Learning points for clinicians.
We recommend continued vigilance and heightened awareness of pulmonary mimics of coronavirus disease 2019 and potential rare sequelae of vaccination. Bronchoalveolar lavage may be useful for full evaluation.
Introduction
The vaccination programme has been the key in controlling the COVID pandemic and is both efficacious and safe.1 Rare neurological and haematological complications have been described with these vaccinations although causality has not been proven.2,3 The reporting of potential adverse effects remains a fundamental responsibility of the medical community.
We describe a case of a patient presenting with respiratory symptoms following coronavirus disease 2019 (COVID-19) vaccination.
Case
A 55-year-old female presented with 11-day history of dry cough, progressive dyspnoea and pleuritic chest pain. She had no known contact with COVID-19. She was a never smoker and had no travel history. She had no exposure to birds, dampness or asbestos. She denied symptoms of connective tissue disease. There was no relevant drug history. Seven weeks prior to the onset of symptoms she had received the first dose of the ChAdOx1 nCov-19 COVID-19 vaccine.
Her temperature was 37.5°C, pulse 100/min, oxygen saturations 96% on air at rest and 92% post-exertion. She had bilateral basal end inspiratory crepitations on auscultation. Chest X-ray (CXR) demonstrated bilateral lower zone opacities. Three days after symptom onset a COVID-19 polymerase chain reaction test was negative and numerous subsequent lateral flow tests all remained negative as were COVID antibodies. White cell count was 9.9, eosinophils 0.9 (109/L), C-reactive protein 124 mg/dl. HIV, Rheumatoid factor, Anti-nuclear antibody (ANA), Anti-nuclear cytoplasmic antibody (ANCA), mycoplasma and strongyloides serology were negative. D-dimer was normal. Urinary antigens for Legionella and pneumococcus and throat swab for respiratory viruses were negative. A course of Doxycycline followed by Levofloxacin did not improve her symptoms.
High-resolution computed tomography (HRCT) chest showed dense bilateral lower lobe dense consolidation (Figure 1).
Figure 1.
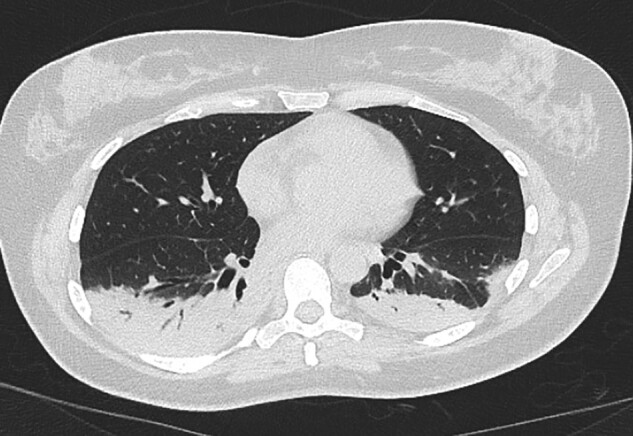
HRCT (axial image) showing dense bilateral consolidation in both lower lobes.
Her forced vital capacity (FVC) was 1.01 l (38% predicted).
Bronchoalveolar lavage (BAL) showed eosinophils of 50%, lymphocytes 14%, neutrophils 10%, macrophages 24% and epithelial cells 1%. No organisms were found.
Based on the overwhelming BAL eosinophilia of 50%, we made a diagnosis of eosinophilic pneumonia, felt likely to have been caused by COVID-19 vaccination. The patient received three doses of intravenous Methylprednisolone 500 mg followed by oral Prednisolone starting at 30 mg per day, tapered and discontinued over 12 weeks. After 3 months her symptoms had resolved, CXR had normalized and FVC improved to 2.68 l (100% predicted).
Discussion
Eosinophilic pneumonia is a rare condition of eosinophil accumulation in the alveolar airspace and interstitium. Causes include vasculitis, parasitic infections, drug reactions and cigarette smoking. BAL is usually required for diagnosis, with eosinophils >25% on differential cell count considered diagnostic.4 CT findings are non-specific but may include randomly distributed ground-glass opacities.
Pulmonary eosinophilia was described during development of respiratory syncytial virus vaccine which was terminated when subjects developed eosinophilic lung infiltration after viral challenge which in some cases was fatal.5 A similar phenomenon was demonstrated in animal models of vaccine development against SARS-CoV-1.6
The temporal relationship between vaccination and the development of eosinophilic pneumonia in the absence of all other known causes led us to associate the two. To the best of our knowledge, this is the first case of eosinophilic pneumonia in association with COVID-19 vaccination.
Conflict of interest. None declared.
Contributor Information
J May, From the Department of Respiratory Medicine, St George’s University Healthcare NHS Trust, London SW17 0QT, UK.
A Draper, From the Department of Respiratory Medicine, St George’s University Healthcare NHS Trust, London SW17 0QT, UK.
R Aul, From the Department of Respiratory Medicine, St George’s University Healthcare NHS Trust, London SW17 0QT, UK.
References
- 1. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci 2021; 428:117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goss A, Samudralwar R, Das R, Nath A. ANA investigates: neurological complications of Covid-19 vaccines. Ann Neurol 2021; 89:856–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. ; American Thoracic Society Committee on BAL in Interstitial Lung Disease. An Official American Thoracic Society Clinical Practice Guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012; 185:1004–14. [DOI] [PubMed] [Google Scholar]
- 5. Kapikian A, Mitchell R, Chanock R, Shvedoff R, Stewart C. An epidemiological study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinate with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89:405–21. [DOI] [PubMed] [Google Scholar]
- 6. Tseng C, Sbrana E, Iwata-Yoshikawa N, Newman P, Garron T, Atmar R, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One 2012; 7:e35421. [DOI] [PMC free article] [PubMed] [Google Scholar]


