Abstract
The vaccine candidate CVnCoV (CUREVAC) showed surprisingly low efficacy in a recent phase 3 trial compared with other messenger RNA (mRNA) vaccines. Here we show that the low efficacy follows from the dose used and the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and is predicted by the neutralizing antibody response induced by the vaccine.
A recent study analyzed the relationship between neutralizing antibody response and protection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection across 8 vaccine platforms [1]. The efficacy results from a phase 2b/3 trial of a ninth vaccine candidate, CVnCoV (CUREVAC), was announced on 16 June 2021 [2]. The low efficacy of this new messenger RNA (mRNA) vaccine, which showed only 48.2% protection from symptomatic SARS-CoV-2 infection [3], was surprising given the high efficacy of 2 previous mRNA-based vaccines [4, 5]. A number of factors have been suggested to play a role in the low efficacy in the CVnCoV study, particularly around the dose and immunogenicity of the vaccine (which uses an unmodified mRNA construct [6, 7]) and the potential role of infection with SARS-CoV-2 variants (which were the dominant strains observed in the CVnCoV trial) [2].
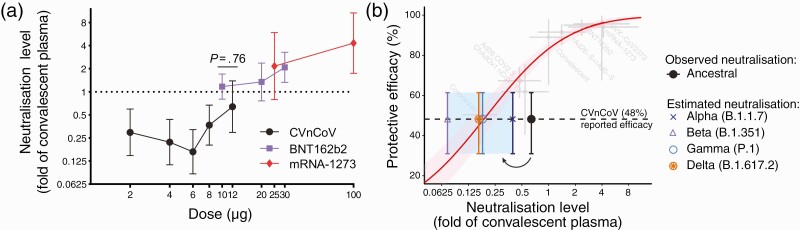
In the recent randomised control trials, the CVnCoV vaccine was administered in a 2 dose regimen with 4 weeks between doses [3], which is very similar to the BNT162b2 and mRNA-1273 vaccine trials, where 2 dose regimens were also used with 3 and 4 week spacing, respectively [4, 5]. However, each dose of the CVnCoV vaccine contained 12 μg of unmodified mRNA, compared with 30 μg and 100 μg of nucleoside-modified mRNA in the BNT162b2 and mRNA-1273, respectively. To investigate the potential effects of dose and immunogenicity in the CVnCoV construct we extracted data on in vitro neutralization titer for 3 reported mRNA vaccines, CVnCoV [7], mRNA-1273 [8], and BNT162b2 [9]. To allow comparison of neutralization levels between studies, we normalized to the average convalescent titer in the same study (recognizing that convalescent groups were not standardized between studies). Figure 1A compares dose and neutralization levels across the 3 vaccines and suggests that the lower neutralization in the CVnCoV study is consistent with the neutralization observed when lower doses of mRNA-1273 [8] and BNT162b2 [9] were administered.
Figure 1.
Potency, immunogenicity, and protection from SARS-CoV-2 infection. A, Relationship between immunization dose (x-axis) and in vitro neutralization titer (expressed as a fold-change over convalescent serum in the same study) (y-axis) is shown for three mRNA-based vaccines, CVnCoV [7], mRNA-1273 [8], and BNT162b2 [9]. Neutralization level of the 12 μg dose of CVnCoV was not significantly different from that of the 10 μg BNT162b2 dose (P = .76, t test). B, Previously reported relationship between neutralization level (normalized to the mean convalescent titer in the same study) and protection from symptomatic SARS-CoV-2 infection is shown in red [1], along with the neutralization and efficacy data from studies of 7 vaccines and convalescent subjects (light-gray points and error bars), which were used to fit this model. Observed mean neutralization level against ancestral SARS-CoV-2 virus in vitro (black) [7], as well as the predicted drop in neutralization level (indicated by arrow and colored points and whiskers) against the alpha, beta, delta and gamma variants [11] are plotted against the protective efficacy observed in the CVnCoV trial [2]. Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Another factor suggested to affect the observed vaccine efficacy was the circulating SARS-CoV-2 variant viruses encountered during the CVnCoV trial. In the primary phase 3 studies used for licensure of the other 8 vaccines, the ancestral virus (which matches the spike protein used as the vaccine immunogen) was the dominant strain in circulation [1]. However, more recent nonrandomized studies have suggested a reduced efficacy of some of these vaccines against SARS-CoV2 variants [10]. In vitro studies have shown that many SARS-CoV-2 variants show a significant reduction in neutralization titer compared to the ancestral virus, and that this effect is observed using serum from both convalescent and vaccinated subjects [11]. In the CVnCoV phase 3 trial the infecting virus was composed almost entirely of a variety of circulating SARS-CoV-2 variants. For example, the alpha (B.1.1.7) and gamma (P.1) variants represented about 53% of infections in the CVnCoV trial and has been shown in a comprehensive meta-analysis to have a 1.6- and 3.5-fold drop in neutralizing titer compared to the ancestral virus for another mRNA vaccine, respectively [11]. Similarly, the beta (B.1.351) and delta (B.1.617.2) variants have been shown to have an 8.8-fold and 3.9-fold drop in neutralizing titer, respectively [11].
To visualize the potential effects of reduced neutralizing level on vaccine efficacy, Figure 1B plots the in vitro neutralizing titer (normalized to the mean convalescent sera) against the ancestral virus observed in the CVnCoV phase 1/2 study [7] along with the observed efficacy in the phase 3 trial. It also shows the predicted effects of the drop in neutralization titer for the different variants listed above [11]. The observed efficacy against variants appears consistent with the initial level of neutralization against the ancestral virus and the expected drop in neutralization titer to variants (as reported for other mRNA vaccines).
This analysis suggests that both a lower dose than the other mRNA vaccines (Figure 1A), as well as the effects of SARS-CoV-2 variants in reducing the neutralizing ability of vaccine-induced serological responses (Figure 1B), were significant contributors to the low efficacy observed in the CVnCoV study.
Notes
Acknowledgments. The authors wish to thank Sarah Sasson for helpful comments and careful reading of the article. This work was approved under the UNSW Sydney Human Research Ethics Committee (approval HC200242). All data and code are freely available at https://github.com/InfectionAnalytics/CVnCoV-CUREVAC.
Authorship statement. All authors contributed to the data collection, design of the study, writing of the manuscript and revision of the manuscript. D. S. K., D. C., A. R., and M. P. D. contributed to the modelling and statistical analysis of the data.
Financial support. This work is supported by an Australian government Medical Research Future Fund awards GNT2002073, MRF2005544, MRF2005760 (to M. P. D.) and MRF2007221 (J. A. T., M. S.) and an NHMRC program grant GNT1149990 (M. P. D.). D. S. K., D. C., and M. P. D. are supported by NHMRC Fellowship/Investigator grants.
Potential conflicts of interest. D. C. reports NSW Health Contract paid to institution for salary support for researcher and research group, Australian Commonwealth Government Contract paid to institution for researcher support, and Australian Research Council paid to institution for research support all outside of the submitted work. D. S. K. reports payments made to the University of New South Wales from National Health and Medical Research Council of Australia (GNT1141921) for the present manuscript and payments made to University of New South Wales from the Australian Research Council (grant number DP180103875) outside of the submitted work. M. P. D. reports payments made to University of New South Wales from National Health and Medical Research Council of Australia (APP1173027) for the present manuscript and is Senior Editor for eLife Journal (receive annual retainer for services). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Deborah Cromer, Kirby Institute, University of New South Wales, Sydney, Australia.
Arnold Reynaldi, Kirby Institute, University of New South Wales, Sydney, Australia.
Megan Steain, School of Medical Sciences, Faculty of Medicine and Health, The University of Sydney, Sydney, New South Wales, Australia; Sydney Institute of Infectious Diseases and Charles Perkins Centre, The University of Sydney, Sydney, New South Wales, Australia.
James A Triccas, School of Medical Sciences, Faculty of Medicine and Health, The University of Sydney, Sydney, New South Wales, Australia; Sydney Institute of Infectious Diseases and Charles Perkins Centre, The University of Sydney, Sydney, New South Wales, Australia.
Miles P Davenport, Kirby Institute, University of New South Wales, Sydney, Australia.
David S Khoury, Kirby Institute, University of New South Wales, Sydney, Australia.
References
- 1. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021. [DOI] [PubMed] [Google Scholar]
- 2. CureVac. CureVac provides update on phase 2b_3 trial of first-generation COVID-19 vaccine candidate, CVnCoV - CureVac. 2021.
- 3. Kremsner PG, Guerrero RAA, Arana E, et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate: results from herald, a phase 2b/3, randomised, observer-blinded, placebo-controlled clinical trial in ten countries in Europe and Latin America. 2021.
- 4. Baden LR, Sahly HM E, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kariko K, Buckstein M, H N, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005; 23:165–75. [DOI] [PubMed] [Google Scholar]
- 7. Kremsner P, Mann P, Bosch J, et al. Phase 1 assessment of the safety and immunogenicity of an mRNA- lipid nanoparticle vaccine candidate against SARS-CoV-2 in human volunteers. medRxiv 2020; doi:2020.11.09.20228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med 2020; 383:1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020; 383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. The Lancet Microbe 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]