ABSTRACT
Testing for mycobacterial lipoarabinomannan (LAM) in urine is a practical but insensitive alternative to sputum testing to diagnose tuberculosis (TB) in people with HIV (PWH). Here, we evaluated urine LAM testing alongside PCR-based tests for Mycobacterium tuberculosis (MTB) DNA in tongue swabs. We hypothesized that the two nonsputum samples would deliver complementary, not redundant, results. The study included 131 South African patients of whom 64 (48.1%) were confirmed to have TB by GeneXpert MTB/RIF Ultra (Xpert Ultra) or culture analysis of sputum. A total of 120 patients (91.6%) were coinfected with HIV and 130 yielded a valid urine LAM result (Alere DETERMINE LAM Ag). Tongue swab samples were tested by IS6110-targeted qPCR with a quantification cycle (Cq) cutoff of 32. Relative to reference sputum testing (TB culture and Xpert Ultra), combined urine LAM and oral swab testing (either sample positive) was significantly more sensitive than either nonsputum sample alone (57% sensitivity for combined testing versus 35% and 39% sensitivity for urine LAM and tongue swabs; P = 0.01 and 0.04, respectively). Specificity of combined testing (neither sample positive) was 97%. On average, tongue swab-positive participants had higher sputum signal strength than urine-LAM positive participants, as measured by sputum Xpert Ultra Cq value (P = 0.037). A subset of tongue swabs (N = 18) was also tested by using Xpert Ultra, which reproduced true positive and true negative IS6110 qPCR results and resolved the two false-positive tongue swabs. With further development, tongue swabs and urine may feasibly serve as complementary nonsputum samples for diagnosis of TB in PWH.
KEYWORDS: tuberculosis, oral swabs, diagnostic, urine, nonsputum, lipoarabinomannan (LAM), OSA, tongue swabs, lipoarabinomannan
INTRODUCTION
Tuberculosis disease (TB), caused by Mycobacterium tuberculosis (MTB), remains a major cause of illness and death in people with HIV (PWH) (1). The standard sample for TB diagnosis is sputum, which can be difficult for PWH to produce and insensitive in some patients. The availability of noninvasive alternatives to sputum testing would substantially improve TB care for PWH (2, 3).
Most TB transmission occurs between development of disease (including asymptomatic disease) and initiation of treatment. Active case finding, in which high-risk populations are actively screened and TB is identified and treated earlier than by relying on symptomatic self-presentation, could reduce transmission in many populations (4, 5). Examples of such populations include contacts of known TB cases, occupants of institutions such as prisons and hospitals, people with HIV, and residents in high-burden areas. Sputum collection is a significant barrier to active screening. For example, only ~20% of participants in a Brazilian prison cohort were able to provide quality sputum specimens (6, 7).
Even in the context of passive TB diagnosis, many patients cannot routinely produce sputum for testing. PWH and children often require induction (nebulization), an invasive procedure. For all these reasons, Fauci and Eisinger (2018) have called for “twenty-first century diagnostic technologies that can detect MTB in a variety of clinical specimens from multiple body sites in addition to sputum” (3). Alternative, noninvasive sampling strategies could help identify the “missing millions” of TB cases that currently go undetected each year (8).
Immunoassays for mycobacterial lipoarabinomannan (LAM) in urine are potential alternatives to sputum testing. However, the single WHO-approved urine LAM test (by Alere/Abbott) lacks sensitivity. Although next-generation urine LAM tests are in development or evaluation, even the best-performing tests of the LAM analyte alone may not be optimally sensitive to detect TB disease in PWH (9–12).
In search of an alternative nonsputum sample type, we and others have shown that MTB DNA can be detected by oral swab analysis (OSA) (13–22). In OSA, the dorsum of the tongue is brushed with a sterile swab, and the collected material is tested for MTB DNA. This noninvasive, nonsputum sample collection approach can be applied to any patient, in any setting, including tertiary hospital, outpatient clinic, remote health point, or home and community settings.
Despite their promise, to date neither OSA nor urine LAM testing has proven to be sensitive enough to detect all TB in PWH. We hypothesize that sensitivity is limited for different reasons in the two methods. For example, pulmonary disease may be required to deposit enough MTB in the mouth for detection by OSA, whereas a very high total mycobacterial burden, including extrapulmonary disease, may be required for transrenal passage of MTB glycolipids into urine. Therefore, a parallel approach using both methods may detect more TB cases in PWH than either method alone. The current study was conducted to assess the complementarity of the two methods.
MATERIALS AND METHODS
Participant enrollment.
South African adults (age ≥16 years) with HIV (regardless of symptoms), or adults with TB symptoms or a positive sputum Xpert Ultra TB test, were consecutively enrolled into the prospective PROVE-TB cohort at Harry Gwala Regional Hospital (formerly Edendale Hospital) and affiliated clinics in Pietermaritzburg, South Africa between October 2019 and February 2021. Persons who had received TB treatment for more than 24 h were excluded.
The PROVE-TB cohort was developed and recruited as a platform for evaluating noninvasive TB diagnostic tests and algorithms in a high-TB-burden setting. Adults receiving usual care were recruited into the cohort at the time of hospital admission if they had HIV, irrespective of TB symptoms or reason for admission. To enrich the cohort for persons with TB disease, in order to conduct early-stage validation tests of novel diagnostic tests, adults in the hospital or affiliated clinics with a new positive sputum Xpert Ultra result were also recruited into the cohort, prior to initiating treatment.
All cohort participants received clinically indicated diagnostic examinations and testing as determined by their treating clinicians, and these tests were used for clinical decision-making. These included sputum Xpert Ultra, urine LAM, chest Xrays, and other blood tests and imaging, as well as other testing directed at the primary admitting diagnosis. For persons in whom clinicians were investigating TB, clinically indicated samples were collected and these results were used to create the TB reference definition. For cohort participants not being clinically investigated for TB, supplemental research sputum and urine samples were collected for Xpert Ultra, culture, and urine LAM testing. Urine LAM testing was repeated for research purposes and interpreted by a single reader, even if a LAM result had been obtained as part of clinical care. Xpert and culture testing were performed in the same laboratory and under the same conditions, regardless of indication (clinical investigation or research screening). Blood was collected for CD4+ T-cell measurement (in persons with HIV), biomarker testing (including C-reactive protein), and biorepository storage for later assays.
Clinical, laboratory, and demographic data were collected from participants and abstracted from clinical charts. Sputum, urine, tongue swab, and blood samples were collected at bedside and transported to the on-site laboratory for processing and analysis. Study data were collected and managed using REDCap electronic data capture tools hosted at the Institute of Translational Health Sciences. All participants provided written informed consent. This study was approved by the ethical committees of the University of KwaZulu-Natal (BREC #BE475/18) and the University of Washington (Study 9092).
Sample collection and analysis.
From each participant, one expectorated sputum sample was tested directly with GeneXpert MTB/RIF Ultra (Xpert Ultra, Cepheid, Sunnyvale, CA, USA) in the South African National Health Laboratory System (NHLS) laboratory at Harry Gwala Regional Hospital. A second expectorated sputum sample for M. tuberculosis complex culture was decontaminated with 1.25% N-acetyl-l-cysteine sodium hydroxide and processed according to the MGIT testing protocol. M. tuberculosis complex liquid culture was conducted in the Provincial NHLS laboratory using the Bactec MGIT960 system (Becton, Dickinson Microbiology System, Sparks, MD, USA).
A TB case was defined as a participant with either a positive sputum Xpert Ultra or a positive sputum TB culture result (reference testing). A non-TB case was defined as a participant with no positive sputum results. Negative sputum testing could comprise either negative sputum Xpert Ultra and TB culture results, or a negative result (either Xpert Ultra or culture) with the second sputum test result unavailable or invalid. Spontaneously voided urine samples were tested for the presence of LAM using the Alere LAM antigen lateral-flow assay (Alere Determine TB LAM Ag test, Abbott Laboratories, Chicago, IL, USA). The LAM test was interpreted according to the manufacturer’s instructions. A result of 1+ or higher according to the manufacturer’s visual read-out card was considered positive. Two tongue dorsum swabs (Copan FLOQSwabs; Copan Italia, Brescia, Italy) were collected into separate tubes as described previously (14, 15). Briefly, study staff ran the swab over the length and breadth of the anterior 2/3 of the subject’s tongue. Pressure was sufficient to slightly bend the swab shafts. Samples were collected for 15 to 20 s while rotating the swab throughout. Swab heads with collected material were snapped off into 2 mL screw cap tubes containing 0.5 mL sterile Tris-EDTA (TE) buffer.
Swabs in buffer were stored frozen at −80°C and transported to the Cangelosi Laboratory for blinded analysis. Persons performing the OSA amplification were blinded to the results of the sputum tests and urine LAM test. Thawed samples were manually processed (Qiagen), concentrated by ethanol precipitation, and tested by IS6110-targeted qPCR. Swab analysis methods were as described previously (14, 15) with the following modifications. Post-boil samples were eluted from the swab head then split, 250 μL reserve and 250 μL for immediate processing by volume-scaled-lysis (300 μL each of Qiagen Buffer AL and 100% ethanol). The qPCR protocol used New England BioLabs Inc Luna Universal Probe qPCR Master Mix, and the ethanol-precipitated samples were rehydrated in 5 μL TE buffer prior to master mix addition. Cq values were recorded, and results were calculated using two Cq thresholds (38 as described previously (14, 15), and a more stringent value of 32).
In addition to manual OSA, a subset of samples was tested by using Xpert Ultra. These samples were defined as “reserve” (leftover half-samples that had been refrozen after boiling) or “duplicate” (separate swab samples collected at the same time and immediately stored). Both reserved and duplicate samples (total, n = 18) were paired with manual OSA data. Samples were tested by Xpert Ultra by using either of two methods described elsewhere (22), depending on whether they were reserve or duplicate. Briefly, in one method (“boil method”), 200 μL half-samples, which were previously boiled and stored frozen, were thawed and received 2.2 mL TE buffer. The samples were shaken on a lab vortexer (GENIE SI-0236 Vortex-Genie 2 Mixer, 120V) on setting 10 for 10 to15 s, then allowed to sit at ambient temperature (20–22°C) for 5 min and then shaken for an additional 10 to 15 s, before allowing them sit for additional 10 min at ambient temperature. The entire recoverable sample volume was transferred into the sample reservoir of the Xpert Ultra cartridge for analysis. Samples were recorded as positive for MTB if GeneXpert software returned any positive result.
Duplicate samples, which were not previously boiled, were processed by the “single swab SR method” described in reference (22). These 0.5-mL samples were thawed at ambient temperature for 30 min, then supplemented with 0.3 mL TE and 1.6 mL of Cepheid Sample Reagent (SR). The samples were vortexed for 10 to 15 s, and incubated for 5 min before vortexing for an additional 10 to 15 s. The samples were further incubated for additional 10 min before the entire sample volume was transferred to the cartridges.
Analysis of results.
Sensitivities and specificities of index methods (swabs, urine LAM, and parallel testing) were calculated relative to the reference method (positive sputum Xpert Ultra or positive sputum culture). When comparing sensitivity and specificity values, significance was calculated by z score (two-tailed, 0.05 significance level).
RESULTS
We enrolled 131 participants (45% female, median age 36 years) who provided all sample types for index and reference testing. A total of 120 (92%) participants had HIV. Participant characteristics are summarized in Table 1. Of the 131 participants, 64 (49%) were TB cases by reference testing (Fig. 1).
TABLE 1.
Participant clinical and laboratory characteristicsa
| Characteristic | Total | TB case | Non-TB case |
|---|---|---|---|
| n = 131 | n = 64 | n = 67 | |
| Gender, n (%) | |||
| Female | 59 (45) | 28 (44) | 31 (46) |
| Male | 72 (55) | 36 (56) | 36 (54) |
| Age, median (IQR) | 36 (31–46) | 35 (28–49) | 37 (33–46) |
| HIV status, n (%) | |||
| Negative | 10 (8) | 10 (16) | 0 (0) |
| Positive | 121 (92) | 54 (84) | 67 (100) |
| CD4+ T-cell count, median (IQR)b | 115 (37–319) | 89 (40–227) | 257 (100–551) |
| Cough present, n (%) | 98 (75) | 56 (88) | 42 (63) |
| Any TB symptom present, n (%)c | 128 (97) | 64 (100) | 64 (96) |
| Urine LF-LAM positive, n (%)d | 22 (17) | 22 (34) | 0 (0) |
| OSA positive (Cq < 32), n (%) | 27 (21) | 25 (39) | 2 (3) |
| OSA positive (Cq < 38), n (%) | 57 (44) | 42 (65) | 15 (22) |
| Xpert Ultra Ct, median (IQR)e | 16.5 (15.9–20.8) |
TB case: reference sputum TB positive (Xpert Ultra and/or culture); Non-TB case: no positive sputum results.
HIV-positive participants only; data available for n = 105.
TB symptoms queried: cough, weight loss, fevers/chills, loss of appetite, fatigue.
LF-LAM, lateral flow lipoarabinomannan test.
Cycle threshold (Ct) values available for 60/62 positive Xpert Ultra results.
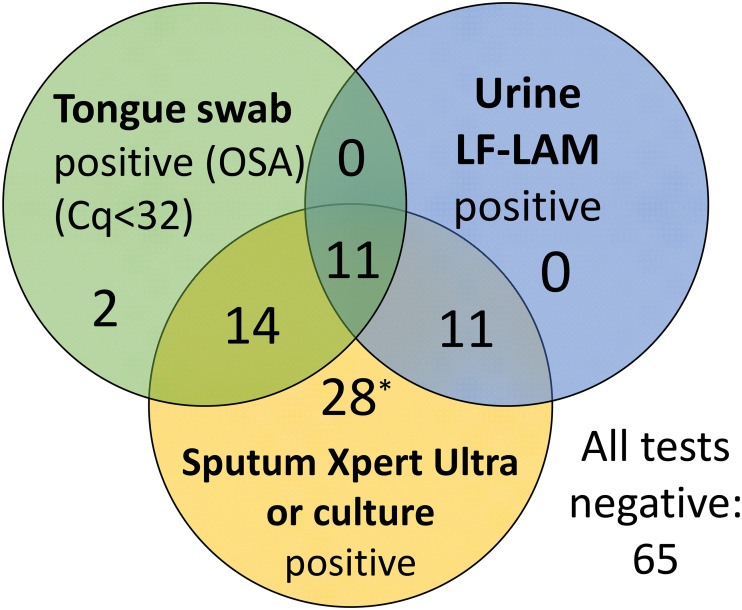
FIG 1.
Venn diagram showing overlap of the two nonsputum tests (tongue swab and urine LAM) and the sputum tuberculosis reference (Xpert Ultra or tuberculosis culture). The number of positive participants falling within each category is shown (n = 131 participants). LF-LAM, lateral flow lipoarabinomannan test; *, one positive sputum reference/tongue swab–participant had an invalid LAM result.
Relative to the sputum reference standard, OSA using manual qPCR conducted on a single swab, with a Cq cutoff of 38, was significantly more sensitive than urine LAM (42/64 [67%] versus 22/63 [35%], respectively; P = 0.005). However, OSA was less specific than urine LAM (52/67 [78%] versus 67/67 [100%], respectively; P < 0.001). When a more stringent Cq cutoff of 32 was applied to define a positive OSA result, OSA and urine LAM performed similarly (respectively, 25/64 [39%] versus 22/63 [35%] sensitive, and 65/67 [97%] versus 67/67 [100%] specific) (Table 2).
TABLE 2.
Sensitivity and specificity of nonsputum tests alone or in combination, compared to TB reference standard (sputum Xpert Ultra or sputum culture)
| Test | Sensitivity | Specificity |
|---|---|---|
| Tongue Swab (OSA) Cq < 32b | 25/64 (39%) | 65/67 (97%) |
| Tongue Swab (OSA) Cq < 38b | 42/64 (67%) | 52/67 (78%) |
| Urine LF-LAMa | 22/63 (35%) | 67/67 (100%) |
| LAM or OSACq<32b | 36/63 (57%) | 65/67 (97%) |
| LAM or OSACq<38b | 45/63 (71%) | 52/67 (78%) |
LF-LAM, lateral flow lipoarabinomannan test.
OSA: Tongue swab tested by manual qPCR using two thresholds of positivity. Cq < 38 (liberal); Cq < 32 (stringent).
When evaluating parallel (combination) nonsputum testing, a positive index test was defined as being either urine LAM-positive or OSA-positive. With parallel testing, sensitivity improved to 36/63 (57%), significantly better than urine LAM alone (P = 0.01) or OSA alone (P = 0.04). Of the 36 participants who were true positive by nonsputum methods, 11 were positive by both methods while 25 were positive by only one or the other (14 by OSA and 11 by urine LAM; Fig. 1). Specificity of the combined method remained high at 65/67 (97%).
In a secondary analysis, we tested the hypothesis that OSA (an airway sample) would correlate more closely with sputum signal strength than urine-LAM (a transrenal sample). Sputum signal strength was based on sputum Xpert Ultra quantitative readouts from the IS6110/IS1081 combined probe (Cq values, which are inversely proportionate to signal strength). In participants who were OSA-positive regardless of urine LAM results, sputum Cq values were slightly lower (stronger signal) than in participants who were urine-LAM positive regardless of OSA results (Table 3; P = 0.037 by unpaired, one-tailed t test). The difference became more significant (P = 0.019 by unpaired, one-tailed t test) when comparing participants who were positive only by OSA to those who were positive only by urine LAM, despite smaller numbers within these mutually exclusive categories (Table 3).
TABLE 3.
Sputum Xpert Ultra signal strength in tongue swab (OSA)-positive versus urine LAM-positive patients
| Value | Urine LAM-positive (regardless of OSA result) | OSA-positive (regardless of urine LAM result) | Urine LAM-positive, OSA-negative | OSA-positive, urine LAM-negative |
|---|---|---|---|---|
| n | 21 | 23 | 11 | 13 |
| Mean sputum Cqa | 17.66 | 16.43 | 18.73 | 16.39 |
| SD | 2.69 | 1.55 | 3.15 | 1.71 |
Value reported by Xpert Ultra, IS6110/IS1081 combined probe.
The manual qPCR method used in Table 1 performed well in past OSA studies (14, 15, 21) but is impractical for routine diagnostic use. Therefore, in an additional secondary analysis we used the semiautomated Cepheid GeneXpert MTB/RIF Ultra platform to test a subset of 18 frozen swab samples from the current study (11 true positives with the strongest qPCR signals by OSA, 5 true negatives by OSA, and the 2 false positives by OSA). The 11 true positive samples selected for this analysis had manual OSA Cq values ranging from 19.9 to 32.0 (average = 27.1 ± 4.0). Xpert Ultra detected 10 of 11 true positives by manual qPCR OSA (the 11th sample had an invalid Xpert Ultra result) and correctly excluded all 5 true negatives. It also correctly excluded the 2 false positives by manual qPCR OSA (Table 4).
TABLE 4.
Diagnostic performance of Xpert Ultra and manual qPCR on 18 tongue swab samples, compared to TB reference sputum testing (ref)a
| Tongue swab subset (n = 18) | TB ref positive | TB ref negative |
|---|---|---|
| OSA Xpert Ultra positive OSA Xpert Ultra negative |
10b 0 |
0 7 |
| OSA qPCRCq<32 positive OSA qPCRCq<32 negative |
11 0 |
2 5 |
Ref: Reference sputum Xpert Ultra or culture positive.
One patient with a positive TB reference sputum sample had an invalid result by tongue swab Xpert Ultra.
DISCUSSION
Many PWH struggle to produce sputum (sputum-scarce) and/or have low MTB cell counts in sputum (paucibacillary) (2, 3). This study tested the hypothesis that parallel testing of two different nonsputum samples can deliver complementary, not redundant diagnostic information, and thereby serve as an alternative to sputum testing for at least some patients.
Recent years have seen advancements in urine LAM testing for sputum-scarce or paucibacillary patients. LAM is an MTB cell wall glycolipid that can pass transrenally into urine. In some patients, most notably PWH with low CD4 counts, LAM is found in the urine in sufficient quantities to enable rapid detection by using lateral flow tests such as the Alere Determine TB LAM Ag. However, the sensitivity of urine LAM tests remains suboptimal (9–12).
We hypothesized that OSA can improve TB diagnosis in paucibacillary and sputum-scarce patients when used in combination with urine LAM testing. The physiological bases for urine LAM testing (transrenal passage of a mycobacterial glycolipid) and OSA (deposition of MTB cells and/or DNA on the tongue dorsum during cough, exhalation, or spontaneous sputum production) are likely to differ from each other. Urine LAM may work best in cases that involve some hematogenous spread of MTB beyond the airways, while OSA may be best in pulmonary TB. The strengths and limitations of the two methods may therefore complement each other.
The results were consistent with this hypothesis. When the more stringent Cq cutoff of 32 was applied, a diagnostic criterion of positivity in either urine LAM or OSA was significantly more sensitive than either method alone. Specificity was acceptable at 97% and was the minimum of the individual test specificities, but not lower in combination.
When using quantitative sputum Xpert Ultra readouts as measures of sputum signal strength, patients who were exclusively OSA-positive were found to have significantly higher loads than patients who were exclusively urine-LAM positive. This is consistent with the view that OSA is a bronchial tree (airway) sample, in contrast to urine LAM which is a bloodstream (circulatory) sample. Thus, the two samples may complement each other because they focus on separate body compartments.
This study focused on the biological question of complementarity. Limitations associated with implementation were only partially addressed. While swab sample collection has minimal resource requirements, the manual qPCR method for OSA requires laboratory facilities, specialized equipment and skilled technical operators, so is not practical for routine clinical laboratory use in its current form. However, analysis of a subset of 18 samples with the near point-of-care Xpert Ultra platform suggested that similar results can be obtained with automated tests (with the caveat that the true-positive samples chosen for this subset were strongly positive by manual qPCR). Therefore, this strategy can in theory be applied in any setting with access to both Alere Determine TB LAM AG and Xpert Ultra.
The Xpert Ultra portion of this study had two limitations: it focused on patients with strong manual OSA signals, and the number of participants was small. Recently, a separate study of OSA using the Xpert Ultra test (22) was conducted in Uganda within a larger cohort of participants with possible TB (n = 183, including 58 PWH). In that study, overall sensitivity of OSA using Xpert Ultra, relative to a sputum microbiology reference standard, was 77.8% (95% CI, 64.4–88.0). Specificity was 100.0% (95% CI, 97.2–100.0). Among the 58 PWH in the Uganda study, sensitivity was 55.6% (95% CI, 21.2–86.3) with no loss of specificity. Those results may better reflect what users of OSA can expect when using Xpert Ultra as a molecular assay, at least in some settings. Other automated amplification platforms intended for near-point-of-care or point-of-care use are also in development and have the potential to be configured for TB OSA.
Another implementation concern is the requirement for two laboratory tests per patient. In context, the same limitation often applies to current sputum-dependent strategies, because paucibacillary and sputum-scarce patients often require multiple sampling and testing attempts to confirm TB. To conserve resources, the two specimens could be collected simultaneously and then tested in a staged reflex algorithm. Swabs, which are easy and inexpensive to collect from any patient, can be collected and stored concurrently (dry or in TE buffer) with the collection of all urine samples. If a patient’s urine is positive for LAM, then that is diagnostic of TB and there is no need to test the stored swab. If the urine LAM test is negative, then the swab can be tested to avoid having to recall the patient for sputum induction.
In conclusion, our results indicate that tongue swabs and urine can serve as complementary nonsputum samples for improved diagnosis of TB in PWH. With further optimization to improve sensitivity, this approach may be considered as a fully nonsputum strategy for detecting TB in patients who are unable to produce adequate sputum for testing, or in settings where sputum collection isn’t practical. Given the diverse spectrum of TB in adults and children, it is unlikely that any single sampling method is ideal for all case types. Thus, it is potentially useful when two nonsputum methods can complement each other as seen here.
ACKNOWLEDGMENTS
This work was supported by the Bill and Melinda Gates Foundation (INV-004527, OPP 1213054), by NIH grants R01AI139254 and U54EB027049. REDCap at ITHS is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR002319. A.E.S. is supported by NIH (NIAID) K23A140918.
We are grateful to Cepheid, Inc., for providing research-use-only Xpert Ultra test kits, and to Copan Italia for providing swabs.
This work was presented in part at virtual TB Science 2021, (Abstract no. TBS-LB-2021-02134).
Contributor Information
Gerard A. Cangelosi, Email: gcang@uw.edu.
Christine Y. Turenne, University of Manitoba
REFERENCES
- 1.World Health Organization. 2020. Global Tuberculosis Report 2020. https://www.who.int/publications/i/item/9789240013131.
- 2.UNITAID. 2017. Tuberculosis diagnostics technology and market landscape. 5th ed. World Health Organization. https://unitaid.org/assets/2017-Unitaid-TB-Diagnostics-Technology-Landscape.pdf. [Google Scholar]
- 3.Fauci AS, Eisinger RW. 2018. Reimagining the research approach to tuberculosis. Am J Trop Med Hyg 98:650–652. 10.4269/ajtmh.17-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro AE, Ross JM, Yao M, Schiller I, Kohli M, Dendukuri N, Steingart KR, Horne DJ. 2021. Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms. Cochrane Database Syst Rev 3:Cd013694. 10.1002/14651858.Cd013694.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks GB, Nguyen NV, Nguyen PTB, Nguyen T-A, Nguyen HB, Tran KH, Nguyen SV, Luu KB, Tran DTT, Vo QTN, Le OTT, Nguyen YH, Do VQ, Mason PH, Nguyen V-AT, Ho J, Sintchenko V, Nguyen LN, Britton WJ, Fox GJ. 2019. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med 381:1347–1357. 10.1056/NEJMoa1902129. [DOI] [PubMed] [Google Scholar]
- 6.Bourdillon PM, Gonçalves CC, Pelissari DM, Arakaki-Sanchez D, Ko AI, Croda J, Andrews JR. 2017. Increase in tuberculosis cases among prisoners, Brazil, 2009–2014. Emerg Infect Dis 23:496–499. 10.3201/eid2303.161006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbone AdSS, Paião DSG, Sgarbi RVE, Lemos EF, Cazanti RF, Ota MM, Junior AL, Bampi JVB, Elias VPF, Simionatto S, Motta-Castro ARC, Pompilio MA, de Oliveira SMdV, Ko AI, Andrews JR, Croda J. 2015. Active and latent tuberculosis in Brazilian correctional facilities: a cross-sectional study. BMC Infect Dis 15:24. 10.1186/s12879-015-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pande T, Vasquez NA, Cazabon D, Creswell J, Brouwer M, Ramis O, Stevens RH, Ananthakrishnan R, Qayyum S, Alphonsus C, Oga-Omenka C, Nafade V, Sen P, Pai M. 2020. Finding the missing millions: lessons from 10 active case finding interventions in high tuberculosis burden countries. BMJ Glob Health 5:e003835. 10.1136/bmjgh-2020-003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broger T, Sossen B, Du Toit E, Kerkhoff AD, Schutz C, Ivanova Reipold E, Ward A, Barr DA, Mace A, Trollip A, Burton R, Ongarello S, Pinter A, Lowary TL, Boehme C, Nicol MP, Meintjes G, Denkinger CM. 2019. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study. Lancet Infect Dis 19:852–861. 10.1016/S1473-3099(19)30001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjerrum S, Schiller I, Dendukuri N, Kohli M, Nathavitharana RR, Zwerling AA, Denkinger CM, Steingart KR, Shah M. 2019. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev 10:CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathavitharana RR, Lederer P, Chaplin M, Bjerrum S, Steingart KR, Shah M. 2021. Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV. Cochrane Database Syst Rev 8:CD014641. 10.1002/14651858.CD014641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muyoyeta M, Kerkhoff AD, Chilukutu L, Moreau E, Schumacher SG, Ruhwald M. 2021. Diagnostic accuracy of a novel point-of-care urine lipoarabinomannan assay for the detection of tuberculosis among adult outpatients in Zambia: a prospective cross-sectional study. Eur Respir J 58:2003999. 10.1183/13993003.03999-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaCourse SM, Seko E, Wood R, Bundi W, Ouma GS, Agaya J, Richardson BA, John-Stewart G, Wandiga S, Cangelosi GA. 2022. Diagnostic performance of oral swabs for non-sputum based TB diagnosis in a TB/HIV endemic setting. PLoS One 17:e0262123. 10.1371/journal.pone.0262123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood RC, Andama A, Hermansky G, Burkot S, Asege L, Job M, Katumba D, Nakaye M, Mwebe SZ, Mulondo J, Bachman CM, Nichols KP, Le Ny A-LM, Ortega C, Olson RN, Weigel KM, Olson AM, Madan D, Bell D, Cattamanchi A, Worodria W, Semitala FC, Somoskovi A, Cangelosi GA, Minch KJ. 2021. Characterization of oral swab samples for diagnosis of pulmonary tuberculosis. PLoS One 16:e0251422. 10.1371/journal.pone.0251422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luabeya AK, Wood RC, Shenje J, Filander E, Ontong C, Mabwe S, Africa H, Nguyen FK, Olson A, Weigel KM, Jones-Engel L, Hatherill M, Cangelosi GA. 2019. Noninvasive Detection of Tuberculosis by Oral Swab Analysis. J Clin Microbiol 57:e01847-18. 10.1128/JCM.01847-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood RC, Luabeya AK, Weigel KM, Wilbur AK, Jones-Engel L, Hatherill M, Cangelosi GA. 2015. Detection of Mycobacterium tuberculosis DNA on the oral mucosa of tuberculosis patients. Sci Rep 5:8668. 10.1038/srep08668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicol MP, Wood RC, Workman L, Prins M, Whitman C, Ghebrekristos Y, Mbhele S, Olson A, Jones-Engel LE, Zar HJ, Cangelosi GA. 2019. Microbiological diagnosis of pulmonary tuberculosis in children by oral swab polymerase chain reaction. Sci Rep 9:10789. 10.1038/s41598-019-47302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valinetz ED, Cangelosi GA. 2021. A look inside: oral sampling for detection of non-oral infectious diseases. J Clin Microbiol 59. 10.1128/JCM.02360-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores JA, Calderon R, Mesman AW, Soto M, Coit J, Aliaga J, Mendoza M, Leon SR, Konda K, Mestanza FM, Mendoza CJ, Lecca L, Murray MB, Holmberg RC, Pollock NR, Franke MF. 2020. Detection of Mycobacterium tuberculosis DNA in buccal swab samples from children in Lima, Peru. Pediatr Infect Dis J 39:e376–e380. 10.1097/INF.0000000000002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesman AW, Calderon R, Soto M, Coit J, Aliaga J, Mendoza M, Franke MF. 2019. Mycobacterium tuberculosis detection from oral swabs with Xpert MTB/RIF ULTRA: a pilot study. BMC Res Notes 12:349–349. 10.1186/s13104-019-4385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Ma Y, Liu R, Shang Y, Ma L, Huo F, Li Y, Shu W, Wang Y, Gao M, Pang Y. 2021. Diagnostic yield of oral swab testing by TB-LAMP for diagnosis of pulmonary tuberculosis. Idr Volume 14:89–95. 10.2147/IDR.S284157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andama A, Whitman GR, Crowder R, Reza TF, Jaganath D, Mulondo J, Nalugwa TK, Semitala FC, Worodria W, Cook C, Wood RC, Weigel KM, Olson AM, Lohmiller Shaw J, Kato-Maeda M, Denkinger CM, Nahid P, Cangelosi GA, Cattamanchi A. 2022. Accuracy of tongue swab testing using Xpert MTB-RIF Ultra for tuberculosis diagnosis. J Clin Microbiol 60:e00421-22. 10.1128/jcm.00421-22. [DOI] [PMC free article] [PubMed] [Google Scholar]