ABSTRACT
Introduction
The vital renal replacement therapy makes it impossible for dialysis patients to distance themselves socially. This results in a high risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and developing coronavuris disease 2019, with excess mortality due to disease burden and immunosuppression. We determined the efficacy of a 100-µg booster of mRNA-1273 (Moderna, Cambridge, MA, USA) 6 months after two doses of BNT162b2 (BioNTech/Pfizer, Mainz, Germany/New York, USA) in 194 SARS-CoV-2-naïve dialysis patients.
Methods
Anti-SARS-CoV-2 spike antibodies were measured with the Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics, Mannheim, Germany) 4 and 10–12 weeks after two doses of BNT162b2 as well as 4 weeks after the mRNA-1273 booster. The presence of neutralizing antibodies was measured by the SARS-CoV-2 Surrogate Virus Neutralization Test (GenScript Biotech, Piscataway, NJ, USA). Two different cut-offs for positivity were used, one according to the manufacturer's specifications and one correlating with positivity in a plaque reduction neutralization test (PRNT). Receiver operating characteristics analyses were performed to match the anti-SARS-CoV-2 spike antibody cut-offs with the cut-offs in the surrogate neutralization assay accordingly.
Results
Any level of immunoreactivity determined by the anti-SARS-CoV-2 spike antibody assay was found in 87.3% (n = 144/165) and 90.6% (n = 164/181) of patients 4 and 10–12 weeks, respectively, after two doses of BNT162b2. This was reduced to 68.5% or 60.6% 4 weeks and 51.7% or 35.4% 10–12 weeks, respectively, when using the ROC cut-offs for neutralizing antibodies in the surrogate neutralization test (manufacturer's cut-off ≥103 U/mL and cut-off correlating with PRNT ≥196 U/mL). Four weeks after the mRNA-1273 booster, the concentration of anti-SARS-CoV-2 spike antibodies increased to 23 119.9 U/mL and to 97.3% for both cut-offs of neutralizing antibodies.
Conclusion
Two doses of BNT162b2 followed by one dose of mRNA-1273 within 6 months in patients receiving maintenance dialysis resulted in significant titres of SARS-CoV-2 spike antibodies. While two doses of mRNA vaccine achieved adequate humoral immunity in a minority, the third vaccination boosts the development of virus-neutralizing quantities of SARS-CoV-2 spike antibodies (against wild-type SARS-CoV-2) in almost all patients.
Keywords: COVID-19, elasomeran, SARS-CoV-2, seroconversion, tozinameran, vaccination
KEY LEARNING POINTS.
What is already known about this subject?
Patients on renal replacement therapy are at high risk for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and demonstrate high hospitalization and mortality rates due to coronavirus disease 2019 (COVID-19).
Vaccination represents the only strategy to prevent infection and a severe course of COVID-19.
Patients with immunosuppression due to therapy and chronic kidney disease are not studied within clinical trials.
What this study adds?
Seroconversion was achieved with BNT162b2 in up to 90% of patients 10–12 weeks after two doses.
Adjusting seroconversion to levels of anti-SARS-CoV-2 spike antibodies, which represent the presence of neutralizing antibodies, resulted in a significant reduction to 50% (positive surrogate virus neutralization test using the manufacturer's cut-off and SARS-CoV-2 wild-type antigen as the target structure) or 35% [positive surrogate virus neutralization test correlating with a plaque reduction neutralization test (PRNT) titre ≥1:20] of patients with adequate immunization success.
A single dose of 100 µg mRNA-1273 6 months after vaccination with BNT162b2 boosts the immune response to 100% seroconversion of anti-SARS-CoV-2 spike antibodies with 97.3% (positive surrogate virus neutralization test using the manufacturer's cut-off) or 97.3% (positive surrogate virus neutralization test correlating with a PRNT titre ≥1:20) of patients with an adequate titre of neutralizing antibodies.
What impact this may have on practice or policy?
A high standard of hygiene in dialysis facilities and vaccination of healthcare, service and transport personnel is recommended, especially if patients are only vaccinated twice.
A single dose of mRNA-1273 within 6 months after two doses of BNT162b2 results in a substantial humoral immune response and should be considered in this cohort.
Further research regarding the role of T-cell-mediated immunity is needed and should be considered in the prospective evaluation of the immunization status.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has to been a great challenge in all areas of life. The reasons for this are infectivity in the pre-symptomatic phase or even in asymptomatic individuals, few therapeutic options and a high mortality rate in people with risk factors [1]. Dialysis patients are particularly at risk from a SARS-CoV-2 infection, as they are mostly dependent on therapy in a nephrology centre on a frequent basis and thus one of the essential non-pharmacological interventions, i.e. social distancing, is limited. Furthermore, the burden of comorbidities in these patients translates into a high hospitalization rate and mortality with coronavirus disease 2019 (COVID-19) [2, 3]. An important step for the protection of this risk group is effective immunization.
Abnormalities in the immune response of uraemic patients are caused by dysfunctional activation and reduced overall function of both the innate and adaptive immune systems. This is aggravated by a reduction in dendritic cells, a shift of T helper 1/T helper 2 T-cell ratios and decreased T-cell activation. These patients also represent a cohort of older people subject to immunosenescence, including hypo reactive T-cells [4]. Some of these patients also have inflammatory systemic diseases, which are additionally treated with immunosuppressive therapies. Correspondingly, only minor vaccination response has been documented in this cohort with most vaccines [5]. The reduced vaccination success consists of a lower rate of seroconversion, lower antibody titres and a shortened presence of the antibody response compared with patients with healthy kidneys [6]. Increased dosing and additional booster doses have reduced the seroconversion failure with some of them.
Randomized clinical trials on SARS-CoV-2 vaccines have been conducted without dialysis patients and none has determined the efficiency of a third dose. Several observational studies on seroconversion after SARS-CoV-2 immunization in dialysis patients have been published. Regardless of the messenger RNA (mRNA) vaccine used, seroconversion rates of ≥90% are found shortly after receiving two doses [7–12]. Only a few studies followed their patients for more than a few weeks. In addition, seroconversion rates were solely based on the positivity of tests used in each study. Cut-offs, however, are mainly determined by testing sera collected before the pandemic and convalescent plasma after COVID-19. This approach results in high sensitivity for antibody detection. However, correlation studies of enzyme-linked immunosorbent assays (ELISAs) for the determination of antibody concentration and virus neutralization tests or surrogate assays lead to significantly increased cut-offs [13, 14]. This results in a discrepancy between strict seroconversion and a concentration of antibodies that effectively inhibits the binding of SARS-CoV-2 to the cell surface. Therefore we analysed a dialysis cohort of 194 patients for their humoral immune response to BNT162b2 (tozinameran; BioNTech/Pfizer, Mainz, Germany/New York, NY, USA) with special regard to neutralization capacity and the duration of adequate antibody levels. Furthermore, we analysed these endpoints after a single additional booster vaccination with mRNA-1273 (elasomeran; Moderna, Cambridge, MA, USA) within 6 months after having received two doses of BNT162b2.
MATERIALS AND METHODS
Patient cohorts
All patients receiving maintenance dialysis during 2021 in the nephrology outpatient department of Grünstadt (Apherese und Dialysezentrum) and Worms (Nierenzentrum), Germany, were informed about the aim of the study and invited to participate. Criteria for enrolment were a minimum age of 18 years and full COVID-19 vaccination with BNT162b2 according to the guidelines of the German Standing Committee on Vaccination (STIKO). Serum samples were screened for SARS-CoV-2-specific anti-nucleocapsid, anti-spike and potentially neutralizing antibodies at 4 weeks and for anti-spike antibodies 10–12 weeks after two vaccine doses (30 µg). A subgroup of patients in the dialysis department of Grünstadt (n = 81) was also analysed ∼4 weeks after having received the first vaccine dose, just before the second immunization. Of 213 individuals, 19 were positive for nucleocapsid antibodies or by SARS-CoV-2 polymerase chain reaction (PCR) and were excluded from the study. In September 2021, the STIKO recommended a third dose of an mRNA vaccine 6 months after having received two doses of an mRNA COVID-19 vaccine in patients with a high risk for a severe course of COVID-19. Accordingly, we offered a single dose of 100 µg mRNA-1273 for booster vaccination within 6 months after the first immunization. Not all patients provided blood samples for all measured time points (n = 165 individuals at 4 weeks, n = 181 individuals at 10–12 weeks after two vaccine doses, n = 166 prior to booster immunization and n = 148 at 4 weeks after booster vaccination). We screened for SARS-CoV-2 infection by PCR testing due to suggestive symptoms and anti nucleocapsid-antibody measurement in every sample. Because only patients on renal replacement therapy (RRT) were analysed, no control group was included. The trial was approved by the Ethics Committee II of the University of Heidelberg at the Medical Faculty Mannheim (2020–590N).
Serological SARS-CoV-2 antibody assays
Immuno assays—electrochemiluminescence double-antigen sandwich immunoassays (ECLIAs)—for antibodies against the nucleocapsid (Elecsys Anti-SARS-CoV-2 or SARS-CoV-2-Nc-Abs), or spike protein (Elecsys Anti-SARS-CoV-2 S) were performed according to the manufacturer's specifications on a cobas e 411 analyser (Roche Diagnostics, Mannheim, Germany). The anti-SARS-CoV-2-S antibody ECLIA (SARS-CoV-2-S-Ab) assay, detects all subclasses of immunoglobulins directed against the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2. The measurement ranges from 0.4 to 250 U/mL. Higher levels of antibodies were determined by dilution according to the manufacturer's instructions. According to the manufacturer's specifications, the cut-off for a reactive test result is defined as ≥0.8 U/mL.
Virus neutralization surrogate ELISA
The ELISA-based GenScript SARS-CoV-2 Surrogate Virus Neutralization Test Kit (GenScript Biotech, Piscataway, NJ, USA) was used according to the manufacturer's protocol. The microtitre plates are coated with the host cell receptor angiotensin-converting enzyme 2 (ACE2). The amount of neutralizing antibodies (SARS-CoV-2-NT-Abs) is measured by the degree of inhibition by using the spike protein–RBD–horseradish peroxidase conjugate as a binding partner. The RBD represents the wild-type SARS-CoV-2. Optical density (OD) was measured at 450 nm using the microplate reader of a VIRCLIA automation system (Vircell Spain, Granada, Spain). The signal:cut-off ratio was calculated and values were expressed and interpreted according to the manufacturer's specifications. The manufacturer-given inhibition of least 30% determined by the virus neutralization test was considered a positive result, indicating the presence of neutralizing antibodies.
Receiver operating characteristics (ROC) analyses
To define the SARS-CoV-2-S-Ab level that defines an adequate inhibition within the SARS-CoV-2-NT-Ab assay, an ROC analysis was performed. An inhibition of ≥30% (cut-off to positivity according to the manufacturer's specifications) within the SARS-CoV-2-NT-Ab assay resulted in a cut-off of 103 U/mL within the SARS-CoV-2-S-Ab assay, indicating the presence of neutralizing antibodies.
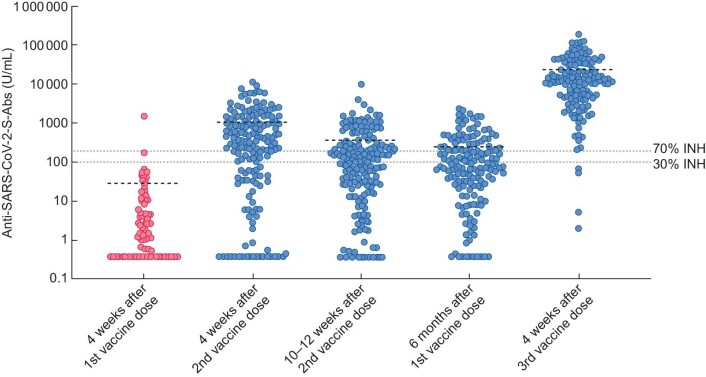
Furthermore, we used a cut-off of ≥70% inhibition in the SARS-CoV-2-NT-Ab assay for the ROC analysis, because at this level of inhibition, the SARS-CoV-2-NT-Ab assay better correlates with the presence of functional neutralizing antibodies with a titre ≥1:20 in the plaque reduction neutralization test (PRNT), as previously described [13]. A cut-off of 196 U/mL was generated for the SARS-CoV-2-S-Ab assay (Table 3, Figure 1).
Table 3.
Seroconversion of patients on RRT after two doses of BNT162b2 and a single dose of 100 µg mRNA-1273 after adjusting for adequate virus neutralization
| All patients | Haemodialysis | Peritoneal dialysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROC ≥ 30% (≥103 U/mL) | n | % | Sero revers., n/N (%) | n | % | Sero revers., n/N (%) | n | % | Sero revers., n/N (%) | P-value |
| After first vaccine dose | 2/81 | 2.5 | n.a. | 1/75 | 1.3 | n.a. | 1/6 | 16.7 | n.a. | .1481 |
| 4 weeks after complete vaccination | 113/165 | 68.5 | 0/2 | 92/141 | 65.2 | 0/1 | 21/24 | 87.5 | 0/1 | .0534 |
| 10–12 weeks after complete vaccination | 94/181 | 51.9 | 24/156 (15.4) | 80/160 | 50 | 20/137 (14.6) | 14/21 | 65.7 | 4/19 (21.1) | .2282 |
| Prior to booster vaccination | 73/166 | 44.0 | 18/160 (11.3) | 65/150 | (31.3) | 15/147 (10.2) | 8/16 | 50 | 3/13 (23) | .8059 |
| 4 weeks after booster vaccination | 144/148 | 97.3 | n.a. | 130/134 | 97.0 | n.a. | 14/14 | 100 | n.a. | >.999 |
| All patients | Haemodialysis | Peritoneal dialysis | ||||||||
| ROC ≥ 70% (≥196 U/mL) | n | % | Sero revers., n/N (%) | n | % | Sero revers., n/N (%) | n | % | Sero revers., n/N (%) | P-value |
| After first vaccine dose | 1/81 | 1.2 | n.a. | 0/75 | 1.3 | n.a. | 1/6 | 16.7 | n.a. | .0741 |
| 4 weeks after complete vaccination | 100/165 | 60.6 | n.a. | 80/141 | 56.7 | n.a. | 20/24 | 83.3 | n.a. | .0252 |
| 10–12 weeks after complete vaccination | 64/181 | 35.4 | 34/156 (21.8) | 57/160 | 35.6 | 25/137 (18.2) | 7/21 | 33.3 | 9/19 (47.4) | >.999 |
| Prior to booster vaccination | 51/166 | 30.7 | 13/140 (9.3) | 44/150 | 29.3 | 1/12 (8.3) | 7/16 | 43.8 | 1/12 (8.3) | .2602 |
| 4 weeks after booster vaccination | 144/148 | 97.3 | n.a. | 130/134 | 97.0 | n.a. | 14/14 | 100 | n.a. | >.999 |
A total of 103 U/mL of SARS-CoV-2-S-Abs demonstrated an inhibition of ≥30% in the SARSCoV-2-NT-Ab assay, which corresponds to the cut-off for positivity recommended by the manufacturer. At least 70% signal reduction in this assay correlates with a higher likelihood of virus neutralization, with a titre ≥1:20 in the PRNT. This resulted in a SARS-CoV-2-S-AB level of 196 U/mL after ROC analysis. Sero-reversion was determined in patients with at least two consecutive measurements of anti-SARS-CoV-2-S-Abs. Sero revers., sero reversion; n.a., not applicable.
aComparison of seroconversion between haemodialysis versus peritoneal dialysis patients.
FIGURE 1:
Scatter plot of anti-SARS-CoV-2-S-Ab levels and mean at the examined time points. The dotted lines represent the corresponding cut-offs of the surrogate virus neutralization test. 30% inhibition (INH), manufacturer's cut-off for positivity; 70% inhibition (INH), cut-off correlating with PRNT positivity (titre ≥1:20).
Statistical analysis
Data were extracted and organized with Excel (Microsoft, Redmond, Washington A, USA). Categorical data were analysed by chi-squared test and non-parametric data by Mann–Whitney U-test. The correlation between examined reactive SARS-CoV-2-S-Ab and positive SARS-CoV-2-NT-Ab assay test results was evaluated by Pearson correlation analysis using SPSS Statistics 27 (IBM, Armonk, NY, USA). ROC analysis and the sensitivity/specificity for the prediction of adequate concentrations of the SARS-CoV-2-S-Ab concentration were calculated using StatsDirect (StatsDirect, Birkenhead, UK). Due to multiple testing, P-values were adjusted to <.01 for significance.
RESULTS
A total of 194 patients on RRT, of whom 110 were male and 84 were female, participated in this study (Table 1). SARS-CoV-2-Nc-Abs were negative in all patients. The haemodialysis (HD) cohort was significantly older compared with the patients treated with peritoneal dialysis (PD) (P = .0011). A total of 10.8% received active immunosuppressive therapy during the duration of the study.
Table 1.
Baseline characteristics of the study population consisting of 194 patients on RRT
| All patients | Haemodialysis | Peritoneal dialysis | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | n | % | n | % | n | % | P-value |
| Gender (Female/male) | 84/110 | 43.3/56.7 | 74/93 | 44.9/55.1 | 10/17 | 37.0/63 | .6182 |
| Active immunosuppressive therapya | |||||||
| Corticosteroids | 19 | 9.8 | 17 | 10.2 | 2 | 7.4 | |
| Mycophenolat mofetil | 2 | 1.0 | 2 | 1.2 | - | - | |
| Rituximab | 3 | 1.5 | 2 | 1.2 | 1 | 3.7 | |
| D.m., mean (SD) | 78 | 41.8 | 68 | 40.7 | 10 | 37.0 | .8804 |
| Age (years), mean (SD) | 69.6 (14.2) | 71.0 (13.9) | 61.6 (13.8) | .0011 | |||
| Time on RRT (years), mean (SD) | 4.2 (5.6) | 4.6 (5.9) | 2.2 (2.2) | .0364 | |||
| Dialysis quality (Kt/V), mean (SD) | 1.25 (0.3) | ||||||
| Dialysis quality (Kt/V weekly), mean (SD) | 2.0 (0.5) | ||||||
| BMI (kg/m2), mean (SD) | 28.8 (5.6) | 28.8 (6.9) | 28.7 (6.6) | .8735 | |||
| CRP (mg/L), mean (SD) | 8.4 (14.1) | 8.8 (15.0) | 6.5 (7.5) | .192 | |||
| Albumin (g/L), mean (SD) | 31.9(4.7) | 31.9 (4.7) | 36.3 (5.1) | .2001 | |||
aActive immunosuppression was defined as therapy with mycophenolate mofetil, mycophenolic acid and systemic corticosteroids during or rituximab within the last 6 months prior to vaccination. BMI, body mass index; CRP, C-reactive protein; SD, standard deviation.
In a subgroup of patients, vaccination-related SARS-CoV-2-S-Ab seroconversion was found in 56.6% (47/81) after the first dose of BNT162b2 (Table 2). Four weeks after two vaccine doses, 85.5% (n = 144/165) had detectable SARS-CoV-2-S-Abs. At least 70 days from the second dose of BNT162b2, 90.5% (n = 164/181) had a level of >0.8 U/mL SARS-CoV-2-S-Abs. Eight of 155 patients showed protracted seroconversion 4 and 10–12 weeks after having received two vaccine doses. Only one patient demonstrated a sero-reversion within this period. Peak antibody levels were found in the overall cohort 4 weeks after two vaccine doses with significantly higher levels of SARS-CoV-2-S-Abs in patients on peritoneal dialysis (P = .0081).
Table 2.
Levels of SARS-CoV-2-S-Abs in BNT162b2 (tozinameran)-vaccinated patients on dialysis at different time points
| All patients | Haemodialysis | Peritoneal dialysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Mean (U/mL) | SD | Resp., n | Mean (U/mL) | SD | Resp., n | Mean (U/mL) | SD | Resp., n | P-valuea |
| After first vaccine dose | 43.9 | 211.4 | 34/81 | 15.4 | 29.5 | 30/75 | 311.6 | 678.9 | 4/6 | .4892 |
| 4 weeks after complete vaccination | 1192.1 | 1873.1 | 144/165 | 1140.0 | 1950.7 | 121/141 | 1473.0 | 1382.6 | 23/24 | .0081 |
| 10–12 weeks after complete vaccination | 396.2 | 928.0 | 164/181 | 416.5 | 416.5 | 144/160 | 244.9 | 285.1 | 20/21 | .5674 |
| Prior to booster vaccination | 261.4 | 426.5 | 147/166 | 271.1 | 436.9 | 131/150 | 230.2 | 369.1 | 16/16 | .2257 |
| 4 weeks after booster vaccination | 23 119.8 | 29 603.4 | 148/148 | 23 982.8 | 30 597.1 | 134/134 | 14 859.4 | 15 830.4 | 14/14 | .3577 |
Patients were vaccinated with a full dose of mRNA-1273 (elasomeran) within 6 months after their first immunization. Resp., responder; SD, standard deviation.
aComparison of mean SARS-CoV-2-S-Ab concentrations between patients on haemodialysis versus peritoneal dialysis for each time point.
In the Pearson correlation analysis, there was a significant correlation (P < .0005, r = .424; weak positive correlation) between the level of neutralizing antibodies (SARS-CoV-2-NT-Abs) and reactive SARS-CoV-2-S-Ab assay results. ROC analysis was performed to define the SARS-CoV-2-S-Ab level that defines an adequate inhibition within the SARS-CoV-2-NT-Ab assay, indicating the presence of neutralizing antibodies. When using this adapted cut-off, sensitivity and specificity were 89.8% [95% confidence interval (CI) 82.9–94.6] and 97.6% (95% CI 87.4–99.9), respectively (Supplementary data, Figure S1A). With this cut-off, the seroconversion rate decreased to only 2/81 individuals after the first vaccine dose (Table 3, Figure 1). Four weeks after two vaccine doses the seroconversion rate was 68.5% (n = 113/165). At least 10 weeks after the second dose, 51.9% (n = 94/181) had adequate levels of SARS-CoV-2-S-Abs (≥103 U/mL, corresponding to the ROC analysed cut-off to positivity in the SARS-CoV-2-NT-Ab assay). Only 3/156 patients examined 4 and 10–12 weeks after vaccination had a protracted humoral immune response, and 24 patients (15.5%) out of this group demonstrated sero reversion 10–12 weeks after two vaccine doses. As 70% inhibition within the SARS-CoV-2-NT-Ab assay correlates with functional virus neutralization within a traditional PRNT with a corresponding titre of ≥1:20 [13], a second ROC analysis was performed to define an additional cut-off for the SARS-CoV-2-S-Ab assay (≥196 U/mL). When using this PRNT-adapted cut-off, sensitivity and specificity were 89.9% (95% CI 82.3–95) and 89.5% (95% CI 78.5–96%) (Supplementary data, Figure S1B), respectively. With this cut-off, the seroconversion rates were further reduced to 1.2%, 60.6% and 35.4% after the first vaccine dose and at 4 weeks and 10–12 weeks after having received two doses, respectively.
Booster vaccination with one dose of mRNA-1273 increased the absolute values of SARS-CoV-2-S-Abs to 23 119.8 U/mL. Seroconversion was achieved in all individuals (Table 2). The significant increase in SARS-CoV-2-S-Ab concentrations dramatically increased the number of patients with adequate humoral immunity in virus neutralization assays. A total of 97.3% of all patients developed virus-neutralizing levels of SARS-CoV-2-S-Abs (positive surrogate virus neutralization test using the manufacturer cut-off and positive surrogate virus neutralization test correlating with a PRNT titre of ≥1:20).
DISCUSSION
Two doses of BNT162b2 followed by one dose of 100 µg mRNA-1273 within 6 months in patients receiving maintenance dialysis resulted in the development of virus-neutralizing quantities of SARS-CoV-2-S-Abs (when using wild-type virus as a target antigen) in almost all of them. While two doses of mRNA vaccine only achieved adequate humoral immunity in a minority of patients, the third dose of mRNA vaccine rendered almost all individuals immune as determined by a surrogate virus neutralization test. While antibodies are the most important mechanism for preventing infection, once the virus is in the cells, it is out of reach. In this case, the T-cell response is crucial, as it can recognize and kill infected cells. In short, T-cells are a kind of ‘key’ in stopping and controlling infection, while antibodies are the ‘key’ to preventing infection. Focussing on the humoral immune response in our study, we were able to demonstrate a good seroconversion rate in dialysis patients of almost 90% after vaccination with BNT162b2 at first glance. However, after adjusting anti-SARS-CoV-2-S-Ab levels to concentrations that achieve sufficient virus neutralization, the proportion of patients who had an adequate humoral immune response against SARS-CoV-2 was substantially reduced. Depending on the time after having received two doses of BNT162b2 and the level of inhibition within the surrogate virus neutralization test, 30% to almost 65% of dialysis patients had insufficient humoral reactivity. A third dose of an mRNA vaccine (mRNA-1273) reversed this course with adequate levels of antibodies in at least 97.3% of patients.
Several studies on seroconversion after SARS-CoV-2 immunization with two doses of an mRNA vaccine in dialysis patients have demonstrated excellent rates for the detection of any immunoreactivity [7–12]. In those trials, also including a control group, substantially reduced levels of antibodies were evident in the dialysis cohort. This difference points to a possible reduced vaccination response in patients on RRT. Although a correlate of protection for antibody concentration is not defined for SARS-CoV-2, vaccine-induced neutralizing antibody activity seems to correlate with vaccine efficacy [15–17]. Immunization trials, as well as clinical observations, clearly point to the correlation of antibody titres against SARS-CoV-2 and immunization efficiency or protection against breakthrough infections. Therefore we performed two ROC analyses, one with regard to the manufacturer's cut-off for positivity and one for high specificity to find a cut-off for an antibody concentration that correlates most likely with the presence of neutralizing antibodies against SARS-CoV-2 in the PRNT [13]. This first adjustment reduced the rate of seroconversion by 17% 4 weeks after and by 38.6% 10 weeks after two vaccine doses. Interestingly, the cut-off of 103 U/mL for the anti-SARS-CoV-2-S assay calculated by the ROC in our study is in the range of the upper margin of the estimated level required to confer a 50% protective neutralization level [16]. Another study by Resman Rus et al. [18] determined the correlation of the anti-SARS-CoV-2-S assay with a virus neutralization test in convalescent plasma and defined a level of 129.2 U/mL as predictive of adequate virus neutralization. Thus this first cut-off from our study appears to be in a meaningful biological range. Interestingly, more than half of the patients with adequate levels of antibodies (>103 U/mL) and a positive result in the surrogate neutralization test demonstrated >90% inhibition, which corresponds to a titre ≥1:160 in the classical PRNT [13]. It should be noted that standardized anti-spike immunoglobulin G (IgG) test results (by using the World Health Organization international standard for SARS-CoV-2 immunoglobulin) are comparable, but not interchangeable [19].
Two other trials also tested for virus neutralization with surrogate virus neutralization assays [7, 8]. Speer et al. [8] found that 82% of their cohort had virus-neutralizing antibodies 3 weeks after receiving two doses of BNT162b2. Stumpf et al. [7] presented an even higher rate of 94% of dialysis patients who were protected 8 weeks after two doses of mRNA-1273. These discrepancies may be attributed to the different vaccines and the different surrogate virus neutralization tests used in the latter trial. Our second cut-off correlating with a minimum low positive titre in a traditional PRNT almost doubled anti-SARS-CoV-2-S-Ab levels, resulting in a further reduction of dialysis patients with adequate humoral immunoreactivity after two doses. Interestingly, Anand et al. [20] found an 11.6 times increased risk for COVID-19 in a large cohort of dialysis patients with pre-breakthrough levels of RBD-IgG <212 U/mL during a time when the Delta variant of concern (VOC) was already dominant in this country. Espi et al. [21] found a significantly higher cut-off for effective virus neutralization through linear regression between PRNT and anti-SARS-CoV-2-S-Abs in haemodialysis patients. Although conversion is difficult, taking into account the correlation between the different assays used, a concentration of almost 2500 U/mL for anti-SARS-CoV-2-S (Roche) could be calculated out of their study [22]. This large difference is probably due to the statistical method and the cut-off selection based on the criterion that all sera have virus neutralizing capacity within the PRNT. It is currently not known which titre in the PRNT correlates with protection from COVID-19. For other viral infections, PRNT levels for protection vary. For example, the level of protection against the measles virus is found at 1:120 [23].
In summary, published data and our cut-offs show an association of SARS-CoV-2-S-Abs in a range of 103–196 U/mL with at least low titres within virus neutralization tests and risk reduction for COVID-19 in dialysis patients. From a hypothetical standpoint in regard to the wild-type SARS-CoV-2, these limits may be irrelevant due to the extreme increase of anti-SARS-CoV-2-S antibodies after a third vaccination in the majority of our patients. How this translates into protection from COVID-19 considering actual and future VOCs has to be addressed in further studies.
In September 2021, the STIKO recommended a third dose of an mRNA vaccine against SARS-CoV-2 in high-risk cohorts. With this recommendation, we offered a third dose of mRNA vaccine to our patients. The mRNA-1273 vaccine was chosen because of its higher seroconversion rate and SARS-CoV-2-S-Ab levels in patients with chronic kidney disease and patients after kidney transplantation [7, 24]. Our approach resulted in an almost 90-fold increase in SARS-CoV-2-S-Ab levels and to >97% of the cohorts with sufficient neutralizing antibodies. Several French groups published the immune response to three doses of BNT162b2 in dialysis patients [21, 25–27]. They were also able to show a dramatic increase in SARS-CoV-2-S-Ab. With the exception of Espi et al. [21] neither of the studies analysed virus neutralization. Bensouna et al. [26] describe an increase in immunization success with an increase in the time to the third vaccination. This timely component as well as the higher mRNA dose with mRNA-1273 may be causative for the significantly higher humoral immune response in our cohort. Further investigations are needed to determine what type of mRNA vaccine and dosage immunocompromised individuals should receive for their (booster) COVID-19 vaccination.
Limitations
Virus neutralization was tested by a surrogate ELISA as a substitute for a PRNT. The SARS-CoV-2-NT-Ab assay used in our study was examined thoroughly by us and other groups [13, 14, 28–30]. Compared with the PRNT, the surrogate ELISA demonstrated good correlation and diagnostic performance in the detection of neutralizing antibodies. New variants of interest (VOIs) and VOCs demonstrate significant mutations within the RBD of the spike protein. Therefore it is possible that surrogate virus neutralization tests that use the wild-type protein can show inappropriate results. Initial data comparing virus neutralization tests and SARS-CoV-2-S-Ab levels for wild-type versus Delta and Omicron VOCs in vaccinated persons indicate reduced effectiveness of immunization with current mRNA vaccines. In this study, 2.2 and 14.4 times the number of SARS-CoV-2-S-Abs were necessary for adequate neutralization of Delta and Omicron, resepctively, in the pseudovirus neutralization test [31]. The same observation can be made for these VOCs in the classic virus neutralization test [32]. Therefore it must be clarified in future studies whether the cut-off found here for seroconversion according to ROC analysis also applies to other quantitative tests for determining the anti-spike antibodies as well as other VOIs and VOCs. Still, we only analysed the humoral-mediated immune response, but cellular immune response also seems to play an important role in SARS-CoV-2 immunity, which is the subject of current research. With the decreasing humoral immunity that we saw in our cohort, T-cells could mediate a more stable and sustained immune response against SARS-CoV-2 and protect patients from COVID-19 [33]. In addition, less is known about the role of memory B cells in COVID-19-vaccinated individuals. Studies showed that SARS-CoV-2-infected individuals mount a broad and durable immunity, including memory compartments continuing to evolve more than 12 months after infection, with an enhancement of serologic responses and B-cell memory with individuals who received an mRNA vaccine [34, 35]. Perhaps this memory compartment is responsible for the high efficacy of a single dose of mRNA-1273 6 months after two doses of BNT162b2.
Conclusion
In this cohort of patients on RRT, two-dose vaccination with BNT162b2 initially resulted in substantial humoral immunization success, with antibody formation in almost all individuals. Adjusting the seroconversion rate with the SARS-CoV-2-NT-Ab test reveals, however, that only a minority has adequate humoral protection against SARS-CoV-2, which further diminished over the following 3 months. However, a single vaccination with mRNA-1273 6 months after two doses of BNT162b2 resulted in complete seroconversion which, due to the significant increase in SARS-CoV-2-S-Ab levels, suggests sufficient humoral immunity in >97.3% of our dialysis cohort.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the Ministry of Science and Health of Rhineland-Palatinate for providing BNT162b2 for vaccination of patients on RRT in early March 2021. We are also grateful for the extraordinary commitment and excellent work of the dialysis staff.
Contributor Information
Niko Kohmer, Institute for Medical Virology, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany.
Holger F Rabenau, Institute for Medical Virology, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany.
Sandra Ciesek, Institute for Medical Virology, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany; German Centre for Infection Research, External Partner Site, Frankfurt, Germany; Fraunhofer Institute for Molecular Biology and Applied Ecology, Branch Translational Medicine and Pharmacology, Frankfurt, Germany.
Bernhard K Krämer, Department of Medicine V, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany; Mannheim Institute for Innate Immunoscience, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany; Center for Preventive Medicine and Digital Health Baden-Württemberg, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
Uwe Göttmann, Department of Medicine V, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany; Nierenzentrum Worms, Germany.
Christine Keller, Praxis für Stoffwechsel- und Nierenerkrankungen, Zentrum für Dialyse und Apherese, Grünstadt, Germany.
Daniela Rose, Department of Medicine V, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany; Praxis für Stoffwechsel- und Nierenerkrankungen, Zentrum für Dialyse und Apherese, Grünstadt, Germany.
Carsten Blume, Praxis für Stoffwechsel- und Nierenerkrankungen, Zentrum für Dialyse und Apherese, Grünstadt, Germany.
Michael Thomas, Praxis für Stoffwechsel- und Nierenerkrankungen, Zentrum für Dialyse und Apherese, Grünstadt, Germany.
Alexander Lammert, Department of Medicine V, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany; Praxis für Stoffwechsel- und Nierenerkrankungen, Zentrum für Dialyse und Apherese, Grünstadt, Germany.
Anne Lammert, Department of Otorhinolaryngology, Head and Neck Surgery, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
FUNDING
Roche Diagnostics, Mannheim, Germany provided SARS-CoV-2 antibody ECLIAs for this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Naicker S, Yang CW, Hwang SJet al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int 2020; 97: 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jager KJ, Kramer A, Chesnaye NCet al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020; 98: 1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thaunat O, Legeai C, Anglicheau Det al. IMPact of the COVID-19 epidemic on the mORTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int 2020; 98: 1568–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eleftheriadis T, Antoniadi G, Liakopoulos Vet al. Disturbances of acquired immunity in hemodialysis patients. Semin Dial 2007; 20: 440–451 [DOI] [PubMed] [Google Scholar]
- 5. Haddiya I. Current knowledge of vaccinations in chronic kidney disease patients. Int J Nephrol Renovasc Dis 2020; 13: 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kausz A, Pahari D. The value of vaccination in chronic kidney disease. Semin Dial 2004; 17: 9–11 [DOI] [PubMed] [Google Scholar]
- 7. Stumpf J, Siepmann T, Lindner Tet al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur 2021; 9: 100178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Speer C, Göth D, Benning Let al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin J Am Soc Nephrol 2021; 16: 1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schrezenmeier E, Bergfeld L, Hillus Det al. Immunogenicity of COVID-19 tozinameran vaccination in patients on chronic dialysis. Front Immunol 2021; 12: 690698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Broseta JJ, Rodríguez-Espinosa D, Rodríguez Net al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis 2021; 78: 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danthu C, Hantz S, Dahlem Aet al. Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol 2021; 32: 2153–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grupper A, Sharon N, Finn Tet al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 2021; 16: 1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohmer N, Rühl C, Ciesek Set al. Utility of different surrogate enzyme-linked immunosorbent assays (sELISAs) for detection of SARS-CoV-2 neutralizing antibodies. J Clin Med 2021; 10: 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubio-Acero R, Castelletti N, Fingerle Vet al. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized oligo-/asymptomatic patients. Infect Dis Ther 2021; 10: 1505–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergwerk M, Gonen T, Lustig Yet al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021; 385: 1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khoury DS, Cromer D, Reynaldi Aet al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27: 1205–1211 [DOI] [PubMed] [Google Scholar]
- 17. Earle KA, Ambrosino DM, Fiore-Gartland Aet al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39: 4423–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resman Rus K, Korva M, Knap Net al. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol 2021; 139: 104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perkmann T, Perkmann-Nagele N, Koller Tet al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr 2021; 9: e0024721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anand S, Montez-Rath ME, Han Jet al. SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann Intern Med 2021; 174: 1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Espi M, Charmetant X, Barba Tet al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int 2022; 101: 390–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giavarina D, Carta M. Improvements and limits of anti- SARS-CoV-2 antibodies assays by WHO (NIBSC 20/136) standardization. Diagnosis (Berl) 2021; doi: 10.1515/dx-2021-0126 [DOI] [PubMed]
- 23. Siennicka J, Częścik A, Trzcińska A. The significance for epidemiological studies anti-measles antibody detection examined by enzyme immunoassay (EIA) and plaque reduction neutralization test (PRNT). Przegl Epidemiol 2014; 68: 417–420 [PubMed] [Google Scholar]
- 24. Hall VG, Ferreira VH, Ku Tet al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385: 1244–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frantzen L, Thibeaut S, Moussi-Frances Jet al. COVID-19 vaccination in haemodialysis patients: good things come in threes…. Nephrol Dial Transplant 2021; 36: 1947–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bensouna I, Caudwell V, Kubab Set al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis 2022; 79: 185–192.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ducloux D, Colladant M, Chabannes Met al. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int 2021; 100: 702–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Müller K, Girl P, von Buttlar Het al. Comparison of two commercial surrogate ELISAs to detect a neutralising antibody response to SARS-CoV-2. J Virol Methods 2021; 292: 114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor SC, Hurst B, Charlton CLet al. A new SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. J Clin Microbiol 2021; 59: e02438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valcourt EJ, Manguiat K, Robinson Aet al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn Microbiol Infect Dis 2021; 99: 115294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia-Beltran WF, St Denis KJ, Hoelzemer Aet al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022; 185: 457–466.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilhelm A, Widera M, Grikscheit Ket al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv 2022; doi: 10.1101/2021.12.07.21267432 [DOI]
- 33. Bonifacius A, Tischer-Zimmermann S, Dragon ACet al. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity 2021; 54: 340–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen KW, Linderman SL, Moodie Zet al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med 2021; 2: 100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Z, Muecksch F, Schaefer-Babajew Det al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021; 595: 426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.