Abstract
Dilated cardiomyopathy (DCM) is one of the important causes of heart failure (HF). With the rapidly evolving technologies for gene analysis and tremendous advances in knowledge of HF genetics, the importance of genetic testing in DCM is currently highlighted. Several genetic variants causing DCM have been identified and this information is used for diagnosis, risk stratification and family screening of DCM patients. However, there are still several challenges in applying genetic testing to real clinical practice. In this review, we will summarize recent understandings in DCM genetics and provide an evidence-based practical guide to the use of genetic testing for DCM patients.
Keywords: Dilated cardiomyopathy, Genetics
INTRODUCTION
Heart failure (HF) is one of the most devastating condition with high mortality and morbidity that is still growing in number world widely as well as in Korea.1),2) Dilated cardiomyopathy (DCM) is a major cause of HF and leading indication for heart transplantation.3),4) Of these DCM patients, 20–50% of patients are estimated to have a genetic predisposition5) and the utilization of genetic testing in cases of DCM has increased to understand the genetic basis of DCM and to provide advanced knowledge of disease pathogenesis and genetic consultation for families. However, the genetic study is not routinely used in clinical field since the genetic basis of DCM has not been fully elucidated and there are some challenges in making a genetic diagnosis in DCM.
In this review, we aimed to provide a practical and evidence-based guide to clinicians and HF specialists to the pragmatic use of genetic testing for DCM patients.
GENETIC CHARACTERISTICS OF DCM
DCM is commonly defined by the presence of left ventricular (LV) or biventricular dilatation and systolic dysfunction in the absence of abnormal loading conditions (hypertension, valve disease) or coronary artery disease sufficient to cause global systolic impairment.6) DCM is classified as idiopathic (idiopathic dilated cardiomyopathy, or IDC) when all detectable causes have been excluded (except genetic causes). Potentially diagnosable causes of DCM include a variety of toxic, metabolic, or infectious agents. A diagnosis of familial dilated cardiomyopathy (FDC) is assigned when IDC occurs in at least 2 closely related family members.7) Family-based studies of first-degree relatives of patients with IDC have established that FDC can be identified in 20% to 50% of patients diagnosed with IDC by clinical screening of family members.5) However, there are still some questions on the practical use of genetic test in DCM8) and these challenges are mostly related to genetic heterogeneity, incomplete or age-related penetrance and variable expressivity DCM. Genetic heterogeneity for DCM means DCM is associated with wide variety of variants in many different genes and the genetic variants are often specific for one family only with extremely low recurrence rate of the same genetic variant in other families.8) Incomplete or age-related penetrance indicates the proportion of genetic variant carriers who exhibit DCM phenotype depends on age and who are healthy on cardiac examination may subsequently develop DCM.9),10) In addition, DCM is characterized by variable expression of disease in terms of disease onset, symptom severity and complication risk. Though there might be some correlation between genotype and phenotype, there can be large differences in relatives of the same family who carry the same genetic variation.11) Historically, rare genetic variants with low allele frequency had been considered potentially related with disease, but recently published researches found that the previously reported rare variants related with cardiomyopathies are not unique to DCM patients but also commonly encountered in general population.12),13) The multigenic models with combined effect of multiple genetic variants are also suggested in recent years to explain phenotypic variation and nonsegregation in DCM.14)
The genetic characteristics of DCM make it complicated to apply genetic tests in real clinical practice. However, this might be changed with development of new technologies and accumulation of large amount of genotypic and phenotypic data of DCM patients.
METHODS OF GENETIC STUDY IN DCM
A traditional method of candidate genetic variant testing, in which small number of specific variants or genes have been tested with Sanger sequencing, is now limitedly used for cascade genetic screening in close family members of DCM with known pathogenic or likely pathogenic gene variant. With development and advance of next-generation sequencing (NGS), targeted gene panels comprising genes (mostly from 50 to 100 genes) relevant to the DCM are mostly widely used in current practice for technical feasibility and the cost. However, the drawbacks of targeted gene panel test are that even though more and more genes have been added to the panel only about a dozen of the genes account for the majority of DCM, the rate of actionable variant detection is less than 20% in DCM probands and in this case it is not useful to find unknown genetic causality.15),16) As the price of NGS plunged, genome wide approaches such as whole-exome or whole-genome sequencing (WES or WGS, respectively) instead of targeted panel sequencing have been considered for diagnostic purposes on any patients irrespective of the disease in question. The advantage of this genome wide approach is that it is possible to find new genetic causality and there is a potential to identify modifying genes. In addition, no additional genetic tests are needed even when new genes have been identified to be associated with DCM or the patients are suspected to have a different disease. However, the interpretation would be more difficult since there must be a flood of variant with unknown significance which might raise the possibility of false positive results and the risk of over-diagnosis.17) More studies are mandatory to find out which methods are better, but it is definitive that genome wide approaches will be replaced panel tests down the road.
GENETIC VARIANTS IN DCM
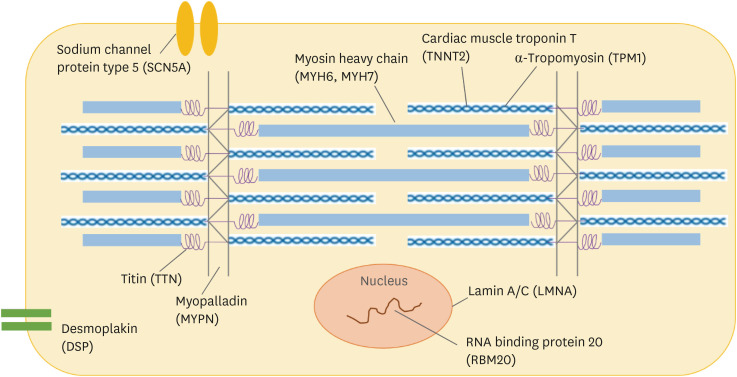
In contrast to many Mendelian disorders, genetic associations present in DCM showed variable penetrance and phenotypical manifestations might occasionally be presented only with an additional insult. The estimated frequency of pathogenic genetic variants was 10–25% in unselected DCM patients and 20–40% in FDC patients.18),19) These genetic variants causing DCM include genes encoding a heterogeneous group of molecules that involve force generation and transmission, sarcomere integrity, cytoskeletal and nuclear architecture, electrolyte homeostasis, mitochondrial function, and gene transcription (Figure 1).
Figure 1. Main genes and protein associated with DCM.
This schematic figure shows cardiomyocyte and cardiac sarcomere. The approximate cellular locations of main proteins with genes associated with DCM is indicated.
DCM = dilated cardiomyopathy.
Table 1 showed the main genetic variants associated with DCM. Many of these genes have been also associated with other forms of cardiomyopathies (hypertrophic cardiomyopathy, restrictive cardiomyopathy, LV noncompaction, and arrhythmogenic right ventricular cardiomyopathy).20) The genetic variants in TTN gene, which encodes sarcomeric protein titin, are the most common variants in DCM, ranging from 10% to 20% of cases.10),20),21),22) Several types of protein-truncating variants (nonsense, short insertion or deletion, or splice-site variants) in TTN (TTNtv) have been identified. The clinical presentation and prognosis of TTNtv are generally similar to IDC, but the patients with TTNtv might have a better response to HF medical therapy than the IDC patients.23) The variants in LMNA gene, which encodes nuclear envelope protein lamin A/C, are the second most common variants related to DCM with a diagnostic yield of 5.5%.19) The LMNA-associated DCM commonly presented as DCM with conduction disorder and the risk of fatal arrhythmia and sudden cardiac death (SCD) is high (up to 46%).5),20),22) The cascade screening of LMNA-associated DCM before the onset of clinical disease, allows individuals to be monitored and timely intervened with a pacemaker or implantable cardioverter defibrillator (ICD).22),24)
Table 1. Main genetic variants associated with DCM.
| Gene | Protein | Function | Estimated prevalence in DCM (%) | Association with other phenotypes |
|---|---|---|---|---|
| TTN | Titin | Sarcomere | 12–25 | LVNC |
| LMNA | Lamin A/C | Nuclear membrane | 4–6 | HCMP, muscular dystrophy |
| MYH7 | β-myosin heavy chain | Sarcomere | 4–10 | HCMP, LVNC, RCMP |
| MYH6 | α-myosin heavy chain | Sarcomere | 4 | HCMP |
| MYPN | Myopalladin | Sarcomere, Z-disc | 3–4 | |
| DSP | Desmoplakin | Desmosome | 3–4 | ARVC |
| RBM20 | RNA-binding protein 20 | RNA-binding protein, spliceosome | 2–5 | LVNC |
| TNNT2 | Cardiac muscle troponin T type 2 | Sarcomere | 2–3 | HCMP, LVNC, RCMP |
| SCN5A | Sodium channel protein type 5, α subunit | Ion channel | 2–3 | Brugada syndrome, LQTS |
| TPM1 | α-tropomyosin | Sarcomere | 0.5–1 | HCMP, LVNC, RCMP |
ARVC = arrhythmogenic right ventricular cardiomyopathy; DCM = dilated cardiomyopathy; HCMP = hypertrophic cardiomyopathy; LQTS = long QT syndrome; LVNC = left ventricular non-compaction; RCMP = restrictive cardiomyopathy.
Although there might be ethnic differences in genetic variations, there are extremely limited reports on genetic variations associated with DCM in Korean. In genetic diagnosis support program launched by Korea National Institute of Health, 88 patients with DCM completed the genetic studies.25) A targeted gene panel with 49 genes (ABCC9, ACTC1, ACTA2, ANKRD1, BAG3, CAV3, CRYAB, CSRP3, CTF1, DES, DMD, DSG2, DSP, EMD, EYA4, FHL2, FKTN, GATAD1, ILK, JUP, LAMA4, LAMP2, LDB3, LMNA, MYBPC3, MYH6, MYH7, MYPN, NEBL, NEXN, PLB1, PLN, PSEN1, PSEN2, RBM20, SCN5A, SDHA, SGCD, TAZ, TCAP, TMPO, TNNC1, TNNC2, TNNI3, TNNT2, TPM1, TTN, TTR and VCL) using NGS technology and Sanger sequencing for the confirmation were used in this program. The variants were most frequently found in sarcomere and cytoskeletal genes. Of the patients, 39.8% have pathogenic/likely pathogenic variants. Twenty-five known variants and 9 novel variants in TTN, MYH6, MYH7, TNNI3, ABCC9, TNNT2 and TAZ were identified in these patients. A 42% of the patients had at least one or more unclassified variants in candidate genes. The variants in MYBPC3 (6.8%), LMNA (5.7%), and MYH7 (5.7%) were most frequently identified. The prevalence of MYBPC3 variant was somewhat higher than previous report26) in this cohort. The prevalence of variant in TTN gene (5%) was relatively lower than the previous report.21) Developing a more comprehensive gene panel with novel DCM genes and using WES can improve the detection rate, and further studies in large sized cohort with detailed clinical information are needed to figure out the exact prevalence and pattern of genetic variation associated with DCM in Korean patients.
CLINICAL USE OF GENETIC TESTING IN DCM
To refine the diagnosis of DCM and identify the specific etiologies
The positive genetic testing in those with cardiomyopathies with unknown cause helps confirming the diagnosis and the etiology, which might result in specific recommendation for the treatment and family screening. The following example is a typical case. A male patient of 55-year-old with frequent ventricular tachycardia and LV ejection fraction of 25% was suspected to have sarcoidosis since there was multiple regional wall motion abnormalities which did not match the coronary territory. However, 18-Fluoro-2-deoxyglucose positron emission tomography revealed no abnormal uptake in myocardium and cardiac biopsy was also negative for sarcoidosis. The genetic analysis showed that he has pathologic variant in MYBPC3 (c.2067+1G>A, splice-donor variant) that has close correlation with hypertrophic cardiomyopathy or less commonly with DCM. The diagnosis of this patient must be cardiomyopathy related to MYBPC3 variant, in regardless of whether his morphologic diagnosis is hypertrophic cardiomyopathy or DCM. He was enrolled for cardiac transplantation for refractory HF symptom and intractable ventricular arrhythmia and his family was screened for cardiomyopathy. In this case, genetic testing confirms the diagnosis of MYBPC3 related cardiomyopathy and if we had the result of genetic test earlier, he could avoid unnecessary tests such as 18-Fluoro-2-deoxyglucose positron emission tomography and endomyocardial biopsy. Furthermore, there are some rare but particular cardiomyopathies where the confirmed diagnosis can lead to a specific therapy. For example, enzyme replacement therapy can be applied for Fabry disease, liver transplantation or tafamidis for transthyretin-related amyloidosis27),28) and arginine and citrulline replacement for cardiomyopathy related to mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome.29) However, in order to reach these specific etiologic diagnoses, patients often go through a bunch of specific tests sequentially for each disease, which is called diagnostic odyssey. Thus, a comprehensive genetic testing can be a time and cost-efficient way to get to the specific diagnosis in cardiomyopathies. Furthermore, a new category of hypokinetic non-dilated cardiomyopathy has been proposed since it is frequent to observe intermediate phenotypes that do not meet standard definition of DCM but has definite causative genetic variants related to DCM or significant myocardial abnormalities on cardiac MRI, radionuclide studies or endomyocardial biopsy.30) Though it is not sure yet whether early treatment in these patients is related with better prognosis, this highlights the importance of genetic testing for early diagnosis of DCM in preclinical state. In these aspects, most guidelines or positional statements recommend genetic testing for DCM with red flags such as neurosensory disorders, skin pigmentation, skeletal myopathy, elevation of creatinine kinase, conduction abnormalities, cardiac hypertrophy and so on.8),31),32),33)
For risk stratification and to guide a specific management: phenotype-genotype association
Positive genetic testing can provide prognostic value in a patient with DCM and help clinical decision making. Currently, ICD is recommended for primary prevention of SCD in DCM patients according to their HF symptoms and LV function, but the benefit is still unclear.34) The risk of ventricular arrhythmia varies according to the etiologies of DCM. For example, patients with HF caused by systemic hypertension might have lower arrhythmic risk while younger patients with some of the malignant genetic variants have a greater risk of SCD and more survival benefit from ICD implantation.35) This is an example of genotype-phenotype association in DCM. DCM related to lamin A/C (LMNA), desmin (DES), filamin C (FLNC) and RNA-binding protein 20 (RBM20) has been shown to be associated with ventricular arrhythmia while sodium channel protein type 5, alpha subunit (SCN5A) is commonly associated with atrial fibrillation but not with ventricular arrhythmia.36),37),38),39),40),41),42),43),44) Though titin (TTN) truncation variant may be in a compensated state and more likely to develop HF under conditions of stress such as pregnancy, alcohol, and cardiac toxic drugs,45),46),47) those with TTN truncation variant still have higher risk of ventricular arrhythmia.48),49) Thus, gene specific management recommendations such as early implantation of ICD could be considered in these patients. Moreover, it has been observed that DCM patients carrying sarcomeric rare variant or RBM20 variant showed a more rapid progression toward death or heart transplantation, which implicates early consideration of more definitive treatment such as heart transplantation.42),50),51) Genotype-phenotype interactions are still an unmet issue and the effects of specific variants on the mechanisms of disease expression remain largely unknown. Extreme genetic heterogeneity and variable penetrance prohibited robust studies on genotype-phenotype correlation and the result of genetic testing does not strongly affect clinical management of DCM. Nevertheless, despite the complex genetic architecture of DCM, an increasing number of actionable prognostic genotype-phenotype associations are emerging and the needs for preemptive treatment are increasing, for example, early ICD implantation for LMNA variant related DCM who has high life-threatening arrhythmic risk.24),52)
Facilitating familial screening
The 2018 Heart Failure Society of America guideline on genetic evaluation of cardiomyopathies24) and the 2018 American College of Medical Genetics and Genomics clinical practice resource on genetic evaluation of cardiomyopathies19) recommended obtaining comprehensive family history of at least 3 generations and phenotypic evaluation of all at-risk first degree relatives. Baseline clinical evaluation for phenotype screening is recommended for all at-risk family members and serial phenotypic screening for the emergence of cardiomyopathy is recommended for clinically unaffected at-risk family members whose genetic status is unknown.24) This screening test is recommended for two reasons: early diagnosis for intervention and refining initial diagnosis of proband by observing the disease in different phase. However, this clinical screening would be an expensive and impractical journey of screening test. For example, if a person found dead in his forties and the autopsy indicates he had DCM and the first manifestation was SCD. If the proband has 5 siblings and 3 children, they all should undergo constant screening tests for cardiomyopathy since the disease can develop later even though initial tests turn out negative. This is the problem of incomplete or age-related penetrance. In this case, genetic test can facilitate familial screening. If the genetic test of the proband has one or more significant disease-causing variants and the relatives do not carry the disease-causing variants, then they can be reassured, and follow-up screening would be no longer required as far as the initial screening tests are negative (negative cascade genetic testing).19),24) If the relative carries the disease-causing variant, then regular clinical follow-up is required in order to detect disease early, and improve the management.19),24)
However, several limitations should be appreciated.8) First, at present there are no tools to prevent disease development in disease-causing variant carriers. Second, even though there are several strategies recommended to take a benefit of an early diagnosis, such as avoidance of intensive and strenuous physical activities, avoidance of environmental hazards, drug therapy (e.g., angiotensin converting enzyme [ACE] inhibitor), precautious for pregnancy and prophylactic ICD implantation, it is important to note that prospective randomized clinical trials on their clinical efficacy are lacking. Third, the identification of the disease-causing variant may result in adverse psychological consequences as the previous psychological burden related to uncertainty might be replaced by the near-certainty of developing the disease and the risk of transmitting the disease to the offspring.8),53),54)
Pharmacogenetics of HF medical treatment
Though the mortality and morbidity of HF have improved during last 2 decades with the continuous progress in HF medical therapy, the HF physicians easily encounter the different individual response to medication and subsequent difference in the patient's prognosis. Modifier genes can aid in pharmacogenetic differences in the individual's response to disease or therapy. The common polymorphic variants of modifier genes include genes of the renin-angiotensin-aldosterone system and adrenergic system could influence drug response in cardiovascular disease in a variety of areas including HF, arrhythmia, hypertension, and dyslipidemia (Table 2).55)
Table 2. The main genetic variations associated with different pharmacological responses in HF.
| Protein (gene) | Polymorphism | Function | Related medications |
|---|---|---|---|
| ACE (ACE) | D/I | D: higher ACE activity and angiotensin II level | ACE inhibitors |
| β-blockers | |||
| Aldosterone synthase (CYP11B2) | Promoter-344 T/C | C: increased transcriptional activity and aldosterone production | ACE inhibitors |
| Aldosterone receptor antagonists | |||
| β1-adrenergic receptors (ADRB1) | Arg389Gly | Arg389: increased adrenergic signal | β-blockers |
| ACE inhibitors | |||
| β2-adrenergic receptors (ADRB2) | Gly49Ser | Gly49: enhanced down regulation | β-blockers |
| Gly16Arg | Receptor down regulation | β-blockers | |
| Gln27Gly | |||
| α-2C receptors (ADRA2C) | α-2C deletion | Decreased uptake of norepinephrine | β-blockers |
| G protein receptor kinase 5 (GRK5) | Gln41Leu | Desensitize β-adrenergic receptor signialing | β-blockers |
| G protein β3 subunit (GNB3) | C825T | Increased α-adrenergic signialing, lower plasma renin | ACE inhibitors |
| Nitric Oxide Synthase (NOS3) | Asp298Glu | Asp: associated with lower NOS3 activity | ACE inhibitors |
| Endothelin 1 (EDN1) | IVS-4 G/A Lys198Asn | Unknown | β-blockers |
ACE = angiotensin converting enzyme; HF = heart failure.
There is large variation in response to beta blocker therapy and the single nucleotide polymorphisms (SNPs) in the β1-adrenergic receptors (ADRB1), β2-adrenergic receptors (ADRB2), α-2C receptors (ADRA2C), and G-protein receptor kinase 5 (GRK5) genes partially explain the variable responsiveness to beta blocker. Regarding ADRB1 genes, the Arg389 homozygotes showed better response to beta blocker therapy and Gly389 variant carriers showed no clinical benefit.56) The Ser49 homozygotes showed worse prognosis compared to Gly49 carriers and decreased sensitivity to beta blocker therapy as well as decreased β1 receptor desensitization. The patients with a homozygous DD genotype of ACE gene which increase ACE activity and angiotensin II levels had a worse prognosis despite treatment with ACE inhibitors, especially in patients did not receive beta blocker therapy.57),58)
Current HF therapies can be tailored to an individual patient with recent advances of genetic information and pharmacogenetics, but challenge remains that; 1) clarifying the inconsistencies between gene-drug response associations which suggest complex genetic and environmental interaction, 2) building clinical data to support a clinical application of pharmacogenetics-guided therapy to improve HF prognosis, 3) more studies to find pharmacogenetics information focused on emerging HF therapies.
CONCLUSIONS
Substantial technical advances in genetic testing and improving understandings of genetic background of DCM make progress in the identification of genetic markers of DCM. However, the genetic background of DCM has not yet been fully elucidated and the diagnostic yield of DCM is not high because of the genetic heterogeneity, incomplete or age-related penetrance and variable expressivity. Nevertheless, identifying the genetic cause of DCM can provide significant implication for the diagnosis of DCM with specific cause, risk stratification, guiding specific treatment and facilitating family screening. For the routine use of genetic testing in real clinical practice, further researches are needed to clarify genotype-phenotype relationship and to make sufficient evidence for the interpretation of genetic test. Recent advances in the genetics of DCM will ultimately improve the management of DCM patients and their families.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Data curation: Lee JH, Lee SE.
- Validation: Lee SE.
- Writing - original draft: Cho MC.
References
- 1.Park JJ, Choi DJ. Current status of heart failure: global and Korea. Korean J Intern Med (Korean Assoc Intern Med) 2020;35:487–497. doi: 10.3904/kjim.2020.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Lim NK, Cho MC, Park HY. Epidemiology of heart failure in Korea: present and future. Korean Circ J. 2016;46:658–664. doi: 10.4070/kcj.2016.46.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SE, Lee HY, Cho HJ, et al. Clinical Characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF) Korean Circ J. 2017;47:341–353. doi: 10.4070/kcj.2016.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SE, Cho HJ, Lee HY, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. doi: 10.1002/ejhf.91. [DOI] [PubMed] [Google Scholar]
- 5.Burkett EL, Hershberger RE. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2005;45:969–981. doi: 10.1016/j.jacc.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 6.Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 7.Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:1641–1649. doi: 10.1016/j.jacc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charron P, Arad M, Arbustini E, et al. Genetic counselling and testing in cardiomyopathies: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2010;31:2715–2726. doi: 10.1093/eurheartj/ehq271. [DOI] [PubMed] [Google Scholar]
- 9.Rankin J, Auer-Grumbach M, Bagg W, et al. Extreme phenotypic diversity and nonpenetrance in families with the LMNA gene mutation R644C. Am J Med Genet A. 2008;146A:1530–1542. doi: 10.1002/ajmg.a.32331. [DOI] [PubMed] [Google Scholar]
- 10.Golbus JR, Puckelwartz MJ, Fahrenbach JP, Dellefave-Castillo LM, Wolfgeher D, McNally EM. Population-based variation in cardiomyopathy genes. Circ Cardiovasc Genet. 2012;5:391–399. doi: 10.1161/CIRCGENETICS.112.962928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman JD, Jacobson Z, Young TL, Marshall JD, Kaplan P. Familial variable expression of dilated cardiomyopathy in Alström syndrome: a report of four sibs. Am J Med Genet A. 2005;135:96–98. doi: 10.1002/ajmg.a.30688. [DOI] [PubMed] [Google Scholar]
- 12.Norton N, Robertson PD, Rieder MJ, et al. Evaluating pathogenicity of rare variants from dilated cardiomyopathy in the exome era. Circ Cardiovasc Genet. 2012;5:167–174. doi: 10.1161/CIRCGENETICS.111.961805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvat C, Johnson R, Lam L, et al. A gene-centric strategy for identifying disease-causing rare variants in dilated cardiomyopathy. Genet Med. 2019;21:133–143. doi: 10.1038/s41436-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan JR, Kinnamon DD, Morales A, Salyer L, Nickerson DA, Hershberger RE. Multigenic disease and bilineal inheritance in dilated cardiomyopathy Is illustrated in nonsegregating LMNA pedigrees. Circ Genom Precis Med. 2018;11:e002038. doi: 10.1161/CIRCGEN.117.002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinnamon DD, Morales A, Bowen DJ, Burke W, Hershberger RE, DCM Consortium Toward genetics-driven early intervention in dilated cardiomyopathy: design and implementation of the DCM precision medicine study. Circ Cardiovasc Genet. 2017;10:e001826. doi: 10.1161/CIRCGENETICS.117.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales A, Kinnamon DD, Jordan E, et al. Variant interpretation for dilated cardiomyopathy: refinement of the American College of Medical Genetics and Genomics/ClinGen Guidelines for the DCM Precision Medicine Study. Circ Genom Precis Med. 2020;13:e002480. doi: 10.1161/CIRCGEN.119.002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzarotto F, Olivotto I, Walsh R. Advantages and perils of clinical whole-exome and whole-genome sequencing in cardiomyopathy. Cardiovasc Drugs Ther. 2020;34:241–253. doi: 10.1007/s10557-020-06948-4. [DOI] [PubMed] [Google Scholar]
- 18.Lakdawala NK, Funke BH, Baxter S, et al. Genetic testing for dilated cardiomyopathy in clinical practice. J Card Fail. 2012;18:296–303. doi: 10.1016/j.cardfail.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershberger RE, Givertz MM, Ho CY, et al. Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2018;20:899–909. doi: 10.1038/s41436-018-0039-z. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum AN, Agre KE, Pereira NL. Genetics of dilated cardiomyopathy: practical implications for heart failure management. Nat Rev Cardiol. 2020;17:286–297. doi: 10.1038/s41569-019-0284-0. [DOI] [PubMed] [Google Scholar]
- 21.Herman DS, Lam L, Taylor MR, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke MA, Cook SA, Seidman JG, Seidman CE. Clinical and mechanistic insights into the genetics of cardiomyopathy. J Am Coll Cardiol. 2016;68:2871–2886. doi: 10.1016/j.jacc.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansweijer JA, Nieuwhof K, Russo F, et al. Truncating titin mutations are associated with a mild and treatable form of dilated cardiomyopathy. Eur J Heart Fail. 2017;19:512–521. doi: 10.1002/ejhf.673. [DOI] [PubMed] [Google Scholar]
- 24.Hershberger RE, Givertz MM, Ho CY, et al. Genetic evaluation of cardiomyopathy-a Heart Failure Society of America practice guideline. J Card Fail. 2018;24:281–302. doi: 10.1016/j.cardfail.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HY. Hereditary dilated cardiomyopathy: recent advances in genetic diagnostics. Korean Circ J. 2017;47:291–298. doi: 10.4070/kcj.2016.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Pavia P, Cobo-Marcos M, Guzzo-Merello G, et al. Genetics in dilated cardiomyopathy. Biomarkers Med. 2013;7:517–533. doi: 10.2217/bmm.13.77. [DOI] [PubMed] [Google Scholar]
- 27.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 28.Suhr OB, Herlenius G, Friman S, Ericzon BG. Liver transplantation for hereditary transthyretin amyloidosis. Liver Transpl. 2000;6:263–276. doi: 10.1053/lv.2000.6145. [DOI] [PubMed] [Google Scholar]
- 29.El-Hattab AW, Almannai M, Scaglia F. Arginine and citrulline for the treatment of MELAS syndrome. J Inborn Errors Metab Screen. 2017;5:10. doi: 10.1177/2326409817697399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto YM, Elliott PM, Arbustini E, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2016;37:1850–1858. doi: 10.1093/eurheartj/ehv727. [DOI] [PubMed] [Google Scholar]
- 31.Rapezzi C, Arbustini E, Caforio AL, et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:1448–1458. doi: 10.1093/eurheartj/ehs397. [DOI] [PubMed] [Google Scholar]
- 32.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Europace. 2011;13:1077–1109. doi: 10.1093/europace/eur245. [DOI] [PubMed] [Google Scholar]
- 34.Køber L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 35.Merlo M, Cannatà A, Gobbo M, Stolfo D, Elliott PM, Sinagra G. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail. 2018;20:228–239. doi: 10.1002/ejhf.1103. [DOI] [PubMed] [Google Scholar]
- 36.Fatkin D, MacRae C, Sasaki T, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 37.Oomen AW, Jones K, Yeates L, Semsarian C, Ingles J, Sy RW. Rare desmin variant causing penetrant life-threatening arrhythmic cardiomyopathy. HeartRhythm Case Rep. 2018;4:318–323. doi: 10.1016/j.hrcr.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor MR, Slavov D, Ku L, et al. Prevalence of desmin mutations in dilated cardiomyopathy. Circulation. 2007;115:1244–1251. doi: 10.1161/CIRCULATIONAHA.106.646778. [DOI] [PubMed] [Google Scholar]
- 39.Hall CL, Akhtar MM, Sabater-Molina M, et al. Filamin C variants are associated with a distinctive clinical and immunohistochemical arrhythmogenic cardiomyopathy phenotype. Int J Cardiol. 2020;307:101–108. doi: 10.1016/j.ijcard.2019.09.048. [DOI] [PubMed] [Google Scholar]
- 40.Begay RL, Graw SL, Sinagra G, et al. Filamin C truncation mutations are associated with arrhythmogenic dilated cardiomyopathy and changes in the cell-cell adhesion structures. JACC Clin Electrophysiol. 2018;4:504–514. doi: 10.1016/j.jacep.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corrado D, Zorzi A. Filamin C: a new arrhythmogenic cardiomyopathy-causing gene? JACC Clin Electrophysiol. 2018;4:515–517. doi: 10.1016/j.jacep.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Brauch KM, Karst ML, Herron KJ, et al. Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy. J Am Coll Cardiol. 2009;54:930–941. doi: 10.1016/j.jacc.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNair WP, Ku L, Taylor MR, et al. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–2167. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 44.Parikh VN, Caleshu C, Reuter C, et al. Regional variation in RBM20 causes a highly penetrant arrhythmogenic cardiomyopathy. Circ Heart Fail. 2019;12:e005371. doi: 10.1161/CIRCHEARTFAILURE.118.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schafer S, de Marvao A, Adami E, et al. Titin-truncating variants affect heart function in disease cohorts and the general population. Nat Genet. 2017;49:46–53. doi: 10.1038/ng.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cresci S, Pereira NL, Ahmad F, et al. Heart failure in the era of precision medicine: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2019;12:458–485. doi: 10.1161/HCG.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 47.Ware JS, Amor-Salamanca A, Tayal U, et al. Genetic etiology for alcohol-induced cardiac toxicity. J Am Coll Cardiol. 2018;71:2293–2302. doi: 10.1016/j.jacc.2018.03.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corden B, Jarman J, Whiffin N, et al. Association of titin-truncating genetic variants with life-threatening cardiac arrhythmias in patients with dilated cardiomyopathy and implanted defibrillators. JAMA Netw Open. 2019;2:e196520. doi: 10.1001/jamanetworkopen.2019.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tayal U, Newsome S, Buchan R, et al. Truncating variants in titin independently predict early arrhythmias in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2017;69:2466–2468. doi: 10.1016/j.jacc.2017.03.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Hoogenhof MM, Beqqali A, Amin AS, et al. RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calcium handling. Circulation. 2018;138:1330–1342. doi: 10.1161/CIRCULATIONAHA.117.031947. [DOI] [PubMed] [Google Scholar]
- 51.Merlo M, Sinagra G, Carniel E, et al. Poor prognosis of rare sarcomeric gene variants in patients with dilated cardiomyopathy. Clin Transl Sci. 2013;6:424–428. doi: 10.1111/cts.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 53.Broadstock M, Michie S, Marteau T. Psychological consequences of predictive genetic testing: a systematic review. Eur J Hum Genet. 2000;8:731–738. doi: 10.1038/sj.ejhg.5200532. [DOI] [PubMed] [Google Scholar]
- 54.Evers-Kiebooms G, Welkenhuysen M, Claes E, Decruyenaere M, Denayer L. The psychological complexity of predictive testing for late onset neurogenetic diseases and hereditary cancers: implications for multidisciplinary counselling and for genetic education. Soc Sci Med. 2000;51:831–841. doi: 10.1016/s0277-9536(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 55.Mestroni L, Begay RL, Graw SL, Taylor MR. Pharmacogenetics of heart failure. Curr Opin Cardiol. 2014;29:227–234. doi: 10.1097/HCO.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oni-Orisan A, Lanfear DE. Pharmacogenomics in heart failure: where are we now and how can we reach clinical application? Cardiol Rev. 2014;22:193–198. doi: 10.1097/CRD.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersson B, Blange I, Sylvén C. Angiotensin-II type 1 receptor gene polymorphism and long-term survival in patients with idiopathic congestive heart failure. Eur J Heart Fail. 1999;1:363–369. doi: 10.1016/s1388-9842(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 58.Huang W, Xie C, Zhou H, Yang T, Sun M. Association of the angiotensin-converting enzyme gene polymorphism with chronic heart failure in Chinese Han patients. Eur J Heart Fail. 2004;6:23–27. doi: 10.1016/j.ejheart.2003.09.004. [DOI] [PubMed] [Google Scholar]