Abstract
Several surrogate biomarkers possess prognostic significance for heart failure (HF), and a decline in their respective values may predict clinical improvement. However, data on the prognostic value of these biomarkers during short-term follow-up after discharge in acute decompensated HF are scarce. We aim to evaluate the prognostic value of short-term follow-up of surrogate biomarkers for predicting the prognosis of hospitalized patients with acute decompensated HF. This multi-center, prospective study will enroll consecutive hospitalized patients with acute decompensated HF. All patients will undergo sampling and comparison of biomarkers, including plasma N-terminal pro-brain natriuretic peptide, growth differentiation factor 15, troponin-T, high-sensitivity C-reactive protein, and urinary albumin/creatinine ratio obtained within 1 month and 6 months after discharge from the index admission. The primary endpoint is a composite of cardiovascular mortality or HF hospitalization during 1 year of follow-up. We will investigate the prognostic value of multiple biomarkers for the primary endpoint. This trial will provide robust evidence for novel multi-biomarker strategies for acute decompensated HF in real-world settings.
Trial Registration
ClinicalTrials.gov Identifier: NCT04437628
Keywords: Biomarkers, Heart failure
INTRODUCTION
The assessment of the clinical signs and symptoms suggestive of heart failure (HF) constitutes the first step in the diagnosis of HF.1),2) The current practice guidelines recommend that natriuretic peptide (NP), the gold standard biomarker, should be measured for the diagnosis and exclusion of HF, owing to the low diagnostic sensitivity and specificity of the clinical symptoms and signs.3),4) NP is also useful for evaluating the severity and prognosis of HF.5),6),7) However, a single biomarker cannot accurately reflect the diverse pathophysiology of HF. Thus, the potential role of multi-biomarker strategies has been suggested to improve the risk-stratification of HF beyond the single-biomarker strategy.3)
The multi-biomarker risk stratification strategy that entails the utilization of a combination of biomarkers enhances risk prediction.8),9) Most previous studies that focused on multi-biomarkers only investigated the prognostic value of the baseline biomarker levels.10),11) There is a need for studies performing repeated measurements of multiple biomarkers to enhance risk prediction beyond the baseline level. Furthermore, NP, which reflects hemodynamic stress and increased ventricular filling pressure, plays a prominent role in HF with reduced ejection fraction (HFrEF); however, the pathophysiology of HF with preserved ejection fraction (HFpEF) is more heterogeneous and involves systemic inflammation and oxidative stress. However, previous studies on multiple biomarkers have usually focused on patients with HFrEF.8),9),10)
Therefore, in the current study, we will endeavor to measure multiple biomarkers including the N-terminal pro-brain NP (NT-proBNP), growth differentiation factor 15 (GDF-15), troponin-T, high-sensitivity C-reactive protein (hs-CRP), and urinary albumin/creatinine ratio (ACR) and the respective changes in these biomarkers in hospitalized patients with acute decompensated HFrEF and HFpEF. Furthermore, we will also assess the incremental prognostic value of multi-marker models and the change during short-term follow-up in a real-world population.
STUDY DESIGN
Objectives of the trial
The main aim of Prognostic value Of Short-Term follow-up of multiple BIOmarkers after discharge in hospitalized patients with acute Heart Failure (POSTBIO-HF) is to investigate the prognostic value of the short-term follow-up of multiple biomarkers including NT-proBNP, troponin T, hs-CRP, urinary ACR, and GDF-15 for patients with acute decompensated HF.
Study design
This trial is a multicenter, nationwide, prospective, observational study. The study population consists of 1,500 hospitalized patients with acute decompensated HF. Written informed consent will be obtained from all patients. The recruitment period is 35 months, commencing from February 2021. The target follow-up duration is 12 months.
Study population
Hospitalized patients with acute decompensated HF will be eligible for enrollment in this study. The diagnostic criteria for HFrEF include 'reduced' ejection fraction (EF) (<40%), accompanied by the presence of the symptoms and/or signs of HF. The diagnosis HFpEF will be made if all of the following criteria are met: a) preservation of EF, defined as EF≥ 40%; b) presence of the symptoms and/or signs of HF; c) elevated levels of NPs (defined as B-type NP [BNP] >100 pg/mL and/or NT-proBNP >300 pg/mL); and d) any structural heart disease (left atrial volume index ≥34 mL/m2, left atrial dimension ≥50 mm, posterior or septal wall thickness ≥11 mm). Patients with any severe condition limiting the life expectancy to <3 months or patients who do not provide permission to be contacted by telephone at the end of the study will be excluded. Table 1 demonstrate the specific inclusion and exclusion criteria, respectively.
Table 1. Inclusion and exclusion criteria.
| Criteria | Detail | |
|---|---|---|
| Inclusion criteria | Patients willing and capable of providing informed consent, and who agree to follow the study protocol | |
| Age >20 years | ||
| Hospitalized patients with acute decompensated HF: the following criteria should be met for the diagnosis of HF: | ||
| 1) HF with reduced EF (i.e., EF <40%): presence of the signs and/or symptoms of HF | ||
| 2) HF with preserved ejection fraction (i.e., EF ≥40%) requires that all of the following parameters are met: a) presence of the signs and/or symptoms of HF, b) elevated levels of natriuretic peptides (defined as BNP >100 pg/mL and/or NT-proBNP >300 pg/mL), and c) any structural heart disease (left atrial volume index ≥34 mL/m2, left atrial dimension ≥50 mm, posterior or septal wall thickness ≥11 mm) | ||
| Exclusion criteria | Any severe condition limiting the life expectancy to <3 months | |
| 1) The patient does not provide permission to be contacted by telephone at the end of the study | ||
HF = heart failure; EF = ejection fraction; BNP = B-type natriuretic peptide; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Study flow
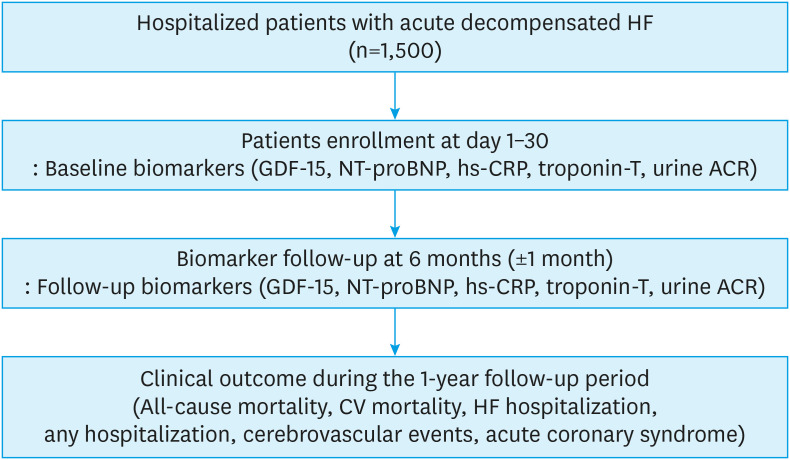
The study flowchart is presented in Figure 1. Patients who meet the inclusion criteria will be screened before discharge from the index admission. After discharge, the informed consent, the clinical characteristics, and baseline biomarkers will be obtained at the first outpatient visit conducted within 1 month. Subsequently, the follow-up biomarkers will be measured 6 months (±1 month) after the index admission. The patients with early mortality within 6 months (before the follow-up sampling) will be dropped out for the final analysis. Patients will be monitored to observe the development of the endpoints during the 1-year follow-up period.
Figure 1. Study flowchart. This figure demonstrates the flowchart of the study population.
GDF-15 = growth differentiation factor 15; NT-proBNP = N-terminal pro-brain natriuretic peptide; hs-CRP = high-sensitivity C-reactive protein; ACR = albumin/creatinine ratio; CV = cardiovascular; HF = heart failure.
Biomarkers and study variables
Plasma and urine sample will be obtained within 1 month and 6 months after discharge from the index admission. Blood samples will be stored at the study centers at −20°C or lower, followed by storage at −80°C at the central laboratory. NT-proBNP, GDF-15, troponin-T, and hs-CRP will be measured using the immunoturbidimetry assay (Roche, Mannheim, Germany). Urinary ACR will be measured using commercially available assays at each center. Each assay will be controlled within 5% of the value of the coefficient of variation every day. The attending physician will record the baseline characteristics, biomarker levels and changes, and development of endpoints in a web-based case report form with the assistance of a clinical research coordinator.
Study outcomes
The primary study outcome is a composite of cardiovascular mortality or HF hospitalization, analyzed as the time to the first event. The secondary outcomes are all-cause and cardiovascular mortality, HF hospitalization, any hospitalization, cerebrovascular events, or acute coronary syndrome.
Statistical analysis
The baseline characteristics, including the values of the biomarkers, will be presented as the mean±SD or median (interquartile range) for continuous variables, and as counts and numbers for categorical variables. The two groups will be compared using Student’s t-test or the χ2 test. The median levels of biomarkers at each time point and changes from baseline will be reported for each patient with paired NT-proBNP, GDF-15, troponin-T, hs-CRP, and urine ACR at the baseline and 6-month follow-up visits. We will assess the association between individual biomarkers and the risk of the endpoints using the Cox proportional hazard regression model. The association between the change in each biomarker and the endpoint(s) will be also evaluated using univariable and multivariable Cox models after adjusting for all baseline characteristics. All biomarkers will be standardized and expressed as 1 SD increase to facilitate intuitive unit-independent comparison. We will use time-dependent receiver operating characteristic (ROC) curves at 1 year to compare the single and multiple biomarker scores to predict the study endpoints. We will quantify improvements in predictive accuracy by comparing the area under the ROC curves (AUC) calculated using Uno’s method at 1 year.12) The net reclassification improvement (NRI) and integrated discrimination improvement (IDI) at 1 year will be calculated to evaluate the incremental value of the multiple-biomarker model compared to a single-biomarker model. We will calculate the standard error estimators for the AUCs, NRIs, and IDIs using the perturbation-resampling method proposed by Uno et al., followed by Wald statistics to test whether the difference between two AUCs, NRIs, or IDIs are equal to zero.12) The reclassification table will be presented according to clinically meaningful risk categories of 0–10%, 10–20%, 20–50%, and over 50%. The Hosmer-Lemeshow goodness-of-fit test will be performed to assess clinical performance with respect to movements across the relevant risk categories. Two-tailed p values <0.05 will be considered statistically significant.
DISCUSSION
NP, the gold standard biomarker of HF, is produced and released mainly by the ventricles of the heart in response to a period of increased mechanical load.13) BNP or NT-proBNP plays a confirmatory role in the diagnosis of HF and can also be used to assign a higher risk for poor prognosis to subpopulations with a specific HF phenotype.2) However, the complex pathophysiology of HF involves the integration of other processes with volume overload. Thus, in addition to NP, multiple biomarkers reflective of systemic inflammation, oxidative stress, myocardial remodeling, and myocardial necrosis have been implicated in HF.3) The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist trial reported that multiple novel plasma biomarkers in key pathophysiological domains augmented the risk stratification of HF.11) A score derived from multiple biomarkers reflecting diverse biological pathways significantly improved the prediction of adverse events.14) However, studies focusing on the serial follow-up of multiple biomarkers are scarce, especially in real-world populations. A multi-time point-based multiple biomarker panel may enhance the predictive value of NT-proBNP in patients with acute decompensated HF. Therefore, the current prospective study with multi-biomarkers will repeatedly measure GDF-15, hs-CRP, urine ACR, and troponin, in addition to NT-proBNP.
GDF-15 is a member of the transforming growth factor-β superfamily, which is associated with adverse outcomes in various cardiovascular diseases and HF.15) GDF-15, which is a cytokine required for myocardial healing and remodeling, is stimulated by angiotensin II, ischemia, inflammation, or oxidative stress.16) In several clinical trials with HFrEF, the baseline GDF-15 levels and subsequent elevation over time were strongly associated with cardiovascular mortality and hospitalization for HF.9),17) Interestingly, GDF-15 plays a prominent role in distinguishing between patients with HFpEF and controls. Therefore, our study with a real-world population that includes patients with both HFrEF and HFpEF may suggest the unique prognostic value of GDF-15 in a population with HFpEF.
The hs-CRP is a marker of low-grade systemic inflammation. The combination of hs-CRP and NT-proBNP can predict the risk of cardiovascular events. Studies have suggested that the combination of inflammatory and neurohumoral biomarkers may optimize the strategy to enhance risk stratification in patients with HF.18) The levels of cardiac troponins, which reflect myocardial injury or necrosis, are elevated in patients with decompensated HF.3) Elevated troponin I or T levels are associated with a worse prognosis in patients with acute decompensated HF.19) Lastly, elevated urinary ACR, reflecting glomerular damage, is a powerful prognostic biomarker in patients with HF, irrespective of the presence of hypertension, diabetes, or chronic kidney disease.20) Our study, which included multiple biomarkers representing various pathophysiologies, may elucidate the crucial role of multi-biomarker strategy for risk stratification in patients with acute decompensated HF, especially in the HFpEF subpopulation. We will also develop multiple biomarker-based scores using NRI and IDI to improve the prediction of cardiovascular events beyond a single biomarker.
CONCLUSION
The current prospective observational study will explore the prognostic value of multiple biomarkers and their short-term alternations in patients with acute decompensated HFrEF and HFpEF. We believe that the findings of this trial will provide robust evidence for novel biomarker strategies, including GDF-15, in a real-world population with acute decompensated HF.
ACKNOWLEDGEMENTS
The authors wish to thank the participants of this study.
Footnotes
Funding: This study is funded by the Roche Company.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Cho DH, Cho HJ, Choi DJ, Yoo BS.
- Data curation: Cho DH, Kim MN, Lee JH, Choi JO, Choi DJ.
- Formal analysis: Cho DH, Son JW, Choi J.
- Funding acquisition: Yoo BS.
- Investigation: Cho DH, Son JW, Lee S, Kim EJ, Choi DJ, Yoo BS.
- Methodology: Cho DH, Son JW, Lee CJ, Choi DJ.
- Project administration: Choi JO, Choi DJ, Yoo BS.
- Resources: Lee CJ, Kim MN, Lee JH, Lee S, Yang DH, Cho HJ, Choi JO, Kim EJ, Choi DJ.
- Validation: Yang DH.
- Visualization: Choi J.
- Writing - original draft: Cho DH.
- Writing - review & editing: Cho DH, Yoo BS.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Kim KJ, Cho HJ, Kim MS, et al. Focused update of 2016 Korean Society of Heart Failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019;1:4–24. doi: 10.36628/ijhf.2019.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 4.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 5.Cheng V, Kazanagra R, Garcia A, et al. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37:386–391. doi: 10.1016/s0735-1097(00)01157-8. [DOI] [PubMed] [Google Scholar]
- 6.Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–2174. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 7.Myhre PL, Vaduganathan M, Claggett B, et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019;73:1264–1272. doi: 10.1016/j.jacc.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demissei BG, Cotter G, Prescott MF, et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur J Heart Fail. 2017;19:1001–1010. doi: 10.1002/ejhf.749. [DOI] [PubMed] [Google Scholar]
- 9.Anand IS, Kempf T, Rector TS, et al. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 10.Lassus J, Gayat E, Mueller C, et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168:2186–2194. doi: 10.1016/j.ijcard.2013.01.228. [DOI] [PubMed] [Google Scholar]
- 11.Chirinos JA, Orlenko A, Zhao L, et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2020;75:1281–1295. doi: 10.1016/j.jacc.2019.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wettersten N. Biomarkers in acute heart failure: diagnosis, prognosis, and treatment. Int J Heart Fail. 2021;3:81–105. doi: 10.36628/ijhf.2020.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ky B, French B, Levy WC, et al. Multiple biomarkers for risk prediction in chronic heart failure. Circ Heart Fail. 2012;5:183–190. doi: 10.1161/CIRCHEARTFAILURE.111.965020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahrenberg R, Edelmann F, Mende M, et al. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail. 2010;12:1309–1316. doi: 10.1093/eurjhf/hfq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wollert KC, Kempf T. Growth differentiation factor 15 in heart failure: an update. Curr Heart Fail Rep. 2012;9:337–345. doi: 10.1007/s11897-012-0113-9. [DOI] [PubMed] [Google Scholar]
- 17.Bouabdallaoui N, Claggett B, Zile MR, et al. Growth differentiation factor-15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2018;20:1701–1709. doi: 10.1002/ejhf.1301. [DOI] [PubMed] [Google Scholar]
- 18.Park JJ, Choi DJ, Yoon CH, et al. Prognostic value of C-reactive protein as an inflammatory and N-terminal probrain natriuretic peptide as a neurohumoral marker in acute heart failure (from the Korean Heart Failure registry) Am J Cardiol. 2014;113:511–517. doi: 10.1016/j.amjcard.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Peacock WF, 4th, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 20.Masson S, Latini R, Milani V, et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail. 2010;3:65–72. doi: 10.1161/CIRCHEARTFAILURE.109.881805. [DOI] [PubMed] [Google Scholar]