Abstract
Objective
People living with long COVID describe a high symptom burden, and a more detailed assessment is needed to inform rehabilitation recommendations. The objectives were to use validated questionnaires to measure the severity of fatigue and compare this with normative data and thresholds for clinical relevance in other diseases; measure and describe the impact of postexertional malaise (PEM); and assess symptoms of dysfunctional breathing, self-reported physical activity, and health-related quality of life.
Methods
This was an observational study with a cross-sectional survey design (data collection from February 2021 to April 2021). Eligible participants were adults experiencing persistent symptoms due to COVID-19 that did not predate the confirmed or suspected infection. Questionnaires included the Functional Assessment of Chronic Illness Therapy–Fatigue Scale and the DePaul Symptom Questionnaire–Post-Exertional Malaise.
Results
After data cleaning, 213 participants were included in the analysis. The total Functional Assessment of Chronic Illness Therapy–Fatigue Scale score was 18 (SD = 10) (where the score can range from 0 to 52, and a lower score indicates more severe fatigue), and 71.4% were experiencing chronic fatigue. Postexertional symptom exacerbation affected most participants, and 58.7% met the PEM scoring thresholds used in people living with myalgic encephalomyelitis/chronic fatigue syndrome.
Conclusion
Long COVID is characterized by chronic fatigue that is clinically relevant and at least as severe as fatigue in several other clinical conditions. PEM is a significant challenge for this patient group. Because of the potential for setbacks and deteriorated function following overexertion, fatigue and postexertional symptom exacerbation must be monitored and reported in clinical practice and in studies involving interventions for people with long COVID.
Impact
Physical therapists working with people with long COVID should measure and validate the patient’s experience. Postexertional symptom exacerbation must be considered, and rehabilitation needs to be carefully designed based on individual presentation. Beneficial interventions might first ensure symptom stabilization via pacing, a self-management strategy for the activity that helps minimize postexertional malaise.
Keywords: COVID-19, Exercise Tolerance, Fatigue, Long COVID, Patient-Reported Outcomes, Rehabilitation
Introduction
Long COVID is a major public health concern. The scale of the COVID-19 pandemic means that even if a small proportion of people infected with SARS-CoV-2 have prolonged symptoms, this translates to millions of people worldwide.1 Estimates suggest that 13.7% of people will continue to have symptoms 12 weeks after infection.2 Even in individuals at low risk of COVID-19 mortality, chronic symptoms can be present and can co-occur with impairment in 1 or more organs.3 Long COVID is a complex, heterogeneous condition that is defined based on an elapsed acute infectious period.4 A clinical case definition has been developed by the World Health Organization5: post COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, and cognitive dysfunction, but there are also others that generally have an impact on everyday functioning. Symptoms may be new-onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms may also fluctuate or relapse over time.5 Persistent (chronic) fatigue is consistently reported to be the most prevalent symptom of long COVID.4,6,7 Chronic fatigue is a distressing, persistent feeling of weariness, tiredness, or exhaustion that is not alleviated by rest and is not proportional to recent activity levels. Chronic fatigue is a hallmark of multiple conditions, where it interferes with usual functioning and negatively impacts quality of life.8
Health care professionals living with long COVID describe an unpredictable and episodic trajectory with a relapsing–remitting nature.9–11 Reports of chronic fatigue alongside fluctuating symptoms that worsen unpredictably or in response to exertion have led to comparisons between long COVID and other postviral conditions, including myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).12–14 A hallmark symptom of ME/CFS is postexertional malaise (PEM),15,16 a worsening of symptoms, and reduction in function after physical, cognitive, or emotional activity that would not have caused a problem before illness.15–17 PEM is often used interchangeably with postexertional symptom exacerbation, though recent use of the latter may include postexertional exacerbation of symptoms in long COVID that may not otherwise be categorized as PEM (eg, exacerbation of breathlessness or tachycardia alone). To date, the identification of PEM in people living with long COVID has been driven by 1 remarkable patient-led effort that asked participants to identify PEM based on a definition.6 However, preliminary studies exploring progressive exercise have not reported PEM at enrollment or monitoring symptom exacerbation in response to exertion.18,19 This may be due to the lack of evidence of PEM in people living with long COVID.
Several studies have focused on persistent symptoms and rehabilitation in hospitalized patients.20,21 In contrast, few studies have included people who were not hospitalized (the majority of individuals affected by COVID-19) or those without laboratory confirmation of infection. There are several explanations for the lack of laboratory confirmation, including lack of access to testing, false negatives,22 inconclusive tests,23 and the numerous disincentives to seeking or accessing testing that can disproportionately affect disadvantaged populations.24 This issue has been consistently raised by patients who may be considered ineligible for long COVID health services and sickness benefits.25 Therefore, studies on symptom burden should include non-hospitalized patients and those who experienced an illness equivalent to the acute symptomatic presentation of COVID-19 and/or had a known exposure to the virus.
The aim of this study was to perform a more detailed assessment of fatigue and PEM in people with long COVID to inform the development of physiotherapy/rehabilitation recommendations. In a sample of adults who identified as living with long COVID, the specific objectives of this study were to use validated questionnaires to measure the severity of fatigue and compare this with normative data and thresholds for clinical relevance in other diseases; assess PEM using screening methods recommended for use in people living with ME/CFS; and describe symptoms of dysfunctional breathing, self-reported physical activity time, and health-related quality of life (HRQL) to compare with normative data.
Methods
Study Design and Setting
This was an observational study with a cross-sectional survey design. The survey was delivered in English and hosted on Qualtrics, a web-based survey tool. Data collection took place from February 11, 2021, to April 25, 2021. The study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB21–0159) and performed according to the Declaration of Helsinki, with the exception of preregistration. A STROBE checklist is available online (https://osf.io/dxu63/).
Participants, Recruitment, and Consent
Eligible participants were adults (≥18 years old) experiencing persistent (≥4 weeks) or long-term symptoms due to COVID-19 that did not predate the confirmed or suspected infection. Participants were eligible if they had a confirmed infection or a strong suspicion of infection based on an illness mimicking the acute phase of COVID-19, having close contact with a confirmed case, or being linked with an outbreak. A recruitment slide was shared with community leaders, patient advocates, and patient support groups via social media and clinical networks of rehabilitation professionals, and voluntary response sampling was used. Participants implied their informed consent before beginning the survey.
Online Survey
Within Qualtrics, identifiable information (email addresses and IP addresses) were excluded to ensure confidentiality, and multiple submissions were prevented via a browser cookie. Participant characteristics, including sociodemographic and medical data, were collected, and participants completed 5 questionnaires that were selected due to their psychometric properties, recommended use, and low participant burden to complete. Detailed information about questionnaires and their scoring thresholds is presented in the Supplementary Material. The Functional Assessment of Chronic Illness Therapy–Fatigue Scale (FACIT-F)26 is a 13-item self-report questionnaire developed for use in people with cancer, is widely recommended for the measurement of fatigue severity and impact,27 and has clinical utility in several other clinical conditions.28–32 In oncology, the FACIT-F has a cutoff point (<34, where the range is 0–52, and a lower score indicates more severe fatigue) designed to operationalize diagnostic fatigue criteria33 and was used in the present study for a comparison of clinical relevance and severity. PEM was measured using the DePaul Symptom Questionnaire–Post-Exertional Malaise (DSQ-PEM).34,35 Scoring step 1 considers a score above a threshold for 1 or more of the first 5 DSQ-PEM items. A threshold score of 2 to 4 for frequency (half the time, most of the time, or all of the time) coupled with a score of 2 to 4 for severity (moderate, severe, or very severe) for the same item is indicative of PEM. This method has been recommended by the National Institute of Neurological Disorders and Stroke (part of the National Institutes of Health) Common Data Elements PEM Working Group.36 Scoring step 2 was designed to operationalize the Common Data Elements recommendations further37 and includes supplementary questions that cover quick recovery, exercise exacerbation, and PEM duration. Item 7 or 8 must have an answer of “yes,” and a response of ≥14 hours is required for item 9. The 25-item Self-Evaluation of Breathing Questionnaire (SEBQ) was used to measure breathing discomfort related to perceptions of air hunger and the work or effort of breathing.38,39 No cutoff point has been validated, but in line with a recent study, we used a strict threshold of >25 as an indicator of significant breathing discomfort.40 HRQL was measured using a 36-item instrument for adults, the RAND 36-Item Short-Form Health Survey (SF-36).41 Self-reported physical activity was measured using the 7-item International Physical Activity Questionnaire–Short Form (IPAQ-SF).42
Data Cleaning
Records were excluded from the dataset if the participant reported a ME/CFS diagnosis or a previous history of postviral fatigue. Records were excluded from the dataset if the participant indicated that they were asymptomatic or experienced 1 symptom during the acute phase of a suspected infection, did not receive a positive test, and provided no free-text elaboration about the suspected infection. Two researchers (R.T., J.G.W.) independently inspected survey responses for evidence of poor data quality (careless responses), non-differentiation of ratings, particularly where items are reversed (eg, FACIT-F and SF-36), and consistency (equivalent responses for similar items both within and between questionnaires). All records that were completed in ≤10 minutes, those that did not include optional comments, and all partially complete records were inspected individually. Partially complete records were included in the analysis if at least 50% of the survey was complete (this was equivalent to the participant completing both the FACIT-F and the DSQ-PEM), at least 1 optional comment regarding the experience of long COVID was provided, and no poor data quality could be identified. In these cases, we assumed that the respondent engaged with the survey before exiting but did not return to complete the survey within a week. Because the survey was anonymized, we were unable to prompt participants to return to complete the survey. Finally, both investigators randomly selected 20 of the remaining records for individual inspection.
Data Analysis
Our primary analysis was descriptive and comparative, and data are presented as mean (SD), median (interquartile range), or frequency (percentage). In exploratory analyses, we checked for differences between groups dichotomized as participants who did and did not receive a laboratory diagnosis of COVID-19; participants who did and did not have 1 or more medical conditions that predated COVID-19 (recoded as yes, no); and participants who did and did not report PEM, based on scoring step 2. Differences in categorical variables between groups were tested using chi-square tests. Differences in questionnaire scores between groups were tested using Mann–Whitney U tests, and the P values were corrected for multiple comparisons using the Holm-Bonferroni correction (PHolm). The relationships between the FACIT-F score and SF-36 subscales and SEBQ score were examined using Spearman correlations, with an adjusted false discovery rate (Pfdr). The threshold to reject the null hypothesis of no difference between groups was set at P < .05. Analysis was performed using Jamovi (V1.8.2)43 and R (V4.0.5).44 Some participant information is presented here in aggregate but was removed from the open dataset to further ensure participant anonymity. An open (quantitative) dataset and analysis are available online (https://osf.io/dxu63/).
Participant Involvement
After data collection, a patient partner (K.F.) provided crucial insight based on lived experience, was involved in the interpretation findings, and provided a critical review of the manuscript.
Results
A total of 280 people implied their consent, and 211 participants (75.4%) completed 100% of the survey. Of the incomplete records, 8 were included. Of the complete records, only 8.2% did not include optional comments, and 6.7% were completed in <10 minutes. Following data cleaning, a total of 213 participants were included in the analysis. A detailed breakdown of exclusions during data cleaning is available online (https://osf.io/dxu63/). Excluding records where the time taken to complete the survey was more than 2 hours (n = 6, assumed to have left the survey open while taking a break) and partially complete records, participants completed the survey in 27.4 (SD = 17.4) minutes.
Participant characteristics are presented in Table 1. Participants primarily were women (85.5%); most were 40 to 49 years old (32.9%), 30 to 39 years old (23.5%), or 50 to 59 years old (22.1%); most were from the United Kingdom (39.5%), Canada (35.2%), or the United States (16.0%); and the sample mainly was White (93.0%). A large proportion of participants had no medical conditions that predated long COVID (46.5%; Tab. 1). The acute and chronic experiences of COVID-19 are shown in Table 2. During the acute phase of the illness, participants identified a median of 7 (6–9) symptoms. The majority of participants had been experiencing symptoms for more than 6 months (72.3%), and one-third of participants (n = 71) indicated that they were not receiving support from their medical/health care team for long COVID symptoms. A large subset of participants indicated that long COVID was preventing their return to work or that they were unable to work (42.3%). A second large subset indicated that long COVID had reduced their capacity to work or reduced the hours they were able to work (41.8%). Only 5.2% of the participants were able to work as usual (Tab. 2).
Table 1.
Participant Characteristics
| Characteristic | No. | % |
|---|---|---|
| Age category, y | ||
| 18–29 | 21 | 9.9 |
| 30–39 | 50 | 23.5 |
| 40–49 | 70 | 32.9 |
| 50–59 | 47 | 22.1 |
| 60–69 | 21 | 9.9 |
| 70–79 | 4 | 1.9 |
| Gender | ||
| Female | 182 | 85.4 |
| Male | 27 | 12.7 |
| Nonbinary | 3 | 1.4 |
| Prefer not to answer | 1 | 0.5 |
| Population group(s) | ||
| White | 198 | 93.0 |
| Black or African American | 3 | 1.4 |
| Hispanic, Latino, Latina, or Latinx | 3 | 1.4 |
| Other (unique responses) | 9 | 4.2 |
| Country of residence | ||
| United Kingdom | 84 | 39.5 |
| Canada | 75 | 35.2 |
| United States | 34 | 16.0 |
| Other Europe | 10 | 4.7 |
| Other (unique responses) | 4 | 1.9 |
| Not reported | 7 | 3.3 |
| Existing medical conditions | ||
| None | 99 | 46.5 |
| Depression or anxiety | 16 | 7.5 |
| Autoimmune disorder | 10 | 4.7 |
| Hypothyroidism | 8 | 3.7 |
| Fibromyalgia | 7 | 3.3 |
| Migraines | 7 | 3.3 |
| Anemia | 6 | 2.8 |
| Asthma (moderate to severe) | 6 | 2.8 |
| Hypertension | 6 | 2.8 |
| Inflammatory bowel disease | 4 | 1.9 |
| Endometriosis | 3 | 1.4 |
Table 2.
Acute and Chronic Experience After Confirmed or Suspected Infection With COVID-19
| Experience | No. | % |
|---|---|---|
| Acute symptoms | ||
| Shortness of breath | 202 | 94.8 |
| Aches and pains | 171 | 80.3 |
| Headache | 170 | 79.8 |
| Tiredness/fatigue | 164 | 77.0 |
| Dry cough | 135 | 63.4 |
| Rash on skin or discoloration of fingers and toes | 129 | 60.6 |
| Sore throat | 128 | 60.1 |
| Fever | 118 | 55.4 |
| Diarrhea | 82 | 38.5 |
| Loss of taste and smell | 37 | 17.4 |
| Chest tightness or pain | 31 | 14.6 |
| Conjunctivitis | 27 | 12.7 |
| Brain fog/confusion/cognitive impairment | 27 | 12.7 |
| Dizziness/light-headedness | 23 | 10.8 |
| Rapid heart rate/tachycardia | 17 | 8.0 |
| Heart palpitations | 16 | 7.5 |
| Loss of appetite/weight loss | 13 | 6.1 |
| Nausea/vomiting | 12 | 5.6 |
| Sinus congestion/pressure | 11 | 5.2 |
| Months experiencing long COVID symptoms | ||
| 1–2 | 20 | 9.4 |
| 3–5 | 39 | 18.3 |
| 6–9 | 30 | 14.1 |
| 10+ | 124 | 58.2 |
| Is long COVID preventing or limiting your ability to work? | ||
| Yes: preventing my return to work/unable to work | 90 | 42.3 |
| Yes: able to work at reduced capacity/reduced hours | 89 | 41.8 |
| No or not applicable: retired, unemployed, or stay-at-home parent | 22 | 10.3 |
| No: able to work as usual | 11 | 5.2 |
| Not reported | 1 | 0.5 |
| Positive test | ||
| Yes | 86 | 40.4 |
| No | 127 | 59.6 |
Fatigue (FACIT-F)
The total FACIT-F score was 18 (SD = 10) (the scores can range from 0 to 52 and a lower score indicates more severe fatigue). As presented in Table 3, scores were low even compared with several other clinical conditions where the FACIT-F was used to measure fatigue.28–32,45–48 More than 90% of the sample were below the cutoff point for clinically relevant fatigue used in people with cancer (Tab. 4), and 71.4% were experiencing chronic fatigue on the basis of this cutoff point and symptoms persisting for ≥3 months.
Table 3.
FACIT-F Scores in Populations With Various Conditions for Comparison With Long COVIDa
| Disease or Clinical Condition | FACIT-F Score b , c | No. of Participants | Age, y b , c | % Womenb | Study |
|---|---|---|---|---|---|
| Long COVIDd | 18 (10) | 213 | 85 | ||
| General population | 44 (9) | 1010 | 46 (17) | 52 | 45 |
| Cancer and anemia | 24 (13) | 2292 | 63 (13) | 65 | 45 |
| Chronic cancer-related fatigue | 27 (7) | 51 | 54 (11) | 65 | 46 |
| Human immunodeficiency virus | 34 (13) | 51 | 40 (7) | 12 | 28 |
| Rheumatoid arthritis | 29 (11) | 631 | 56e | 79 | 30 |
| Psoriatic arthritis | 36 (12) | 135 | 52 (13) | 42 | 29 |
| Iron deficiency anemia | 24 (12) | 608 | 45 (14) | 89 | 31 |
| Chronic obstructive pulmonary disease | 42 (9) | 564 | 68 (10) | 68 | 47 |
| Parkinson disease | 34 (10) | 118 | 64 (10) | 46 | 32 |
| Chronic immune thrombocytopenia | 36 (12) | 207 | 50e | 67 | 48 |
| Stroke | 38 (10) | 51 | 63 (14) | 51 | 28 |
a COVID = coronavirus disease; FACIT-F = Functional Assessment of Chronic Illness Therapy–Fatigue Scale.
b Values have been rounded.
c Values are reported as the mean (SD) unless otherwise indicated.
d See Table 1 for ages of people with long COVID.
e Value is reported as the median.
Table 4.
Patient-Reported Outcome Measuresa
| Outcome Variable | Result |
|---|---|
| Fatigue (FACIT-F; n = 213) | |
| Total score, mean (SD) | 18 (10) |
| Total score, median (IQR) | 17 (11–24) |
| Score < 34, no. (%) | 194 (91.1) |
| PEM (DSQ-PEM; n = 213) | |
| Above threshold for frequency and severity (statements are abbreviated), no. (%) | |
| 1. Dead, heavy feeling after starting to exercise | 145 (68.1) |
| 2. Next-day soreness or fatigue after nonstrenuous, everyday activities | 163 (76.5) |
| 3. Mentally tired after the slightest effort | 153 (71.8) |
| 4. Minimum exercise makes you physically tired | 182 (85.4) |
| 5. Physically drained or sick after mild activity | 173 (81.2) |
| 6. [No] recovery within an hour or 2 after exhausting activity | 191 (8 9.7) |
| 7. Worsening of fatigue after minimal physical effort | 188 (88.3) |
| 8. Worsening of fatigue after minimal mental effort | 178 (83.6) |
| 9. Feel worse after activities, and this lasts ≥14 h | 148 (69.5) |
| 10. Do not exercise because it makes symptoms worse | 191 (91.8)b |
| DSQ-PEM scoring step 1, yes, no. (%) | 202 (94.8) |
| DSQ-PEM scoring step 2, yes, no. (%) | 125 (58.7) |
| Breathing (SEBQ; n = 210) | |
| Total score, mean (SD) | 30 (17) |
| Total score, median (IQR) | 29 (16–43) |
| Score ≥ 25, no. (%) | 116 (55.2) |
| HRQL (SF-36; n = 210), score as a %, mean (SD) | |
| Physical functioning | 40 (24) |
| Role limitations due to physical health problems | 3 (10) |
| Role limitations due to emotional problems | 38 (42) |
| Energy/fatigue | 18 (16) |
| Emotional well-being | 56 (21) |
| Social functioning | 31 (24) |
| Pain | 49 (26) |
| General health | 45 (21) |
| Health change | 6 (14) |
| Physical activity (IPAQ-SF; n = 205 unless otherwise indicated) | |
| No vigorous activity, no. (%) | 159 (77.6) |
| No moderate activity, no. (%) | 120 (58.5) |
| No walking, no. (%) | 40 (19.5) |
| Category = none/low activity, no. (%) | 122 (59.8)c |
| Category = moderate activity, no. (%) | 64 (31.4)c |
| Category = high activity, no. (%) | 18 (8.8)c |
| Sitting (min/d), median (IQR) | 525 (383–620)d |
a DSQ-PEM = DePaul Symptom Questionnaire–Post-Exertional Malaise; FACIT-F = Functional Assessment of Chronic Illness Therapy–Fatigue Scale; HRQL = health-related quality of life; IPAQ-SF = International Physical Activity Questionnaire–Short Form; IQR = interquartile range; PEM = postexertional malaise; SEBQ = Self-Evaluation of Breathing Questionnaire; SF-36 = 36-Item Short-Form Health Survey.
b n = 208.
c n = 204.
d n = 168.
Postexertional Malaise (DSQ-PEM)
Summary data for the DSQ-PEM are presented in Table 4. For items 1 to 5, the proportion of participants meeting the scoring threshold ranged from 68.1% (dead, heavy feeling after starting to exercise) to 85.4% (minimum exercise makes you physically tired). Overall, 94.8% of the participants met the threshold for at least 1 of the first 5 items (scoring step 1). In fact, nearly one-half (46.9%) of the participants met the threshold for all 5 items. Using scoring step 2, which incorporates the supplementary DSQ-PEM, 58.7% of participants in this sample met the scoring thresholds used for people with ME/CFS.
Breathing (SEBQ)
The total SEBQ score was 30 (SD = 17) (the scores can range from 0 to 75 and a higher score indicates more severe symptoms), and 55.2% of the sample had a score of >25,40 indicating significant breathing discomfort. For comparison, in a sample of 180 participants from the general population, the mean was reported to be 15.5 (SD = 11.5).38
Health-related Quality of Life (SF-36)
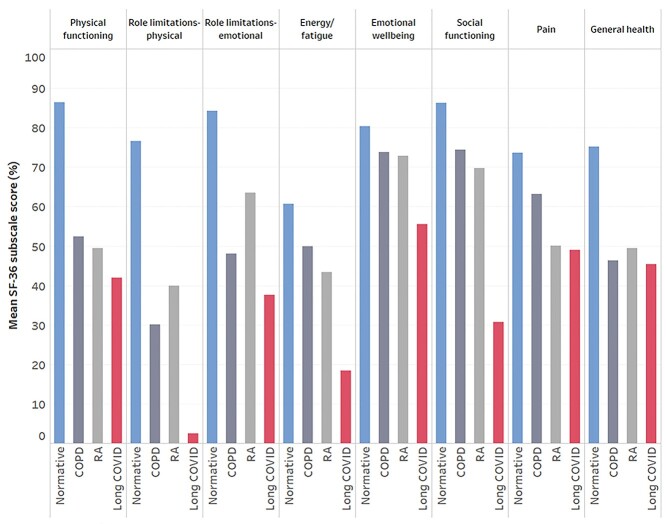
Summary data for SF-36 subscales can be found in Table 4. As visualized in Figure 1, HRQL was severely impaired in people living with long COVID compared with normative data from the general population49 and 2 chronic diseases.50,51 The health concepts most impacted by long COVID were role limitations due to physical health problems (3% [SD = 10%], where all scores range from 0% to 100%, and a higher percentage indicates better health) and energy/fatigue (18% [SD = 16%]). The health change item asks participants, “Compared with 1 year ago, how would you rate your health in general now?” Here, the score was also strikingly low (6% [SD = 14%]), with 79.5% answering “Much worse now than 1 year ago” and 18.6% answering “Somewhat worse now than 1 year ago.” Nine participants (4.3%) left free-text comments to express uncertainty about whether to answer questions 33 to 36 (which all contribute to the general health subscale) based on their current health or health prior to COVID-19. Removing these participants’ responses due to this uncertainty did not change the descriptive statistics for the general health subscale, so they remain in our analysis.
Figure 1.
Health-related quality of life (HRQL). Visualization of the impact of long coronavirus disease (COVID) on HRQL measured using eight 36-Item Short-Form Health Survey (SF-36) subscales. Mean scores from the present study are presented alongside data from the general population (normative),49 people with rheumatoid arthritis,50 and people with chronic obstructive pulmonary disease.51
Physical Activity (IPAQ-SF)
Most participants reported some walking for a duration of at least 10 minutes (80.4%), and this took place on 4 (2–7) days per week for 30 (20–60) minutes. We intended to use the IPAQ-SF as a self-reported estimate of moderate and vigorous physical activity levels. However, because some participants rated activities of daily living as moderate or vigorous physical activity, the data were difficult to interpret, and later exploratory analysis used only the walking variable as a measure of physical activity. Additional information for the IPAQ-SF is provided in Table 4 and in the Supplementary Material.
Exploratory Statistical Analysis
Laboratory Diagnosis
The only sample characteristic that differed between groups dichotomized based on laboratory diagnosis of COVID-19 was age (χ2 = 14.1; P = .015), where participants who were 40 to 69 years seemed less likely to have a laboratory confirmation compared with other age categories (Tab. 1). Participants with a laboratory confirmation did not have a higher occurrence of PEM (χ2 = 0.97; P = .325), a higher fatigue severity (U = 5138; PHolm = 1.000; r = 0.06), or more breathing discomfort (U = 4938; PHolm = 1.000; r = 0.06). The only SF-36 subscale that differed between groups was general health (U = 4044; PHolm = .033; r = 0.24), where people with laboratory confirmation rated their general health approximately 10% better than those without. This was not due to differences in the proportion of people receiving support from their medical/health care team for long COVID symptoms (χ2 = 0.24; P = .621).
Exploratory Relationships
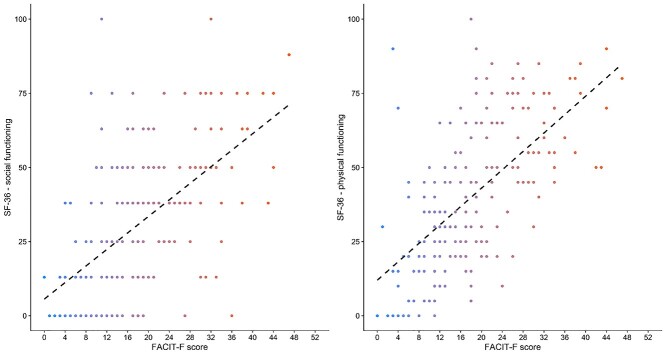
More severe fatigue was significantly associated with worse HRQL (all SF-36 subscales) and more breathing discomfort (ρ = −0.24; Pfdr = .002). The strongest SF-36 correlations were with physical functioning (ρ = 0.65; Pfdr = 0.002) (Fig. 2), social functioning (ρ = 0.56; Pfdr = .002) (Fig. 2), pain (ρ = 0.40; Pfdr = .002), and health change (ρ = 0.33; Pfdr = .002) subscales.
Figure 2.
Relationship between fatigue (Functional Assessment of Chronic Illness Therapy–Fatigue Scale [FACIT-F] score, with a lower score representing more severe fatigue), social functioning (left panel; ρ = 0.56; Pfdr [false discovery rate adjusted P value] = .002), and physical functioning (right panel; ρ = 0.40; Pfdr = .002). Dashed lines are a graphical representation of Spearman correlations.
Figure 3.
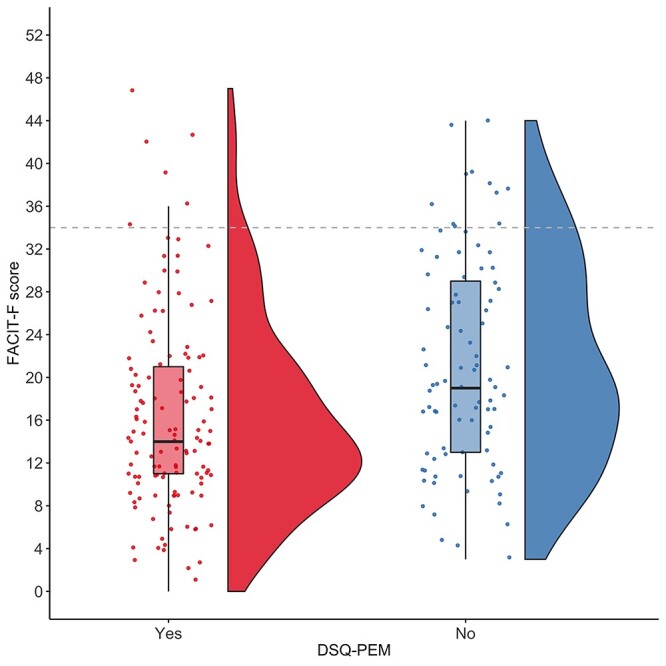
Fatigue and postexertional malaise (PEM). Rain cloud plot52 of fatigue (Functional Assessment of Chronic Illness Therapy–Fatigue Scale [FACIT-F] score, with a lower score representing more severe fatigue) in people with PEM, identified using step 2 scoring criteria for the DePaul Symptom Questionnaire–Post-Exertional Malaise (DSQ-PEM). Participants with PEM reported more severe fatigue (PHolm [Holm-Bonferroni adjusted P value] = .011). As a crude marker of severity, the dashed line shows the threshold score of clinical relevance in oncology, recommended for the diagnosis of cancer-related fatigue.
Medical/Health Conditions That Predated COVID-19
The only sample characteristic that differed between groups dichotomized based on health conditions that predated COVID-19 was support for long COVID symptoms (χ2 = 5.44; P = .020); more participants with a comorbidity reported receiving support from their health care team. Participants with a comorbidity did not report more severe fatigue (U = 4487; PHolm = .080; r = 0.20) but did report more breathing discomfort (U = 3997; PHolm = .011; r = 0.26). Presence of any comorbidity was reflected in approximately 10% lower physical functioning (U = 3624; PHolm = .011 r = 0.34) and general health (U = 3712; PHolm = .011; r = 0.32). Participants with a comorbidity had a higher occurrence of PEM compared with those with none (64.9 vs 51.5%; χ2 = 3.92; P = .048).
Postexertional Malaise
Groups dichotomized based on the presence of PEM differed based on work status/limitations (χ2 = 13.0; P = .023). A large proportion (71.1%) of participants who reported that long COVID was limiting their usual capacity to work were experiencing PEM. Similarly, a large proportion (68.9%) of participants who reported no/low physical activity were experiencing PEM. Participants with PEM reported more severe fatigue (U = 3888; PHolm = .011; r = 0.29) (Fig. 352), reduced physical functioning (U = 3809; PHolm = .011; r = 0.29), reduced social functioning (U = 3731; PHolm = .011; r = 0.30), and worse health compared with 1 year ago (U = 4479; PHolm = .028; r = 0.16). In total, 95.2% of people with PEM had scores of <34 on the FACIT-F compared with 85.2% of those without PEM (above the step 2 scoring threshold). Because comorbidities increase the prevalence of PEM by approximately 13%, we repeated all of the above analyses for participants reporting no comorbidities (n = 99) as a robustness check, and none of the above findings were altered.
Discussion
In adults who identified as living with long COVID, our main findings were that the overwhelming majority were living with chronic fatigue that was clinically relevant and appeared at least as severe as fatigue in several other clinical conditions; the impact on HRQL was substantial despite a relatively young sample where almost one-half have no comorbidities; and the overwhelming majority were living with some level of postexertional symptom exacerbation, and many meet the threshold criteria for PEM using a self-report tool validated in people with ME/CFS. In this sample, people experiencing worsening of symptoms with exertion reported a reduced capacity to work and reduced physical and social functioning. Many participants were also living with breathing discomfort (air hunger and increased sensations of breathing effort), were unable to be physically active, had role limitations due to physical health problems, and rated their health as much worse compared with 1 year ago. Overall, symptom burden was not higher in people who received laboratory confirmation of COVID-19 compared with those with only an acute illness that was reasonably attributable to infection (in line with other data53), and comorbidities alone do not explain long COVID symptoms.
Chronic fatigue is difficult for patients to articulate and easy for others to dismiss.54,55 Our data offer insight into the severity of the most commonly reported symptom of long COVD based on comparison with several other clinical conditions where the FACIT-F has been validated (Tab. 3) and comparison with a clinical cutoff point recommended for the diagnosis of fatigue in people with cancer.33 Fatigue is not only extremely common in long COVID, but for many, its severity and persistence are life-altering. Our exploratory analyses are in line with data from other populations on the associations between fatigue, reduced function, increased disability, and reduced HRQL.8,56 The measurement of fatigue should be considered, and validation of a fatigue scale (such as the FACIT-F, which has demonstrated clinical utility in several populations) in people with long COVID is a priority. It is not currently understood how to treat chronic fatigue in people living with long COVID. Evidence from other conditions may offer some insight while data is being collected in long COVID. For example, at least some people with chronic fatigue after cancer treatment can benefit from exercise.57–59 The mechanisms for the improvement in chronic cancer-related fatigue remain under investigation, but considering that the biological effects of exercise are multiple and interacting, reversal of deconditioning is unlikely to be the only pathway.60
In contrast, exercise therapy is not a route to recovery for everyone with chronic cancer-related fatigue,57,61 and in people with ME/CFS, exercise (and other types of exertion) can cause serious setbacks and deterioration in function.17 PEM is not caused by general deconditioning: PEM rarely occurs outside of the context of ME/CFS and is associated with impairments measured during a 2-day cardiopulmonary exercise test protocol that are not present in sedentary controls.62 We found that a large proportion of people living with long COVID are experiencing PEM, and this corroborates many testimonials from patients/health care professionals with long COVID describing “relapses” after return to work and exercise.14,63 The natural trajectory of PEM is unknown, but for more than 60% of those experiencing PEM in this sample, more than 40 weeks had passed since the confirmed/suspected SARS-CoV-2 infection. This aligns with recent UK data showing that after an initial decline in symptoms between 4 to 12 weeks, the prevalence of persistent symptoms plateaus between 12 to 22 weeks. Based on our findings, symptom exacerbation must be considered in rehabilitation and exercise interventions for people living with long COVID. In people with PEM, an activity plan needs to be carefully designed based on individual presentation with input from each patient.17 The DSQ-PEM might be useful as a screening measure and to facilitate a discussion with patients about postexertional symptom exacerbation. Beneficial interventions might first ensure symptom stabilization, with a long-term goal of improved function (eg, return to roles, daily activities, or work64) and HRQL. In ME/CFS, pacing is a self-management strategy for activity that helps minimize PEM.65 Improvements may be aided by careful tailoring, pacing, prioritization, and modest goal setting.66 However, PEM will not be an issue for everyone with chronic fatigue, and the presence of PEM is not sufficient for an ME/CFS diagnosis. However, the presence of chronic fatigue and PEM that persists after COVID-19, alongside a substantial reduction in the ability to engage in pre-illness activities, unrefreshing sleep, cognitive impairment, or orthostatic intolerance, should lead to a comprehensive assessment to exclude or diagnose ME/CFS,67 because this may help individuals access appropriate care.
A study of over half a million people in the UK identified 2 distinct long COVID symptom profiles: a smaller cluster with predominantly respiratory symptoms and a larger “tiredness” cluster. We found that more than one-half of our sample reported significant breathlessness and other respiratory symptoms. In the absence of physiological respiratory or cardiac disease, long COVID may involve chronic changes in breathing patterns that result in this breathing discomfort.68 Respiratory physiotherapy and breathing retraining may be helpful for people with breathing discomfort, considering improvements in symptoms in people living with postural orthostatic tachycardia syndrome and asthma.69,70 More broadly, due to differences in the clinical presentation of long COVID, simple rehabilitation interventions may be inadequate; complex and multidisciplinary interventions that are tailored to symptom profiles may be more effective. Effective rehabilitation is urgently needed considering that HRQL is severely impacted by long COVID, and role limitations due to physical health problems appear to be worse in long COVID compared with 2 chronic diseases (Fig. 1).
Currently, there is limited information about whether exercise is beneficial for people living with long COVID, especially considering the heterogeneous range of symptoms. Although seeking insight from studies in other clinical conditions is valuable, the methodological/reporting inadequacies of rehabilitation literature59,71 should not be repeated in studies of long COVID. Whereas exercise is likely to be beneficial for some, there are many unknowns, including whether all patients with persistent symptoms should undergo screening for respiratory and cardiac complications before beginning exercise; whether exercise rehabilitation needs to be medically supervised; what level of tailoring is required; what frequency, intensity, duration, and type of exercise can be recommended; and the trajectory of recovery for people living with long COVID. It is essential that future clinical trials (including pilot/feasibility studies) report modifications, symptom exacerbation, and other adverse events. More generally, transparent reporting using published guidelines and open-access repositories 72,73 will ensure progress in optimizing care for people with long COVID. Furthermore, research may be more impactful and meaningful when it is deeply collaborative and involves patients as partners.74
Limitations
One of the main limitations of survey designs such as ours is selection bias. People living with long COVID who were experiencing fatigue, PEM, or breathlessness may have been more inclined to participate than people living with long COVID who were not experiencing any of these specific symptoms. Although we were primarily interested in these concepts, we acknowledge the constellation of signs and symptoms that make up the long COVID experience that were not assessed here, including symptoms related to cognitive impairment and orthostatic intolerance. The self-selection of participants into the study may mean that the prevalence of symptoms is overestimated (if those with severe symptoms were more likely to take part due to perceived importance) or underestimated (if those with severe symptoms were less likely to take part due to energy limitations). Prevalence data should be interpreted with caution because the direction and magnitude of the impact of selection bias is difficult to determine. Strict data cleaning procedures excluded approximately 24% of records after informed consent. Participants who did not indicate a confirmed or probable infection SARS-CoV-2 and those with a history of ME/CFS or postviral fatigue were excluded, and existing medical conditions were accounted for within the analyses. However, because this survey was anonymous, the confirmed or suspected infection with SARS-CoV-2 was self-reported, and this is a study limitation. Methods of verification of laboratory-confirmed or probable SARS-CoV-2 infection should be included and reported in future studies. We made a priori decisions about the duration of data collection in February 2021 to April 2021 because data (on PEM in particular) were considered time-sensitive due to the emerging discussion around exercise rehabilitation. However, this resulted in a sample size of 213 participants, which is significantly smaller than a long COVID survey with >3500 respondents that had a much broader scope6. The estimated prevalence of fatigue, PEM, and respiratory symptoms are higher in the aforementioned survey6, but rather than a limitation of the present study, this is likely due to the different objectives and validated questionnaires/scoring thresholds used herein. A limitation of our cross-sectional design is that it does not include a matched cohort or control group for comparison of the prevalence of clinically relevant symptoms. Furthermore, a cross-sectional design does not capture the natural trajectory of recovery, which may limit the extent that these data inform specific rehabilitation programs. Finally, our sample was composed of primarily White participants from North America or Europe, and our data may not be generalizable to other racial/ethnic groups or other world regions.
Long COVID is characterized by reduced HRQL and chronic fatigue that is clinically relevant and is at least as severe as fatigue in several other clinical conditions. PEM seems to be a common and significant challenge for the majority of this patient group and occurs alongside a reduced capacity to work, be physically active, and function both physically and socially. Because people with long COVID report setbacks and deterioration in function following overexertion, fatigue, and postexertional symptom exacerbation must be monitored and reported in studies involving interventions for people with long COVID.
Supplementary Material
Author Contributions
Concept/idea/research design: R. Twomey, J. DeMars, J.G. Wrightson
Writing: R. Twomey, S.N. Culos-Reed, J. Weatherald, J.G. Wrightson
Data collection: R. Twomey
Data analysis: R. Twomey, J.G. Wrightson
Project management: R. Twomey
Providing institutional liaisons: R. Twomey
Consultation (including review of manuscript before submitting): J. DeMars, K. Franklin, S.N. Culos-Reed, J. Weatherald
K. Franklin contributed in the role of patient partner and provided guidance based on her own lived experience of long COVID. R. Twomey also provided software and data curation and wrote the original draft. J.G. Wrightson provided visualization.
Ethics Approval
The study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB21–0159).
Data Availability
An anonymized dataset is available at https://osf.io/dxu63/.
Disclosure and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
This manuscript was posted before peer review on the Medrxiv preprint server on June 24, 2021.
Funding
This study was not funded. However, during the conduct of this study, RT was supported by the O'Brien Institute of Public Health and Ohlson Research Initiative, Cumming School of Medicine, University of Calgary and Canadian Institutes of Health Research Fellowship. NCR was NCR was funded by the Canadian Cancer Society, Canadian Institutes of Health Research, Alberta Cancer Foundation, and University of Calgary funding support. JW was supported by the Libin Cardiovascular Institute at the University of Calgary, Heart & Stroke Foundation of Canada, and Canadian Institutes of Health Research. JGW was supported by the Hotchkiss Brain Institute and the Cumming School of Medicine, University of Calgary.
Conflict of interests
JDM is the owner of Breath Well Physio (Alberta, Canada) and has been treating people living with long COVID in private practice since August, 2020. JDM delivered a paid course for rehabilitation professionals working with people with long COVID in April 2021. The authors have no other conflicts of interest to disclose.
References
- 1. Johns Hopkins Coronavirus Resource Center. COVID-19 map . Accessed May 19, 2021. https://coronavirus.jhu.edu/map.html.
- 2. Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. Accessed May 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021.
- 3. Dennis A, Wamil M, Alberts J, et al. . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rando HM, Bennett TD, Byrd JB, et al. . Challenges in defining long COVID: striking differences across literature, electronic health records, and patient-reported information. medRxiv. 2021;2021.03.20.21253896. 10.1101/2021.03.20.21253896. [DOI] [Google Scholar]
- 5. World Health Organization . A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. Accessed October 6, 2021.https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
- 6. Davis HE, Assaf GS, McCorkell L, et al. . Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tabacof L, Tosto-Mancuso J, Wood J, et al. . Post-acute COVID-19 syndrome negatively impacts health and wellbeing despite less severe acute infection. medRxiv. 2020;2020.11.04.20226126. doi: 10.1101/2020.11.04.20226126. [DOI] [Google Scholar]
- 8. Penner I, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol. 2017;13:662–675. 10.1038/nrneurol.2017.117. [DOI] [PubMed] [Google Scholar]
- 9. Gorna R, MacDermott N, Rayner C, et al. . Long COVID guidelines need to reflect lived experience. Lancet 2021;397:455–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown DA, O’Brien KK, Josh J, et al. . Six lessons for COVID-19 rehabilitation from HIV rehabilitation. Phys Ther 2020;100:1906–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor AK, Kingstone T, Briggs TA, et al. . Reluctant pioneer: a qualitative study of doctors’ experiences as patients with long COVID. Health Expect. 2021;24:833–842. 10.1111/hex.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (me/cfs)—a systemic review and comparison of clinical presentation and symptomatology. Medicina 2021;57:418. doi: 10.3390/medicina57050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kedor C, Freitag H, Meyer-Arndt L, et al. . Chronic COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) following the first pandemic wave in Germany—a first analysis of a prospective observational study. medRxiv. 2021;2021.02.06.21249256. 10.1101/2021.02.06.21249256. [DOI] [Google Scholar]
- 14. Décary S, Gaboury I, Poirier S, et al. . Humility and acceptance: working within our limits with long covid and myalgic encephalomyelitis/chronic fatigue syndrome. J Orthop Sports Phys Ther 2021;51:197–200. [DOI] [PubMed] [Google Scholar]
- 15. Chu L, Valencia IJ, Garvert DW, Montoya JG. Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: a patient-centered, cross-sectional survey. PLoS One 2018;13:e0197811. doi: 10.1371/journal.pone.0197811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holtzman CS, Bhatia S, Cotler J, Jason LA. Assessment of post-exertional malaise (PEM) in patients with myalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS): a patient-driven survey. Diagnostics. 2019;9:26. doi: 10.3390/diagnostics9010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Treating the most disruptive symptoms first and preventing worsening of symptoms. Accessed March 6, 2021. https://www.cdc.gov/me-cfs/healthcare-providers/clinical-care-patients-mecfs/treating-most-disruptive-symptoms.html. [Google Scholar]
- 18. Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition – a cohort study. Chron Respir Dis. 2021;18:14799731211015692. 10.1177/14799731211015691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Putrino D, Tabacof L, Tosto-Mancuso J, et al. . Autonomic therapy reduces fatigue and improves global impression of change in individuals with post-acute COVID-19 syndrome. Research Square. 2021. [preprint]. doi: 10.21203/rs.3.rs-440909/v1. [DOI]
- 20. Li J, Xia W, Zhan C, et al. . Effectiveness of a telerehabilitation program for COVID-19 survivors (TERECO) on exercise capacity, pulmonary function, lower limb muscle strength, and quality of life: a randomized controlled trial. medRxiv. 2021;2021.03.08.21253007. 10.1101/2021.03.08.21253007. [DOI] [Google Scholar]
- 21. Group PCC, Evans RA, McAuley H, et al. . Physical, cognitive and mental health impacts of COVID-19 following hospitalisation – a multi-centre prospective cohort study. medRxiv. 2021;2021.03.22.21254057. doi: 10.1101/2021.03.22.21254057. [DOI] [Google Scholar]
- 22. Public Health Agency of Canada. Understanding COVID-19 testing. 2020. Accessed May 16, 2021. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/understanding-covid-19-testing.html. [Google Scholar]
- 23. Centers for Disease Control and Prevention (CDC). COVID-19 and your health. 2020. Accessed May 16, 2021. https://www.cdc.gov/coronavirus/2019-ncov/testing/serology-overview.html. [PubMed] [Google Scholar]
- 24. Alwan NA, Johnson L. Defining long COVID: going back to the start. Med (N Y) 2021;2:501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alwan NA. A negative COVID-19 test does not mean recovery. Nature 2020;584:170–170. [DOI] [PubMed] [Google Scholar]
- 26. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manag 1997;13:63–74. [DOI] [PubMed] [Google Scholar]
- 27. Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol 2008;20:17–25. [DOI] [PubMed] [Google Scholar]
- 28. Butt Z, Lai J,. Shei, Rao D, Heinemann AW, Bill A, Cella D. Measurement of fatigue in cancer, stroke, and HIV using the functional assessment of chronic illness therapy – fatigue (FACIT-F) scale. J Psychosom Res 2013;74:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chandran V, Bhella S, Schentag C, Gladman DD. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheum Dis 2007;66:936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811–819. [PubMed] [Google Scholar]
- 31. Acaster S, Dickerhoof R, DeBusk K, Bernard K, Strauss W, Allen LF. Qualitative and quantitative validation of the FACIT-fatigue scale in iron deficiency anemia. Health Qual Life Outcomes. 2015;13:60. 10.1186/s12955-015-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hagell P, Höglund A, Reimer J, et al. . Measuring fatigue in Parkinson’s disease: a psychometric study of two brief generic fatigue questionnaires. J Pain Symptom Manag 2006;32:420–432. [DOI] [PubMed] [Google Scholar]
- 33. Van Belle S, Paridaens R, Evers G, et al. . Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer 2005;13:246–254. [DOI] [PubMed] [Google Scholar]
- 34. Cotler J, Holtzman C, Dudun C, Jason L. A brief questionnaire to assess post-exertional malaise. Diagnostics 2018;8:66. doi: 10.3390/diagnostics8030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jason L, Evans M, Porter N, et al. . The development of a revised Canadian myalgic encephalomyelitis chronic fatigue syndrome case definition. Am J Biochem Biotechnol. 2010;6:120–135. [Google Scholar]
- 36. NINDS Common Data Elements (CDE) Group Post-Exertional Malaise Subgroup Summary . Myalgic encephalomyelitis/chronic fatigue syndrome. 2017. Accessed October 29, 2019. https://www.commondataelements.ninds.nih.gov/sites/nindscde/files/Doc/MECFS/PEM_Subgroup_Summary.pdf.
- 37. Cotler J, Holtzman C, Dudun C, Jason LA. A brief questionnaire to assess post-exertional malaise. Diagnostics 2018;8:36. doi: 10.3390/diagnostics8030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitchell AJ, Bacon CJ, Moran RW. Reliability and determinants of self-evaluation of breathing questionnaire (SEBQ) score: a symptoms-based measure of dysfunctional breathing. Appl Psychophysiol Biofeedback 2016;41:111–120. [DOI] [PubMed] [Google Scholar]
- 39. Courtney R, Greenwood KM. Preliminary investigation of a measure of dysfunctional breathing symptoms: the self evaluation of breathing questionnaire (SEBQ). Inter J Osteopat Med. 2009;12:121–127. [Google Scholar]
- 40. Kiesel K, Rhodes T, Mueller J, Waninger A, Butler R. Development of a screening protocol to identify individuals with dysfunctional breathing. Int J Sports Phys Ther 2017;12:774–786. [PMC free article] [PubMed] [Google Scholar]
- 41. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 42. Silsbury Z, Goldsmith R, Rushton A. Systematic review of the measurement properties of self-report physical activity questionnaires in healthy adult populations. BMJ Open 2015;5:e008430. doi: 10.1136/bmjopen-2015-008430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. The Jamovi Project (2021). Jamovi (Version 1.6). Accessed March 21, 2022. https://www.jamovi.org.
- 44. R Core Team . R: A Language and Environment for Statistical Computing (Version 3.4.0). R Foundation for Statistical Computing. 2017; Vienna, Austria. Accessed March 21, 2022. https://www.R-project.org. [Google Scholar]
- 45. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002;94:528–538. [DOI] [PubMed] [Google Scholar]
- 46. Twomey R, Lavigne C, Martin T, Culos-Reed SN, Millet GY. Cardiopulmonary exercise testing for the measurement of maximal oxygen uptake in people with cancer-related fatigue: a retrospective analysis. SportRxiv 2020. [Preprint]. doi: 10.31236/osf.io/db7uy. [DOI]
- 47. Stridsman C, Müllerova H, Skär L, Lindberg A. Fatigue in COPD and the impact of respiratory symptoms and heart disease—a population-based study. COPD: J Chron Obstruct Pulmon Dis 2013;10:125–132. [DOI] [PubMed] [Google Scholar]
- 48. Signorovitch J, Brainsky A, Grotzinger KM. Validation of the FACIT-fatigue subscale, selected items from FACT-thrombocytopenia, and the SF-36v2 in patients with chronic immune thrombocytopenia. Qual Life Res 2011;20:1737–1744. [DOI] [PubMed] [Google Scholar]
- 49. Garratt AM, Stavem K. Measurement properties and normative data for the Norwegian SF-36: results from a general population survey. Health Qual Life Outcomes 2017;15:51. doi: 10.1186/s12955-017-0625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wolfe F, Michaud K, Li T, Katz RS. EQ-5D and SF-36 quality of life measures in systemic lupus erythematosus: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, and fibromyalgia. J Rheumatol 2010;37:296–304. [DOI] [PubMed] [Google Scholar]
- 51. Bentsen SB, Rokne B, Wahl AK. Comparison of health-related quality of life between patients with chronic obstructive pulmonary disease and the general population. Scand J Caring Sci 2013;27:905–912. doi: 10.1111/scs.12002. [DOI] [PubMed] [Google Scholar]
- 52. Allen M, Poggiali D, Whitaker K, Marshall TR, Langen J, Kievit RA. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Res. 2021;4:63. 10.12688/wellcomeopenres.15191.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ziauddeen N, Gurdasani D, O’Hara ME, et al. . Characteristics of long Covid: findings from a social media survey. medRxiv. 2021;2021.03.21.21253968. doi: 10.1101/2021.03.21.21253968. [DOI] [Google Scholar]
- 54. Pertl MM, Quigley J, Hevey D. “I’m not complaining because I’m alive”: barriers to the emergence of a discourse of cancer-related fatigue. Psychol Health 2014;29:141–161. [DOI] [PubMed] [Google Scholar]
- 55. McManimen S, McClellan D, Stoothoff J, Gleason K, Jason LA. Dismissing chronic illness: a qualitative analysis of negative health care experiences. Health Care Women Int 2019;40:241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jones JM, Olson K, Catton P, et al. . Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv 2016;10:51–61. [DOI] [PubMed] [Google Scholar]
- 57. Sandler CX, Goldstein D, Horsfield S, et al. . Randomized evaluation of cognitive-behavioral therapy and graded exercise therapy for post-cancer fatigue. J Pain Symptom Manag 2017;54:74–84. [DOI] [PubMed] [Google Scholar]
- 58. Sheehan P, Denieffe S, Murphy NM, Harrison M. Exercise is more effective than health education in reducing fatigue in fatigued cancer survivors. Support Care Cancer 2020;28:4953–4962. [DOI] [PubMed] [Google Scholar]
- 59. Twomey R, Yeung S, Wrightson JG, et al. . Physical activity in adults with fatigue after cancer treatment: a systematic review of randomized trials with fatigue as an eligibility criterion. Communications in Kinesiology 2021;1. doi: 10.51224/cik.v1i3.40. [DOI]
- 60. Twomey R, Martin T, Temesi J, Culos-Reed SN, Millet GY. Tailored exercise interventions to reduce fatigue in cancer survivors: study protocol of a randomized controlled trial. BMC Cancer 2018;18:757. doi: 10.1186/s12885-018-4668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Twomey R, Yeung ST, Wrightson JG, Millet GY, Culos-Reed SN. Post-exertional malaise in people with chronic cancer-related fatigue. J Pain Symptom Manag. 2020;2:407–416. [DOI] [PubMed] [Google Scholar]
- 62. Lim EJ, Kang EB, Jang ES, Son CG. The prospects of the two-day cardiopulmonary exercise test (CPET) in ME/CFS patients: a meta-analysis. J Clin Med 2020;9:4040. doi: 10.3390/jcm9124040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown D (Host). The long COVID physio podcast [multiple audio podcast episodes]. 2021. Accessed May 2021. https://longcovid.physio/podcast.
- 64. Humphreys H, Kilby L, Kudiersky N, Copeland R. Long COVID and the role of physical activity: a qualitative study. BMJ Open 2021;11:e047632. doi: 10.1136/bmjopen-2020-047632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Centers for Disease Control and Prevention . Treatment of ME/CFS. 2021. Accessed June 8, 2021. https://www.cdc.gov/me-cfs/treatment/.
- 66. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 67. US ME/CFS Clinician Coalition . ME/CFS treatment recommendations. Version 1 February 20. 2021. Accessed May, 2021. https://mecfscliniciancoalition.org/clinical-management/.
- 68. Boulding R, Stacey R, Niven R, Fowler SJ. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 2016;25:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reilly CC, Floyd SV, Lee K, et al. . Breathlessness and dysfunctional breathing in patients with postural orthostatic tachycardia syndrome (POTS): the impact of a physiotherapy intervention. Auton Neurosci. 2020;223:102601. 10.1016/j.autneu.2019.102601. [DOI] [PubMed] [Google Scholar]
- 70. Santino TA, Chaves GS, Freitas DA, Fregonezi GA, Mendonça KM. Breathing exercises for adults with asthma. Cochrane Database Syst Rev 2020;3:CD00127. doi: 10.1002/14651858.CD001277.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wormgoor MEA, Rodenburg SC. The evidence base for physiotherapy in myalgic encephalomyelitis/chronic fatigue syndrome when considering post-exertional malaise: a systematic review and narrative synthesis. J Transl Med 2021;19:1. doi: 10.1186/s12967-020-02683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hoffmann TC, Glasziou PP, Boutron I, et al. . Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 73. Altman DG, Moher D. Importance of transparent reporting of Health Research. EQUATOR Network. 2014. Accessed September 15, 2020. https://www.equator-network.org/wp-content/uploads/2016/10/AltmanMoher-Chapter-1-Guidelines-for-Reporting-Health-Research-A-Users-Manual.pdf. [Google Scholar]
- 74. Government of Canada CI of HR . Strategy for patient-oriented research. 2018. Accessed May 28, 2021. https://cihr-irsc.gc.ca/e/41204.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
An anonymized dataset is available at https://osf.io/dxu63/.