Abstract
Analysis of the sequence data obtained from the 5′ portion of the Streptococcus pneumoniae type 19A capsular polysaccharide biosynthesis locus (cps19a) revealed that the first seven genes are homologous to the first seven genes in the type 19F (cps19f) locus. The former genes were designated cps19aA to -G and were 70 to 90% identical to their cps19f counterparts. Southern hybridization analysis of the cps loci from various S. pneumoniae serotypes with probes specific for the cps19aC, cps19aD, and cps19aE genes indicated a hybridization pattern complementary to that previously reported for cps19fC, cps19fD, and cps19fE. That is, all serotypes tested contained high-stringency homologues of either the cps19aC to -E genes or the cps19fC to -E genes, but not both. On this basis S. pneumoniae cps loci can be divided into two distinct classes. Long-range PCR was used to amplify the cps regions between cpsB and aliA from a variety of pneumococcal serotypes. Direct sequencing of the 5′ end of these PCR products, and phylogenetic analysis of the sequence data, confirmed the presence of the two distinct classes of cpsC. Whereas members within one class are greater than 95% identical to each other, the DNA sequence identity between the two classes is only approximately 70%.
Streptococcus pneumoniae (the pneumococcus) is an important cause of invasive disease in human populations throughout the world, resulting in high morbidity and mortality. Control of pneumococcal disease is being complicated by the increasing prevalence of antibiotic-resistant strains and the suboptimal clinical efficacy of existing vaccines. S. pneumoniae produces a polysaccharide capsule which is essential for virulence because it protects the pneumococcus from the nonspecific immune defenses of the host during an infection (2).
There are now 90 recognized serotypes of S. pneumoniae (9), each of which produces a structurally distinct capsular polysaccharide (CPS). Classical genetic studies carried out by Austrian et al. (3) demonstrated that the S. pneumoniae genes required for biosynthesis and expression of CPS are closely linked on the pneumococcal chromosome. This fact enabled us to clone and sequence the complete capsule locus from S. pneumoniae type 19F (designated cps19f) (8, 17). Our studies were concentrated on S. pneumoniae type 19F because it is one of the commonest causes of invasive disease in children, and the type 19F CPS is one of the poorest immunogens in this group (6). Type 19F belongs to serogroup 19, which also contains the immunologically cross-reactive types 19A, 19B, and 19C. S. pneumoniae type 19A is also an important cause of disease, whereas types 19B and 19C are rare causes of disease (25).
We have previously examined the distribution of individual cps19f genes among other pneumococcal serotypes, including the other members of serogroup 19, by Southern hybridization analysis (17). Only homologues to cps19fA and -B, the first two genes in the cps locus, were present in all serotypes examined. Cps19fA is a putative transcriptional attenuator, but the function of Cps19fB is unknown. The next two genes in the cps locus, cps19fC and -D, encode proteins which are predicted to be involved in chain length regulation and export of CPS (8, 17). Moreover, Cps19fC and -D are essential for CPS expression in S. pneumoniae type 19F, as in-frame deletion mutations in either cps19fC or cps19fD result in the loss of CPS production (16a). Thus, cps19fC and -D homologues are probably essential for CPS production in all S. pneumoniae serotypes which are synthesized via lipid-linked repeat unit intermediates in a fashion similar to type 19F CPS. To date, this would include all pneumococcal serotypes which have been characterized except type 3, which is synthesized by a processive transferase (1, 5, 21). Surprisingly, however, 10 of the 21 serotypes tested in previous hybridization studies, including type 19A, did not contain high-stringency homologues of cps19fC and -D.
The structures of the CPS for types 19F and 19A are almost identical, consisting of the same rhamnose→N-acetyl mannosamine→glucose trisaccharide repeat units joined by different glycosidic linkages (α[1→2] for 19F and α[1→3] for 19A) (10, 20). Thus the only predicted functional difference between the protein products expressed by the cps19f and cps19a loci would be that of the polysaccharide polymerase. However, the type 19A cps locus appears to be more divergent, with high-stringency homologues of only eight of the cps19f genes present, compared to homologues of 13 out of the 15 cps19f genes present in types 19B and 19C (17). This study investigates the basis for this apparent diversity.
Bacterial strains.
S. pneumoniae Rx1-19F has been described previously (8). A clinical isolate of S. pneumoniae type 19A, strain 1777/39, was obtained from Jorgen Henrichsen, Statens Seruminstitut, Copenhagen, Denmark. All other S. pneumoniae strains were clinical isolates from the Women’s and Children’s Hospital, North Adelaide, Australia. Pneumococci were routinely grown either in Todd-Hewitt broth (Oxoid Limited, Basingstoke, England) supplemented with 0.5% (wt/vol) yeast extract (Difco Laboratories, Detroit, Mich.) or on blood agar (Oxoid) and serotyped by the quellung reaction using sera obtained from the Statens Seruminstitut.
Characterization of the 5′ portion of cps19a.
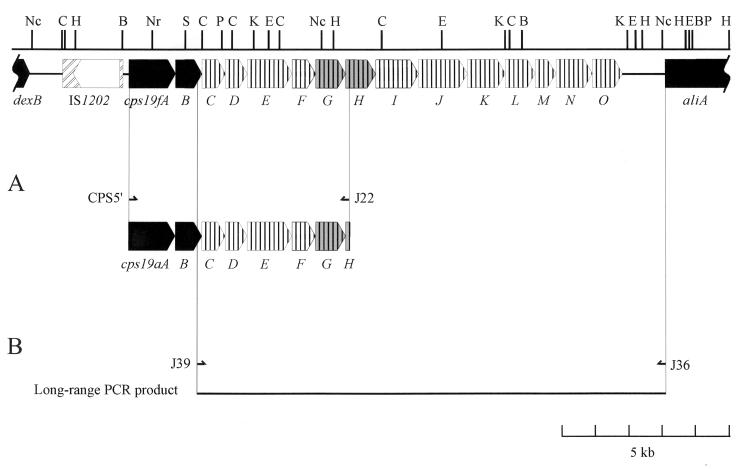
Genes homologous to cps19fA, -B, -G, and -H were predicted to be present in the cps19a locus based on previous Southern hybridization data obtained with the cps19f genes as probes (17). Thus, the 5′ portion of cps19a was amplified by long-range PCR using the Expand Long Template PCR system (Boehringer, Mannheim, Germany), according to the manufacturer’s instructions, and was performed in a Hybaid Touchdown Thermal Cycler. The two primers used to amplify this region (CPS5′ and J22) (Fig. 1A) were based on regions of the cps19f sequence which are predicted to be homologous to the cps19a locus. The resultant PCR product was sequenced by using dye-terminator chemistry with specifically designed primers on an Applied Biosystems model 373A automated DNA sequencer. The sequences were analyzed with DNASIS software (version 7.0; Hitachi Software Engineering, South San Francisco, Calif.). Analysis of the compiled DNA sequence revealed that the cps19f and cps19a loci are indeed very closely related. There are seven open reading frames (ORFs) in this portion of the cps19a locus, designated cps19aA to -G, which are organized in an order identical to those in cps19f, with similarities to the cps19f genes ranging from 70.1 to 90.9% identity. The sizes, G+C content, and percent identity of the cps19a and cps19f protein products are shown in Table 1, and the arrangement of the cps19aA to -G genes compared to those from cps19f is shown in Fig. 1A. The arrangement of the genes in this region of the two loci are remarkably similar; even the intergenic gaps between the cps19a genes and the cps19f genes are similar in size. The most significant difference between the two loci is the start codon used for cps19G. Whereas the start codon for both cps19aG and cps19aH is TTG, only cps19fH has a TTG start codon in the cps19f locus.
FIG. 1.
The dexB-to-aliA region of the S. pneumoniae type 19F chromosome. The regions conserved among all pneumococcal serotypes are indicated in black, the cps19G and -H genes are shaded grey to indicate a higher degree of identity between cps19f and cps19a in this region. (A) Arrangement of the cps19a locus between the CPS5′ and J22 primers. CPS5′ (5′-TGATGTTCAAGGTATAGGTGTTAATCA) is homologous to nucleotides 146 to 169 of the cps19f sequence (17), immediately preceding the cps19fA gene, and J22 (5′-AATTGAATTCTTTTATAGATTTAACACAAG) is complementary to nucleotides 6743 to 6772 of the cps19f sequence, in the 5′ region of cps19fH. (B) The portion of the cps locus between cpsB and aliA from various pneumococcal serotypes was amplified by using the two primers J39 and J36. The positions of the two primers J39 (5′-TAGTTCATGTAGTTGCAAGTGACATGCACAA, homologous to nucleotides 2190 to 2220 of the cps19f sequence, in the 3′ region of cps19fB) and J36 (5′-CAATAATGTCACGCCCGCAAGGGCAAGT, complementary to nucleotides 16463 to 16490 of the cps19f sequence, located just after the start of aliA) are indicated with half arrows. Abbreviations for restriction sites are as follows: B, BamHI; C, ClaI; E, EcoRI; H, HindIII; K, KpnI; Nc, NcoI; Nr, NruI; P, PstI; S, SphI.
TABLE 1.
Comparison of cps19a and cps19f ORFs
| cps19a ORF | Predicted protein product
|
%G+Ca | cps19f ORF | Predicted protein product
|
%G+C | % Identityc
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Molecular mass (Da) | No. of aab | Molecular mass (Da) | No. of aa | DNA | aa | ||||
| cps19aA | 53,576 | 481 | 39.5 | cps19fA | 53,572 | 481 | 38.1 | 90.5 | 92.3 |
| cps19aB | 28,138 | 243 | 41.3 | cps19fB | 28,352 | 243 | 38 | 82 | 85.2 |
| cps19aC | 25,473 | 230 | 42.1 | cps19fC | 25,497 | 230 | 38.2 | 70.1 | 71.7 |
| cps19aD | 25,155 | 229 | 41 | cps19fD | 24,947 | 227 | 34.5 | 73 | 80.2 |
| cps19aE | 51,971 | 453 | 37.7 | cps19fE | 52,595 | 455 | 33.2 | 71.2 | 70.5 |
| cps19aF | 28,273 | 247 | 34.1 | cps19fF | 28,155 | 247 | 33.6 | 78.9 | 82.9 |
| cps19aG | 31,195 | 266 | 37.2 | cps19fG | 31,647 | 269 | 36.3 | 90.9 | 93.6 |
Percent guanine plus cytosine content of coding region.
aa, amino acids.
Percent identity between cps19a and cps19f ORFs.
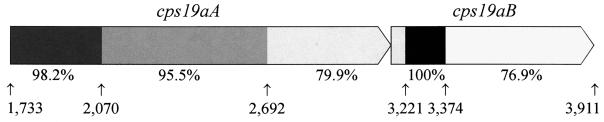
Interestingly, even though cps19aA and -B sequences hybridize to cps19fA- and cps19fB-specific probes (17), the overall identity between the genes is lower than expected (90.5 and 82%, respectively), with no clearly identifiable point from which downstream sequences diverge. Instead, the cps19aAB genes present a mosaic pattern with small regions of varying degrees of identity to the cps19fAB genes, ranging from 76.6 to 100%, as shown in Fig. 2. This suggests that the cps19a locus and the type 19A serotype may be the result of several recombination events between the ancestral cps locus and exogenous DNA. Some of these recombination events may have involved small DNA fragments that did not affect the serotype, while others resulted in the exchange of larger regions of the capsule locus, which may have altered the structure and hence serotype of the expressed CPS. A small region of cps19aB (nucleotides 3,221 to 3,374) has 100% identity to cps19fB. This region presumably accounts for the high-stringency hybridization of the cps19aB DNA to a cps19fB probe (17), as there is only 76.7% identity between the remainder of the cps19aB and cps19fB genes. The highly conserved region either may encode a functionally important domain in the cps19B gene product or may simply be the result of a recombination event.
FIG. 2.
Diagrammatic representation of the similarity of the cps19aAB genes to the cps19fAB genes. There are several possible recombination points in this region of the locus. Increasing similarity is represented by progressively darker shades of grey, and the percent identity is shown under the individual shaded regions. The arrows indicate the points of divergence, and the number below each arrow corresponds to the nucleotide number of the cps19a sequence.
Southern hybridization analysis.
Previous Southern hybridization data have shown that high stringency homologues of cps19fA and -B are present in all serotypes tested, whereas cps19fF and -G are serogroup specific. However, high-stringency cps19fC, -D, and -E homologues were present in some serotypes tested but not others (17). The presence of homologues to the divergent cps19aC, cps19aD, and cps19aE genes in the cps loci of various S. pneumoniae serotypes was therefore examined by Southern hybridization. Digoxigenin (DIG)-labelled DNA fragments corresponding to the cps19aC, cps19aD, and cps19aE genes were used to probe, at high stringency, ClaI-restricted chromosomal DNA from representative pneumococci belonging to serotypes 2, 3, 4, 6A, 6B, 7F, 8, 9N, 9V, 12, 14, 16, 17, 18C, 19F, 19B, 19C, 20, 22, 23F, and 24. The hybridization data for the type 19A cps19aC, cps19aD, and cps19aE gene probes and previous data obtained for the type 19F cps19fC, cps19fD, and cps19fE gene probes (17) are compared in Table 2.
TABLE 2.
Hybridization of cps19fC to -E and cps19aC to -E genes with other pneumococcal serotypesa
| Serotype | DIG-labelled DNA probe
|
|||||
|---|---|---|---|---|---|---|
| cps19fC | cps19fD | cps19fE | cps19aC | cps19aD | cps19aE | |
| 2 | − | − | − | + | + | + |
| 3 | + | + | − | − | − | − |
| 4 | + | + | − | + | − | − |
| 6A | − | − | − | + | + | + |
| 6B | − | − | − | + | + | + |
| 7F | + | + | + | − | − | − |
| 8 | − | − | − | + | + | + |
| 9N | + | + | + | − | − | − |
| 9V | − | − | − | + | + | + |
| 12 | − | − | − | + | + | + |
| 14 | + | + | + | − | − | − |
| 16 | + | + | + | − | − | − |
| 17 | − | − | − | + | + | + |
| 18C | + | + | + | − | − | − |
| 19F | + | + | + | − | − | − |
| 19A | − | − | − | + | + | + |
| 19B | + | + | + | − | − | − |
| 19C | + | + | + | − | − | − |
| 20 | + | + | + | − | − | − |
| 22 | − | − | − | + | + | + |
| 23F | − | − | − | + | + | + |
| 24 | + | + | + | − | − | − |
DNA fragments equivalent to nucleotides 3932 to 4356, 4356 to 4820, and 5594 to 6480 of the cps19a sequence were labelled with DIG and used as probes for the cps19aC, -D, and -E genes, respectively. The results for cps19fC, cps19fD, and cps19fE have been published previously (17).
The most remarkable feature seen in Table 2 is that all the serotypes tested contained high-stringency homologues of either cps19fC to -E or cps19aC to -E, except types 3 and 4, which do not have a high-stringency homologue of either cps19fE or cps19aE (the gene encoding the glucose-1-phosphate transferase which catalyzes the addition of glucose-1-phosphate to the lipid carrier, a common first step in biosynthesis of the lipid-linked repeat unit [12, 17]). The absence of a cpsE homologue in types 3 and 4 is not surprising, because the type 4 CPS does not contain glucose, and the mode of type 3 CPS biosynthesis is atypical, occurring via a processive transferase (1, 5). Type 4 also contains a hybrid cpsC gene, hybridizing to both the cps19fC and the cps19aC probes, as described below. Thus, these Southern hybridization data suggest that S. pneumoniae cps loci can be divided into two distinct classes, designated class I and class II, where class I loci contain high-stringency cps19fC to -E homologues and class II loci contain high-stringency cps19aC to -E homologues.
Amplification of capsule loci by long-range PCR.
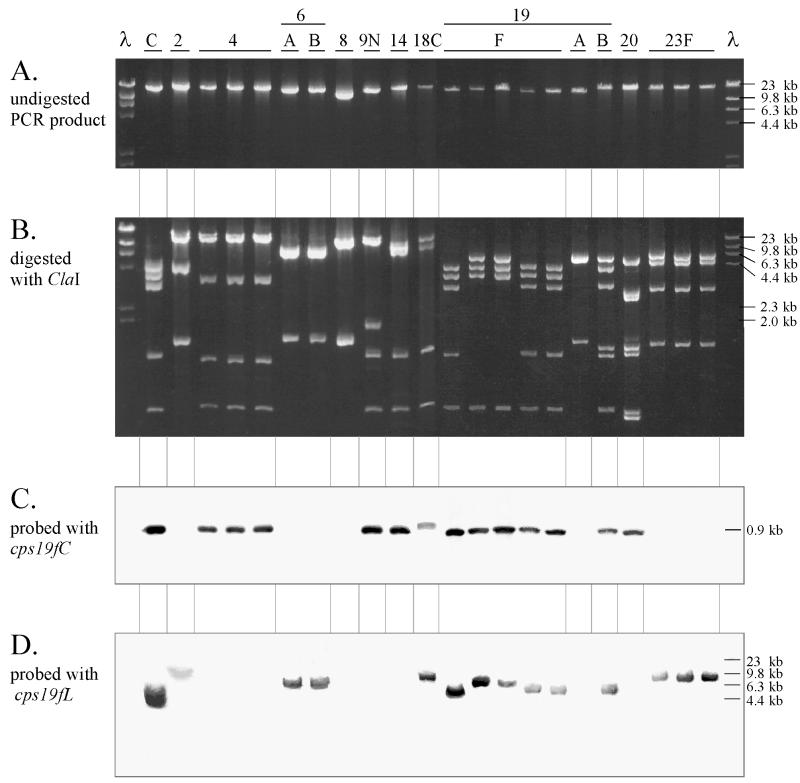
In order to directly characterize the two classes of cpsC gene, long-range PCR was used to amplify the portion of the cps loci between cpsB and aliA (Fig. 1B) from several S. pneumoniae serotypes so that DNA sequencing could be undertaken. DNAs prepared from serotypes or groups 2, 4, 6A, 6B, 7F, 8, 9N, 9V, 12, 14, 16, 17, 18C, 19F, 19A, 19B, 19C, 20, 22, 23F, and 24 were used as templates for long-range PCR. PCR products were obtained from at least one pneumococcal isolate of types 2, 4, 6A, 6B, 8, 9N, 14, 18C, 19F, 19A, 19B, 20, and 23F but not from types 7F, 9V, 12, 16, 17, 19C, 22, and 24. Analysis of the DNA fragments reveals that the PCR products ranged in size from 15 to 20 kb, as shown in Fig. 3A. The PCR products were digested with the restriction endonuclease ClaI and electrophoresed on a 1% agarose gel in a Tris-borate-EDTA (TBE) buffer system as described by Maniatis et al. (16) (Fig. 3B). Identical restriction patterns were obtained for three different isolates of serotypes 4 and 23F. However, a restriction site polymorphism was observed in two of the five PCR products from different type 19F strains (Fig. 3B).
FIG. 3.
Long-range PCR products. PCR products, not digested (A) or digested with ClaI (B), were electrophoresed on a 1% agarose gel in the presence of ethidium bromide. ClaI-restricted PCR product was subjected to Southern hybridization analysis using DIG-labelled probes specific for cps19fC (C) or cps19fL (D). The probes specific for cps19fC and cps19fL correspond to nucleotides 2380 to 2998 and 11539 to 12493 of the cps19f sequence (17). The molecular size standards are shown on the right-hand side of the figure and correspond to HindIII-digested λ phage DNA.
Southern hybridization analysis of long-range PCR products.
In order to confirm that they contained cps-related sequences, the long-range PCR products from the various S. pneumoniae serotypes were restricted with ClaI and subjected to Southern hybridization analysis using probes specific for two different type 19F gene probes, cps19fC (located in the 5′ region of the cps19f locus) and cps19fL (located in the 3′ region of the cps19f locus) (Fig. 3C and D).
The cps19fC probe hybridized at high stringency with a 0.9-kb DNA fragment in types 4, 9N, 14, 18C, 19F, 19B, and 20. Both the hybridization pattern and the size of the DNA fragment which hybridized with the cps19fC probe are consistent with the Southern hybridization data obtained when probing ClaI-restricted chromosomal DNA with the cps19fC probe (data not shown).
The cps19fL probe hybridized with DNA fragments ranging in size from 4 to 10 kb in the ClaI-restricted PCR products from types 2, 6A, 6B, 18C, 19F, 19B, and 23F. Hybridization was consistent with that obtained from Southern hybridization with ClaI-restricted chromosomal DNA from these isolates, although the sizes of the restriction fragments differ (data not shown). The size of this ClaI fragment is affected because there is no ClaI site between cps19fL and the end of the PCR product in type 19F.
DNA sequencing of the 5′ portion of cpsC in the long-range PCR products.
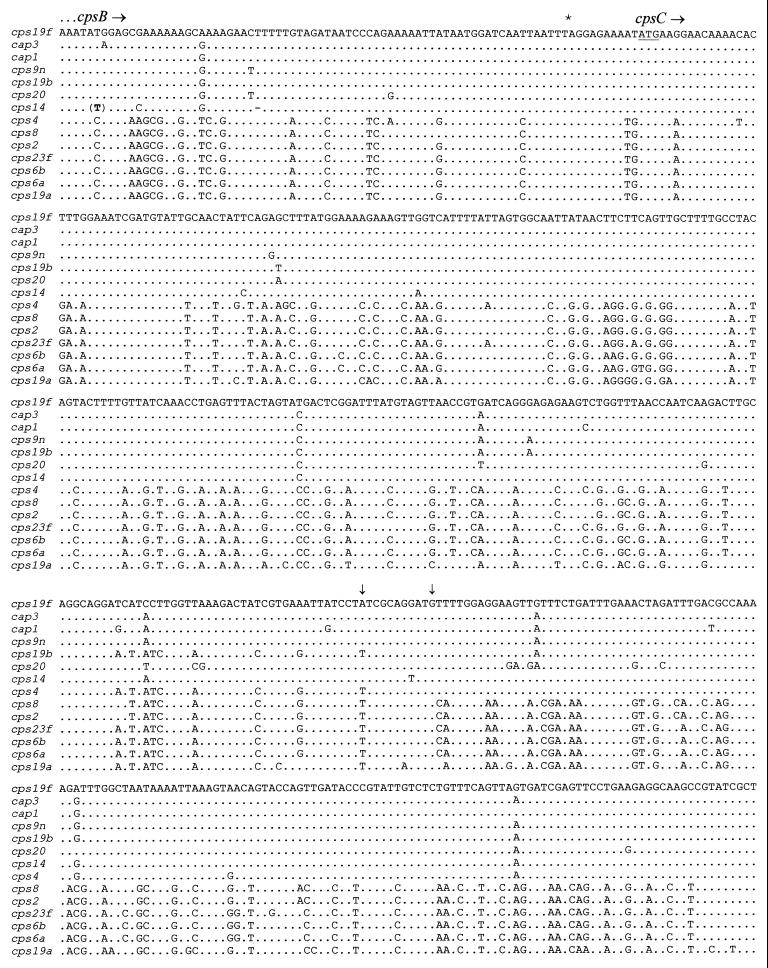
The long-range PCR products were subjected to one round of sequence analysis with the J39 primer (located at the 5′ end of the PCR product) in order to determine the presence of a cpsC homologue. No sequence data were obtained from the type 18C PCR template, presumably due to the low yield of the PCR product obtained. Analysis of the sequence data obtained from all the other templates and that available for types 1, 3, and 14 (1, 13, 18) showed that, indeed, there were two distinct cpsC genes in these loci. Types 1, 3, 9N, 14, 19F, 19B, and 20 have class I cpsC genes which exhibit >95% nucleotide sequence identity to cps19fC, whereas types 2, 6A, 6B, 8, 19A, and 23F have class II cpsC genes which exhibit 72 to 74% identity to cps19fC and >95% identity to cps19aC (Fig. 4). The sequences obtained from the PCR products also included the last 75 nucleotides of cpsB; this region can also be separated into the same two classes as described above (Fig. 4).
FIG. 4.
Comparison of class I and class II cps sequences. The first 500 nucleotides of the sequence obtained are shown (100 nucleotides per line). Dots indicate nucleotides which are identical to that for cps19f. The stop codon of the cpsB gene is indicated with an asterisk. The start codon of cpsC is underlined. (T) denotes an extra nucleotide, and - denotes the absence of a nucleotide in the cps14 DNA sequence. The vertical arrows indicate the region where the crossover between class I and class II sequences has occurred in cps4.
An interesting exception is found in type 4, the cps4C gene of which is a hybrid consisting of a class II 5′ region and a class I 3′ region, with a distinct crossover point in the vicinity of nucleotide 345 of the cps4 sequence (Fig. 4). Comparison of the type 4 cps sequence data (available from the TIGR microbial database) with the cps19f sequence showed another point of divergence within the cpsB gene. The cps4B gene is almost identical (except for the first 42 nucleotides) to the cps19aB gene and shows the same point of sequence divergence from the cps19fB gene (nucleotide 3374 in Fig. 2). Thus, in the cps4 locus a region of 852 nucleotides, including most of cps4B and part of cps4C, has approximately 74% identity to cps19f, whereas the remainder of the cps4A to -D region exhibits >95% identity to cps19fA to -D. This may have arisen as a consequence of recombination between a class I cps locus and a DNA fragment (approximately 852 nucleotides long) from a class II cps locus, resulting in a mosaic cpsB-cpsC region. Analysis of the available type 23F sequence data (4, 22) indicated that the class II cps23f locus also diverges from the class I cps19f locus within the cpsB gene, but 98 nucleotides further downstream from the point of divergence for cps19a and cps4. This suggests that the point of sequence divergence from class I to class II within cpsB may vary between different serotypes.
Phylogenetic analysis.
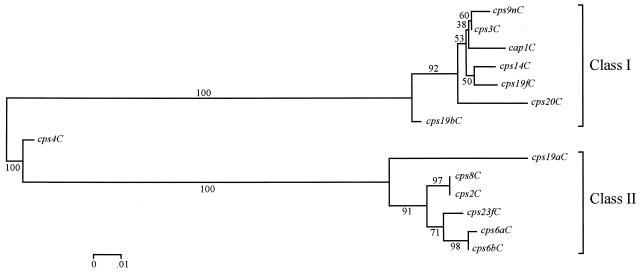
To further confirm the presence of two distinct classes of cpsC sequences, their phylogenetic relationship was investigated. An alignment of the partial cpsC sequences was generated by using CLUSTAL W (24) (data not shown), and this alignment was used to generate a phylogenetic tree by the neighbor-joining method and the distance measure of Tamura and Nei (23), as implemented in the program MEGA (15). The tree in Fig. 5 shows two highly significant clusters of cpsC sequences (based on a bootstrapping test with 500 replications) and confirms the observations initially made on the basis of sequence homology that the cpsC genes are divided into two classes. The cps4C sequence forms a third cluster; as described above, this gene is a hybrid of the two cpsC classes and has a recombination crossover point at or near nucleotide 345 (as shown in Fig. 4) within the cpsC gene. The cps19bC gene is also separated from the other class I cpsC sequences; cps19bC also appears to have a mosaic structure with a small region of class II sequence (nucleotides 409 to 444 in Fig. 4), which is presumably the result of a recombination event.
FIG. 5.
Phylogenetic tree of cpsC sequences. The cpsC gene sequences were aligned by using CLUSTAL W (24), and the phylogenetic tree was generated by using MEGA (15), as described in the text. The numbers associated with the branches are bootstrapping confidence limits, resulting from 500 replications, as defined in MEGA. The scale represents the number of nucleotide substitutions per site.
Conclusions.
The 5′ portion of the cps locus from S. pneumoniae type 19A is similar to cps19f, in that it has the same number of genes arranged in the same order. However, many of these genes demonstrate only 70 to 80% nucleotide sequence identity with their cps19f counterpart, suggesting either that the two loci diverged a long time ago or that portions of these loci have separate origins. Some regions within the cps19aA, -B, and -G genes do have >90% identity to those in cps19f, which may be a consequence of recombination between the two loci or perhaps is due to a requirement for a higher degree of conservation in regions encoding functionally important domains.
Southern hybridization analysis identified two classes of cpsC, cpsD, and cpsE genes in S. pneumoniae cps loci, which were designated as either class I or class II. Class I pneumococcal cps loci contain high-stringency cps19fC to -E homologues, whereas class II loci contain high-stringency cps19aC to -E homologues. Direct sequencing of the long-range PCR products obtained confirmed the presence of two classes of cpsC gene. Phylogenetic analysis of the sequence data also confirmed that the pneumococcal cpsC gene is divided into two closely related classes. The presence of the cpsC and cpsD genes in all cps loci examined is consistent with the important role of CpsC and CpsD in pneumococcal CPS production. Both are predicted to be involved in chain length regulation and export of the CPS (8, 17). At this stage, it is not possible to determine whether the differences between class I and class II cpsC and cpsD gene products are functionally significant. Translation of the genes indicates a similar degree of amino acid sequence divergence between class I and class II CpsC proteins (approximately 70% identity). Interestingly, even small differences between the functionally homologous Rol (Wzz) proteins of Shigella species has previously been shown to affect the modal chain length of the lipopolysaccharide O antigen (11).
The cpsE gene was also present in all S. pneumoniae serotypes tested which contain glucose in their CPS, except type 3, which has a different mode of CPS biosynthesis (1, 5). This gene is also separated into either class I or class II, along with the preceding cpsC and cpsD genes. However, the two different classes do not appear to affect the function of CpsE, which is a glucose-1-phosphate transferase. Kolkman et al. (14) demonstrated glucose-1-phosphate transferase activity in several pneumococcal serotypes now known to contain either class I or class II cpsE genes. In all S. pneumoniae cps loci sequenced to date, the gene which follows cpsE is serotype or serogroup specific (21).
All S. pneumoniae cps loci examined contain highly conserved cpsA and cpsB genes, indicating that they probably evolved from a common ancestor. However, their cpsC, cpsD, and cpsE genes can be separated into either class I or class II sequences, suggesting that recombination between the original S. pneumoniae ancestor (either class I or class II) and exogenous DNA resulted in the formation of two distinct clonal S. pneumoniae strains from which all subsequent serotypes have evolved. The presence of DNA homologous to cps19fA to -D, even though these genes are not functional in type 3 pneumococci (7), probably reflects the common origin between type 3 and other class I pneumococci.
The type 4 and 19B cpsC sequences both show evidence of recombination within the cps loci. Two recent studies have demonstrated that natural recombination events involving exchange of entire cps loci (or major portions thereof) have resulted in switching of capsule type (e.g., from 23F to 19F) by multiply drug-resistant pneumococcal clones on numerous occasions (4, 19). The current study indicates that recombination events involving small fragments within pneumococcal cps loci may also be common in nature and may represent a mechanism whereby additional serotype diversity is generated.
Nucleotide sequence accession numbers.
The cps19a sequence has been deposited with GenBank under accession no. AF094575. The sequences for the 5′ region of cpsC from serotypes 2, 6A, 6B, 8, 9N, 19B, and 20 are available under GenBank accession no. AF106132, AF106133, AF106134, AF106135, AF106136, AF106137, and AF106138, respectively.
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Arrecubieta C, García E, López R. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene. 1995;167:1–7. doi: 10.1016/0378-1119(95)00657-5. [DOI] [PubMed] [Google Scholar]
- 2.Austrian R. Some observations on the pneumococcus and on the current status of pneumococcal disease and its prevention. Rev Infect Dis. 1981;3(Suppl.):S1–S17. doi: 10.1093/clinids/3.supplement_1.s1. [DOI] [PubMed] [Google Scholar]
- 3.Austrian R, Bernheimer H P, Smith E E B, Mills G T. Simultaneous production of two capsular polysaccharides by pneumococcus. II. The genetic and biochemical bases of binary capsulation. J Exp Med. 1959;110:585–602. doi: 10.1084/jem.110.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffey T J, Enright M C, Daniels M, Morona J K, Morona R, Hryniewicz V, Paton J C, Spratt B G. Recombinational exchanges at the capsular polysaccharide biosynthesis locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol Microbiol. 1998;27:73–83. doi: 10.1046/j.1365-2958.1998.00658.x. [DOI] [PubMed] [Google Scholar]
- 5.Dillard J P, Vandersea M W, Yother J. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J Exp Med. 1995;181:973–983. doi: 10.1084/jem.181.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas R M, Paton J C, Duncan S J, Hansman D. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 7.García E, López R. Molecular biology of the capsular genes of Streptococcus pneumoniae. FEMS Microbiol Lett. 1997;149:1–10. doi: 10.1111/j.1574-6968.1997.tb10300.x. [DOI] [PubMed] [Google Scholar]
- 8.Guidolin A, Morona J K, Morona R, Hansman D, Paton J C. Nucleotide sequence of an operon essential for capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 19F. Infect Immun. 1994;62:5384–5396. doi: 10.1128/iai.62.12.5384-5396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzenellenbogen E, Jennings H J. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 19A (57) Carbohydr Res. 1983;124:235–245. doi: 10.1016/0008-6215(83)88459-6. [DOI] [PubMed] [Google Scholar]
- 11.Klee S R, Tzschaschel B D, Timmis K N, Guzman C A. Influence of different rol gene products on the chain length of Shigella dysenteriae type 1 lipopolysaccharide O antigen expressed by Shigella flexneri carrier strains. J Bacteriol. 1997;179:2421–2425. doi: 10.1128/jb.179.7.2421-2425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolkman M A B, Morrison D A, van der Zeijst B A M, Nuijten P J M. The capsule polysaccharide synthesis locus of Streptococcus pneumoniae serotype 14: identification of the glycosyl transferase gene cps14E. J Bacteriol. 1996;178:3736–3741. doi: 10.1128/jb.178.13.3736-3741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolkman M A B, van der Zeijst B A M, Nuijten P J M. Functional analysis of glycosyltransferases encoded by the capsular polysaccharide biosynthesis locus of Streptococcus pneumoniae serotype 14. J Biol Chem. 1997;272:19502–19508. doi: 10.1074/jbc.272.31.19502. [DOI] [PubMed] [Google Scholar]
- 14.Kolkman M A B, van der Zeijst B A M, Nuijten P J M. Diversity of capsular polysaccharide synthesis gene clusters in Streptococcus pneumoniae. J Biochem (Tokyo) 1998;123:937–945. doi: 10.1093/oxfordjournals.jbchem.a022028. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Comput Appl Biosci. 1994;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 16.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 16a.Morona, J. K. Unpublished observations.
- 17.Morona J K, Morona R, Paton J C. Characterization of the locus encoding the Streptococcus pneumoniae type 19F capsular polysaccharide biosynthetic pathway. Mol Microbiol. 1997;23:751–763. doi: 10.1046/j.1365-2958.1997.2551624.x. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz R, Mollerach M, López R, García E. Molecular organization of the genes required for the synthesis of type 1 capsular polysaccharide of Streptococcus pneumoniae: formation of binary encapsulated pneumococci and identification of cryptic dTDP-rhamnose biosynthesis genes. Mol Microbiol. 1997;25:79–92. doi: 10.1046/j.1365-2958.1997.4341801.x. [DOI] [PubMed] [Google Scholar]
- 19.Nesin M, Ramirez M, Tomasz A. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J Infect Dis. 1998;177:707–713. doi: 10.1086/514242. [DOI] [PubMed] [Google Scholar]
- 20.Ohno N, Yadomae T, Miyazaki T. The structure of the type specific polysaccharide of pneumococcus type XIX. Carbohydr Res. 1980;80:297–304. doi: 10.1016/s0008-6215(00)84638-8. [DOI] [PubMed] [Google Scholar]
- 21.Paton J C, Morona J K. Streptococcus pneumoniae capsular polysaccharide. In: Fischetti V, Novick R, Ferretti J, Portnoy D, Rood J, editors. Gram-positive pathogens, in press. Washington D.C: ASM Press; 1999. [Google Scholar]
- 22.Ramirez M, Tomasz A. Molecular characterization of the complete 23F capsular polysaccharide locus of Streptococcus pneumoniae. J Bacteriol. 1998;180:5273–5278. doi: 10.1128/jb.180.19.5273-5278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamara K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 24.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dam J E G, Fleer A, Snippe H. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Leeuwenhoek. 1990;58:1–47. doi: 10.1007/BF02388078. [DOI] [PubMed] [Google Scholar]