Abstract
Purpose
To report incidence of acute versus delayed presentations of bleeding requiring embolization after focal liver biopsy, in correlation with angiographic findings and treatment success rates. The available literature will be reviewed as well.
Materials and Methods
HIPAA-compliant, institutional review board approved retrospective review of 2180 consecutive patients undergoing 2335 targeted liver biopsies at a tertiary care cancer center. Hepatic arterial embolization episodes within 30 days from biopsy were identified via Radiology PACS. Electronic medical record review was performed for indication of embolization and post-embolization clinical course.
Results
The incidence of post-biopsy bleeding requiring embolization was 0.5% (12/2335 biopsies). In those with bleeding, 1/12 (8%) had no hepatic arterial findings at angiography. Angiographic hepatic arterial findings resolved after embolization in 11/11 patients (100% technical success). Bleeding ceased after embolization in 10/12 patients (83% clinical success). Complications were seen in 2/12 (17%) patients: cholecystitis and hepatic infarct respectively. Delayed presentation of bleeding (defined as >24 hours post-biopsy) occurred in 5/12 (42%) patients; the longest latency was 12 days.
Conclusion
The overall incidence of bleeding requiring embolization in our population was 0.5%. This complication rate compares favorably to the 0% to 4.2% (median 0.29%) rate quoted in the available, heterogeneous, literature on this topic. Delayed presentation occurred in almost half of patients. Arterial embolization carries excellent technical and clinical success rates.
Keywords: Image-Guided Biopsy; Hemorrhage; Embolization, Therapeutic; Radiology, Interventional
Introduction
Patients with cancer in the United States are living longer [1, 2]. Surveillance imaging performed during survivorship will detect suspicious liver lesions prompting biopsy for diagnostic and prognostic purposes. Additionally, as “personalized medicine” becomes a reality, tumor biopsy is becoming increasingly important to allow molecular analysis of tumor cells. Bleeding is the main clinically significant complication of this procedure. Significant bleeding may necessitate arterial embolization. An understanding of the risks associated with imaging guided needle biopsy is essential for medical and surgical oncologists to weigh the costs and benefits in deciding whether or not to recommend a biopsy, for interventional radiologists to present accurate risk estimates to their patients and for patients to determine whether or not to proceed with a recommended procedure. We hope that our experience will help provide data useful in those pursuits.
Materials and Methods
Patient Population
Institutional Review Board approval was obtained. All patients were contained within a single electronic medical record system belonging to the institution, a tertiary care dedicated cancer hospital providing longitudinal patient care. The electronic medical record and PACS systems were retrospectively queried for all patients who underwent imaging guided percutaneous liver biopsy followed within 30 days by arterial embolization between January 2004 and December 2010. Demographic and laboratory data were obtained. Tumor biology, number of passes, and needle gauge, were not consistently documented in the retrospective cohort and this information could not be ascertained for all patients. Patients in whom embolization was performed to treat a tumor, or to treat bleeding related to subsequent biliary drainage were excluded from this analysis. For the patients who did have hepatic embolization performed for bleeding related to their biopsy, biopsy and embolization technique and images were reviewed, and clinical presentation and course was assessed.
Biopsy Technique
Pre-biopsy bloodwork was performed within 30 days for all patients. The institutional permissive coagulation parameters were INR <1.5 and platelet count >50,000. The institutional guidelines regarding holding anticoagulants were consistent with the Society of Interventional Radiology guidelines on this topic. Patients outside of these parameters either underwent transfusion or biopsy at the discretion of the operator. Imaging guidance and post-biopsy imaging (CT/ultrasound), biopsy device (needle gauge, coaxial vs bare introduction), and type of biopsy (core vs. fine needle) were determined based on clinical indication and operator preference. All patients had some type of post-procedure imaging; nearly all had post procedure CT images through the level of the biopsy. Post-biopsy monitoring lasted at least 2 hours. Signs and symptoms of hemorrhage at any time after biopsy (pain, hypotension, tachycardia) prompted consideration for CT to evaluate for hemorrhage or other adverse event.
Diagnostic Angiography and Embolization
Embolization was considered in patients with symptomatic hemorrhage and/or significant findings on CT. Decision for angiography was based on clinical acuity and operator preference. Decision for embolization, selectivity of embolization and choice of embolic agent (gelfoam/coils/PVA/particles) were based on angiographic findings and operator preference.
Results
In total, 2335 percutaneous focal liver biopsies were identified for 2180 patients during the evaluation period.
Bleeding requiring embolization occurred after 12/2335 of biopsies (0.5% incidence). (Table 1a, 1b, 1c, Figures 1–3) Angiograms demonstrated hepatic arterial findings in 11/12 (92%) patients including arteriovenous fistula (5/12), pseudoaneurysm (2/12), extravasation or blush (6/12). One patient had no angiographic findings (1/12). This patient presented 12 days after biopsy with a 5.7-point hemoglobin drop and CT evidence of subcapsular and intrahepatic hematoma.
Table 1a.
Clinical-lnterventional debriefing analysis for all patients with major hemorrhage after focal liver biopsy. HCC= hepatocellular carcinoma.
| Patient | Age (yrs) | Gender | Biopsy indication (Resulting diagnosis) | Underlying liver disease | Hgb/Hct/PIt/PTT/INR | Tumor Size (cm) | Couinaud Segment |
|---|---|---|---|---|---|---|---|
| 1 | 55 | F | Leukemia, new liver lesion (no malignant cells) |
None | 8.9/26.5/276/34.2/1.1 morning of procedure |
1.2 | VIII |
| 2 | 44 | F | Lymphoma, enlarging liver lesion (lymphoma) |
None | 13.5/40/107/45.4/1.09 morning of procedure |
6.1 | VIII |
| 3 | 45 | F | Remote history of breast cancer, new liver lesions (breast cancer metastasis) |
Diffuse liver metastases with pseudocirrhosis | 12.1/36.9/151/31.9/1.05 4 days before procedure |
6.1 | II |
| 4 | 59 | M | Hepatitis B, cirrhosis with multiple liver lesions (granulomatous process) |
Hepatitis B and cirrhosis | 12.7/39.1/138/31.4/1.33 22 days before procedure |
3 | V |
| 5 | 63 | F | Breast cancer with liver lesions (breast cancer metastasis) |
None | 9.0/27/140/24.1/0.99 12 days before procedure |
1.5 | IVa |
| 6 | 65 | F | Lymphoma with multiple diffuse liver lesions (HCC) |
Hepatitis C, idiopathic thrombocytopenic purpura | 8.6/27.8/37/29.8/1.0 morning of procedure |
11 | V |
| 7 | 56 | M | Rectum cancer, liver lesions (rectum cancer metastasis) |
None | 8.8/28.9/344/26.2/1.12 16 days before procedure |
10.2 | III |
| 8 | 68 | F | Breast cancer, ovary cancer, multiple liver lesions (ovary cancer metastasis) |
None | 11.3/32.7/234/28.7/0.94 2 days before procedure |
1.7 | VI |
| 9 | 65 | M | Prostate cancer, liver lesions (prostate cancer metastasis) |
Diffuse liver metastases | 12.9/39.4/244/30.2/0.96 17 days before procedure |
8.3 | VI |
| 10 | 51 | F | Liver lesions, diagnosis (HCC) |
Fatty liver | 10.8/35.4/314/24.8/0.94 13 days before procedure |
6.8 | V |
| 11 | 22 | M | Adrenal cancer, liver lesions (adrenal cancer metastasis) |
None | 14.9/45.7/456/31.5/0.99 3 days before procedure |
1.8 | VIII |
| 12 | 61 | F | Hepatitis B, liver lesion indeterminate at imaging (well-differentiated hepatic neoplasm, likely adenoma) |
Hepatitis B, cirrhosis, portal vein thrombus | 10.2/31.1/152/30/15 1 day before procedure |
3.5 | V/VI |
Table 1b.
Clinical-lnterventional debriefing analysis for all patients who underwent ultrasound-guided focal liver biopsy and developed hemorrhage requiring embolization. MAC=monitored anesthesia care. CECT=contrast enhanced computed tomography. NECT = Non-enhanced computed tomography. US-ultrasound.
| Patient | Needle Size (coaxial) | Needle passes | Tissue Samples | Minimal Transparenchymal Trajectory (mm) | Imaging Guidance | Anesthesia | Imaging findings at completion of study | Time to post-biopsy hemorrhage diagnosis (hours/days) | Symptoms leading to hemorrhage diagnosis | Hemoglobin drop before embolization performed | Imaging findings at hemorrhage diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20–22G | 3 | 2 | 26 | CT | MAC | No bleeding | 1 d | Dyspnea | 1.9 | CECT: Intrahepatic and subcapsular hematoma; hemoperitoneum; right pleural effusion |
| 2 | 19.5G | 1 | 1 | 22 | CT | MAC | No breeding | 5 d | Pain | 4.1 | CECT: Perihepatic hematoma, intratumoral bleeding; hemoperitoneum |
| 3 | 18G | 1 | 1 | 0 | CT | MAC | No bleeding | 3 h | Pain, hypotension | 4 | NECT: Perihepatic hematoma, hemoperitoneum |
| 4 | 19.5G | 4 | 3 | 30 | CT | MAC | No breeding | 8 d | Fever, pain | 0.2 | CECT: Subcapsular hematoma, right pleural effusion and ascites |
| 5 | 20G(19G) | 5 | 3 | 12 | US | MAC | Subcapsular hematoma | Immediate | Pain, hypotension | 2.3 | NECT: Large subcapsular hematoma |
| 6 | 20G | 1 | 1 | 3 | CT | MAC | No breeding | 5 h | Hypotension | 2.9 | CECT: Intra- and perihepatic hematoma, pseudoaneurysm, hemoperitoneum |
| 7 | 1SG(17G) | 2 | 1 | 18 | CT | MAC | Subcapsular hematoma | Immediate | Orthostatic hypotension | 1.3 | NECT: Perihepatic hematoma, hemoperitoneum |
| 8 | 22G(20G) | 4 | 4 | 43 | CT | MAC | Subcapsular hematoma | Immediate | Pain, hypotension | 3.6 | NECT: Subcapsular and intrahepatic hematoma; hemoperitoneum |
| 9 | 19.5G | 1 | 1 | 15 | CT/US | MAC | Free fluid in pelvis | Immediate | Hypotension | 2.7 | NECT: Hemoperitoneum |
| 10 | 18G | 2 | 1 | 0 | CT | MAC | Subcapsular hematoma | 4 d | Pain | 3.7 | NECT: Hemobilia in the gallbladder |
| 11 | 22G | 2 | 2 | 3 | CT | MAC | No bleeding | 12 d | Pain | 5.7 | NECT: Subcapsular and intra hepatic hematoma |
| 12 | 18G(17G) | 1 | 2 | 26 | CT | MAC | No breeding | 10 d | Abdominal distension, hypotension | 3.5 | CECT: Hemoperitoneum, extravasation, pleural effusion |
Table 1c.
Clinical-Interventional debriefing analysis for all patients who underwent ultrasound-guided focal liver biopsy and developed hemorrhage requiring embolization. PVA=polyvinyl alcohol particles. AVF = Arterial-Venous fistula. PSA = Pseudoaneurysm
| Patient | Angiography findings | Embolization materials | Catheter position | Result |
|---|---|---|---|---|
| 1 | AVF | 100 μm PVA, microcoils | Segmental (focal / sub-selective not possible) | Effective |
| 2 | Extravasation | 40–120 μm microspheres, PVA, microcoils | Segmental, lobar (focal / sub-selective not possible) | Effective |
| 3 | AVF, PSA | 500–700 μm microspheres, microcoils | Focal sub-selective/subsegmental | Effective |
| 4 | AVF | 300 μm PVA, microcoils | Focal sub-selective and segmental | Effective |
| 5 | AVF | 300–500 μm PVA | Lobar | Effective |
| 6 | PSA, hypervascular tumor | 50 μm PVA, microcoils | Focal sub-selective and subsegmental | Effective |
| 7 | Transient blush | Gelfoarr particles and slurry | Lobar | Effective |
| 8 | AVF, Extravasation | Microcoils | Focal sub-selective | Effective |
| 9 | Transient blush | Gelfoam particles | Sectoral | Post-embolization transfusion was given for hemoglobin drop by 1 point. |
| 10 | Hepatic arterial-biliary fistula | 100–300 μm microspheres, 100 μm PVA | Lobar | Persistent slow bleeding for 8 days after embolization. |
| 11 | None | 50 μm PVA | Lobar | Effective |
| 12 | Extravasation | Coils, 300–500 μm PVA | Sectoral | Effective |
Figure 1.
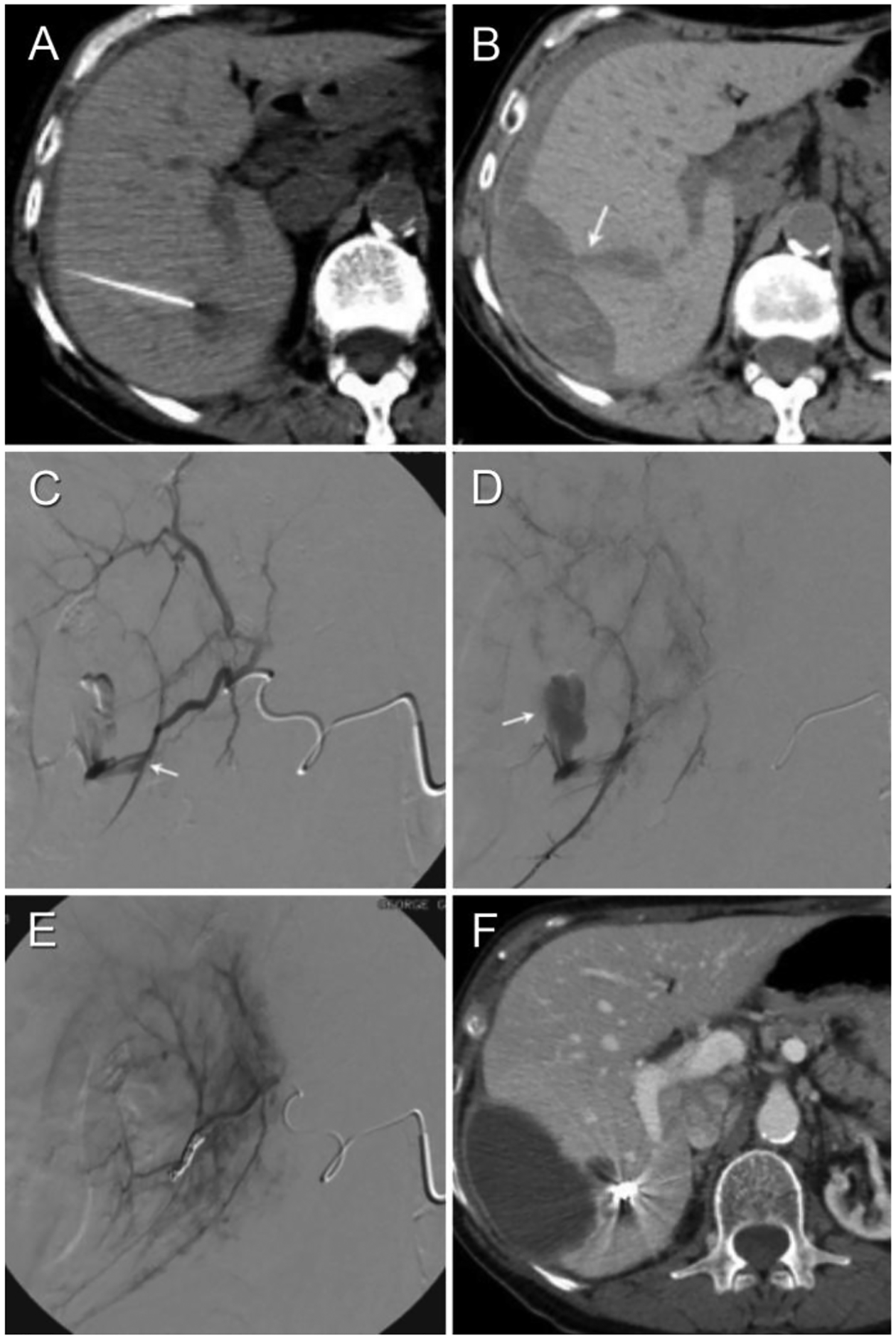
69-year-old female with history of breast and ovary cancer presents with multiple liver lesions. (Patient #8 in Tables 1a–c) (A) Non-enhanced interventional CT shows target lesion and biopsy needle vector and tip. There is a thin crescentic subcapsular hematoma laterally. (B) Follow-up non-enhanced CT performed 3 hours post-biopsy demonstrates enlarged subcaspular hematoma. (C,D) Diagnostic arteriogram demonstrates extravasation from right posterior hepatic artery branch. (E) Post-embolization arteriogram demonstrates coil mass and no further extravasation (technical success). (F) Contrast-enhanced axial CT performed 4 weeks post-biopsy demonstrates expected evolutionary changes of subcapsular hematoma, as well as radiodense coil mass. Note the location of the coil mass respective to needle tip in Figure 1a.
Figure 3.
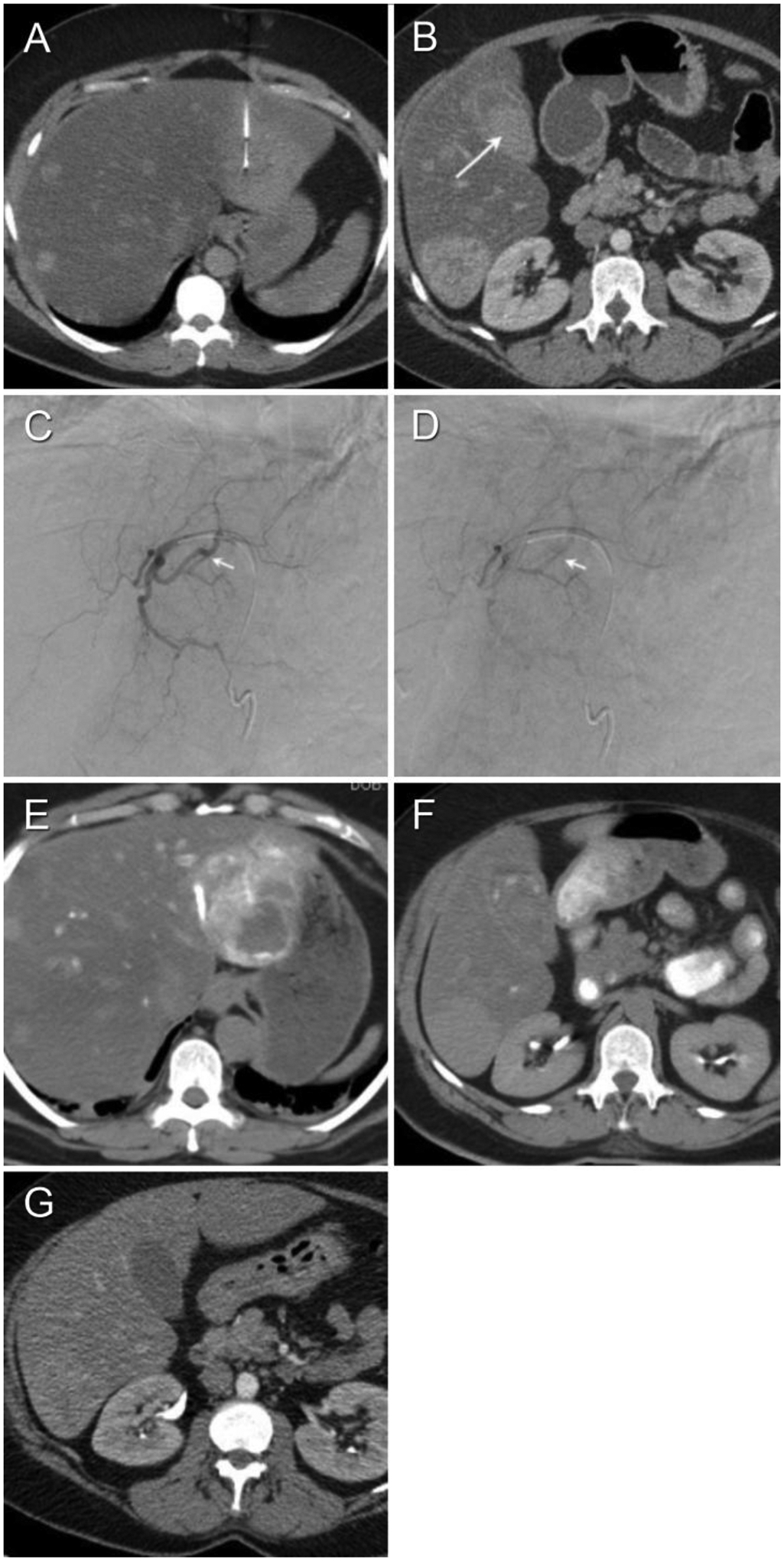
A 51-year-old female with presents with multiple liver lesions (Patient #10 in Tables 1a–c). (A) Non-enhanced interventional CT shows target lesion and biopsy needle vector and tip. (B) Contrast-enhanced axial CT on day 4 post-biopsy to evaluate severe right upper quadrant pain demonstrates hyperdense gallbladder contents compatible with hemorrhage. (C, D) Diagnostic arteriogram performed on day 14 post-biopsy for severe persistent pain shows arterio-venous fistula without active extravasation. The left hepatic artery was embolized to stasis with 6 cc 100–300 μm embospheres and 1 cc 100 μm PVA (not shown). (E, F) Non-enhanced post-embolization CT hyperdensity of the tumor (indicating successful embolization of the tumor) as well as the lumen of the gallbladder, duodenum and stomach (indicating hemobilia with enterogastric reflux). (G) Contrast-enhanced axial CT performed 21 days post-biopsy shows interval evolution and near-complete resolution of subcapsular hematoma.
Hepatic arterial findings resolved after embolization in 11/11 patients (100% technical success). Bleeding ceased after finding-directed or empiric embolization in 10/12 patients (83% clinical success). One patient had persistent slow bleeding for 8 days after embolization and was considered a clinical failure. The other patient received a blood transfusion after embolization but did not require further treatment. The one patient (1/12) with no angiographic abnormality underwent empiric lobar embolization without complication, and had resulting in clinical success. Complications were seen in 2/12 (17%) patients: cholecystitis and hepatic infarct respectively. Delayed presentation (defined as >24 hours post-biopsy) occurred in 5/12 (42%) patients; the longest latency was 12 days. Of the remaining 7 patients, four (4/12) presented immediately while the other 3/12 presented at 3, 5, and 24 hours respectively.
In five separate outlier patients, an embolization was performed within the 30 day period but the embolization was unrelated to biopsy (spontaneous tumor hemorrhage remote to the biopsy site in 3/5 patients, bleeding related to biliary drainage catheter in 2/5 patients).
Patients who presented with delayed bleeding did not exhibit unifying clinical characteristics nor characteristics consistently diverging them from patients with acute presentation. (Table 1a, 1b, 1c) For example, the patients with delayed bleeding underwent biopsy with a variety of needle sizes for lesions of a variety of locations with various angiographic findings. (Table 1a–c)
Discussion
Personalized medicine will be driven by molecular analysis of biosamples such as tumor tissue. It will require more material than needed for conventional tumor diagnosis [3] and may require multiple biopsies over the course of treatment for research protocols or to look for new mutations to explain a change in response to therapy or a difference in response of a particular tumor relative to others in the same patient, even in the same organ [3, 4].
The risks and benefits must be weighed prior to recommending or agreeing, to undergo, any procedure; the risks must therefore be known and discussed. The incidence of hemorrhage requiring embolization in our study, one of the largest such series to date, was 0.5% [5]. This value is comparable to the relevant literature on this topic from the last thirty years where the rate of major bleeding ranges from 0% to 24% with a median of 0.29% (Table 2). Of note, the institutional guidelines followed in this study are concordant with the Society of Interventional Radiology guidelines for bleeding parameter management (INR < 1.5; Platelets > 50,000). [6]
Table 2.
Review of the literature regarding major bleeding after focal image-guided liver biopsy.
| Year | Patient Population | Number of Focal Liver Biopsies | Core vs FNA/Gauge/Passes | Image Guidance /Tract Embolized? | Permissive Coagulative Parameters | Bleeding Requiring Intervention (IR/ surgery) | Bleeding Requiring Transfusion | Bleeding Not Requiring Transfusion or Intervention | Overall Major Hemorrhage Rate | |
|---|---|---|---|---|---|---|---|---|---|---|
| Luening [11] | 1991 | 510 | 510 | FNA and core/14–21 gauge/3–4 passes | CT/Not specified | Inclusion parameters not specified | 0 | 1 | 0 | 0% (0/510) |
| Zins [12] | 1992 | 72 | 24 | Core/18 Gauge/1–2 passes | US/Gelatin and Thrombin | Inclusion parameters for focal biopsy patients not specified | 1 patient died of hemorrhage; specific treatments not reported | 4.2% (1/241) | ||
| Little [13] | 1996 | 476 | 296 | 18–20G/1–3 passes | 277 US and 19 CT/Not reported | Plt >50K PT <15 PTT <45 |
8 US 2 CT |
15 US 1 CT |
0 | 3.4% (10/296) |
| Riemann [14] | 2000 | 321 | 47 | Core/18g or 20g/2 Passes | US/Not reported | Quicktime >50% Plt >50K |
0 | 1 | 0 | 0% (0/47) |
| Giorgio [15] | 2003 | 12,962 | 16,648 | 2320 19G Core; 14,328 22G FNA/ not reported | US/Not reported | Plt >45K INR<1.7 Held anticoagulants 1 week prior, aspirin 2–3 days prior |
0 | 0 | 36/16,648 | 0% (0/16,648) |
| Terjung [16] | 2003 | 629 | 100 | Core/Menghini (Gauge not reported)/Not reported | US/Not reported | INR <1.4 Plt >50K |
1 patient died of hemorrhage; specific treatments not reported | 1% (1/100) | ||
| Kim [17] | 2007 | 352 | 201 | Core/10–18 Gauge/2 passes usually | US/Not specified | Inclusion parameters not specified | 1 | 1 | 0 | 0.5% (1/20) |
| El-Osta [18] | 2011 | 155 | 46 | Core/18g/not reported | CT vs US, exact number not specified /Not reported | Inclusion parameters not specified | 0 | 0 | 0 | 0% (0/46) |
| Westheim [19] | 2012 | 275 Pediatric Patients | 25 | Not Specified/18–22 gauge/1–8 passes | US/Not specified | Inclusion parameters not specified | 1 | 5 | 0 | 4% (1/25) |
| Aribas [20] | 2012 | 1300 | 1300 | Core and FNA/up to 20G/Four or less | US/Not Specified | Inclusion parameters not specified | 1 | 0 | Not specified | 0.08% (1/1300) |
| Median rate of major hemorrhage across all major studies in the last 30 years: | 0.29% | |||||||||
The diagnostic yield of angiography performed with intent to embolize was 11/12 (92%). One patient (1/12) did not have angiographic findings (Table 1). Despite the lack of angiographic findings, the patient’s clinical improvement after lobar hepatic arterial embolization points to a likely spasmodic culprit artery as opposed to a hepatic or portal vein laceration.
Embolization was effective and safe. Angiographic abnormalities were effectively managed with embolization in 11/11 patients (100% technical success). Bleeding ceased after finding-directed or empiric embolization in 10/12 patients (83% clinical success). Complications (cholecystitis and hepatic infarct respectively) were seen in 2/12 (17%) patients.
It is interesting that so many of the patients who bled and required embolization presented with delayed bleeding. We defined “delayed presentation” as more than 24 hours post-biopsy in keeping with the literature on this topic [7]. Among patients with bleeding requiring embolization, delayed presentation was seen in 5/12 patients (42%) with the longest latency lasting 12 days. The diagnosis was established immediately in 4/12 cases (33%), and within 24 hours in 7/12 patients (59%). Specific to delayed bleeding episodes, Terjung et al [8] reported an incidence 70% (439/629 patients), which continues to be the highest reported rate of delayed bleeding in this setting. At our institution, the standard monitoring period is approximately 2 hours post-biopsy. Piccinino studied this duration and reported that 61% of complications are found within two hours after biopsy and 96% within 24 hours [9].
Limitations
Retrospective reviews carry certain limitations. We report only on patients who underwent embolization at our institution, a tertiary care, cancer hospital in a major metropolitan area. It is possible that some of our patients who bled after discharge presented to, and were treated at, their local hospital. Further, we do not know the number of patients who bled enough to require transfusion, but did not get embolized. Our general practice is to embolize patients if we are aware that they have bled significantly. However, it is conceivable that a patient who re-presented after discharge post biopsy might have been managed by the referring service without notifying the Interventional Radiology service of the admission or the complication. It is possible as well that the number of patients receiving intervention may potentially be underestimated due to patient wishes for DNR or supportive care. For example, in their study of 15,181 patients (including focal and non-focal biopsies), Atwell [10] found that all three patients who died due to hemorrhagic complications after liver biopsy had care withheld or withdrawn at the request of the family.
Still, despite these limitations, we have found these data to be useful in our practice when helping patients and referring clinicians weigh the risks and benefits of proceeding with imaging guided needle biopsy of the liver.
Conclusions
The overall incidence of bleeding requiring embolization in our population was 0.5%. This complication rate compares favorably to the 0% to 4.2% (median 0.29%) rate quoted in the available, heterogeneous, literature on this topic. Delayed presentation occurred in almost half of patients. Arterial embolization carries excellent technical and clinical success rates.
Figure 2.
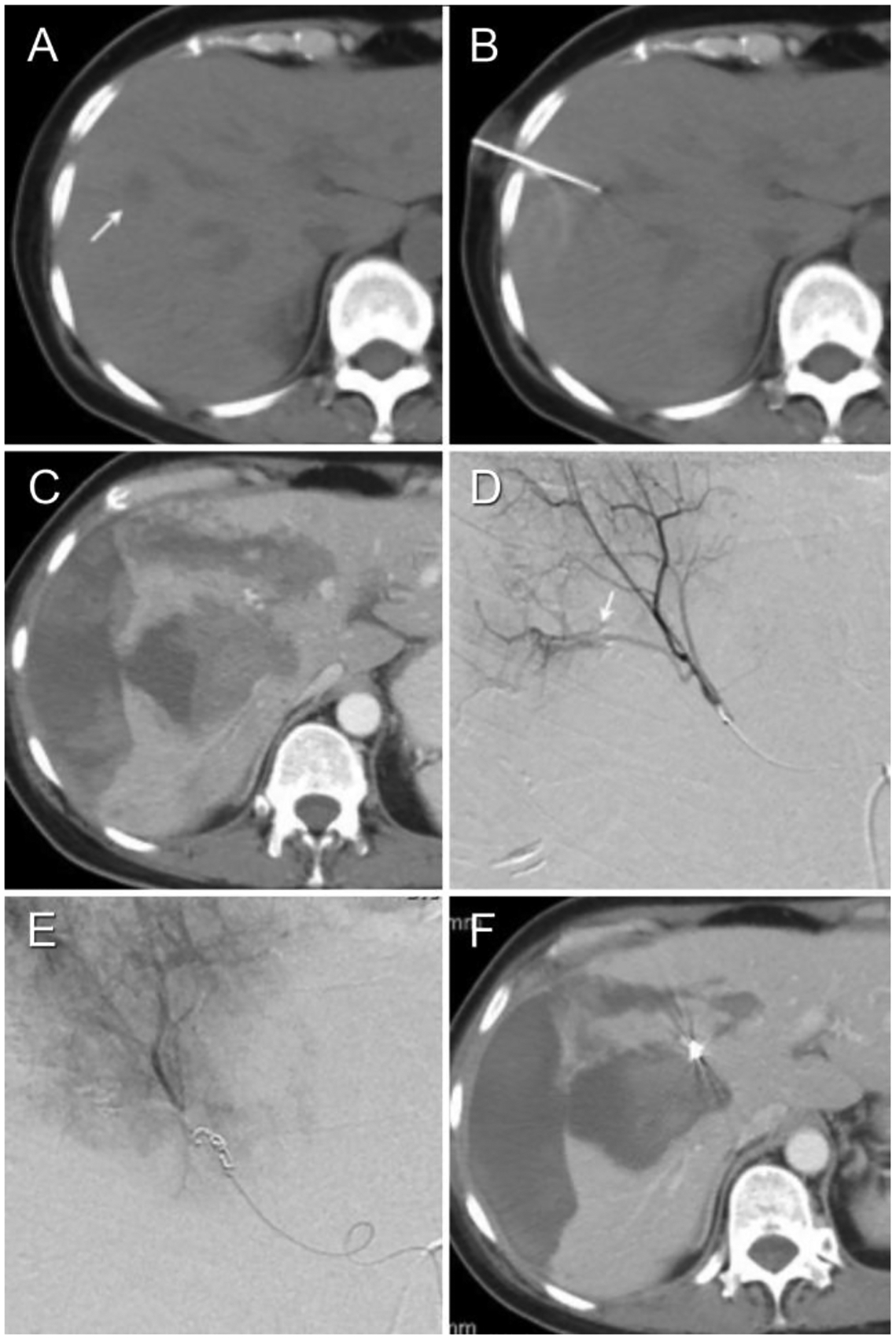
A 55-year-old female with history of leukemia presents with new liver lesions. (Patient #1 in Tables 1a–c). (A) Pre-biopsy non-enhanced axial CT shows target lesion. (B) Non-enhanced interventional CT shows target lesion and biopsy needle vector and tip. (C) Contrast-enhanced axial CT on day 2 post-biopsy to evaluate for dyspnea and dropping hematocrit demonstrates a large peri-hepatic and intrahepatic hematoma. (D) Diagnostic arteriogram shows arterio-venous fistula but no extravasation. (E) Post-embolization arteriogram after intra-arterial injection of 100 μm PVA to stasis followed by two 3 mm microcoils. The arteriovenous fistula is no longer evident (technical success). (F) Contrast-enhanced axial CT on day 7 post-biopsy week later demonstrates expected evolutionary changes of subcapsular hematoma, as well as radiodense coil mass.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1.].Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(13):1715–1721. [DOI] [PubMed] [Google Scholar]
- [2.].Wilson RJ, Ryerson AB, Zhang K, Dong X. Relative survival analysis using the Centers for Disease Control and Prevention’s National Program of Cancer Registries Surveillance System Data, 2000–2007. Journal of registry management. 2014;41(2):72–76. [PMC free article] [PubMed] [Google Scholar]
- [3.].Chabner BA. New results will change the paradigm for phase I trials and drug approval. The oncologist. 2010;15(10):1023–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4.].El-Osta H, Hong D, Wheler J, et al. Outcomes of research biopsies in phase I clinical trials: the MD anderson cancer center experience. The oncologist. 2011;16(9):1292–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5.].Atwell TD, Smith RL, Hesley GK, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR American journal of roentgenology. 2010;194(3):784–789. [DOI] [PubMed] [Google Scholar]
- [6.].Patel IJ, Davidson JC, Nikolic B, et al. Consensus Guidelines for Periprocedural Management of Coagulation Status and Hemostasis Risk in Percutaneous Image-guided Interventions. Journal of Vascular and Interventional Radiology;23(6):727–736. [DOI] [PubMed] [Google Scholar]
- [7.].Minuk GY, Sutherland LR, Wiseman DA, MacDonald FR, Ding DL. Prospective study of the incidence of ultrasound-detected intrahepatic and subcapsular hematomas in patients randomized to 6 or 24 hours of bed rest after percutaneous liver biopsy. Gastroenterology. 1987;92(2):290–293. [DOI] [PubMed] [Google Scholar]
- [8.].Terjung B, Lemnitzer I, Dumoulin FL, et al. Bleeding complications after percutaneous liver biopsy. An analysis of risk factors. Digestion. 2003;67(3):138–145. [DOI] [PubMed] [Google Scholar]
- [9.].Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. Journal of hepatology. 1986;2(2):165–173. [DOI] [PubMed] [Google Scholar]
- [10.].Atwell TD, Smith RL, Hesley GK, et al. bad Mixes Focal and Nonfocal Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR American journal of roentgenology. 2010;194(3):784–789. [DOI] [PubMed] [Google Scholar]
- 11.Lüning M, Schröder K Fau - Wolff H, Wolff H Fau - Kranz D, Kranz D Fau - Hoppe E, Hoppe E Percutaneous biopsy of the liver. (0174–1551 (Print) [DOI] [PubMed]
- 12.Zins M, Vilgrain V Fau – Gayno S, Gayno S Fau - Rolland Y, Rolland Y Fau - Arrive L, Arrive L Fau - Denninger MH, Denninger MH Fau - Vullierme MP, Vullierme MP Fau - Najmark D, Najmark D Fau - Menu Y, Menu Y Fau - Nahum H, Nahum H US-guided percutaneous liver biopsy with plugging of the needle t rack: a prospective study in 72 higlh-risk patients. {0033–8419 (Print)) [DOI] [PubMed]
- 13.Little AF, Ferris JV, Dodd GD 3rd, Baron RL (1996) Image-guided percutaneous hepatic biopsy: effect of ascites on the complication rate. Radiology 199 (1):79–S3. doi: 10.114S/radiology.199.1.8633176 [DOI] [PubMed] [Google Scholar]
- 14.Riemann E, Menzel J, Schiemann U, Domschke W, Konturek JW (2000) Lltrasound-guided biopsies of abdominal organs with an automatic biopsy system. A retrospective analysis of the quality of biopsies and of hemorrhagic complications. Scandinavian journal of gastroenterology 35 (1): 102–107 [DOI] [PubMed] [Google Scholar]
- 15.Giorgio A, Tarantino L, de Stefano G, Francica G, Esposito F, Perrotta A, Aloisio V, Farelia N, Mariniello N, Goppola C, Caturelli E (2003) Complications after interventional sonography of focal liver lesions: a 22-year single-center experience. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 22 (2):193–205 [DOI] [PubMed] [Google Scholar]
- 16.Terjung B, Lemnitzer I, Dumoulin FL, Effenberger W, Brackmann HH, Sauerbruch T, Spengler U (2003) In Bleeding Complications after Percutaneous Liver Biopsy. Digestion 67 (3): 133–145. doi: 10.1159/000071293 [DOI] [PubMed] [Google Scholar]
- 17.Kim KW, Kim MJ, Kim HC, Park SH, Kim SY, Park MS, Kim TK (2007) Value of “patent track” sign on Doppler sonography after percutaneous liver biopsy in detection ofpostbiopsy bleeding: a prospective study in 352 patients. AJR American journal of roentgenology 189 (1):109–116. doi : 10.2214/ajr.07.2071 [DOI] [PubMed] [Google Scholar]
- 18.El-Osta HHD, Wheler J et al. Outcomes of research biopsies in phase I clinical trials: the MD anderson cancer center experience. The Oncologist 2011:16:1292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westheim BH, Ostensen Ab Fau - Aagenaes I, Aagenaes I Fau - Sanengen T, Sanengen T Fau - Almaas R, Almaas R Evaluation of risk factorsfor bleeding after liver biopsy in children. (1536–4801 (Electronic))
- 20.Aribaş BK, Arda K, Çiledağ N, Aktaş E, Yakut F, Kavak Ş, Şahin G, Kaygusuz H (2012) Accuracy and safety of percutaneous US-guided needle biopsies in specific focal liver lesions: Comparison of large and small needles in 1300 patients. Panminerva Medica 54 (3):233–240 [PubMed] [Google Scholar]


