Abstract
Background
In the Greater Mekong Subregion of Southeast Asia, Plasmodium vivax malaria is endemic and causes significant morbidity. In this study, the efficacy of chloroquine for treating uncomplicated P. vivax malaria at the eastern and western borders of Myanmar was investigated.
Methods
A total of 197 participants with microscopically confirmed P. vivax infection were enrolled from three townships of the southeastern (Thanbyuzayat and Kawthoung) and western (Kyauktaw) borders of Myanmar. Patients were treated with chloroquine according to the national malaria treatment guidelines and followed for 28 days.
Results
Among the 197 enrollments, 172 completed the 28-day follow-up. Twelve recurrent P. vivax infections, all occurring in the third and fourth week, were detected, resulting in an overall cumulative rate of recurrence of 4.7% [95% confidence interval (CI): 1.5% – 7.8%]. The incidence rate of recurrence varied among the three sites. In Thanbyuzayat township, no patients had recurrent parasitemia between days 7 and 28. In contrast, Kyauktaw township had a day 28 cumulative incidence rate of recurrence of 7.2% (95% CI 0.6%–13 .9%) compared to 6.9% (95% CI: 0.6%–13.2%) in Kawthoung township.
Conclusion
While this study confirmed the relatively high clinical efficacy of chloroquine for treating P. vivax in Myanmar with modest rates of recurrent infections within 28 days of the treatment, it also revealed considerable geographical heterogeneity of chloroquine efficacy, which warrants continuous surveillance efforts.
Keywords: Chloroquine, Plasmodium vivax, clinical efficacy, recurrent parasitemia, Myanmar
Introduction
Among the five Plasmodium species infecting humans, Plasmodium vivax is the most geographically widespread species outside Africa [1]. Although it is generally considered less virulent than Plasmodium falciparum, P. vivax can cause multiple relapsing episodes of the disease, increasing its morbidity substantially [2]. In recent years, especially under malaria elimination settings, the resilience of P. vivax to conventional control measures has resulted in increased proportions of this parasite in many endemic areas [3], stressing the importance of effective management of vivax malaria. In the Greater Mekong Subregion (GMS), the ongoing malaria elimination campaign, aiming to eliminate P. falciparum by 2025 and all malaria by 2030, has also led to a dramatic change in the landscape of malaria in the region [4]. In the last decade, the dominant parasite has shifted to P. vivax in all the GMS countries [5]. Spatially, malaria is concentrated along the international borders [6–8]. With a disproportional distribution of malaria across these borders, cross-border spread and introduction of the parasites by migrating human or mosquito populations have become a paramount concern. Especially, the aptitude of P. vivax to form silent hypnozoites in the host liver enables the persistence of infections and facilitates the concealed migration of the parasites, presenting a major challenge to malaria elimination.
Chemotherapy plays a key role in the effective management of malaria. Chloroquine (CQ) combined with primaquine (PQ) has been used as the frontline drug for the radical cure of P. vivax malaria for several decades. CQ-resistant P. vivax parasites have been detected globally.[9] In Indonesia, the high prevalence of CQ-resistant parasites led to the replacement of CQ with an artemisinin combination therapy (ACT) as the first-line treatment for P. vivax [10]. The first CQ-resistant P. vivax cases in the GMS were reported in Myanmar almost thirty years ago [11], followed by additional reports [12, 13]. In recent years, although the efficacy of CQ/PQ for uncomplicated P. vivax remained high in Myanmar and its border areas [14, 15], there have been increasing numbers of studies showing clinical failures in various regions of Myanmar [16–19], indicative of RI or even RII type resistance according to the WHO defined criteria [20]. Despite the increasing trend of CQ resistance in P. vivax, the molecular mechanisms of resistance are poorly understood [21], making molecular surveillance of resistance difficult. Thus, management of drug resistance in P. vivax relies heavily on monitoring the clinical efficacy of the drugs. Yet, clinical efficacy studies also have limitations, including variable CQ pharmacokinetics in different patients and the inability to distinguish recrudescence due to treatment failure, relapse due to activation of hypnozoites, and reinfection [22].
Efficacy studies conducted in different vivax-endemic areas of Myanmar showed that the clinical failure rates of CQ in treating P. vivax had been relatively low (below 10%), but there was substantial spatial heterogeneity with some areas (e.g., southern Myanmar) showing increased failure rates. Thus, the objective of this study was to continuously monitor the efficacy of CQ for treating uncomplicated cases of vivax malaria at sentinel sites located at the eastern and western borders of Myanmar. We showed that although CQ generally remained effective, the day-28 cumulative incidence rate of recurrence at two study sites reached ~7%.
Materials and methods
Ethical Consideration
The research protocol of this study was approved by the Institutional Review Board at the Department of Medical Research under the auspice of the Ministry of Health (10-a/Ethnics 2010). Written informed consent/assent was obtained from all participants and guardians in case of minors after a full explanation of the research activities.
Sample size consideration
Sample size, based on a conserved 28-day treatment failure rate of 4%, a precision of 5% and a 95% confidence level (CI), was estimated to be 59. If assuming a 5% lost-to-follow-up rate, 62 patients were planned to enroll at each site for the study.
Study sites and populations
This study was conducted near the western Myanmar-Bangladesh border (Kyauktaw Township, Rakhine State) and eastern Myanmar-Thailand border (Thanbyuzayat Township, Mon State and Kawthoung Township, Tanintharyi Region) in 2012 (Fig. 1). While they were among the townships with the highest malaria burden in Myanmar, the two eastern townships also had disproportionally high vivax malaria incidence. The study was performed at the local township hospitals, where febrile patients with axillary temperature ≥37.5 °C or history of fever within the last 24 h were diagnosed for malaria by microscopic examination of Giemsa-stained thick and thin blood smears. Inclusion criteria included patients with P. vivax mono-infection with a parasite density of ≥250 asexual parasites per microliter (method of calculation detailed below), aged ≥ five years old, able to swallow oral medication, and able to fulfill the regular follow-up visits. Exclusion criteria included patients with underlying chronic diseases, patients with symptoms of severe malaria, those younger than five years, and pregnant or lactating women. For women of reproductive ages, pregnancy was diagnosed with a rapid human chorionic gonadotropin urine test. In addition, patients with a recent malaria history and receiving malaria treatment in the previous month were also excluded.
Fig. 1.
Locations of the three study sites in Myanmar.
Study procedures
A general physical examination was performed at the enrollment, and a questionnaire was used to record the demographic and clinical information of the patients (e.g., age, sex, axillary temperature). Participants who met inclusion criteria and gave written consent/assent were given 3-day directly observed treatment with CQ at 25 mg base/kg, divided into 10, 10, and 5 mg base/kg on days 0, 1, and 2, respectively, according to the Myanmar National Malaria Control Program (NMCP) guidelines. CQ phosphate tables (150 mg chloroquine base/tablet, Chlorofos®), manufactured by Burma Pharmaceutical Industry, was administered to patients in three age groups as follow: 5–9 years (2 tablets each on days 0 and 1, and 1 tablet on day 2), 10–14 years (3 tablets each on days 0 and 1, and 1.5 tablets on day 2), and ≥ 15 years (4 tablets each on days 0 and 1, and 2 tablets on day 2). After drug administration, each patient was monitored for at least half an hour for vomiting, allergy to treatment, or any adverse reactions. If no adverse reaction occurred, patients were requested to return for the follow-up visits on days 1, 2, 3, 7, 14, 21, and 28, and at any time during the follow-up period when they felt ill. When a patient came for the follow-up or unscheduled visits, finger-prick blood was taken to prepare thick and thin blood smears for microscopic examination and blood spots on filter paper for molecular identification of Plasmodium species. In addition, a general physical examination was given, and adverse events were recorded on each visit. Because of PQ’s schizonticidal activity, PQ treatment was postponed after completion of day 28 follow-up according to World Health Organization (WHO) 28-day protocol for determining CQ clinical efficacy [23]. The primary outcome of the study was the risk of P. vivax recurrence between day 7 and day 28 as a measure of adequate clinical and parasitological response and late parasitological failure. The secondary outcomes were the duration of parasite and fever clearance to identify cases of early treatment failure.
Microscopy examinations and species identification by PCR
Microscopic identification of Plasmodium species and parasite counting were done by two qualified microscopists independently at the field sites and confirmed by an expert whenever necessary. A blood smear with no asexual parasite detected after counting 1,000 white blood cells (WBCs) was considered negative. Parasite density, the number of asexual parasites per μl of blood, was determined as follows, assuming 6,000 WBCs/μl of blood: Parasite density = Number of asexual parasites counted/200 WBCs × 6,000 WBCs/μl. The parasite densities measured by two microscopists were averaged. If they differed by >50%, a third microscopist was required, and the parasite density was deliberated by averaging the two most concordant parasite counts. For day 0 and recurrent parasitemia during the follow-up period, Plasmodium parasite species were confirmed by nested PCR of the 18S rRNA gene [24].
Data analysis
Statistical Package for the Social Sciences (SPSS, IBM Corp. 2015 for Macintosh, Version 23.0. Armonk, NY) was used for statistical analysis. The Mann-Whitney U test and Fisher’s exact test were used to compare asexual parasite densities and patients with adequate clinical and parasitological response (ACPR) and patients with recurrent parasitemia. The risk of recrudescence during the follow-up was estimated by the Log-rank Mantel-Cox test. The cumulative incidence of treatment failures and 95% CI were calculated as described earlier after correction for the dropouts [10].
Results
Baseline characteristics of the patients
A total of 62, 67, and 68 patients with P. vivax mono-infection diagnosed by microscopy were enrolled at the township hospitals of Kyauktaw, Thanbyuzayat, and Kawthoung, respectively (Fig. 2). All were P. vivax mono-infections except for a single P.vivax/P. falciparum mixed infection that was later confirmed by PCR (Fig. 2). The study population had 65.8% male patients, mainly due to the recruitment of male-biased mobile and migrant worker populations from a rubber plantation area of Thanbyuzayat Township (Table 1). There were also differences in the age of patients among the three study sites. According to the treatment regimen, the participants were divided into three age groups: 5–9, 10–14, and ≥15 years (Table 1). The ≥15 years age group accounted for 57.1% of the study population. This age group also had significantly higher asexual parasite density than the younger age groups (P < 0.010, Mann Whitney U Test) (Table 1). This difference was even more prominent at the Thanbyuzayat site, where the ≥15 years group had a mean parasite density of 11,861 asexual parasites/μl blood. At enrollment, 42.3% of the patients had detectable gametocytes by microscopy (Table 1). During the entire study, 12 participants were lost to follow-up, 8 of which occurred in the Thanbyuzayat Township, where many patients were migrant workers.
Fig. 2.
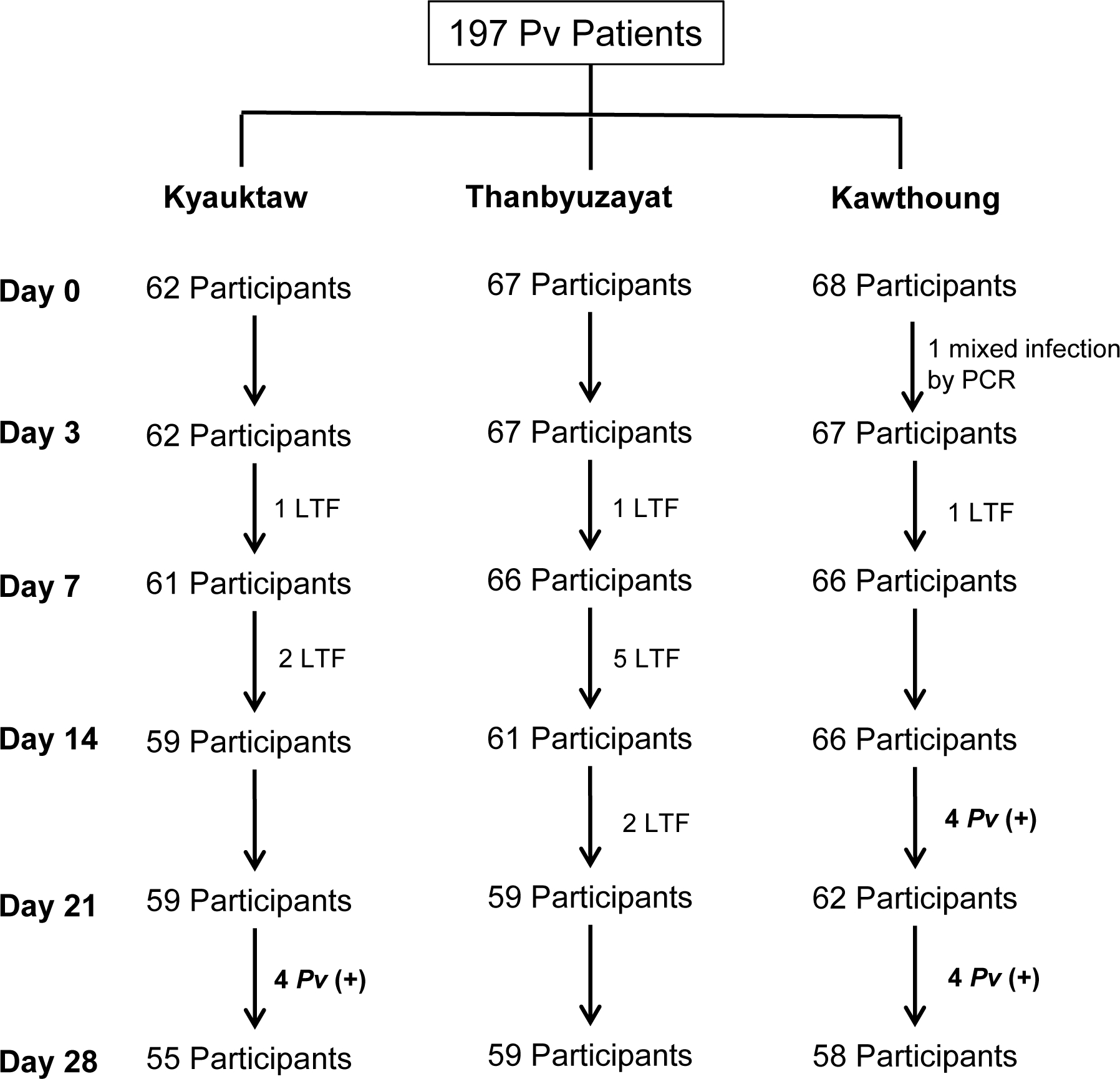
Flow chart of the study on the therapeutic efficacy of chloroquine for uncomplicated P. vivax malaria in three study sites of Myanmar. LTF, lost to follow-up; Pv (+), P. vivax recurrent parasitemia.
Table 1.
Demographic and clinical features of the P. vivax patients at enrollment
| Characteristics | Kyauktaw | Thanbyuzayat | Kawthoung | Total* |
|---|---|---|---|---|
|
| ||||
| Patient number (% of male) | 62 (48.5%) | 67 (85.0%) | 67 (57.4%) | 196 (65.8%) |
| Age in years [mean (range)] | 9.3 (6–43) | 28.6 (6.5–60) | 21.9 (5–55) | 20 (5–60) |
| Febrile patients (> 37.5 °C) [n (%)] | 23 (37.1%) | 63 (94.0%) | 31 (46.3%) | 117 (59.2%) |
| Axillary temperature, °C [mean (range)] | 37.5 (36.5–40.6) | 38.7 (36.8–40.0) | 37.7 (36.1–41.2) | 38.0 (36.1–41.2) |
| Asexual parasite density (/μl) [geometric mean (range)] | ||||
| 5–9 years (n = 65) | 5,341 (323–21,101) | 5,733 (4,015–8,318) | 2,535 (394–8,209) | 4,810 (323–21,101)a |
| 10–14 years (n = 20) | 5,660 (515–18,712) | 7,157 (488–17,619) | 1,440 (500–4,860) | 4,271 (488–18,712)a |
| ≥15 years (n = 112) | 4,141 (537–14,908) | 11,861 (262–62,221) | 6,955 (263–29,739) | 9,320 (262–62,221)b |
| Patients with gametocytemia [n (%)] | 37 (59.7%) | 24 (35.8%) | 22 (32.8%) | 83 (42.3%) |
For parasite density, age groups marked with the different letters (a,b) were significantly different (P < 0.01, Mann-Whitney U test).
Fever and parasite clearance
There were differences in the treatment response and clinical efficacy of CQ among the three study sites. Parasite clearance was rapid at the Kyauktaw and Kawthoung townships, with 6% of patients remaining parasitemic on day 2 and all being aparasitemic on day 3. However, parasite clearance was slow in Thanbyuzayat, where adult patients had significantly higher parasitemia at enrollment, with ~51% and 15% remaining parasite positive on day 2 and day 3, respectively (Fig. 3A). At all sites, no patients had parasitemia on day 7. In addition, the dynamics of fever clearance were also different among the sites (Fig. 3B). In Thanbyuzayat Township, the proportion of febrile patients was sharply reduced from 94% to 9% within 24 h of CQ treatment. In Kawthoung, although only 46.3% of patients had fever at enrollment, 35.8% remained febrile 24 h after the treatment. Nevertheless, all patients cleared fever within 48 h of CQ treatment. No serious adverse events occurred.
Fig. 3.
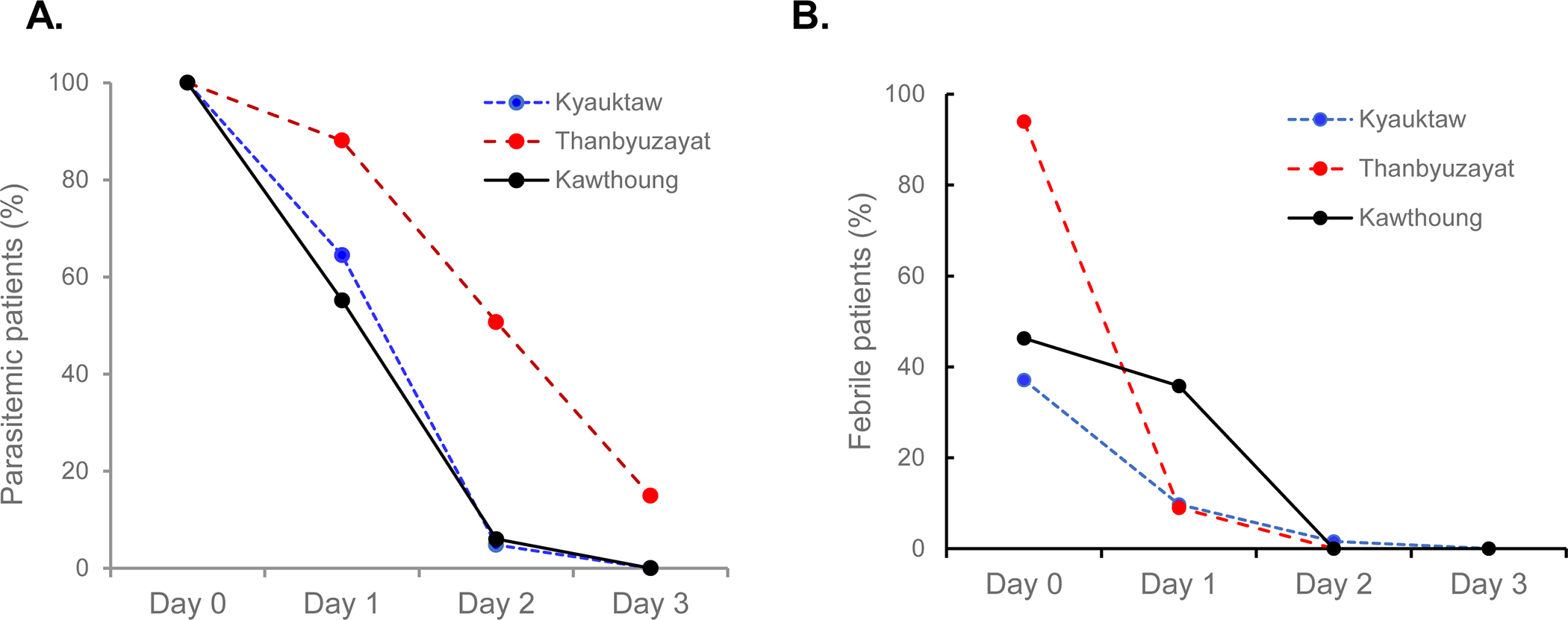
Percentages of patients with parasitemia (A) or fever (B) within the first three days of CQ treatment at the three study sites.
Parasite recurrence within 28 days
Among the 197 total enrollments, 12 patients had recurrent parasitemia, all occurring after day 14 (4 on day 21 and 8 on day 28), which resulted in an overall cumulative incidence rate of recurrence of 4.7% on day 28 (95% CI: 1.5% – 7.8%) (Fig. 4A). According to the WHO definition, these recurrent infections between follow-up days 7 and 28 were regarded as late parasitological failures (LPF). Interestingly, despite the slow parasite clearance rate in Thanbyuzayat, no patients had recurrent parasitemia between day 7 and day 28. In comparison, the day 28 cumulative incidence rates of parasite recurrence were 7.2% (95% CI 0.6%–13 .9%) in Kyauktaw and 6.9% (95% CI: 0.6%–13.2%) in Kawthoung. Next, the potential influences of demographic factors on CQ efficacy were compared between the LPF and ACPR cases (Table 2). Though the mean age of the LPF cases (15.9 years) was younger than that of the ACPR cases (20.5 years), the differences were not statistically significant (P < 0.05). Given the difference in the CQ regimens among the three age groups, a more detailed analysis of the cumulative hazard of recurrent parasitemia was performed. This analysis revealed that the 10–14 years age group had no recurrent parasitemia, whereas the younger and older groups had a similar cumulative hazard (Fig. 4B). However, the difference was not statistically different (P = 0.229, Log-rank Mantel-Cox test). Although the slower parasite clearance curve of the patients from Thanbyuzayat may imply the impact of parasitemia, a comparison of the initial parasitemia between LPF and ACPR patients did not reveal a significant difference (Table 2).
Fig. 4.
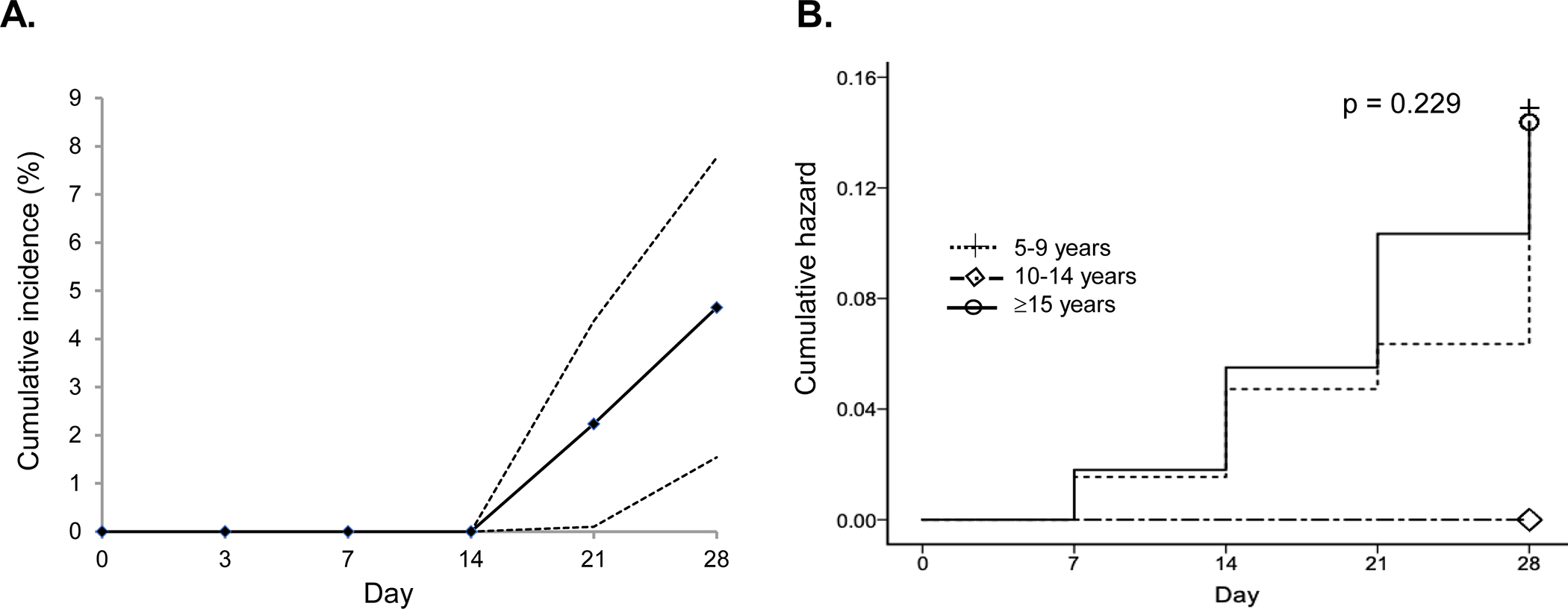
A. The overall cumulative incidence rate of recurrent P. vivax parasitemia during the 28-day follow-up after CQ monotherapy. B. The cumulative hazards of recurrent parasitemia among the three age groups.
Table 2.
Comparison between patients with recurrent parasitemia and the ACPR patients
| Characteristics | Recurrent (n=12) | ACPR (n=173) | P value |
|---|---|---|---|
|
| |||
| Age (years): mean, median (range) | 15.9, 12.5 (6–36) | 20.5, 18 (5–60) | 0.188# |
| Sex (% male) | 7 (58.3%) | 113 (65.3%) | 0.419* |
| Asexual parasite density (/μl) [geometric mean (range)] | 5,607 (263–27,029) | 7,243 (261–62,221) | 0.734# |
Mann-Whitney U test
Fisher’s exact test.
Discussion
The lack of molecular markers for detecting CQ-resistant P. vivax demands continuous monitoring of the clinical efficacy of CQ at sentinel sites [9]. This study compared the clinical efficacy of CQ for P. vivax in three sites located in the western and eastern borders of Myanmar. The overall efficacy of CQ for treating uncomplicated vivax malaria remained relatively high, with a cumulative incidence rate of recurrence within 28 days below 5%. However, for the intent-to-treat patient populations, CQ treatment showed 100% efficacy in Thanbyuzayat, 93.5% in Kyauktaw, and 88.1% in Kawthoung. Such a remarkable heterogeneity also reverberates with other CQ efficacy studies conducted across vivax-endemic areas of Myanmar [16, 17, 19]. In southern Myanmar (Tanintharyi Region), substantial evidence shows the deterioration of CQ efficacy and the potential emergence of CQ-resistant P. vivax parasites. While an earlier study in 2002–2003 in Dawei showed a worrisome 66% CQ efficacy [12], several subsequent surveys in the southern tip township Kawthoung in 2009–2012 consistently revealed lower than 90% CQ efficacy [17, 18]. These results suggest that the existence of hotspot areas in Myanmar where the deterioration of CQ efficacy was more substantial.
While the variations in CQ efficacy may reflect intrinsic genetic differences among the parasite isolates, demographic, immunological, and epidemiological settings may also contribute to these variations. Patients in Thanbyuzayat with a much higher proportion of adults had a significantly higher parasitemia than in other sites, which may be responsible for the observed delayed parasite clearance, with 10 out of 67 patients (14.9%) remaining parasitemic on day 3. The adult population in Thanbyuzayat was made up mostly of migratory workers at the rubber plantations. Delayed healthcare-seeking behavior, often associated with the mobile population [25], may account for the higher parasitemia in adult patients. In addition, since the mobile worker populations carried a significantly higher risk of repeated malaria infection [26, 27], which increases their immunity against malaria, it is also possible that malaria symptoms in these patients occurred at much higher parasitemia. Nevertheless, CQ treatment demonstrated 100% efficacy in the population from Thanbyuzayat, suggesting that delayed parasite clearance on day 3 may not be a useful surrogate marker of recurrence after CQ treatment [9, 28]. In contrast, the patient cohorts from Kyauktaw and Kawthoung townships presented with recurrent P. vivax infections within 28 days of CQ treatment, even though no patients had persistent parasitemia on day 3. Regardless of whether the recurrent parasitemia may represent relapse, recrudescence, or reinfection, they may be CQ-resistant since the 28-day in vivo efficacy test presumes that an effective blood level of CQ persists for at least 28 days [22]. Alternatively, the recurrent parasitemia may be due to CQ blood levels falling below the minimum inhibitory concentration, since all recurrences occurred in weeks 3 and 4 after CQ treatment. In line with this assumption, a recent study conducted in Cambodia detected 60% of the recurrences within 28 days occurred when CQ blood concentrations fell below the therapeutic level [29]. Thus, the CQ level in the blood on the day of recurrence should be measured in the future to identify true CQ resistance.
Previous reports from other areas revealed that children had higher chances to have recurrent parasitemia of P. vivax malaria [30–33]. The underlying mechanism is ill-defined but might be due to the incomplete development of their immune system, limiting the ability to eliminate the residual parasites that lead to recurrence. CQ underdosing is especially prevalent in children and may also be associated with the increased incidence of recurrent parasitemia in children [34, 35]. In this study, patients with LPF were much younger than those with ACPR, although the difference was not statistically significant. More robust studies to consider bodyweight and blood CQ levels are needed to determine the factors associated with recurrent P. vivax infections.
To evaluate the clinical response of P. vivax to CQ treatment, PQ treatment was postponed after completion of day 28 follow-up, thus eliminating the complication from the additional schizonticide activity of PQ [36, 37]. There is clear evidence showing that PQ noticeably enhances the therapeutic efficacy of CQ in the treatment of P. vivax infections [9, 14, 38]. Thus, it is expected that CQ/PQ combination would result in lower recurrence rates. Considering this, two recent studies conducted at the northeastern border of Myanmar detected early recurrent P. vivax infections after CQ/PQ treatment [16, 19]. Together with the much earlier reports of sporadic CQ resistance in Myanmar [11–13], these studies suggested the decline of the CQ/PQ efficacy and the emergence of CQ-resistant strains in Myanmar, which warrants follow-up studies to ensure the efficacy of the frontline treatment of vivax malaria.
Conclusion
This study confirmed the relatively high clinical efficacy of CQ for the treatment of vivax malaria in the eastern and western borders of Myanmar and, at the same time, revealed geographical heterogeneity in CQ efficacy. The detection of recurrent P. vivax infections after CQ or CQ/PQ treatment in the eastern (Kawthoung and Laiza) and western (Kyauktaw) borders, together with other longitudinal monitoring efforts of CQ efficacy in Myanmar and neighboring regions, indicates the emergence of CQ-resistant P. vivax.
Acknowledgments
We thank all the medical assistants from the township hospitals for their commitment to complete this study.
Funding
This research was supported by the President’s Malaria Initiative for the GMS (MPK) and a grant (U19AI089672) from the National Institute of Allergy and Infectious Diseases, NIH, USA (LC).
Footnotes
Competing interests
The authors declare that there is no conflict of interest.
Consent for publication
Not applicable.
References
- 1.Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, et al. : A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White NJ, Imwong M: Relapse. Adv Parasitol. 2012;80:113–150. [DOI] [PubMed] [Google Scholar]
- 3.Cui L, Brashear A, Menezes L, Adams J: Elimination of Plasmodium vivax malaria: problems and solutions. In Current Topics and Emerging Issues in Malaria Elimination. IntechOpen; 2021: 27 [Google Scholar]
- 4.WHO: Eliminating malaria in the Greater Mekong Subregion: United to end a deadly disease. https://wwwwhoint/malaria/publications/atoz/eliminating-malaria-greater-mekong/en/. 2016. [Google Scholar]
- 5.WHO: Countries of the Greater Mekong ready for the “last mile” of malaria elimination. WHO Technical Bulletin #9. 2020. [Google Scholar]
- 6.Socheat D, Denis MB, Fandeur T, Zhang Z, Yang H, Xu J, Zhou X, Phompida S, Phetsouvanh R, Lwin S, et al. : Mekong malaria. II. Update of malaria, multi-drug resistance and economic development in the Mekong region of Southeast Asia. Southeast Asian J Trop Med Public Health. 2003;34 Suppl 4:1–102. [PubMed] [Google Scholar]
- 7.Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, Fan Q, Fang Q, Jongwutiwes S, Parker D, et al. : Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delacollette C, D’Souza C, Christophel E, Thimasarn K, Abdur R, Bell D, Dai TC, Gopinath D, Lu S, Mendoza R, et al. : Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health. 2009;40:674–691. [PubMed] [Google Scholar]
- 9.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ: Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird JK, Wiady I, Fryauff DJ, Sutanihardja MA, Leksana B, Widjaya H, Kysdarmanto, Subianto B: In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am J Trop Med Hyg. 1997;56:627–631. [DOI] [PubMed] [Google Scholar]
- 11.Myat Phone K, Myint O, Myint L, Thaw Z, Kyin Hla A, Nwe Nwe Y: Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans R Soc Trop Med Hyg. 1993;87:687. [DOI] [PubMed] [Google Scholar]
- 12.Guthmann JP, Pittet A, Lesage A, Imwong M, Lindegardh N, Min Lwin M, Zaw T, Annerberg A, de Radigues X, Nosten F: Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop Med Int Health. 2008;13:91–98. [DOI] [PubMed] [Google Scholar]
- 13.Marlar T, Myat Phone K, Aye Yu S, Khaing Khaing G, Ma S, Myint O: Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans R Soc Trop Med Hyg. 1995;89:307–308. [DOI] [PubMed] [Google Scholar]
- 14.Chu CS, Phyo AP, Lwin KM, Win HH, San T, Aung AA, Raksapraidee R, Carrara VI, Bancone G, Watson J, et al. : Comparison of the cumulative efficacy and safety of chloroquine, artesunate, and chloroquine-primaquine in Plasmodium vivax malaria. Clin Infect Dis. 2018;67:1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Yang HL, Tang LH, Li XL, Huang F, Wang JZ, Li CF, Wang HY, Nie RH, Guo XR, et al. : Monitoring Plasmodium vivax chloroquine sensitivity along China-Myanmar border of Yunnan Province, China during 2008–2013. Malar J. 2014;13:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan L, Wang Y, Parker DM, Gupta B, Yang Z, Liu H, Fan Q, Cao Y, Xiao Y, Lee MC, et al. : Therapeutic responses of Plasmodium vivax malaria to chloroquine and primaquine treatment in northeastern Myanmar. Antimicrob Agents Chemother. 2015;59:1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyunt MH, Han JH, Wang B, Aye KM, Aye KH, Lee SK, Htut Y, Kyaw MP, Han KT, Han ET: Clinical and molecular surveillance of drug resistant vivax malaria in Myanmar (2009–2016). Malar J. 2017;16:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Htun MW, Mon NCN, Aye KM, Hlaing CM, Kyaw MP, Handayuni I, Trimarsanto H, Bustos D, Ringwald P, Price RN, et al. : Chloroquine efficacy for Plasmodium vivax in Myanmar in populations with high genetic diversity and moderate parasite gene flow. Malar J. 2017;16:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu S, Zeng W, Ngassa Mbenda HG, Liu H, Chen X, Xiang Z, Li C, Zhang Y, Baird JK, Yang Z, Cui L: Efficacy of directly-observed chloroquine-primaquine treatment for uncomplicated acute Plasmodium vivax malaria in northeast Myanmar: A prospective open-label efficacy trial. Travel Med Infect Dis. 2020;36:101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chemotherapy of malaria: Report of the WHO Scientific Group. WHO Technical Report Series No. 375. [https://apps.who.int/iris/bitstream/handle/10665/40671/WHO_TRS_375.pdf]
- 21.Buyon LE, Elsworth B, Duraisingh MT: The molecular basis of antimalarial drug resistance in Plasmodium vivax. Int J Parasitol Drugs Drug Resist. 2021;16:23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird JK: Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev. 2009;22:508–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO: Methods for surveillance of antimalarial drug efficacy. pp. 852009:85. [Google Scholar]
- 24.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA: A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg. 1999;60:687–692. [DOI] [PubMed] [Google Scholar]
- 25.Hein KT, Maung TM, Htet KKK, Shewade HD, Tripathy JP, Oo SM, Lin Z, Thi A: Low uptake of malaria testing within 24 h of fever despite appropriate health-seeking among migrants in Myanmar: a mixed-methods study. Malar J. 2018;17:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peeters Grietens K, Gryseels C, Dierickx S, Bannister-Tyrrell M, Trienekens S, Uk S, Phoeuk P, Suon S, Set S, Gerrets R, et al. : Characterizing types of human mobility to inform differential and targeted malaria elimination strategies in northeast Cambodia. Sci Rep. 2015;5:16837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TM, Zhang SS, Feng J, Xia ZG, Luo CH, Zeng XC, Guo XR, Lin ZR, Zhou HN, Zhou SS: Mobile population dynamics and malaria vulnerability: a modelling study in the China-Myanmar border region of Yunnan Province, China. Infect Dis Poverty. 2018;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratcliff A, Siswantoro H, Kenangalem E, Wuwung M, Brockman A, Edstein MD, Laihad F, Ebsworth EP, Anstey NM, Tjitra E, Price RN: Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans R Soc Trop Med Hyg. 2007;101:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popovici J, Pierce-Friedrich L, Kim S, Bin S, Run V, Lek D, Hee KHD, Lee Soon UL, Cannon MV, Serre D, Menard D: Recrudescence, reinfection, or relapse? A more rigorous framework to assess chloroquine efficacy for Plasmodium vivax malaria. J Infect Dis. 2019;219:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy GS, Basri H, Purnomo, Andersen EM, Bangs MJ, Mount DL, Gorden J, Lal AA, Purwokusumo AR, Harjosuwarno S, et al. : Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet. 1993;341:96–100. [DOI] [PubMed] [Google Scholar]
- 31.Graf PC, Durand S, Alvarez Antonio C, Montalvan C, Galves Montoya M, Green MD, Santolalla ML, Salas C, Lucas C, Bacon DJ, Fryauff DJ: Failure of Supervised Chloroquine and Primaquine Regimen for the Treatment of Plasmodium vivax in the Peruvian Amazon. Malar Res Treat. 2012;2012:936067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruebush TK 2nd, Zegarra J, Cairo J, Andersen EM, Green M, Pillai DR, Marquino W, Huilca M, Arevalo E, Garcia C, et al. : Chloroquine-resistant Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2003;69:548–552. [PubMed] [Google Scholar]
- 33.Phyo AP, Lwin KM, Price RN, Ashley EA, Russell B, Sriprawat K, Lindegardh N, Singhasivanon P, White NJ, Nosten F: Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial. Clin Infect Dis. 2011;53:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Commons RJ, Simpson JA, Thriemer K, Humphreys GS, Abreha T, Alemu SG, Anez A, Anstey NM, Awab GR, Baird JK, et al. : The effect of chloroquine dose and primaquine on Plasmodium vivax recurrence: a WorldWide Antimalarial Resistance Network systematic review and individual patient pooled meta-analysis. Lancet Infect Dis. 2018;18:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Sena LWP, Mello A, Ferreira MVD, de Ataide MA, Dias RM, Vieira JLF: Doses of chloroquine in the treatment of malaria by Plasmodium vivax in patients between 2 and 14 years of age from the Brazilian Amazon basin. Malar J. 2019;18:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pukrittayakamee S, Imwong M, Chotivanich K, Singhasivanon P, Day NP, White NJ: A comparison of two short-course primaquine regimens for the treatment and radical cure of Plasmodium vivax malaria in Thailand. Am J Trop Med Hyg. 2010;82:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pukrittayakamee S, Vanijanonta S, Chantra A, Clemens R, White NJ: Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J Infect Dis. 1994;169:932–935. [DOI] [PubMed] [Google Scholar]
- 38.Baird JK, Basri H, Subianto B, Fryauff DJ, McElroy PD, Leksana B, Richie TL, Masbar S, Wignall FS, Hoffman SL: Treatment of chloroquine-resistant Plasmodium vivax with chloroquine and primaquine or halofantrine. J Infect Dis. 1995;171:1678–1682. [DOI] [PubMed] [Google Scholar]


