Abstract
Background and Objectives
Lower body mass index (BMI) is considered a poor prognostic factor in patients with heart failure (HF). We aimed to investigate the clinical impact of BMI on the risk of mortality in patients with acute HF (AHF) across various phenotypes.
Methods
We retrospectively identified 4,146 registry patients with AHF and BMI data. The study population was categorized according to the WHO Asia-Pacific BMI classification: BMI <18.5 kg/m2 (underweight; n=418), BMI 18.5–23 kg/m2 (ideal; n=1,620), BMI 23–25 kg/m2 (overweight; n=828), BMI 25–30 kg/m2 (obesity I; n=1,047), and BMI ≥30 kg/m2 (obesity II; n=233). The risk of all-cause mortality was compared between these 5 groups.
Results
During a median follow-up of 32 months, 1,732 patients (41.8%) died. Compared to patients with obesity II, those with overweight, ideal BMI or underweight status had a higher risk of mortality (overweight: hazard ratio [HR], 1.606; 95% confidence interval [CI], 1.016–2.539; p=0.042) (ideal BMI: HR, 1.744; 95% CI, 1.112–2.734; p=0.015) (underweight: HR, 2.729; 95% CI, 1.686–4.417; p<0.001). Higher risk of mortality among patients with lower BMI was observed regardless of age, sex, hypertension, diabetes, ischemic heart disease, atrial fibrillation, and HF phenotype. Furthermore, low muscle index (total muscle mass/height2), calculated using serum cystatin C data in a subset of 579 patients, was associated with higher mortality risk.
Conclusions
A lower BMI is associated with a higher risk of mortality in patients with AHF. This obesity paradox is observed in AHF regardless of comorbidities and HF phenotype.
Keywords: Body mass index, Obesity, Sarcopenia, Heart failure, Mortality
INTRODUCTION
The obesity paradox in patients with heart failure (HF) refers to the observation of a lower risk of mortality and cardiovascular events in those with higher body mass index (BMI), while those with lower BMI have a worse prognosis.1),2),3) This phenomenon contrasts with well-established associations between obesity and cardiovascular risk factors, but is observed in several chronic diseases. In particular, the association between obesity and a better prognosis in HF has been reported in many studies, even though patients with obesity are at higher risk for the development of HF.4),5) The obesity paradox in HF can be explained by a higher prevalence of co-morbidities and catabolic status in patients with low BMI. As shown in several studies, the occurrence of weight loss is associated with high mortality and morbidity, and worse symptom status and quality of life in patients with HF.6),7),8) Therefore, catabolic status in patients with HF should be regarded as an important risk factor for mortality.
Despite evidence supporting the obesity paradox in patients with HF, several studies have suggested that the association between lower BMI and a worse prognosis is inconsistent, varying according to age, sex, presence of diabetes mellitus (DM), or HF phenotype (i.e. HF with reduced ejection fraction [HFrEF], mildly reduced ejection fraction [HFmrEF], or preserved ejection fraction [HFpEF]).2),9),10),11),12) Furthermore, the impact of body composition or skeletal muscle mass on the risk of mortality in relation to BMI has not been fully investigated. In the present study, we aimed to assess the relationship between BMI and mortality risk in patients admitted for acute HF (AHF), investigate whether this association differs according to comorbidities and HF phenotype, and assess the potential impact of the total muscle mass on the mortality risk.
METHODS
Patients
The STrain for Risk Assessment and Therapeutic Strategies in patients with Acute Heart Failure) (STRATS-AHF) registry enrolled 4,312 patients hospitalized for AHF at three tertiary university hospitals from January 2009 to December 2016. Detailed information regarding the registry and primary outcomes has been reported elsewhere.3),13) In brief, patients with signs or symptoms of HF, and either lung congestion or objective findings of left ventricular (LV) systolic dysfunction or structural heart disease, were eligible for the study. Patients who presented with acute coronary syndrome were excluded. The study protocol was approved by the ethics committee at each hospital and complied with the Declaration of Helsinki. Informed consent was waived, given the retrospective nature of the study.
Body mass index and its classification
Body weight and height were measured using an electronic scale, during the index hospitalization for AHF. BMI was calculated as the body weight (kg) divided by the height (m) squared. Participants were divided into five groups according to the World Health Organization Asia-Pacific BMI classification: BMI <18.5 kg/m2 (underweight), BMI 18.5–23 kg/m2 (ideal), BMI 23–25 kg/m2 (overweight), BMI 25–30 kg/m2 (obesity class I), and BMI ≥30 kg/m2 (obesity class II).14)
Measurement of muscle mass and muscle index
Among the total study population (n=4,146), serum levels of cystatin C were available in a subset of 579 patients. Given that cystatin C is independent of muscle mass, and that the discrepancy between creatinine and cystatin C-based glomerular filtration rate (GFR) can be explained by the muscle mass, the total body muscle mass can be estimated by the serum levels of creatinine and cystatin C, using the equations suggested by Kim et al.15) Details regarding the calculation of the total muscle mass are provided in the Supplementary Data 1.
Then, in order to estimate the total muscle mass according to the body size of patients, we adopted the muscle index, calculated as the total muscle mass/height (m)2, considering that the skeletal muscle index (appendicular skeletal muscle mass/height2)) is used to diagnose sarcopenia.16)
Echocardiography and strain analysis
All images were obtained using a standard ultrasound machine with a 2.5-MHz probe. Standard techniques were used to obtain M-mode, 2-dimensional, Doppler, and strain measurements, in accordance with the guidelines of the American Society of Echocardiography.17),18)
Study outcomes
The primary outcome was the 5-year all-cause mortality from the index hospitalization for AHF. The data regarding the vital status of all patients were collected from the National Insurance database or National Death Records.
Statistical analysis
Continuous variables are presented as the mean±standard deviation (SD) or median with interquartile range (IQR); categorical variables are presented as frequencies. Differences between groups were evaluated using the Student’s t-test, one-way analysis of variance, Mann-Whitney U test, or Kruskal-Wallis test for continuous variables, and the χ2 test or Fisher exact test for categorical variables, as appropriate.
As patients exclusively underwent assessment of either the B-type natriuretic peptide (BNP) level or the N-terminal proBNP (NT-proBNP) level, the natriuretic peptide level could not be applied as a continuous variable. Thus, given the statistically equal distribution of these two parameters and their comparable prognostic values, BNP and NT-proBNP levels were each categorized into 10 deciles. The cut-off values for the 10 deciles of BNP and NTproBNP levels are shown in Supplementary Figure 1.
Survival analyses were performed using the Cox proportional hazard regression model to determine the independent predictors of the study outcome. Multivariable Cox proportional hazard regression analysis, with the stepwise backward elimination method, was performed using variables with p values of <0.1 on univariable analysis. Data were analyzed using SPSS version 22 (IBM, Chicago, IL, USA), and R programming software version 4.1.1 (The R Foundation for Statistical Computing, Vienna, Austria). A two-sided p value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics
The median age of the study participants was 73 years, 53% were male, and the median BMI was 23.0 kg/m2 (Table 1). The median age showed a gradual decrease with higher BMI, whereas the prevalence of comorbidities, including hypertension (HTN), DM, and ischemic heart disease (IHD), was more frequent in patients with higher BMI. Accordingly, cholesterol and glycated hemoglobin (HbA1c) levels gradually increased with higher BMI category. Additionally, the hemoglobin level tended to be lower among those with lower BMI.
Table 1. Baseline characteristics.
| Characteristics | Total (n=4,146) | Underweight (n=418) | Ideal BMI (n=1,620) | Overweight (n=828) | Obesity class I (n=1,047) | Obesity class II (n=233) | p value | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 73 (63–80) | 78 (70–84) | 75 (65–81) | 73 (63–80) | 71 (60–78) | 68 (50–77) | <0.001 | |
| Male sex | 2,207 (53.2) | 186 (44.4) | 855 (52.7) | 483 (58.3) | 570 (54.4) | 113 (48.5) | <0.001 | |
| BMI (kg/m2) | 23.0 (20.7–25.7) | 17.0 (16.1–18.0) | 21.0 (20.0–22.0) | 24.0 (23.5–24.4) | 26.6 (25.8–27.9) | 32.3 (31.0–34.0) | <0.001 | |
| Systolic blood pressure (mmHg) | 125 (110–144) | 120 (106–140) | 122 (109–141) | 125 (109–142) | 129 (112–148) | 139 (119–154) | <0.001 | |
| Diastolic blood pressure (mmHg) | 72 (62–83) | 70 (60–80) | 71 (61–81) | 70 (61–81) | 74 (64–85) | 78 (68–90) | <0.001 | |
| NYHA functional class IV | 1,566 (37.8) | 161 (38.4) | 623 (38.4) | 305 (36.8) | 397 (37.9) | 80 (35.9) | 0.803 | |
| Hypertension | 2,386 (57.5) | 190 (45.3) | 866 (53.4) | 500 (60.4) | 671 (64.1) | 159 (71.3) | <0.001 | |
| Diabetes mellitus | 1,432 (34.5) | 101 (24.1) | 500 (30.9) | 309 (37.3) | 417 (39.8) | 105 (47.1) | <0.001 | |
| Ischemic heart disease | 1,348 (32.5) | 106 (25.3) | 540 (33.3) | 290 (35.0) | 337 (32.2) | 75 (33.6) | 0.013 | |
| Atrial fibrillation | 1,233 (29.7) | 128 (30.5) | 465 (28.7) | 238 (28.7) | 329 (31.4) | 73 (32.7) | 0.539 | |
| Laboratory tests | ||||||||
| Total cholesterol (mg/dL) | 150.0 (123.0–180.0) | 145.5 (117.9–174.0) | 149.0 (123.0–177.0) | 148.0 (123.0–180.0) | 154.0 (126.0–185.0) | 152.0 (126.0–180.0) | 0.001 | |
| Hemoglobin (g/dL) | 12.0 (10.0–14.0) | 11.0 (10.0–13.0) | 12.0 (10.0–14.0) | 12.0 (10.0–14.0) | 12.0 (11.0–14.0) | 13.0 (11.0–15.0) | <0.001 | |
| Blood urea nitrogen (mg/dL) | 21.0 (15.9–31.0) | 22.0 (16.0–34.0) | 22.0 (16.0–32.0) | 20.0 (15.5–30.2) | 20.2 (15.0–29.0) | 19.0 (15.0–28.0) | 0.012 | |
| Creatinine (mg/dL) | 1.1 (0.8–1.5) | 1.0 (0.8–1.4) | 1.1 (0.8–1.6) | 1.1 (0.9–1.5) | 1.1 (0.9–1.5) | 1.1 (0.8–1.6) | 0.737 | |
| GFR (mL/min/1.73m2) | 61.3 (38.2–82.7) | 63.0 (38.6–82.7) | 58.8 (37.5–81.8) | 60.3 (37.6–83.2) | 62.9 (40.0–83.4) | 65.4 (38.7–84.1) | 0.207 | |
| Hemoglobin A1c (%) | 6.1 (5.8–7.0) | 6.0 (5.7–6.6) | 6.0 (5.7–7.0) | 6.2 (5.7–7.0) | 6.3 (5.9–7.2) | 6.7 (6.0–7.8) | <0.001 | |
| BNP (pg/mL) | 980.0 (463.0–2,069.0) | 1,422.0 (533.0–3,177.0) | 1,043.5 (523.0–2,495.0) | 828.0 (381.0–1,752.0) | 902.5 (462.0–1,720.0) | 599.0 (172.5–1,350.5) | <0.001 | |
| NT-proBNP (pg/mL) | 4,490.3 (1,698.0–11,060.0) | 9,381.5 (3,514.0–18,572.6) | 5,615.0 (2,141.0–13,814.5) | 4,299.8 (1,546.0–10,374.0) | 2,746.0 (1,101.0–6,274.8) | 2,473.1 (908.5–6,250.9) | <0.001 | |
| Echocardiographic parameters | ||||||||
| LV-EDD (mm) | 53.0 (47.0–59.1) | 51.0 (45.0–57.0) | 53.0 (47.0–60.0) | 53.0 (47.0–59.0) | 53.0 (47.0–60.0) | 53.2 (48.0–61.0) | <0.001 | |
| LV-ESD (mm) | 40.0 (32.0–48.7) | 39.0 (32.0–47.6) | 41.0 (33.0–49.3) | 40.0 (32.5–48.0) | 38.7 (31.0–48.0) | 37.0 (30.2–50.0) | 0.005 | |
| LV-MI (g/m2) | 127.2 (103.7–156.7) | 131.6 (105.2–169.1) | 129.0 (105.5–162.1) | 127.2 (101.7–155.0) | 123.7 (101.4–150.1) | 123.7 (105.0–150.1) | <0.001 | |
| LV-EDV (mL) | 109.2 (76.0–152.8) | 100.0 (72.0–140.0) | 112.0 (78.0–154.0) | 106.4 (75.5–146.8) | 111.0 (75.0–156.0) | 109.5 (74.6–171.0) | <0.001 | |
| LV-ESV (mL) | 67.0 (37.7–107.0) | 65.9 (36.6–97.0) | 70.0 (40.7–109.0) | 64.0 (37.1–104.0) | 64.0 (36.0–109.0) | 60.7 (31.0–120.0) | 0.012 | |
| LV-EF (%) | 39.0 (27.0–54.0) | 37.0 (27.0–52.8) | 37.0 (26.1–51.2) | 40.0 (28.0–54.0) | 41.3 (29.0–56.0) | 44.3 (28.5–59.1) | <0.001 | |
| E velocity (m/sec) | 0.98 (0.66–1.00) | 0.97 (0.60–1.02) | 1.00 (0.69–1.00) | 0.95 (0.65–1.00) | 0.98 (0.66–1.00) | 0.96 (0.74–1.03) | 0.379 | |
| A velocity (m/sec) | 0.86 (0.57–1.00) | 0.91 (0.64–1.00) | 0.84 (0.54–1.00) | 0.88 (0.58–1.00) | 0.86 (0.59–1.00) | 0.84 (0.46–1.00) | 0.887 | |
| e’ velocity (cm/sec) | 5.0 (3.8–6.2) | 5.0 (3.6–6.1) | 4.8 (3.6–6.0) | 4.9 (3.8–6.0) | 5.0 (4.0–6.6) | 5.1 (4.0–7.0) | <0.001 | |
| a’ velocity (cm/sec) | 6.7 (5.0–8.2) | 7.0 (5.6–8.2) | 6.0 (4.8–8.0) | 7.0 (5.0–8.5) | 6.9 (5.0–9.0) | 7.1 (4.8–8.9) | 0.015 | |
| s’ velocity (cm/sec) | 5.1 (4.0–6.6) | 5.0 (4.0–6.4) | 5.0 (4.0–6.4) | 5.0 (4.0–6.4) | 5.4 (4.3–7.0) | 5.8 (4.4–7.0) | <0.001 | |
| E/e’ ratio | 16.6 (11.7–23.2) | 15.9 (11.3–22.0) | 17.2 (12.0–24.3) | 16.1 (11.4–23.2) | 15.9 (11.4–22.2) | 15.7 (12.2–21.8) | <0.001 | |
| Left atrial dimension (mm) | 44.0 (39.0–50.9) | 41.4 (34.9–48.0) | 44.0 (38.4–50.0) | 44.0 (39.0–50.0) | 46.0 (41.0–52.0) | 48.8 (43.2–54.9) | <0.001 | |
| LAVI (mL/m2) | 51.8 (38.0–70.4) | 55.2 (38.3–80.5) | 54.7 (40.0–74.0) | 49.2 (36.4–68.8) | 49.3 (37.0–67.0) | 48.9 (35.7–62.3) | <0.001 | |
| LARS (%) | 12.0 (7.0–19.5) | 12.0 (6.3–17.9) | 11.5 (7.0–18.5) | 13.0 (8.0–21.0) | 13.0 (8.0–17.0) | 12.0 (7.0–16.0) | 0.002 | |
| LV-GLS (%) | 10.0 (7.0–14.0) | 10.1 (7.0–13.7) | 9.9 (7.0–13.7) | 10.0 (7.3–14.0) | 11.0 (7.0–14.4) | 10.1 (6.7–14.7) | 0.011 | |
| RV strain (%) | 13.0 (8.0–17.0) | 12.0 (8.0–16.0) | 13.0 (8.0–17.0) | 13.0 (8.0–17.0) | 13.0 (8.0–17.0) | 12.0 (7.0–16.0) | 0.198 | |
| TR Vmax (m/sec) | 3.0 (2.5–3.4) | 3.0 (2.6–3.4) | 3.0 (2.6–3.4) | 2.9 (2.5–3.3) | 2.9 (2.5–3.3) | 3.0 (2.5–3.3) | <0.001 | |
| PASP (mmHg) | 41.5 (32.0–52.9) | 43.0 (33.6–54.0) | 43.4 (34.0–54.0) | 41.0 (30.0–50.7) | 39.1 (29.5–51.0) | 41.7 (30.6–51.0) | <0.001 | |
| Medication at discharge | ||||||||
| Use of beta-blockers | 2,533 (61.1) | 245 (58.5) | 924 (57.0) | 507 (61.2) | 702 (67.0) | 155 (69.5) | <0.001 | |
| Use of RAS blockers | 2,833 (68.3) | 258 (61.6) | 1,078 (66.5) | 566 (68.4) | 756 (72.2) | 175 (78.5) | <0.001 | |
| Use of MRA | 1,858 (44.8) | 174 (41.6) | 713 (44.0) | 355 (42.9) | 506 (48.3) | 110 (49.3) | 0.255 | |
| Event | ||||||||
| 5-Year mortality | 1,732 (41.8) | 258 (61.7) | 747 (46.1) | 336 (40.6) | 333 (31.8) | 58 (26.0) | <0.001 | |
| Follow-up duration (months) | 32.0 (12.0–54.8) | 22.0 (4.9–43.2) | 30.0 (10.2–54.2) | 33.6 (13.7–54.1) | 36.3 (17.1–58.6) | 39.2 (22.0–60.6) | <0.001 | |
Values are given as the median with interquartile range or as a number (percentage).
BMI = body mass index; NYHA = New York Heart Association; GFR = glomerular filtration rate; BNP = B-type natriuretic peptide; NT-proBNP = N-terminal pro B-type natriuretic peptide; LV = left ventricular; EDD = left ventricular end-diastolic dimension; ESD = end-systolic dimension; MI = mass index; EDV end-diastolic volume; ESV = end-systolic volume; LAVI = left atrial volume index; GLS = global longitudinal strain; LARS = left atrial reservoir strain; RV = right ventricular; TR = tricuspid regurgitation; PASP = pulmonary artery systolic pressure; RAS = renin-angiotensin system; MRA = mineralocorticoid antagonist.
During a median follow-up of 32 months (IQR 12–55 months), 1,732 patients (41.8%) died. The annualized mortality rate was 14.4% (per 100 person-years) in the total study population, 27.8% among patients with underweight status, 16.5% among patients with ideal BMI, 13.6% among patients with overweight status, 9.9% among patients with obesity class I, and 7.4% among patients with obesity class II.
Predictors of all-cause mortality
Multivariable Cox regression analysis with the stepwise backward elimination method was performed using variables with p values of <0.1 on univariable analysis (Table 2 and Supplementary Table 1). Advanced age, male sex, lower systolic blood pressure, presence of DM, lower hemoglobin and GFR levels, and higher natriuretic peptide level were associated with higher mortality risk. The use of beta-blockers and renin-angiotensin system blockers, and higher LV global longitudinal strain were associated with lower mortality risk.
Table 2. Multivariable predictors of all-cause mortality.
| Variables | Multivariable model #1 | Multivariable model #2 | |||||
|---|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | p value | Adjusted HR | 95% CI | p value | ||
| Age (per +1 year) | 1.045 | 1.038–1.053 | <0.001 | 1.045 | 1.038–1.052 | <0.001 | |
| Male sex | 1.313 | 1.143–1.508 | <0.001 | 1.340 | 1.161–1.546 | <0.001 | |
| Systolic blood pressure (per +1 mmHg) | 0.996 | 0.994–0.999 | 0.003 | 0.996 | 0.994–0.999 | 0.002 | |
| Diabetes mellitus | 1.304 | 1.135–1.498 | <0.001 | 1.326 | 1.150–1.528 | <0.001 | |
| Hemoglobin (per +1 g/dL) | 0.919 | 0.889–0.951 | <0.001 | 0.922 | 0.891–0.954 | <0.001 | |
| Glomerular filtration rate (per +1 mL/min/1.73 m2) | 0.995 | 0.992–0.998 | 0.001 | 0.994 | 0.991–0.997 | <0.001 | |
| Natriuretic peptide level (decile) | 1.006 | 1.003–1.009 | <0.001 | 1.055 | 1.025–1.086 | <0.001 | |
| Use of beta-blockers | 0.672 | 0.585–0.770 | <0.001 | 0.647 | 0.562–0.745 | <0.001 | |
| Use of RAS blockers | 0.648 | 0.562–0.747 | <0.001 | 0.646 | 0.559–0.747 | <0.001 | |
| LV-GLS (per +1%) | 0.941 | 0.926–0.957 | <0.001 | 0.942 | 0.926–0.959 | <0.001 | |
| BMI (per +1 kg/m2) | 0.951 | 0.932–0.970 | <0.001 | - | - | - | |
| WHO Asia-Pacific BMI classification | |||||||
| Obesity II (reference) | - | - | - | Reference | |||
| Obesity I | - | - | - | 1.345 | 0.852–2.124 | 0.204 | |
| Overweight | - | - | - | 1.606 | 1.016–2.539 | 0.042 | |
| Ideal BMI | - | - | - | 1.744 | 1.112–2.734 | 0.015 | |
| Underweight | - | - | - | 2.729 | 1.686–4.417 | <0.001 | |
Multivariable model #1 was performed using BMI as a continuous variable, and multivariable model #2 was performed using BMI as a categorical variable: BMI categories were defined according to the World Health Organization Asia-Pacific BMI classification: BMI <18.5 kg/m2 (underweight), BMI 18.5–23 kg/m2 (ideal BMI), BMI 23–25 kg/m2 (overweight), BMI 25–30 kg/m2 (obesity class I), and BMI ≥30 kg/m2 (obesity class II).14)
HR = hazard ratio; CI = confidence interval; RAS = renin-angiotensin system; LV = left ventricular; GLS = global longitudinal strain; BMI = body mass index.
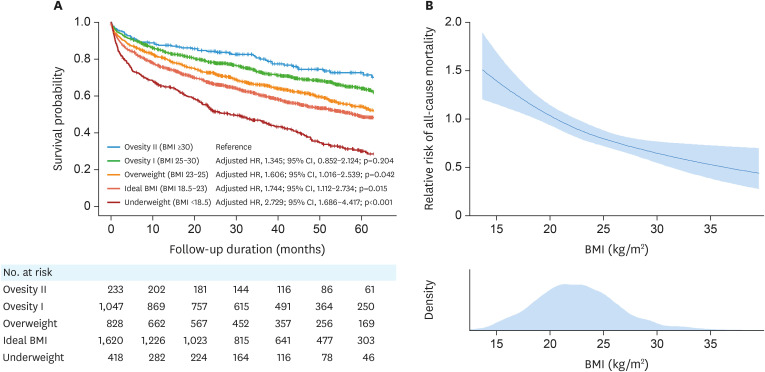
Higher BMI was associated with a lower risk of mortality (adjusted hazard ratio [HR], 0.951; 95% confidence interval [CI], 0.932–0.970; p<0.001). Using the obesity class II BMI category as a reference, lower BMI category was significantly associated with higher mortality risk (obesity I: adjusted HR, 1.345; 95% CI, 0.852–2.124; p=0.204) (overweight: adjusted HR, 1.606; 95% CI, 1.016–2.539; p=0.042) (ideal BMI: adjusted HR, 1.744; 95% CI, 1.112–2.734; p=0.015) (underweight: adjusted HR, 2.729; 95% CI, 1.686–4.417; p<0.001) (Table 2 and Figure 1).
Figure 1. Risk of all-cause mortality according to the BMI. (A) All-cause mortality-free survival is shown according to the BMI category: BMI <18.5 kg/m2 (underweight), BMI 18.5–23 kg/m2 (ideal BMI), BMI 23–25 kg/m2 (overweight), BMI 25–30 kg/m2 (obesity class I), and BMI ≥30 kg/m2 (obesity class II). (B) The association of the risk of all-cause mortality with BMI is shown in the spline curve.
BMI = body mass index; HR = hazard ratio; CI = confidence interval.
Prognostic value of the body mass index across subgroups
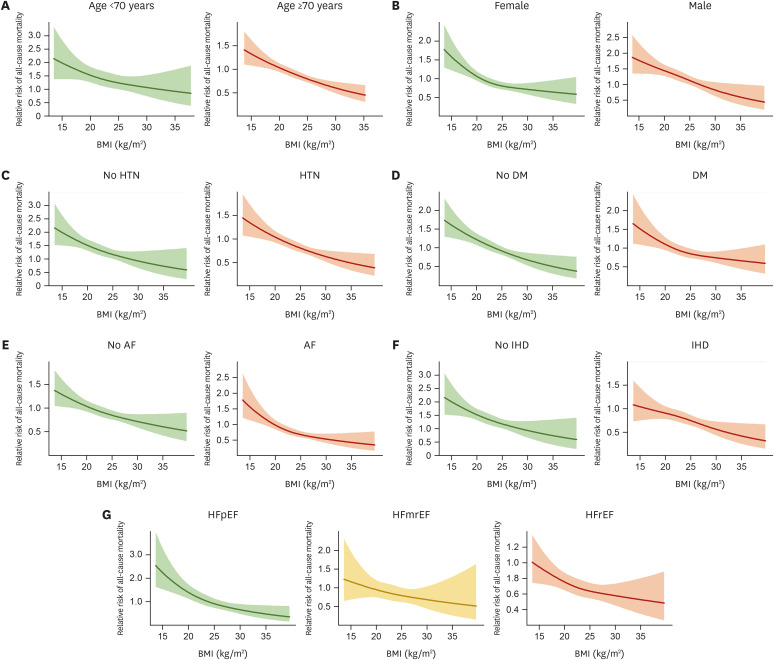
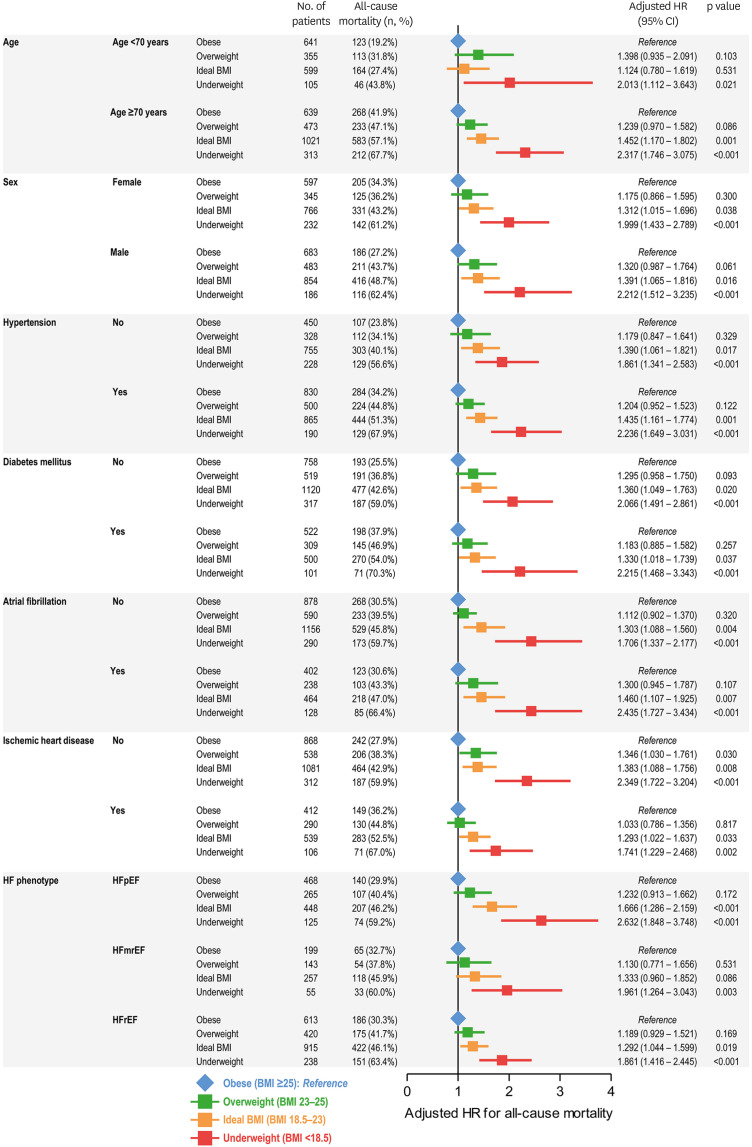
In order to assess the relationship between BMI and the risk of mortality in various subgroups of patients with AHF, we divided the study population according to age, sex, HTN, DM, atrial fibrillation (AF), IHD, and HF phenotype (HFpEF, HFmrEF, and HFrEF) (Figures 2 and 3). An association between lower BMI and higher mortality risk was observed across subgroups (Figure 2). Compared to that in patients with obesity class II, patients with underweight status had a significantly higher risk of mortality in all subgroups, followed by those with ideal BMI, overweight status, and obesity class I (Figure 3).
Figure 2. Spline curves for all-cause mortality in evaluated subgroups. Multivariable-adjusted spline curves for the all-cause mortality are shown according to the BMI in subgroups divided by (A) age, (B) sex, (C) HTN, (D) DM, (E) AF, (F) IHD, and (G) phenotypes of HF.
BMI = body mass index; HTN = hypertension; DM = diabetes mellitus; AF = atrial fibrillation; IHD = ischemic heart disease; HFpEF = heart failure with preserved ejection fraction; HFmrEF = heart failure with mildly reduced ejection fraction; HFrEF = heart failure with reduced ejection fraction.
Figure 3. Subgroup analyses for all-cause mortality according to the BMI classification. The adjusted HRs are shown for all-cause mortality in subgroups based on age, sex, hypertension, diabetes mellitus, atrial fibrillation, ischemic heart disease, and phenotype of HF. Using the patients with BMI >25 kg/m2 (obese) as reference (blue), the HRs of patients with overweight (green), ideal BMI (orange), and underweight (red) are summarized.
HR = hazard ratio; CI = confidence interval; BMI = body mass index.
Impact of muscle index on the mortality risk
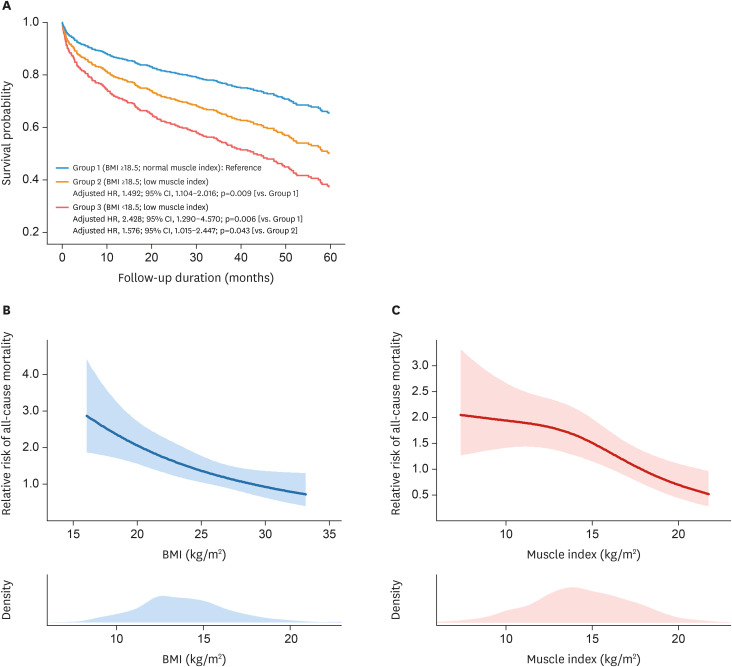
The total body muscle mass was calculated in a subset of 579 patients, using the serum levels of cystatin C and creatinine.15) Patients in this subset were categorized into three groups according to BMI (using the cutoff for underweight status, i.e. 18.5 kg/m2) and muscle index (total body muscle mass/height2; using sex-specific median values): BMI ≥18.5 kg/m2 and muscle index ≥15.7 kg/m2 for men or ≥12.9 kg/m2 for women (group 1; n=271), BMI ≥18.5kg/m2 but reduced muscle mass (group 2, n=256), and BMI <18.5 kg/m2 (group 3; n=52).Of note, most of the patients (92%) in the group 3 had reduced muscle mass. Compared to those with normal BMI and muscle index (group 1), the mortality risk was significantly higher in group 3 (adjusted HR, 2.428; 95% CI, 1.290–4.570; p=0.006) as well as in group 2 (adjusted HR, 1.492; 95% CI, 1.104–2.016; p=0.009) (Figure 4A). In addition, the mortality risk was higher in group 3 compared to that in group 2 (adjusted HR, 1.576; 95% CI, 1.015–2.447; p=0.043).
Figure 4. Associations between BMI, muscle index, and mortality risk. (A) Cumulative incidence curves are shown for all-cause mortality according to the subgroups divided by BMI and muscle index, among the subset of 579 patients with cystatin C data. The associations of the risk of all-cause mortality with (B) BMI and (C) muscle index are shown in the adjusted spline curves.
BMI = body mass index; HR = hazard ratio; CI = confidence interval.
As in the total study population, the significant association between the lower BMI and higher risk of mortality was consistent in this subset of 579 patients with cystatin C data (Figure 4B). In addition, there was an inverse relationship between the muscle index and mortality risk, even after adjusting for age, sex, blood pressure, DM, hemoglobin, GFR, LV global longitudinal strain, and BMI classification (Figure 4C).
DISCUSSION
In the present study, we showed that lower BMI is associated with higher risk of all-cause mortality in patients with AHF, and that this association (known as the obesity paradox) is observed regardless of age, sex, comorbidities, and HF phenotype. Furthermore, among a subset of patients with available total body muscle mass data, those with low muscle index had higher mortality risk, even in those with ideal or higher BMI, suggesting sarcopenia as the main cause of the obesity paradox in patients with AHF.
Obesity paradox in HF
The obesity paradox indicates that, despite well-established negative impacts of obesity on health-related outcomes and cardiovascular risk factors, the presence of obesity is associated with lower mortality risk in patients with chronic diseases, such as myocardial infarction, stroke, HF, renal disease, and DM.19),20) As obesity leads to worsened cardiovascular risk factors, including lipid profile, blood pressure, glycemic control, and systemic inflammation, which are associated with the risk of developing cardiovascular diseases, including HF, better survival in patients with obesity with cardiovascular diseases is contradictory to the conventional view.
The survival advantage of obesity in cardiovascular diseases may be explained by several mechanisms.19) First, higher BMI may indicate greater metabolic reserves, enabling patients to endure catabolic consequences from chronic diseases. Second, patients with high BMI may have greater muscle mass, with greater muscular strength, implicating better cardiorespiratory fitness. Third, patients with obesity have an attenuated response to the renin-angiotensin-aldosterone system and a lower natriuretic peptide level. Fourth, the mode of disease presentation or underlying etiology may differ between patients with higher and lower BMI.
However, the interpretation of this phenomenon is still under debate. One noted skepticism of the obesity paradox in HF is the lead-time bias; HF in patients with low BMI may be detected at a more advanced stage, with longer cachexia duration and non-purposeful weight loss, whereas HF may be detected earlier in patients with high BMI.21) Another important point is the possibility of selection or collider stratification bias, which results from unmeasured confounding factors.22),23) Because obesity serves as a risk factor for the development of HF, the presence of obesity and the mortality risk may collide in HF. More specifically, if there are unmeasured factors that have a larger impact on mortality than the presence of obesity, the failure to include these “unmeasured factors” in the analysis can lead to an overestimation of the association between obesity and lower mortality risk. Nonetheless, from a practical viewpoint, the obesity paradox indicates that underweight status in patients with HF implicates a higher risk of mortality, and recognizing this phenomenon in patients admitted for HF can serve as the basis for providing appropriate treatment options. In other words, aside from the debate on the intrinsic effect of obesity on the mortality risk, underweight status can be considered as an indicator of high mortality risk in AHF.3)
HF, body mass index, and prognosis
In the present study, among patients hospitalized for AHF, the obesity paradox was observed across various subgroups. Specifically, a concordant relationship between lower BMI and higher mortality risk was demonstrated regardless of age, sex, HTN, DM, AF, IHD, and HF phenotype (HFrEF, HFmrEF, and HFpEF). These findings may seem to contradict several previous studies that reported a lack of the obesity paradox in certain subgroups, such as those with younger age (<75 years),12) female,24) those with DM,10) those with HFrEF,25) or conversely, those with HFpEF.2) The lack of the obesity paradox in certain subgroups in the above-mentioned studies might be due to the sample size or the characteristics of the included study population. Indeed, in studies that suggested an attenuated, or absent, obesity paradox in several subgroups, it was noted that the overall event-free survival showed a tendency for higher mortality risk in patients with lower BMI.24),25) In the present study, which had a large study population, the obesity paradox was observed across various HF phenotypes. Additionally, although the shape of the spline curves and relative risk ratio slightly differed between subgroups, the association between lower BMI and higher mortality risk was concordant. Regarding the potential impact of sex on the obesity paradox, a study by Lee et al.24) suggested the lack of obesity paradox in female patients among the 2,616 patients hospitalized for HF. However, a more frequent occurrence of all-cause death and composite events (all-cause death and HHF) was observed in female patients with lower BMI, compared to those with higher BMI, and the multivariable analysis for all-cause mortality in female patients showed borderline statistical significance. Thus, it may be inferred that the lack of the obesity paradox in specific subgroups reported in a few previous studies might be due to a small study population.
Furthermore, in the study by Shah et al.,12) which confirmed the obesity paradox in elderly patients (>75 years) but not in younger patients (≤75 years), patients with BMI <18.5 kg/m2 were excluded from the main analyses. In the present study, among patients aged <70 years, the mortality risk in patients with overweight status or ideal BMI was not significantly higher than that in patients with obesity; however, there was a significantly higher mortality risk in patients with underweight status, and the spline curve for mortality risk showed an obvious trend for higher mortality risk as the BMI decreased. Thus, the lack of the obesity paradox in younger patients reported by Shah et al.12) may be due to the study inclusion/exclusion criteria. Additionally, the presence of comorbidities could impact the relationship between BMI and mortality risk. Adamopoulos et al.10) reported a lack of the obesity paradox in patients with DM and HF; however, the study population in the previous study showed significant differences compared to our registry. In the study by Adamopoulos et al.10), the mean age of the final study population was 63 years, more than 70% had an ischemic HF etiology, and only 3% had New York Heart Association (NYHA) class IV symptoms. In contrast, the present study population had a median age was 73 years, 32.5% had an ischemic HF etiology, and 37.8% had NYHA class IV symptoms.
There is also a debate regarding the presence of the obesity paradox according to HF phenotype. In the study by Powell-Wiley et al.2), which analyzed approximately 40,000 patients with HF from the Get With The Guidelines-Heart Failure (GWTG-HF) registry, the obesity paradox for 30-day mortality was observed across all BMI levels in patients with HFrEF, but not in those with HFpEF; the differential slopes of obesity and mortality for HFpEF and HFrEF were different. In particular, the authors found a small, but statistically significant, increase in the 30-day mortality rate in patients with HFpEF and BMI >30 kg/m2 compared to that in patients with HFpEF and lower BMI. This finding may be explained by the well-established association between obesity and the development of HFpEF, the distinct phenotype of obesity-related HFpEF,26) and the diagnostic role of BMI for the probability of HFpEF as assessed by the H2FPEF score.27) In contrast, in the present study, the spline curve for all-cause mortality in patients with HFpEF showed a gradual decrease across all BMI levels (Figure 2). Nonetheless, it was noted that, among patients with HFpEF, the slope for mortality risk was steeper in those with BMI <25 kg/m2 than in those with BMI >25 kg/m2, suggesting that the relationship between BMI and mortality risk is relatively attenuated in patients with overweight status or obesity, consistent with the results of GWTG-HF registry study.2)
Due to differences in the baseline characteristics of the study population between studies, a consensus regarding the obesity paradox in HF might be unachievable. However, given the concordant trends showing higher mortality risk with lower BMI, our findings support the presence of the obesity paradox in HF regardless of comorbidities and HF phenotype.
Sarcopenia and mortality risk in HF
Although the obesity paradox is an important feature for the prognostication of patients with HF, BMI-based therapeutic intervention requires caution. In particular, despite the higher mortality risk in patients with HF and lower BMI, simple weight gain as a therapeutic measure is not recommended. Previous studies have shown that higher BMI is associated with a significant risk of developing de novo HF and a higher risk of hospitalization for HF, especially in patients with HFpEF.28),29),30) Thus, advising simple weight gain cannot be justified, despite the robust tendency of the obesity paradox in HF. Moreover, body composition indices, including the skeletal muscle mass and body fat, have more relevant clinical values than BMI.31) Recent studies have focused on the importance of the presence of catabolic status, which can be defined as cachexia and sarcopenia. These conditions reflect the burden of comorbidities and chronic illness. Sarcopenia is defined as a progressive loss of muscle mass and strength, with a risk of adverse outcomes, such as disability, poor quality of life and death, whereas cachexia is defined as a multifactorial syndrome characterized by a severe loss of body weight, fat, and muscle mass due to an underlying illness.16),32) Several studies have suggested that, even in patients with similar low BMI values, patients with cachexia or sarcopenia have a higher mortality risk than those with lower BMI but sufficient muscle mass.6),8),33)
In the present study, we identified a subset of patients with available data for calculating body muscle mass using the difference between serum creatinine and cystatin C levels.15) Patients in this subset were categorized according to BMI and muscle index, comprising subgroups reflecting normal BMI with normal muscle mass, normal BMI with low muscle mass, and low BMI with low muscle mass. Although different from the standard definition and diagnostic criteria, the clinical condition of normal BMI with low muscle mass reflects sarcopenia, and the condition of low BMI with low muscle mass reflects cachexia, given that sarcopenia indicates a condition of decreased muscle mass, and cachexia indicates the loss of both fat and muscle mass. These two conditions are prevalent in patients with HF, and are known to be associated with increased mortality risk.32) In this subset analysis, we found that patients with low BMI and low muscle index (i.e. cachexia) had the highest mortality risk, and among those with preserved BMI, patients with low muscle index had a higher mortality risk. This finding indicates that reduced muscle mass may be the key component in the obesity paradox. Further, our findings support the importance of caloric restriction with appropriate exercise training in patients with HF, which has been recommended in recent guidelines,32) based on the benefits of these therapeutic interventions in clinical trials.34),35) Thus, it can be inferred that, among patients hospitalized for AHF, those with low BMI need intensive care, including nutritional support with appropriate protein intake, and the application of tolerable exercise and rehabilitation. Additionally, even in patients who are not underweight, the estimation of skeletal muscle mass would be helpful for risk stratification as well as for determining the appropriate therapeutic intervention.
The present study has several limitations. First, muscle mass was calculated using serum creatinine and cystatin C levels,15) not by direct measures, and cystatin C levels were available only in 579 of 4,146 patients. Additionally, fluid retention was not considered in this analysis. Second, we assumed that the clinical conditions of low muscle mass and low BMI reflect sarcopenia and cachexia, respectively, but these assumptions are different from the guideline-directed diagnostic criteria. Therefore, the interpretation of our findings based on the estimation of muscle mass requires caution. Third, we focused on the mortality risk in patients with established HF, and did not assess the impact of BMI on the development of HF. Fourth, whether therapeutic intervention for BMI and body composition is associated with a better prognosis cannot be inferred from our findings. Future studies are required to assess whether such interventions have beneficial impacts on the prognosis. Finally, the present study did not reveal the underlying pathophysiology of the obesity paradox in patients with AHF. Instead, we focused on the situation in which hospitalization for HF has occurred, and showed a robust association between lower BMI and higher mortality risk, suggesting that more attention should be applied to patients with AHF and low BMI.
Lower BMI is associated with higher mortality risk in patients with AHF. This obesity paradox was observed in patients with AHF regardless of age, sex, comorbidities, and HF phenotype. Furthermore, our subset analysis suggested that a lack of muscle mass is the main pathophysiology of the obesity paradox. Further studies are warranted to assess the potential benefits of interventions targeting optimal weight control in patients with HF.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Hwang IC, Park JB, Cho GY.
- Data curation: Hwang IC, Choi HM, Park JH, Lee SP, Kim HK, Kim YJ, Cho GY.
- Formal analysis: Hwang IC, Choi HM, Park JB.
- Investigation: Hwang IC, Choi HM, Yoon YE, Cho GY.
- Methodology: Hwang IC, Choi HM, Yoon YE, Park JJ, Park JB, Park JH, Lee SP, Kim HK, Cho GY.
- Software: Hwang IC, Lee SP.
- Supervision: Hwang IC, Yoon YE, Park JH, Kim HK, Kim YJ, Cho GY.
- Validation: Hwang IC.
- Visualization: Hwang IC.
- Writing - original draft: Hwang IC.
- Writing - review & editing: Hwang IC, Choi HM, Yoon YE, Park JJ, Park JB, Park JH, Lee SP, Kim HK, Kim YJ, Cho GY.
SUPPLEMENTARY MATERIALS
Calculation of total muscle mass.
Risk of mortality according to the natriuretic peptide levels. Risk of all-cause mortality is shown according to the deciles of BNP (grey dot) and NT-proBNP (yellow dot).2)
Univariable predictors of all-cause mortality
References
- 1.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 2.Powell-Wiley TM, Ngwa J, Kebede S, et al. Impact of body mass index on heart failure by race/ethnicity from the get with the guidelines-heart failure (GWTG-HF) registry. JACC Heart Fail. 2018;6:233–242. doi: 10.1016/j.jchf.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang IC, Cho GY, Choi HM, et al. Derivation and validation of a mortality risk prediction model using global longitudinal strain in patients with acute heart failure. Eur Heart J Cardiovasc Imaging. 2020;21:1412–1420. doi: 10.1093/ehjci/jez300. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61:151–156. doi: 10.1016/j.pcad.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Fülster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur Heart J. 2013;34:512–519. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 7.Bielecka-Dabrowa A, Ebner N, Dos Santos MR, Ishida J, Hasenfuss G, von Haehling S. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur J Heart Fail. 2020;22:2314–2326. doi: 10.1002/ejhf.2011. [DOI] [PubMed] [Google Scholar]
- 8.Emami A, Saitoh M, Valentova M, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) Eur J Heart Fail. 2018;20:1580–1587. doi: 10.1002/ejhf.1304. [DOI] [PubMed] [Google Scholar]
- 9.Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The heart failure overweight/obesity survival paradox: the missing sex link. JACC Heart Fail. 2015;3:917–926. doi: 10.1016/j.jchf.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Adamopoulos C, Meyer P, Desai RV, et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity-matched study. Eur J Heart Fail. 2011;13:200–206. doi: 10.1093/eurjhf/hfq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamora E, Lupón J, Enjuanes C, et al. No benefit from the obesity paradox for diabetic patients with heart failure. Eur J Heart Fail. 2016;18:851–858. doi: 10.1002/ejhf.576. [DOI] [PubMed] [Google Scholar]
- 12.Shah R, Gayat E, Januzzi JL, Jr, et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–785. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 13.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947–1957. doi: 10.1016/j.jacc.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Regional Office for the Western P. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 15.Kim SW, Jung HW, Kim CH, Kim KI, Chin HJ, Lee H. A new equation to estimate muscle mass from creatinine and cystatin C. PLoS One. 2016;11:e0148495. doi: 10.1371/journal.pone.0148495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. 2014;60:294–305. doi: 10.1159/000356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):S276–S281. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–150. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol. 2015;25:342–349. doi: 10.1016/j.annepidem.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Charnigo R, Guglin M. Obesity paradox in heart failure: statistical artifact, or impetus to rethink clinical practice? Heart Fail Rev. 2017;22:13–23. doi: 10.1007/s10741-016-9577-0. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Kim HL, Kim MA, et al. Obesity paradox in Korean male and female patients with heart failure: a report from the Korean Heart Failure Registry. Int J Cardiol. 2021;325:82–88. doi: 10.1016/j.ijcard.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson F, Kragelund CB, Torp-Pedersen C, et al. DIAMOND study group Effect of obesity and being overweight on long-term mortality in congestive heart failure: influence of left ventricular systolic function. Eur Heart J. 2005;26:58–64. doi: 10.1093/eurheartj/ehi022. [DOI] [PubMed] [Google Scholar]
- 26.Packer M, Kitzman DW. Obesity-related heart failure with a preserved ejection fraction: the mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium-glucose cotransporter-2. JACC Heart Fail. 2018;6:633–639. doi: 10.1016/j.jchf.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Hwang IC, Cho GY, Choi HM, et al. H2FPEF score reflects the left atrial strain and predicts prognosis in patients with heart failure with preserved ejection fraction. J Card Fail. 2021;27:198–207. doi: 10.1016/j.cardfail.2020.09.474. [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Han KD, Jung JH, et al. Weight change and the incidence of heart failure in the Korean population: data from the National Health Insurance Health checkup 2005–2015. Eur J Prev Cardiol. 2022;28:1767–1773. doi: 10.1093/eurjpc/zwaa049. [DOI] [PubMed] [Google Scholar]
- 29.Packer M, Lam CS, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020;22:1551–1567. doi: 10.1002/ejhf.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandviwala TM, Basra SS, Khalid U, et al. Obesity and the paradox of mortality and heart failure hospitalization in heart failure with preserved ejection fraction. Int J Obes. 2020;44:1561–1567. doi: 10.1038/s41366-020-0563-1. [DOI] [PubMed] [Google Scholar]
- 31.Piepoli MF, Corrà U, Veglia F, et al. Exercise tolerance can explain the obesity paradox in patients with systolic heart failure: data from the MECKI Score Research Group. Eur J Heart Fail. 2016;18:545–553. doi: 10.1002/ejhf.534. [DOI] [PubMed] [Google Scholar]
- 32.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 33.Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail. 2017;4:492–498. doi: 10.1002/ehf2.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor RS, Walker S, Smart NA, et al. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol. 2019;73:1430–1443. doi: 10.1016/j.jacc.2018.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calculation of total muscle mass.
Risk of mortality according to the natriuretic peptide levels. Risk of all-cause mortality is shown according to the deciles of BNP (grey dot) and NT-proBNP (yellow dot).2)
Univariable predictors of all-cause mortality