Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a novel severe postinfectious condition associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The purpose of this report is to describe nationwide trends in the evolving clinical management of MIS-C.
Methods
Patients with MIS-C were reported from state and local jurisdictions to the Centers for Disease Control and Prevention’s (CDC’s) MIS-C national surveillance system. Patients’ case reports were reviewed to ensure that they met the CDC MIS-C case definition and had sufficient data for analysis. The prevalence of use of treatments for MIS-C, temporal trends in use of these treatments, and frequency of administration of different treatment combinations were analyzed.
Results
There were 4470 patients meeting the MIS-C case definition with onset dates from 19 February 2020 to 31 July 2021. The proportion of patients admitted to an intensive care unit (ICU) has declined over time, from 78.7% in April 2020 to 57.5% in June 2021 (P = .001). The most common treatments were intravenous immunoglobulin (IVIG), given to 85.6% of patients; steroids (77.7%), and antiplatelet medications (73.7%); use of each of these treatments has increased over time, particularly in patients not requiring admission to an ICU (all P < .001). Older patients and non-Hispanic Black patients were more likely to receive additional modes of therapy including vasoactive medication, noninvasive respiratory support, anticoagulation medication, and intubation/mechanical ventilation.
Conclusions
IVIG, steroids, and antiplatelet medication have become increasingly utilized as standard treatment for MIS-C patients, while the use of other treatments may be contingent on the type and severity of clinical findings.
Keywords: multisystem inflammatory syndrome in children, MIS-C, SARS-CoV-2, treatment, surveillance
In patients with MIS-C reported to CDC surveillance, IVIG, steroids, and antiplatelet medication have become increasingly utilized as standard treatment regardless of disease severity. Older and non-Hispanic Black patients were more likely to receive additional treatments.
First reported in April 2020, multisystem inflammatory syndrome in children (MIS-C) is a severe postinfectious hyperinflammatory condition linked to previous infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1–5]. Alternately named pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS), MIS-C has been described as having clinical features overlapping with Kawasaki disease (KD), toxic shock syndrome (TSS), and other inflammatory disorders.
From the first identified patients, management of MIS-C has often included use of intravenous immunoglobulin (IVIG) and steroids such as methylprednisolone to reduce inflammation, sometimes augmented by additional immune modulators such as infliximab or anakinra [6–15]. Aspirin has been frequently utilized, typically at low doses (3–5 mg/kg/day) as a platelet antiaggregant or sometimes at higher doses (30–80 mg/kg/day) for its anti-inflammatory effect [9, 10, 12, 14]. Vasoactive medications such as milrinone and epinephrine have been used to manage MIS-C patients with hypotension and shock [7, 9–15]. Additional MIS-C patient management may include anticoagulants such as enoxaparin or warfarin for treating thrombosis or cardiac dysfunction, and interventions such as high-flow nasal cannula or mechanical ventilation to treat patients with respiratory distress [6–13].
Existing guidelines on MIS-C clinical management draw from a combination of recommendations for similar clinical syndromes, experiences from management of early MIS-C patients, and consensuses of experts in medical fields including pediatrics, cardiology, rheumatology, and infectious diseases. On 17 June 2020, the American College of Rheumatology (ACR) first released a set of recommendations [16–18] that included IVIG (2 g/kg) and aspirin as standard treatment, with low-moderate dose glucocorticoids (1–2 mg/kg/day) for patients with shock or organ threatening disease. Recommendations also included high dose glucocorticoids (10–30 mg/kg/day) or anakinra (>4 mg/kg/day) for MIS-C patients refractory to first-line treatment. On 17 July 2020, the United Kingdom’s PIMS-TS National Consensus Management Study Group released its recommendations for management [19, 20]; some differences from the ACR guidelines include use of methylprednisolone alongside IVIG in KD-like patients under 12 months old or with coronary artery changes, consideration of a second dose of IVIG for refractory patients, and preference of infliximab over anakinra for patients with KD-like phenotype. Additional information that may have influenced MIS-C patient management includes CDC’s 16 July 2020 clinician outreach and communication activity (COCA) webinar on MIS-C, which summarized current knowledge and treatment practices [21], and the 28 October 2020 publication of a study indicating more rapid cardiac recovery for patients treated with methylprednisolone [22]. Clinical trials on MIS-C interventions are underway [23, 24], but robust information on the efficacy of treatments for this novel syndrome are not expected for some time.
In the current article, we present results from the largest patient database thus far reported with MIS-C treatment information. These results include trends in use of treatments, combinations of treatments used, and assessment of treatment use by age group and whether patients were admitted to an intensive care unit (ICU). Although this observational report cannot assess treatment effectiveness, details of MIS-C management practices over time may be useful for helping to guide future clinical recommendations.
METHODS
The US Centers for Disease Control and Prevention (CDC) published a Health Advisory describing MIS-C on 14 May 2020 and asked for suspected cases to be reported to state, local, and territorial health departments. Information on patients with suspected MIS-C were reported to the CDC by these health departments using CDC’s MIS-C case report form, which captured data on patient demographics, underlying medical conditions, clinical findings, laboratory tests, imaging results and treatment [25, 26]. Patient records were assessed to determine if they met the CDC case definition for MIS-C [2], which includes: patients aged <21 years hospitalized with fever, involvement of at least 2 organ systems (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurological), either laboratory evidence of current or previous SARS-CoV-2 infection or exposure to coronavirus disease 2019 (COVID-19), and lack of an alternative diagnosis. The full MIS-C case definition is included in supplemental materials. To assess temporal trends, patients required an estimated date of MIS-C symptom onset: if the date of symptom onset was not recorded, date of fever onset was used, or if both were missing, then date of hospital admission was used. This analysis includes patients who met the case definition and were reported to the CDC as of 4 August 2021, had laboratory confirmation of SARS-CoV-2 infection (patients without a positive test were excluded due to potential for misclassification) and who had an estimated date of MIS-C symptom onset were included in the analysis.
Information on the following methods of treatment were captured in the case report form: non-invasive respiratory treatment (low flow or high flow oxygen nasal cannula, noninvasive ventilation); intubation/mechanical ventilation; extracorporeal membrane oxygenation (ECMO); vasoactive medications (eg, epinephrine, milrinone, norepinephrine, or vasopressin); steroids; immune modulators (eg, anakinra, tocilizumab); antiplatelets (eg, aspirin, clopidogrel); anticoagulants (eg, heparin, enoxaparin, warfarin); dialysis; and intravenous immunoglobulin (IVIG), both single dose and 2 doses. Use of each category of treatment was recorded as a binary yes/no response; information on specific medications was collected for vasoactive medications, immune modulators, anticoagulants, and antiplatelets, but additional information such as dose and duration were not systematically collected. In addition, given particular interest in the use of certain treatments commonly utilized as first-line therapies in patients with MIS-C (IVIG and steroids), patients were divided into 4 mutually exclusive categories of anti-inflammatory treatments received. Those categories were (1) patients who received IVIG and steroids; (2) patients who received IVIG but not steroids; (3) patients who received steroids but not IVIG; and (4) patients who received neither steroids nor IVIG.
To assess trends over time, the proportion of MIS-C patients receiving each type of treatment was determined by month of symptom onset. Treatment trends were broken down by whether patients were admitted to an ICU, which was defined as having a documented date of ICU admission or known length of ICU stay or having received ICU-level treatment, including mechanical ventilation, vasoactive medication, or ECMO. The case report form did not contain information on the timing of treatments and clinical findings; as such, we cannot determine whether treatments preceded clinical findings, and any associations are not intended to make inferences about treatment effectiveness. Statistical comparisons were performed using Cochran-Armitage tests for trend in proportions receiving treatments by month and age group, and Fisher exact tests for difference in proportions receiving treatments by race/ethnicity. Few MIS-C patients had onset date in the months at the beginning of the study period (February 2020 and March 2020), although the final month in our study period (July 2021) also had smaller numbers due to expected delays in case reporting; proportions in these 3 months are not described due to potentially imprecise results, but those data are included in the trend tests. All analyses were conducted using R version 4.0.2 [27]. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy (eg, 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C.§241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
RESULTS
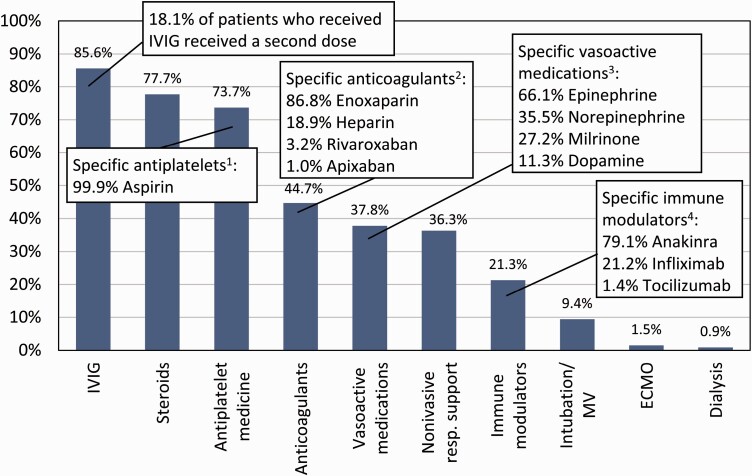
There were 4901 patients with suspected MIS-C reported to the CDC from 49 states along with the District of Columbia, New York City, and Puerto Rico, with dates of symptom onset between 19 February 2020 and 29 July 2021 [28]. Of those, 4470 (91.2%) met study inclusion criteria and were included in the analysis. The most commonly utilized treatments were IVIG (administered to 85.6% of patients, out of which 18.1% received a second dose), steroids (77.7%), and antiplatelet medication (73.7%) (Figure 1). Other common treatments included anticoagulants (received by 44.7% of patients with MIS-C), vasoactive medications (37.8%), noninvasive respiratory support (36.3%), and immune modulators (21.3%). Fewer than one tenth (9.4%) received intubation/mechanical ventilation, 1.5% received ECMO, and 0.9% received dialysis. For patients receiving anticoagulants, information on specific anticoagulants was recorded for 1760 of 2000 patients (88.0%): of those, 86.8% received enoxaparin, 18.9% received heparin, 3.2% received rivaroxaban, and 1.0% received apixaban (some patients received more than one medication and less common medications were not listed). Specific medications were recorded for 1456 of 1690 (86.2%) patients receiving vasoactive treatment: the most common were epinephrine (66.1%), norepinephrine (35.5%), milrinone (27.2%), and dopamine (11.3%). For immune modulators, information on specific medications was recorded for 795 of 954 (83.3%) patients: the most common were anakinra (79.1%), infliximab (21.2%), and tocilizumab (1.4%). Specific medications were recorded for 2928 of 3293 (88.9%) patients receiving antiplatelet medications: almost all (99.9%) received aspirin. The most common combinations of treatments received is shown in Supplementary Figure 1.
Figure 1.
Proportion of patients with MIS-C given different treatments, CDC MIS-C national surveillance, February 2020 to July 2021. Abbreviations: CDC, Centers for Disease Control and Prevention; ECMO, extracorporeal membrane oxygenation; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children; MV, mechanical ventilation. 1Percentages are for the 2928 (88.9%) patients with information on specific antiplatelets; some patients had multiple types listed. 2Percentages are for the 1760 (88.0%) patients with information on specific anticoagulants; some patients had multiple types listed. 3Percentages are for the 1456 (86.2%) patients with information on specific vasoactive medications; some patients had multiple types listed. 4Percentages are for the 761 (79.8%) patients with information on specific immune modulators; some patients had multiple types listed.
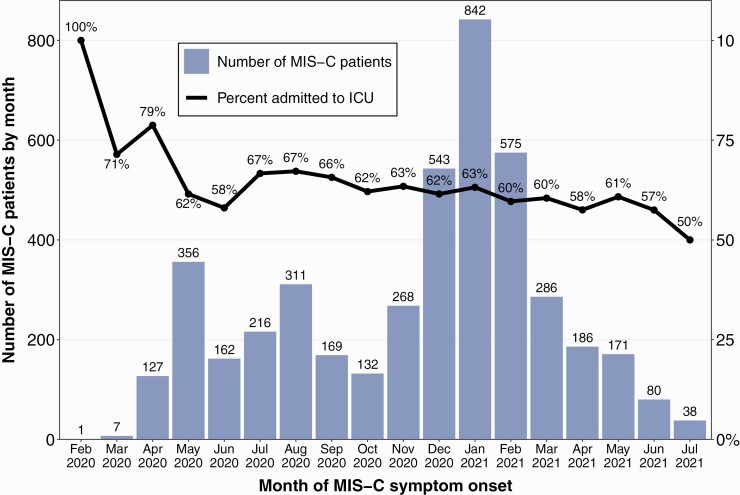
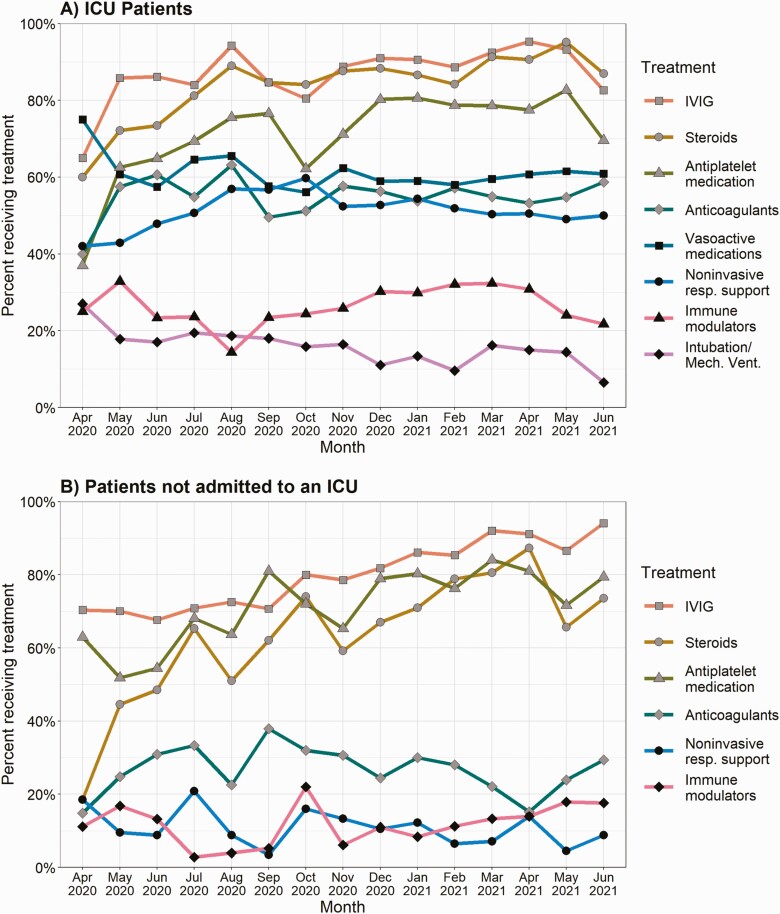
Figure 2 shows the number of MIS-C patients and proportion of patients admitted to an ICU by month. The overall proportion of patients admitted to an ICU was 62.5%, which decreased over time from 78.7% in April 2020 to 57.5% in June 2021 (P = .001) data from February-March 2020 and July 2021 not described due to imprecise proportions from smaller samples. Among patients with MIS-C admitted to an ICU, use of IVIG increased from 65.0% in April 2020 to 82.6% in June 2021 (P < .001), and similar increases were observed with the use of steroids (60.0% to 87.0%, P < .001) and antiplatelet medication (37.0% to 69.6%, P < .001) (Figure 3A). Use of vasoactive medications decreased from 75.0% in April 2020 to 60.9% in June 2021 (P = .028). The use of IVIG, steroids, and antiplatelet medications rose more considerably for MIS-C patients who were not admitted to an ICU: use of IVIG increased from 70.4% in April 2020 to 94.1% in June 2021 (P < .001), use of steroids increased from 18.5% to 73.5% (P < .001), and use of antiplatelet medication increased from 63.0% to 79.4% (P < .001) (Figure 3B).
Figure 2.
Number of MIS-C patients and percent of patients admitted to an ICU by month, CDC MIS-C national surveillance, February 2020 to July 2021. Abbreviations: CDC, Centers for Disease Control and Prevention; ICU, intensive care unit; MIS-C, multisystem inflammatory syndrome in children.
Figure 3.
Trends in treatments for patients with MIS-C by month for (A) patients admitted to an ICU during course of the hospitalization, and (B) patients not admitted to an ICU, CDC MIS-C national surveillance, April 2020 to June 2021. February 2020 (n = 1 for ICU, n = 0 for non-ICU), March 2020 (n = 5 for ICU, n = 2 for non-ICU), and July 2021 (n = 19 for ICU, n = 19 for non-ICU) not included due to imprecise proportions from smaller samples. Abbreviations: CDC, Centers for Disease Control and Prevention; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children.
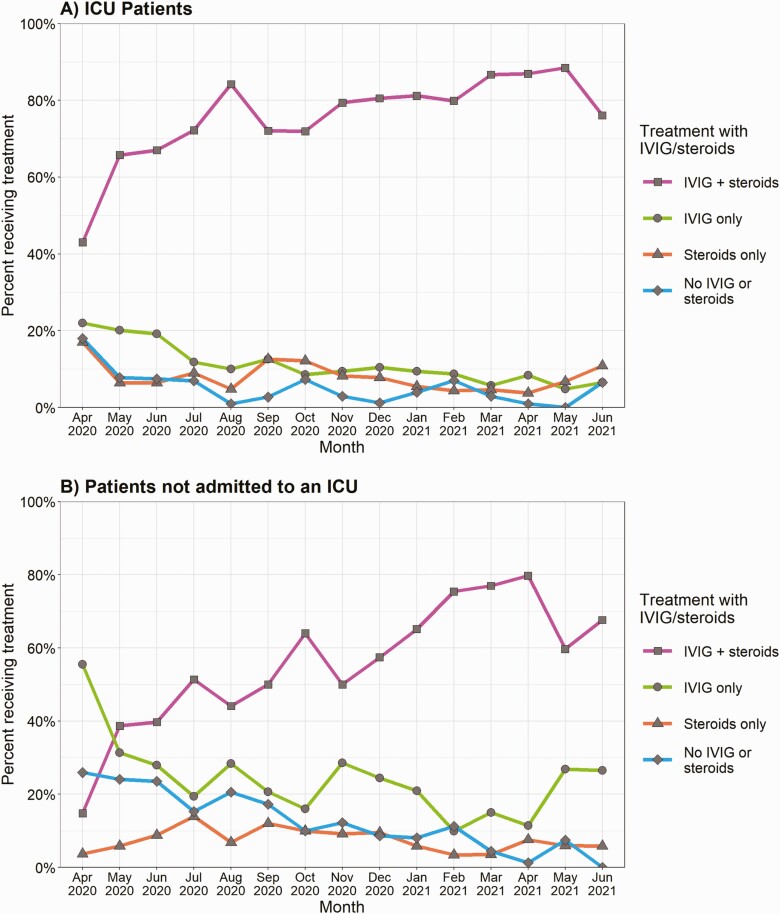
For patients admitted to an ICU, the proportion of patients who received a combination of IVIG and steroids increased over time (43.0% in April 2020 to 76.1% in June 2021), whereas the proportion of patients who received IVIG but not steroids (22.0% to 6.5%) and patients who received neither IVIG nor steroids (18.0% to 6.5%) declined (Figure 4). Among patients not admitted to an ICU, there was a substantial increase in the proportion of patients who received both IVIG and steroids (14.8% in April 2020 to 67.6% in June 2021), although there were decreases in patients who received IVIG but not steroids (55.6% to 26.5%) and patients who received neither IVIG nor steroids (25.9% to 0.0%) (Figure 4B).
Figure 4.
Trends in anti-inflammatory treatment combination categories for patients with MIS-C by month for (A) patients admitted to an ICU during course of the hospitalization, and (B) patients not admitted to an ICU, CDC MIS-C national surveillance, April 2020 to June 2021.Categories are mutually exclusive and add up to 100%. February 2020 (n = 1 for ICU, n = 0 for non-ICU), March 2020 (n = 5 for ICU, n = 2 for non-ICU), and July 2021 (n = 19 for ICU, n = 19 for non-ICU) not included due to imprecise proportions from smaller samples. Abbreviations: CDC, Centers for Disease Control and Prevention; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children.
The proportion of patients receiving a given treatment was lower among older children for IVIG (received by 72.5% in patients aged 16–20) and antiplatelet medication (55.6% in patients aged 16–20) (Table 1). Conversely, the proportion of patients receiving treatments increased with age for other treatments: patients aged 0–4 generally had the lowest prevalence of use of steroids (69.1%), anticoagulation medication (31.5%), vasoactive medications (22.9%), noninvasive respiratory treatment (22.9%), immune modulators (17.8%), intubation/mechanical ventilation (6.5%), ECMO (1.1%), and dialysis (0.2%). Compared to non-Hispanic White patients, non-Hispanic Black patients were significantly more likely to receive a 2nd dose of IVIG (19.7% vs 14.5%, P = .001), anticoagulants (49.1% vs 41.0%, P < .001), vasoactive medications (44.9% vs 33.3%, P < .001), noninvasive respiratory treatment (42.3% vs 34.6%, P < .001), and intubation/mechanical ventilation (13.2% vs 6.7%, P < .001) (Table 2).
Table 1.
Proportion of Patients With MIS-C Receiving Different Treatments and Combinations of Treatments by Age Group a , CDC MIS-C National Surveillance, February 2020 to July 2021
| Treatments | Age 0–4 n = 1087 | Age 5–11 n = 2016 | Age 12–15 n = 888 | Age 16–20 n = 477 | P-Valueb |
|---|---|---|---|---|---|
| IVIG | 934 (85.9%) | 1773 (87.9%) | 772 (86.9%) | 346 (72.5%) | <.001 |
| 2nd dose IVIG | 166 (15.3%) | 307 (15.2%) | 155 (17.5%) | 62 (13%) | .871 |
| Steroids | 751 (69.1%) | 1590 (78.9%) | 749 (84.3%) | 384 (80.5%) | <.001 |
| Antiplatelet medication | 834 (76.7%) | 1554 (77.1%) | 638 (71.8%) | 265 (55.6%) | <.001 |
| Anticoagulation medication | 342 (31.5%) | 857 (42.5%) | 512 (57.7%) | 288 (60.4%) | <.001 |
| Vasoactive medications | 249 (22.9%) | 763 (37.8%) | 462 (52%) | 215 (45.1%) | <.001 |
| Noninvasive respiratory treatment | 249 (22.9%) | 744 (36.9%) | 401 (45.2%) | 227 (47.6%) | <.001 |
| Immune modulators | 193 (17.8%) | 420 (20.8%) | 225 (25.3%) | 116 (24.3%) | <.001 |
| Intubation/mechanical ventilation | 71 (6.5%) | 163 (8.1%) | 108 (12.2%) | 77 (16.1%) | <.001 |
| ECMO | 12 (1.1%) | 21 (1%) | 20 (2.3%) | 16 (3.4%) | <.001 |
| Dialysis | 2 (0.2%) | 14 (0.7%) | 11 (1.2%) | 15 (3.1%) | <.001 |
Abbreviations: CDC, Centers for Disease Control and Prevention; ECMO, extracorporeal membrane oxygenation; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children.
2 (0.0%) patients were missing information on age.
P Values are for Cochran-Armitage tests for trend by age group.
Table 2.
Proportion of Patients With MIS-C Receiving Different Treatments and Combinations of Treatments by Race and Ethnicity a , CDC MIS-C National Surveillance, February 2020 to July 2021
| Non-Hispanic White n = 1217 | Non-Hispanic Black n = 1307 | Hispanic n = 1306 | Other n = 377 | ||||
|---|---|---|---|---|---|---|---|
| Treatments | n (%) | n (%) | P Valueb | n (%) | P Valueb | n (%) | P Valueb |
| IVIG | 1060 (87.1%) | 1102 (84.3%) | .047 | 1121 (85.8%) | .383 | 332 (88.1%) | .659 |
| 2nd dose IVIG | 177 (14.5%) | 258 (19.7%) | .001 | 183 (14%) | .733 | 49 (13%) | .499 |
| Steroids | 970 (79.7%) | 1048 (80.2%) | .766 | 980 (75%) | .006 | 291 (77.2%) | .310 |
| Antiplatelet medication | 911 (74.9%) | 967 (74%) | .648 | 977 (74.8%) | 1.000 | 281 (74.5%) | .892 |
| Anticoagulation medication | 499 (41%) | 642 (49.1%) | <.001 | 597 (45.7%) | .018 | 165 (43.8%) | .370 |
| Vasoactive medications | 405 (33.3%) | 587 (44.9%) | <.001 | 468 (35.8%) | .180 | 129 (34.2%) | .755 |
| Noninvasive respiratory treatment | 421 (34.6%) | 553 (42.3%) | <.001 | 473 (36.2%) | .405 | 119 (31.6%) | .290 |
| Immune modulators | 254 (20.9%) | 293 (22.4%) | .359 | 284 (21.7%) | .593 | 78 (20.7%) | 1.000 |
| Intubation/mechanical ventilation | 82 (6.7%) | 173 (13.2%) | <.001 | 116 (8.9%) | .046 | 29 (7.7%) | .562 |
| ECMO | 18 (1.5%) | 24 (1.8%) | .535 | 17 (1.3%) | .736 | 7 (1.9%) | .636 |
| Dialysis | 10 (0.8%) | 21 (1.6%) | .102 | 8 (0.6%) | .638 | 2 (0.5%) | .742 |
Abbreviations: CDC, Centers for Disease Control and Prevention; ECMO, extracorporeal membrane oxygenation; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children.
263 (5.9%) patients were missing information on race/ethnicity.
Fisher exact P values testing for differences in proportions compared to non-Hispanic White patients.
DISCUSSION
This study described the frequency of treatments used for patients with MIS-C, how the use of those treatments has changed over time, and common combinations of treatments. Most notably, the use of IVIG, steroids, and antiplatelet medication has become commonplace, regardless of disease severity, with each treatment being administered to over 70% of patients not admitted to an ICU in more recent months.
The development and publication of MIS-C clinical management guidelines may have helped the adoption of more standardized treatment regimens. Both the ACR and the UK PIMS-TS guidelines recommend IVIG and aspirin for all MIS-C patients [16–20]. These guidelines suggest methylprednisolone for use in patients who remain refractory after IVIG treatment or as initial treatment in patients with shock and/or organ threatening disease (ACR) or for KD-like patients under 12 months old or with coronary artery changes (UK PIMS-TS). It remains unclear how closely clinicians followed the ACR or UK PIMS-TS guidelines, as many institutions had their own protocols [29, 30]. Although some institutions administered IVIG, steroids, and aspirin to all patients with MIS-C, many other institutions utilized a tiered approach, with the use of immunomodulatory and antiplatelet medications based on disease severity [31]. Our data show that the use of IVIG, antiplatelet medication, and steroids did not suddenly rise after the release of these clinical management guidelines; rather, routine adoption of these treatments has been more gradual. Additionally, the current study did not contain information on specific type of steroid, dose, or duration, making it difficult to determine if treatment followed published guidelines. The increases in use of IVIG, antiplatelet medication, and steroids were most pronounced in non-ICU MIS-C patients, indicating a substantial shift toward these treatments in all MIS-C patients, regardless of disease severity.
Results from additional observational studies may continue to influence management of MIS-C patients. A recent study from Son et al found that compared to IVIG alone, use of IVIG with glucocorticoids decreased risk of cardiovascular dysfunction, shock, and need for adjunctive therapy [32]. Our results showed that since 1 January 2021, over 70% of MIS-C patients who were not admitted to an ICU received both IVIG and steroids; adoption of the recommendation from the aforementioned study might cause that number to further increase. However, McArdle et al found no difference in risk of inotropic or ventilator support for patients receiving IVIG with glucocorticoids compared to patients receiving IVIG alone; differences in study populations, patient inclusion criteria, and methods to control for confounding variables may have contributed to the dissimilar results [33]. Ongoing clinical trials are aiming to test the effectiveness of infliximab, methylprednisolone, anakinra, and tocilizumab in MIS-C patients [23, 24]; these studies will be invaluable for improving clinical management and reducing risk of severe outcomes.
From the first encounters with MIS-C, clinical management of MIS-C has been strongly influenced by recommended management for KD patients, for whom IVIG and aspirin are standard first-line treatments, while corticosteroids and infliximab are among the established treatments for adjunctive therapy in patients believed to be at high risk for cardiac complications or for patients not responding to first-line IVIG treatment [34]. A study using data from 49 large US pediatric hospitals found that roughly 19% of KD patients received a second dose of IVIG, while fewer than 6% received steroids and fewer than 3% received infliximab [35]. In the current study on patients with MIS-C, the proportion of IVIG recipients who received a second dose was similar (18.1%), but the proportion of patients receiving steroids (77.7%) and immune modulators (21.3%) was substantially higher. Compared to patients with KD, patients with MIS-C have been shown to have greater levels of inflammation [14, 15, 36], have involvement of more organ systems, and have been more likely to have severe outcomes like shock, decreased cardiac function, myocarditis, pneumonia, and acute kidney injury (Godfred-Cato, submitted), which may explain the trend towards more aggressive treatment of patients.
Proportions of MIS-C patients receiving specific types of treatments differed by age group. Younger patients were more likely to receive IVIG and antiplatelet medication: KD is most common in children aged 0-4, and the common use of IVIG and antiplatelets in MIS-C patients of similar age likely reflects experience with treatment of clinically similar KD patients [34]. Conversely, all other treatments captured in the case report form were more commonly administered to older children. Previous analyses from MIS-C cases submitted to CDC have noted more severe disease in older patients including cardiac damage, shock, and pneumonia [37, 38]; the clinical manifestations would have required more thorough clinical management. This current study also found that non-Hispanic Black patients were more likely to receive a second dose of IVIG, anticoagulants, vasoactive medications, noninvasive respiratory treatment, immune modulators, and intubation/mechanical ventilation; this is consistent with research showing that non-Hispanic Black MIS-C patients were at greater risk of decreased cardiac function and ICU admission [37]. Although the exact cause of these racial disparities is unknown, factors such as insufficient access to healthcare, increased prevalence of underlying medical conditions, and increased exposure to environmental pollutants can affect a wide range of health risks and outcomes [39].
Previous publications using the same CDC MIS-C surveillance database have found that the percentage of patients reported with severe outcomes has decreased over time [37, 38]. Although changes in clinical management for MIS-C have been noted in this study, the extent to which these changes may have improved patient outcomes cannot be determined. Increased recognition and published guidelines for MIS-C may have also resulted in more rapid administration of treatments. However, this hypothesis also cannot be verified through this study: there was no observable difference in days from MIS-C symptom onset to hospital admission over time, and the database did not include information on when treatments were first administered.
The primary limitation of this study is that the results cannot be used to make inferences about the effectiveness of specific treatments. As an observational study, confounding by indication for treatment would likely be a major source of bias; this is compounded by the lack of timing information on most clinical findings and treatments, making it difficult to determine if treatments necessarily preceded outcomes for analyzed patients. Preliminary attempts at controlling for this bias through stratification or through propensity score matching were not promising—even after these controls were applied, use of most treatments was still associated with greater probability of severe outcomes, suggestive of residual confounding. Additional limitations regarding data availability include (1) lack of information on additional interventions such as antivirals/antibiotics or fluid resuscitation; (2) no information on vaccination status; (3) lack of information on medication dose and duration; and (4) lack of trended laboratory marker data. Access to SARS-CoV-2 testing and other case identification and reporting considerations may have varied over time and jurisdiction. Without a specific diagnostic test for MIS-C, this study may have included patients with other inflammatory syndromes who had clinical pictures overlapping with MIS-C.
CONCLUSION
The present study, using a large nationwide data set of patients going back to the beginning of the MIS-C outbreak, provided important information characterizing patterns of clinical management of patients with MIS-C across institutions and over time. This information may help augment the evolving understanding of best practices in the clinical management of MIS-C and inform future considerations on the care of children with this serious condition.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Most common combinations of six different treatments received for patients with MIS-C, CDC MIS-C national surveillance, February 2020—July 2021.aTreatment combinations are listed below each column; for example, the first column shows that 13% of patients with MIS-C received IVIG, steroids, antiplatelet medication, and none of the other listed treatments.
IVIG = intravenous immunoglobulin; Y = Yes; N = No
aFigure displays the 30 most common treatment combinations which are used in 94.3% of patients; an additional 29 combinations are not shown.
Notes
Acknowledgments. The authors thank local and state health departments for submitting reports of patients with suspected multisystem inflammatory syndrome in children to the national surveillance system.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Financial support. This work was supported by the US Centers for Disease Control and Prevention.
Potential conflicts of interest. M. E. O. reports payment to the institution where they have clinical responsibilities from the NIH (MUSIC Study) outside of the conduct of the study. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Joseph Y Abrams, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Ermias D Belay, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Shana Godfred-Cato, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Angela P Campbell, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Laura D Zambrano, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Amber Kunkel, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Allison D Miller, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Michael J Wu, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Lu Meng, CDC COVID-19 Response Team, Atlanta, Georgia, USAand; Apex Systems Affiliated with General Dynamics Information Technology, Atlanta, Georgia, USA.
Ami B Shah, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
Matthew E Oster, CDC COVID-19 Response Team, Atlanta, Georgia, USAand.
References
- 1. Guidance—Paediatric multisystem inflammatory syndrome temporally associated with COVID-19: Royal College of Paediatrics and Child Health, 2020. Available at: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19. [Google Scholar]
- 2. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention Health Alert Network, 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. [Google Scholar]
- 3. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. World Health Organization, 2020. Available at: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. [Google Scholar]
- 4. Health advisory: pediatric multi-system inflammatory syndrome potentially associated with coronavirus disease (COVID-19) in children. New York State Department of Health, 2020. 5/6/2020. Available at: https://health.ny.gov/press/releases/2020/docs/2020-05-13_health_advisory.pdf. [Google Scholar]
- 5. Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief, 15 May 2020. World Health Organization, 2020. Available at: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. [Google Scholar]
- 6. Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142:429–36. [DOI] [PubMed] [Google Scholar]
- 7. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020; 79:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramcharan T, Nolan O, Lai CY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol 2020; 41:1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riollano-Cruz M, Akkoyun E, Briceno-Brito E, et al. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York city experience. J Med Virol 2020; 93:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020; 369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henderson LA, Canna SW, Friedman KG, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol 2020; 72:1791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson LA, Canna SW, Friedman KG, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol 2021; 73:e13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19: American College of Rheumatology MIS-C and COVID-19 Related Hyperinflammation Task Force. 2020; 7:69. Available at: https://www.rheumatology.org/Portals/0/Files/ACR-COVID-19-Clinical-Guidance-Summary-MIS-C-Hyperinflammation.pdf?ver=2020-07-02-140939-180. [Google Scholar]
- 19. Harwood R, Allin B, Jones CE, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2 (PIMS-TS): the results of a national Delphi process. medRxiv 2020; 385:23–34. doi: 10.1016/S2352-4642(20)30304-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harwood R, Allin B, Jones CE, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health 2021; 5:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ermias DB, Eva WC, Matthew EO, Adriana HT.. Clinical management of multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Clinician Outreach and Communication Activity: Centers for Disease Control and Prevention; July 16, 2020. Available at: https://emergency.cdc.gov/coca/calls/2020/callinfo_071620.asp. . [Google Scholar]
- 22. Belhadjer Z, Auriau J, Méot M, et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation 2020; 142:2282–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adriana HT. MIS-C Comparative Effectiveness Study (MISTIC): Clinical trial ID NCT04898231. ClinicalTrials.gov. San Diego: University of California, 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04898231. [Google Scholar]
- 24. Peter WH. A randomised trial of treatments to prevent death in patients hospitalised with COVID-19 (coronavirus): Clinical Trial ISRCTN50189673. ISRCTN registry: University of Oxford, 2020. Available at: https://www.isrctn.com/ISRCTN50189673. [Google Scholar]
- 25. Multisystem inflammatory syndrome associated with COVID-19 case report form. Centers for Disease Control and Prevention, 2020. Available at: https://www.cdc.gov/mis-c/pdfs/hcp/mis-c-form-fillable.pdf. [Google Scholar]
- 26. Godfred-Cato S, Bryant B, Leung J, et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. Morb Mortal Wkly Rep 2020; 69:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Team RC. R: a language and environment for statistical computing computer program, version 4.0.2. Vienna, Austria: R Core Team, 2020. [Google Scholar]
- 28. Miller AD, Zambrano LD, Yousaf AR, et al. Multisystem inflammatory syndrome in children—United States, February 2020–July 2021. Clin Infect Dis 2021:ciab1007. doi: 10.1093/cid/ciab1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jonat B, Gorelik M, Boneparth A, et al. Multisystem inflammatory syndrome in children associated with coronavirus disease 2019 in a children’s hospital in New York city: patient characteristics and an institutional protocol for evaluation, management, and follow-up. Pediatr Crit Care Med 2021; 22:e178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dove ML, Jaggi P, Kelleman M, et al. Multisystem inflammatory syndrome in children: survey of protocols for early hospital evaluation and management. J Pediatr 2021; 229:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children—initial therapy and outcomes. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med 2021; 385:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–99. [DOI] [PubMed] [Google Scholar]
- 35. Dominguez SR, Birkholz M, Anderson MS, et al. Diagnostic and treatment trends in children with Kawasaki disease in the United States, 2006–2015. Pediatr Infect Dis J 2019; 38:1010–4. [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez-Smith JJ, Verweyen EL, Clay GM, et al. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: a cohort study. TheLancet Rheumatology 2021; 3:e574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abrams JY, Oster ME, Godfred-Cato SE, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc. Health 2021; 5:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belay ED, Abrams J, Oster ME, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr 2021; 178:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Social Determinants of Health: Know What Affects Health. Available at: https://www.cdc.gov/socialdeterminants/. Accessed 26 June 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.