Abstract
Background
The burden of long-term symptoms (ie, long COVID) in patients after mild COVID-19 is debated. Within a cohort of healthcare workers (HCWs), frequency and risk factors for symptoms compatible with long COVID are assessed.
Methods
Participants answered baseline (August/September 2020) and weekly questionnaires on SARS-CoV-2 nasopharyngeal swab (NPS) results and acute disease symptoms. In January 2021, SARS-CoV-2 serology was performed; in March, symptoms compatible with long COVID (including psychometric scores) were asked and compared between HCWs with positive NPS, seropositive HCWs without positive NPS (presumable asymptomatic/pauci-symptomatic infections), and negative controls. The effect of time since diagnosis and quantitative anti-spike protein antibodies (anti-S) was evaluated. Poisson regression was used to identify risk factors for symptom occurrence.
Results
Of 3334 HCWs (median, 41 years; 80% female), 556 (17%) had a positive NPS and 228 (7%) were only seropositive. HCWs with positive NPS more frequently reported ≥1 symptom compared with controls (73% vs 52%, P < .001); seropositive HCWs without positive NPS did not score higher than controls (58% vs 52%, P = .13), although impaired taste/olfaction (16% vs 6%, P < .001) and hair loss (17% vs 10%, P = .004) were more common. Exhaustion/burnout was reported by 24% of negative controls. Many symptoms remained elevated in those diagnosed >6 months ago; anti-S titers correlated with high symptom scores. Acute viral symptoms in weekly questionnaires best predicted long-COVID symptoms. Physical activity at baseline was negatively associated with neurocognitive impairment and fatigue scores.
Conclusions
Seropositive HCWs without positive NPS are only mildly affected by long COVID. Exhaustion/burnout is common, even in noninfected HCWs. Physical activity might be protective against neurocognitive impairment/fatigue symptoms after COVID-19.
Keywords: long COVID, serology, asymptomatic, healthcare workers, risk factors
In this prospective healthcare worker cohort, participants with SARS-CoV-2–positive nasopharyngeal swab were most likely to report long-COVID symptoms, whereas seropositive participants without positive swab were only mildly affected. Baseline physical activitywas negatively associated with neurocognitive impairment and fatigue.
Emerging evidence points to different long-lasting symptoms after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, also called long coronavirus disease (COVID) [1]. The most commonly reported symptoms are fatigue, shortness of breath, chest pain, olfactory dysfunction, and neurocognitive impairment. Data suggest that long COVID is a complex multisystem illness and that individual long-COVID symptoms are caused by different biological mechanisms that are not mutually exclusive [2–5]. Symptoms seem to decrease over the first 3 months without a significant decline thereafter, and follow-up durations of up to 12 months have been reported [4, 6–10]. Risk factors for long COVID include female sex, high body mass index (BMI), and severity of coronavirus disease 2019 (COVID-19) [4, 6–8]. Most data on long COVID come from cohorts of hospitalized patients [5]. However, the burden of long COVID in patients with mild or even asymptomatic SARS-CoV-2 infection is largely unknown. Also, adequate control groups are often lacking, which is crucial in the light of the nonspecific nature of many long-COVID symptoms [2, 11, 12].
In this prospective multicenter healthcare worker (HCW) cohort, we compared the frequency of symptoms compatible with long COVID (including several established psychometric scores) between HCWs with positive nasopharyngeal swab (NPS), HCWs with presumably asymptomatic or pauci-symptomatic (ie, only seropositive) COVID-19, and negative controls. Furthermore, the effect of time since diagnosis and the concentration of antibodies against the viral spike protein was assessed. We also sought to identify potentially modifiable factors associated with the occurrence of these symptoms.
METHODS
Study Design, Population, and Procedures
This cohort was initiated in late summer 2020, after the first COVID-19 wave in Switzerland (Supplementary Figure 1). Hospital employees from 23 healthcare institutions located in northern and eastern Switzerland were eligible; study enrollment took place in July and August 2020 [13]. The study was approved by the Ethics Committee of Eastern Switzerland (#2020-00502). Participants answered a baseline questionnaire upon inclusion and were then prospectively followed. They answered weekly electronic questionnaires, including symptoms compatible with viral respiratory infection, date/result of any SARS-CoV-2 NPS, and—starting from January 2021—receipt of any COVID-19 vaccine. In January/February 2021, SARS-CoV-2 nucleocapsid antibodies were measured and participants were informed about their serology results. Finally, participants answered a questionnaire in March 2021, which asked about the presence of symptoms associated with long COVID and contained 4 psychometric scores (see below). Participants vaccinated in the week before the long-COVID questionnaire were excluded.
SARS-CoV-2 Diagnostics
Healthcare workers were requested to get tested in case of compatible symptoms according to national recommendations. These included fever and/or the presence of any respiratory symptom (ie, shortness of breath, cough, or sore throat). Detection of SARS-CoV-2 was made by polymerase chain reaction (PCR) or rapid antigen test (RAT), depending on the method used in the participating institutions. Self-reported swab results were validated as described previously [14].
Anti-nucleocapsid protein antibodies (anti-N) were determined to assess seropositivity against SARS-CoV-2, as described previously [13]. For most of anti-N–positive HCWs, anti-spike protein antibodies (anti-S) were measured.
Predictor Variables and Outcomes
Our main predictor variable was SARS-CoV-2 positivity, which was further subdivided into HCWs with positive self-reported NPS irrespective of serology result (suggesting symptomatic infection) and those with positive anti-N and either negative or no NPS result (suggesting asymptomatic/pauci-symptomatic infection). SARS-CoV-2–negative HCWs (ie, seronegative without positive NPS) served as the control group.
As the main outcome, we calculated a sum score of self-reported symptoms (among cough, dyspnea, headache, muscle/limb pain, fever/feverish feeling, chills, impaired olfaction, chest pain, heart palpitations, dizziness, exhaustion, and tiredness). Individual symptoms were also analyzed. Furthermore, we calculated the sum score of 4 well-established psychometric measurements, including the 16-item Rivermead Post-Concussion Questionnaire (RMEAD) score as a measure for neurocognitive impairment, the 9-item Fatigue Severity Scale (FSS), the 8-item Patient Health Questionnaire (PHQ) (without the question on sleep quality) for depression, and the 7-item General Anxiety Disorder (GAD) score [15, 16] (Supplementary Table 1). Because of the considerable number of missing values for the psychometric scores, a complete case analysis was also performed.
Influence of Time Since Diagnosis and Anti-S Titers
For HCWs with SARS-CoV-2–positive NPS, we further determined the influence of the time elapsed since diagnosis on all outcomes. Healthcare workers were therefore classified into the following 4 groups, according to the time of positive swab: 0 to 4 weeks (“acute infection”), 5 to 12 weeks (“acute post-COVID”), 13 to 24 weeks (“long post-COVID”), and more than 24 weeks (“persistent post-COVID”) prior to the questionnaire [17]. Again, SARS-CoV-2–negative HCWs served as the control group.
Similarly, for those with positive NPS and available anti-S results, we determined the influence of the anti-S titer on the symptom sum score and the psychometric measurements. Healthcare workers were classified into 4 groups, according to the quartile of their anti-S titer. Because this analysis was adjusted for time since positive NPS, we did not include a negative control group.
Statistical Analyses
The proportion of HCWs reporting any and reporting individual long-COVID symptoms was related to SARS-CoV-2 status (NPS-positive/only seropositive/negative) using chi-square tests and the calculation of odds ratios (ORs) and to time interval since positive swab, with chi-square tests for trend in proportions.
The symptom sum score and scores of the psychometric measurements were analyzed using univariable quasi-Poisson regression to estimate mean scores per patient group. Quasi-Poisson models include an overdispersion parameter to account for increased error variation. For the association between anti-S and each of the scores, we used a generalized additive model to adjust for time since positive NPS (included as a smooth term). Multivariable quasi-Poisson regression was used to assess the independent association of SARS-CoV-2 positivity with symptom scores. Potential confounders included baseline health data, social determinants, job details, cultural and physical activities at baseline, and receipt of any COVID-19 vaccine. As a measure of disease severity, the total number of acute viral symptoms reported during the 4 follow-up weeks with the highest number of symptoms was included as a covariable. For definitions and further details, including variable selection for multivariable analysis and handling of missing data, see the Supplementary Methods.
RESULTS
Study Population
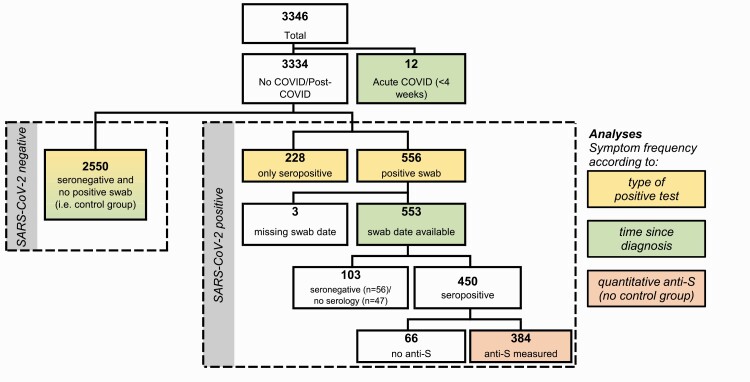
We included 3346 participants, representing 20% of the eligible population of the participating hospitals. Twelve HCWs with recent SARS-CoV-2 diagnosis (within 4 weeks before the long-COVID questionnaire) were only included for the analysis on symptom frequency according to time elapsed since diagnosis, but not for the other analyses (Figure 1). For the remaining 3334 HCW, the median age was 40.5 years, 80% were female, 47% were nurses and 16% were physicians, and patient contact was reported by 83%. These characteristics were similar to the entire eligible population [13]. Baseline characteristics by SARS-CoV-2 positivity are shown in Table 1.
Figure 1.
Study flow showing the total population and the subpopulations analyzed for specific analyses. Abbreviations: anti-S, anti-spike protein antibodies; COVID, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Characteristics of Participants With SARS-CoV-2–Positive Swab, Seropositive Participants (No Positive Swab), and Seronegative Participants (No Positive Swab)
| Variable | Levels | Positive NPS (n = 556) | Only Seropositive (n = 228) | Negative (n = 2550) |
|---|---|---|---|---|
| Anthropometrics | ||||
| Age (years) | Median (range) | 38.9 (16.8–63.5) | 37.9 (17.1–63.9) | 41.0 (16.5–72.6) |
| Sex | Male | 98 (18%) | 45 (20%) | 529 (21%) |
| Female | 453 (81%) | 182 (80%) | 2003 (79%) | |
| Body mass index (kg/m2) | Median (range) | 23.5 (16.5–47.3) | 23.8 (17.7–40.6) | 23.3 (14.3–50.0) |
| Pregnancy (females) | Yes | 24 (5%) | 9 (5%) | 67 (3%) |
| No | 386 (85%) | 158 (87%) | 1825 (91%) | |
| Social determinants (at baseline) | ||||
| Ethnicity | Caucasian | 550 (99%) | 225 (99%) | 2496 (98%) |
| Other | 6 (1%) | 3 (1%) | 54 (2%) | |
| Children ≤6 years in household | Yes | 97 (17%) | 34 (15%) | 429 (17%) |
| No | 459 (83%) | 194 (85%) | 2121 (83%) | |
| Social leisure activities | Sports club, yoga, gym | 240 (43%) | 103 (45%) | 1141 (45%) |
| Only other(s) | 178 (32%) | 68 (30%) | 810 (32%) | |
| None | 138 (25%) | 57 (25%) | 599 (23%) | |
| Risk profile (at baseline) | ||||
| Comorbidity | Yes (any disease) | 199 (36%) | 78 (34%) | 929 (36%) |
| Medication intake | Yes (any drug) | 262 (47%) | 111 (49%) | 1162 (46%) |
| Active smoking | Yes | 61 (11%) | 25 (11%) | 413 (16%) |
| No | 495 (89%) | 203 (89%) | 2137 (84%) | |
| Alcohol intake | >1 Drink/week | 219 (39%) | 85 (37%) | 1172 (46%) |
| ≤1 Drink/week | 337 (61%) | 143 (63%) | 1378 (54%) | |
| Work-related factors (at baseline) | ||||
| Profession | Nurse | 329 (59%) | 131 (57%) | 1033 (41%) |
| Physician | 70 (13%) | 25 (11%) | 428 (17%) | |
| Other | 141 (25%) | 59 (26%) | 980 (38%) | |
| Patient contact | Yes | 482 (87%) | 183 (80%) | 1924 (75%) |
| No | 52 (9%) | 25 (11%) | 473 (19%) | |
| Contacts with COVID-19 patients | >20 | 227 (41%) | 103 (45%) | 637 (25%) |
| 1−20 | 177 (32%) | 58 (25%) | 869 (34%) | |
| None | 79 (14%) | 40 (18%) | 806 (32%) | |
| Working in intensive care | Yes | 40 (7%) | 17 (7%) | 187 (7%) |
| No | 516 (93%) | 211 (93%) | 2363 (93%) | |
| Workload >80% | Yes | 304 (55%) | 140 (61%) | 1329 (52%) |
| No | 252 (45%) | 88 (39%) | 1221 (48%) | |
| COVID-19 history | ||||
| Serology | Positive | 453 (81%) | 228 (100%) | 0 |
| Negative | 56 (10%) | 0 | 2550 (100%) | |
| Nasopharyngeal swab(s) | Any positive | 556 (100%) | 0 | 0 |
| All negative | 0 | 113 (50%) | 1475 (58%) | |
| None reported | 0 | 115 (50%) | 1075 (42%) | |
| Mean no. of symptoms/week | Median (range) | 0.3 (0.0–4.2) | 0.1 (0.0–5.0) | 0.1 (0.0–4.0) |
| SARS-CoV-2 vaccine | Yes | 70 (13%) | 75 (33%) | 1123 (44%) |
| No | 486 (87%) | 153 (67%) | 1427 (56%) |
Abbreviations: COVID-19, coronavirus disease 2019; NPS, nasopharyngeal swab; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
COVID-19 was diagnosed in 784 (24%) HCWs, either by positive NPS and any serology result (n = 556, 71%) or by positive serology only (n = 228, 29%); 2550 HCWs (76%) were seronegative and reported either a negative or no NPS (Figure 1).
Frequency of Long-COVID Symptoms
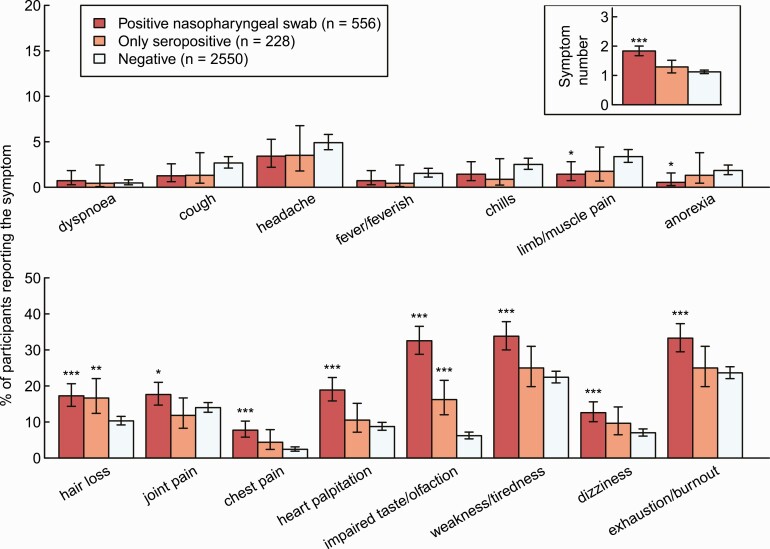
The proportion of HCWs reporting 1 or more symptom was higher for HCWs with a positive NPS compared with negative controls (73% vs 52%, P < .001), but not for seropositive HCWs without a positive NPS (58% vs 52%, P = .13). The most common symptoms were exhaustion/burnout (33% in NPS-positive vs 25% in only seropositive vs 24% in negative controls) and weakness/tiredness (34% vs 25% vs 22%). Impaired taste/olfaction (33% vs 16% vs 6%) and hair loss (17% vs 17% vs 10%) were the only symptoms that were significantly more common in only seropositive HCWs compared with negative controls. Symptoms of acute infection, such as fever, chills, headache, or dyspnea, were not more common in SARS-CoV-2–positive participants (Figure 2, Supplementary Table 2).
Figure 2.
Percentage of HCWs reporting symptom and total number of reported symptoms (upper right corner) by HCWs with a positive SARS-CoV-2 swab (red), seropositive HCWs without a positive swab (orange), and seronegative HCWs without a positive swab (light blue). Statistics: Positive HCWs are compared with negative HCWs, respectively (***P < .001; **P = .001–.01; *P = .01–.05). Abbreviations: HCW, healthcare worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
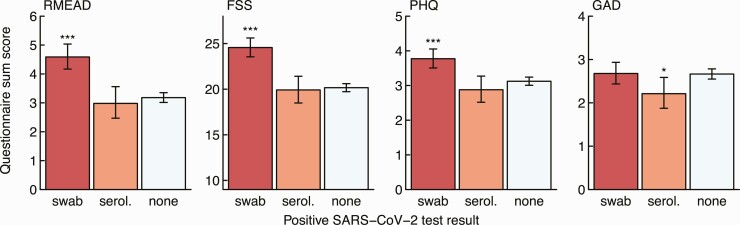
Similarly, NPS-positive participants (but not seropositive participants without positive swab) showed significantly higher scores in the RMEAD, FSS, and PHQ compared with negative controls, whereas this was not the case for the anxiety (GAD) score (Figure 3). The complete case analysis showed similar results (Supplementary Figure 2). A fatigue score of 36 or more, indicating clinically relevant fatigue, was obtained for 260 (10.6%) SARS-CoV-2–negative participants, for 22 (10.0%) seropositive participants without positive swab (OR, .9; 95% confidence interval [CI]: .6–1.5; P = .86), and for 130 (23.7%) NPS-positive participants (OR, 2.6; 95% CI: 2.1–3.3; P < .001).
Figure 3.
Sum score for the post-concussion (RMEAD), fatigue severity (FSS), depression (PHQ), and anxiety (GAD) scores by HCWs with a positive SARS-CoV-2 swab (red), seropositive HCWs without a positive swab (orange), and seronegative HCWs without a positive swab (light blue). Statistics: Positive HCWs are compared with negative HCWs, respectively (***P < .001; *P = .01–.05). Abbreviations: FSS, Fatigue Severity Scale; GAD, General Anxiety Disorder; HCW, healthcare worker; PHQ, Patient Health Questionnaire; RMEAD, Rivermead Post-Concussion Questionnaire; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; serol., serology.
Time Since Diagnosis
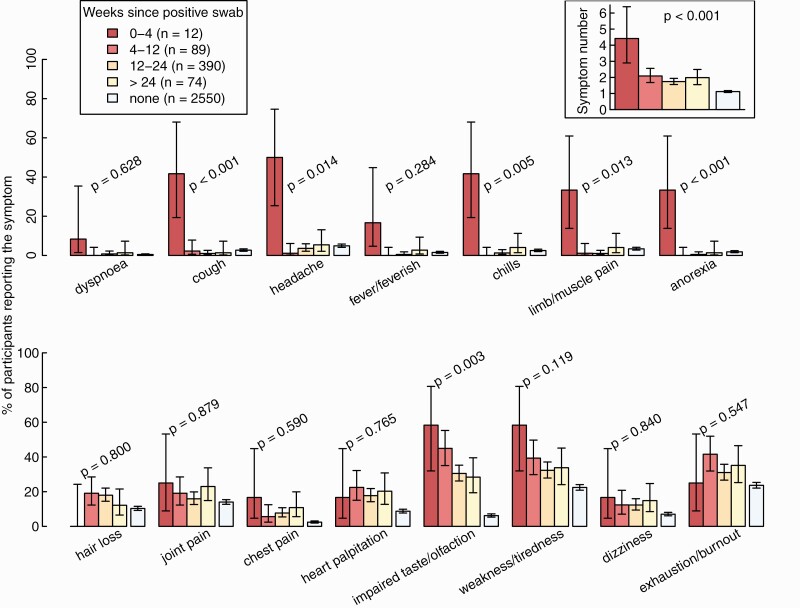
For this analysis, we included 565 participants with a positive NPS after a median of 117 days (interquartile range, 93–147 days) after infection and 2550 SARS-CoV-2–negative controls (Figure 1). The total number of symptoms declined after acute infection (P < .001), but was still higher after 24 weeks compared with noninfected controls (Figure 4). Cough, dyspnea, headache, chills, limb/muscle pain, and anorexia were mainly reported within 4 weeks after acute infection, but rarely thereafter. Other symptoms, such as impaired taste and weakness/tiredness, were frequent during acute infection and remained significantly more common after 24 weeks compared with the control; hair loss was not reported within 4 weeks after acute infection but only in the post-acute phase.
Figure 4.
Percentage of healthcare workers reporting individual symptoms and total number of reported symptoms (upper right corner) according to weeks since positive SARS-CoV-2 swab, compared with negative controls. Statistics: P values from tests for trends over the 4 categories, without negative controls. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
For the psychometric measurements, there was no correlation between sum scores and time since positive swab, such that scores remained significantly higher in the RMEAD, FSS, and PHQ for those with an infection history more than 24 weeks in the past (Supplementary Figure 3).
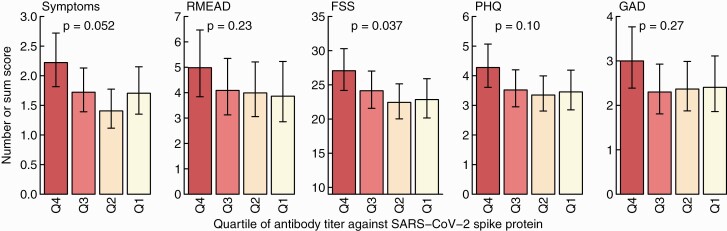
Antibody Concentration Against the Viral Spike Protein (Anti-S Titers)
We included 384 participants with positive NPS and available anti-S results for this analysis (Figure 1). Adjusted for time since positive swab, the quartile with the highest anti-S titers consistently scored highest for symptom numbers and all psychometric measurements. However, statistical significance was only reached for the FSS when evaluating the trend across all quartiles (Figure 5).
Figure 5.
Sum score for symptoms, the post-concussion (RMEAD), fatigue severity (FSS), depression (PHQ), and anxiety (GAD) scores according to quartile of SARS-CoV-2 anti-spike titers. Results are adjusted for time since positive swab. Statistics: P values from tests for linear trends over the 4 categories. Abbreviations: FSS, Fatigue Severity Scale; GAD, General Anxiety Disorder; PHQ, Patient Health Questionnaire; Q, quartile; RMEAD, Rivermead Post-Concussion Questionnaire; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Multivariable Analyses
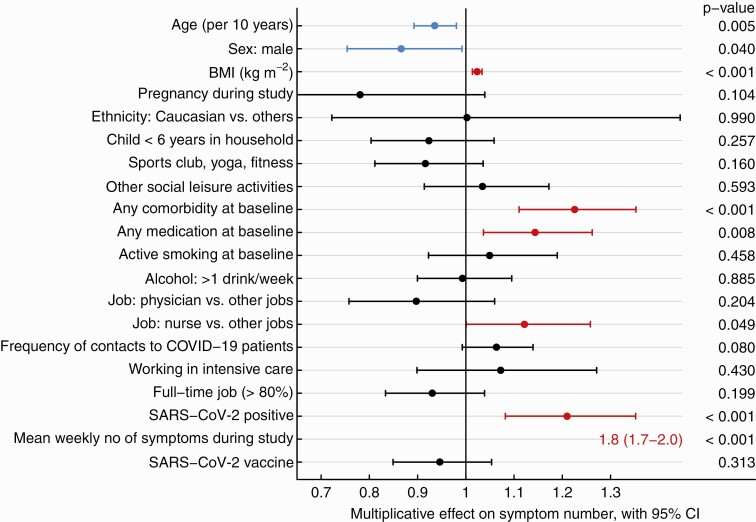
Results of univariable analysis regarding sum score of the long-COVID questions are shown in Supplementary Table 3. In multivariable analysis, SARS-CoV-2 positivity, younger age, female sex, increased BMI, any comorbidity, and any medication at baseline as well as working as a nurse were significantly associated with the symptom sum score. The strongest risk factor was the number of acute viral symptoms reported in the weekly questionnaires; COVID-19 vaccination (mostly administered after SARS-CoV-2 infection, where applicable) was not associated with the outcome (Figure 6).
Figure 6.
Forest plot showing the multiplicative effect of potential influential factors on the number of long-COVID symptoms, as determined through multivariable Poisson regression. Factors in blue are negatively and those in red are positively associated with the number of symptoms. Abbreviation: BMI, body mass index; CI, confidence interval; COVID, coronavirus disease; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Multivariable analyses for the RMEAD and FSS score showed similar results. However, SARS-CoV-2 positivity was not associated with the RMEAD score after accounting for all potential confounders. Having children younger than 6 years in the same household and physical activities at baseline were negatively associated with both scores, while working in intensive care was positively associated with the FSS score (Supplementary Figure 4).
DISCUSSION
In this prospective cohort of SARS-CoV-2–infected and noninfected HCWs, symptoms compatible with long COVID were most common in HCWs with a positive NPS, whereas seropositive HCWs without a positive NPS were only mildly affected. For most symptoms, we did not observe a significant attenuation with increasing time since diagnosis. Physical activity at baseline showed a negative association with neurocognitive impairment and fatigue symptoms. The main strengths of our study are the inclusion of a noninfected control group, the prospective follow-up with weekly questionnaires capturing acute viral symptoms, and the large number of serologies performed, which allowed us to identify HCWs with asymptomatic seroconversion and to ascertain truly negative controls with high reliability.
The main finding of our study is that seropositive participants without a positive swab, representing presumable asymptomatic or pauci-symptomatic disease, showed similar symptom frequency as the negative control group, both for the symptom sum score as well as for the psychometric scores. This is in line with a study evaluating the physical endurance of young recruits after a COVID-19 outbreak, where those with asymptomatic infection—in contrast to symptomatic recruits—did not show reduced maximal aerobic capacities [18]. In an Italian cohort assessing post-COVID symptoms at 6 months after infection, 40% of those with mild disease reported post-COVID symptoms (similar to those with severe disease), whereas only 5% of asymptomatic participants reported post-COVID symptoms [19].
Impaired taste/olfaction and hair loss were the only symptoms being more prevalent in seropositive participants without a positive swab compared with negative controls. Impaired taste/olfaction was also the only symptom showing a significant attenuation over time, but was still frequently reported by those infected more than 24 weeks previously. This is in accordance with multiple other reports, which classifies impaired taste/olfaction as a cardinal symptom for long COVID. Of note, loss of taste has been associated with a lower quality of life and increased risk for depression [20, 21], which underlines the pathological significance of this symptom. A possibly underrecognized symptom of long COVID is hair loss, which has, in fact, been reported as one of the most common symptoms in some studies, with more than 25% of patients being affected [22, 23]. In a case series of almost 200 patients from Spain, a prior history of fever was the only factor associated with hair loss after SARS-CoV-2 infection [24]. In our study, hair loss was reported by 17% of positive participants.
The symptoms most commonly reported were weakness and exhaustion. This is in line with other reports showing that up to 55% of patients with COVID-19 can be affected by these long-term sequelae [15]. However, these symptoms were also reported by almost one-fourth of noninfected controls. This shows that including a control group to assess these nonspecific symptoms is of utmost importance. Irrespective of SARS-CoV-2 infection, many HCWs are exhausted 1 year into the pandemic, which is also reflected in the results of the multivariable analysis for the fatigue score, where working in intensive care was identified as an independent risk factor. Several other studies have reported similar findings [11, 25, 26].
The number of acute viral symptoms was the strongest risk factor for long-COVID symptoms, as shown previously [6]. In line with this finding, anti-S concentrations, which is a proxy for the severity of acute disease [27], were associated with the presence of long-COVID symptoms. Similar results have been reported previously [12, 19]. These results are plausible and anti-S titers could therefore be used to identify high-risk populations, which could particularly benefit from early intervention therapies. However, acute viral symptoms were associated with long COVID independent of documented SARS-CoV-2 infection, which can be explained by the fact that probably not all symptomatic HCWs underwent testing for SARS-CoV-2; at the same time, the sensitivity of the serology test used in our study is only approximately 90% [28]. Other viral diseases have also been postulated to cause either long-term respiratory symptoms, in the case of respiratory syncytial virus [29]; chronic fatigue after infection with influenza or Epstein-Barr virus [30, 31]; or long-COVID clinical features after influenza [32, 33]. However, we do not know to what extent other infections might have played a role in our cohort.
Other risk factors, such as female sex, increased BMI, or baseline comorbidities have also been identified by others as risk factors for long COVID [9]. For age, data are conflicting. Some studies have reported more long-COVID symptoms with increasing age [6], whereas others have reported an association with younger age, being in line with our data [34]. Of note, SARS-CoV-2 vaccination (administered after SARS-CoV-2 infection in the large majority of our patients) did not reduce the number of long-COVID symptoms, as has been suggested in a previous study [35]. However, a recent study showed that, if people are vaccinated before SARS-CoV-2 infection, the risk for long COVID seems to be decreased [36].
Interestingly, we found physical activity at baseline to be negatively associated with the neurocognitive impairment and fatigue scores, suggesting a protective role in the prevention of these symptoms. The facts that (1) other social activities were not associated with decreased risk and that (2) “paced” physical activity is also part of successful treatment bundles for long COVID both suggest a true effect, although residual confounding is possible [37]. Further study is needed to evaluate the potential role of physical activity in the prevention as well as therapy for long-COVID.
We also found having children younger than 6 years to be negatively associated with neurocognitive impairment and fatigue, a finding that is not readily intuitive. However, increased physical activity during COVID-19 in HCWs living with children has been reported, which may also explain the positive effects found in our study [38].
Our study has limitations. First, assessing the presence of long-COVID symptoms at only 1 time point might have led to underestimation of their prevalence as symptoms are reported to be fluctuating [5]. Second, we cannot exclude that certain individuals with COVID-19 did not show any seroconversion or that anti-N had again waned below the detection level at the time of our blood draw. However, we consider this effect to be small, as most individuals, even after mild/moderate infection, show robust and prolonged antibody reaction [39]; also, individuals were asked to report any positive NPS, which further reduces the risk of misclassification. Third, participants knew about their SARS-CoV-2 status (both for serology and NPS) at the time of the long-COVID questionnaire, which might be a source of recall bias. However, the fact that participants with the highest concentration of anti-S (titers not known to participants) had higher symptom scores suggests a true effect. Fourth, the Delta variant, which was not yet the predominant strain when the study was conducted, might differently impact the occurrence of long-COVID symptoms [40]. Fifth, we did not assess long-COVID symptoms at baseline, which precludes us from estimating the true impact of the pandemic on the prevalence of these symptoms. Nevertheless, the inclusion of a noninfected control group allowed us to assess the independent impact of SARS-CoV-2 on the occurrence of these, often nonspecific symptoms. Finally, no additional clinical or laboratory workup was performed, which could potentially help to better understand the determinants of the ongoing symptoms.
In conclusion, we show that SARS-CoV-2–seropositive HCWs without a positive NPS are only mildly affected by long COVID. However, if present, most symptoms including neurocognitive and fatigue symptoms persist, even for those with a diagnosis more than 6 months ago. The main predictor for long COVID is the severity of acute disease, also reflected in the highest symptom frequency among those with high anti-S titers. Exhaustion and tiredness are commonly reported among HCWs, also in those without SARS-CoV-2 infection. This is probably a consequence of the general burden that the pandemic is placing on this particularly exposed population. Further studies should evaluate the potential protective role of physical activity in the prevention of post-concussion and fatigue symptoms after SARS-CoV-2 infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the employees of the participating healthcare institutions who either took part in this study themselves or supported it. Furthermore, we thank the laboratory staff for shipment, handling, and analysis of the blood samples.
Financial support. This work was supported by the Swiss National Science Foundation (grant number 31CA30_196544; grant number PZ00P3_179919 to P. K.), the Federal Office of Public Health (grant number 20.008218/421-28/1), and the Health Department of the Canton of St Gallen.
Potential conflicts of interest. F. A. reports an honorarium for a lecture from Mediweek Davos. S. P. K. reports participation on an Advisory Board for Moderna. D. V.-G. reports that for the analyses of serum antibodies in study participants at the Spital Thurgau, Roche Diagnostics (Schweiz) has provided the kits and supplementary material free of charge. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Carol Strahm, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Marco Seneghini, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Sabine Güsewell, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Thomas Egger, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Onicio Leal-Neto, Epitrack, Recife, Brazil; Department of Economics, University of Zurich, Zurich, Switzerland.
Angela Brucher, Psychiatry Services of the Canton of St Gallen (South), St Gallen, Switzerland.
Eva Lemmenmeier, Clienia Littenheid AG, Private Clinic for Psychiatry and Psychotherapy, Littenheid, Switzerland.
Dorette Meier Kleeb, Division of Occupational Health, Kantonsspital Baden, Baden, Switzerland.
J Carsten Möller, Center for Neurological Rehabilitation, Zihlschlacht, Switzerland.
Philip Rieder, Hirslanden Clinic, Zurich, Switzerland.
Markus Ruetti, Fuerstenland Toggenburg Hospital Group, Wil, Switzerland.
Remus Rutz, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Hans Ruedi Schmid, Central Laboratory, Kantonsspital Baden, Baden, Switzerland.
Reto Stocker, Hirslanden Clinic, Zurich, Switzerland.
Danielle Vuichard-Gysin, Division of Infectious Diseases and Hospital Epidemiology, Thurgau Hospital Group, Muensterlingen, Switzerland.
Benedikt Wiggli, Division of Infectious Diseases and Hospital Epidemiology, Kantonsspital Baden, Baden, Switzerland.
Ulrike Besold, Geriatric Clinic St Gallen, St Gallen, Switzerland.
Stefan P Kuster, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland; Federal Office of Public Health, Bern, Switzerland.
Allison McGeer, Sinai Health System, Toronto, Canada.
Lorenz Risch, Labormedizinisches Zentrum Dr Risch Ostschweiz AG, Buchs, Switzerland; Private Universität im Fürstentum Liechtenstein, Triesen, Liechtenstein; Center of Laboratory Medicine, Institute of Clinical Chemistry, University of Bern, Inselspital, Bern, Switzerland.
Andrée Friedl, Division of Infectious Diseases and Hospital Epidemiology, Kantonsspital Baden, Baden, Switzerland.
Matthias Schlegel, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Dagmar Schmid, Clinic for Psychosomatic and Consultation Psychiatry, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Pietro Vernazza, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Christian R Kahlert, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland; Department of Infectious Diseases and Hospital Epidemiology, Children’s Hospital of Eastern Switzerland, St Gallen, Switzerland.
Philipp Kohler, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St Gallen, Switzerland.
References
- 1. Nalbandian A, Sehgal K, Gupta A, et al. . Post-acute COVID-19 syndrome. Nat Med 2021; 27:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silva Andrade B, Siqueira S, de Assis Soares WR, et al. . Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021; 13:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Proal AD, VanElzakker MB.. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 2021; 12:698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ayoubkhani D. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 1 April 2021. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021. Accessed 22 July 2021.
- 5. Fernandez-de-Las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, et al. . Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med 2021; 92:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sudre CH, Murray B, Varsavsky T, et al. . Attributes and predictors of long COVID. Nat Med 2021; 27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daugherty SE, Y G, Heath K, et al. . Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021; 373:n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis HE, Assaf GS, McCorkell L, et al. . Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wynberg E, van Willigen HDG, Dijkstra M, et al. . Evolution of COVID-19 symptoms during the first 12 months after illness onset. Clin Infect Dis 2021. doi: 10.1093/cid/ciab759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitaker M, Elliott J, Chadeau-Hyam M, et al. . Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508 707 people. medRxiv, doi: 10.1101/2021.06.28.21259452, 2021, preprint: not peer reviewed. [DOI] [Google Scholar]
- 11. Lai J, Ma S, Wang Y, et al. . Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open 2020; 3:e203976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blomberg B, Mohn KG, Brokstad KA, et al. . Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27:1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahlert CR, Persi R, Gusewell S, et al. . Non-occupational and occupational factors associated with specific SARS-CoV-2 antibodies among hospital workers—a multicentre cross-sectiona l study. Clin Microbiol Infect 2021; 27:1336–44. doi: 10.1101/2020.11.10.20229005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohler P, Güsewell S, Seneghini M, et al. . Impact of baseline SARS-CoV-2 antibody status on syndromic surveillance and the risk of subsequent COVID-19—a prospective multicenter cohort study. BMC Med 2021; 19:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nasserie T, Hittle M, Goodman SN.. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 2021; 4:e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong TL, Weitzer DJ.. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-A systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas) 2021; 57. doi: 10.3390/medicina57050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez-de-Las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, Cuadrado ML, Florencio LL.. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health 2021; 18. doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crameri GAG, Bielecki M, Zust R, Buehrer TW, Stanga Z, Deuel JW.. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill 2020; 25. doi: 10.2807/1560-7917.ES.2020.25.36.2001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peghin M, Palese A, Venturini M, et al. . Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect 2021; 27:1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kollndorfer K, Reichert JL, Bruckler B, Hinterleitner V, Schopf V.. Self-esteem as an important factor in quality of life and depressive symptoms in anosmia: a pilot study. Clin Otolaryngol 2017; 42:1229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Croy I, Nordin S, Hummel T.. Olfactory disorders and quality of life—an updated review. Chem Senses 2014; 39:185–94. [DOI] [PubMed] [Google Scholar]
- 22. Xiong Q, Xu M, Li J, et al. . Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. . More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moreno-Arrones OM, Lobato-Berezo A, Gomez-Zubiaur A, et al. . SARS-CoV-2-induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol 2021; 35:e181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. P S, Wang M, Song T, et al. . The psychological impact of COVID-19 pandemic on health care workers: a systematic review and meta-analysis. Front Psychol 2021; 12:626547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgantini LA, Naha U, Wang H, et al. . Factors contributing to healthcare professional burnout during the COVID-19 pandemic: a rapid turnaround global survey. PLoS One 2020; 15:e0238217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia-Beltran WF, Lam EC, Astudillo MG, et al. . COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021; 184:476–88, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahase E. Covid-19: Two antibody tests are “highly specific” but vary in sensitivity, evaluations find. BMJ 2020; 369:m2066. [DOI] [PubMed] [Google Scholar]
- 29. Piedimonte G. Respiratory syncytial virus and asthma: speed-dating or long-term relationship?. Curr Opin Pediatr 2013; 25:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magnus P, Gunnes N, Tveito K, et al. . Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine 2015; 33:6173–7. [DOI] [PubMed] [Google Scholar]
- 31. Petersen I, Thomas JM, Hamilton WT, White PD.. Risk and predictors of fatigue after infectious mononucleosis in a large primary-care cohort. QJM 2006; 99:49–55. [DOI] [PubMed] [Google Scholar]
- 32. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ.. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273 618 survivors of COVID-19. PLoS Med 2021; 18:e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al-Aly Z, Xie Y, Bowe B.. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021; 594:259–64. [DOI] [PubMed] [Google Scholar]
- 34. Sigfrid L, Drake TM, Pauley E, et al. . Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. Lancet Reg Health Eur 2021; 8:100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW.. Symptoms after COVID-19 vaccination in patients with persistent symptoms after acute infection: a case series. Ann Intern Med 2021; 174:1335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antonelli M, Penfold RS, Merino J, et al. . Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 2022; 22:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19 (NG188): evidence review 5: interventions. NICE Guideline, No. 188. London: National Institute for Health and Care Excellence (UK), 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK567264/. Accessed 13 October 2021. [PubMed] [Google Scholar]
- 38. F Bu, Bone JK, Mitchell JJ, Steptoe A, Fancourt D.. Longitudinal changes in physical activity during and after the first national lockdown due to the COVID-19 pandemic in England. Sci Rep 2021; 11:17723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wajnberg A, Amanat F, Firpo A, et al. . Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020; 370:1227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crook H, Raza S, Nowell J, Young M, Edison P.. Long Covid—mechanisms, risk factors, and management. BMJ 2021; 374:n1648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.