Abstract
While much has been learned regarding the genetic basis of host-pathogen interactions, less is known about the molecular basis of a pathogen’s survival in the environment. Biofilm formation on abiotic surfaces represents a survival strategy utilized by many microbes. Here it is shown that Vibrio cholerae El Tor does not use the virulence-associated toxin-coregulated pilus to form biofilms on borosilicate but rather uses the mannose-sensitive hemagglutinin (MSHA) pilus, which plays no role in pathogenicity. In contrast, attachment of V. cholerae to chitin is shown to be independent of the MSHA pilus, suggesting divergent pathways for biofilm formation on nutritive and nonnutritive abiotic surfaces.
The genetic basis of colonization of biotic surfaces by bacterial pathogens has been intensively investigated in the context of host-pathogen interactions. In contrast, genetic determinants of survival of bacterial pathogens outside a host have been less well scrutinized. One strategy which is important in environmental survival of many bacteria is entry into any one of a variety of dormant states (3, 8, 14). An alternative survival strategy is the formation of surface-attached bacterial communities known as biofilms. In this note, we begin to address the role of biofilm formation in survival in a nutrient-limited environment. Vibrio cholerae is an ideal pathogen for a study of environmental survival because of its ability to survive outside the human host for long periods of time.
Vibrio cholerae is the causative agent of cholera, a human diarrheal disease that rapidly leads to dehydration and sometimes, death. Cholera is characterized epidemiologically by the ability to cause pandemics. In order to cause pandemics, which begin with ingestion of contaminated, poorly cooked seafood and are propagated through ingestion of fecally contaminated drinking water (7), pathogenic strains of V. cholerae must be able to survive in both marine and freshwater aquatic environments for extended periods of time. Environmental studies have shown that attachment to the surfaces of zooplankton, crustaceans, insects, and water plants is an integral part of V. cholerae’s aquatic lifestyle (10, 20, 22). Biofilm formation, therefore, may be an important mechanism used by V. cholerae to survive in the environment.
Biofilms are microbial communities formed by initial attachment to a surface, followed by cell-cell interactions to form multiple layers and development of three-dimensional structures, including water channels through which nutrients diffuse in and waste products diffuse out (5). In aquatic environments, diverse surfaces are available for formation of a biofilm. These surfaces include suspended mineral particulates, of which negatively charged silicates are a major component; plants, whose surface includes organic polymers such as cellulose; and the exoskeletons of crustaceans and zooplankton, which are comprised primarily of chitin, a polymer of N-acetylglucosamine. Chitin is distinct from the other surfaces listed because many Vibrio species are able to use it as a sole carbon and nitrogen source (4).
To determine whether attachment to abiotic surfaces and to the intestinal epithelium utilized the same or different adhesion factors, we first focused on the role of one of the most well-studied intestinal adhesion factors of V. cholerae, the toxin-coregulated pilus (TCP), in biofilm formation on an abiotic surface. TCP, a type IV bundle-forming pilus found in pathogenic V. cholerae, is an essential intestinal colonization factor for all types of V. cholerae (1, 9, 21, 23). Biofilm formation assays were done as previously described with minor modifications (17). Briefly, borosilicate glass tubes were utilized as surfaces for bacterial attachment. Three hundred microliters of the indicated medium, inoculated with a 1:100 dilution of overnight cultures grown in Luria-Bertani (LB) broth, were placed in each tube. These were allowed to incubate at the indicated temperature for 24 h. Tubes were then rinsed vigorously with distilled water to remove nonadherent cells, filled with 350 μl of a 0.1% crystal violet solution (Sigma), allowed to incubate for 30 min, and again rinsed vigorously with water. Biofilm formation was quantitated by measuring the optical density at 570 nm (OD570) of a solution produced by extracting cell-associated dye with 400 μl of dimethyl sulfoxide (DMSO).
We compared experimental conditions which maximize in vitro expression of TCP in V. cholerae El Tor with experimental conditions which maximize biofilm formation and also studied the biofilm formation properties of an El Tor TCP-deficient mutant. In V. cholerae El Tor, expression of TCP in vitro is maximized by anaerobic growth in AKI medium (a rich medium) at 37°C (11). However, biofilm formation by V. cholerae El Tor in AKI medium at 37°C was inferior to biofilm formation in LB broth at room temperature.
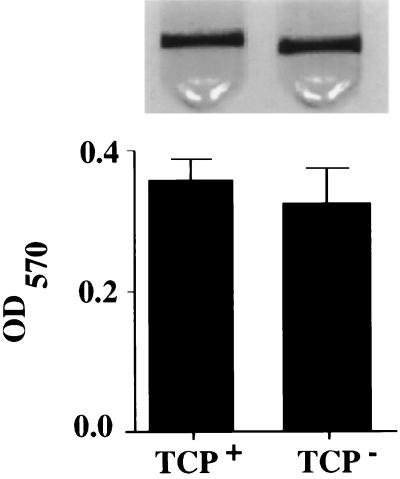
To confirm that TCP does not play a role in biofilm formation, we compared the biofilms formed by a TCP-deficient mutant (an in-frame deletion [6]) with the biofilms formed by a wild-type El Tor strain at room temperature in LB broth. In Fig. 1, the biofilm made by the El Tor TCP mutant is compared both qualitatively and quantitatively with that made by wild-type V. cholerae El Tor. We conclude from these data that the genetic pathways and environmental cues which promote adhesion to the intestinal surface are different from those which promote biofilm formation on abiotic surfaces and are highly specific for survival in these very different niches.
FIG. 1.
Biofilm formation ability on borosilicate of V. cholerae El Tor strain N16961 (TCP+) and its respective TCP-deficient derivative strain (TCP−) grown overnight in LB broth at room temperature. Biofilms stained with crystal violet are shown above, and the biofilm-associated dye is quantitated below by extraction with DMSO and measurement of the OD570 of the resulting solution. Experiments were done in triplicate. Error bars represent 1 standard deviation.
V. cholerae El Tor can be distinguished from classical V. cholerae by its ability to cause mannose-sensitive hemagglutination of erythrocytes (19). Mannose-sensitive hemagglutination is mediated by yet another type IV pilus, called the mannose-sensitive hemagglutinin (MSHA), whose structural pilin subunit is encoded by the mshA gene (12, 13). MSHA has been extensively investigated as a potential colonization factor but appears to play no role in virulence (1, 21, 23). Because type IV pili have previously been implicated in biofilm formation by other bacterial species (16), we hypothesized that MSHA might be involved in biofilm formation by V. cholerae on abiotic surfaces. An MSHA-deficient mutant of N16961Sm (KFV11 [this study]), constructed as previously described for El Tor strain C6706 (23), was used for these experiments. This mutant was shown to be defective for hemagglutination of human and sheep erythrocytes.
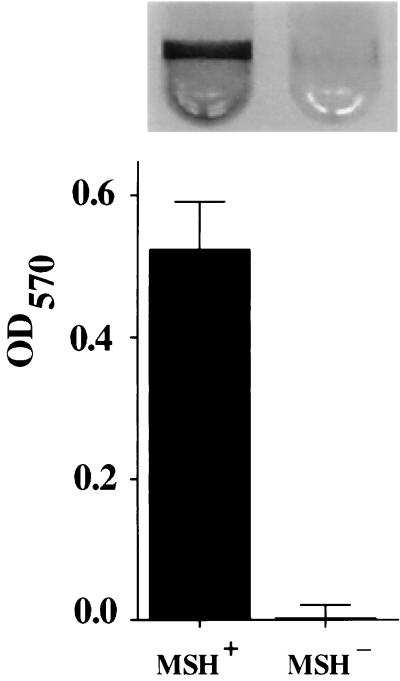
In Fig. 2, we compare qualitatively and quantitatively the biofilm formation abilities of V. cholerae El Tor strain N16961 with its respective MSHA mutant derivative. These biofilms were formed at room temperature over a period of 24 h in LB broth. As illustrated in Fig. 2, the El Tor MSHA mutant is unable to make a biofilm under the conditions of this experiment. Similar results were observed for El Tor strain C6706 and its MSHA mutant derivative (not shown). Because mannose has previously been shown to interfere with biofilm formation by Escherichia coli on abiotic surfaces, which is mediated by mannose-sensitive type I pili (18), we assayed the effect of α-methyl d-mannoside, a nonmetabolizable derivative of mannose, on biofilm formation. Addition of 100 mM α-methyl d-mannoside abolished biofilm formation by wild-type V. cholerae El Tor, while 100 mM fucose did not (not shown). These two results demonstrate that MSHA plays an essential role in biofilm formation by V. cholerae El Tor.
FIG. 2.
Biofilm formation ability on borosilicate of V. cholerae El Tor strain N16961 (MSH+) and its respective MSHA-deficient derivative strain (MSH−) grown overnight in LB broth at room temperature. Biofilms stained with crystal violet are shown above, and the biofilm-associated dye is quantitated below.
We also tested attachment of the parent El Tor strain and MSHA mutant to two surfaces which are abundant in the aquatic environment: cellulose, a key structural component of plants, and chitin, an important component of the exoskeletons of crustaceans and insects. These experiments were done with V. cholerae El Tor (N16961) and its derivative MSHA mutant strain transformed with pSMC2, a plasmid which includes a β-lactamase gene and a constitutively expressed gene for green fluorescent protein (2). For experiments in rich media, bacterial cultures were harvested in the exponential phase, and 10 to 50 μl of each culture was placed on a glass slide alone or covered with a small amount of cellulose fibers or chitin. A coverslip was placed on top of the sample to minimize desiccation. For experiments in minimal medium with lactate (15), cells were grown for 4 to 8 h, harvested at an OD600 of approximately 0.1 to 0.2, and then incubated with several granules of purified chitin on a glass slide. All images shown in this work were acquired at a ×400 magnification after approximately 30 min of incubation on the relevant surface.
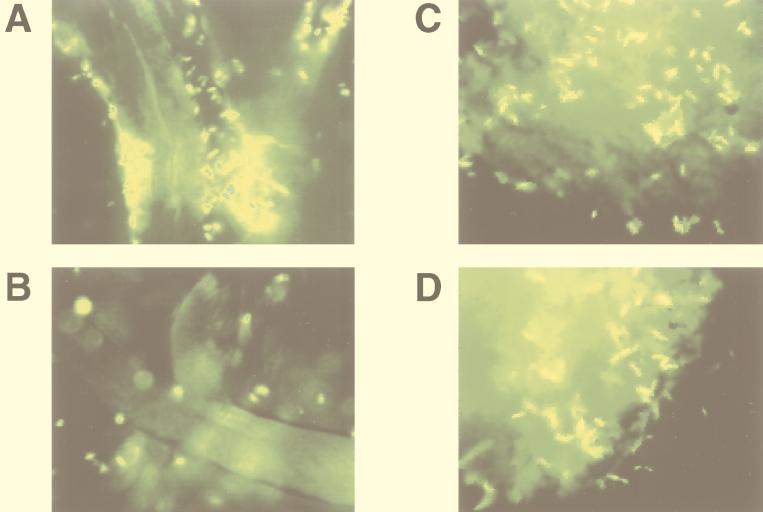
Figure 3A and B illustrate V. cholerae El Tor (N16961) and the MSHA mutant, respectively, in the presence of cellulose fibers. Although a few MSHA mutant cells are observed in Fig. 3B, most are not associated with the surface of the cellulose fibers.
FIG. 3.
Attachment to cellulose and chitin by a V. cholerae El Tor strain and its MSHA mutant derivative grown to mid-log phase in LB media. (A) Wild type [N16961(pSMC2)] with cellulose. (B) MSHA mutant derivative with cellulose. (C) Wild type [N16961(pSMC2)] with chitin. (D) MSHA mutant derivative with chitin.
Chitin is a polymer of N-acetyl-d-glucosamine. Although chitin and cellulose are both polysaccharides, chitin differs from cellulose in that it is a nutritive surface for V. cholerae. Figure 3C and D show no observable difference in the chitin-binding ability of the parent strain and the MSHA mutant, respectively. Furthermore, while attachment to glass is greatly decreased in minimal media (not shown), we find no difference between adherence of V. cholerae to chitin in rich medium and that in minimal medium. These data demonstrate that both the external triggers and genetic bases of biofilm formation on nutritive and nonnutritive surfaces differ.
Cholera is distinguished as a disease that has caused multiple epidemics and pandemics worldwide. The ability of V. cholerae to cause epidemics and pandemics is intimately related to its ability to survive in the environment of the human intestine and in the aquatic environment. Scavenging nutrients is a critical aspect of survival in both of these environments. We have shown that the environmental signals and cellular structures required for adhesion to the intestinal epithelium, nonnutritive abiotic surfaces, and nutritive, abiotic surfaces are distinct. We propose, therefore, that the regulation and genetic bases of the pathways leading to biofilm formation on the intestinal epithelium, nonnutritive abiotic surfaces, and nutritive abiotic surfaces are divergent. The independence of these pathways may maximize V. cholerae’s access to nutrients in each of these environments. For instance, biofilm formation on nonnutritive surfaces occurs in a rich medium only, whereas adhesion to nutritive surfaces occurs in both rich and minimal medium. These divergent pathways may maximize nutrient scavenging as follows. In a nutrient-rich microenvironment, bacteria that form biofilms on any available surface are able to remain in the hospitable environment with a minimum of energy expenditure. In contrast, in a nutrient-poor microenvironment, a bacterium adhering selectively to a surface that is edible is guaranteed a constant food source. In conclusion, biofilm formation on abiotic surfaces is a surface-specific process that may maximize nutrient scavenging and, thus, survival in the aquatic environment.
Acknowledgments
We thank R. Taylor and J. J. Mekalanos for helpful discussions and for providing us with plasmids and strains and George O’Toole, Leslie Pratt, Steven Finkel, Dianne Newman, and other members of the Kolter and Mekalanos laboratories for many helpful discussions.
P.I.W. was a Howard Hughes Medical Institute Postdoctoral Fellow. K.J.F. was funded by NRSA Postdoctoral Training grant A107410-06. This work was supported by NIH grant GM58213 to R.K.
REFERENCES
- 1.Attridge S R, Manning P A, Holmgren J, Jonson G. Relative significance of mannose-sensitive hemagglutinin and toxin-coregulated pili in colonization of infant mice by Vibrio cholerae El Tor. Infect Immun. 1996;64:3369–3373. doi: 10.1128/iai.64.8.3369-3373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloemberg G V, O’Toole G A, Lugtenberg B J J, Kolter R. Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol. 1997;63:4543–4551. doi: 10.1128/aem.63.11.4543-4551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colwell R R, Huq A. Vibrios in the environment: viable but nonculturable Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: American Society for Microbiology; 1994. pp. 117–133. [Google Scholar]
- 4.Colwell R R, Spira W M. The ecology of Vibrio cholerae. In: Barua D, Greenough W B I, editors. Cholera. New York, N.Y: Plenum; 1992. pp. 107–127. [Google Scholar]
- 5.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 6.Fullner K J, Mekalanos J J. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect Immun. 1999;67:1393–1404. doi: 10.1128/iai.67.3.1393-1404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass R I, Black R E. The epidemiology of cholera. In: Barua D, Greenough W B I, editors. Cholera. New York, N.Y: Plenum; 1992. pp. 129–154. [Google Scholar]
- 8.Grossman A D. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 9.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huq A, Huq S A, Grimes D J, O’Brien M, Chu K H, McDowell Capuzzo J, Colwell R R. Colonization of the gut of the blue crab (Callinectes sapidus) by Vibrio cholerae. Appl Environ Microbiol. 1986;52:586–588. doi: 10.1128/aem.52.3.586-588.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microb Immunol. 1986;30:1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 12.Jonson G, Holmgren J, Svennerholm A M. Identification of a mannose-binding pilus on Vibrio cholerae El Tor. Microb Pathog. 1991;11:433–441. doi: 10.1016/0882-4010(91)90039-d. [DOI] [PubMed] [Google Scholar]
- 13.Jonson G, Lebens M, Holmgren J. Cloning and sequencing of Vibrio cholerae mannose-sensitive haemagglutinin pilin gene: localization of mshA within a cluster of type 4 pilin genes. Mol Microbiol. 1994;13:109–118. doi: 10.1111/j.1365-2958.1994.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 14.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 15.Myers C R, Nealson K H. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J Bacteriol. 1990;172:6232–6238. doi: 10.1128/jb.172.11.6232-6238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 18.Pratt L A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 19.Sakazaki R. Bacteriology of Vibrio and related organisms. In: Barua D, Greenough W B I, editors. Cholera. New York, N.Y: Plenum; 1992. pp. 37–55. [Google Scholar]
- 20.Shukla B N, Singh D V, Sanyal S C. Attachment of non-culturable toxigenic Vibrio cholerae O1 and non-O1 and Aeromonas spp. to the aquatic arthropod Gerris spinolae and plants in the River Ganga, Varanasi. FEMS Immunol Med Microbiol. 1995;12:113–120. doi: 10.1111/j.1574-695X.1995.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 21.Tackett C O, Taylor R K, Losonsky G, Lim Y, Nataro J P, Kaper J B, Levine M M. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect Immun. 1998;66:692–695. doi: 10.1128/iai.66.2.692-695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamplin M L, Gauzens A L, Huq A, Sack D A, Colwell R R. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol. 1990;56:1977–1980. doi: 10.1128/aem.56.6.1977-1980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thelin K H, Taylor R K. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]