Abstract
The presence and severity of functional mitral regurgitation (FMR) is associated with worse outcomes in patients with heart failure and reduced ejection fraction. Prior to the availability of percutaneous mitral valve repair, management for FMR has been limited to medical therapy, cardiac resynchronization therapy for a specific subset of patients and surgery which has yet to demonstrate mortality benefits. Transcatheter edge-to-edge repair (TEER) of the mitral valve has emerged in the past decade as an invaluable member of the armamentarium against FMR with the 2 landmark randomized controlled trials providing deep insights on patient selection. In addition, TEER has spurred the rapid advancement in our understanding of FMR. This article seeks to provide an overview as well as our current understanding on the role of TEER in FMR.
Keywords: Mitral regurgitation, Heart failure
INTRODUCTION
Functional mitral regurgitation (FMR) frequently accompanies patients with heart failure (HF) and reduced ejection fraction (EF).1) Its presence and severity portend a worse prognosis with increased mortality and HF hospitalizations.2),3),4),5) Management of this complex entity has thus far been limited to medical therapy, cardiac resynchronization therapy (CRT) and surgery. Most recently, transcatheter edge-to-edge repair (TEER) of the mitral valve has joined the armamentarium in an effort to improve the prognosis of this debilitating condition. This article seeks to provide an overview as well as our current understanding on the role of TEER in FMR.
PATHOPHYSIOLOGY OF FMR
Primary mitral regurgitation (MR) occurs due to the disruption of one or more components of the mitral valve apparatus. In secondary or functional MR, the problem lies with the left ventricle (LV) while the mitral valve apparatus remains intact. It occurs due to an imbalance in the tethering (papillary muscle and chords) and closing forces (LV contraction) resulting in incomplete coaptation of the mitral valve leaflets and hence MR.6) There are a few situations that can result in this. Firstly, the LV can be markedly dilated resulting in symmetrical displacement of the papillary muscles and dilatation of the mitral annulus. This increases the tethering forces on the mitral valve leaflets and causes incomplete coaptation. Secondly, the LV may not be markedly dilated but there is localized LV or papillary muscle dysfunction resulting in asymmetrical tethering and mitral valve leaflet closure. Thirdly, in the setting of left bundle branch block, there is delayed electrical and hence mechanical activation of the anterolateral papillary muscle compared to the posteromedial papillary muscle.7) This results in an unsynchronized contraction of the papillary muscles and closure of the mitral valve leaflets.
There is another clinical entity known as atrial FMR that has recently gained awareness and clinical interest. The LV size and function is normal but MR occurs due to left atrial (LA) and mitral annular dilatation.8) This frequently occurs in the setting of atrial fibrillation but can occur in the setting of HF with preserved EF with LA dilatation.
SEVERITY OF FMR
Echocardiographic quantification of MR has been based on a combination of qualitative and quantitative parameters. In 2003, the American Society of Echocardiography (ASE)9) defined severe MR (both primary and secondary) with the following cut-offs: regurgitant volume (RVol) ≥60 mL/beat, regurgitant fraction (RF) ≥50% and effective regurgitant orifice area (EROA) ≥40 mm2. These values were based on an observational single-centre study published in 1997 which compared the echocardiographic and angiographic grading of MR in 180 patients (96 primary MR and 84 secondary MR).10) Since then, these cut-off values underwent a few controversial revisions in both the American College of Cardiology (ACC)/American Heart Association (AHA) and European Society of Cardiology (ESC) guidelines. In the ESC 201211) and ACC/AHA 201412) guidelines, FMR was defined as severe if EROA was ≥20 mm2 or RVol ≥30 mL/beat. The rationale for this change was predominantly due to the prognostic implications of EROA in FMR. Several studies demonstrated that an EROA ≥20 mm2 in FMR was an independent predictor of mortality. 5),13),14) But it is important to note that for many of these patients, prognosis is determined by the degree of LV dysfunction rather than from the MR. However, ACC/AHA in 201715) and subsequently ESC in 202116) reverted back to the original severity cut-offs. ESC added a qualification that an EROA ≥30 mm2 may be considered as severe in the setting of an elliptical regurgitant orifice area as well as a RVol ≥45 mL in the setting of low flow conditions.
There are a few aspects to be mindful about. Firstly, severity of MR by EROA should take into account the LV end-diastolic volume (LVEDV). This will bring us to the conceptual framework of proportion and disproportionate FMR proposed by Grayburn et al.17) which we will elaborate later. Secondly, there are several limitations on echocardiographic assessment of EROA in FMR. In FMR, the regurgitant orifice is frequently crescenteric, which can lead to an under-estimation of the true EROA when calculation is done by the proximal isovelocity surface area (PISA) method which assumes a circular regurgitant orifice. In addition, rather than being holosystolic, FMR frequently has a biphasic pattern. The regurgitant orifice is largest in early and late systole with a nadir in midsystole. Measuring the EROA via PISA method may hence be over or under-estimated depending on which time point it was taken. Thirdly, FMR is dynamic and the severity can vary greatly with loading conditions.
Therefore, with awareness on the limitations and inaccuracies of individual severity parameters, it is important to make an overall assessment on the severity of MR based on multiple parameters in the context of the clinical status of the patient. If there are inconsistencies, alternative modalities such as cardiac magnetic resonance (CMR) imaging or LV angiography may be useful.
MANAGEMENT OF FMR
Prior to the development of percutaneous mitral valve repair therapies, options for the management of FMR have been medical therapy, CRT and surgery. Medical therapy has been the backbone of HF management and the pharmaceutical options available are growing. CRT best serves a specific subset of patient with ventricular dyssynchrony. As for mitral valve surgery for FMR, this has been done mainly for patients who are undergoing concomitant coronary artery bypass grafting (CABG) or other cardiac surgery. TEER is the most recent addition to the list of therapies for FMR. The Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) and Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA-FR) trials together helped define the subset of patients who would derive the greatest benefit from TEER. Most importantly, a multi-disciplinary Heart Team compromising of HF specialists, imaging physicians, interventionists, surgeons and electrophysiologists is critical to select and tailor the appropriate therapy for the individual patient.
TEER FOR FMR
Early experience of TEER for FMR
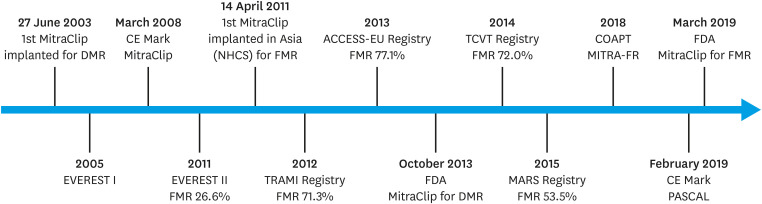
In 1991, Ottavio Alfieri developed the surgical edge-to-edge technique by suturing the mitral valve leaflet edges in a patient with anterior mitral valve prolapse to create a double-orifice mitral valve.18) Subsequently, the first-in-human MitraClip was deployed in a lady with degenerative MR (DMR).19) Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) was the landmark study that pitted MitraClip against surgery for patients with 3–4+ MR.20) In this group of patients with normal surgical risk, MitraClip compared to surgery was less efficacious in reducing MR but had superior safety and comparable mortality as well as symptom improvement. Importantly, it showed that the MitraClip was a viable option not just for patients with DMR but also for those with FMR, with approximately 27% of the study patients having FMR. However, this was insufficient to establish its role in treating patients with FMR as mitral valve surgery has not convincingly shown to be beneficial as standard of care for FMR. With the approval for commercial use, many registries provided data on the early real-world experience with the MitraClip system.21),22),23) They showed dramatic improvement in acute procedural success (>90% vs. 77% in EVEREST II) and symptomatic benefits with the MitraClip. Building on the EVEREST II trial, the European registries which had predominantly (>70%) FMR patients, demonstrated the feasibility and safety of the MitraClip in this group. However, mortality was high at 1 year (approximately 15–20%) given the challenging profiles of patients with FMR—older, sicker, depressed LVEF and more co-morbidities. Kicking start in Asia, the first MitraClip was implanted in National Heart Centre Singapore (NHCS) in a patient with FMR.24) The MitraClip Asia-Pacific Registry subsequently described the early outcomes of MitraClip in the Asia-Pacific region.25) Similar to the European registries, acute procedural success was high (>90%) and there was no difference in 30-day outcomes of mortality, New York Heart Association (NYHA) functional class and MR severity ≤2+ between the DMR and FMR cohorts. Figure 1 shows the timeline of key milestones while Table 1 gives a summary on the important publications for TEER on FMR.
Figure 1. Timeline for TEER in FMR.
TEER = transcatheter edge-to-edge repair; FMR = functional mitral regurgitation; DMR = degenerative mitral regurgitation; EVEREST = Endovascular Valve Edge-to-Edge Repair Study; CE Mark = Conformité Européene Mark; NHCS = National Heart Centre Singapore; TRAMI = transcatheter mitral valve interventions; FDA = Food and Drug Administration; TCVT = Transcatheter Valve Treatment Sentinel Pilot Registry; MARS = MitraClip Asia-Pacific Registry; COAPT = Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; MITRA-FR = Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation.
Table 1. Summary of publications for TEER in FMR.
| Publication | Study type | Location | Numbers | FMR | Baseline characteristics | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|
| EVEREST II20) | RCT | • US and Canada | 184 | FMR 26.6% | • Age 67.3±12.8 years | • Acute procedural success=77.0% | |||
| • 37 sites | DMR 73.4% | • STS score 5.0±4.0% | • Mortality at 1-year=6.1% | ||||||
| • September 2005 to November 2008 | • NYHA I–II at 1-year=98.0% | ||||||||
| • MR ≤2+ at 1-year=79.0% | |||||||||
| • Similar rate of mortality and symptom improvement between TEER and surgery but better MR reduction with surgery and superior safety with TEER | |||||||||
| TRAMI21) | Registry | • Germany | 749 | FMR 71.3% | • Age 76.0 (71.0–81.0) years | • Acute procedural success=97.0% | |||
| • 21 sites | DMR 27.8% | • Log EuroSCORE 20.0 (12.0–31.0)% | • Mortality at 1-year=20.3% | ||||||
| • August 2010 to July 2013 | • STS score 6.0 (4.0–11.0)% | • NYHA I–II at 1-year=63.3% | |||||||
| ACCESS-EU22) | Registry | • European | 567 | FMR 77.1% | • Age 73.7±9.6 years | • Acute procedural success=91.0% | |||
| • 14 sites | DMR 22.9% | • Log EuroSCORE 23.0±18.3% | • Mortality at 1-year=17.3% | ||||||
| • April 2009 to April 2011 | • NYHA I–II at 1-year=71.4% | ||||||||
| • MR ≤2+ at 1-year=78.9% | |||||||||
| TCVT23) | Registry | • European | 628 | FMR 72.0% | • Age 74.2±9.7 years | • Acute procedural success=95.4% | |||
| • 25 sites | DMR 28.0% | • Log EuroSCORE 20.4±16.7% | • Mortality at 1-year=15.3% | ||||||
| • January 2011 to December 2012 | • NYHA I–II at 1-year=74.2% | ||||||||
| • MR ≤2+ at 1-year=94.0% | |||||||||
| MARS25) | Registry | • Singapore, Australia, Malaysia, Indonesia, China | 163 | FMR 54.0% | FMR patients | FMR patients | |||
| • 8 sites | DMR 46.0% | Age 70.4±10.1 years | Acute procedural success=95.5% | ||||||
| • February 2011 to March 2014 | STS score 8.8±9.3% | Mortality at 30-day=4.5% | |||||||
| Logistic EuroSCORE 19.0±14.4% | MR ≤2+ at 30-day=83.0% | ||||||||
| NYHA I–II at 30-day=78.2% | |||||||||
| No difference in outcomes between DMR and FMR patients who underwent TEER | |||||||||
| MITRA-FR26) | RCT | • France | 152 | FMR 100% | • Age 70.1±10.1 years | • Acute procedural success=95.8% | |||
| • 37 sites | • EuroSCORE II 6.6 (3.5–11.9)% | • Mortality at 1-year=24.3% | |||||||
| • December 2013 to March 2017 | • No difference in rate of all-cause mortality or HF hospitalization between TEER + GDMT and GDMT alone at 1 year | ||||||||
| COAPT27) | RCT | • US and Canada | 302 | FMR 100% | • Age 71.7±11.8 years | • Acute procedural success=95.0% | |||
| • 78 sites | • STS score 7.8±5.5% | • Mortality at 1-year=19.1% | |||||||
| • December 2012 to June 2017 | • NYHA I–II at 1-year=72.2% | ||||||||
| • MR ≤2+ at 1-year=94.8% | |||||||||
| • TEER + GDMT had significantly lower rate of all-cause mortality and HF hospitalization compared to GDMT alone at 24 months | |||||||||
| EuroSMR28) | Registry | • European | 1,233 | FMR 100% | • Age 73±10 years | • Procedural success=93.2% (Female), 94.6% (Male) | |||
| • 8 sites | • EuroSCORE II 9±8% | • Mortality at 1-year=18.9% (Female), 19.9% (Male) | |||||||
| • November 2008 to December 2018 | • Logistic EuroSCORE 21±16% | • Mortality at 2-year=26.5% (Female), 25.4% (Male) | |||||||
| • MR ≤2+ at follow-up: median 13 months (8–29)=90.4% (Female), 90.0% (Male) | |||||||||
| STS-ACC TVT29) | Registry | • US | 33,873 (2019=10,460) | 2019 | 2019 (All patients) | 2019 (FMR) | |||
| • 403 sites | FMR 15.1% (1,576) | Age 79.0 (72.0–85.0) years | Mortality at 30-day=4.9% | ||||||
| • 2014 to March 2020 | STS score 4.91 (2.84–8.55) | NYHA I–II at 30-day=76.7% | |||||||
Shown as mean ± standard deviation or median (interquartile range) as according to primary paper.
TEER = transcatheter edge-to-edge repair; FMR = functional mitral regurgitation; DMR = degenerative mitral regurgitation; MR = mitral regurgitation; RCT = randomized controlled trial; STS = Society of Thoracic Surgeons; NYHA = New York Heart Association; EuroSCORE = European System for Cardiac Operative Risk Evaluation; HF = heart failure; GDMT = guideline-directed medical therapy; EuroSMR = European Registry of Transcatheter Repair for Secondary Mitral Regurgitation; STS-ACC TVT = Society of Thoracic Surgeons-American College of Cardiology Transcatheter Valve Therapy.
COAPT and MITRA-FR
Next came the MITRA-FR and COAPT trials which were presented 2 months apart at ESC Congress August 2018 and Transcatheter Cardiovascular Therapeutics September 2018, respectively. It created a roller-coaster ride for the community but settled down with a deeper analysis into the trials. Both studies randomized patients who had symptomatic (NYHA class ≥2) moderate-severe FMR on optimal medical therapy to either MitraClip with medical therapy or medical therapy alone. MITRA-FR was an investigator-initiated European study involving 304 patients from 37 French sites and was the first of the 2 studies to be released.26) COAPT was an industry-sponsored North American study that involved 614 patients from 78 centres in US and Canada.27) On the surface, the results of the 2 trials were dramatically different. In MITRA-FR, there was no difference in the primary composite end-point of freedom from all-cause death or HF hospitalization, as well as the secondary outcomes of all-cause death, cardiovascular death, HF hospitalization or major adverse cardiovascular events at 1 year between the 2 groups. In COAPT, MitraClip with guideline-directed medical therapy (GDMT) significantly reduced the primary efficacy end-point of HF hospitalization at 2 years as compared to GDMT alone (annualized rate of HF hospitalizations 35.8% with MitraClip and GDMT group vs. 67.9% with GDMT alone; hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.40–0.70; p<0.001), yielding a number needed-to-treat (NNT) of 3.1. In addition, the 2-year all-cause mortality, a powered, pre-specified secondary end-point, was significantly lower among MitraClip-treated patients (29.1% among MitraClip-treated patients vs. 46.1% for GDMT only patients; HR, 0.62; 95% CI, 0.46–0.82; p<0.001) yielding a NNT of 5.9. The mortality benefit garnered from MitraClip therapy in the COAPT trial was unprecedented compared with prior trials of other HF therapies such as beta-blockers, angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB), angiotensin receptor-neprilysin Inhibitor (ARNi), mineralocorticoid receptor antagonists (MRA), implantable cardioverter-defibrillator and CRT. All other powered secondary endpoints including NYHA status, 6-minute walk distance (6MWD) and quality of life (QOL) measures showed significant improvements with MitraClip therapy.
These were seemingly similar studies but with contrasting results. However, careful analysis reveals very different patient populations. Firstly, in MITRA-FR, severe FMR was defined by European guidelines with EROA ≥20 mm2 or RVol ≥30 mL/beat. In COAPT, severe FMR was defined by US guidelines and a tiered set of criteria adapted from the 2003 ASE guidelines to quantify MR severity was used. Hence the mean EROA was larger in COAPT (41±15 mm2) as compared to that in MITRA-FR (31±10 mm2). Secondly, the inclusion criteria for COAPT limited the LVEF to 20–50% and LV end-systolic diameter (LVSED) ≤70 mm while MITRA-FR allowed for an LVEF of 15–40% and had no restrictions on LV size. Therefore, patients in the MITRA-FR study had more dilated LV with mean LVEDV 135±35 mL/m2 as compared to 101±34 mL/m2 in COAPT patients. Thirdly, the COAPT study had a central eligibility committee which adjudicated that patient were on maximally tolerated GDMT and remained symptomatic before offering the MitraClip therapy. In the MITRA-FR trial, GDMT was up to individual heart teams, which more accurately reflected real-world practice. Lastly, there was a difference in technical results between both studies. Acute procedural results of MR ≤2+ was 95% in COAPT as compared to 91% in MITRA-FR. The procedural complication rate for COAPT was 8.5% and the primary safety endpoint of freedom from device-related complications at 12 months was 96.6%. At 1-year, 95% of patients in COAPT had MR ≤2+ as compared to 83% in MITRA-FR. These results reflected the superiority of procedural outcomes in the COAPT study. Some reasons arose in an attempt to explaining the disparity in procedural results. Procedural experience was one as MITRA-FR allowed centres to participate in the study as long as they had performed 5 prior MitraClip procedures. The dilated LV among MITRA-FR patients could possibly make it more challenging for clip deployment and maintain optimal coaptation over time.
On the basis of the results of the COAPT trial, the US Food and Drug Administration (FDA) approved the use of MitraClip for patients with severe FMR despite maximally tolerated GDMT on 14th March 2019. In both the ACC/AHA 202030) and ESC 202116) guidelines, TEER has a class 2a recommendation for patients with severe, symptomatic FMR fulfilling COAPT inclusion criteria. Similarly in the Asia-Pacific region, the Asian Pacific Society of Cardiology (APSC) published a consensus document in 2021 which recommend consideration of MitraClip for patients with 3–4+ FMR who remained symptomatic despite optimal GDMT and CRT-defibrillator (CRT-D) if indicated.31) Table 2 provides a summary on the evolution of guidelines and consensus statements on TEER for FMR.
Table 2. Evolution of guidelines and consensus statements on TEER for FMR.
| Title | Year | Type | Recommendation | Class |
|---|---|---|---|---|
| AHA/ACC: VHD Focused Update15) | 2017 | Guideline | None for TEER for FMR as clinical use in US has yet been approved in 2017. | NA |
| ESC/EACTS: VHD32) | 2017 | Guideline | When revascularization is not indicated and surgical risk is not low, a percutaneous edge-to-edge procedure may be considered in patients with severe SMR and LVEF >30% who remain symptomatic despite optimal medical management (including CRT if indicated) and who have a suitable valve morphology by echocardiography, avoiding futility. | 2b |
| In patients with severe SMR and LVEF <30% who remain symptomatic despite optimal medical management (including CRT if indicated) and who have no option for revascularization, the Heart Team may consider a percutaneous edge-to-edge procedure or valve surgery after careful evaluation for a ventricular assist device or heart transplant according to individual patient characteristics. | 2b | |||
| ACC/AHA: VHD30) | 2020 | Guideline | In patients with chronic severe SMR related to LV systolic dysfunction (LVEF <50%) who have persistent symptoms (NYHA class II, III, or IV) while on optimal GDMT for HF (stage D), TEER is reasonable in patients with appropriate anatomy as defined on TEE and with LVEF between 20% and 50%, LVESD ≤70 mm, and pulmonary artery systolic pressure ≤70 mmHg. | 2a |
| APSC: MitraClip for MR31) | 2021 | Consensus recommendations | MitraClip should be considered for (≥3+) symptomatic FMR patients who are already on GDMT. FMR patients should receive at least 1 month of optimized GDMT, with reasonable attempts to uptitrate treatment, as well as CRT-D if indicated, before being evaluated for further intervention or MitraClip use. | NA |
| ESC/EACTS: VHD16) | 2021 | Guideline | Patients without concomitant coronary artery or other cardiac disease requiring treatment. | 2a |
| TEER should be considered in selected symptomatic patients, not eligible for surgery and fulfilling criteria suggesting an increased chance of responding to the treatment (COAPT criteria). | ||||
| Patients without concomitant coronary artery or other cardiac disease requiring treatment. | 2b | |||
| In high-risk symptomatic patients not eligible for surgery and not fulfilling the criteria suggesting an increased chance of responding to TEER, the Heart Team may consider in selected cases a TEER procedure or other transcatheter valve therapy if applicable, after careful evaluation for ventricular assist device or heart transplant. | ||||
| Patients with concomitant coronary artery or other cardiac disease requiring treatment. | 2a | |||
| In symptomatic patients, who are judged not appropriate for surgery by the Heart Team on the basis of their individual characteristics, PCI (and/or TAVI) possibly followed by TEER (in case of persisting severe SMR) should be considered. |
TEER = transcatheter edge-to-edge repair; FMR = functional mitral regurgitation; NA = not applicable; AHA = American Heart Association; ACC = American College of Cardiology; VHD = valvular heart disease; ESC = European Society of Cardiology; EACTS = European Association for Cardio-Thoracic Surgery; SMR = secondary mitral regurgitation; APSC = Asian Pacific Society of Cardiology; LVEF = left ventricular ejection fraction; CRT = cardiac resynchronization therpy; CRT-D = cardiac resynchronization therapy-defibrillator; LV = left ventricular; NYHA = New York Heart Association; GDMT = guideline-directed medical therapy; HF = heart failure; TEE = transesophageal echocardiogram; LVESD = left ventricular end-systolic diameter; MR = mitral regurgitation; PCI = percutaneous coronary intervention; TAVI = transcatheter aortic valve implantation; COAPT = Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation.
Most recently, the 3-year outcomes of the COAPT study were reported which continued to demonstrate durable benefits with TEER in their patient cohort.33) Firstly, the curves for the composite end-point of all-cause death or HF hospitalization continued to diverge from 2 to 3 years in favour of TEER and GDMT compared to GDMT alone. TEER resulted in a NNT of 3.4 to prevent 1 death or HF hospitalization within 3 years compared to 4.5 within 2 years. Secondly, there were sustained improvement in the outcomes of all-cause mortality, HF hospitalization, QOL measures and functional capacity with TEER at 3 years. Thirdly, among patients who were originally from the GDMT only group that crossed over and underwent TEER after 2 years had lower subsequent compositive outcome of death or HF hospitalization as well as HF hospitalizations compared with those who remained on GDMT only. Lastly, TEER was safe with no device-related complications between 30 days and 3 years.
Conceptual framework of disproportionate and proportionate MR
Although both studies set off from the start with similar goals to compare MitraClip with GDMT against GDMT alone in FMR, the slight differences in inclusion criteria generated drastically different results which improved our understanding on patient selection.
Before the COAPT and MITRA-FR trials, Grayburn et al.34) published an article in 2014 demonstrating the importance of taking into account the contribution of LV volume (LVEDV), LV function (LVEF) and pressure (LV-LA pressure gradient) when assessing FMR severity using EROA. Based on Gorlin’s hydraulic formula, there is a linear relationship between EROA and LVEDV for a given LVEF, with increasing MR EROA expected as the LV dilates. An EROA of 20 mm2 can be associated with severe MR (RF >50%) when LVEDV is smaller at approximately 150 mL. However, if the LV is severely dilated at 300 mL, larger values of EROA of at least 40 mm2 is required to identify severe MR.
After the COAPT and MITRA-FR trials, Grayburn et al.35) reconciled the difference in results with a conceptual framework of proportionate and disproportionate MR. In patients with proportionately severe MR, the severity of MR is expected based on the degree of LV dilatation (echo-derived EROA is approximately similar to the Gorlin formula-derived EROA). With marked LV dilatation, both papillary muscles are displaced symmetrically resulting in impaired mitral leaflet coaptation. In such cases, medical therapy with neurohumoral antagonists to reduce LV volumes will in turn reduce MR severity. As for those with disproportionately severe MR, the severity of MR is disproportionate (greater than expected) to the degree of LV dilatation (echo-derived EROA is greater than the Gorlin formula-derived EROA). Localized LV dysfunction or ventricular dyssynchrony result in uncoordinated papillary muscle contraction and hence MR. Procedures that restore mitral valve function, such as mitral valve interventions or ventricular resynchronization, will hence provide the greatest benefit in such cases. Therefore, based on this conceptual framework, patients from the COAPT trial with larger EROA and smaller LV fell into the category of disproportionate MR while those in the MITRA-FR trial with smaller EROA and larger LV had more proportionate MR. The results from the COAPT trial support the benefit of TEER in disproportionate MR.
Subsequent work was done to test this conceptual framework among existing studies and registries.
1. In a sub-group analysis of the COAPT trial, patients were divided into 6 sub-groups based on EROA (≤30, >30–40 and >40 mm2) and LVEDV index (LVEDVi, ≤96 or >96 mL/m2).7) Disproportionate MR was identified when EROA/LVEDV ratio was greater than 0.14. Out of the 6 sub-groups, 1 sub-group had proportionate MR and they had similar efficacy to TEER with patients from the MITRA-FR trial in terms of combined all-cause mortality or HF hospitalization at 12 months. Another important finding is that for patients with EROA ≤30 mm2, those with small LV volumes responded better to TEER compared with those with larger LV volumes, reflecting the importance of LV size over MR severity in determining response to TEER. However, this is a post hoc analysis and numbers are small (56 patients in the sub-group with proportionate MR).
2. Another study from 3 European centres yielded similar findings. The 344 patients with 3–4+ FMR and on at least 3 months of GDMT underwent TEER with MitraClip.36) Outcomes were compared between those with a COAPT-like profile and those with a non-COAPT-like profile. All of 3 criteria must be fulfilled to define a COAPT-like profile: (1) absence of severe LV dysfunction with LVEF ≥20% and LVESD ≤70 mm, (2) absence of right ventricular impairment and/or severe pulmonary hypertension (PH) and (3) absence of haemodynamic instability. Patients with a COAPT-like profile had greater freedom from all-cause mortality and composite end-point of cardiovascular death and HF hospitalization at 2-year and 5-year compared with non-COAPT-like patients.
3. However, Orban et al.37) tested this conceptual framework with patients from the EuroSMR registry using the EROA/LVEDV ratio but was unable to provide convincing results to support this. The 1,016 patients with FMR who underwent the MitraClip procedure in 8 European centres were stratified into 3 groups based on EROA/LVEDV ratio: (1) MR-dominant, MD or ‘disproportionate MR’ (high EROA/LVEDV ratio ≥0.165 cm2/100 mL LVEDV), (2) MR-LV-co-dominant, MLCD or ‘proportionate MR’ (intermediate EROA/LVEDV ratio <0.165 and ≥0.115 cm2/100 mL LVEDV) and (3) LV-dominant, LD or ‘non-severe MR’ (low EROA/LVEDV ratio <0.115 cm2/100 mL LVEDV). There was no difference in 2-year mortality between patients with MD and those with MLCD treated with the MitraClip. Survival was better compared to those with LD. Nevertheless, all patients regardless of EROA/LVEDV ratio derived symptomatic improvement in terms of NYHA functional class, QOL and 6MWD.
4. From a different perspective, Bartko et al.38) looked at 291 patients with HF and reduced LVEF on GDMT (93% renin-angiotensin-aldosterone system [RAAS] inhibitors, 86% on beta-blockers and 63% on MRA). Median LVEF was 25% and LVEDV of 214 mL. At 84 months, patients with disproportionate MR had a near 2-fold higher mortality as compared to those with proportionate and non-severe MR. Therefore, in patients with disproportionate MR, GDMT only may be insufficient and interventions on the mitral valve is likely required to improve outcomes.
5. Similarly, Sannino et al.39) evaluated 177 patients with symptomatic 3–4+ FMR for which 43 patients (27%) have a 1+ reduction in MR severity with medical therapy alone. Responders to medical therapy, when compared with non-responders, were less likely to have QRS duration >120 ms and had lower EROA/LVEDV ratios, which are features of proportionate FMR.
Although the conceptual framework of disproportionate and proportionate MR derived from the relationship between EROA and LVEDV seems logical to reconcile the difference in outcomes after TEER in the COAPT and MITRA-FR studies, subsequent analyses put its validity to question. Instead of an EROA proportionality analysis (EROA:LVEDV ratio), Gaasch et al.40) reported with a RVol proportionality analysis (RVol:LVEDV ratio) that both studies had similarly low proportionality coefficients (0.18 in MITRA-FR and 0.15 in COAPT) that were similar to values reported in FMR studies (<0.20). Hence it was suggested that from a volume-based analysis, both trials had proportionate MR. However, a major limitation of this analysis was the use of estimated RF in the COAPT study due to the absence of reported RVol data. In both the EROA:LVEDV and RVol:LVEDV hypothesis, the Achilles heel still lies with the limitations of measurements with 2D echocardiography. Both PISA-derived EROA and RVol as well as LV volume measurements are frequently under-estimated with 2D echocardiography. 3D echocardiography41) and cardiac MRI42) could help overcome these limitations.
In our opinion, FMR proportionality is a conceptual framework to understand the underlying pathophysiology driving the MR and help us select the most appropriate therapy for the patient. However, it should and must not be the be-all and end-all in the selection or exclusion of patients for TEER. By dichotomizing FMR into proportionate or disproportionate may deprive patients from the benefits of TEER. In another post hoc analysis of the COAPT study based on EROA and LVEDVi relationship, patients were evaluated in 2 groups—group 1 had characteristics similar to the MITRA-FR cohort (EROA ≤0.30 cm2 and LVEDVi >96 mL/m2) while the rest were in group 2 (EROA >0.30 cm2 and/or LVEDVi ≤96 mL/m2).43) In group 1 (n=56), although there was no significant benefit in all-cause mortality and HF hospitalization at 24 months with TEER, there was however significant improvement in patient-centred outcomes (QOL and 6MWD) at 12 months. Outcomes from the MitraBridge registry further raises caution in routinely excluding patients based on the disproportionate and proportionate MR hypothesis.44) In this registry, 119 patients with advanced HF and 3–4+ FMR who were potential heart transplant candidates underwent MitraClip as a bridging therapy. Of note, majority of the patients would be ineligible for MitraClip based on the COAPT criteria—84% had Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles of 1 to 6. In addition, they had mostly proportionate FMR with median LVEDVi of 122.5 mL/m2, EROA of 30 mm2 and RVol of 41 mL/beat. Nonetheless, beneficial results were demonstrated with MitraClip with nearly a quarter (23.5%) of patients removed from heart transplant consideration due to clinical improvement and two thirds (64%) remained free from adverse events at 1 year (death, urgent heart transplant or left ventricular assist device [LVAD] implantation, first rehospitalization for HF).
Clinical considerations for TEER in FMR
Beyond severity of MR and LV size, the quest to identify the optimal candidate for TEER continues. Advance right ventricle (RV) dysfunction as defined by RV-pulmonary artery (PA) uncoupling and LV fibrosis have been of interest in risk stratifying patients with FMR.
Impact of the right heart on TEER in FMR
RV dysfunction,45) PH46),47) and severe tricuspid regurgitation48) have been associated with worse outcomes after TEER for FMR. However, these are individual parameters which evaluate the RV and PA circulations as separate units. RV-PA coupling assesses RV systolic performance according to the afterload and its prognostic utility in various HF and valvular heart disease populations have been demonstrated.49),50),51),52) Thus far, 2 studies have demonstrated the negative prognostic impact of abnormal RV-PA coupling on patients with FMR who underwent TEER. From the EuroSMR registry, 817 patients with FMR who underwent TEER were evaluated according to RV-PA coupling assessed by tricuspid annular plane systolic excursion-to-systolic pulmonary artery pressure (TAPSE/sPAP) ratio.53) Patients with impaired RV-PA coupling (TAPSE/sPAP ≤0.274 mm/mmHg; n=211, 25.8%) compared to those with normal RV-PA coupling (TAPSE/sPAP >0.274 mm/mmHg; n=606, 74.2%) had lower 1-year and 2-year survival (70.2% vs. 84.0%, respectively; p<0.001; and 53.4% vs. 73.1%, respectively; p<0.001). In a post hoc analysis of the COAPT trial, patients were compared based on RV-PA coupling assessed by ratio of RV free wall longitudinal strain and RV systolic pressure (RVFWLS/RVSP) instead.54) At 2 years, impaired RV-PA coupling (RVFWLS/RVSP ≤0.5%/mmHg; n=261, 70.2%) compared to normal RV-PA coupling (RVFWLS/RVSP >0.5%/mmHg; n=111, 29.8%) had higher rate of all-cause death or HF hospitalization (61.8% vs. 39.3% respectively; HR, 1.90; 95% CI, 1.35–2.66; p=0.0002), all-cause death (42.6% vs. 19.3% respectively; HR, 2.62; 95% CI, 1.64–4.19; p<0.0001), HF hospitalization (49.8% vs. 32.0% respectively; HR, 2.16; 95% CI, 1.34–3.48; p=0.002) and percentage of patients with NYHA class III–IV (57.9% vs. 40.0%; relative risk, 1.43; 95% CI, 1.11–1.88; p=0.007). In both treatment arms of the COAPT trial, impaired RV-PA coupling was associated with worse outcomes but TEER with GDMT compared to GDMT alone improved clinical outcomes to a consistent effect regardless of RV-PA uncoupling.
CMR imaging and FMR
Echocardiography is the workhorse imaging modality for the assessment of FMR. Although not mandatory in routine practice, CMR has emerged as a valuable tool in complementing echocardiography in the evaluation of FMR and refining clinical management. It provides a comprehensive assessment of MR severity, LV volumes and function as well as myocardial fibrosis. Firstly, CMR obtains excellent and reproducible measurements of LV volumes and function. Secondly, in the assessment of MR severity, CMR quantifies MR volume from the difference between LV stroke volume and total forward aortic flow volume.55) With these values, MR RF can be calculated and hence provide additional quantitative assessment of MR severity to overcome the shortcomings of echocardiography. Thirdly and most unique to CMR, characterization and identification of myocardial fibrosis has prognostic impact in FMR as well as treatment outcomes. Cavalcante et al.56) elegantly described the interaction of ischaemic MR (IMR) severity and myocardial infarct size (MIS) on prognosis. In this single-centre observation study, 578 patients with IMR who underwent CMR were followed-up over a median duration of 4.9 years. Patients with significant IMR (MR fraction ≥35%) and large MIS (≥30% LV mass) had the highest risk of all-cause mortality or heart transplant (HR, 5.16) while those with less significant IMR (MR fraction <35%) and small MIS (<15% LV mass) had the best outcomes (HR, 0.95). On multivariable analysis, the 2-way interaction between IMR severity and IMS was found to be a strong predictor of worse outcomes (p=0.008). In a sub-group analysis of patients who underwent mitral valve surgery, those with significant IMR and large MIS had the worse outcomes while those with significant IMR and small IMS had the best outcomes. Specific to TEER, Velu et al.57) look at the impact of myocardial fibrosis on outcomes. Among those with FMR (n=11) who underwent TEER, those with myocardial fibrosis had a higher rate of adverse outcomes (NYHA class III/IV or death at 1 month) compared to those without myocardial fibrosis.
Technical considerations for TEER in FMR
There are a few technical considerations for TEER in FMR that we would like to highlight.
1. The wider NTW clip has become our workhorse clip due to its ability to achieve greater horizontal coaptation for broad FMR jets. However, if there is difficulty getting the clip arm under the leaflet, there could be chords blocking the access of the wider clip. Slight medial or lateral translation may be required to overcome this obstacle and in certain circumstances, changing out to the narrower NT clip may be necessary. It is to bear in mind that the COAPT trial was performed with the NT size and excellent results were achieved.
2. Not infrequently, the posterior leaflet is severely restricted and tethered which makes grasping difficult. Entering the LV with a slight aorta hugger trajectory may help get the clip arm under the posterior leaflet. The controlled gripper actuation enhancement in the MitraClip G4 system which allows for both simultaneous and independent grasping may be useful in this situation. The anterior leaflet can be grasped first before gently torquing posteriorly to scoop under the posterior leaflet. However, this should be done as a last resort and performed with extreme caution due to the risk of leaflet deformation and tear during the maneuver.
3. After MR correction with TEER, LVEF may deteriorate and could lead to acute HF or hypotension.58) Intra-aortic balloon pump and right heart catheterization may be required, especially if TEER is performed during a decompensated state.
4. FMR is dynamic and its severity depends on the loading conditions.59) Assessment of MR severity may be best done with transthoracic echocardiogram instead of during transesophageal echocardiogram for which the latter is performed in a sedated state. Similarly, during anaesthesia when performing TEER, the severity of MR may not be reflective of the awake and ambulatory states. It is hence important to increase the blood pressure after grasping to assess the severity of residual MR and determine if further clips are required.
5. It is important to reduce MR severity to ≤2+. In the COAPT trial, achieving MR ≤2+ at 30 days was associated with lower all-cause mortality, HF hospitalization and QOL at 2-year compared with MR 3–4+.60) It was found that the MR severity of 0–1+ and 2+ at 30-day had similar prognosis. However, reduction of MR severity has to be balanced with the increase in mitral valve gradient (MVG) created. In general, MVG of 5 mmHg after TEER has been considered the upper limit. In a post hoc analysis of COAPT patients who underwent TEER, outcomes were evaluated based on discharge echocardiographic MVG.61) There was no difference in outcomes (2-year all-cause mortality, HF hospitalization, NYHA functional class, QOL and 6MWD) across all 4 quartiles with mean MVG of 2.1 mmHg, 3.0 mmHg, 4.2 mmHg and 7.2 mmHg respectively. In addition, when analyzed with a MVG cut-off value of 5 mmHg, discharge MVG ≥5 mmHg was not associated with worse outcomes. However, the number of patients in each group were small as well as those with MVG >5 mmHg. In our opinion, we should still avoid having an elevated post-procedural MVG but can possibly accept a slightly higher MVG if a greater reduction of MR severity can be achieved in FMR patients.
PASCAL
The PASCAL is another TEER device that received CE Mark approval for treatment of MR in February 2019. Mitral valve repair with the PASCAL device is performed similarly like the MitraClip system by approximation of the anterior and posterior leaflets but with the use of paddles and clasps instead of clip arms and grippers. There are a few differentiating features of the PASCAL device that may be of advantage in FMR pathologies with wide jets and coaptation gaps. Firstly, the broad contoured paddles maximize leaflet coaptation and distribute the stresses on the leaflet over a larger area. Secondly, the central spacer fills the regurgitant orifice area and reduces traction on the leaflets especially if there is a wide coaptation gap.
Thus far, early studies on the use of PASCAL for the treatment of FMR has been promising. CLASP is a prospective, single-arm study involving 124 patients (FMR 69%, n=85) who underwent TEER of the mitral valve with the PASCAL device.62) In the FMR cohort, the 2-year mortality and freedom from HF hospitalizations were comparable with that from the COAPT trial (CLASP: 27.7% and 77.5%, respectively; COAPT: 29.1% and 64.3%, respectively). In addition, at 2-year, there was excellent MR reduction with 95% having MR ≤2+ (99% in COAPT) and symptom improvement with 88% at NYHA class I–II compared to 35.3% at baseline. The pivotal CLASP IIF randomized controlled trial comparing PASCAL and MitraClip among patients with FMR is currently underway. It will be exciting to see if this trial will shed light on the nuances, if any, on the selection between the 2 TEER devices.
MEDICAL THERAPY FOR FMR
Guideline-directed or maximally tolerated medical therapy has always been the foundation in HF management. Neurohumoral antagonists such as beta-blockers, RAAS inhibition with ACEi, ARB and MRA have been essential and mandatory. More recently, newer agents such as ARNi and sodium-glucose co-transporter-2 inhibitors have expanded the therapeutic options.
Pharmacotherapy works to reverse LV remodeling and both beta-blockers63),64) and RAAS inhibitors65),66),67) have shown to reduce LV volumes and MR severity. In the PRIME trial, neprilysin inhibition demonstrated that it can enhance the effect of RAAS inhibition on reducing MR severity.68) 118 patients with HF and FMR from 4 Korean sites were randomized to sacubitril/valsartan or valsartan. Although the absolute values were small, sacubitril/valsartan compared to valsartan significantly reduced MR EROA (−0.058±0.095 cm2 vs. −0.018±0.105 cm2; p=0.032) and RVol (mean difference, −7.3 mL; 95% CI, −12.6 to −1.9; p=0.009) at 1 year.
Learning from the COAPT trial, it is critical for patients to be on maximally tolerated GDMT before considering TEER for MR in those that remained persistently symptomatic. Firstly, this will select for patients who have predominantly disproportionate MR that will derive the greatest benefit from TEER. Secondly, reducing the LV size will facilitate the procedure by allowing greater vertical coaptation during grasping and reduce tension on the leaflets.
After TEER in FMR, aggressive medical therapy should be maintained or even better, optimized further if MR reduction improves baseline blood pressure. Ikenaga et al.69) evaluated 41 patients who underwent repeat MV intervention for recurrent MR out of 478 consecutive patients treated with MitraClip. Seventeen patients had FMR as the original aetiology prior to MitraClip. Progressive LV dilatation with worsened tenting was the predominant reason resulting in recurrent MR. In our opinion, the results brought about 2 key points. Firstly, after TEER, patients still need to be closely monitored and have their medical therapy obsessively scrutinized and maximized. This is to maintain LV size and preserve the ability of the MitraClip to control MR. Secondly, going back to the concept of proportionate and disproportionate MR and patient selection, those with severe FMR from marked global LV dilatation will benefit less from TEER. In such cases, the underlying LV disease will continue to drive LV dilatation even after TEER and result in MR recurrence. For those with FMR who has a more ‘primary’ mechanism of MR, addressing MR should theoretically halt further LV dilatation and hence garner the most benefit from TEER. In an observational study, down-titration of GDMT after TEER was associated with higher risk of death or heart transplantation compared with those who had unchanged or up-titration of GDMT.70)
CRT FOR FMR
CRT has been shown to reduce the severity of FMR. However, various parameters (example, jet area, ERO, vena contracta) have been used in different studies which make it challenging to consolidate and quantify the degree of MR reduction from CRT.71),72),73),74),75),76),77),78),79) Nonetheless, achieving 1 or more grade in MR reduction was demonstrated in approximately 46% to 56% of patients with CRT.
The predominant mechanism of MR reduction with CRT is a more coordinated contraction of the papillary muscles and hence allowing for symmetrical coaptation of mitral valve leaflets. In addition, CRT restores LV synchrony and contraction efficiency, thereby increasing the closing forces on the mitral valve leaflets. Two phases of MR reduction have been described80)—an acute reduction of MR from coordinated contraction of papillary muscles after CRT initiation,81),82) as well as a longer-term response from the beneficial effects of CRT on reverse LV remodeling and reduction of LV size to reduce tethering forces on the mitral valve leaflets.
Most importantly, the persistence of moderate-to-severe FMR after CRT has been shown to be a negative prognostic factor with increased mortality.79),83) In such CRT nonresponders, clinical benefits have been demonstrated with TEER. The PERMIT-CARE registry was the first to describe the benefit of TEER in this subset of patients.84) 51 patients who remained symptomatic (NYHA III–IV) with significant FMR (grade ≥2) derived improvement in functional class, LVEF and reduction in LV volumes with TEER. Giaimo et al.85) described similar functional class, LVEF and LV volume improvement in 30 patients who underwent TEER after failing CRT. The latest was a post hoc analysis of patients from the COAPT trial based on the presence or absence of prior CRT.86) Regardless of prior CRT, TEER improved the clinical outcomes of patients with 3–4+ FMR who remained symptomatic despite maximally tolerated GDMT compared to GDMT alone. Clinical benefits included lower 2-year composite of death and HF hospitalization, as well as the individual components of death, HF hospitalization, QOL measures and functional capacity.
However, the optimal timing to determine if one is a non-responder to CRT remains to be defined. Di Biase et al.77) evaluated 794 patients who underwent CRT implantation and found that baseline MR severity and change in MR severity at 3 months predicted response to CRT. They hence suggested a 3-month duration for re-evaluation after CRT. Of note, the mean time between CRT and TEER was 32.9±25.7 months for the PERMIT-CARE registry and 31.7±20.2 months for patients from the study by Giaimo et al.85) This duration was not defined in the COAPT trial but patients are required to be on CRT for at least 1 month before consideration for TEER.
The current strategy in the management of symptomatic FMR despite GDMT include implantation of CRT as per guidelines before considering TEER. However, refinement on this algorithm can be made as we gain deeper insights on the factors that predict a favourable or poor response of FMR to CRT as well as on the optimal timing of mitral valve intervention after CRT implantation.
SURGERY FOR FMR
Mitral valve surgery is the gold standard for treatment of DMR but its evidence on FMR has been weak. In patients with severe FMR undergoing CABG or other cardiac surgery, in line with the guidelines, we would recommend concomitant mitral valve surgery. However, in those who have isolated severe symptomatic FMR despite GDMT, the lack of strong surgical evidence together with robust data from the COAPT trial tips in favor for TEER for mortality and functional benefits.
TRANSCATHETER MITRAL VALVE REPLACEMENT (TMVR) FOR FMR
While evidence for MV surgery for isolated severe FMR remains sparse, TEER has established its role in this cohort. However, there are certain patients with anatomical features that make TEER challenging or not feasible while surgical risk remains high and prohibitive. In such cases, TMVR has recently emerged as an attractive option.
Several early feasibility studies on TMVR devices recruited predominantly patients with FMR and demonstrated its efficacy in MR reduction, symptom improvement and safety.87) However, there are still multiple challenges of TMVR in the mitral space which limits its widespread use for now. Pivotal trials are currently ongoing.
CONCLUSION
TEER has been an invaluable addition to the therapies for FMR. In appropriately selected patients, TEER is safe and has a powerful impact on survival, reduction of HF hospitalizations and symptom improvement. Not only that, it has driven forward a deeper understanding of FMR with the anatomical, pathophysiological, imaging and interventional aspects experiencing rapid growth in recent years.
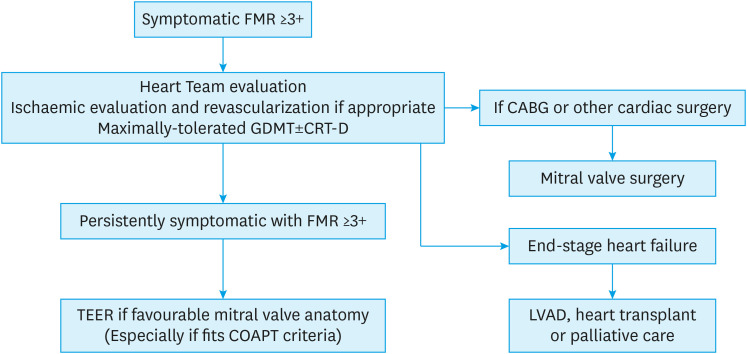
Figure 2 shows a practical algorithm on the evaluation and management of patients with symptomatic FMR taking into consideration the guidelines published and our current understanding based on available evidence. In patients with symptomatic significant FMR, we would recommend a Heart Team assessment, ischaemic evaluation and revascularization if appropriate, maximally-tolerated GDMT, and CRT-D if indicated. For patients who have end-stage HF—LVAD, heart transplant or palliation should be considered. For those undergoing CABG or other cardiac surgeries—concomitant mitral valve surgery will be recommended. In the remaining patients who are persistently symptomatic despite maximally-tolerated GDMT, TEER should be considered if mitral valve anatomy is favourable and especially so if the COAPT inclusion criteria is fulfilled (LVEF 20–50%, LVESD ≤70 mm and PA systolic pressure ≤70 mmHg). However, this clinical decision pathway will likely evolve as our understanding of FMR advances and with the development of other treatment options such as TMVR. It is exciting and hopeful times ahead for FMR.
Figure 2. Practical algorithm for symptomatic FMR management.
FMR = functional mitral regurgitation; GDMT = guideline-directed medical therapy; CRT-D = cardiac resynchronization therapy-defibrillator; CABG = coronary artery bypass graft; TEER = transcatheter edge-to-edge repair; COAPT = Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; LVAD = left ventricular assist device.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Wong N, Yeo KK.
- Writing - original draft: Wong N, Yeo KK.
- Writing - review & editing: Wong N, Yeo KK.
References
- 1.Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10:285–291. doi: 10.1016/j.cardfail.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Bartko PE, Arfsten H, Heitzinger G, et al. Natural history of bivalvular functional regurgitation. Eur Heart J Cardiovasc Imaging. 2019;20:565–573. doi: 10.1093/ehjci/jey178. [DOI] [PubMed] [Google Scholar]
- 3.Dziadzko V, Clavel MA, Dziadzko M, et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391:960–969. doi: 10.1016/S0140-6736(18)30473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sannino A, Smith RL, 2nd, Schiattarella GG, Trimarco B, Esposito G, Grayburn PA. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a systematic review and meta-analysis. JAMA Cardiol. 2017;2:1130–1139. doi: 10.1001/jamacardio.2017.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 6.Silbiger JJ. Mechanistic insights into ischemic mitral regurgitation: echocardiographic and surgical implications. J Am Soc Echocardiogr. 2011;24:707–719. doi: 10.1016/j.echo.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Packer M, Grayburn PA. New evidence supporting a novel conceptual framework for distinguishing proportionate and disproportionate functional mitral regurgitation. JAMA Cardiol. 2020;5:469–475. doi: 10.1001/jamacardio.2019.5971. [DOI] [PubMed] [Google Scholar]
- 8.Deferm S, Bertrand PB, Verbrugge FH, et al. Atrial functional mitral regurgitation: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2465–2476. doi: 10.1016/j.jacc.2019.02.061. [DOI] [PubMed] [Google Scholar]
- 9.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 10.Dujardin KS, Enriquez-Sarano M, Bailey KR, Nishimura RA, Seward JB, Tajik AJ. Grading of mitral regurgitation by quantitative Doppler echocardiography: calibration by left ventricular angiography in routine clinical practice. Circulation. 1997;96:3409–3415. doi: 10.1161/01.cir.96.10.3409. [DOI] [PubMed] [Google Scholar]
- 11.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS) Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63:e57–185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 13.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 14.Lancellotti P, Troisfontaines P, Toussaint AC, Pierard LA. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation. 2003;108:1713–1717. doi: 10.1161/01.CIR.0000087599.49332.05. [DOI] [PubMed] [Google Scholar]
- 15.Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97:1675–1680. doi: 10.1136/hrt.2011.225789. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2021:ehab395 [Google Scholar]
- 18.Grayburn PA, Thomas JD. Basic principles of the echocardiographic evaluation of mitral regurgitation. JACC Cardiovasc Imaging. 2021;14:843–853. doi: 10.1016/j.jcmg.2020.06.049. [DOI] [PubMed] [Google Scholar]
- 19.Maisano F, Torracca L, Oppizzi M, et al. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg. 1998;13:240–245. doi: 10.1016/s1010-7940(98)00014-1. [DOI] [PubMed] [Google Scholar]
- 20.Condado JA, Acquatella H, Rodriguez L, Whitlow P, Vélez-Gimo M, St Goar FG. Percutaneous edge-to-edge mitral valve repair: 2-year follow-up in the first human case. Catheter Cardiovasc Interv. 2006;67:323–325. doi: 10.1002/ccd.20603. [DOI] [PubMed] [Google Scholar]
- 21.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 22.Baldus S, Schillinger W, Franzen O, et al. MitraClip therapy in daily clinical practice: initial results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail. 2012;14:1050–1055. doi: 10.1093/eurjhf/hfs079. [DOI] [PubMed] [Google Scholar]
- 23.Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62:1052–1061. doi: 10.1016/j.jacc.2013.02.094. [DOI] [PubMed] [Google Scholar]
- 24.Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol. 2014;64:875–884. doi: 10.1016/j.jacc.2014.06.1166. [DOI] [PubMed] [Google Scholar]
- 25.Yeo KK, Ding ZP, Chua YL, et al. Percutaneous mitral valve repair with MitraClip for severe functional mitral regurgitation. Singapore Med J. 2013;54:e9–12. [PubMed] [Google Scholar]
- 26.Tay E, Muda N, Yap J, et al. The MitraClip Asia-Pacific registry: differences in outcomes between functional and degenerative mitral regurgitation. Catheter Cardiovasc Interv. 2016;87:E275–E281. doi: 10.1002/ccd.26289. [DOI] [PubMed] [Google Scholar]
- 27.Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 28.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 29.Park SD, Orban M, Karam N, et al. EuroSMR investigators. sex-related clinical characteristics and outcomes of patients undergoing transcatheter edge-to-edge repair for secondary mitral regurgitation. JACC Cardiovasc Interv. 2021;14:819–827. doi: 10.1016/j.jcin.2020.12.042. [DOI] [PubMed] [Google Scholar]
- 30.Mack M, Carroll JD, Thourani V, et al. Transcatheter mitral valve therapy in the United States: a report from the STS-ACC TVT registry. J Am Coll Cardiol. 2021;78:2326–2353. doi: 10.1016/j.jacc.2021.07.058. [DOI] [PubMed] [Google Scholar]
- 31.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143:e72–227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 32.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2021:ehab395 [Google Scholar]
- 33.Yeo KK, Tan JW, Muller DW, et al. Asian Pacific Society of Cardiology consensus recommendations on the use of MitraClip for mitral regurgitation. Eur Cardiol. 2021;16:e25. doi: 10.15420/ecr.2021.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 35.Mack MJ, Lindenfeld J, Abraham WT, et al. 3-year outcomes of transcatheter mitral valve repair in patients with heart failure. J Am Coll Cardiol. 2021;77:1029–1040. doi: 10.1016/j.jacc.2020.12.047. [DOI] [PubMed] [Google Scholar]
- 36.Grayburn PA, Carabello B, Hung J, et al. Defining “severe” secondary mitral regurgitation: emphasizing an integrated approach. J Am Coll Cardiol. 2014;64:2792–2801. doi: 10.1016/j.jacc.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc Imaging. 2019;12:353–362. doi: 10.1016/j.jcmg.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Packer M, Grayburn PA. New evidence supporting a novel conceptual framework for distinguishing proportionate and disproportionate functional mitral regurgitation. JAMA Cardiol. 2020;5:469–475. doi: 10.1001/jamacardio.2019.5971. [DOI] [PubMed] [Google Scholar]
- 39.Adamo M, Fiorelli F, Melica B, et al. COAPT-like profile predicts long-term outcomes in patients with secondary mitral regurgitation undergoing MitraClip implantation. JACC Cardiovasc Interv. 2021;14:15–25. doi: 10.1016/j.jcin.2020.09.050. [DOI] [PubMed] [Google Scholar]
- 40.Orban M, Karam N, Lubos E, et al. Impact of proportionality of secondary mitral regurgitation on outcome after transcatheter mitral valve repair. JACC Cardiovasc Imaging. 2021;14:715–725. doi: 10.1016/j.jcmg.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 41.Bartko PE, Heitzinger G, Arfsten H, et al. Disproportionate functional mitral regurgitation: advancing a conceptual framework to clinical practice. JACC Cardiovasc Imaging. 2019;12:2088–2090. doi: 10.1016/j.jcmg.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Sannino A, Sudhakaran S, Milligan G, et al. Effectiveness of medical therapy for functional mitral regurgitation in heart failure with reduced ejection fraction. J Am Coll Cardiol. 2020;76:883–884. doi: 10.1016/j.jacc.2020.05.079. [DOI] [PubMed] [Google Scholar]
- 43.Gaasch WH, Aurigemma GP, Meyer TE. An appraisal of the association of clinical outcomes with the severity of regurgitant volume relative to end-diastolic volume in patients with secondary mitral regurgitation. JAMA Cardiol. 2020;5:476–481. doi: 10.1001/jamacardio.2019.5980. [DOI] [PubMed] [Google Scholar]
- 44.Shanks M, Siebelink HM, Delgado V, et al. Quantitative assessment of mitral regurgitation: comparison between three-dimensional transesophageal echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2010;3:694–700. doi: 10.1161/CIRCIMAGING.110.947176. [DOI] [PubMed] [Google Scholar]
- 45.Uretsky S, Aldaia L, Marcoff L, et al. The effect of systolic variation of mitral regurgitation on discordance between noninvasive imaging modalities. JACC Cardiovasc Imaging. 2019;12:2431–2442. doi: 10.1016/j.jcmg.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Lindenfeld J, Abraham WT, Grayburn PA, et al. Association of effective regurgitation orifice area to left ventricular end-diastolic volume ratio with transcatheter mitral valve repair outcomes: a secondary analysis of the COAPT trial. JAMA Cardiol. 2021;6:427–436. doi: 10.1001/jamacardio.2020.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godino C, Munafò A, Scotti A, et al. MitraClip in secondary mitral regurgitation as a bridge to heart transplantation: 1-year outcomes from the International MitraBridge Registry. J Heart Lung Transplant. 2020;39:1353–1362. doi: 10.1016/j.healun.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Giannini C, Fiorelli F, Colombo A, et al. Right ventricular evaluation to improve survival outcome in patients with severe functional mitral regurgitation and advanced heart failure undergoing MitraClip therapy. Int J Cardiol. 2016;223:574–580. doi: 10.1016/j.ijcard.2016.08.189. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto T, Nakamura M, Yeow WL, et al. Impact of pulmonary hypertension on outcomes in patients with functional mitral regurgitation undergoing percutaneous edge-to-edge repair. Am J Cardiol. 2014;114:1735–1739. doi: 10.1016/j.amjcard.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 50.Tigges E, Blankenberg S, von Bardeleben RS, et al. Implication of pulmonary hypertension in patients undergoing MitraClip therapy: results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail. 2018;20:585–594. doi: 10.1002/ejhf.864. [DOI] [PubMed] [Google Scholar]
- 51.Truong VT, Ngo TNM, Mazur J, et al. Right ventricular dysfunction and tricuspid regurgitation in functional mitral regurgitation. ESC Heart Fail. 2021;8:4988–4996. doi: 10.1002/ehf2.13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosch L, Lam CS, Gong L, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–1671. doi: 10.1002/ejhf.873. [DOI] [PubMed] [Google Scholar]
- 53.Guazzi M, Dixon D, Labate V, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. doi: 10.1016/j.jcmg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 54.Lurz P, Orban M, Besler C, et al. Clinical characteristics, diagnosis, and risk stratification of pulmonary hypertension in severe tricuspid regurgitation and implications for transcatheter tricuspid valve repair. Eur Heart J. 2020;41:2785–2795. doi: 10.1093/eurheartj/ehaa138. [DOI] [PubMed] [Google Scholar]
- 55.Sultan I, Cardounel A, Abdelkarim I, et al. Right ventricle to pulmonary artery coupling in patients undergoing transcatheter aortic valve implantation. Heart. 2019;105:117–121. doi: 10.1136/heartjnl-2018-313385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karam N, Stolz L, Orban M, et al. Impact of right ventricular dysfunction on outcomes after transcatheter edge-to-edge repair for secondary mitral regurgitation. JACC Cardiovasc Imaging. 2021;14:768–778. doi: 10.1016/j.jcmg.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 57.Brener MI, Grayburn P, Lindenfeld J, et al. Right ventricular-pulmonary arterial coupling in patients with HF secondary MR: analysis from the COAPT trial. JACC Cardiovasc Interv. 2021;14:2231–2242. doi: 10.1016/j.jcin.2021.07.047. [DOI] [PubMed] [Google Scholar]
- 58.Uretsky S, Argulian E, Narula J, Wolff SD. Use of cardiac magnetic resonance imaging in assessing mitral regurgitation: current evidence. J Am Coll Cardiol. 2018;71:547–563. doi: 10.1016/j.jacc.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Cavalcante JL, Kusunose K, Obuchowski NA, et al. Prognostic impact of ischemic mitral regurgitation severity and myocardial infarct quantification by cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2020;13:1489–1501. doi: 10.1016/j.jcmg.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Velu JF, Hirsch A, Boekholdt SM, et al. Myocardial fibrosis predicts adverse outcome after MitraClip implantation. Catheter Cardiovasc Interv. 2019;93:1146–1149. doi: 10.1002/ccd.27993. [DOI] [PubMed] [Google Scholar]
- 61.Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation: implications for management. Circulation. 2008;118:2298–2303. doi: 10.1161/CIRCULATIONAHA.107.755942. [DOI] [PubMed] [Google Scholar]
- 62.Wong N, Tan WC, Widodo WA, et al. Dynamic mitral regurgitation treated with MitraClip. Ann Acad Med Singapore. 2021;50:280–282. [PubMed] [Google Scholar]
- 63.Kar S, Mack MJ, Lindenfeld J, et al. Relationship between residual mitral regurgitation and clinical and quality-of-life outcomes after transcatheter and medical treatments in heart failure: COAPT trial. Circulation. 2021;144:426–437. doi: 10.1161/CIRCULATIONAHA.120.053061. [DOI] [PubMed] [Google Scholar]
- 64.Halaby R, Herrmann HC, Gertz ZM, et al. Effect of mitral valve gradient after MitraClip on outcomes in secondary mitral regurgitation: results from the COAPT trial. JACC Cardiovasc Interv. 2021;14:879–889. doi: 10.1016/j.jcin.2021.01.049. [DOI] [PubMed] [Google Scholar]
- 65.Szerlip M, Spargias KS, Makkar R, et al. 2-year outcomes for transcatheter repair in patients with mitral regurgitation from the CLASP study. JACC Cardiovasc Interv. 2021;14:1538–1548. doi: 10.1016/j.jcin.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Waagstein F, Strömblad O, Andersson B, et al. Increased exercise ejection fraction and reversed remodeling after long-term treatment with metoprolol in congestive heart failure: a randomized, stratified, double-blind, placebo-controlled trial in mild to moderate heart failure due to ischemic or idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2003;5:679–691. doi: 10.1016/s1388-9842(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 67.Lowes BD, Gill EA, Abraham WT, et al. Effects of carvedilol on left ventricular mass, chamber geometry, and mitral regurgitation in chronic heart failure. Am J Cardiol. 1999;83:1201–1205. doi: 10.1016/s0002-9149(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 68.Seneviratne B, Moore GA, West PD. Effect of captopril on functional mitral regurgitation in dilated heart failure: a randomised double blind placebo controlled trial. Br Heart J. 1994;72:63–68. doi: 10.1136/hrt.72.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He Z, Sun Y, Gao H, et al. Efficacy and safety of supramaximal titrated inhibition of renin-angiotensin-aldosterone system in idiopathic dilated cardiomyopathy. ESC Heart Fail. 2015;2:129–138. doi: 10.1002/ehf2.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keren G, Pardes A, Eschar Y, et al. One-year clinical and echocardiographic follow-up of patients with congestive cardiomyopathy treated with captopril compared to placebo. Isr J Med Sci. 1994;30:90–98. [PubMed] [Google Scholar]
- 71.Kang DH, Park SJ, Shin SH, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–1365. doi: 10.1161/CIRCULATIONAHA.118.037077. [DOI] [PubMed] [Google Scholar]
- 72.Ikenaga H, Makar M, Rader F, et al. Mechanisms of mitral regurgitation after percutaneous mitral valve repair with the MitraClip. Eur Heart J Cardiovasc Imaging. 2020;21:1131–1143. doi: 10.1093/ehjci/jez247. [DOI] [PubMed] [Google Scholar]
- 73.Stolfo D, Castrichini M, Biagini E, et al. Modifications of medical treatment and outcome after percutaneous correction of secondary mitral regurgitation. ESC Heart Fail. 2020;7:1753–1763. doi: 10.1002/ehf2.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 75.St John Sutton MG, Plappert T, Abraham WT, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 76.Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol. 2002;40:111–118. doi: 10.1016/s0735-1097(02)01932-0. [DOI] [PubMed] [Google Scholar]
- 77.Cabrera-Bueno F, Molina-Mora MJ, Alzueta J, et al. Persistence of secondary mitral regurgitation and response to cardiac resynchronization therapy. Eur J Echocardiogr. 2010;11:131–137. doi: 10.1093/ejechocard/jep184. [DOI] [PubMed] [Google Scholar]
- 78.Verhaert D, Popović ZB, De S, et al. Impact of mitral regurgitation on reverse remodeling and outcome in patients undergoing cardiac resynchronization therapy. Circ Cardiovasc Imaging. 2012;5:21–26. doi: 10.1161/CIRCIMAGING.111.966580. [DOI] [PubMed] [Google Scholar]
- 79.van Bommel RJ, Marsan NA, Delgado V, et al. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation. 2011;124:912–919. doi: 10.1161/CIRCULATIONAHA.110.009803. [DOI] [PubMed] [Google Scholar]
- 80.Di Biase L, Auricchio A, Mohanty P, et al. Impact of cardiac resynchronization therapy on the severity of mitral regurgitation. Europace. 2011;13:829–838. doi: 10.1093/europace/eur047. [DOI] [PubMed] [Google Scholar]
- 81.Sitges M, Vidal B, Delgado V, et al. Long-term effect of cardiac resynchronization therapy on functional mitral valve regurgitation. Am J Cardiol. 2009;104:383–388. doi: 10.1016/j.amjcard.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 82.Cipriani M, Lunati M, Landolina M, et al. Prognostic implications of mitral regurgitation in patients after cardiac resynchronization therapy. Eur J Heart Fail. 2016;18:1060–1068. doi: 10.1002/ejhf.569. [DOI] [PubMed] [Google Scholar]
- 83.Spartera M, Galderisi M, Mele D, et al. Role of cardiac dyssynchrony and resynchronization therapy in functional mitral regurgitation. Eur Heart J Cardiovasc Imaging. 2016;17:471–480. doi: 10.1093/ehjci/jev352. [DOI] [PubMed] [Google Scholar]
- 84.Ypenburg C, Lancellotti P, Tops LF, et al. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J Am Coll Cardiol. 2007;50:2071–2077. doi: 10.1016/j.jacc.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 85.Kanzaki H, Bazaz R, Schwartzman D, Dohi K, Sade LE, Gorcsan J., 3rd A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol. 2004;44:1619–1625. doi: 10.1016/j.jacc.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 86.van der Bijl P, Khidir M, Ajmone Marsan N, et al. Effect of functional mitral regurgitation on outcome in patients receiving cardiac resynchronization therapy for heart failure. Am J Cardiol. 2019;123:75–83. doi: 10.1016/j.amjcard.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 87.Cipriani M, Lunati M, Landolina M, et al. Prognostic implications of mitral regurgitation in patients after cardiac resynchronization therapy. Eur J Heart Fail. 2016;18:1060–1068. doi: 10.1002/ejhf.569. [DOI] [PubMed] [Google Scholar]
- 88.Auricchio A, Schillinger W, Meyer S, et al. Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol. 2011;58:2183–2189. doi: 10.1016/j.jacc.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 89.Giaimo VL, Zappulla P, Cirasa A, et al. Long-term clinical and echocardiographic outcomes of Mitraclip therapy in patients nonresponders to cardiac resynchronization. Pacing Clin Electrophysiol. 2018;41:65–72. doi: 10.1111/pace.13241. [DOI] [PubMed] [Google Scholar]
- 90.Kosmidou I, Lindenfeld J, Abraham WT, et al. Transcatheter mitral valve repair in patients with and without cardiac resynchronization therapy: the COAPT trial. Circ Heart Fail. 2020;13:e007293. doi: 10.1161/CIRCHEARTFAILURE.120.007293. [DOI] [PubMed] [Google Scholar]
- 91.Di Biase L, Auricchio A, Mohanty P, et al. Impact of cardiac resynchronization therapy on the severity of mitral regurgitation. Europace. 2011;13:829–838. doi: 10.1093/europace/eur047. [DOI] [PubMed] [Google Scholar]
- 92.Scott EJ, Rotar EP, Charles EJ, Lim DS, Ailawadi G. Surgical versus transcatheter mitral valve replacement in functional mitral valve regurgitation. Ann Cardiothorac Surg. 2021;10:75–84. doi: 10.21037/acs-2020-mv-217. [DOI] [PMC free article] [PubMed] [Google Scholar]