Abstract
Objective:
Adopted children tend to show an increased risk for a variety of psychopathological outcomes, even when adoption occurs at birth, which some suggest is a result of non-random assignment of adoptees and parents. This study utilizes a nonhuman primate model, in which adoptions were randomly assigned, to investigate the behavioral and physiological outcomes associated with at-birth adoption.
Method:
Immediately following birth, rhesus monkey infants were randomly assigned to be reared by either their biological mother (n=113) or by an unrelated, lactating, adoptive mother (n=34). At six months of age, infant behavior and physiology were assessed during a stressful series of mother-infant separations. Four years later, stress-related behaviors were measured following confrontation by an unfamiliar intruder, an ecologically meaningful stressor.
Results:
When compared to infants reared by their biological mothers, adopted infants exhibited more behavioral withdrawal and higher plasma adrenocorticotropic hormone (ACTH) concentrations in response to separation. These behavioral differences persisted four years later during a stressful intruder challenge, with adoptees exhibiting more behavioral withdrawal, stereotypies, and impulsive approaches of the potentially aggressive intruder.
Conclusion:
Compared to infants reared by their biological mothers, adopted infants exhibited more behavioral inhibition, impulsivity, and higher ACTH concentrations, even when subjects were randomly assigned to be adopted or to remain with their biological mother. To the extent that these findings generalize to humans, they suggest that the overall risk for psychopathology in adopted individuals persists even after random assignment to adoption conditions.
Keywords: adoption, adrenocorticotrophic hormone, early experience, mother-infant, rhesus monkey
Introduction
Although there are wide individual differences, in general, when compared to children raised by their biological parents, a disproportionate number of adopted children exhibit poorer long-term developmental outcomes and meet criteria for clinical diagnoses1–4. Some have suggested that these outcome differences may be, in part, due to genetic factors, with adopted infants at greater risk for inheriting psychopathology from their biological parents5–6. Others suggest that temperament differences between adoptive parents and adopted offspring may lead to increased parent-child conflict7. While both may play a role in adoptee outcomes, certain differences between biological and adoptive parents make assessing outcome disparities between adopted and non-adopted individuals difficult. For example, parents who adopt tend to be older, have high SES, high educational attainment, and strongly desire children, which may impact their parenting practices and involvement8–10. Adopted children tend to be born into in low SES environments to biological parents who are more likely to have underlying mental health diagnoses and are less likely to seek prenatal care11–12. In such uncontrolled situations, it is difficult to disentangle the etiological mechanisms that contribute to outcome differences among adopted individuals. Furthermore, the age at which an infant is adopted, as well as preadoption history, are important variables to consider when assessing the effects of adoption. For example, those that are adopted later may have experienced adverse conditions leading to the adoption and such experiences are known to contribute to developmental outcomes (for example, see 13). While the impact of adverse conditions on adoption and development is an important line of research, this study will focus on the relationship between at-birth adoptions and developmental variation.
A first step in understanding the etiology of psychopathology in adopted individuals is to establish whether differences in adoption outcomes persist when adopted individuals are randomly assigned to adoptive parents. Although it is unethical to conduct a study with this design in a human sample, animal models are commonly utilized to investigate questions related to adoption (also termed “cross-fostering”). For example, seminal studies in rodents indicate profound differences in pups that are randomly fostered to high- or low-quality mothers (as measured by rates of maternal licking)14. Others show the effects of at-birth adoption on other outcomes, for example, rodents adopted at birth tend to show differences in sleep architecture15. While valuable, rodent studies may fail to capture the complexities of human development that are better modeled in a more-closely related species. While they have not been widely utilized as a model of adoption, rhesus monkeys (Macaca mulatta) have a long history of being utilized for modeling human development, including behavior16. While difficult to perform in primates because they only give birth once a year and, consequently, sample sizes tend to be small, rhesus monkeys have been used in adoption studies to disentangle the effects of maternal treatment (for example, see 17. Rhesus monkeys have been instrumental in establishing maternal influences on outcomes such as alcohol intake18, neurotransmitter functioning19, and infant abuse20. Given the rich history of using nonhuman primates to study developmental outcomes on other variables, we propose to utilize a translational rhesus monkey model to investigate the impact of at-birth adoption on developmental outcomes.
The nonhuman primate model allows for increased control over variables (such as random assignment) that make adoption difficult to study in humans. Furthermore, as rhesus monkeys develop 3–4 times more quickly than humans21, longitudinal assessments of developmental outcomes can be performed in a relatively rapid fashion. Rhesus monkeys possess marked similarities to humans, including genetic similarities22, as well as similarities in the HPA axis23, and, like humans, they live in complex social groups, requiring extended time in to acquire the skills necessary to achieve social competence24. While others have assessed the specific effects of at-birth adoption in rhesus monkeys25–27, the sample sizes were small (n<10 subjects) and the study designs lacked random assignment. What follows is a nonhuman primate model assessing the effects of adoption on infant outcomes. It is, to the authors’ knowledge, the first large-scale comparison of adopted infants and those reared by their biological mothers and utilizes randomized adoption assignments. As such, it is an important step in establishing a nonhuman primate model of human adoption that could be utilized to identify the underlying etiology of aberrant developmental outcomes in adoptees.
This longitudinal study seeks to establish a useful nonhuman primate model of adoption by comparing the developmental outcomes among adopted individuals and those reared by their biological mothers during infancy and again as young adults. The study first examines stress-induced behaviors and the neuroendocrine response to a social separation stressor in infancy and then evaluates differences in the behavioral response to the stress of an unfamiliar intruder later in life. Specifically, as infants, subjects underwent a series of mother-infant social separation stressors and the time spent in behavioral withdrawal (sometimes termed freezing), a widely accepted measure of anxiety-like behaviors in rhesus monkeys28–30 was recorded. To identify differences in behavioral withdrawal during short- and long-term periods of stress, behavioral withdrawal was assessed during both acute and chronic maternal separation. Approximately four years later, as young adults, subjects were exposed to the stressor of the Intruder Challenge test31 a standardized, ecologically-meaningful method of inducing stress in nonhuman primates via the introduction of an unfamiliar age- and sex-matched intruder to the homecage.
Given the evidence of deleterious outcomes for human adoptees, it was hypothesized that, under conditions of stress, adopted infants would, on average, exhibit heighted anxiety-like behaviors and an increased neuroendocrine response to a repeated social separation stressor, when compared to infants reared by their biological mothers. It was further hypothesized that these differences would persist into young adulthood, with the adoptees exhibiting more aberrant responses to an intruder, when compared to infants that were reared by their biological mothers.
Method
Subjects
Subjects were N=147 socially-housed rhesus macaque (Macaca mulatta) infants (74 female monkeys, 73 male monkeys) living with their biological or adoptive mothers and housed in mixed-sex social groups containing 2 adult male monkeys and 8–10 unrelated adult female monkeys, and approximately 2–5 same-age infant monkeys, allowing for species-typical socialization and peer-socialization in a setting that approximates the natural social composition of rhesus macaques32. Subjects were housed in indoor-outdoor enclosures (indoor: 2.44 X 3.05 X 2.21 m; outdoor: 2.44 X 3.0 X 2.44 m), with a 12-hour (0700–1900) light-dark cycle for the indoor enclosure and natural seasonal light cycles for the outdoor enclosure. The overall genetic diversity of the colony was maintained by selective out-breeding, and other studies from our laboratory have shown the overall degree of relatedness (identity-by-descent) is 1.68%, which is about the level of third-degree cousins, which is considered sufficiently unrelated in human33 nonhuman primate34 studies. The sample used in this study included monkeys from ten birth-year cohorts (born between 1996–2005), with procedures remaining consistent over the years. For each year of the study, about 20% of the subjects were randomly assigned to be adopted. Adoptees (n=34; 17 female infants and 17 male infants) were fostered to an unrelated, lactating female monkey within 48 hours of birth. Other than the adoption procedures, adoptees and infants reared by their biological mothers (n=113; 57 female infants, 56 male infants) received identical treatment, including a newborn examination by a veterinarian and a check of the mothers to assure lactation. A power analysis was performed to estimate necessary sample size using the main variables hypothesized to differ between the two groups. With an alpha set at .05 and power set at .90, the sample size needed for between-group comparisons is approximately n=29 for each group. Thus, the sample size of this study is more than adequate for the study objectives. All animals were fed Purina® high-protein monkey chow (#5043), and had access to water ad libitum. Supplementary fruit and other food-based enrichment were provided daily. All protocols for the use of experimental animals were approved by the Institutional Animal Care and Use Committee of the National Institute of Alcohol Abuse and Alcoholism.
Adoption Procedures
Given the length of time it took to obtain a sufficient sample of adopted infants, it is important to note that, as much as possible, between-year variability was minimized by utilizing same personnel and procedures, with the same two technicians planning and performing all of the adoption procedures and the same laboratory coordinator training all of the technicians on the behavior coding system. Furthermore, the animal care staff maintained the same personnel and the same attending veterinarian provided care during the study period. Adopted infants were randomly assigned to adoptive mothers that met the following criteria:
must have given birth within a week of the adoption (the mother’s biological infant was separated the mother and raised in the neonatal nursery)
must be unrelated to their adopted infant
must be living in a separate social group to avoid interactions with the biological mother
must be multiparous
lactation must be assured via medical evaluation
The adoptions were conducted utilizing common procedures from many nonhuman primate laboratories and piloted in this laboratory before beginning the study. Using an established protocol over all the years of the study, the following procedure was utilized for each adoption: at 0700, the adoptive mother was separated from her social group into a temporary holding cage. She was restrained and lactation was assessed to ensure that the infant would not suffer from dehydration. Following that assessment, infants to be adopted were removed from their sedated biological mother and placed into a transport cage. The transport cage was then fastened to an opening on the adoptive mother’s holding cage and the transport cage door was opened, allowing the lactating female monkey to retrieve the infant, which most did immediately. The dyad’s behavior was observed hourly over the first day and checked again the following morning. If the adoptive mother retrieved the infant, successfully nursed it, and showed no signs of rejection, the adoption was considered successful. If the lactating female monkey failed to nurse the infant or showed signs of rejecting the infant, the infant was retrieved and returned to its biological mother or placed in a neonatal nursery for rearing (fewer than 10% of total attempts). None of the adoptive mothers showed evidence of recognizing the adoptive infant as different from their biological infant, exhibiting the typical maternal care for the newborn, including restrictiveness, retrievals, cleaning and grooming, and if there was a recognition by the other group members that infant was different, they showed no evidence of such awareness as far as could be observed. Mothers social dominance rank was obtained using established procedures35. To assure that mothers’ rank was unrelated to whether they reared an adopted or biological infant for this study, preliminary analyses assessed for distributions of dominance rank (high, medium, or low) and rearing status (adoptive mother or biological mother). Results showed no significant relationship between dominance and rearing status (x2= 0.08, p=.96). Mothers rearing an adopted or a biological infant showed no significant differences in average parity (adoptive mothers: M=4.45 offspring, biological mothers: M=4.34 offspring; t(174)=1.97, p=.13). Furthermore, 40% of the adoptive mothers were peer- or surrogate-reared, compared to 30% of the infants reared by their biological mothers, a difference that is unlikely to be meaningful, and if it was, it would likely lead to more problems in mother for the biological mother group. As the infants were randomly assigned to mothers, in some cases, the sex of the infant that she adopted was different than the sex of her biological infant. We used a chi-square to assess whether there was a significant number of infants being raised by mothers that had a biological infant of the opposite sex and found no effect (x2=2.31, p=.13). As noted earlier, like the adopted infants, the infants reared by their biological mothers were also removed from their mothers for a veterinary examination and the biological mothers were also assessed to assure that the they were lactating.
Separation Paradigm
The procedure for the separations is well established and was used in our laboratory for at a number of years before the onset of this study36.To assure continuity between years, the senior researcher and laboratory coordinator oversaw all of the social separations. When the infants reached six months of age, mothers and infants were captured and separated for four sequential, 4-day-long social separations, each followed by three days of mother-infant reunion. The social separation procedure began on Monday afternoon at 1300, with the mothers’ removal from the social group. She was isolated from her social group so neither she nor her infant could see or hear one another. The infant remained in the homecage with the rest of the social group. On day-5 (Friday), the mother was reunited with her infant in the homecage and a three-day mother-infant reunion ensued. This procedure was repeated for a period of four weeks. As weaning occurs around six months of age in the natural setting37, following the separation paradigm, all infants were removed from their mothers and placed into a large pen with 25–40 other same-aged infants. They remained in this larger peer group until they were young adults, with all subjects receiving identical treatment until the Intruder Challenge test was conducted.
Infant Behavior
During each of the four, 4-day separations, trained observers blind to the rearing conditions of the subjects collected behavioral observations of the infants interacting in their homecage. For day-1 (D1), three 5-minute behavior coding sessions were conducted during the first two hours of the separation. For days 2–4 (D2-D4), two 5-minute behavior coding sessions were conducted each day. Consistent with previous work38, the data were aggregated into an Acute and Chronic phase, with D1 of each separation period recorded as the Acute phase and the D2-D4 recorded as the Chronic phase. Thus, for each of the separations, infant behavioral scores for each week were averaged across D1 to produce the Acute phase and across D2-D4 for the Chronic phase. Behavioral withdrawal, defined as the amount of time the infant remained inactive and not engaging their environment or interacting with other individuals, during the separations was measured. The time spent withdrawn was recorded in seconds over a 5-minute observation period. Behavior was recorded using handheld by the senior researcher rater reliability of r≥0.85 and were reassessed twice a year to assure that there was no inter-rater drift.
Physiology
To investigate rearing differences in HPA axis activity, two 2mL blood samples were obtained from the femoral vein of the infants 1 and 2 hours after each social separation on D1, between 1300–1500. Samples were obtained while the infants were awake and restrained, immediately following hand capture, as in39. Blood samples were obtained while the infants were awake to allow for behavior coding following the sampling. After the blood samples were obtained, they were immediately placed on wet ice and centrifuged at 4°C for 20 minutes. The plasma was aliquoted and frozen in liquid nitrogen, after which the samples were stored in an ultracold freezer at −70° C until they were assayed. Samples were assayed for plasma cortisol and adrenocorticotropic hormone (ACTH) using standard radioimmunoassay described elsewhere38, with an intra- and inter-assay reliability greater than .90 for cortisol and .85 for ACTH.
Intruder Challenge Test
All subjects were maintained in mixed-sex group housing until approximately three years of age, when the male and female monkeys were separated to avoid unplanned pregnancies. When subjects were approximately four years old (M=4.14±0.25 years), a subset (n=74; 47 female monkeys, 27 male monkeys) was tested in the stress-eliciting Intruder Challenge test32, a paradigm designed to assess the behavioral responses to an ecologically meaningful challenge (an unfamiliar intruder). Of this subset, n=58 were reared by their biological mothers and n=16 were adoptees. The Intruder Challenge test has been previously utilized to answer questions related to biological and environmental factors40–41.
For this test, subjects are exposed to an unfamiliar age- sex- and size-matched conspecific (the intruder) in a controlled manner. First, the intruder was removed from its homecage and placed into a holding cage (.76X.63X.91 m) for a 30-min acclimation period. The holding cage had a mesh front and top so that the intruder was visible from all directions. Three randomly-selected same-sex test subjects from the same social group were segregated from the larger social group and placed into the outdoor portion of their home enclosure for a 10-minute acclimation period. After the acclimation period, the intruder’s holding cage was moved to the front of the outdoor portion of the test subjects’ home enclosure so that the test subjects could approach the intruder and interact through the mesh of the cage. Full body contact was prevented to avoid injury to any of the animals and none of the animals were injured during testing
The test subjects’ behavior was recorded for 30 minutes by observers that were blind to subjects’ rearing conditions. Separate observers were assigned to each of the three subjects. Behavior was recorded using handheld computers equipped with Observer™ software (Noldus, Leesburg, Virginia). The software allows each observer to record the frequency and duration of various behaviors performed by the test subject. Behavioral withdrawal, latency to approach the intruder, locomotion, environmental exploration, social contact, stereotypy, and threat vocalizations were recorded based on a standard behavioral ethogram (See Table 1), which has been used in previous studies41. When meeting an unfamiliar conspecific, behavioral withdrawal is indicative of increased anxiety, rapid approach is considered a measure of impulsivity, with aggression relatively frequent. All three behaviors could be considered disadvantageous to survival in the presence of an intruder threat. All behavior coders were trained by the laboratory coordinator to an interobserver reliability r≥0.85 and were reassessed twice a year to assure that there was no inter-rater drift.
Table 1.
Intruder Challenge Behavioral Ethogram
| Behavior | Description |
|---|---|
|
| |
| Aggression | Focal subject gives/receives aggression to/from the intruder/cagemates. Contact (e.g., bites, slaps) and non-contact (eg, threats, lunges) are counted. |
| Latency to approach intruder | Time from the start of test until the focal subject first enters proximity (1m) to the intruder. |
| Approach intruder | Number of times the focal subject enters proximity (1m) to the intruder. |
| Activity | Focal subject moves across the substrate (eg, walking, running, swinging across cage, etc.). |
| Environmental exploration | Focal subject engages in active manual, oral, or pedal examination, exploration, or manipulation of the environment. |
| Social contact | Focal subject is within arm’s reach of the cagemates. |
| Behavioral withdrawal | Focal subject remains motionless, and does not engage in social interaction, activity, or environmental exploration. |
| Stereotypy | Focal subject engages in repetitive, nonadaptive, rhythmic movement (e.g., pacing, flipping, saluting). |
| Threat vocalizations | Focal subject emits aggressive barks. |
Note. Behaviors were scored as duration (seconds), with the exception of threat vocalizations, aggression, and approach intruder, which were scored as frequencies.
Data Analysis
All analyses were conducted in SPSS, version 26 (IBM, 2019), with alpha set to p<.05 and all tests were two-tailed. Results are reported as mean (M) ± the standard error of the mean. When significant interactions were found, post-hoc tests were conducted. Preliminary independent t-tests showed no significant sex differences in variables of interest (p<.05; see Tables S1–S2, available online). As is the case with many stress-inducing paradigms, the behavioral and physiological data were not normally distributed. While numerous studies indicate that ANOVA is robust to violations of normality (for example see 42). Nevertheless, to address this, the data were log-transformed and identical analyses were performed, producing results that were similarly significant and in the same direction as the results using the raw data. To increase the interpretability of this work, we report the results of analyses using the raw data below.
Infant Behavior.
A mixed design, repeated measures ANOVA was utilized to statistically compare the relationships between rearing condition, week of separation, and behavioral withdrawal, with rearing condition (adopted or reared by biological mother) as the independent variable and behavioral withdrawal (seconds) as the dependent variable and week of separation (1–4) as the repeated measure.
Infant Physiology.
Two mixed design, repeated measures ANOVAs were utilized to statistically compare the relationship between rearing condition, week of separation (1–4), and hour the sample was taken (hour 1 or hour 2) as the two repeated measures and ACTH or cortisol concentrations as the dependent variables.
Young Adult Behavior.
A MANCOVA was used to assess the relationship between rearing condition and the behaviors exhibited by the now-adult subjects during the Intruder Challenge test, with rearing condition (adopted or reared by biological mother) as the independent variable and total behavioral withdrawal (seconds), stereotypies (seconds), locomotion (seconds), socializing with familiar conspecifics (seconds), aggression (frequency), aggressive vocalizations (frequency), and latency to approach the intruder (seconds) as the dependent variables. As preliminary analyses indicated that age of subject during testing was significantly related to the duration of certain behaviors (p<.05), age of subject was included as a covariate in the model.
Results
Infants’ Separation Behavior
Results showed a significant main effect of rearing on the duration of behavioral withdrawal (F(1,134)=6.00, p= .02), with adopted infants spending more time in behavioral withdrawal (M=86.49±5.24 seconds), when compared to infants reared by their biological mothers (M=71.88±2.85 seconds).
There was a significant main effect of week on the time in behavioral withdrawal (F(1,134)=7.20, p=.008), with infants spending significantly more time in behavioral withdrawal during week 1, when compared to the other weeks (week 1: M=86.85±3.80, week 2: M=76.85±3.64, week 3: M=75.36±3.30, week 4: M=77.69±3.43).
There was also a significant main effect of separation phase (F(1,134)=7.20, p<.0001), with subjects showing more time in behavioral withdrawal in the Acute phase (M=86.53±4.02 seconds), when compared to the Chronic phase (M=71.84±2.54 seconds).
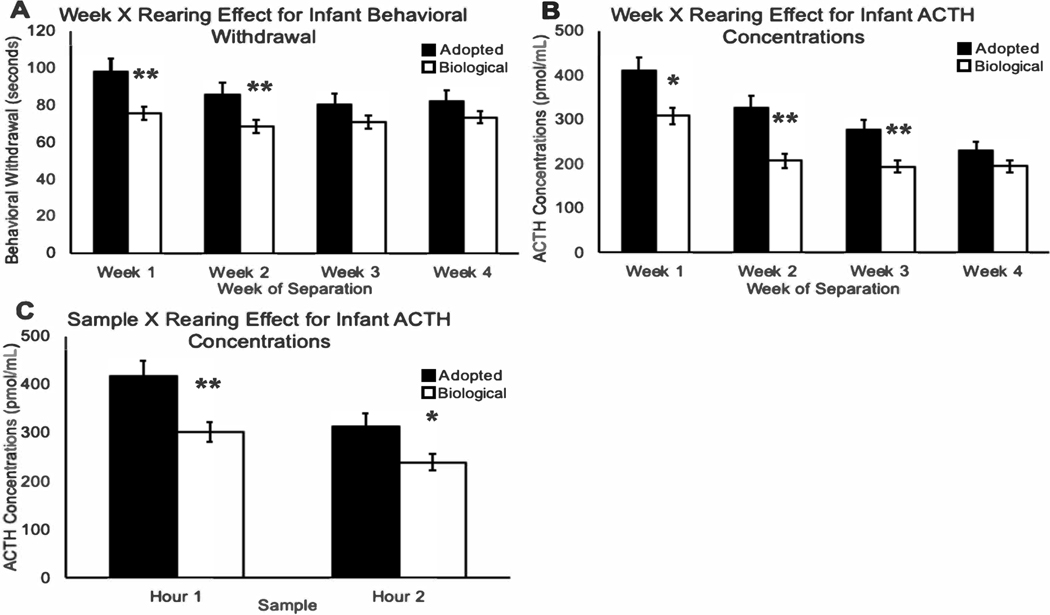
There was a significant two-way week-by-rearing-condition interaction (F(1,134)=5.16, p=.03), with adopted infants exhibiting more time in behavioral withdrawal during separations, when compared to the infants reared by their biological mothers (see Figure 1A). A posteriori testing indicated that adopted infants exhibited significantly more behavioral withdrawal in weeks 1 (p=.001) and 2 (p=.004), when compared to infants reared by their biological mothers. Infants from either rearing condition showed no differences in behavioral withdrawal in weeks 3 (p=.10) and 4 (p=.12). See Table 2. Further analysis of behavioral withdrawal showed a linear trend for the adopted infants (p=.01), but not the infants reared by their biological mothers (p=.41), with the adopted infants beginning at high level, and then showing a significant linear decline across weeks.
Figure 1. Differences in Adopted Infants and Those Reared by Their Biological Mothers.
Note: Depiction of the behavioral and neuroendocrine differences among adopted infants (portrayed by the black bars) and those reared by their biological mothers (portrayed by the white bars). Adopted infants exhibited more time in behavioral withdrawal during separations, when compared to infants reared by their biological mothers (F(1,134)=5.16, p=.03; Panel 1A). Adopted infants exhibited a progressive decline in plasma ACTH concentrations over the four separations, while infants reared by their biological mothers had higher plasma ACTH concentrations on week 1, compared to weeks 2–4 (F(1,132)=5.01, p=.03; Panel 1B). Adopted infants also exhibited higher ACTH concentrations in both hour-1 and hour-2, when compared to infants that were raised by their biological mother (F(1,132)=6.60, p=.01; Panel 1C). Error bars represent standard error of the mean.
Table 2.
Assessing Rearing Differences in Infant Behavioral Withdrawal and ACTH Concentrations During the Separation Paradigm
| Mean | SEM | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Adopted | Biological | Adopted | Biological | p | ||
|
| ||||||
| Behavioral withdrawal | ||||||
|
| ||||||
| Week 1 | 102.16 | 76.77 | 6.80 | 3.68 | .001 | |
| Week 2 | 83.86 | 65.01 | 5.73 | 3.10 | .004 | |
| Week 3 | 76.92 | 66.70 | 5.43 | 2.94 | .10 | |
| Week 4 | 79.97 | 69.64 | 5.72 | 3.10 | .12 | |
|
| ||||||
| ACTH (Week) | ||||||
|
| ||||||
| Week 1 | 493.93 | 388.15 | 41.35 | 21.25 | .02 | |
| Week 2 | 380.89 | 253.58 | 33.90 | 17.43 | .001 | |
| Week 3 | 310.29 | 223.22 | 27.62 | 14.20 | .006 | |
| Week 4 | 259.59 | 232.10 | 28.41 | 14.60 | .39 | |
|
| ||||||
| ACTH (Hour) | ||||||
|
| ||||||
| Hour 1 | 414.08 | 305.67 | 32.60 | 16.75 | .004 | |
| Hour 2 | 308.70 | 242.83 | 26.70 | 13.72 | .03 | |
Note. Boldface type indicates a significant difference (p < .05).
Infant Physiology
There was a significant main effect of rearing on ACTH concentrations (F(1,132)=7.18, p=.008), with the adopted infants exhibiting higher plasma ACTH in response to separation (M=361.18±28.85 pmol/mL), when compared to infants reared by their biological mothers (M=274.26±14.83 pmol/mL).
There was a significant main effect of week on ACTH concentrations (F(1,132)=107.17, p<.0001), with a significant linear decline in ACTH concentrations as the weeks progressed (week 1: M=441.04±23.25 pmol/mL, week 2: M=317.23±19.06 pmol/mL, week 3: M=266.75±15.53 pmol/mL, week 4: M=245.84±15.97 pmol/mL).
There was also a main effect of hour (F(1,132)=101.73, p<.001), with ACTH concentrations at hour-1 significantly higher (M=359.89±18.32 pmol/mL) than at hour-2 (M=275.55±15.01 pmol/mL).
There was a significant week-by-rearing-condition interaction (F(1,132)=5.01, p=.03), with adopted infants exhibiting a progressive linear decline in stress-induced plasma ACTH concentrations over the four separations (see Figure 1B). A posteriori testing indicated that adopted infants exhibited significantly more ACTH concentrations in week 1 (p= 02), week 2 (p=.001), and week 3 (p=.006), when compared to infants reared by their biological mothers. Infants from either rearing condition showed no differences in ACTH in week 4 (p=.39). See Table 2. Further analysis of ACTH concentrations showed a linear trend for the adopted infants (p<.001) and the infants reared by their biological mothers (p<.0001). Adopted infants began at a high level and showed a significant linear decline across weeks, while infants reared by their biological mothers showed higher levels of ACTH in week 1 compared to all other weeks.
There was also a significant hour-by-rearing-condition interaction (F(1,132)=6.60, p=.01; see Figure 1C). A posteriori testing indicated that adopted infants exhibited significantly higher ACTH concentrations in both hour-1 (p=.004) and hour-2 (p=.03). See Table 2.
There was a significant main effect of week on cortisol concentrations (F(1,159)=15.84, p < .0001), with a significant linear decline in cortisol concentrations as the weeks progressed. There was also a main effect of hour (F(1,159)=209.60, p<.0001), with cortisol concentrations at hour-1 significantly lower, when compared to hour-2. There was also a significant week-by-hour interaction (F(1,150)=26.30, p < .0001), with hour-2 cortisol concentrations exhibiting a significant linear decline across weeks. See Table S3, available online. There were no rearing differences for cortisol during any phases of the separation stressor. See Table S4, available online.
Young Adult Behavior
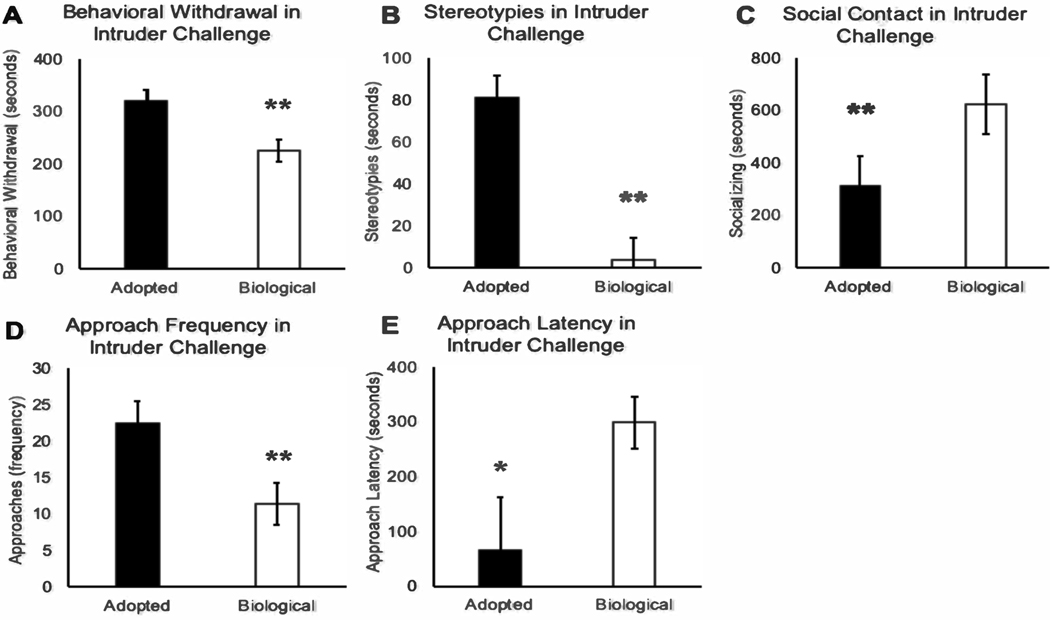
There was a significant multivariate effect for rearing condition (adopted or reared by biological mother) (F(9,50)=.4.77, p<.0001; Wilk’s Λ=0.538). Significant rearing condition differences were found for time spent in behavioral withdrawal (F(1,58)=9.80, p=.003), stereotypies (F(1,58)=10.20, p=.002), social contact with others (F(1,58)=8.12, p=.006), frequency of approaching the intruder (F(1,58)=11.81, p=.001), and latency to approach the intruder (F(1,58)=4.64, p=.04). Compared to subjects that were reared by their biological mothers, adopted subjects spent more time in behavioral withdrawal and stereotypies and less time in social contact with their homecage conspecifics (see Figure 2A–C). When compared to subjects reared by their biological mothers, adopted subjects also engaged in more frequent approaches of the intruder and exhibited a shorter latency to approach the intruder (see Figure 2D–E). There were no significant relationships between rearing condition and time spent in locomotion, environmental exploration, aggression, or number of aggressive vocalizations (p>.05; See Table 3).
Figure 2. Differences in Adopted Young Adults and Those Reared by their Biological Mothers.
Note: Depiction of the behavioral differences among adopted young adults (portrayed by the black bars) and those reared by their biological mothers (portrayed by the white bars). Compared to subjects that were reared by their biological mothers, adopted subjects spent more time in behavioral withdrawal (F(1,58)=9.80, p=.003; Panel 2A), stereotypies (F(1,58)=10.20, p=.002; Panel 2B), social contact with others (F(1,58)=8.12, p=.006; Panel 2C), and they approached the intruder more often (F(1,58)=11.81, p=.001; Panel 2D), exhibiting a shorter latency to approach the intruder (F(1,58)=4.64, p=.04; Panel 2E). Error bars represent standard error of the mean.
Table 3.
Rearing Differences in Behaviors During Intruder Challenge
| Mean | SEM | ||||
|---|---|---|---|---|---|
|
| |||||
| Adopted | Biological | Adopted | Biological | p | |
|
| |||||
| Aggression | 0.19 | 0.48 | 0.14 | 0.18 | .34 |
| Activity | 349.21 | 329.84 | 68.92 | 27.78 | .78 |
| Approach intruder (frequency) | 22.53 | 11.38 | 2.89 | 1.40 | .001 |
| Approach intruder (latency) | 65.76 | 299.03 | 96.53 | 46.89 | .04 |
| Behavioral withdrawal | 321.28 | 225.70 | 27.21 | 13.22 | .003 |
| Environmental exploration | 8.87 | 14.93 | 6.92 | 3.52 | .57 |
| Social contact with peers | 313.94 | 674.74 | 112.81 | 54.80 | .006 |
| Stereotypies | 80.99 | 3.82 | 21.53 | 10.46 | .002 |
| Threat vocalizations | 26.00 | 38.92 | 11.67 | 14.65 | .63 |
Note. Boldface type text indicates a significant difference (p < .05).
Discussion
In support of the hypotheses, randomly-assigned adopted infants exhibited more stress-induced behavioral withdrawal and higher stress-induced plasma ACTH concentrations during weeks 1–3 of a social separation paradigm, when compared to infants reared by their biological mothers. Four years later, these behavioral differences persisted, with adopted subjects continuing to exhibit more stress-induced behavioral withdrawal. Adoptees also showed evidence of impulse control deficits, approaching the potentially aggressive intruder more rapidly, as well as more frequently. They were less likely to engage in social interactions with their peers and exhibited more repetitive stereotypies during the intruder challenge, when compared to subjects reared by their biological mothers.
Mother-infant social separation tends to elicit high levels of stress in infant rhesus monkeys29,43, although there are individual differences in coping and resilience44. It is notable, that even after random assignment of both offspring and mothers, the adopted infants exhibited more behavioral withdrawal and higher stress-induced plasma ACTH than that observed in infants raised by their biological mothers, suggesting that adopted infants are less resilient to both mild and chronic stress, when compared to subjects reared by their biological mothers. During the separation stressor, though infants from both conditions remained in their homecage while their mothers were removed, none of the other members of the social group carried or consoled them. Consequently, it is unlikely that the stress-induced differences in behavior and plasma ACTH concentrations are a result of infants from one condition being cared for by others in their mothers’ absence. As studies in humans show that adoptees are at risk for psychopathology45, it is important to point out that in human infants and children high levels of stress-induced behavioral inhibition and HPA axis output predict psychopathology later in life, particularly anxiety disorders and socioemotional deficits30,46–48. While there were significant differences in stress-induced plasma ACTH concentrations between adopted infants and those reared by their biological mothers, there were no differences in stress-induced plasma cortisol concentrations. While formal tests assessing the underlying reason for this HPA axis response fractionization are beyond the scope of this study, it is worth noting that other nonhuman primate studies show similar response fractionization (high ACTH concentrations and no differences in cortisol) in infant rhesus monkeys following long-term chronic stress, such as social subordination49 and early-life adverse conditions50.
Consistent with adoption outcome studies in humans, early adoption was associated with long-term developmental deficits, as demonstrated by the high levels of stress-induced behavioral withdrawal that persisted into young adulthood. Moreover, as young adults, the adoptees approached the intruder more often and more frequently, an indication of impulsivity in the adoptees. The adoptees also showed high rates of repetitive stereotypies and low rates of social affiliation with their familiar peers, suggesting that early adoption also led to social deficits that persisted into adulthood, at least under conditions of stress. The results of the study are consistent with studies in humans, indicating that adopted individuals show behavioral, socioemotional and impulse control deficits, even when they are adopted at birth and randomly assigned to the adoption condition.
As is common in longitudinal work, the sample size of adopted subjects that were also assessed in young adulthood was smaller than required by the power analysis. Consequently, our ability to detect significant differences between adopted subjects and those reared by their biological mothers may have been reduced. Nevertheless, even with this small sample size, the adopted infants exhibited persistent stress-induced behavioral withdrawal from infancy into young adulthood, which may indicate the strength of the effect of at-birth adoption on developmental outcomes.
It is also worth noting that the results from this study assess extremes in behavioral outcomes under conditions of stress and, in humans, studies that investigate stress-induced differences between those that are adopted at birth and those reared by their biological parents are surprisingly lacking. Consequently, the extent to which the stress-induced differences seen in this study generalize to humans should be interpreted with caution. While a variety of studies assess the outcomes of institutionalized children, studies assessing the outcomes of children, adolescents, and young adults that were adopted at birth are sorely needed, particularly longitudinal studies. Nevertheless, to the extent that the findings generalize to humans, they suggest the behavioral deficits seen in human adoptees are not solely a result of high genetic loading in adoptees, but are instead a result of the early experience of adoption.
While the assignments to adoption were random, to assure that the differences in the behaviors measured were not the result of a random over-representation of half-siblings that had high or low dependent variable values, we performed a post hoc assessment of half-sibling values on the dependent variables. Each of the dependent variables were sorted from high to low and a mean split was used to compare the distribution of offspring from fathers and then mothers. No differences were found in the high or low distributions between offspring with the same fathers or mothers.
Given the randomly-assigned adoptions in this study, it is likely that some factor other than genetic loading for psychopathology, access to prenatal and perinatal care, or parental characteristics contribute to the development of psychopathological behavior and outcomes in adoptees. This study represents an important first step in establishing a nonhuman primate model to assess adoption outcomes. Hence, one limitation is that maternal treatment was not measured; nor were the temperaments of the infant or mother assessed. Subsequent studies will assess the early mother-infant relationship, including comparing the maternal behaviors of mothers rearing biological or adopted infants. It should also be noted that the model used in this study does not generalize to all types of adoption, but is limited to at-birth adoptions. Nevertheless, to the extent that these results generalize to humans, the current study suggests that the increased risk for behavioral pathology and other atypical developmental outcomes in adopted infants is not likely to be a product of genetic predispositions toward psychopathology in adoptees, as adoptions were randomly assigned. Because of the many similarities between rhesus monkeys and humans, and given the random assignment of infants to adoption conditions, this study provides a powerful methodology to investigate the impact of adoption on human development humans, which may contribute to the development of improved strategies for preventing or alleviating the risk for psychopathology among adoptees.
Supplementary Material
Clinical Vignette.
One of the foundational components of attachment theory is that a secure attachment is rooted in parents providing a secure base to reduce their infants’ arousal. This occurs when parents exhibit appropriate sensitivity to their infants’ needs, an area that has received much attention using nonhuman primate models. The senior author, who has three decades of experience coding mother and infant behavior in rhesus monkeys, could not tell the difference between the mother-infant dyads that involved an adoptee and those that did not involve an adoptee during general behavioral observations of the dyads. He notes that, when observing the adopted infants and the infants reared by their biological mothers, the differences were subtle, more likely to emerge during stress, and that they were unlikely to be a consequence of overt maltreatment or neglect.
Acknowledgments
This work was supported by the intramural programs of the National Institute on Alcohol Abuse and Alcoholism and the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by small mentoring grants from Brigham Young University.
The authors would like to thank the many post-docs, graduate and undergraduate students, as well as the animal care staff for their contributions to the data collection that made this project possible.
Footnotes
Disclosure: Drs. Wood, Schwandt, Barr, Suomi, Higley, Ms. Espinel, Mr. Hunter, Mss. Emmett, Skowbo, Shannon, and Mr. Lindell have reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth K. Wood, Brigham Young University, Provo, Utah..
Whitney F. Espinel, Brigham Young University, Provo, Utah..
Jacob Hunter, Brigham Young University, Provo, Utah..
Alexa Emmett, Brigham Young University, Provo, Utah..
Andrea N. Skowbo, Brigham Young University, Provo, Utah..
Melanie L. Schwandt, Laboratory of Clinical Studies, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland..
Courtney Shannon, Section of Comparative Behavioral Genomics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Rockville, Maryland..
Stephen G. Lindell, Laboratory of Clinical Studies, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland.; Section of Comparative Behavioral Genomics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Rockville, Maryland.
Christina S. Barr, Laboratory of Clinical Studies, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland.; Section of Comparative Behavioral Genomics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Rockville, Maryland.
Stephen J. Suomi, Section of Comparative Ethology, Eunice Shriver Kennedy National Institute of Child Health and Human Development, National Institutes of Health, Poolesville, Maryland..
J. Dee Higley, Brigham Young University, Provo, Utah..
References
- 1.Brodzinsky DM. Long-term outcomes of adoption. Future Child. 1993;3(1):153–166. 10.2307/1602410 [DOI] [Google Scholar]
- 2.Wierzbicki M Psychological adjustment of adoptees: A meta-analysis. J Clin Child Psychol. 1993;22(4):447–454. 10.1207/s15374424jccp2204_5 [DOI] [Google Scholar]
- 3.Brodzinsky DM, Radice C, Huffman L, Merkler K. Prevalence of clinically significant symptomatology in a nonclinical sample of adopted and nonadopted children. J Clin Child Psychol. 1987;16(4):350–356. 10.1207/s15374424jccp1604_9 [DOI] [Google Scholar]
- 4.Kim WJ, Davenport C, Joseph J, Zrull J, Woolford E. Psychiatric disorder and juvenile delinquency in adopted children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1988;27(1):111–115. 10.1097/00004583-198801000-00017 [DOI] [PubMed] [Google Scholar]
- 5.Tully EC, Iacono WG, McGue M. An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. American Journal of Psychiatry. 2008;165(9):1148–1154. 10.1176/appi.ajp.2008.07091438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leerkes EM, Gedaly LR, Zhou N, Calkins S, Henrich VC, Smolen A Further evidence of the limited role of candidate genes in relation to infant–mother attachment outcomes. Attachment & human development. 2017;19(1):76–105. 10.1080/14616734.2016.1253759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlomer GL, Del Giudice M, Ellis BJ. Parent–offspring conflict theory: An evolutionary framework for understanding conflict within human families. Psychological Review. 2011;118(3):496. DOI: 10.1037/a0024043 [DOI] [PubMed] [Google Scholar]
- 8.Ishizawa H, Kubo K. Factors affecting adoption decisions: Child and parental characteristics. journal of Family Issues. 2014;35(5):627–653. 10.1177/0192513X13514408 [DOI] [Google Scholar]
- 9.Malm K, Welti K. Exploring motivations to adopt. Adoption Quarterly. 2010;13(3–4):185–208. 10.1080/10926755.2010.524872 [DOI] [Google Scholar]
- 10.Hansen ME. Adoptive family structure. Center for Adoption Research;2006. doi: 10.1080/15548730802523216 [DOI] [Google Scholar]
- 11.Ge X, Conger RD, Cadoret RJ, et al. The developmental interface between nature and nurture: a mutual influence model of child antisocial behavior and parent behaviors. Developmental Psychology. 1996;32(4):574. Doi: 10.1037/0012-1649.32.4.574 [DOI] [Google Scholar]
- 12.Simmel C, Brooks D, Barth RP, Hinshaw SP. Externalizing symptomatology among adoptive youth: Prevalence and preadoption risk factors. Journal of abnormal child psychology. 2001;29(1):57–69. DOI: 10.1023/a:1005251513130 [DOI] [PubMed] [Google Scholar]
- 13.Sonuga-Barke EJ, Kreppner J. The development and care of institutionally reared children. Child Development Perspectives. 2012;6(2):174–80. 10.1111/j.1750-8606.2011.00231.x [DOI] [Google Scholar]
- 14.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–8. DOI: 10.1126/science.286.5442.1155 [DOI] [PubMed] [Google Scholar]
- 15.Santangeli O, Lehtikuja H, Palomäki E, Wigren HK, Paunio T, Porkka-Heiskanen T Sleep and behavior in cross-fostering rats: Developmental and sex aspects. Sleep. 2016;39(12):2211–21 doi: 10.5665/sleep.6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow HF, Harlow MK. The affectional systems. In: Schrier AM, Harlow HF, Stollinitz F, eds. Behavior of Nonhuman Primates. Vol 2. New York: Academic Press; 1965:287–334. [Google Scholar]
- 17.Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ (2002) Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry, 7, 1058–1063. DOI: 10.1038/sj.mp.4001157 [DOI] [PubMed] [Google Scholar]
- 18.Higley JD, Hasert MF, Suomi SJ, & Linnoila M (1991) A new nonhuman primate model of alcohol abuse: Effects of early experience, personality, and stress on alcohol consumption. Proceedings of the National Academy of Science USA, 88, 7261–7265. 10.1073/pnas.88.16.7261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, & Higley JD (2002) Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular Psychiatry, 7, 118–122. 10.1038/sj.mp.4000949 [DOI] [PubMed] [Google Scholar]
- 20.Maestripieri D (2005). Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proceedings of the National Academy of Sciences, 102(27), 9726–9729. 10.1073/pnas.0504122102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305(5689):1423–1426. DOI: 10.1126/science.1102541 [DOI] [PubMed] [Google Scholar]
- 22.Gibbs RA, Rogers J, Katze M, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316(5822):222–234. DOI: 10.1126/science.1139247 [DOI] [PubMed] [Google Scholar]
- 23.Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Hormones and behavior. 2006;50(4):623–631. DOI: 10.1016/j.yhbeh.2006.06.012 [DOI] [PubMed] [Google Scholar]
- 24.Capitanio JP. Early experience and social processes in rhesus macaques (Macaca mulatta): II. Complex social interaction. Journal of Comparative Psychology. 1985;99(2):133–144. 10.1037/0735-7036.99.2.133 [DOI] [PubMed] [Google Scholar]
- 25.Champoux M, Boyce WT, Suomi SJ. Biobehavioral comparisons between adopted and nonadopted rhesus monkey infants. Journal of Developmental and Behavioral Pediatrics. 1995;16:6–13. Doi: 10.1097/00004703-199502000-00002 [DOI] [PubMed] [Google Scholar]
- 26.Deets AC, Harlow HF. Adoption of single and multiple infants by rhesus monkey mothers. Primates. 1974;15(2–3):193–203. Doi: 10.1007/BF01742280 [DOI] [Google Scholar]
- 27.Ellsworth JA, Andersen C. Adoption by captive parturient rhesus macaques: Biological vs. adopted infants and the cost of being a “twin” and rearing “twins”. American Journal of Primatology. 1997;43(3):259–264. Doi: [DOI] [PubMed] [Google Scholar]
- 28.Biederman J, Rosenbaum JF, Bolduc-Murphy EA, et al. A 3-year follow-up of children with and without behavioral inhibition. J Am Acad Child Adolesc Psychiatry. 1993;32(4):814–821. DOI: 10.1097/00004583-199307000-00016 [DOI] [PubMed] [Google Scholar]
- 29.Kalin NH, Shelton SE, Rickman M, Davidson RJ. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav Neurosci. 1998;112(1):251–254. Doi: 10.1037/0735-7044.112.1.251 [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum JF, Biederman J, Bolduc-Murphy EA, et al. Behavioral inhibition in childhood: A risk factor for anxiety disorders. Harv Rev Psychiatry. 1993;1(1):2–16. DOI: 10.3109/10673229309017052 [DOI] [PubMed] [Google Scholar]
- 31.Fairbanks LA Individual differences in response to a stranger: Social impulsivity as a dimension of temperament in vervet monkeys (Cercopithecus aethiops sabaeus). Journal of Comparative Psychology. 2001;115:22–28. DOI: 10.1037/0735-7036.115.1.22 [DOI] [PubMed] [Google Scholar]
- 32.Lindburg DG. The rhesus monkey in North India: An ecological and behavioral study. In: Rosenblum LA, ed. Primate Behavior: Developments in Field and Laboratory Research. Vol 2. New York: Academic Press; 1971:1–106. [Google Scholar]
- 33.Newman TK, Syagailo YV, Barr CS, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57(2):167–172. DOI: 10.1016/j.biopsych.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 34.Robin RW, Chester B, Rasmussen JK, Jaranson JM, Goldman D. Prevalence and characteristics of trauma and posttraumatic stress disorder in a southwestern American Indian community. Am J -Psychiatry. 1997;154(11):1582–1588. DOI: 10.1176/ajp.154.11.1582 [DOI] [PubMed] [Google Scholar]
- 35.Bastian ML, Sponberg AC, Sponberg AC, Suomi SJ, Higley JD. Long term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta). Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology. 2003;42(1):44–51. DOI: 10.1002/dev.10091 [DOI] [PubMed] [Google Scholar]
- 36.Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology. 1991. Apr;103(4):551–6. DOI: 10.1007/BF02244258 [DOI] [PubMed] [Google Scholar]
- 37.Prescott MJ, Nixon ME, Farningham DAH, Naiken S, Griffiths MA. Laboratory macaques: When to wean? Appl Anim Behav Sci. 2012;137(3–4):194–207. 10.1016/j.applanim.2011.11.001 [DOI] [Google Scholar]
- 38.Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32(2):127–145.38. DOI: 10.1016/0006-3223(92)90016-s [DOI] [PubMed] [Google Scholar]
- 39.Gunnar MR, Gonzalez CA, Goodlin BL, Levine S. Behavioral and pituitary-adrenal responses during a prolonged separation period in infant rhesus macaques. Psychoneuroendocrinology. 1981;6(1):65–75. 10.1016/0306-4530(81)90049-4 [DOI] [PubMed] [Google Scholar]
- 40.Fairbanks LA, Newman TK, Bailey JN, Jorgensen MJ, Breidenthal SE, Ophoff RA, Comuzzie AG, Martin LJ, Rogers J. Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biological psychiatry. 2004;55(6):642–7. DOI: 10.1016/j.biopsych.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 41.Schwandt M, Lindell SG, Sjoberg RL, et al. Gene-environment interactions and response to social intrusion in male and female rhesus macaques. Biol Psychiatry. 2010;67(4):323–330. DOI: 10.1016/j.biopsych.2009.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanca MJ, Alarcón R, Arnau J, Bono R, & Bendayan R (2017). Non-normal data: Is ANOVA still a valid option?. Psicothema, 29(4), 552–557 DOI: 10.7334/psicothema2016.383 [DOI] [PubMed] [Google Scholar]
- 43.Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain Behav. 2008;7(4):463–469. doi: 10.1111/j.1601-183X.2007.00381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frigerio A, Ceppi E, Rusconi M, Giorda R, Raggi ME, Fearon P. The role played by the interaction between genetic factors and attachment in the stress response in infancy. J Child Psychol Psychiatry. 2009;50(12):1513–1522. DOI: 10.1111/j.1469-7610.2009.02126.x [DOI] [PubMed] [Google Scholar]
- 45.Clark DB, Cornelius J, Wood DS, Vanyukov M. Psychopathology risk transmission in children of parents with substance use disorders. American Journal of Psychiatry. 2004;161(4):685–691. doi: 10.1176/appi.ajp.161.4.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158(10):1673–1679. DOI: 10.1176/appi.ajp.158.10.1673 [DOI] [PubMed] [Google Scholar]
- 47.Kagan J, Snidman N. Early childhood predictors of adult anxiety disorders. Biol Psychiatry. 1999;46(11):1536–1541. DOI: 10.1016/s0006-3223(99)00137-7 [DOI] [PubMed] [Google Scholar]
- 48.Smider NA, Essex MJ, Kalin NH, et al. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73(1):75–92. DOI: 10.1111/1467-8624.00393 [DOI] [PubMed] [Google Scholar]
- 49.Saltzman W, Hogan BK, Abbott DH. Diminished cortisol levels in subordinate female marmosets are associated with altered central drive to the hypothalamic-pituitary-adrenal axis. Biological psychiatry. 2006;60(8):843–9. DOI: 10.1016/j.biopsych.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 50.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ Rearing condition and rh5-HTTLPR interact to influence limbichypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological psychiatry. 2004;55(7):733–8. DOI: 10.1016/j.biopsych.2003.12.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.