 | 23 | 0.14 | 0.12 | |
| L mid-cingulate | 24 | − 3 | − 6 | 28 | 0.15 | 0.12 | |
| L caudate | − 6 | 8 | 16 | 0.13 | 0.12 | ||
| L caudate | − 13 | 11 | 9 | 0.12 | 0.12 | ||
| R mid-cingulate | 24 | 10 | 8 | 33 | 0.11 | 0.10 |
Clusters are italicized
BA Brodmann Area, x, y, z coordinates in Montreal Neurological Institute (MNI) space, k cluster size in mm3, max maximum value within cluster, mean average value within cluster, L left, R right
Fig. 1.
Regions of significant, reliable functional activations differing by age across affect. P values represent thresholds with FWE-corrections for multiple comparisons
Next, we examined brain regions that more frequently activated in OA > YA across affect (192/553 points; 31/72 contrasts). Here, we did not observe any significant clusters of activation at p < .01. However, at p < .02, we observed one large cluster centered on the right dACC (11, 46, − 1; k = 1149), that also included a large cluster in the medial prefrontal cortex (primarily dmPFC but also some vmPFC), with some activation also in the rostral superior frontal cortex, right anterior insula, and left and right caudate. See Table 4 and Fig. 1.
Table 4.
Coordinates for older > younger adult affect, k-threshold of p < .02, FWE-corrected for multiple comparisons
| Region | BA | x | y | z | k | Max | Mean |
|---|---|---|---|---|---|---|---|
| R anterior cingulate (cluster) | 32 | 11 | 46 | − 1 | 1149 | 0.16 | 0.10 |
| R anterior cingulate | 32 | 11 | 46 | − 1 | 0.16 | 0.11 | |
| R anterior cingulate | 32 | 14 | 39 | 9 | 0.16 | 0.11 | |
| L superior frontal gyrus | 10 | − 29 | 60 | 5 | 0.16 | 0.11 | |
| L caudate body | − 13 | 27 | 8 | 0.15 | 0.10 | ||
| R medial frontal gyrus | 10 | 11 | 47 | 7 | 0.15 | 0.10 | |
| R middle frontal gyrus | 10 | 25 | 43 | − 2 | 0.13 | 0.11 | |
| R caudate head | 20 | 29 | 2 | 0.13 | 0.10 | ||
| R superior frontal gyrus | 11 | 21 | 44 | − 14 | 0.13 | 0.10 | |
| R medial frontal gyrus | 10 | 10 | 38 | − 6 | 0.13 | 0.10 | |
| L superior frontal gyrus | 10 | − 15 | 60 | 9 | 0.12 | 0.10 | |
| L medial frontal gyrus | 9 | − 4 | 42 | 22 | 0.12 | 0.10 | |
| L anterior cingulate | 32 | − 7 | 36 | 14 | 0.12 | 0.09 | |
| R anterior insula | 13 | 33 | 18 | 5 | 0.12 | 0.09 | |
| L caudate body | − 3 | 14 | 11 | 0.12 | 0.09 | ||
| R medial frontal gyrus | 9 | 8 | 49 | 18 | 0.12 | 0.09 | |
| L anterior cingulate | 32 | − 7 | 35 | − 2 | 0.12 | 0.09 | |
| R inferior frontal gyrus | 47 | 40 | 26 | 1 | 0.09 | 0.09 | |
| R inferior frontal gyrus | 47 | 38 | 35 | 0 | 0.09 | 0.09 | |
| R medial frontal gyrus | 10 | 15 | 60 | − 5 | 0.09 | 0.09 | |
| R medial frontal gyrus | 10 | 12 | 60 | 12 | 0.09 | 0.09 | |
| R medial frontal gyrus | 10 | 8 | 57 | 20 | 0.09 | 0.09 |
Clusters are italicized
BA Brodmann area, x, y, z coordinates in Montreal Neurological Institute (MNI) space, k cluster size in mm3, max maximum value within cluster, mean average value within cluster, L left, R right
Age Differences in Valence
Next, we examined brain regions that more frequently activated in YA > OA during negative and positive affect. Negative affect contrasts (197/553 points; 20/72 contrasts) included study contrasts wherein negative > neutral or negative > positive affect. Here, we observed one cluster in the left amygdala (− 18, − 5, − 16, k = 388, p < .01), which also included the hippocampus, rostral parahippocampal gyrus, parts of the entorhinal and perirhinal cortices, and the sublobar lateral geniculum body in the thalamus. There were only a few positive affect contrasts for younger > older adults (71/553 points; 8/72 contrasts including contrasts with positive > neutral or positive > negative affect). We found no significant clusters, even at exploratory p < .02 and p < .05 thresholds, although it should be noted that this analysis is likely underpowered due to the small sample of contrasts. See Table 5.
Table 5.
Coordinates for younger > older adult negative and high arousal affect, k-threshold of p < .01 and .02, FWE-corrected for multiple comparisons
| Region | BA | x | y | z | k | Max | Mean |
|---|---|---|---|---|---|---|---|
| Negative affect | |||||||
| L amygdala (cluster) | − 18 | − 5 | − 16 | 388 | 0.29 | 0.21 | |
| L amygdala | − 18 | − 5 | − 16 | 0.29 | 0.23 | ||
| L parahippocampal gyrus | 28 | − 20 | − 28 | − 10 | 0.26 | 0.21 | |
| L hippocampus | − 33 | − 12 | − 17 | 0.26 | 0.18 | ||
| L parahippocampal gyrus | 27 | − 27 | − 29 | − 3 | 0.26 | 0.19 | |
| L parahippocampal gyrus | 35 | − 20 | − 21 | − 9 | 0.21 | 0.21 | |
| L sublobar lateral geniculum body | − 24 | − 21 | − 2 | 0.16 | 0.16 | ||
| High arousal affect | |||||||
| R mid-cingulate (cluster) | 24 | 3 | − 3 | 27 | 467 | 0.25 | 0.18 |
| R mid-cingulate | 24 | 3 | − 3 | 27 | 0.25 | 0.18 | |
| R mid-cingulate | 24 | 7 | 5 | 23 | 0.25 | 0.18 | |
| L mid-cingulate | 24 | − 7 | 0 | 28 | 0.25 | 0.18 | |
| L caudate body | − 16 | − 9 | 23 | 0.25 | 0.20 | ||
| L caudate body | − 13 | 11 | 14 | 0.20 | 0.17 | ||
| L anterior mid-cingulate | 33 | − 3 | 18 | 16 | 0.20 | 0.16 | |
| L posterior cingulate | 23 | − 3 | − 11 | 33 | 0.20 | 0.17 | |
| L thalamus | − 22 | − 15 | 16 | 0.20 | 0.15 | ||
| L caudate body | − 16 | 2 | 21 | 0.15 | 0.15 | ||
| R mid-cingulate | 24 | 10 | 0 | 39 | 0.15 | 0.15 | |
| R medial frontal gyrus (cluster) | 10 | 15 | 54 | 1 | 477 | 0.25 | 0.15 |
| R medial frontal gyrus | 10 | 15 | 54 | 1 | 0.25 | 0.17 | |
| R anterior cingulate | 24 | 4 | 38 | 3 | 0.25 | 0.18 | |
| R medial frontal gyrus | 10 | 4 | 57 | 9 | 0.20 | 0.17 | |
| R medial frontal gyrus | 9 | 8 | 42 | 14 | 0.20 | 0.15 | |
| L anterior cingulate | 32 | − 8 | 39 | 14 | 0.20 | 0.17 | |
| R anterior cingulate | 0 | 47 | 1 | 0.20 | 0.16 | ||
| L medial frontal gyrus | 9 | − 4 | 44 | 24 | 0.19 | 0.16 | |
| R medial frontal gyrus | 10 | 19 | 62 | − 7 | 0.15 | 0.15 | |
| R medial frontal gyrus | 9 | 12 | 47 | 21 | 0.15 | 0.15 | |
| R medial frontal gyrus | 10 | 8 | 60 | 17 | 0.15 | 0.15 | |
| R medial frontal gyrus | 10 | 18 | 41 | 9 | 0.15 | 0.15 | |
Clusters are italicized
Nothing was significant for YA > OA Positive Affect at p < .01, .02, or .05. There were insufficient contrasts to examine YA > OA low arousal affect
BA Brodmann Area, x, y, z coordinates in Montreal Neurological Institute (MNI) space, k cluster size in mm3, max maximum value within cluster, mean average value within cluster, L left, R right
Turning to older adults, we examined brain regions that more frequently activated in OA > YA for contrasts where negative affect was the target (60/553 points; 13/72 contrasts). We observed one cluster in the right somewhat more dorsal ACC (14, 39, 9, k = 435, p < .01) that extended primarily into the dmPFC but also included some dorsolateral prefrontal cortex (dlPFC) and rostral parts of the superior and middle frontal gyrus. For positive affect (68/553 points; 8/72 contrasts), there were no significant clusters of activation at p < .01, again likely due to the small number of contrasts. At p < .02, we found a very large cluster of activation centered in the left caudate head and left ACC (− 10, 23, 5, k = 5093), but this large cluster also extended into the retrosplenial cortex, putamen, postcentral gyrus which included the primary somatosensory cortex, the pulvinar, thalamus, and the dorsolateral prefrontal cortex along with other activations in the medial and superior frontal cortex. Note that as a general rule of thumb, an MKDA with 10 contrasts or fewer is considered relatively unreliable, so such results should be taken as provisional. See Table 6, but also Table S7 for additional exploratory analyses with positive affect.
Table 6.
Coordinates for older > younger adult negative and high arousal affect, k-threshold of p < .01, FWE-corrected for multiple comparisons
| Region | BA | x | y | z | k | Max | Mean |
|---|---|---|---|---|---|---|---|
| Negative affect | |||||||
| R anterior cingulate (cluster) | 32 | 14 | 39 | 9 | 435 | 0.23 | 0.15 |
| R anterior cingulate | 32 | 14 | 39 | 9 | 0.23 | 0.14 | |
| R medial frontal gyrus | 9 | 19 | 41 | 23 | 0.21 | 0.14 | |
| R superior frontal gyrus | 9 | 15 | 41 | 36 | 0.21 | 0.15 | |
| R middle frontal gyrus | 11 | 20 | 37 | −1 | 0.16 | 0.16 | |
| R middle frontal gyrus | 9 | 35 | 45 | 36 | 0.15 | 0.15 | |
| High arousal affect | |||||||
| R middle frontal gyrus (cluster) | 10 | 25 | 43 | − 2 | 561 | 0.23 | 0.15 |
| R middle frontal gyrus | 10 | 25 | 43 | − 2 | 0.23 | 0.17 | |
| R anterior cingulate | 32 | 15 | 46 | − 1 | 0.23 | 0.17 | |
| R caudate head | 20 | 29 | 2 | 0.23 | 0.15 | ||
| R inferior frontal gyrus | 13 | 32 | 9 | − 13 | 0.16 | 0.13 | |
| R medial frontal gyrus | 10 | 15 | 51 | − 10 | 0.16 | 0.16 | |
| R inferior frontal gyrus | 47 | 40 | 26 | 1 | 0.16 | 0.16 | |
| R inferior frontal gyrus | 47 | 38 | 35 | 0 | 0.16 | 0.16 | |
| R medial frontal gyrus | 10 | 19 | 47 | 11 | 0.16 | 0.16 | |
| R inferior frontal gyrus | 47 | 42 | 14 | − 8 | 0.12 | 0.12 | |
Clusters are italicized
There were insufficient contrasts to examine OA > YA positive affect and low arousal affect
BA Brodmann Area, x, y, z coordinates in Montreal Neurological Institute (MNI) space, k cluster size in mm3, max maximum value within cluster, mean average value within cluster, L left, R right
Age Differences in Arousal
Next, we examined brain regions that reliably activated in younger > older adults during high and low arousal affect. For YA > OA contrasts where high arousal affect was the target relative to low and medium arousal (238/553 points; 20/72 contrasts), there were no significant clusters of activation at the a priori threshold of p < .01. Exploratory analyses at p < .02 revealed two significant clusters. The first cluster centered in the right mid-cingulate cortex (MCC; 3, −3, 27, k = 467, p < .02) extending into the anterior MCC (aMCC), left and right caudate body, and left thalamus. The second cluster centered in the ventromedial prefrontal cortex (vmPFC; 15, 54, 1, k = 477, p < .02) extending into aMCC, dACC, and dmPFC. For YA > OA low arousal affect, there were insufficient contrasts (i.e., only 2) to compute this MKDA comparison. See Table 5.
For OA > YA contrasts where high arousal affect was the target relative to low and medium arousal (132/553 points; 18/72 contrasts), there was one significant cluster of activation centered in the middle frontal gyrus and anterior portions of the right vmPFC (25, 43, − 2, k = 561, p < .01) extending into the dACC, right caudate, and more dorsal, anterior parts of the insula. For OA > YA low arousal affect, there were insufficient contrasts (i.e., only 1) to compute this MKDA comparison. See Table 6.
Secondary Meta-Analytic Contrasts
Finally, we contrasted both sets of age contrasts against each other, i.e., [(YA > OA) > (OA > YA)] and [(OA > YA) > (YA > OA)] in order to determine the specificity of contrast effects observed by subtracting out anything due to chance within each age group. Results largely replicated those reported for the YA > OA and OA > YA contrasts. See Tables S3-S4 in SMs. For example, when YA > OA was the target contrast (relative to OA > YA), activations in the left and right amygdala and parahippocampal gyrus predominated across affect as well as within negative affect. On the other hand, when OA > YA was the target contrast (relative to YA > OA), a single cluster of activation centered in the dmPFC and dACC emerged as significant across affect. For negative affect when OA > YA was the target, we observed a single cluster with its peak of activation in the superior frontal gyrus spanning the dmPFC into the dACC and inferior frontal gyrus. With a few more contrasts afforded by this less conservative analysis, we also observed greater activation in the left caudate and right thalamus for positive affect when YA > OA was the target. However, we still did not find any significant effects for positive affect when OA > YA was the target.
For high arousal affect when YA > OA was the target, significant peak activation was also observed in the left lentiform nucleus, caudate, and thalamus. In high arousal contrasts where OA > YA was the target, significant clusters emerged in the left prefrontal cortex and caudate. Importantly, with this less conservative analysis, we were also able to examine age differences in low arousal affect. For low arousal contrasts with YA > OA as the target, one large cluster occurred in the left uncus extending throughout the superior temporal gyrus and parts of the vmPFC as well as a second large cluster with its peak of activation in the right culmen. For low arousal contrasts wherein OA > YA was the target, activation centered in the dorsal and pregenual ACC extending into the vmPFC. Analyses with positive and low arousal affect still include fewer contrasts relative to contrasts with negative and high arousal affect; therefore, we caution that the positive and low arousal affect findings should be viewed as provisional.
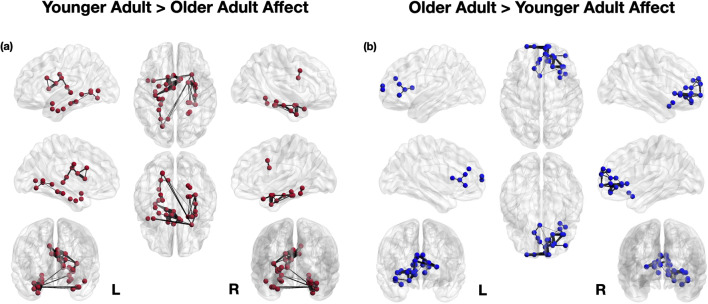
Age Differences in Functional Coactivation During Affect
Using the whole-brain meta-analytic coordinates identified in the primary YA > OA affect and OA > YA affect neural reference spaces, we next analyzed patterns of functional coactivation between these meta-analytically thresholded regions (Fig. 2). For YA > OA, nodes in the amygdala and parahippocampal gyrus, inferior and superior temporal gyrus, posterior insula, thalamus, lingual gyrus, caudate, and MCC were most reliably coactivated during affect, even after correcting for multiple comparisons. In particular, younger adults showed greater coactivation between multiple nodes within the parahippocampus (extending into amygdala and hippocampus). There was also a cluster of coactivating nodes centered in the mid-cingulate and caudate, as well as coactivations between the posterior insula with the thalamus, MCC, and caudate.
Fig. 2.
Meta-analytic coactivation of older and younger adult brain regions during affect, corrected for false discovery rate
On the other hand, for OA > YA, nodes in the ACC, caudate, anterior insula, and frontal gyrus were most reliably coactivated during affect, even when correcting for multiple comparisons. In particular, nodes in the ACC, dmPFC, mid-frontal, superior frontal, and caudate tended to co-activate together during affect. To a lesser extent, there were also coactivations between the inferior frontal gyrus, anterior insula, and parts of the medial and mid-frontal cortex. Full coactivation correlation matrices are reported in the SMs.
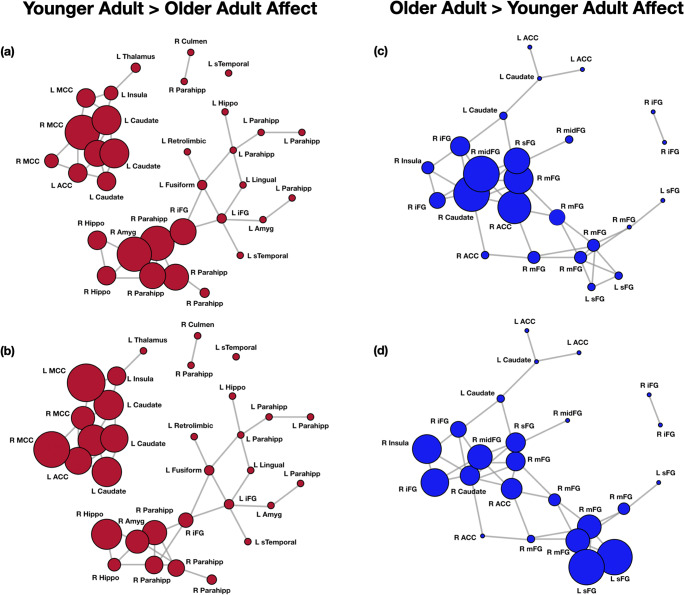
Finally, to better identify important nodes within the YA > OA and OA > YA networks for affect, we computed the eigenvector centrality, betweenness centrality, and local efficiency of nodes within each network. See Fig. 3 and relevant tables in the SMs. For the YA > OA network, nodes with the highest eigenvector centrality (EC) values were the right MCC (x = 7, y = 5, z = 23; EC = 0.48), left caudate (− 16, 8, 17; − 16, 9, 23; − 6, 8, 16; − 13, 11, 9; ECs = 0.42, 0.40, 0.37, 0.25 respectively), anterior MCC (− 4, 15, 23; EC = 0.28), left MCC (− 3, − 6, 28; EC = 0.26), and left posterior insula (− 34, − 19, 12; EC = 0.23). Nodes with highest betweenness centrality (BC) were the right inferior frontal gyrus (35, 6, − 13; 30, 14, − 20; BC = 0.17, 0.12 respectively), right amygdala (29, 0, − 25; BC = 0.11), left fusiform (− 22, − 66, − 4; BC = 0.11), and left parahippocampus (− 25, − 57, 3; BC = 0.10). Nodes with the highest local efficiency (LE) were the left and right MCC (− 3, − 6, 28; 10, 8, 33; both LE = 1.00), right hippocampus (34, − 8, − 19; LE = 0.83), several nodes in the left caudate (LE = 0.83–0.80), as well as the right parahippocampus (22, 0, − 11; LE = 0.73) and left anterior MCC (− 4, 15, 23; LE = 0.72).
Fig. 3.
Network depictions of meta-analytic regions varying by centrality and efficiency in older vs. younger adult brains during affect. a Eigenvector centrality for YA > OA affect, with greater centrality in the right and left mid-cingulate cortex (MCC), left caudate, right amygdala (amyg), and parts of right parahippocampus (parahipp). b Local efficiency for YA > OA affect, with greater efficiency in the right and left mid-cingulate cortex (MCC), left caudate and insula, right amygdala (amyg), and parts of right hippocampus and parahippocampus (hippo; parahipp). c Eigenvector centrality for OA > YA affect, with greater centrality revealed for right caudate, mid-frontal gyrus (midFG), medial frontal gyrus (mFG), and right anterior cingulate (ACC). d Local efficiency for OA > YA affect, with greater efficiency in right anterior insula, inferior, medial, and mid-frontal gyrus (iFG, mFG, midFG), as well as left superior frontal gyrus (sFG)
For the OA > YA network, nodes with the highest eigenvector centrality values were nodes in the right caudate (20, 29, 2, EC = 1.00), right mid-frontal gyrus (25, 43, − 2; EC = 0.99), right ACC (11, 46, − 1; EC = 0.95), right medial frontal gyrus (10, 38, − 6; EC = 0.87), and the right superior frontal gyrus (21, 44, − 14; EC = 0.79). Nodes with the highest betweenness centrality were the right medial frontal gyrus (10, 38, − 6; BC = 0.28), left caudate (− 3, 14, 11; BC = 0.25), right ACC S1 (11, 46, − 1; BC = 0.19), right medial frontal gyrus (11, 47, 7; BC = 0.19), and the right and left caudate S7 S4 (20, 29, 2; − 13, 27, 8; BC = 0.16, 0.15 respectively). Finally, the nodes with greatest local efficiency were the left superior frontal gyrus S3 S10 (− 29, 60, 5; − 15, 60, 9; both LE = 1.00), right anterior insula (33, 18, 5; LE = 0.83), right inferior frontal gyrus (38, 35, 0; LE = 0.83), right mid-frontal gyrus (25, 43, − 2; LE = 0.76), and the right medial frontal gyrus (12, 60, 12; LE = 0.7). See SMs for additional tables.
Discussion
Age Differences in the Neural Reference Space for Affect
We present the first known meta-analysis that examines age differences between younger and older adults’ functional brain activation and coactivation during affect. When pooling across the experience and perception of affective valence and arousal, we found that younger adults exhibited more reliable activation than older adults in subcortical structures such as the bilateral amygdala, hippocampus, and thalamus, whereas older adults had more reliable activation in prefrontal cortical regions such as the medial (especially dmPFC), middle, superior, and inferior frontal gyrus. Relative to older adults, younger adults also exhibited more posterior activation both within specific brain structures and with respect to the whole brain. For instance, younger adults had more reliable activity in the posterior insula and MCC during affect, whereas older adults had more reliable activity in the anterior insula and dACC during affect. These findings may be consistent with evidence on age-related changes in domain-general brain function, insofar as they may indicate a possible posterior-to-anterior shift in aging from sensory processing regions to prefrontal regions with increasing age (i.e., PASA; Davis et al., 2008; McCarthy et al., 2014). As such, our findings are consistent with some effects found in cognitive aging neuroscience.
We also found that younger adults had more reliable activity than older adults in regions that form a central autonomic network such as the amygdala, thalamus, and posterior insula. Younger adults exhibited reliable coactivation between subcortical and limbic structures such as parahippocampal gyrus (extending into amygdala and hippocampus), the posterior insula, thalamus, and MCC. The MCC and amygdala were particularly central and locally efficient hubs amongst this group of regions, and younger adults’ coactivation regions were more likely to cluster in segregated neighbors than older adults. In contrast, older adults had more reliable activity in regions that comprise the dorsal salience network (i.e., dACC) and default mode network (i.e., dmPFC; Benarroch, 1993; Cersosimo & Benarroch, 2013; Goswami, Frances, & Shoemaker, 2011; see Ding et al., 2020; Sie, Chen, Shiau, & Chu, 2019 for recent work). Older adults showed more reliable coactivations amongst the dACC, dmPFC, and mid-to-superior frontal cortex as well as the inferior frontal gyrus, anterior insula, and more anterior parts of the caudate. Amongst these regions, the dACC, caudate, and middle frontal gyrus were more central, locally efficient nodes, and older adults' patterns of coactivation were less segregated than those of younger adults. Collectively, these findings have implications for theories of age-related differences in affect.
First, our findings lend provisional support for the theory of maturational dualism. The finding that younger adults had more reliable activation within and coactivation amongst brain regions associated with autonomic control and interoceptive representations may suggest that younger adults’ affective experiences and perceptions are characterized by relatively greater involvement of visceral signals than those of older adults. In contrast, the finding that older adults had more reliable activation within and coactivation amongst brain regions comprising the dorsal salience network and default mode network may suggest that older adults are engaging in relatively more mentalizing, autobiographical memory, and self-regulation during affective experiences and perceptions than younger adults. Indeed, proponents of SST have predicted such outcomes as evidence that older adults are using different forms of emotion regulation than younger adults (e.g., Martins & Mather, 2016).
It may be tempting to interpret these results as evidence that older adults are engaging in emotion regulation to a greater extent than younger adults during these in-scanner affect tasks. We did not include studies of explicit emotion regulation in our database but cannot rule out that older adults may be either engaging in emotion regulation or approaching affective tasks differently than younger adults (e.g., due to motivational differences, increased semantic knowledge, greater cognitive effort, greater expertise, etc.). Notably, in our meta-analysis of negative affect, older adults exhibited more reliable activity than younger adults in regions associated with the frontoparietal control network (e.g., dlPFC). In prior work with younger adults, the dlPFC shows consistent increases during emotion regulation (see Buhle et al., 2014 for meta-analysis). Yet, individual studies of emotion regulation have shown that older adults engage dlPFC to a similar extent as younger adults (Winecoff, LaBar, Madden, Cabeza, & Huettel, 2011). The dlPFC is also commonly activated in younger adults across studies of emotion in which participants are not explicitly engaging in emotion regulation (Lindquist et al., 2012). Consequently, we caution against the reverse inference that activation of dlPFC in older adults implies that older adults are engaging in emotion regulation to a greater extent than younger adults.
Taken together, both maturational dualism and SST fit the overall pattern of findings and may suggest compatible (rather than competing) neural mechanisms of affective aging. Nevertheless, the above meta-analytic interpretations are reverse inferences that warrant verification in future studies. Another caveat is that greater activity in prefrontal regions for older adults could be unrelated to psychological processes but may instead reflect prefrontal structural declines resulting in stronger BOLD activity in these regions due to more diffuse, less efficient neuronal activity (Morcom & Henson, 2018). This alternative explanation should be addressed in the future.
Age Differences in Neural Representations of Valence
We separately examined the neural representation of valence to further weigh in on predictions from different theories of affective aging. The findings for negative affect largely replicate the overall affect findings. This is unsurprising given that there were relatively more studies of negative than positive affect in our database. During negative affect, younger adults had the most reliable activity (relative to older adults) in the left amygdala, with some activation extending throughout the left and right parahippocampal gyrus. As in the overall neural reference space findings, the amygdala is linked to the generation of autonomic states, consistent with maturational dualism. SST also suggests that younger adults should experience relatively more robust negative affect. To the extent that the left amygdala reflects the intensity of negative affect, these findings would also support SST. Of note, our prior meta-analytic work also found that the left amygdala was most frequently activated during negative relative to positive affect in the literature on young adults (Lindquist et al., 2016).
Although most studies in the database tested for age differences in positivity (20/27 studies), it is striking that so few studies reported significant age differences in positive affect, especially given predictions from SST and the large number of behavioral studies testing the positivity effect in affective aging. We found no significant regions of activation for younger > older adult positive affect, regardless of corrected thresholds explored (ps < .01, .02, .05). Note that we did observe significant activation for older > younger adults at the exploratory FWE-corrected threshold of p < .02 in the left caudate, which is sometimes associated with reward learning (Haruno & Kawato, 2006) and motivated behavior (Delgado, 2004). Given the lesser threshold, we caution against strong inferences, but these findings may be provisional support for SST. This finding should be interpreted against the backdrop of the null effects of OA > YA comparisons for positivity in the broader literature however. Future studies should prioritize evaluating age differences in positive affect with larger samples and more diverse methods of targeting positive affect in the scanner.
Age Differences in Neural Representations of Arousal
There were a large number of high arousal contrasts in the database but almost no low arousal contrasts, due to the kinds of studies and stimuli that predominate in the existing literature. It is also worth noting that many studies in the meta-analytic database examined negative valence that was mid-to-high arousal, so it is likely that arousal and valence are confounded in our findings (see Lindquist et al., 2016 for a discussion). When examining high arousal contrasts, findings replicate the general pattern of findings observed for negative affect but include several additional regions not observed in the overall and negative affect contrasts. In line with maturational dualism, younger adults again showed reliable activation relative to older adults in regions implicated in visceromotor control and autonomic nervous system representations (Beissner, Meissner, Bar, & Napadow, 2013; Gianaros et al., 2017; Kleckner et al., 2017; Satpute et al., 2019; Touroutoglou et al., 2019) such as the mid-cingulate (including aMCC), caudate, thalamus and the posterior vmPFC/ACC.
In contrast, older adult high arousal affect was again more related to dorsal, anterior parts of the salience network such as the middle frontal gyrus, anterior portions of the vmPFC, and the dACC and more dorsal, anterior parts of the insular cortex. Interestingly, in healthy young adults, the dorsal salience subsystem is related to executive function, whereas the ventral salience subsystem is related to subjective arousal (Touroutoglou et al., 2018; Touroutoglou, Hollenbeck, Dickerson, & Barrett, 2012). One might assume that relatively greater activation of the dorsal salience network in older adults during high arousal affect may reflect greater regulation efforts. A separate interpretation is that due to age-related decreases in dorsal salience functional connectivity (as found in Touroutoglou et al., 2018), older adults have more diffuse (less efficient) neuronal processing in the dorsal salience network, resulting in greater activity during states of heightened arousal. Again, these findings should be addressed in future research.
Strengths, Limitations, and Future Directions for the Affective Neuroscience of Aging
In the present study, we used functional neuroimaging meta-analytic techniques to summarize three decades of literature on age-related neural differences during affective experience and perception. This work sheds light on what we know to date about how the aging brain and affect are related. However, this work is not without limitations. Below, we discuss both merits and limitations and then highlight critical future directions that will help further the affective neuroscience of aging.
The Power and Limits of Meta-Analysis
This meta-analysis identified the most consistent (i.e., reliable) effects in the affective neuroscience of aging literature. Doing so is critical, given that any one study is prone to false positives, sampling biases, and method-specific confounds (Wager et al., 2007). However, due to the nature of the existing literature, our meta-analysis best speaks to reliable age differences in the neural bases of negative and high arousal affect. The published literature contained too few significant age differences in positive affect, despite the fact that nearly three-quarters of included studies did test for age differences in positive affect. Furthermore, low arousal affect was especially underrepresented in the meta-analytic database, due to its infrequent inclusion in study designs. Given recent behavioral work showing that older adults are more likely to report experiencing and may even prefer low arousal affective experiences and stimuli (Bjalkebring, Västfjäll, & Johansson, 2015; MacCormack et al., 2019; Sands & Isaacowitz, 2017), future neuroimaging studies should strive to understand age differences in arousal, not just valence.
In addition to biases or limitations in the existing literature, coordinate-based neuroimaging meta-analyses further limit the scope of possible data that can be included. Coordinate-based neuroimaging meta-analyses remain the gold standard for assessing the reliability of functional brain activity but require that coordinates be derived from contrasts rather than correlations or functional connectivity approaches. Consequently, we focused on studies with functional coordinates that contrasted age groups but did not include studies that treated age as a continuous variable or that focused exclusively on functional connectivity. We were able to produce coactivation analyses to approximate network function, yet meta-analytic coactivation is not equivalent to within-study functional connectivity. Nevertheless, these findings help reveal which brain regions were reliably coactivating as a unit together across aging studies during affect, which may prove useful for future functional connectivity studies of affective aging.
Generalizability and Specificity
One strength of our meta-analysis is that the adults included are relatively generalizable to healthy community samples. On average across studies, young adults were frequently from the community (rather than university students), ranged in age from 21 to 26 years, and were often matched with older adults on key demographics such as sex and education. Of course, our findings generalize to the population only insofar as most Western scientific studies do—participants in our sample were mostly North American adults and it is unclear how these neuroimaging findings (or even affective aging findings) replicate and generalize across cultures and ethnicities (see Grossmann, Karasawa, Kan, & Kitayama, 2014).
In addition to generalizability across populations, it remains unknown how findings might generalize across other psychological domains vs. might be specific to affect. Some of the age-related differences we observed (e.g., older adult greater dmPFC and superior frontal activity) parallel findings in the cognitive aging neuroscience literature. This may suggest that there are domain-general aging processes—such as structural declines—that in turn impact multiple psychological functions. As such, it is unclear how the present findings are driven by domain-general vs. affect-specific neural aging. Although this meta-analysis cannot quantitatively speak to shared vs. unique patterns of neural aging for “cognitive” and “affective” tasks, future work should build on these findings to investigate domain-general vs. domain-specific patterns associated with aging during tasks that are relatively more vs. less affect-laden.
Mapping Within-Person Trajectories of Affective Aging
Another crucial limitation is that all included studies were cross-sectional in nature and, as such, cannot account for within-person changes, individual differences (e.g., different rates of aging), or generational differences (Hoffman & Stawski, 2009; Salthouse, 2012; Sliwinski, Hoffman, & Hofer, 2010). This means that the present findings only reveal age differences rather than age-related changes, which would require within-person longitudinal assessments across ideally three or more timepoints. For instance, it is possible that effects observed herein are confounded with cohort or generational effects (e.g., older adults who grew up in a different historical context compared to young adult samples). Hence, it remains unclear if the neural differences found between older vs. younger adults are due to the process of aging itself or due to generational, historical, or cultural shifts across time. Similarly, even if the effects observed are due to aging, the exact causal relation between brain aging and affective aging remains unclear. Although in this meta-analysis we assume that age-related changes in brain morphology and function likely precede and give rise to age-related changes in affective processes, this need not be the case. For example, shifts in affect over time may also influence the nature and time course of brain aging (e.g., chronic stressors and depression likely accelerate brain aging whereas more positive social affective experiences may help buffer against certain forms of brain aging). Multi-site studies like the Midlife in the United States Study (Brim et al., 1996) and the Lifespan Human Connectome Project in Aging (Bookheimer et al., 2019) represent important next steps for clarifying how within-person aging might causally predict downstream affective processes in later life and vice versa.
Linking Structure with Function and Brain with Body
Lastly, we do not yet know how these observed age differences in functional brain activation and coactivation during affect are grounded in age-related structural changes to the central and peripheral nervous system. A broader challenge facing neuroscience is to link brain function such as in functional connectivity fMRI with brain structure using techniques such as diffusion tensor imaging to map structural connectivity (Damoiseaux, 2017; Meier et al., 2016; Poldrack, 2010; Snyder & Bauer, 2019; Vázquez-Rodríguez et al., 2019). Indeed, although functional connectivity must be grounded in the structural limitations of the brain’s architecture, structural connectivity alone is insufficient for explaining functional connectivity (Friston, 2011).
Many studies in both humans and non-human animals already document aging effects on brain structure, broadly finding that even in healthy aging, the brain shrinks in volume (especially in the frontal and temporal cortices, as well as the putamen, thalamus, and accumbens), an effect that is partially explained by neuronal shrinkage, shortening of synaptic spines, and fewer synapses (J. S. Allen et al., 2005; Bagarinao et al., 2018; Fjell & Walhovd, 2010; Fjell et al., 2013; Good et al., 2001; Meunier, Achard, Morcom, & Bullmore, 2009; Raz et al., 2005; Scheibel, Lindsay, Tomiyasu, & Scheibel, 1975; Smith et al., 2007). Furthermore, myelination deteriorates throughout the central and peripheral nervous systems, leading to slower, less efficient neuronal signaling (Hill, Li, & Grutzendler, 2018; Marner, Nyengaard, Tang, & Pakkenberg, 2003; Melcangi et al., 2000; Palve & Palve, 2018; Peters, 2002, 2009; Verdú et al., 2000). Despite these broad findings, there remain many mixed and contradictory results, as neuroscientists seek to understand how diverse markers of brain structure and function shift and relate to each other in multidimensional ways, be they markers of structural integrity and connectivity, functional activity and connectivity, amyloid deposition, glucose metabolism, or neuroinflammation.
Finally, it remains unclear how peripheral nervous system aging interacts with central nervous system aging, and what implications these together may hold for affective aging. We interpret some of our findings as potential evidence for shifts in the neural representation of visceral and interoceptive processing, but ultimately, future research must measure and examine these relations between central and peripheral aging. Bridging measures of the aging brain and body will further clarify how health and disease in later life may mitigate vs. accelerate affective aging, and in turn, how affective aging could itself drive late-life health and disease. In the future, an affective neuroscience of aging should account for both within- and between-system changes in the periphery and brain, bringing us one step closer to understanding how the aging nervous system interfaces with affective aging.
Conclusions
Our meta-analysis revealed reliable differences in neural activation and coactivation between younger and older adults during affect. Meta-analytic findings were consistent with multiple theories of affective aging and do not provide evidence in clear favor for one model over other models. This may be in part because affective aging is a heterogeneous process, driven by multiple temporal, situational, and individual factors. Future experimental neuroimaging work should explicitly articulate and falsify hypotheses associated with each model of affective aging and rule out alternative interpretations that may be confounded with observed effects. For example, does greater prefrontal activity in late life during affect reflect structural declines in these regions, increased cognitive effort during in-scanner tasks, improved regulation strategies, greater reliance on semantic knowledge and accumulated predictions, or compensation for structural and functional age-related changes happening elsewhere in the brain? This meta-analysis cannot adjudicate between these possible mechanisms. Instead, careful study design and experimentation are needed in future work. In the meantime, these findings begin to pinpoint specific neuronal patterns of affective aging across the healthy adult lifespan and shore up behavioral findings demonstrating that even processes as basic as affective experience and perception do not remain stable across adulthood.
Electronic Supplementary Material
(PDF 615 kb)
Additional Information
Funding
This work was supported by a Ruth L. Kirschstein National Research Service Award predoctoral fellowship from the National Institute on Aging to JKM (1F31AG055265-01A1) and by a T32 postdoctoral fellowship to JKM from the National Heart, Lung, and Blood Institute (5T32HL007560-37) via the University of Pittsburgh Department of Psychiatry.
Data Availability
Data are available on OSF at: https://osf.io/wsya5/
Conflict of Interests
The authors declare that there are no conflicts of interest.
Ethical Approval
All studies in the meta-analytic database were approved by IRBs at their respective institutions.
Informed Consent
All participants in studies completed informed consent in accordance with ethics guidelines.
Contributor Information
Jennifer K. MacCormack, Email: maccormack@pitt.edu
Kristen A. Lindquist, https://orcid.org/0000-0002-5368-9302
References
- Allard ES, Kensinger EA. Age-related differences in neural recruitment during the use of cognitive reappraisal and selective attention as emotion regulation strategies. Frontiers in Psychology. 2014;5:296. doi: 10.3389/fpsyg.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, E. A., Erhardt, E. B., Damaraju, E., Gruner, W., Segall, J. M., Silva, R. F., … Calhoun, V. D. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2. 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagarinao E, Watanabe H, Maesawa S, Mori D, Hara K, Kawabata K, et al. An unbiased data-driven age-related structural brain parcellation for the identification of intrinsic brain volume changes over the adult lifespan. NeuroImage. 2018;169:134–144. doi: 10.1016/j.neuroimage.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Bagarinao E, Watanabe H, Maesawa S, Mori D, Hara K, Kawabata K, et al. Reorganization of brain networks and its association with general cognitive performance over the adult lifespan. Scientific Reports. 2019;9:11352. doi: 10.1038/s41598-019-47922-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. The theory of constructed emotion: An active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience. 2017;12:1–23. doi: 10.1093/scan/nsw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, L. F., & Bar, M. (2009). See it with feeling: Affective predictions during object perception. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364, 1325–1334. 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed]
- Barrett LF, Bliss-Moreau E. Affect as a psychological primitive. In: Zanna MP, editor. Advances in experimental social psychology. Burlington: Academic Press; 2009. pp. 167–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. Large-scale brain networks in affective and social neuroscience: Towards an Tntegrative functional architecture of the brain. Current Opinion in Neurobiology. 2013;23:361–372. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. Historical pitfalls and new directions in the neuroscience of emotion. Neuroscience Letters. 2019;693:9–18. doi: 10.1016/j.neulet.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar K-J, Napadow V. The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. Journal of Neuroscience. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings. 1993;68:988–1001. doi: 10.1016/S0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistcal Society: Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Berntson GG, Gianaros PJ, Tsakiris M. Interoception and the autonomic nervous system: Bottom-up meets top-down. In: Tsakiris M, De Preester H, editors. Mind and interoception. New York: Oxford University Press; 2018. pp. 3–25. [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage. 2014;102:345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Birditt KS, Fingerman KL. Do we get better at picking our battles? Age group differences in descriptions of behavioral reactions to interpersonal tensions. Journals of Gerontology - Series B Psychological Sciences and Social Sciences. 2005;60:121–128. doi: 10.1093/geronb/60.3.P121. [DOI] [PubMed] [Google Scholar]
- Birditt KS, Fingerman KL, Almeida DM. Age differences in exposure and reactions to interpersonal tensions: A daily diary study. Psychology and Aging. 2005;20:330–340. doi: 10.1037/0882-7974.20.2.330. [DOI] [PubMed] [Google Scholar]
- Bjalkebring, P., Västfjäll, D., & Johansson, B. E. A. (2015). Happiness and arousal: Framing happiness as arousing results in lower happiness ratings for older adults. Frontiers in Psychology, 6, 706. 10.3389/fpsyg.2015.00706. [DOI] [PMC free article] [PubMed]
- Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, et al. The lifespan human connectome project in aging: An overview. NeuroImage. 2019;185:335–348. doi: 10.1016/j.neuroimage.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti SP. Centrality and network flow. Social Networks. 2005;27:55–71. doi: 10.1016/j.socnet.2004.11.008. [DOI] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brassen S, Gamer M, Büchel C. Anterior cingulate activation is related to a positivity bias and emotional stability in successful aging. Biological Psychiatry. 2011;70:131–137. doi: 10.1016/j.biopsych.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, Shweder RA. Midlife in the United States (MIDUS 1), 1995–1996. Ann Arbor, MI: Inter-University Consortium for Political and Social Research; 1996. [Google Scholar]
- Brooks JA, Shablack H, Gendron M, Satpute AB, Parrish MH, Lindquist KA. The role of language in the experience and perception of emotion: A neuroimaging meta-analysis. Social Cognitive and Affective Neuroscience. 2017;12:169–183. doi: 10.1093/scan/nsw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose A, Scheibe S, Schmiedek F. Life contexts make a difference: Emotional stability in younger and older adults. Psychology and Aging. 2013;28:148–159. doi: 10.1037/a0030047. [DOI] [PubMed] [Google Scholar]
- Bruine de Bruin W, van Putten M, van Emden R, Strough J. Age differences in emotional responses to monetary losses and gains. Psychology and Aging. 2018;33:413–418. doi: 10.1037/pag0000219. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M. What is emotion? Behavioural Processes. 2002;60:69–83. doi: 10.1016/S0376-6357(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, et al. Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience. 2018;19:701–710. doi: 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, Young AW. Facial expression recognition across the adult life span. Neuropsychologia. 2003;41:195–202. doi: 10.1016/S0028-3932(02)00149-5. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Grady CL, Ng C, Hasher L. Age differences in the frontoparietal cognitive control network: Implications for distractibility. Neuropsychologia. 2012;50:2212–2223. doi: 10.1016/j.neuropsychologia.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. The James-Lange theory of emotions: A critical examination and an alternative theory. The American Journal of Psychology. 1927;39:106. doi: 10.2307/1415404. [DOI] [PubMed] [Google Scholar]
- Cao, W., Luo, C., Zhu, B., Zhang, D., Dong, L., Gong, J., et al. (2014). Resting-state functional connectivity in anterior cingulate cortex in normal aging. Frontiers in Aging Neuroscience, 6, 280. 10.3389/fnagi.2014.00280. [DOI] [PMC free article] [PubMed]
- Carstensen LL, DeLiema M. The positivity effect: A negativity bias in youth fades with age. Current Opinion in Behavioral Sciences. 2018;19:7–12. doi: 10.1016/j.cobeha.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037/0003-066X.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. doi: 10.1111/j.0963-7214.2005.00348.x. [DOI] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. doi: 10.1037/0022-3514.79.4.644. [DOI] [PubMed] [Google Scholar]
- Cassidy BS, Leshikar ED, Shih JY, Aizenman A, Gutchess AH. Valence-based age differences in medial prefrontal activity during impression formation. Social Neuroscience. 2013;8:462–473. doi: 10.1080/17470919.2013.832373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo, M. G., & Benarroch, E. E. (2013). Central control of autonomic function and involvement in neurodegenerative disorders. In Handbook of clinical neurology (pp. 45–57). 10.1016/B978-0-444-53491-0.00005-5. [DOI] [PubMed]
- Charles ST, Piazza JR. Age differences in affective well-being: Context matters. Social and Personality Psychology Compass. 2009;3:711–724. doi: 10.1111/j.1751-9004.2009.00202.x. [DOI] [Google Scholar]
- Charles ST, Reynolds CA, Gatz M. Age-related differences and change in positive and negative affect over 23 years. Journal of Personality and Social Psychology. 2001;80:136–151. doi: 10.1037/0022-3514.80.1.136. [DOI] [PubMed] [Google Scholar]
- Cheng S-T. Age and subjective well-being revisited: A discrepancy perspective. Psychology and Aging. 2004;19:409–415. doi: 10.1037/0882-7974.19.3.409. [DOI] [PubMed] [Google Scholar]
- Coats AH, Blanchard-Fields F. Emotion regulation in interpersonal problems: The role of cognitive-emotional complexity, emotion regulation goals, and expressivity. Psychology and Aging. 2008;23:39–51. doi: 10.1037/0882-7974.23.1.39. [DOI] [PubMed] [Google Scholar]
- Cole MW, Repovš G, Anticevic A. The frontoparietal control system. The Neuroscientist. 2014;20:652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel — Now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Wager TD, Yarkoni T. The relation between statistical power and inference in fMRI. PLoS One. 2017;12:e0184923. doi: 10.1371/journal.pone.0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csardi, G., & Nepusz, T. (2006). The igraph software package for complex network research. InterJournal: Complex Systems, 1695.
- Damasio AR. The feeling of what happens: Body and emotion in the making of consciousness. San Diego, CA: Harcourt; 1999. [Google Scholar]
- Damoiseaux JS. Effects of aging on functional and structural brain connectivity. NeuroImage. 2017;160:32–40. doi: 10.1016/j.neuroimage.2017.01.077. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Seven sins in the study of emotion: Correctives from affective neuroscience. Brain and Cognition. 2003;52:129–132. doi: 10.1016/S0278-2626(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14:1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Tarumi T, Wang C, Vernino S, Zhang R, Zhu DC. Central autonomic network functional connectivity: Correlation with baroreflex function and cardiovascular variability in older adults. Brain Structure and Function. 2020;225:1575–1585. doi: 10.1007/s00429-020-02075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, Christoff K. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences. 2018;115:E1598–E1607. doi: 10.1073/pnas.1715766115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Thiruchselvam R, Todd R, Christoff K. Emotion and the prefrontal cortex: An integrative review. Psychological Bulletin. 2017;143:1033–1081. doi: 10.1037/bul0000096. [DOI] [PubMed] [Google Scholar]
- Dolcos, S., Katsumi, Y., & Dixon, R. A. (2014). The role of arousal in the spontaneous regulation of emotions in healthy aging: A fMRI investigation. Frontiers in Psychology, 5, 681. 10.3389/fpsyg.2014.00681. [DOI] [PMC free article] [PubMed]
- Duncan S, Barrett LF. Affect is a form of cognition: A neurobiological analysis. Cognition & Emotion. 2007;21:1184–1211. doi: 10.1080/02699930701437931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durso GRO, Luttrell A, Way BM. Over-the-counter relief from pains and pleasures alike: Acetaminophen blunts evaluation sensitivity to both negative and positive stimuli. Psychological Science. 2015;26:750–758. doi: 10.1177/0956797615570366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK, Fischer H. Neural mechanisms of reading facial emotions in young and older adults. Frontiers in Psychology. 2012;3:223. doi: 10.3389/fpsyg.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR. In sickness and in health: The co-regulation of inflammation and social behavior. Neuropsychopharmacology. 2017;42:242–253. doi: 10.1038/npp.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman, P., & Cordaro, D. (2011). What is meant by calling emotions basic. Emotion Review, 3, 364–370. 10.1177/1754073911410740.
- English, T., & Carstensen, L. L. (2014). Emotional experience in the mornings and the evenings: Consideration of age differences in specific emotions by time of day. Frontiers in Psychology, 5, 185. 10.3389/fpsyg.2014.00185. [DOI] [PMC free article] [PubMed]
- Esposito, F., Aragri, A., Pesaresi, I., Cirillo, S., Tedeschi, G., Marciano, E., … Di Salle, F. (2008). Independent component model of the default-mode brain function: Combining individual-level and population-level analyses in resting-state fMRI. Magnetic Resonance Imaging, 26, 905–913. 10.1016/j.mri.2008.01.045. [DOI] [PubMed]
- Everaerd D, Klumpers F, Oude Voshaar R, Fernández G, Tendolkar I. Acute stress enhances emotional face processing in the aging brain. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2:591–598. doi: 10.1016/j.bpsc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Khalsa SS, Salomons TV, Prkachin KM, Frey-Law LA, Lee JE, et al. Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Structure and Function. 2016;221:1499–1511. doi: 10.1007/s00429-014-0986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fere C. Note on changes in electrical resistance under the effect of sensory stimulation and emotion. Comptes Rendus des Seances de la Societe de Biologie. 1888;5:217–219. [Google Scholar]
- Fischer H, Nyberg L, Bäckman L. Age-related differences in brain regions supporting successful encoding of emotional faces. Cortex. 2010;46:490–497. doi: 10.1016/j.cortex.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Bäckman L. Age-differential patterns of brain activation during perception of angry faces. Neuroscience Letters. 2005;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: Courses, causes, and cognitive consequences. Reviews in the Neurosciences. 2010;21:187–221. doi: 10.1515/REVNEURO.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, et al. Critical ages in the life course of the adult brain: Nonlinear subcortical aging. Neurobiology of Aging. 2013;34:2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LC. A set of measures of centrality based on betweenness. Sociometry. 1977;40:35–41. doi: 10.2307/3033543. [DOI] [Google Scholar]
- Friedman BH. Feelings and the body: The Jamesian perspective on autonomic specificity of emotion. Biological Psychology. 2010;84:383–393. doi: 10.1016/j.biopsycho.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: A review. Brain Connectivity. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Software: Practice and Experience. 1991;21:1129–1164. [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Garfinkel, S. N., & Critchley, H. D. (2013). Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on: “Anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al. (2012). Social Cognitive and Affective Neuroscience, 8, 231–234. 10.1093/scan/nss140. [DOI] [PMC free article] [PubMed]
- Garfinkel, S. N., Minati, L., Gray, M. A., Seth, A. K., Dolan, R. J., & Critchley, H. D. (2014). Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34, 6573–6582. 10.1523/JNEUROSCI.3507-13.2014. [DOI] [PMC free article] [PubMed]
- Gianaros PJ, Sheu LK, Uyar F, Koushik J, Jennings JR, Wager TD, Verstynen TD. A brain phenotype for stressor-evoked blood pressure reactivity. Journal of the American Heart Association. 2017;6:e006053. doi: 10.1161/JAHA.117.006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goswami R, Frances MF, Shoemaker JK. Representation of somatosensory inputs within the cortical autonomic network. NeuroImage. 2011;54:1211–1220. doi: 10.1016/j.neuroimage.2010.09.050. [DOI] [PubMed] [Google Scholar]
- Grady C, Maisog J, Horwitz B, Ungerleider L, Mentis M, Salerno J, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. The Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, M. A., Beacher, F. D., Minati, L., Nagai, Y., Kemp, A. H., Harrison, N. A., & Critchley, H. D. (2012). Emotional appraisal is influenced by cardiac afferent information. Emotion, 12, 180–191. 10.1037/a0025083. [DOI] [PubMed]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Skorpen CG, Hsu AY. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12:590–599. doi: 10.1037/0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Grossmann I, Karasawa M, Kan C, Kitayama S. A cultural perspective on emotional experiences across the life span. Emotion. 2014;14:679–692. doi: 10.1037/a0036041. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, et al. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–295. doi: 10.1016/S0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2010;30:12878–12884. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. Journal of Neurophysiology. 2006;95:948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- He, X., Qin, W., Liu, Y., Zhang, X., Duan, Y., Song, J., … Yu, C. (2013). Age-related decrease in functional connectivity of the right fronto-insular cortex with the central executive and default-mode networks in adults from young to middle age. Neuroscience Letters, 544, 74–79. 10.1016/j.neulet.2013.03.044. [DOI] [PubMed]
- He, X., Qin, W., Liu, Y., Zhang, X., Duan, Y., Song, J., … Yu, C. (2014). Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Human Brain Mapping, 35, 3446-3464. 10.1002/hbm.22414. [DOI] [PMC free article] [PubMed]
- Hill RA, Li AM, Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nature Neuroscience. 2018;21:683–695. doi: 10.1038/s41593-018-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6:97–120. doi: 10.1080/15427600902911189. [DOI] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, Yonekura Y. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York: Henry Holt and Company; 1890. [Google Scholar]
- Kan IP, Garrison SL, Drummey AB, Emmert BE, Rogers LL. The roles of chronological age and time perspective in memory positivity. Aging, Neuropsychology, and Cognition. 2018;25:598–612. doi: 10.1080/13825585.2017.1356262. [DOI] [PubMed] [Google Scholar]
- Kehoe EG, Toomey JM, Balsters JH, Bokde ALW. Healthy aging is associated with increased neural processing of positive valence but attenuated processing of emotional arousal: An fMRI study. Neurobiology of Aging. 2013;34:809–821. doi: 10.1016/j.neurobiolaging.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Winocur G, Grady CL. Age-related differences in brain activity underlying identification of emotional expressions in faces. Social Cognitive and Affective Neuroscience. 2007;2:292–302. doi: 10.1093/scan/nsm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults’ emotional memories. Journal of Cognitive Neuroscience. 2008;20:1161–1173. doi: 10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- Kessler E-M, Staudinger UM. Affective experience in adulthood and old age: The role of affective arousal and perceived affect regulation. Psychology and Aging. 2009;24:349–362. doi: 10.1037/a0015352. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Tranel D. Interoceptive awareness declines with age. Psychophysiology. 2009;46:1130–1136. doi: 10.1111/j.1469-8986.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner, I. R., Zhang, J., Touroutoglou, A., Chanes, L., Xia, C., Simmons, W. K., et al. (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour, 1. 10.1038/s41562-017-0069. [DOI] [PMC free article] [PubMed]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical–subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Wager TD. Meta-analysis of neuroimaging data. Wiley Interdisciplinary Reviews: Cognitive Science. 2010;1:293–300. doi: 10.1002/wcs.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynak TE, Marsland AL, Wager TD, Gianaros PJ. Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2018;94:76–92. doi: 10.1016/j.neubiorev.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Physical Review Letters. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:153–164. doi: 10.3758/CABN.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related valence-based reversal in recruitment of medial prefrontal cortex on a visual search task. Social Neuroscience. 2010;5:560–576. doi: 10.1080/17470910903512296. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Neural processing of emotional pictures and words: A comparison of young and older adults. Developmental Neuropsychology. 2011;36:519–538. doi: 10.1080/87565641.2010.549864. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Brown R. A higher-order theory of emotional consciousness. Proceedings of the National Academy of Sciences. 2017;114:E2016–E2025. doi: 10.1073/pnas.1619316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV, Ekman P. Emotion, physiology, and expression in old age. Psychology and Aging. 1991;6:28–35. doi: 10.1037/0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. Influence of age and gender on affect, physiology, and their interrelations: A study of long-term marriages. Journal of Personality and Social Psychology. 1994;67:56–68. doi: 10.1037/0022-3514.67.1.56. [DOI] [PubMed] [Google Scholar]
- Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P. A., … Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine, 6, e1000100. 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed]
- Lindquist KA, Barrett LF. A functional architecture of the human brain: Emerging insights from the science of emotion. Trends in Cognitive Sciences. 2012;16:533–540. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex. 2016;26:1910–1922. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, K. A., Wager, T. D., Kober, H., Bliss-Moreau, E., & Barrett, L. F. (2012). The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences, 35, 121–143. 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed]
- Livingstone, K. M., & Isaacowitz, D. M. (2019). Age similarities and differences in spontaneous use of emotion regulation tactics across five laboratory tasks. Journal of Experimental Psychology: General. 10.1037/xge0000556. [DOI] [PMC free article] [PubMed]
- MacCormack, J. K., Armstrong-Carter, E. L., Gaudier-Diaz, M. M., Meltzer-Brody, S., Sloan, E. K., Lindquist, K. A., & Muscatell, K. A. (2020). Beta-adrenergic contributions to emotion and psychophysiology during acute psychosocial stress. Under Review. [DOI] [PMC free article] [PubMed]
- MacCormack, J. K., Henry, T. R., Davis, B. M., Oosterwijk, S., & Lindquist, K. A. (2019). Aging bodies, aging emotions: Interoceptive differences in emotion representations and self-reports across adulthood. Emotion, advance online publication. 10.1037/emo0000699 [DOI] [PMC free article] [PubMed]
- MacCormack JK, Lindquist KA. Bodily contributions to emotion: Schachter’s legacy for a psychological constructionist view on emotion. Emotion Review. 2017;9:36–45. doi: 10.1177/1754073916639664. [DOI] [Google Scholar]
- MacCormack JK, Muscatell KA. The metabolic mind: A role for leptin and ghrelin in affect and social cognition. Social and Personality Psychology Compass. 2019;13:e12496. doi: 10.1111/spc3.12496. [DOI] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. The Journal of Comparative Neurology. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Martins B, Mather M. Default mode network and later-life emotion regulation: Linking functional connectivity patterns and emotional outcomes. In: Ong AD, Löckenhoff CE, editors. Bronfenbrenner series on the ecology of human development. Emotion, aging, and health. Washington: American Psychological Association; 2016. pp. 9–29. [Google Scholar]
- Mather M. The emotion paradox in the aging brain. Annals of the New York Academy of Sciences. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. The affective neuroscience of aging. Annual Review of Psychology. 2016;67:213–238. doi: 10.1146/annurev-psych-122414-033540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- McCarthy, P., Benuskova, L., & Franz, E. A. (2014). The age-related posterior-anterior shift as revealed by voxelwise analysis of functional brain networks. Frontiers in Aging Neuroscience, 6, 301. 10.3389/fnagi.2014.00301. [DOI] [PMC free article] [PubMed]
- McDowell CL, Harrison DW, Demaree HA. Is right hemisphere decline in the perception of emotion a function of aging? The International Journal of Neuroscience. 1994;79:1–11. doi: 10.3109/00207459408986063. [DOI] [PubMed] [Google Scholar]
- Meier J, Tewarie P, Hillebrand A, Douw L, van Dijk BW, Stufflebeam SM, Van Mieghem P. A mapping between structural and functional brain networks. Brain Connectivity. 2016;6:298–311. doi: 10.1089/brain.2015.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Magnaghi V, Martini L. Aging in peripheral nerves: Regulation of myelin protein genes by steroid hormones. Progress in Neurobiology. 2000;60:291–308. doi: 10.1016/S0301-0082(99)00028-3. [DOI] [PubMed] [Google Scholar]
- Mendes WB. Weakened links between mind and body in older age: The case for maturational dualism in the experience of emotion. Emotion Review. 2010;2:240–244. doi: 10.1177/1754073910364149. [DOI] [Google Scholar]
- Menon, V. (2015). Salience network. In Brain mapping (pp. 597–611). 10.1016/B978-0-12-397025-1.00052-X.
- Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. NeuroImage. 2009;44:715–723. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Mikkelsen, M. B., O’Toole, M. S., Lyby, M. S., Wallot, S., & Mehlsen, M. (2019). Emotional reactivity and interoceptive sensitivity: Exploring the role of age. Psychonomic Bulletin & Review, 26, 1440–1448. 10.3758/s13423-019-01603-y. [DOI] [PubMed]
- Mogilner C, Kamvar SD, Aaker J. The shifting meaning of happiness. Social Psychological and Personality Science. 2011;2:395–402. doi: 10.1177/1948550610393987. [DOI] [Google Scholar]
- Morcom AM, Henson RNA. Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. The Journal of Neuroscience. 2018;38:7303–7313. doi: 10.1523/JNEUROSCI.1701-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C, Borod JC, Welkowitz J, Alpert M. The perception of facial emotion across the adult life span. Developmental Neuropsychology. 1993;9:305–314. doi: 10.1080/87565649309540559. [DOI] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Eickhoff SB. Ten simple rules for neuroimaging meta-analysis. Neuroscience & Biobehavioral Reviews. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:207–233. doi: 10.3758/CABN.3.3.207. [DOI] [PubMed] [Google Scholar]
- Murphy J, Geary H, Millgate E, Catmur C, Bird G. Direct and indirect effects of age on interoceptive accuracy and awareness across the adult lifespan. Psychonomic Bulletin & Review. 2018;25:1193–1202. doi: 10.3758/s13423-017-1339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan H-Y, Callicott JH, Goldberg TE, et al. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. Journal of Cognitive Neuroscience. 2009;21:1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell, K. A., Moieni, M., Inagaki, T. K., Dutcher, J. M., Jevtic, I., Breen, E. C., … Eisenberger, N. I. (2016). Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain, Behavior, and Immunity, 57, 21–29. 10.1016/j.bbi.2016.03.022. [DOI] [PMC free article] [PubMed]
- Nashiro K, Sakaki M, Braskie MN, Mather M. Resting-state networks associated with cognitive processing show more age-related decline than those associated with emotional processing. Neurobiology of Aging. 2017;54:152–162. doi: 10.1016/j.neurobiolaging.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiss MB, Leigland LA, Carlson NE, Janowsky JS. Age differences in perception and awareness of emotion. Neurobiology of Aging. 2009;30:1305–1313. doi: 10.1016/j.neurobiolaging.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert SD, Almeida DM, Charles ST. Age differences in reactivity to daily stressors: The role of personal control. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62:216–225. doi: 10.1093/geronb/62.4.P216. [DOI] [PubMed] [Google Scholar]
- Northoff, G. (2012). From emotions to consciousness – A neuro-phenomenal and neuro-relational approach. Frontiers in Psychology, 3, 303. 10.3389/fpsyg.2012.00303. [DOI] [PMC free article] [PubMed]
- Onoda K, Ishihara M, Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. Journal of Cognitive Neuroscience. 2012;24:2186–2198. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- Palve SS, Palve SB. Impact of aging on nerve conduction velocities and late responses in healthy individuals. Journal of Neurosciences in Rural Practice. 2018;9:112–116. doi: 10.4103/jnrp.jnrp_323_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S, Robinson RG, Andreasen NC, Downhill JE, Davidson RJ, Kirchner PT, Hichwa RD. Emotional activation of limbic circuitry in elderly normal subjects in a PET study. American Journal of Psychiatry. 1997;154:384–389. doi: 10.1176/ajp.154.3.384. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Robinson RG, Boles Ponto LL, Watkins GL, Hichwa RD. Regional cerebral blood flow changes during visually induced subjective sadness in healthy elderly persons. The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:35–44. doi: 10.1176/jnp.15.1.35. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-J, Friston K. Structural and functional brain networks: From connections to cognition. Science. 2013;342:1238411–1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: A review. Journal of Neurocytology. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters, A. (2009). The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Frontiers in Neuroanatomy, 3, 2009. 10.3389/neuro.05.011.2009. [DOI] [PMC free article] [PubMed]
- Petersen SE, Sporns O. Brain networks and cognitive architectures. Neuron. 2015;88:207–219. doi: 10.1016/j.neuron.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectrums. 2004;9:258–266. doi: 10.1017/S1092852900009196. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Mapping mental function to brain structure: How can cognitive neuroimaging succeed? Perspectives on Psychological Science. 2010;5:753–761. doi: 10.1177/1745691610388777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Russell JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Development and Psychopathology. 2005;17:715–734. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz, N., Lindenberger, U., Rodrigue, K. M., Kennedy, K. M., Head, D., Williamson, A., … Acker, J. D. (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15, 1676–1689. 10.1093/cercor/bhi044. [DOI] [PubMed]
- Ritchey M, Bessette-Symons B, Hayes SM, Cabeza R. Emotion processing in the aging brain is modulated by semantic elaboration. Neuropsychologia. 2011;49:640–650. doi: 10.1016/j.neuropsychologia.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Pruis TA, Stevens AA, Janowsky JS. More is less: Emotion induced prefrontal cortex activity habituates in aging. Neurobiology of Aging. 2011;32:1634–1650. doi: 10.1016/j.neurobiolaging.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Human Brain Mapping. 2009;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, et al. Youthful memory capacity in old brains: Anatomic and genetic clues from the northwestern SuperAging project. Journal of Cognitive Neuroscience. 2013;25:29–36. doi: 10.1162/jocn_a_00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roski, C., Caspers, S., Langner, R., Laird, A. R., Fox, P. T., Zilles, K., … Eickhoff, S. B. (2013). Adult age-dependent differences in resting-state connectivity within and between visual-attention and sensorimotor networks. Frontiers in Aging Neuroscience, 5, 67. 10.3389/fnagi.2013.00067. [DOI] [PMC free article] [PubMed]
- Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neuroscience & Biobehavioral Reviews. 2008;32:863–881. doi: 10.1016/j.neubiorev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, Junqué C. Reorganization of brain networks in aging: A review of functional connectivity studies. Frontiers in Psychology. 2015;6:663. doi: 10.3389/fpsyg.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: A comparison of image-based and coordinate-based pooling of studies. NeuroImage. 2009;45:810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Are individual differences in rates of aging greater at older ages? Neurobiology of Aging. 2012;33:2373–2381. doi: 10.1016/j.neurobiolaging.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin, G. R., & Carstensen, L. L. (2011). Socioemotional functioning and the aging brain. In J. Decety & J. T. Cacioppo (Eds.), Oxford library of psychology. The Oxford handbook of social neuroscience (p. 507–521). Oxford University Press.
- Sands M, Garbacz A, Isaacowitz DM. Just change the channel? Studying effects of age on emotion regulation using a TV watching paradigm. Social Psychological and Personality Science. 2016;7:788–795. doi: 10.1177/1948550616660593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands M, Isaacowitz DM. Situation selection across adulthood: The role of arousal. Cognition and Emotion. 2017;31:791–798. doi: 10.1080/02699931.2016.1152954. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y, Suzuki H. Aging effects on conduction velocities of myelinated and unmyelinated fibers of peripheral nerves. Neuroscience Letters. 1985;53:15–20. doi: 10.1016/0304-3940(85)90090-4. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Kang J, Bickart KC, Yardley H, Wager TD, Barrett LF. Involvement of sensory regions in affective experience: A meta-analysis. Frontiers in Psychology. 2015;6:1860. doi: 10.3389/fpsyg.2015.01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute AB, Kragel PA, Barrett LF, Wager TD, Bianciardi M. Deconstructing arousal into wakeful, autonomic and affective varieties. Neuroscience Letters. 2019;693:19–28. doi: 10.1016/j.neulet.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute AB, Lindquist KA. The default mode network’s role in discrete emotion. Trends in Cognitive Sciences. 2019;23:851–864. doi: 10.1016/j.tics.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe S, Blanchard-Fields F. Effects of regulating emotions on cognitive performance: What is costly for young adults is not so costly for older adults. Psychology and Aging. 2009;24:217–223. doi: 10.1037/a0013807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe S, English T, Tsai JL, Carstensen LL. Striving to feel good: Ideal affect, actual affect, and their correspondence across adulthood. Psychology and Aging. 2013;28:160–171. doi: 10.1037/a0030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Experimental Neurology. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Schwarz, N., & Clore, G. L. (1983). Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology, 45, 513–523. https://doi.org/10.1037/0022-3514.45.3.513.
- Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed]
- Seth, A. K. (2018). Consciousness: The last 50 years (and the next). Brain and Neuroscience Advances, 2. 10.1177/2398212818816019. [DOI] [PMC free article] [PubMed]
- Shallcross AJ, Ford BQ, Floerke VA, Mauss IB. Getting better with age: The relationship between age, acceptance, and negative affect. Journal of Personality and Social Psychology. 2013;104:734–749. doi: 10.1037/a0031180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw EE, Schultz AP, Sperling RA, Hedden T. Functional connectivity in multiple cortical networks is associated with performance across cognitive domains in older adults. Brain Connectivity. 2015;5:505–516. doi: 10.1089/brain.2014.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie J-H, Chen Y-H, Shiau Y-H, Chu W-C. Gender- and age-specific differences in resting-state functional connectivity of the central autonomic network in adulthood. Frontiers in Human Neuroscience. 2019;13:369. doi: 10.3389/fnhum.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski M, Hoffman L, Hofer SM. Evaluating convergence of within-person change and between-person age differences in age-heterogeneous longitudinal studies. Research in Human Development. 2010;7:45–60. doi: 10.1080/15427600903578169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: A voxel-based morphometric study in healthy elderly. Neurobiology of Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Snyder AZ, Bauer AQ. Mapping structure-function relationships in the vrain. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2019;4:510–521. doi: 10.1016/j.bpsc.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neuroscience & Biobehavioral Reviews. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- St. Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures. Psychological Science. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. 2010;31:315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, Dickerson BC. Youthful brains in older adults: Preserved neuroanatomy in the default mode and salience networks contributes to youthful memory in superaging. Journal of Neuroscience. 2016;36:9659–9668. doi: 10.1523/JNEUROSCI.1492-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svärd, J., Fischer, H., & Lundqvist, D. (2014). Adult age-differences in subjective impression of emotional faces are reflected in emotion-related attention and memory tasks. Frontiers in Psychology, 5, 423. 10.3389/fpsyg.2014.00423. [DOI] [PMC free article] [PubMed]
- Tarchanoff J. Galvanic phenomena in the human skin during stimulation of the sensory organs and during various forms of mental activity. Pflüger’s Archiv Für Die Gesamte Physiologogie Des Men-Schen Und Der Tiere. 1890;46:46–55. doi: 10.1007/BF01789520. [DOI] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research: Neuroimaging. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD, Åhs F. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Molecular Psychiatry. 2012;17:549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Andreano JM, Adebayo M, Lyons S, Barrett LF. Motivation in the service of allostasis: The role of the anterior mid-cingulate cortex. In: Elliot AJ, editor. Advances in motivation science. 6. Cambridge, MA: Academic Press; 2019. pp. 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett LF. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Lindquist KA, Dickerson BC, Barrett LF. Intrinsic connectivity in the human brain does not reveal networks for ‘basic’ emotions. Social Cognitive and Affective Neuroscience. 2015;10:1257–1265. doi: 10.1093/scan/nsv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou, A., Zhang, J., Andreano, J. M., Dickerson, B. C., & Barrett, L. F. (2018). Dissociable effects of aging on salience subnetwork connectivity mediate age-related changes in executive function and affect. Frontiers in Aging Neuroscience, 10. 410. 10.3389/fnagi.2018.00410. [DOI] [PMC free article] [PubMed]
- Tsai JL, Levenson RW, Carstensen LL. Autonomic, subjective, and expressive responses to emotional films in older and younger Chinese Americans and European Americans. Psychology and Aging. 2000;15:684–693. doi: 10.1037/0882-7974.15.4.684. [DOI] [PubMed] [Google Scholar]
- Turner BO, Paul EJ, Miller MB, Barbey AK. Small sample sizes reduce the replicability of task-based fMRI studies. Communications Biology. 2018;1:62. doi: 10.1038/s42003-018-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Executive functions and neurocognitive aging: Dissociable patterns of brain activity. Neurobiology of Aging. 2012;33:826.e1–826.e13. doi: 10.1016/j.neurobiolaging.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Birmingham W, Berg CA. Are older adults less or more physiologically reactive? A meta-analysis of age-related differences in cardiovascular reactivity to laboratory tasks. The Journals of Gerontology: Series B. 2010;65B:154–162. doi: 10.1093/geronb/gbp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BCM, Stam CJ, Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PLoS One. 2010;5:e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Rodríguez, B., Suárez, L. E., Markello, R. D., Shafiei, G., Paquola, C., Hagmann, P., … Misic, B. (2019). Gradients of structure–function tethering across neocortex. Proceedings of the National Academy of Sciences, 116, 21219-21227. 10.1073/pnas.1903403116. [DOI] [PMC free article] [PubMed]
- Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. Journal of the Peripheral Nervous System. 2000;5:191–208. doi: 10.1111/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: A voxel-based meta-analysis. Journal of Cognitive Neuroscience. 2010;22:2864–2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: A meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: Current and future directions. Social Cognitive and Affective Neuroscience. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. NeuroImage. 2009;45:S210–S221. doi: 10.1016/j.neuroimage.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/S1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wang, Liang, LaViolette, P., O’Keefe, K., Putcha, D., Bakkour, A., Van Dijk, K. R. A., … Sperling, R. A. (2010). Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. NeuroImage, 51, 910–917. 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed]
- Wang, Lubin, Su L, Shen H, Hu D. Decoding lifespan changes of the human brain using resting-state functional connectivity MRI. PLoS One. 2012;7:e44530. doi: 10.1371/journal.pone.0044530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM, Mormino EC, Huijbers W, Schultz AP, Hedden T, Sperling RA. Relationships between default-mode network connectivity, medial temporal lobe structure, and age-related memory deficits. Neurobiology of Aging. 2015;36:265–272. doi: 10.1016/j.neurobiolaging.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, C. G. (2018). Graph theory analysis of brain MRI data. CRAN.
- Winecoff A, LaBar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience. 2011;6:165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiology of Aging. 2006;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Xia C, Touroutoglou A, Quigley KS, Barrett LF, Dickerson BC. Salience network connectivity modulates skin conductance responses in predicting arousal experience. Journal of Cognitive Neuroscience. 2017;29:827–836. doi: 10.1162/jocn_a_01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, T., Zhang, S., Lee, L.-E., Chao, H. H., van Dyck, C., & Li, C.-S. R. (2018). Exploring age-related changes in resting state functional connectivity of the amygdala: From young to middle adulthood. Frontiers in Aging Neuroscience, 10, 209. 10.3389/fnagi.2018.00209. [DOI] [PMC free article] [PubMed]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Andreano, J. M., Dickerson, B. C., Touroutoglou, A., & Barrett, L. F. (2020). Stronger functional connectivity in the default mode and salience networks is associated with youthful memory in superaging. Cerebral Cortex, 30, 72–84. 10.1093/cercor/bhz071. [DOI] [PMC free article] [PubMed]
- Zhang L, Zuo X-N, Ng KK, Chong JSX, Shim HY, Ong MQW, et al. Distinct BOLD variability changes in the default mode and salience networks in Alzheimer’s disease spectrum and associations with cognitive decline. Scientific Reports. 2020;10:6457. doi: 10.1038/s41598-020-63540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsoldos I, Cousin E, Klein-Koerkamp Y, Pichat C, Hot P. Age-related differences in brain activity during implicit and explicit processing of fearful facial expressions. Brain Research. 2016;1650:208–217. doi: 10.1016/j.brainres.2016.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 615 kb)
Data Availability Statement
Data are available on OSF at: https://osf.io/wsya5/