Abstract
Background
Rare cases of thrombosis and thrombocytopenia (thrombosis with thrombocytopenia syndrome [TTS]) have been associated with 2 coronavirus disease 2019 adenovirus vector vaccines: the ChAdOx1 nCoV-19 Vaxzevria vaccine (Oxford/AstraZeneca) and the JNJ-7836735 Johnson & Johnson vaccine (Janssen). It is unknown if TTS is a class-mediated effect of adenovirus-based vaccines or if it could worsen known hypercoagulable states. Since most cases of TTS happen in women of childbearing age, pregnancy is a crucial risk factor to assess. Understanding these risks is important for advising vaccine recipients and future adenovirus vector vaccine development.
Methods
To explore the potential associations of adenovirus-based vaccine components with symptoms of TTS in the general clinical trial population and in pregnant women in clinical trials, we conducted a systematic review and meta-analysis of adenovirus-based vector vaccines to document cases of thrombocytopenia, coagulopathy, and or pregnancy from 1 January 1966 to 9 August 2021.
Results
We found 167 articles from 159 studies of adenovirus vector–based vaccines, 123 of which targeted infectious diseases. In the general population, 20 studies reported an event of thrombocytopenia and 20 studies indicated some coagulopathy. Among pregnant women, of the 28 studies that reported a total of 1731 pregnant women, thrombocytopenia or coagulopathy were not reported.
Conclusions
In this systematic review and meta-analysis, there was no class-wide effect of adenovirus vector vaccines toward thrombocytopenia or coagulopathy events in the general population or in pregnant women.
Keywords: COVID-19, adenovirus, platform-based vaccines, thrombosis, pregnancy
In this systematic review and meta-analysis, there was no class-wide effect of adenovirus vector vaccines toward thrombocytopenia or coagulopathy events in the general population or in pregnant women.
As vaccination against coronavirus disease 2019 (COVID-19) has begun across the globe, in rare cases the administration of adenovirus-based COVID-19 vaccines has been linked with unusual cases of thrombosis such as cerebral venous sinus thrombosis and portal vein thrombosis [1]. ChAdOx1 nCoV-19 Vaxzevria (Oxford/AstraZeneca), a chimpanzee adenovirus vaccine, encodes the nonstabilized severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein whereas the JNJ-7836735 Johnson & Johnson vaccine (Janssen) uses an adenovirus (Ad) 26 viral vector to encode the SARS-CoV-2 spike protein.
The syndrome, called thrombosis with thrombocytopenia syndrome (TTS), or vaccine-induced thrombotic thrombocytopenia (VITT), is broadly defined as any venous or arterial thrombosis associated with thrombocytopenia (platelet count <150 × 109/L), elevated D-dimer (>4 times the upper limit of normal), and positive PF4 heparin-induced thrombocytopenia enzyme-linked immunosorbent assay within 42 days of COVID-19 vaccination [2, 3]. There has been some variability in the definition of this new syndrome, including slight variations in laboratory parameters and timing of presentation [4–7]. The term TTS will be used for this article in line with the Centers for Disease Control and Prevention (CDC), US Food and Drug Administration (FDA), and World Health Organization. Unusual sites of thrombosis after vaccination have been noted such as cerebral venous sinus and splanchnic vein thrombosis, which occur in the general population at 0.22–1.57 cases per 100 000 person-years [1, 8]. More common sites of thrombosis in the general population, such as lower-extremity deep venous thrombosis and pulmonary embolism, as well as arterial thrombosis, have also been reported [3, 9, 10].
TTS is triggered by complexes of PF4 and anti-PF4 antibodies that activate platelets via FcγIIa receptors. This mirrors the mechanism of heparin-induced thrombocytopenia, though the trigger for antibody formation is not known [11, 12]. Platelet activation with endothelial cell injury results in thrombocytopenia and thrombosis.
Most cases of TTS are described in women of childbearing age [1]. Pregnancy and the postpartum period are themselves prothrombotic states due to the Virchow triad of (1) increased venous stasis of the lower extremities, (2) intrinsic hypercoagulability of pregnancy, and (3) endothelial injury of delivery [13, 14].
In first-generation Ad5 viral vectors, used mostly for gene therapy experiments in rhesus macaques, thrombocytopenia was one of many side effects, along with increased fibrinogen and von Willebrand factor, and clotting time prolongation [15]. Thrombocytopenia was thought to be related to increased platelet clearance after vaccine administration [16, 17].
The COVID-19 pandemic marks the first time the adenovirus vector vaccines are being administered globally. With large numbers of people receiving these vaccines, rare side effects are more likely to surface. If TTS is a class-wide effect, examining the adverse effects reported from prior adenovirus vector vaccine trials could speak to the risk of thrombosis with adenovirus vector COVID-19 vaccines, both in the general population and in the hypercoagulable state of pregnancy. We conducted a systematic review and meta-analysis of pregnancy and thrombotic outcomes in adenovirus vector vaccine trials.
METHODS
Search Strategy, Information Sources, and Eligibility Criteria
PubMed was searched for clinical trials of adenovirus vector vaccines using the terms “(Adenovirus vaccine) AND (Clinical trial)” and separately “Adenovirus vaccine” and the clinical trials filter. Articles were included from 1 January 1966 to 9 August 2021. The last search was on 24 August 2021. All relevant citations were explored. Gray literature was searched from FDA documents for COVID-19 vaccine trials with adenovirus vector vaccines, and the company web pages of Johnson & Johnson and AstraZeneca were queried for further publications. Titles and abstracts were reviewed and included if they reported human phase 1–4 clinical trials or postmarketing studies. Studies were excluded if they did not report primary clinical trial results, were not reported in English, or involved autologous dendritic cell trails transduced with adenovirus, inactivated or oral vaccine trials against adenovirus from the 1960s to 1970s (this vaccine was thought to be sufficiently distinct from the adenovirus viral vector vaccines). Preclinical vaccine trials in animals were excluded, as were vaccine targeting infectious diseases of nonhuman animals. Studies were grouped based on outcomes of thrombocytopenia, coagulopathy (defined as bleeding, prolonged prothrombin time [PT], or activated partial thromboplastin time [aPTT], or report of thrombosis including transient ischemic attacks), pregnancy, and pregnancy outcomes. This systematic review and meta-analysis were completed prior to registration and otherwise was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Table 1).
Search and Selection Process
One author (L. P.) reviewed studies for eligibility criteria. Two authors (L. P. and K. M. P.) reviewed full articles and supplements for cases of thrombocytopenia, thrombosis, coagulopathy, pregnancy, and pregnancy outcomes, as well as number of participants in each study arm. If agreement could not be met between the 2 authors, a third author (S. B. O.) was available to resolve the discrepancy. No automated tools were used in the search or selection process.
Data Collection Process
Grade of laboratory abnormality was noted. Coagulopathy and thrombocytopenia events were used as a proxy for TTS. Pregnancy outcomes were grouped into (1) healthy term births and elective abortions and (2) all other events: spontaneous abortions, therapeutic abortions, preterm birth, congenital abnormality, fetal distress in labor, and/or hemorrhage in labor. As a pregnant woman may be pregnant with multiple gestations (eg, twins), the number of pregnancies does not equal the number of pregnancy outcomes. If no outcomes were reported, the outcomes of interest were marked as zero. If the exact number of outcomes was unclear, this was noted, and the nearest estimate was included in meta-analysis if possible. If specified that the adverse event occurred before adenovirus vector administration and in response to another vaccine, this event was counted toward a non–adenovirus vector event even if the person was in the adenovirus-based vaccine arm.
Synthesis Methods and Risks of Bias
Clinical trials were divided into studies that targeted infectious diseases and those that targeted cancers. For those studies that targeted infectious diseases, 3 meta-analyses were conducted for thrombocytopenia, coagulopathy, and pregnancy outcomes. Studies included in systematic review and meta-analyses that contributed to the relative risk were assessed for reporting bias. Meta-analyses was planned for the cancer targeted clinical trials, but as nearly all participants received an adenovirus vector vaccine, these results were simply described. For the meta-analysis, the Paule and Mandel method was used for binary outcomes and forest plots were generated. Relative risk (RR) with 95% confidence interval (CI) was used as an effect measure. A fixed-effects model was used if I2 < 25 and a random-effects model if I2 > 50. If I2 was between 25 and 50, both models were reported. Funnel plots and linear regression test of funnel plot asymmetry were generated to assess reporting bias, and asymmetry of funnel plots was evaluated by linear regression via Harbord method with a P value of < .05 noted as significant. Sensitivity analysis was performed with removal of trials where the comparison arm was another viral vector. Data extracted from included studies, data for analysis, and code are available upon request. All analysis was performed in R (version 4.0.2) software with the meta package [18].
RESULTS
Search Results
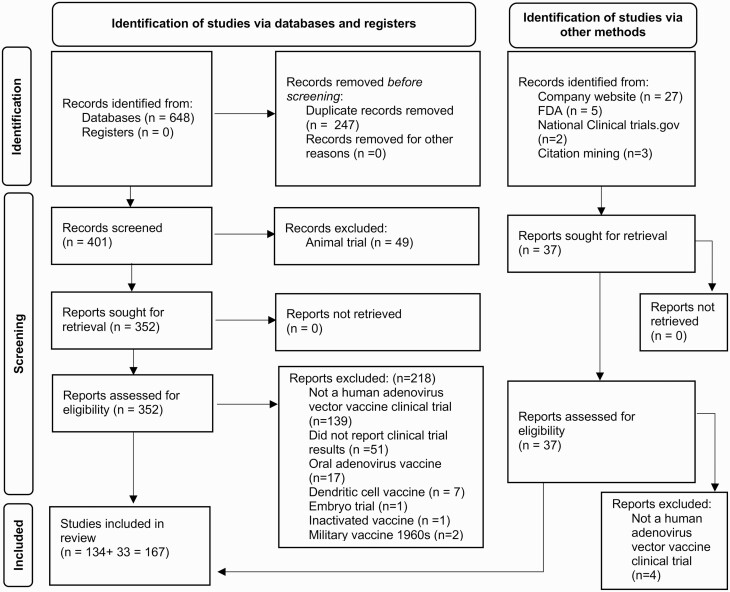
The PubMed search returned 648 articles. After duplicates were removed, 401 articles were screened for outcomes (Figure 1). Of these, 49 were animal trials and excluded. The remaining 352 articles were assessed for eligibility, and 218 met inclusion criteria. Thirty-seven additional articles were identified from the gray literature: 27 from company websites, 5 from the FDA, 3 from back-tracing citations from already included articles, and 2 from ClinicalTrials.gov. Of these, 4 were excluded as they were not adenovirus vector–based vaccine clinical trials [19–22]. A total of 167 articles spanning 159 trials were included in the final analysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram: identification, screening, and inclusion of manuscripts reviewed for adenovirus vector–based vaccines. Adapted from: Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. doi:10.1136/bmj.n71. For more information, visit http://www.prisma-statement.org/. Abbreviation: FDA, US Food and Drug Administration.
Adenovirus Vaccines Targeting Infectious Diseases
For adenovirus vector–based vaccines targeting infectious diseases, there were 123 trials for 17 infectious diseases using 17 adenovirus vector types and 157 907 total participants. Of those with known arm allocation (n = 92 056), 65.3% (n = 60 154) received an adenovirus vector vaccine and the remainder received placebo or another vaccine. The most common vectors used were Ad5 (30.1% of studies) and chimpanzee adenovirus 63 (ChAd63) (16.2% of studies). Studies targeting human immunodeficiency virus (HIV) and Ebola virus disease were the most common, making up 29.3% and 16.2% of studies, respectively.
Adenovirus Vaccines Targeting Noninfectious Diseases
For adenovirus-based vaccines for malignancies or noninfectious disease targets, 36 trials were found covering 13 diseases. Nine hundred five participants were enrolled, of whom 96.9% (n = 877) received an adenovirus-based vaccine. The most common vector used was Ad5 (55.6% of studies), following by chimeric Ad2/Ad5 vector (25% of studies). The most common target was metastatic or advanced malignancy (5 studies).
Thrombocytopenia Outcomes
Twenty studies reported thrombocytopenia events (Supplementary Table 2). One study did not report exact numbers of thrombocytopenia events [23]. Six studies were testing vaccination dosage, and all participants received at least 1 dose of adenovirus vector vaccine [24–29]. ChAd3 and Ad5 were the most common viral vectors, used in 32% and 26% of studies, respectively. The most frequent diseases targeted were Ebola virus (7 studies) and Plasmodium falciparum (5 studies). Of 33 events in the adenovirus vector vaccine group, 22 (67%) had grades associated with them: 9 grade 1, 7 grade 2, and 6 grade 3. Of the 20 episodes of thrombocytopenia in the non–adenovirus vector vaccine group, 6 (30%) had associated grades: 1 grade 1, 4 grade 2, 1 grade 3. Across all studies, the incidence of thrombocytopenia was 0% (interquartile range [IQR], 0%–0%) in the adenovirus and nonadenovirus arms.
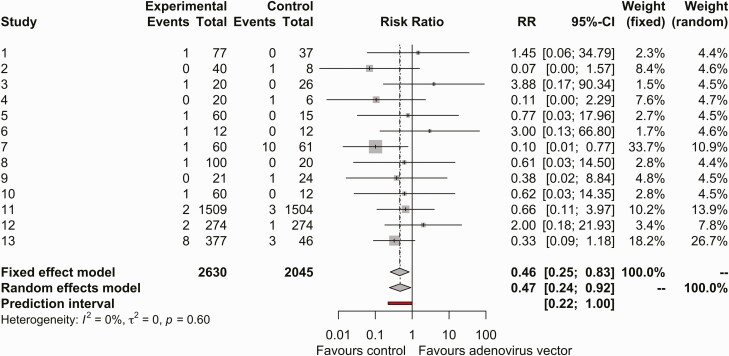
After removing studies where all participants received an adenovirus vector vaccine or exact numbers of thrombocytopenia were not reported, 13 studies were included in the meta-analysis (Figure 2). Using a fixed-effects model, the risk ratio for thrombocytopenia events in adenovirus vector arms compared with non–adenovirus vector arms was 0.460 (95% CI, .255–.830; I2 = 0%). The funnel plot was symmetric (Supplementary Figure 1).
Figure 2.
Forest plot of thrombocytopenia events in the adenovirus vector vaccine arm or placebo arm. Abbreviations: CI, confidence interval; RR, relative risk.
Sensitivity Analysis
For sensitivity analysis, thrombocytopenia events that occurred after receipt of other viral vectors or vaccines were removed. In the study by Tamminga et al, the episode of thrombocytopenia in the trial arm occurred after the DNA prime dose but before the adenovirus boost [30]. In the study by Ogwang et al, participants in the active arm received ChAd63 encoding multiple epitopes-Thrombospondin-Related Adhesion Protein (ME-TRAP) followed by Modified Vaccinia Virus Ankara (MVA) ME-TRAP vaccine whereas placebo recipients received 2 doses of rabies vaccine. There was 1 episode of thrombocytopenia after ChAd63, 4 episodes after the MVA ME-TRAP, and 6 after the rabies vaccine [31]. After the removal of these 2 articles, the RR was 0.568 via fixed-effects model, though this was not a significant difference (95% CI, .293–1.102; I2 = 0%) (Supplementary Figure 2).
Coagulopathy Outcomes
Twenty studies also reported at least 1 prolongation of PT, aPTT, thrombotic event, or coagulopathic event (Supplementary Table 3). One study did not report exact numbers of prolongations but rather that the aPTT was increased in the ChAd3 group and decreased in the recombinant vesicular stomatitis virus group [29]. ChAd3, Ad35, and Ad5 were the most common viral vectors (20% each). The most frequent diseases targeted were Ebola (40%) and HIV (25%). Four of the studies were testing dosage of vaccination and all participants were allocated to receive at least 1 dose of adenovirus vector vaccine [26, 32–34]. Seventy episodes of coagulopathy were reported in the adenovirus arms and 16 in the nonadenovirus arms. Overall, there was 0% (IQR, 0%–0%) incidence of thrombocytopenia in the adenovirus and nonadenovirus arms.
Of 48 events of PT or aPTT prolongation in the adenovirus vector vaccine group, 27 reported grades: 18 grade 1, 6 grade 2, 1 grade 3, and 2 grade 4. Of the 8 episodes of PT or aPTT prolongation in the non–adenovirus vector vaccine group, 3 reported grades: 2 grade 1 and 1 grade 3. Of the participants in the adenovirus arm, 0.08% had a prolongation of PT/aPTT compared to 0.03% in the nonadenovirus arm. Overall, this was a rare event, with 0% (IQR, 0%–0%) occurring in both adenovirus vector and non–adenovirus vector recipients. The prolongation of aPTT was frequently attributed to a positive antiphospholipid antibody/positive lupus anticoagulant [34, 35].
There were 20 thrombotic events in the adenovirus vector vaccine arm (8 deep vein thrombosis [DVT], 8 pulmonary emboli [PE], 2 cerebral venous sinus thrombosis, 1 thrombosis not otherwise specified, and 1 ischemic stroke) and 5 in the non–adenovirus vector vaccine arm (4 deep vein thrombosis, 1 pulmonary embolism). Six of the DVTs were reported in JNJ-78436735/Ad26.COV2.S along with 4 PEs [36, 37]. One DVT, PE, and ischemic stroke occurred in the adenovirus arm for ChAdOx1 nCoV-19 compared with 1 DVT and 1 hemolytic anemia in the placebo group [38]. Two cerebral sinus thromboses were reported, 1 in JNJ-78436735 and 1 in Ad26.ZEBOV, although these were not attributed to vaccination [36, 37]. One hemorrhagic stroke was reported in the placebo arm of Gam-COVID-Vac [39]. There was no overlap of thrombocytopenic events in these cases with thrombotic events.
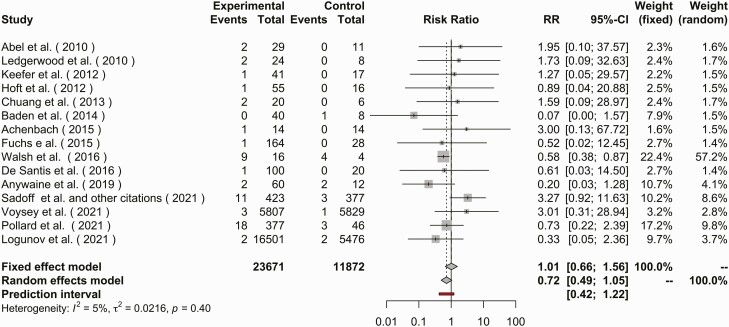
In the meta-analysis of coagulopathy outcomes, 16 publications were included. The risk ratio for coagulopathy via a fixed-effects model was 1.013 (95% CI, .659–1.557; I2 = 4.6%) comparing adenovirus-based vectors to non-adenovirus-based vectors or controls (Figure 3). Visually there was heterogeneity of publications via funnel plot with an absence of small studies with an RR <1, although test for heterogenicity failed to reject the null hypothesis of publication bias (P = .076) (Supplementary Figure 3).
Figure 3.
Forest plot of coagulopathy events in the adenovirus vector vaccine arm or placebo arm. Coagulopathy events described as elevated prothrombin, partial thromboplastin time, bleeding event, or thrombosis event. Abbreviations: CI, confidence interval; RR, relative risk.
Pregnancy Outcomes
Twenty-eight studies reported at least 1 pregnancy for a total of 1731 pregnancies (Supplementary Table 4). Of these, the majority of 1199 pregnancies did not have full information fully reported [40, 41]. In trials where arm allocation was described and full results were reported, 285 received an adenovirus vector–based vaccines (54.5%) and 238 received a placebo or another vaccine. Ad5 and Ad26 were the most common viral vectors used (27.6% and 24.1%, respectively). HIV and Ebola trials were again the most frequent diseases target and comprised 48.3% and 20.7% of the studies, respectively. These were larger trials, did not have pregnancy as an exclusion criterion, or were specifically studying pregnant individuals. Of the 80 pregnancies in the adenovirus vector vaccine group where the pregnancy outcomes were reported, 57.5% resulted in healthy full-term births. Of the 76 pregnancies in placebo or non–adenovirus vector arms, 61.8% resulted in healthy full-term births. This is overall consistent with the established background risk of major birth defects of 2%–4% and miscarriage of 15%–20% in the general population of the United States [40]. No cases of DVT, cerebral sinus thrombosis, or other coagulopathy was reported with these pregnancies, although 1 placebo recipient had postpartum hemorrhage [42].
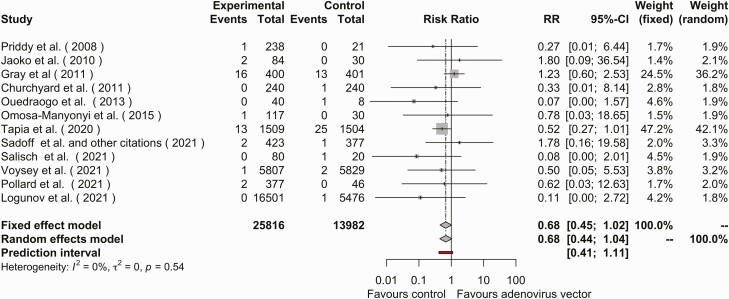
Thirteen studies reported adverse pregnancy events. In 1 of these studies, all participants received study vaccine [43]. Using a fixed-effects model, the RR of adverse event in pregnancy was 0.675 (95% CI, .448–1.017; I2 = 0%) (Figure 4). The funnel plot of publications was grossly symmetric (test for heterogeneity P > .05). (Supplementary Figure 4). Across all pregnancy events, the RR for pregnancy was not different between adenovirus and nonadenovirus arms (Supplementary Figure 5).
Figure 4.
Forest plot of adverse pregnancy events in the adenovirus vector vaccine arm or placebo arm. Abbreviations: CI, confidence interval; RR, relative risk.
Of the 36 clinical trials of adenovirus vector therapy for malignancy or gene therapy, 5 reported thrombocytopenia [44–48] and 5 studies reported a coagulopathy [45, 49–52] (Supplementary Table 5). There were no pregnant individuals in these trials. In majority of these trials (34 of 36), all participants received an adenovirus vector–based vaccine. In the 2 trials where all participants did not receive an adenovirus vector–based vaccine [53, 54], there were no outcomes of interest.
DISCUSSION
To our knowledge, this is the largest systematic review and meta-analysis examining the adverse effects of thrombocytopenia, coagulopathy, or adverse birth outcomes of adenovirus vector vaccines. We found (1) no increased risk for thrombocytopenia, coagulopathy, or adverse pregnancy outcome and (2) no evidence of coagulopathy in pregnant individuals. By aggregating clinical trials of adenovirus vector vaccines in a systematic review and meta-analysis, we were able to analyze for class-adverse effects of thrombocytopenia, coagulopathy, and adverse pregnancy outcome.
Events of thrombocytopenia and coagulopathy were quite rare, with a median of 0 participants having these outcomes in both the adenovirus and nonadenovirus arms [55]. For thrombocytopenia, it initially appeared that adenovirus-based vector vaccines had a lower risk for thrombocytopenic events compared with control (RR, 0.460 [95% CI, .255–.830]; I2 = 0%). This effect was no longer significant after removal of studies that had other viral vectors or rabies vaccines in the control arm (RR, 0.569 [95% CI, .293–1.102]; I2 = 0%). In addition to the coagulopathy associated with the Vaxzevria and Johnson & Johnson COVID-19 vaccines, it should be noted that the Ad26.ZeBOV phase 2 trial was paused briefly due to 2 neurologic events, 1 of which was a cerebral venous sinus thrombosis.
Understanding potential risks of vaccination in pregnancy remains paramount for best advising pregnant women in the decision of vaccination during pregnancy. From this meta-analysis there was no increased risk of thrombocytopenia, coagulopathy, or adverse birth outcome associated with receipt of adenovirus vaccination. Furthermore, the hypercoagulability of pregnancy does not seem to predispose toward the more phenotypically severe spectrum of thrombotic disease seen in TTS. Altogether, these findings are in line with the CDC’s recommendations for all pregnant persons to receive a COVID-19 vaccine [56].
This systematic review and meta-analysis has several limitations. Primarily, though this study examined all available clinical trials, with an estimated 10.6 cases of TTS per million doses in women aged 30–39 years (and 3.8 cases per million doses in the general population), it may be underpowered to detect a different in TTS rates given the low baseline population incidence [57]. Second, we did not include in our case definition a D-dimer >4 times the upper limit of normal, which is a part of the American Society for Hematology TTS definition [2]. This was to cast as wide a net as possible and not exclude potential cases, as TTS was not defined when most of these studies were conducted. Furthermore, several studies used nonexact statements about the number or grade of adverse effects. For pregnancy, outcomes had not yet been fully reported for several studies. Some data were not publicly available; for example, in FDA documents, 1522 pregnancies were reported as related to the AD26.ZEBOV vaccine [40], whereas our search yielded only 1087 pregnancies. Finally, the review was limited to English-language literature.
CONCLUSIONS
Though rare cases of TTS have been associated with COVID-19 adenovirus vector vaccines, in this systematic review and meta-analysis of clinical trial data there was no increased risk of thrombocytopenia or coagulopathy when comparing adenovirus vector vaccines to placebo arms. Additionally, there was no increase of adverse fetal outcome across these studies and no report of thrombosis or coagulopathy in pregnant women.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant number 5T32AI007517-20 to L. P. and K. M. P.).
Contributor Information
Lauren Pischel, Section of Infectious Diseases, Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA; Yale School of Public Health, New Haven, Connecticut, USA.
Kavin M Patel, Section of Infectious Diseases, Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
George Goshua, Section of Hematology, Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA; Department of Health Policy and Management, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Saad B Omer, Section of Infectious Diseases, Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA; Yale School of Public Health, New Haven, Connecticut, USA; Yale Institute of Global Health, New Haven, Connecticut, USA; Yale School of Nursing, Orange, Connecticut, USA.
References
- 1. Cines DB, Bussel JB.. SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. N Engl J Med 2021; 384:2254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bussel JB, Connors JM, Cines DB, et al. Thrombosis with thrombocytopenia syndrome (also termed vaccine-induced thrombotic thrombocytopenia). Available at: https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia. Accessed 10 November 2021.
- 3. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S.. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021; 384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med 2021; 385:1680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Society on Thrombosis and Haemostasis. ISTH interim guidance for the diagnosis and treatment on vaccine-induced immune thrombotic thrombocytopenia. 2021. Available at: https://www.isth.org/news/561406/. Accessed 6 January 2022.
- 6. Favaloro EJ, Pasalic L, Lippi G.. Review and evolution of guidelines for diagnosis of COVID-19 vaccine induced thrombotic thrombocytopenia (VITT). Clin Chem Lab Med 2022; 60:7–17. [DOI] [PubMed] [Google Scholar]
- 7. Task Force for Global Health. Interim case definition of thrombosis with thrombocytopenia syndrome (TTS). Available at: https://brightoncollaboration.us/thrombosis-with-thrombocytopenia-syndrome-interim-case-definition/. Accessed 6 January 2020.
- 8. Abboud CS, Wey SB, Baltar VT.. Risk factors for mediastinitis after cardiac surgery. Ann Thorac Surg 2004; 77:676–83. [DOI] [PubMed] [Google Scholar]
- 9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384:2124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384:2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baker AT, Boyd RJ, Sarkar D, et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci Adv 2021; 7:eabl8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I.. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021; 596:565–9. [DOI] [PubMed] [Google Scholar]
- 13. Marik PE, Plante LA.. Venous thromboembolic disease and pregnancy. N Engl J Med 2008; 359:2025–33. [DOI] [PubMed] [Google Scholar]
- 14. Devis P, Knuttinen MG.. Deep venous thrombosis in pregnancy: incidence, pathogenesis and endovascular management. Cardiovasc Diagn Ther 2017; 7:S309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lozier JN, Csako G, Mondoro TH, et al. Toxicity of a first-generation adenoviral vector in rhesus macaques. Hum Gene Ther 2002; 13:113–24. [DOI] [PubMed] [Google Scholar]
- 16. Gaggar A, Shayakhmetov DM, Lieber A.. CD46 is a cellular receptor for group B adenoviruses. Nat Med 2003; 9:1408–12. [DOI] [PubMed] [Google Scholar]
- 17. Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D.. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 2007; 109:2832–9. [DOI] [PubMed] [Google Scholar]
- 18. Balduzzi S, Rücker G, Schwarzer G.. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barasheed O, Rashid H, Alfelali M, et al. Viral respiratory infections among Hajj pilgrims in 2013. Virol Sin 2014; 29:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tatsis N, Blejer A, Lasaro MO, et al. A CD46-binding chimpanzee adenovirus vector as a vaccine carrier. Mol Ther 2007; 15:608–17. [DOI] [PubMed] [Google Scholar]
- 21. Shukarev G, Callendret B, Luhn K, Douoguih MA.. Two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Hum Vaccin Immunother 2017; 13:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitonsa J, Ggayi AB, Anywaine Z, et al. Implementation of accelerated research: strategies for implementation as applied in a phase 1 Ad26.ZEBOV, MVA-BN-Filo two-dose Ebola vaccine clinical trial in Uganda. Glob Health Action 2020; 13:1829829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kibuuka H, Kimutai R, Maboko L, et al. A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-uninfected East Africans (RV 172). J Infect Dis 2010; 201:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020; 395:1845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 2020; 396:887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ewer K, Rampling T, Venkatraman N, et al. A monovalent chimpanzee adenovirus ebola vaccine boosted with MVA. N Engl J Med 2016; 374:1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tapia MD, Sow SO, Lyke KE, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2016; 16:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tapia MD, Sow SO, Mbaye KD, et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: a randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2020; 20:719–30. [DOI] [PubMed] [Google Scholar]
- 29. Kennedy SB, Bolay F, Kieh M, et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamminga C, Sedegah M, Maiolatesi S, et al. Human adenovirus 5-vectored Plasmodium falciparum NMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection. Hum Vaccin Immunother 2013; 9:2165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogwang C, Kimani D, Edwards NJ, et al. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci Transl Med 2015; 7:286re–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crank MC, Wilson EM, Novik L, et al. Safety and immunogenicity of a rAd35-EnvA prototype HIV-1 vaccine in combination with rAd5-EnvA in healthy adults (VRC 012). PLoS One 2016; 11:e0166393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu L, Zhang Z, Gao H, et al. Open-label phase I clinical trial of Ad5-EBOV in Africans in China. Hum Vaccin Immunother 2017; 13:2078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ledgerwood JE, DeZure AD, Stanley DA, et al. Chimpanzee adenovirus vector Ebola vaccine. N Engl J Med 2017; 376:928–38. [DOI] [PubMed] [Google Scholar]
- 35. De Santis O, Audran R, Pothin E, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 2016; 16:311–20. [DOI] [PubMed] [Google Scholar]
- 36. Muir K-L, Kallam A, Koepsell SA, Gundabolu K.. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 2021; 384:1964–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang R, Hefter Y.. FDA review of efficacy and safety of the Janssen COVID-19 vaccine emergency use authorization request. 2021. Available at: https://www.fda.gov/media/146267/download. Accessed 1 February 2021. [Google Scholar]
- 38. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021; 397:671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. US Food and Drug Administration. Briefing document. Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee Meeting, 2021. Available at: https://www.fda.gov/media/146217/download. Accessed 10 February 2022. [Google Scholar]
- 41. US Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee, February 26, 2021 meeting briefing document—sponsor. 2021. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-february-26-2021-meeting-announcement. Accessed 10 February 2022. [Google Scholar]
- 42. Tapia MD, Sow SO, Ndiaye BP, et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in adults in Africa: a randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2020; 20:707–18. [DOI] [PubMed] [Google Scholar]
- 43. Koblin BA, Casapia M, Morgan C, et al. Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: a randomized clinical trial. PLoS One 2011; 6:e24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herman JR, Adler HL, Aguilar-Cordova E, et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum Gene Ther 1999; 10:1239–49. [DOI] [PubMed] [Google Scholar]
- 45. Yoo GH, Moon J, Leblanc M, et al. A phase 2 trial of surgery with perioperative INGN 201 (Ad5CMV-p53) gene therapy followed by chemoradiotherapy for advanced, resectable squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx: report of the Southwest Oncology Group. Arch Otolaryngol Head Neck Surg 2009; 135:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sung MW, Yeh HC, Thung SN, et al. Intratumoral adenovirus-mediated suicide gene transfer for hepatic metastases from colorectal adenocarcinoma: results of a phase I clinical trial. Mol Ther 2001; 4:182–91. [DOI] [PubMed] [Google Scholar]
- 47. Li JL, Liu HL, Zhang XR, et al. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther 2009; 16:376–82. [DOI] [PubMed] [Google Scholar]
- 48. Raper SE, Yudkoff M, Chirmule N, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther 2002; 13:163–75. [DOI] [PubMed] [Google Scholar]
- 49. Malaeb BS, Gardner TA, Margulis V, et al. Elevated activated partial thromboplastin time during administration of first-generation adenoviral vectors for gene therapy for prostate cancer: identification of lupus anticoagulants. Urology 2005; 66:830–4. [DOI] [PubMed] [Google Scholar]
- 50. Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther 2004; 10:958–66. [DOI] [PubMed] [Google Scholar]
- 51. Hamid O, Varterasian ML, Wadler S, et al. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J Clin Oncol 2003; 21:1498–504. [DOI] [PubMed] [Google Scholar]
- 52. Mulvihill S, Warren R, Venook A, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther 2001; 8:308–15. [DOI] [PubMed] [Google Scholar]
- 53. Diaz CM, Chiappori A, Aurisicchio L, et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors. J Transl Med 2013; 11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cappuccini F, Bryant R, Pollock E, et al. Safety and immunogenicity of novel 5T4 viral vectored vaccination regimens in early stage prostate cancer: a phase I clinical trial. J Immunother Cancer 2020; 8:e000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pollard AJ, Launay O, Lelievre J-D, et al. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2021; 21:493–506. [DOI] [PubMed] [Google Scholar]
- 56. Centers for Disease Control and Prevention. COVID-19 vaccines while pregnant or breastfeeding. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html. Accessed 10 July 2021.
- 57. See I;, Advisory Committee on Immunization Practices. Updates on thrombosis with thrombocytopenia syndrome (TTS). Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf. Accessed 6 January 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.