Abstract
Introduction.
Nationally representative studies of the combined impact of drinking and body mass (BMI) on mortality outcomes are unavailable. We investigate whether both act together to elevate risk of all-cause or liver mortality.
Methods.
We obtained self-reported histories of drinking and BMI from 129 098 women (mean age 47.2 years) and 102 568 men (mean age 45.6 years) ≥18 years interviewed from 1997 to 2004 in the National Health Interview Survey and related these data to the deaths that occurred by 31 December 2006 (women = 8486; men = 7819 deaths). Death hazards among current drinkers in different BMI groups were adjusted for age, education, race and smoking.
Results.
Obese (≥30 kg m−2) adults with consumption of >40 g day−1 (women) or >60 g day−1 (men) pure ethanol were at risk of increased mortality from all-cause and chronic liver disease (P trend <0.0001). For heavy drinkers with BMI ≥30 kg m−2, each 5 kg m−2 higher BMI was associated with an elevated all-cause mortality in men (hazard ratios 1.27, 95% confidence interval [CI]: 1.16–1.40) and women (1.12, [1.02–1.24]). The excess risk due to interaction was more pronounced in men (7.30, [3.60–11.00]) than women (2.90, [0.50–5.30]).
Discussion and Conclusions.
Obesity and excess alcohol are both related to all-cause and liver mortality—the latter with evidence of a supra-additive interaction between the risk factors. The presence of both factors in the same population and their impact should inform treatment, public health policies and research.
Keywords: high body mass index, heavy drinking, interaction effect, obesity, National Health Interview Survey
Introduction
Individual habits and behaviours can have a major influence on health, and among the especially damaging behaviours are alcohol use, nutrition and exercise habits that contribute to obesity [1]. Consequently, these risk factors have been included as indicators in the United Nations efforts to reduce premature mortality of non-communicable diseases [2] and in the indicators for Sustainable Development Goals [3]. For the USA, estimates from the Global Burden of Disease 2017 Study suggest that in this year for the population under age 70 years of age, 16.3% of US deaths were caused by high body mass index (BMI) (referred to here as obesity) and 7.5% by alcohol use [4].
One specific cause of death important in this context has been chronic liver disease, in particular liver cirrhosis [5]. This cause of death, closely related to alcohol and BMI, has shown markedly increased mortality rates over the past years and has been identified as one of the main reasons for stagnating life expectancy in North America [6,7].
The present paper aims to examine the impact of the two risk factors—alcohol use and obesity—on all-cause and liver disease mortality. For all-cause mortality, alcohol use has been linked to more than 200 disease and injury conditions [8], but four broad causes of death cover the majority of alcohol-attributable mortality (cancer, cardiovascular disease, chronic liver disease and injury), all with a multitude of different pathways. Obesity is mainly linked to non-communicable disease, in particular cardiovascular disease [1]. While some of the pathways may interact, overall, the effects of both risk factors on all-cause mortality seem to be additive.
As for chronic liver disease, in particular liver cirrhosis, there are established pathways via heavy drinking [9] and obesity [10], but there are indications that both pathways interact [9]. If we use BMI as the indicator for obesity, there have been four longitudinal cohorts studied on a potential interaction in predicting liver hospitalisations or mortality [11–14], all with large sample sizes (up to more than 1.2 million participants in the million women study) [13].
The mechanisms by which alcohol consumption and obesity affect the liver are not completely understood, but biochemical and pathological evidence suggests a presence of a common pathway [15]. One possible mechanism of the synergistic effect of alcohol and obesity could be linked to raised serum alanine aminotransferase and aspartate aminotransferase levels, a marker for liver injury in the general population [16]. Second, studies have suggested that there is an integral role of cytochrome (CYP2E1), a catalytic enzyme, in the pathogenesis of fatty liver disease due to alcohol and obesity [17]. It is well accepted that both obesity and chronic alcohol consumption result in oxidative stress leading to adipokine dys-regulation and subsequent progression of alcoholic liver disease, which may explain the interaction effect of both factors in causing liver injury [18,19]. Third, through its effect of hepatic insulin sensitivity, obesity leads to steatohepatitis and the lipid solubility of alcohol makes adipose tissue a main target for its effects [15]. Contrary, alcoholic fatty liver induces insulin resistance and promotes obesity. This could be mediated through the appetite enhancing effect of ethanol [20] or inability to make up for the extra energy obtained from alcohol by decreasing other food intake [21].
A better understanding of the effect of alcohol consumption and obesity on all-cause, and in particular liver disease, mortality may help physicians and health professionals in treating their patients and establishing strategies to reduce the burden. We aim to evaluate whether alcohol and obesity interact to increase the risk of mortality, based on a large cohort study of US adults.
Methods
Study design
We examined data from a cohort of 242 397 adults in the US National Health Interview Survey (NHIS) between 1997 and 2004 [22,23] that were linked to the National Death Index [24,25]. The NHIS is a cross-sectional nationally representative health survey of the civilian, noninstitutionalised population of the USA. The survey uses a stratified and multistage sample design and allows representative sampling of the households. One adult (18 years or older) is randomly selected from each household for a detailed interview on socio-demographic and health behaviours. The NHIS samples were drawn from each of the 50 states and the District of Columbia. Response rates were in the range of 87% in 2004 to 92% in 1997.
Each year, approximately 35 000 households and 87 500 persons are enrolled in the survey. Black and Hispanic population were deliberately oversampled, but the sample weights ensure that the final totals conform to national ethnic proportions. The NHIS sampling frame excludes only approximately 7 million adults (primarily patients in long-term care, prisons and active-duty military facilities) from the total US domestic population of 226 million adults in 2004. Mortality among survey participants until 31 December 2006 was assessed by means of periodic matching of their records to the National Death Index [24,25], which includes information from death-certificates for all deaths in the USA since 1986. Matching of records was performed for a combination of name, date of birth and Social Security number with a success rate over 95%.
Rates of enrolment for women exceeded those for men. A total of 136 808 women and 105 589 men aged 18 years or older participated in the NHIS between 1997 and 2004. Of these participants, 7710 women (561 of whom died) and 3021 men (273 of whom died) were excluded because of missing data (e.g. educational level, smoking status, drinking status, BMI status or cause of death). We ensured NHIS’ complex sample survey design was taken into account prior to analysis. Additional details about the survey can be obtained elsewhere [26].
Statistical analysis
Information on height, weight, drinking alcohol, smoking, education and race was self-reported in the survey. BMI (kg m−2) was calculated from the weight in kilograms divided by the square of the height in metres. Drinking status was divided into lifetime abstainer, former drinker and current drinker. Lifetime abstainers were those who reported not having 12+ drinks in any year in their lifetime. Former drinkers were those who reported lifetime drinking of at least 12+ drinks in any year but not drinking in the past year. Current drinkers are those who drink at least one drink in the past year. The amount of pure ethanol in a standard drink across all types of alcoholic beverages was approximately 14 g. Drinking frequency and average number of drinks per drinking day were converted into average daily consumption of ethanol in grams per day (up to 20, >20 to 40, >40 to 60 and >60 g day−1). We calculated hazard ratios for drinkers in low and high BMI groups (<23.5 kg m−2 defined as weight category I lower BMI, 23.5 to 29.9 kg m−2 defined as weight category II higher, served as reference, or ≥30 as obese) with the use of an sex-stratified Cox proportional hazards model [27], adjusted for educational level (less than high school, equal to high school, or more than high school), race (White, Black or Hispanic and other) and smoking tobacco (non-smoker, former smoker or current smoker). Proportional hazards assumption was assessed through Schoenfeld residuals. As a sensitivity analysis, Cox regression using multivariable fractional polynomial [28] was used with BMI ≥25 to fit the risk curves on a continuous scale. Overall hazard ratios (HR) for all cause and chronic liver diseases excluding deaths in the first two years of follow-up were also analysed.
To investigate any supra-additive interaction [see also; 29,30] between BMI and alcohol consumption, the relative excess risks due to interaction (RERI) and the synergy index (SI) were obtained, using the methodology described in Andersson et al [31]. RERI indicates the extent to which the relative excess risk, when both factors are present, is greater than the sum of the relative excess risks for each of the factors individually. The SI is the ratio of the combined effects and the individual effects. We hypothesised that the combined effect of excess alcohol consumption and obesity would outweigh the simple additive effects of each factor separately and would be indicated by a RERI value greater than zero and an SI greater than one. For this analysis, obesity was defined as BMI ≥30 kg m−2 (with 23.5 to 29.9 as reference) and excess alcohol drinking [32,33] was defined as drinking >60 g pure alcohol/day for men and >40 g day−1 for women. P-values were calculated using two-sided tests. All estimates were weighted according to the NHIS sample weights [24]. All analyses were performed in stata SE (Release 14) [34].
Results
Characteristics of the study participants
Among 129 098 women and 102 568 men, aged 18 years or older, who were followed for a mean of 7 years (1.5 million person-years), a total of 16 305 deaths (8486 deaths in women and 7819 in men) were recorded (see Table 1). Chronic liver diseases constituted 1.3% of all deaths (76 women and 135 men) (See Table S1, Supporting Information, for details). The proportions of lower weight and obesity were 29.9% and 22.6%, respectively. Overweight people (BMI ≥30 kg m−2) were more likely to be of Black ethnicity, socially more stable, had more pre-existing conditions and more commonly drank alcohol; lower weight people (BMI < 23.5 kg m−2) were more likely to be younger, lived without family, had lower education levels and more commonly smoked tobacco. The proportion of overweight people increased with age until age 60, whereas lower weight people increased with age after age 60 (see Figure 1).
Table 1.
Baseline characteristics of 231 666 adult US men and women according to BMI categoriesa
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| BMI (kg m−2) |
BMI (kg m−2) |
|||||||
| Characteristics | Weight category I lower: <23.5 | Weight category II higher: 23.5–29.9 | Obese: ≥30.0 | Overall | Weight category I lower: <23.5 | Weight category II higher: 23.5–29.9 | Obese: ≥30.0 | Overall |
| Number of participants surveyed | 21 568 | 58 586 | 22 414 | 102 568 | 47 622 | 51 448 | 30 028 | 129 098 |
| Deaths due to all causes | 2335 | 4012 | 1472 | 7819 | 3432 | 3290 | 1764 | 8486 |
| Deaths due to liver diseases | 35 | 74 | 26 | 135 | 26 | 23 | 27 | 76 |
| Mean (SD) age at baseline, years | 43.2 (19.6) | 46.2 (16.8) | 46.1 (15.2) | 45.6 (17.2) | 44.6 (19.3) | 49.2 (18.3) | 48.0 (16.6) | 47.2 (18.4) |
| Mean (SD) weight, kg | 69.2 (6.2) | 83.3 (8.6) | 104.2 (11.0) | 84.7 (14.3) | 57 (5.8) | 69.9 (7.3) | 89.7 (11.0) | 69.4 (14.4) |
| Marital status (%) b | ||||||||
| Married or living with partner | 16.7 (9973) | 59.1 (35159) | 24.2 (14222) | 100 (59354) | 38.1 (23345) | 40.5 (25907) | 21.3 (13837) | 100 (63089) |
| Unmarried, widowed or separated | 29.5 (11595) | 51.9 (23427) | 18.5 (8192) | 100 (43214) | 39.6 (24277) | 37.0 (25541) | 23.3 (16191) | 100 (66009) |
| Race (%) | ||||||||
| White | 20.3 (14632) | 57.6 (40684) | 22.1 (15007) | 100 (70323) | 41.5 (34864) | 38.5 (32722) | 20.0 (17088) | 100 (84656) |
| Black | 21.1 (2556) | 52.1 (6438) | 26.7 (3132) | 100 (12126) | 23.0 (4395) | 40.8 (7998) | 36.2 (7084) | 100 (19477) |
| Other | 23.7 (4380) | 55.7 (11464) | 20.6 (4275) | 100 (20119) | 37.6 (8381) | 41.4 (10728) | 20.9 (5856) | 100 (24965) |
| Educational level (%) b | ||||||||
| <High school graduate | 25.0 (4113) | 51.6 (9356) | 23.4 (4021) | 100 (17490) | 29.1 (6318) | 42.1 (10131) | 28.8 (6909) | 100 (23358) |
| High school graduate | 24.7 (550) | 51.1 (1312) | 24.2 (569) | 100 (2431) | 34.4 (994) | 41.6 (1340) | 23.9 (784) | 100 (3118) |
| >High school graduate | 20.1 (16905) | 57.8 (47918) | 22.1(17824) | 100 (82647) | 40.5 (40310) | 38.6 (39977) | 20.8 (22335) | 100 (102662) |
| Alcohol consumption (%) b,c | ||||||||
| Lifetime abstainer | 27.1 (3772) | 52.2 (7911) | 20.7 (3007) | 100 (14690) | 35.6 (12940) | 40.1 (15966) | 24.3 (9859) | 100 (38765) |
| Formerly drank | 18.6 (3207) | 55.0 (9037) | 26.4 (4236) | 100 (16480) | 30.9 (5953) | 39.6 (7923) | 29.5 (6000) | 100 (19876) |
| Current drinkerd | 20.4 (14589) | 58.3 (41638) | 21.2 (15171) | 100 (71398) | 40.8 (28729) | 39.1 (27559) | 20.1 (14169) | 100 (70457) |
| Up to 20 g day−1 | 19.6 (11704) | 58.2 (34296) | 22.2 (12709) | 100 (58709) | 42.0 (26913) | 38.6 (26003) | 19.3 (13580) | 100 (66496) |
| 20–40 g day− | 21.0 (1462) | 59.9 (4141) | 19.1 (1296) | 100 (6899) | 49.3 (1069) | 39.2 (880) | 11.5 (277) | 100 (2226) |
| >40–60 g day−1 | 23.4 (612) | 57.2 (1502) | 19.4 (489) | 100 (2603) | 49.9 (274) | 37.7 (205) | 12.4 (78) | 100 (557) |
| >60 g day−1 | 28.2 (479) | 49.9 (878) | 21.9 (371) | 100 (1728) | 37.5 (108) | 39.3 (105) | 23.2 (64) | 100 (277) |
| Smoking status (%) b | ||||||||
| Current smoking | 27.4 (7450) | 53.6 (14801) | 18.9 (5019) | 100 (27270) | 43.1 (11350) | 37.6 (10405) | 19.3 (5595) | 100 (27350) |
| Formerly smoked | 14.8 (4356) | 59.1 (16345) | 26.1 (7040) | 100 (27741) | 34.3 (7952) | 40.7 (9817) | 25.0 (6271) | 100 (24040) |
| Never smoked | 20.8 (9762) | 57.1 (27440) | 22.1 (10355) | 100 (47557) | 38.6 (28320) | 39.3 (31226) | 22.1 (18162) | 100 (77708) |
| Pre-existing chronic disease (%) b,e | ||||||||
| Diabetes mellitus | 16.3 (139) | 51.5 (388) | 32.2 (226) | 100 (753) | 25.0 (197) | 38.0 (334) | 37.0 (337) | 100 (868) |
| Hypertension | 24.1 (174) | 50.1 (359) | 25.8 (170) | 100 (703) | 38.0 (413) | 41.0 (466) | 21.0 (234) | 100 (1113) |
Women and men who were 18 years of age or older were included in the study cohort. This analysis excluded 1614 men (2%) and 5769 women (5%) for whom data on variables characteristics were missing; percentages reported (row) may not total 100 because of rounding or missing data.
Prevalence (expressed as percentages) was weighted by the National Health Interview Survey weights.
Lifetime abstainers were those who reported not having 12+ drinks in any year in their lifetime. Formerly drank were those who reported lifetime drinking of at least 12+ drinks in any year but not having drunk in the past year.
Current drinkers are those who drink at least one drink in the past year.
The chronic diseases are self-reported. BMI, body mass index.
Figure 1.
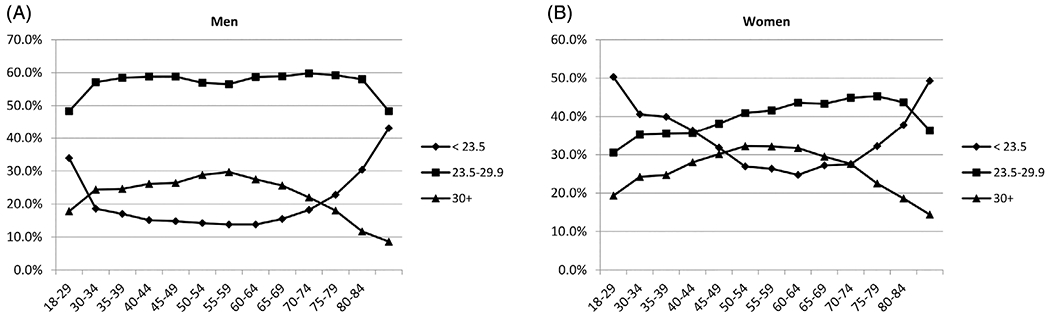
The proportions of NHIS participants who were lower weight (category I: BMI <23.5 kg m−2), higher weight (category II: 23.5–29.9 kg m−2) and obese (BMI ≥30 kg m−2) are shown for men and for women.
Impact of alcohol consumption and BMI on all-cause and liver mortality
Higher levels of daily alcohol consumption were strongly associated with increased all-cause and liver mortality. When past drinkers or people with pre-existing conditions relevant to the outcomes were excluded, the HRs for all-cause mortality associated with increasing alcohol consumption strengthened, rather than weakened (P for trend <0.0001; see Table 2). Similarly, strong dose–response associations were observed when analysis was restricted to the obese population (BMI ≥30 kg m−2) in both all-cause and liver mortality (for details of all-cause see Table S2, Supporting Information). Obese women, in particular, showed eightfold risk at 5 drinks per day as opposed to obese men little under fivefold (Figure 2, see also Appendix S1, Supporting Information, for risk functions). Alcoholic liver mortality, in particular, was notable with an increase in alcohol dose where hazards were manifold (P for trend = 0.0145). Analyses excluding deaths in the first two years of follow-up data yielded results similar to those reported here (see Table S3, Supporting Information). A stratified analysis of obese drinkers by age at baseline showed HRs per 5 kg increment were inversely associated with age (Figure 3); and men [1.27, 95% confidence interval (CI) 1.16–1.40] were more vulnerable than women (1.12, 95% CI 1.02–1.24). Findings were contrary for drinkers in the BMI <30 kg m−2 group (Table S4, Supporting Information).
Table 2.
Adjusteda hazard ratios (95% CI) for all-cause and chronic liver disease mortality according to BMI by daily alcohol consumption
| All |
Weight category Ib |
Obeseb |
||||||
|---|---|---|---|---|---|---|---|---|
| COD (ICD-10) | Alcohol consumption | No. of deaths | Overallc | Overall (pre-existing conditions and former drinkers removed) | Lower BMI <23.5 BMI kg m−2 | Lower BMI (pre-existing conditions and former drinkers removed) | Obese ≥30 BMI kg m−2 | Obese (pre-existing conditions and former drinkers removed) |
| All causes | Lifetime abstainer | 4825 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Former | 5019 | 1.09 (1.05–1.14) | (..) | 1.06 (1.01–1.11) | (..) | 1.25 (1.12–1.39) | (..) | |
| Up to 20 g day−1 | 5214 | 0.71 (0.68–0.74) | 0.79 (0.75–0.83) | 0.72 (0.69–0.77) | 0.76 (0.71–0.80) | 0.80 (0.71–0.89) | 0.81 (0.72–0.91) | |
| >20–40 g day−1 | 566 | 0.83 (0.76–0.91) | 1.03 (0.93–1.14) | 0.85 (0.77–0.94) | 0.91 (0.82–1.02) | 0.99 (0.75–1.29) | 1.0 (0.76–1.31) | |
| >40–60 g day−1 | 281 | 1.05 (1.0–1.19) | 1.40 (1.23–1.60) | 1.10 (1.0–1.25) | 1.16 (1.01–1.34) | 1.52 (1.06–2.18) | 1.55 (1.08–2.22) | |
| >60 g day−1 | 191 | 1.42 (1.23–1.65) | 1.86 (1.58–2.18) | 1.47 (1.24–1.74) | 1.54 (1.29–1.84) | 1.48 (1.0–2.28) | 1.50 (1.0–2.31) | |
| Test for trend | χ2 | 3209.8 | 4266.4 | 491.5 | ||||
| P-value | <0.0001 | <0.0001 | <0.0001 | |||||
| Chronic liver disease and cirrhosis (K70, K73-K74) | Lifetime abstainer | 37 | 1.00 | 1.00 | 1.00 | |||
| Former | 42 | 1.22 (0.77–1.94) | 1.54 (0.79–2.99) | 1.08 (0.49–2.38) | ||||
| Up to 20 g day−1 | 61 | 0.77 (0.49–1.19) | 1.50 (0.83–2.73) | 0.40 (0.16–0.97) | ||||
| >20–40 g day−1 | 20 | 2.55 (1.41–4.63) | 3.54 (1.61–7.79) | 4.11 (1.38–12.22) | ||||
| >40–60 g day−1 | 17 | 5.41 (2.89–10.14) | 8.27 (3.74–18.33) | 4.08 (0.83–19.93) | ||||
| >60 g day−1 | 22 | 10.25 (5.64–18.62) | 14.89 (6.86–32.29) | 12.33 (3.85–39.46) | ||||
| Test for trend | χ2 | 319.9 | 319.9 | 63.0 | ||||
| P-value | <0.001 | <0.001 | 0.1930 | |||||
| Alcoholic liver disease (K70) | Lifetime abstainer | 7 | 1.00 | 1.00 | 1.00 | |||
| Former | 12 | 1.71 (0.65–4.49) | 2.48 (0.75–8.18) | 0.61 (0.05–7.18) | ||||
| Up to 20 g day−1 | 25 | 1.43 (0.60–3.46) | 2.07 (0.68–6.31) | 1.12 (0.18–6.94) | ||||
| >20–40 g day−1 | 13 | 6.60 (2.44–17.81) | 7.41 (2.11–25.98) | 9.65 (1.23–75.48) | ||||
| >40–60 g day−1 | 9 | 10.77 (3.72–31.21) | 9.19 (2.29–36.89) | 17.92 (1.92–167.57) | ||||
| >60 g day−1 | 17 | 26.39 (9.92–70.17) | 26.20 (7.55–90.93) | 51.26 (7.38–356.24) | ||||
| Test for trend | χ2 | 385.9 | 13.2 | 148.8 | ||||
| P-value | 0.0003 | 0.0003 | 0.0145 | |||||
Adjusted for age at baseline, sex, education, race/ethnicity and smoking status.
Weight category I: Higher BMI 23.5–29.9 kg m−2 served as reference (due to relatively smaller number of deaths, analyses following removal of pre-existing conditions and former drinkers were not performed for liver disease mortality).
Evaluation of proportional hazards assumption was based on Schoenfeld residuals. Based on the global test of the overall model (all-cause mortality) with χ2 value of 14.98 (df = 14) and P-value = 0.3796, we would not reject the null hypothesis meaning the hazards are in fact proportional for this model. Similar observations were noted for liver mortality (χ2 = 12.54, df = 14, P = 0.5629).
BMI, body mass index; CI, confidence interval; COD, cause of death; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th revision.
Figure 2.
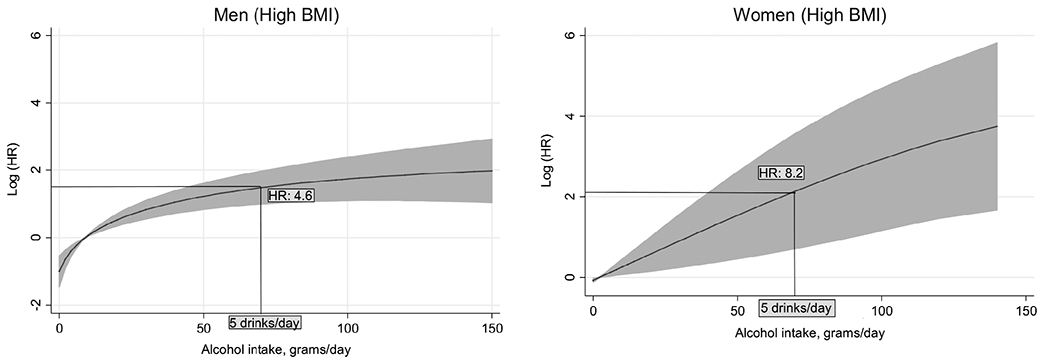
Multivariable adjusted* hazard ratios (95% CI) of chronic liver disease mortality by daily alcohol intake (grams/day) in obesity.
*Adjusted for age at baseline, education, race/ethnicity and smoking status.
Figure 3.
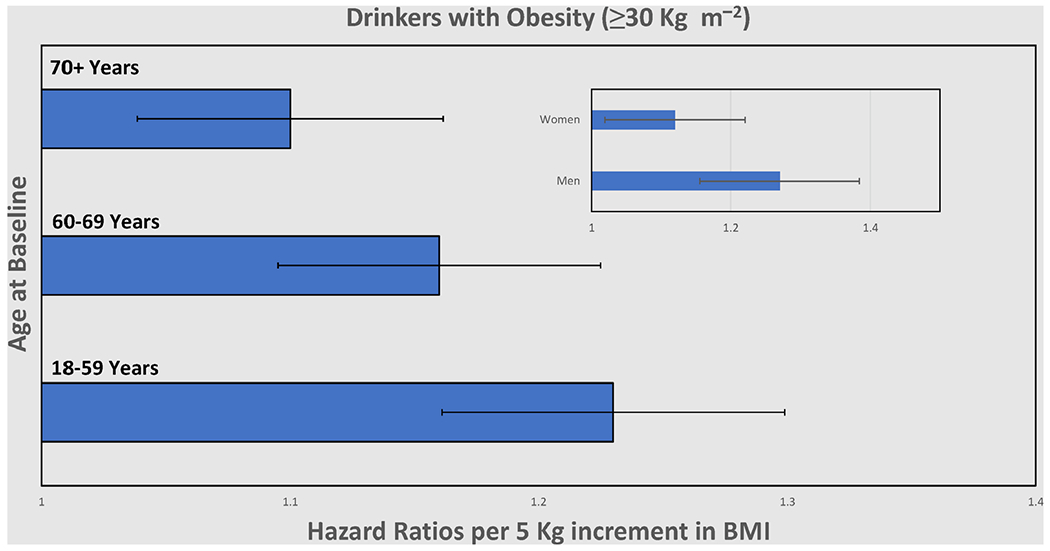
All-cause mortality of drinkers with obesity (≥30 kg m−2) at different age at baseline. Hazard ratio per 5 kg increment in BMI. All analyses adjust for age at baseline, sex, education, race/ethnicity and smoking status.
Interaction effect of obesity and heavy drinking on liver and all-cause mortality
We found evidence of a supra-additive interaction between obesity and heavy drinking of alcohol on liver mortality. Figure 4 shows, by sex, the excess risks due to obesity, heavy alcohol consumption, and their interaction in an analysis of liver disease mortality adjusted for all risk factors. The excess risk due to obesity was relatively small and significant only for women (men 0.93, 95% CI 0.44–1.95; women 3.39, 95% CI 1.62–7.10) compared with that due to alcohol (men 8.09, 95% CI 3.43–19.10; women 5.41, 95% CI 2.29–10.77). As for interaction indices, both were larger in men than women (RERI: men 7.30, 95% CI 3.60–11.00; women 2.90, 95% CI 0.50–5.30; SI: men 2.04, 95% CI 1.27 to 3.27; women 1.43, 95% CI 1.01–2.05). In other words: the effect of the combination of obesity and heavy drinking of alcohol was larger than the additive effect of the two separately; being both overweight or obese and consuming over 60 g day−1 (40 g day−1 in women) of alcohol led to a greater risk of dying of chronic liver disease and cirrhosis. In contrast, the interaction analysis on all-cause mortality did not show any significant excess risk (Table S5, Supporting Information).
Figure 4.
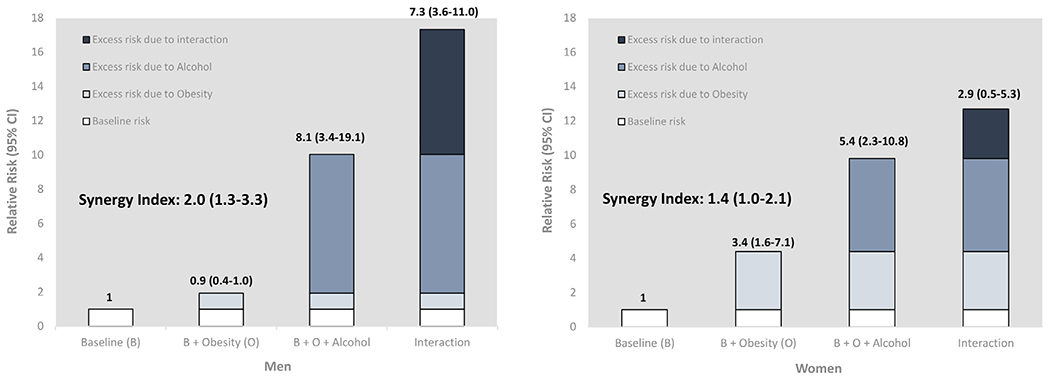
Relative risks of contribution of obesity and excess drinking of alcohol to liver disease mortality. Note: Obesity was defined as ≥30 BMI kg m−2 (BMI 23.5–29.9 kg m−2 was assigned a reference for this analysis). Lifetime abstention, as reference to high-risk drinking, is defined as having less than 12 drinks in entire life. Excess drinking was defined as drinking alcohol >60 g day−1 for men (>40 g day−1 for women). Adjusted for age at baseline, sex, education, race/ethnicity and smoking status. Delta method was used to calculate uncertainty around interaction indices (covariance matrix of main and interaction variables were obtained from overall sample size). BMI, body mass index; RR, relative risk.
Discussion
In this large, prospective, nationally representative study, we investigated the joint effects of obesity and heavy alcohol consumption on all-cause and liver mortality. We noted the impact of both risk factors on these outcomes and a supra-additive interaction effect on chronic liver disease and cirrhosis deaths—suggesting the combination of obesity and heavy alcohol consumption is greater than the additive effect of these two factors separately.
Findings in relation to other studies
Our findings on the association between obesity and liver cirrhosis mortality are largely consistent with previous prospective studies across sex [12,13,35,36], but the magnitude of the risk was relatively higher. It could be due to the fact that our study uniquely examined mortality data unlike other studies [12,13,36] that examined hospital admission and death, altogether. An Australian cohort study [37] found that obese individuals with 40 g+ day−1 alcohol intake had abnormally higher level of serum liver enzymes and obesity accounted for half of the raised level and alcohol accounted approximately 10%. Contrary to this, we observed that among obese participants who were excess drinkers, alcohol accounted for over 47% (8.1/17.3) of the total effect on mortality in men; and obesity accounted for 27% (3.4/12.7) of the total effect on mortality in women (see Figure 4). This difference could be attributed to differences in study design and settings between countries. Consistent with the Million Women Study [13] and Prospective Studies Collaboration [35], we also observed an increased risk of liver mortality with higher alcohol dose (40 g day−1 or more) among individuals in the lower BMI category (Table 2). Interpreting the relevance of the association between lower BMI and cirrhosis is difficult, but it is likely that early liver disease may trigger reduced appetite or cause malabsorption.
Strengths and limitations
Our study has some limitations. First, there could be some unknown confounding factors other than the variables recorded in the NHIS. Second, the NHIS excludes the prison population (who tend to have an increased prevalence of drinking [38]). This should not materially affect the observed differences between current drinkers (or their body mass) and those who never drank, among the adults surveyed in the NHIS. Third, the number of deaths in our study was lower than that noted in other studies [39], but this is offset by the fact that the surveyed individuals in this study are representative of the US population [24]. Fourth, misclassification of the causes of death, including hospital death certificates, particularly of older patients [40,41], might affect the observed hazards for liver disease mortality, although this may not affect our analysis on all-cause risks. Fifth, data on self-reported alcohol consumption and body mass might have been underestimates of their true values. Since these data were collected only at baseline, some of the surveyed drinkers may have subsequently quit or their body mass may have changed, thereby somewhat reducing their risk, but, with 7 years of follow-up, any distortion of the hazard ratios might be insignificant. Similarly, the excess mortality among former drinkers might be overestimated due to reverse-causality (i.e. the sick-quitters effect) [42]. To address this issue, we explored analyses excluding deaths in the first 2 years of follow-up data, which did not materially change the results reported here (data not shown). However, a single measurement of the exposure at baseline may lead to regression dilution bias, thus leading to an underestimate of the real relationships [43]. Lastly, it is possible that deaths may have been slightly underestimated due to incomplete matching of records to the National Death Index.
Conclusion
Our findings have very important research, clinical and public health implications. It reiterates that alcohol use should be considered in clinical practice for all diagnoses of chronic liver disease, irrespective of whether they have been labelled as alcoholic liver cirrhosis or not [44]. In fact, the classification of liver disease into distinct categories—such as alcoholic—may hinder both clinical practice and research [45]. Moreover, our findings raise the question of whether there are thresholds for alcohol use and its impact on liver disease, or whether there is an impact of alcohol use below the level of heavy drinking, as usually defined [9]. If the latter is the case, there may also be interactions with other risk factors. In terms of public health implications, we need to be aware of the population health implications of current trends of increased alcohol consumption [46] and BMI [47], not only visible at the global level, but also in many countries, including the USA [48,49]. These trends likely have contributed to the increase in chronic liver deaths in the USA. At least for alcohol, there are proven effective and cost-effective policies to reverse such trends [50]. While such policies should be implemented, as they also reduce any interaction with other risk factors, better measures to reduce the trend of increasing obesity should be developed.
Supplementary Material
Table S1. All-cause and cause-specific deaths by BMI categories among US adults 18 years and older in National Health Interview Survey, 1997-2004.
Table S2. Adjusted hazard ratios (95% confidence interval) of all-cause and cause-specific deaths in relation to BMI for men and all women (18 years and older)*.
Appendix S1. Univariate risk functions to plot predicted probabilities of dying due to liver cirrhosis (in log scale).
Table S3. Adjusted* hazard ratios (95% confidence interval) for all-cause and chronic liver disease mortality by daily alcohol consumption following exclusion of first two years of follow-up data.
Table S4. All-cause mortality among current drinkers in low and high body mass index.
Table S5. Interaction effect of excess drinking† and obesity on all-cause and chronic liver disease and cirrhosis mortality.
Acknowledgements
This research was conducted as part of the Calibrated Agent Simulations for Combined Analysis of Drinking Etiologies project and the authors would like to thank the whole team for their input to wider discussions in generating the research reported in this paper. This study was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (R01AA024443).
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
References
- [1].GBD 2017 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization. NCD Global Monitoring Framework: Ensuring progress on noncommunicable diseases in countries; 2017. Available at: http://www.who.int/nmh/global_monitoring_framework/en/ (accessed 23 March 2020).
- [3].United Nations. Sustainable development goals: 17 goals to transform our world; 2017. Available at: http://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed 7 October 2018).
- [4].Institute for Health Metrics and Evaluation (IHME). GBD Compare:Viz Hub; 2020. Available at: https://vizhub.healthdata.org/gbd-compare/ (accessed 6 March 2020).
- [5].Lopez AD, Williams TN, Levin A et al. Remembering the forgotten noncommunicable diseases. BMC Med 2014;12:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Case A, Deaton A. Mortality and morbidity in the 21(st) century. Brookings Pap Econ Act 2017;2017:397–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rehm J, Probst C. Decreases of life expectancy despite decreases in non-communicable disease mortality: The role of substance use and socioeconomic status. Eur Addict Res 2018;24:53–9. [DOI] [PubMed] [Google Scholar]
- [8].Rehm J, Gmel GE Sr, Gmel G et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017;112:968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roerecke M, Vafaei A, Hasan OSM et al. Alcohol consumption and risk of liver cirrhosis: A systematic review and meta-analysis. Am J Gastroenterol 2019;114:1574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010;51:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Åberg F, Färkkilä M, Männistö V. Interaction Between Alcohol Use and Metabolic Risk Factors for Liver Disease: A Critical Review of Epidemiological Studies. Alcohol Clin Exp Res 2020;44:384–403. [DOI] [PubMed] [Google Scholar]
- [12].Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey SG. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu B, Balkwill A, Reeves G, Beral V, Million Women Study C. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ 2010;340:c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yi SW, Hong JS, Yi JJ, Ohrr H. Impact of alcohol consumption and body mass index on mortality from nonneoplastic liver diseases, upper aerodigestive tract cancers, and alcohol use disorders in Korean older middle-aged men: Prospective cohort study. Medicine (Baltimore) 2016;95:e4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diehl AM. Obesity and alcoholic liver disease. Alcohol 2004;34:81–7. [DOI] [PubMed] [Google Scholar]
- [16].Loomba R, Bettencourt R, Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment Pharmacol Ther 2009;30:1137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci 2007;32:453–68. [DOI] [PubMed] [Google Scholar]
- [18].Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004;34:9–19. [DOI] [PubMed] [Google Scholar]
- [19].Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 2008;44:723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Westerterp-Plantenga MS, Verwegen CR. The appetizing effect of an apéritif in overweight and normal-weight humans. Am J Clin Nutr 1999;69:205–12. [DOI] [PubMed] [Google Scholar]
- [21].Tremblay A, Wouters E, Wenker M, St-Pierre S, Bouchard C, Després JP. Alcohol and a high-fat diet: a combination favoring overfeeding. Am J Clin Nutr 1995;62:639–44. [DOI] [PubMed] [Google Scholar]
- [22].Mehta N, Preston S. Continued increases in the relative risk of death from smoking. Am J Public Health 2012;102:2181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rostron B. Smoking-attributable mortality in the United States. Epidemiology 2011;22:350–5. [DOI] [PubMed] [Google Scholar]
- [24].Ingram DD, Lochner KA, Cox CS. Mortality experience of the 1986-2000 National Health Interview Survey Linked Mortality Files participants. Vital Health Stat 2 2008;147:1–37. [PubMed] [Google Scholar]
- [25].National Center for Health Statistics. Office of Analysis and Epidemiology. The National Health Interview Survey (1986-2004) Linked Mortality Files, mortality follow-up through 2006: Matching Methodology; 2009. Available at: http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhis_final.pdf (accessed 20 March 2020).
- [26].Blewett LA, Rivera Drew JA, King ML, Williams KCW. IPUMS Health Surveys: National Health Interview Survey, Version 6.4 [dataset]; 2019. Available at: 10.18128/D070.V6.4; https://www.nhis.ipums.org (accessed 24 March 2020). [DOI]
- [27].Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72. [DOI] [PubMed] [Google Scholar]
- [28].Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: Parsimonious parametric modelling. J R Stat Soc Ser C Appl Stat 1994;43:429–53. [Google Scholar]
- [29].Rothman KJ. Epidemiology: an introduction. Oxford: Oxford University Press, 2002. [Google Scholar]
- [30].de Mutsert R, Jager KJ, Zoccali C, Dekker FW. The effect of joint exposures: examining the presence of interaction. Kidney Int 2009;75:677–81. [DOI] [PubMed] [Google Scholar]
- [31].Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–9. [DOI] [PubMed] [Google Scholar]
- [32].European Medicines Agency. Guideline on the development of medicinal products for the treatment of alcohol dependence. London: European Medicines Agency, 2010. Available at:. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-medicinal-products-treatment-alcohol-dependence_en.pdf (accessed 18 December 2020). [Google Scholar]
- [33].World Health Organization. International guide for monitoring alcohol consumption and related harm; 2000. Available at: https://apps.who.int/iris/handle/10665/66529 (accessed 24 September 2018).
- [34].StataCorp. Stata Statistical Software: Release, 14th edn. StataCorp LP: College Station, 2015. [Google Scholar]
- [35].Prospective Studies C. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ioannou GN, Weiss NS, Boyko EJ et al. Is central obesity associated with cirrhosis-related death or hospitalization? A population-based, cohort study. Clin Gastroenterol Hepatol 2005;3:67–74. [DOI] [PubMed] [Google Scholar]
- [37].Adams LA, Knuiman MW, Divitini ML, Olynyk JK. Body mass index is a stronger predictor of alanine aminotransaminase levels than alcohol consumption. J Gastroenterol Hepatol 2008;23:1089–93. [DOI] [PubMed] [Google Scholar]
- [38].Compton WM, Dawson D, Duffy SQ, Grant BF. The effect of inmate populations on estimates of DSM-IV alcohol and drug use disorders in the United States. Am J Psychiatry 2010;167:473–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Thun MJ, Carter BD, Feskanich D et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013;368:351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rampatige R, Mikkelsen L, Hernandez B, Riley I, Lopez A. Systematic review of hospital based cause of death statistics: strengthening evidence for policy. Bull World Health Organ 2014;92:807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mieno MN, Tanaka N, Arai T et al. Accuracy of death certificates and assessment of factors for misclassification of underlying cause of death. J Epidemiol 2016;26:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet 1988;332:1267–73. [DOI] [PubMed] [Google Scholar]
- [43].Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
- [44].European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- [45].Lange S, Roerecke M, Rehm J. For most fully alcohol-attributable diagnoses in the ICD, the etiological specification should be removed. Adicciones 2020;32:90–3. [DOI] [PubMed] [Google Scholar]
- [46].Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 2019;393:2493–502. [DOI] [PubMed] [Google Scholar]
- [47].NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 populationbased measurement studies in128· 9 million children, adolescents, and adults. Lancet 2017;390:2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dawson DA, Goldstein RB, Saha TD, Grant BF. Changes in alcohol consumption: United States, 2001–2002 to 2012–2013. Drug Alcohol Depend 2015;148:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Inoue Y, Qin B, Poti J, Sokol R, Gordon-Larsen P. Epidemiology of obesity in adults: latest trends. Curr Obes Rep 2018;7:276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chisholm D, Moro D, Bertram M et al. Are the “Best Buys” for alcohol control still valid? An update on the comparative cost-effectiveness of alcohol control strategies at the global level. J Stud Alcohol Drugs 2018;79:514–22. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. All-cause and cause-specific deaths by BMI categories among US adults 18 years and older in National Health Interview Survey, 1997-2004.
Table S2. Adjusted hazard ratios (95% confidence interval) of all-cause and cause-specific deaths in relation to BMI for men and all women (18 years and older)*.
Appendix S1. Univariate risk functions to plot predicted probabilities of dying due to liver cirrhosis (in log scale).
Table S3. Adjusted* hazard ratios (95% confidence interval) for all-cause and chronic liver disease mortality by daily alcohol consumption following exclusion of first two years of follow-up data.
Table S4. All-cause mortality among current drinkers in low and high body mass index.
Table S5. Interaction effect of excess drinking† and obesity on all-cause and chronic liver disease and cirrhosis mortality.


