Abstract
Introduction
We used a longitudinal cohort of US adults who were current or former smokers to explore how three participant-reported factors—general stress, coronavirus disease of 2019 (COVID-19) distress, and perceived risk of complications from COVID-19 related to smoking—were associated with changes in smoking status.
Methods
Smoking status was assessed at three time points. Timepoint 1 status was assessed at a prior study completion (2018–2020). Timepoint 2 (start of the pandemic), and Timepoint 3 (early phase of the pandemic) statuses were assessed using an additional survey in 2020. After classifying participants into eight groups per these time points, we compared the means of participant-reported factors and used a linear regression model to adjust for covariates.
Results
Participants (n = 392) were mostly female (73.9%) and non-Hispanic White (70.1%). Between Timepoints 2 and 3, abstinence rates decreased by 11%, and 40% of participants reported a smoking status change. Among those reporting a change and the highest general stress levels, newly abstinent participants had higher perceived risk of complications from COVID-19 related to smoking than those who relapsed during pandemic (mean (SD): 14.2 (3.3) vs. 12.6 (3.8)). Compared to participants who sustained smoking, those who sustained abstinence, on average, scored 1.94 less on the general stress scale (βeta Coefficient (β): −1.94, p-value < .01) and 1.37 more on the perceived risk of complications from COVID-19 related to smoking scale (β: 1.37, p-value .02).
Conclusions
Decreased abstinence rates are concerning. Patterns of reported factors were as expected for individuals who sustained their smoking behavior but not for those who changed.
Implications
We observed an increase in smoking rates during the COVID-19 pandemic. In exploring how combinations of general stress levels, COVID-19 distress levels, and perceived risk of complications from COVID-19 related to smoking were associated with changes in smoking, we observed expected patterns of these factors among individuals who sustained abstinence or smoking. Among individuals who changed smoking status and reported high stress levels, those who reported a higher perceived risk of complications from COVID-19 related to smoking abstained from smoking. In contrast, those who reported a lower perceived risk of complications from COVID-19 related to smoking, started smoking. An intersectional perspective may be needed to understand smokers’ pandemic-related behavior changes.
Introduction
Changes in smoking behavior have been observed since the onset of the coronavirus disease of 2019 (COVID-19) pandemic.1 Amongst a US cohort of smokers, about 28% of respondents decreased the number of cigarettes they smoked while 30% increased the amount smoked.2 Similar variability in smoking was observed in studies conducted in Spain3, the UK4, and Poland.5 Characterizing changes in smoking behavior is essential to understanding the full scope of COVID-19 on smoking behavior.6 Few reports have used longitudinal data to evaluate smoking status before and after the pandemic onset or explored factors associated with continued smoking or abstinence.
Multiple and conflicting pandemic-related factors may have influenced smoking behavior. Studies have reported increased anxiety, depression, and stress-related concerns about COVID-19.7–11. Typically, these factors are associated with increased smoking.12 Smoking behaviors could also have been influenced by differing perceptions on the risk of complications from COVID-19. These perceptions may have been shaped by inconsistent scientific debates regarding the association between COVID-19 and smoking. Early evidence indicated that COVID-19 patients with any history of smoking were more likely to experience severe or critical COVID‐19 health outcomes, in‐hospital mortality, and the need for mechanical ventilation when compared to individuals without any history of smoking.13,14 Such reports may have increased motivation to quit or reduce smoking among some smokers.15 Other reports indicated no association or even suggested a protective effect of nicotine against COVID-19 infection,16-18 which may have led to increased consumption or relapse among some individuals.
The COVID-19 era could represent a “transition” phase for smoking behavior.19 Understanding how conflicting factors associated with the pandemic influenced changes in smoking behaviors is important to design effective smoking cessation interventions. The current report provides a longitudinal description of changes in smoking status. It explores how different combinations of three participant-reported factors—general stress, COVID-19 distress, and perceived risk of complications from COVID-19 related to smoking—influenced those changes.
Methods
Study Design
The study design is a longitudinal cohort of adults from across the United States with exposure to tobacco (ie, active smoking or abstinent) ascertained in 2019 and then with follow-up during the COVID-19 pandemic in June 2020.
Study Setting and Participants
The longitudinal cohort of adults was recruited from participants of a prior smoking cessation randomized control trial (n = 1487) conducted between 2017 and 2020. Briefly, the trial evaluated the effectiveness of an enhanced computer tailoring messaging approach that used machine learning compared to a standard messaging system.20 We recruited the trial participants using advertisements (on Google and Facebook) and Research Match.21 Both the intervention and comparison groups in the trial received messages through email for 6 months. The primary outcome was 7-day point prevalence cessation at 6 months. Details of the recruitment approach and protocol for the smoking cessation behavioral trial have been published.20
A subset of the participants in the above smoking cessation randomized controlled trial had accepted to be contacted for future research inquiries (n = 701). In June 2020, we distributed an additional COVID-19 related survey to assess the impact of the pandemic on smoking to this subset. Of those, 392 responded to the survey. We compared the distribution of participant characteristics for individuals who responded and those who did not respond to the additional survey. The proportion of participants who identified as Asian, American Indian, or Alaska Native, and Native was higher in those who responded to the additional survey (n = 30, 8.1%) compared to those who did not respond (n = 9%, 3.1) (see Supplemental Table 1). Participants who responded were compensated with a $10 Amazon online gift card. All surveys were administered via the Research Electronic Data Capture web application system.22 This study was approved by the Institutional Review Board at the University of Massachusetts Chan Medical School.
Smoking Status Assessed at Three Time Points
Smoking status was assessed at three time points. At the end of the prior smoking study (Timepoint 1), smoking status was ascertained by asking the question, “Have you smoked cigarettes (even one puff) in the last 7 days?” Participants who responded with “yes” were classified as smoking and those who responded with a “no” were classified as abstinent.
Smoking status at the next two time points (Timepoint 2 and 3) was assessed using the additional COVID-19 related survey distributed in June 2020. To assess smoking at the start of the pandemic (Timepoint 2), we asked, “Has your cigarette smoking status changed since the start of the COVID-19 pandemic?” Response options included: (1) “no change; I was not smoking before, and I have not started smoking”; (2) “no change; I was smoking before and have not stopped”; (3) “yes, I started smoking”; 4) “yes, I stopped smoking”. We classified participants who indicated response options #2 and #4 as smoking and those who indicated response options #1 and #3 were classified as abstinent at Timepoint 2. To assess smoking status during the early phase of the pandemic (Timepoint 3), we used the question (in the additional survey), “Do you now smoke cigarettes every day, some days, or not at all?” Those who responded with “every day” or “some days” were classified as smoking, and those who responded “not at all” were classified as abstinent.
Assessment of the Three Participant-Reported Factors (General Stress Levels, COVID-19 Related Distress Levels, and Perceived Risk of Complications from COVID-19 Related to Smoking) in the Additional COVID-19 Related Survey
General stress was measured using the 4-item Perceived Stress Scale (PSS-4), 23 a self-reported scale that generates a global stress score, based on general questions rather than specific experiences.23 The PSS-4 measures the degree to which situations in one’s life over the past month are appraised as stressful.23 Sample questions include “In the last month, how often have you felt that you were unable to control the important things in your life?” and “In the last month, how often have you felt confident about your ability to handle your personal problems?” (See Supplemental Table 2 for a complete list of questions). Responses (0 = never, 1 = almost never, 2 = sometimes, 3 = fairly often, and 4 = very often) to each statement were summed up to form a continuous variable, with higher scores indicating greater stress levels (scores ranged from 0 to16).
COVID-19 related distress was measured in the additional survey using the Impact of Event Scale-6 (IES-6), a self-reported scale assessing subjective distress related to a specific life event (eg, COVID-19).24 Participants responded to statements such as “In the past 7 days, I thought about COVID-19 when I did not mean to” and “In the past 7 days, I felt watchful or on-guard” (see Supplemental Table 2 for a complete list of questions). The response format measures severity (0 = not at all, 1 = a little bit, 2 = moderately, 3 = quite a bit, 4 = extremely). Higher scores on the IES-6 scale indicate higher levels of COVID-19 related distress (scores on this scale ranged from 0 to 24).
Guided by the emerging research on the associations between COVID-19 health complications and smoking,13-18 we developed five statements to capture the level of perceived risk of complications from COVID-19 related to smoking on a Likert scale ranging from 1 = strongly agree to 5 = strongly disagree). These statements included: (1) “Compared with people who have never smoked, smokers are more likely to get COVID-19”; (2) “Compared with people who have never smoked, smokers are more likely to be hospitalized if they get COVID-19”; (3) “Compared with people who have never smoked, smokers more likely to die if they get COVID-19”; (4) “I think quitting smoking now will lead to better COVID-19 health outcomes (less severe, fewer complications, shorter hospital time, etc.)”; and (5) “I think continuing to smoke will lead to better COVID-19 health outcomes (such as fewer complications or shorter hospital stay).” Responses to questions 1 to 4 were reverse coded such that high values indicated a higher perceived risk of COVID-19 related to smoking. After reverse coding the variables, perceived risk of complications from COVID-19 related to smoking was assessed as a continuous variable by summing up responses for all the statements (range of scores: 5 to 25).
Participant Characteristics Assessed in the Additional COVID-19 Related Survey
Data were collected on sociodemographic factors, geographical zip codes, number of cigarettes smoked per day, and past 30-day electronic cigarette use. Changes in the amount of smoking were assessed using the question, “Since the start of COVID-19, compared to the period before, do you feel that you smoke: more than usual, about the same, or less than usual?” Overall health status was assessed using the question, “How would you describe your own health?” Participants responded on a five-point scale (excellent, very good, good, fair, poor). Self-assessed overall health status has been validated as a useful indicator of health for a variety of populations and allows for broad comparisons across different conditions and populations.25 We collected information on the presence of comorbidities that were known to be associated with worse outcomes in COVID-19 infections based on the list released by the Center for Disease Control and Prevention (C.D.C.),26 including respiratory issues, diabetes, hypertension, any type of cancer, or kidney failure. The number of days since last contact (date of completion of the 6-month survey) was operationalized as the number of days between the calendar date on which the participant completed the 6-month survey and the date they completed the survey in June 2020.
Analysis
All data analysis was performed using STATA 15. We calculated the percent and mean distributions of participants’ characteristics and estimated the prevalence of smoking abstinence at the three time points. We categorized study participants into eight groups based on transitions from active smoking to abstinence and vice versa at these time points. This resulted into eight smoking states ranging from sustained abstinent (participants who were abstinent at all three time points) to sustained smokers (participants who smoked at all three time points) (Supplemental Table 3).
The following steps were taken to explore how different combinations of the three participant-reported factors (general stress and COVID-19 distress levels and perceived risk of complications from COVID-19 related to smoking) were related to changes in smoking. This approach was used because of the exploratory nature of the analysis, the considerable variation between study groups, and small sample sizes in some study groups.
We calculated overall and groups means (and SD) of the three participant-reported factors (presented in Table 1).
We compared mean differences between groups using two-sample t-tests (for groups with n > 30) and a Wilcoxon rank-sum test (for groups with n < 30) for all three factors.
We used linear regression to confirm group mean differences of the three participant-reported factors and changes in smoking behaviors. Given the small sample sizes in some study groups, we further combined participants into four study groups: smokers who transitioned to abstinence (n = 35), those who transitioned to smoking (n = 70), those who sustained smoking (n = 210), and those who sustained abstinence (n = 74). We used a two-step criterion to assess confounding. First, we used bivariate analysis to assess differences between study groups for each covariate. Bivariate relationships were examined using the chi-square test for categorical variables and one-way analysis of variance for continuous variables. Second, significantly different variables between study groups (p-value < .05) were included in the final model as covariates. We assessed for differences between groups for the following variables: age, gender, race, education level, marital status, overall health status, presence of a comorbidity, number of adults and children in the household, the number of cigarettes smoked per day, current electronic cigarette use, number of days since last contact, and geographical region (Northeast, Midwest, South, and West) identified using the zip codes. Based on the criteria, we included the number of days since last contact, age, gender, number of children in household, and change in employment status during COVID-19 as covariates in the final model.
We also assessed using analysis of variance whether the means of the three participant-reported factors were significantly different between subgroups of sustained smokers (n = 182) based on their changes in the amount of smoking (smoking less than usual [n = 34], more than usual [n = 93], or about the same amount [n = 54]).
Table 1.
Mean Scores and SD of General Stress Levels, COVID-19 Related Distress Levels, and Perceived Risk of Complications From COVID-19 Related to Smoking by Smoking Status Transitions
| Study groupsa | n | General stress levels Mean (SD) |
COVID-19 related distress levels Mean (SD) |
Perceived risk of complications from COVID-19 related to smoking Mean (SD) |
|
|---|---|---|---|---|---|
| Overall | 392 | 7.2 (3.4) | 9.5 (6.1) | 13.2 (4.0) | |
| 1 | Sustained Abstinence (A1,A2,A3) | 53 | 5.8 (3.3) | 8.2 (6.0) | 14.0 (3.7) |
| 2 | Abstinent pre-COVID (S1,A2,A3) | 21 | 6.2 (3.6) | 10.1 (6.7) | 14.7 (3.6) |
| 3 | Newly abstinent (A1,S2,A3) | 15 | 8.4 (3.5) | 10.0 (7.0) | 14.2 (3.3) |
| 4 | Newly abstinent (S1,S2,A3) | 20 | 7.6 (3.6) | 10.3 (6.6) | 12.6 (5.9) |
| 5 | Relapsed during pandemic (A1,A2,S3) | 33 | 7.1 (3.0) | 10.9 (7.3) | 13.5 (3.9) |
| 6 | Relapsed during pandemic (S1,A2,S3) | 37 | 8.1 (2.6) | 12.5 (6.2) | 12.6 (3.8) |
| 7 | Smoking pre-COVID (A1,S2,S3) | 28 | 7.2 (2.9) | 9.5 (5.7) | 12.1 (4.9) |
| 8 | Sustained smoking (S1,S2,S3) | 182 | 7.5 (3.6) | 9.0 (5.6) | 12.9 (3.8) |
General stress levels measured using the 4-item Perceived Stress Scale (PSS-4), a self-reported scale that generates a global stress score that is based on general questions rather than focusing on specific experiences. COVID-19 related distress was measured using the Impact of Event Scale -6 (IES-6), a self-reported scale assessing subjective distress related to a specific life event. Specific life event used in this study was COVID-19.
aEach letter in parentheses represents the smoking status at each of the three time points: A represented abstinence from smoking, S represents smoking at each of the time points. Timepoints are represented as numbers: 1 (Timepoint 1), 2 (Timepoint 2), and 3 (Timepoint 3).
Results
Participant Characteristics
Participants were mostly female (73.9%), non-Hispanic White (70.1%), and had college or higher-level education (80.0%). Most (94%) had an existing medical comorbidity, and 42% reported overall good health. A mean number of cigarettes smoked per day was 19 (SD: 2.4). Participants in the study were from 38 US states (Table 2).
Table 2.
Sociodemographic Characteristics of Study Participants (N = 392 adults)
| Characteristics | n/N | % | |
|---|---|---|---|
| Gender | Female | 290/392 | 73.9 |
| Age | 19–34 | 137/392 | 35.0 |
| 35–54 | 155/392 | 39.5 | |
| 55 and older | 100/392 | 25.5 | |
| Race/ethnicitya | Non-Hispanic White | 260/371 | 70.1 |
| Non-Hispanic Black | 60/371 | 16.2 | |
| Hispanic | 21/371 | 5.7 | |
| Other | 30/371 | 8.1 | |
| Educationa | High school/less than high school | 77/385 | 20.0 |
| Some college | 198/385 | 51.4 | |
| Bachelors/advanced degree | 110/385 | 28.6 | |
| Marital status | Married/unmarried couple | 181/392 | 46.2 |
| Divorced/separated | 100/392 | 25.5 | |
| Widowed | 17/392 | 4.3 | |
| Never married/single | 94/392 | 24.0 | |
| Overall health statusa | Excellent | 27/391 | 6.9 |
| Very good | 94/391 | 24.0 | |
| Good | 166/391 | 42.5 | |
| Fair | 90/391 | 23.0 | |
| Poor | 14/391 | 3.6 | |
| Presence of comorbidityb | 368/392 | 93.9 | |
| Adults in householda | Mean (SD) | 3.3 (1.1) | |
| Children in householda | Mean (SD) | 2.0 (1.5) | |
| Changes in employment statusa | No changes: Employed before and after pandemic onset | 87/353 | 24.7 |
| No changes: Unemployed before and after pandemic onset | 101/353 | 28.6 | |
| Working from home | 87/353 | 24.7 | |
| Unemployment was pandemic-related | 78/353 | 22.1 | |
| Cigarettes per day | Mean (SD) | 19 (2.4) | |
| Currently vape or use electronic cigarettesa | Yes | 79/390 | 20.3 |
| No | 311/390 | 79.7 | |
| US Census regionsa | Northeast | 39/283 | 13.8 |
| Midwest | 111/283 | 39.2 | |
| South | 85/283 | 30.0 | |
| West | 48/283 | 17.0 |
aMissing data: race/ethnicity (n = 21), education (n = 7), overall health status (n = 1), adults in household (n = 2), children in household (n = 6), current electronic cigarette use (n = 3), US census regions (n = 109), and changes in employment status (n = 39).
bComorbidities assessed included the following conditions or clinical characteristics: respiratory issues, diabetes, hypertension, any type of cancer, or kidney failure.
Changes in Smoking Status Comparing the Three Timepoints
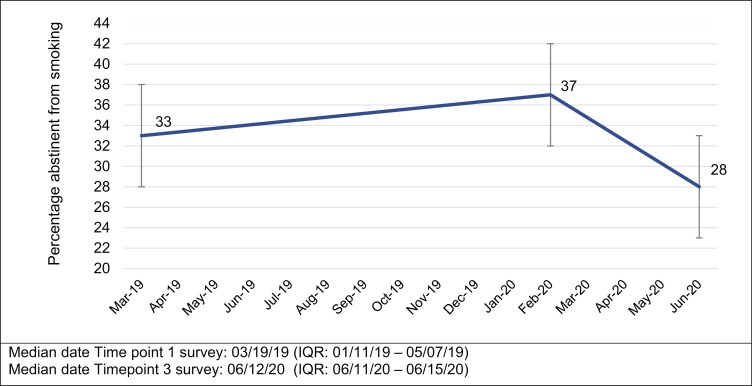
Abstinence rates increased from 33% (n = 130) at Timepoint 1 to 36% (Timepoint 2), but then decreased to 28% at Timepoint 3. (Figure 1). Close to half of participants (46%, n = 182) smoked at all time points, 13% (n = 53) had sustained abstinence throughout, and 40% (n = 157) transitioned between smoking states. About 25% reported resuming smoking, and 9% reported stopping at Timepoint 3 (Table 1).
Figure 1.
Percentage of individuals reporting abstinence from smoking before and after the COVID-19 outbreak (July 2019–June 2020).
Comparison of the Three Participant-Reported Factors (General Stress Levels, COVID-19 Related Distress Levels, and Perceived Risk of Complications From COVID-19 Related to Smoking) Among the Eight Groups
Overall, the mean level of general stress was 7.2 (SD: 3.4), COVID-19 related distress was 9.5 (SD: 6.1), and perceived risk of complications from COVID-19 related to smoking was 13.2 (SD: 4.0). Below, we explored the different combinations of these participant-reported in individuals who sustained smoking during the COVID-19 pandemic and those who transitioned between smoking states.
Combinations of the Three Participant-Reported Factors Among Participants Who Sustained Their Smoking Status (Sustained Abstainers Versus Sustained Smokers)
Participants who sustained abstinence at all three time points (group 1 in Table 1) and those who were smoking at Timepoint 1 and abstinent at Timepoints 2 and 3 (group 2 in Table 1) had a similar combination of low general stress levels (means (SD): 5.8 (3.3)group 1 vs. 6.2 (3.6)group 2, p-value > .05) and high perceived risk of complications from COVID-19 related to smoking (means (SD): 14.0 (3.7)group 1 vs., 14.7 (3.6)group 2, p-value > .05). Participants who sustained smoking (group 8 in Table 1) and those who were abstinent at Timepoint 1 but smoked at Timepoints 2 and 3 (group 7 in Table 1) also had a similar combination of high general stress (means (SD): 7.2 (2.9)group 8 vs. 7.5(3.6) group 7, p-value > .05) and low perceived risk of complications from COVID-19 related to smoking (means (SD): 12.9 (3.8) group 8 vs. 12.1(4.9)group 7, p-value > .05) (Table 1).
Given the similarity in the combinations of these factors, we combined participants in group 1 with those in group 2 (sustained abstainers_combined, n = 74) and combined participants in group 7 with those in group 8 (sustained smokers_combined, n = 210). Compared to participants in the sustained abstainers_combined group, those in the newly formed sustained smoking group had higher general stress levels (means (SD): 7.4 (3.5) sustained smokers_combined vs. 5.9 (3.3)sustained abstainers_combined, p-value < .05) and lower perceived risk of complications from COVID-19 related to smoking (means (SD): 12.8 (3.9)sustained smokers_combined vs. 14.2 (3.7)sustained abstainers_ combined, p-value < .05). COVID-19 distress levels were similar between the two groups (means (SD): 9.0 (5.6)sustained smokers_combined vs. 8.7(6.7)sustained abstainers_combined, p-value > .05).
Combinations of the Three Participant-Reported Factors Among Participants Who Changed Smoking States (Transitioned to Smoking Versus Transitioned to Abstinence)
We compared the two newly abstained (groups 3 and 4 in Table 1) and the two relapsed during the pandemic groups (groups 5 and 6 in Table 1). In the newly abstained groups, group 3 individuals (smoked at Timepoint 1, relapsed at Timepoint 2, and abstained at Timepoint 3) reported higher general mean stress levels (means (SD): 8.4(3.5)group 3 vs. 7.6(3.6)group 4,p-value > .05) and a higher perceived risk of complications from COVID-19 related to smoking (means (SD): 14.2 (3.3)group 3 vs. 12.6 (5.9)group 4,p-value > .05) than group 4 individuals (smoking at Timepoints 1 and 2 and abstinent at Timepoint 3). The COVID-19 distress levels were similar between these two groups. In the relapsed during pandemic groups comparison, group 5 individuals (abstinent at Timepoints 1 and 2 but had relapsed by Timepoint 3) reported lower general stress levels (means (SD): 7.1(3.0)group 5 vs. 8.1 (2.6) group 6,p-value > .05), lower COVID-19 distress levels (means (SD): 10.9 (7.3)group 5 vs. 12.5 (6.2)group 6,p-value > .05), and higher perceived risk of complications from COVID-19 (means (SD): 13.5 (3.9)group 5 vs. 12.6 (3.8)group 6,p-value > .05) than group 6 individuals (smoking at Timepoint 1, Abstinent at Timepoint 2, and Smoking at Timepoint 3). Among individuals in the two groups that reported the highest general stress levels (new abstinent group 3 and relapsed during pandemic group 6), group 3 had higher perceived risk of complications from COVID-19 related to smoking, as compared to the group 6 individuals (means (SD): 14.2 (3.3)group 3 vs. 12.6 (3.8)group 7, p-value > .05).
We combined participants in groups 5 and 6 to form a group of participants who relapsed during the pandemic (relapsed during pandemic_combined, n = 70) and combined participants in groups 3 and 4 to form a group of participants who were newly abstinent (newly abstinent_combined, n = 35). Compared to relapsed during pandemic_combined, those in the newly abstinent_combined had slightly lower COVID-19 distress levels (means (SD): 10.2 (6.6)newly abstinent_combined vs. 11.7 (6.7)relapsed during the pandemic_combined, p-value > .05). However, the mean difference was not statistically significant. General stress level (means (SD): 7.9 (3.5)newly abstinent_combined vs. 7.6 (2.8)relapsed during the pandemic_combined,p-value > .05) and perceived risk of complications from COVID-19 related to smoking (means (SD): 13.1 (4.9)newly abstinent_combined vs. 13.0 (3.8)relapsed during the pandemic_combined,p-value > .05) was also similar between these two groups.
Linear Regression Estimates of the Three Participant-Reported Factors
The newly formed study groups were as follows; smokers who transitioned to abstinence (newly abstinent_combined, n = 35), those who transitioned to smoking (relapsed during the pandemic_combined, n = 70), those who sustained smoking (sustained smokers_combined, n = 210), and those who sustained abstinence (sustained abstainers_ combined, n = 74). Using the sustained smokers_combined as a reference group, sustained abstainers_combined, on average, scored 1.94 points less on the general stress scale (β: −1.94, p-value: < .01) and 1.37 more points on the perceived risk of complications from COVID-19 related to smoking scale (β: 1.37, p-value: .02). We did not observe significant differences between these two groups regarding COVID-19 related distress levels (β: −1.10, p-value: .21) (Supplemental Table 4)
Changes in Amount of Smoking Among Participants Who Sustained Smoking (Study Group 8 as Shown in Table 1)
In addition, half of all sustained smokers reported smoking more at Timepoint 3 (50.2%). Compared to sustained smokers who reported no changes in amount smoked, those who smoked more had a higher perceived risk of complications from COVID-19 related to smoking (mean (SD): 13.3 (3.6)), higher general stress (mean (SD): 10.4 (5.4)), and higher levels of COVID-19 related distress (mean (SD): 8.3 (3.5)). Similarly, sustained smokers who smoked more had a higher perceived risk of complications from COVID-19 related to smoking (mean (SD): 13.4 (3.7)), higher general stress (mean (SD): 9.4 (6.1)), and higher levels of COVID-19 related distress (mean (SD): 7.6(3.0)) than sustained smokers who reported no changes in amount smoked. those. The differences between groups were significant for general stress and COVID-19 distress levels, but not for perceived risk of complications from COVID-19 related to smoking (Table 3).
Table 3.
Change in Amount of Smoking Among Sustained Smokers Since the Start of COVID-19 Pandemic (N = 182)
| Change in amount of smoking | |||
|---|---|---|---|
| Participant-reported factors | More than usual 93 (51.4%) |
About the samea 54 (29.8%) |
Less than usual 34 (18.9%) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Perceived risk of complications from COVID-19 related to smoking | 13.3 (3.6) | 12.0 (4.1) | 13.4 (3.7) |
| COVID-19 related distressb | 10.4 (5.4)* | 6.2 (4.7) | 9.4 (6.1)* |
| General Stress levelb | 8.3 (3.5)* | 5.9 (3.6) | 7.6 (3.0)* |
aReference group.
bHigher values indicate higher levels of stress, higher distress levels, and higher perceived risk. Missing (n = 1).
*p-value < .05.
Discussion
In a longitudinal study of US former and current smokers, smoking abstinence increased from 33% at the completion of the prior study (Timepoint 1) to 37% at the start of the pandemic (Timepoint 2) but decreased to 28% during early phase of the pandemic (Timepoint 3). In our exploration of whether different combinations of three participant-reported factors (general stress levels, COVID-19 related distress levels, and perceived risk of complications from COVID-19 related to smoking) were associated with changes in smoking behavior, we found expected patterns for individuals who sustained their smoking status (ie, sustained abstainers or sustained smokers) but not for those who transitioned between smoking status (ie, became newly abstinent or relapsed). We also found that the increase or decrease in the amount smoked during the pandemic was both associated with higher general stress levels and higher COVID-19 distress levels.
Since 2000, U.S. adult abstinence rates have increased and were at 86% in 2019.27 Given that the abstinence rate decreased even early on in the pandemic could indicate reversal of some of the successes of tobacco cessation efforts.28 We found that individuals who sustained abstinence had a combination of low general stress levels and high perceived risk of complications from COVID-19 related to smoking. Those who sustained smoking had high general stress levels and low perceived risk of complications from COVID-19 related to smoking. One other study has reported that higher stress levels deterred smoking cessation during the COVID-19 pandemic.29 Studies from past pandemics, such as the H1N1 pandemic, have also shown that a higher perceived personal risk is associated with positive lifestyle behaviors.30 Crafting effective public health narratives to provide consistent, clear messaging despite dealing with uncertainties of a novel disease is an area that needs further research.25
Combinations of the three participant-reported factors were not apparent among the four groups that transitioned between smoking states. In two of the four groups that transitioned (newly abstinent or relapsed during pandemic) and reported the highest general stress, those who reported higher perceived risk of complications from COVID-19 related to smoking transitioned to abstinence while those who reported a low perceived risk of complications from COVID-19 related to smoking transitioned to smoking. However, this pattern was not present in the two other groups that transitioned but reported low general stress levels. Further exploration of the smoker’s decision to quit smoking during the pandemic may be better understood from an intersectional perspective of multiple pandemic-related factors since smokers are likely experiencing a combination of risk and protective factors of smoking during the COVID-19 pandemic.
Consistent with earlier studies,2–4 we found that among individuals who sustained smoking, some smoked more during the pandemic while others smoked less. In the current study, both these contrasting behaviors were associated with higher levels of all three factors. Some individuals may have used smoking as a coping mechanism to deal with the threat of COVID-19,31 thus increasing amount smoked despite the high perceived risk. Others may have reduced their smoking as a consideration for others’ health - for example, when spending more time at home with loved ones, including children, due to social distancing/work-from-home measures.19 Future studies exploring why individuals changed the amount they smoked during the pandemic are needed.
Limitations
This study had limitations. Since we did not specify a calendar date when collecting data on smoking status at Timepoint 2, participants may have interpreted the “start of the pandemic” differently, for example, in January 2020 (when cases of COVID-19 were first reported in the United States) or March 2020 when COVID-19 was declared a pandemic by the World Health Organization. We did not account for these differing interpretations. We did not have information on current or past psychiatric diagnoses and use of alcohol or other illicit drugs, both of which are likely to have a confounding effect on the relationships explored in the current study. Given that we assessed changes in smoking status at three time points and assessed participant-reported factors at one time point (Timepoint 3), we were unable to conduct a temporal assessment of the association between perceived risk of complications from COVID related to smoking, general distress levels, and COVID-19 related distress and changes in smoking status or changes in amount smoked. Finally, the response rate of 55.9% likely had minimal impact on our findings. The distribution of participant characteristics was similar between individuals who responded to the additional survey and those who did not respond were similar except for race/ethnicity.
In conclusion, our findings indicate a cause for concern as the abstinence rates declined even in the early phase of the pandemic. Many of the current smokers also reported an increase in the amount smoked, potentially increasing their dependence on tobacco. We found that among individuals who sustained abstinence or smoking, the combination of stress and perceived risk of complications from COVID related to smoking were as expected. That is, individuals who sustained abstinence had a combination of low general stress levels and high perceived risk of complications from COVID-19 and those who sustained smoking had high general stress levels and low perceived risk of complications from COVID-19 related to smoking. However, these patterns were not as predictable for those who transitioned between smoking states. Future research should explore an intersectional perspective to understand the behavior of those whose smoking behavior transitioned during the pandemic. Understanding these perspectives is important for supporting future cessation efforts of these individuals who smoke.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Contributor Information
Catherine S Nagawa, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Mayuko Ito Fukunaga, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA; Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, MA, USA; Meyers Primary Care Institute, Worcester, MA, USA.
Jamie M Faro, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Feifan Liu, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Ekaterina Anderson, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA; Center for Healthcare Organization and Implementation Research (CHOIR), VA Bedford Healthcare System, Bedford, MA, USA.
Ariana Kamberi, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Elizabeth A Orvek, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Maryann Davis, Department of Psychiatry, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Lori Pbert, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Sarah L Cutrona, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA; Meyers Primary Care Institute, Worcester, MA, USA.
Thomas K Houston, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Rajani S Sadasivam, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Funding
This project was supported by grants (P50 CA244693-01) from the NIH/National Cancer Institute. CSN also received funding support from NIH/National Cancer Institute grant 1F31CA263974-01.
Role of the Funder/Sponsor
The NIH/NIC had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIH.
Declaration of Interests
None declared.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the project principal investigator, Dr. Sadasivam Rajani.
References
- 1. Cucinotta D, Vanelli M. WHO declares COVID-19 a PANDEMIC. Acta Biomed. 2020;91(1):157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klemperer EM, West JC, Peasley-Miklus C, Villanti AC. Change in tobacco and electronic cigarette use and motivation to quit in response to COVID-19. Nicotine Tob Res. 2020;22(9):1662–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. López-Bueno R, Calatayud J, Casaña J, et al. . COVID-19 Confinement and health risk behaviors in Spain. Front Psychol. 2020;11:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen DT-H. The psychosocial impact of the COVID-19 pandemic on changes in smoking behavior: evidence from a nationwide survey in the U.K. Tob. Prev. Cessation;. 2020;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sidor A, Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12(6):1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yingst JM, Krebs NM, Bordner CR, Hobkirk AL, Allen SI, Foulds J. Tobacco use changes and perceived health risks among current tobacco users during the COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(4):1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banerjee D. The COVID-19 outbreak: Crucial role the psychiatrists can play. Asian J Psychiatr. 2020;50:102014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moghanibashi-Mansourieh A. Assessing the anxiety level of Iranian general population during COVID-19 outbreak. Asian J Psychiatr. 2020;51:102076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed MZ, Ahmed O, Aibao Z, Hanbin S, Siyu L, Ahmad A. Epidemic of COVID-19 in China and associated psychological problems. Asian J Psychiatr. 2020;51:102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salari N, Hosseinian-Far A, Jalali R, et al. . Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kashdan TB. Social anxiety spectrum and diminished positive experiences: theoretical synthesis and meta-analysis. Clin Psychol Rev. 2007;27(3):348–365. [DOI] [PubMed] [Google Scholar]
- 12. Slopen N, Kontos EZ, Ryff CD, Ayanian JZ, Albert MA, Williams DR. Psychosocial stress and cigarette smoking persistence, cessation, and relapse over 9-10 years: a prospective study of middle-aged adults in the United States. Cancer Causes Control. 2013;24(10):1849–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2021;93(2):1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chertok IRA. Perceived risk of infection and smoking behavior change during COVID-19 in Ohio. Public Health Nurs. 2020;37(6):854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bommele J, Hopman P, Walters BH, et al. . The double-edged relationship between COVID-19 stress and smoking: Implications for smoking cessation. Tob Induc Dis. 2020;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction. 2021;116(6):1319–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caponnetto P, Inguscio L, Saitta C, Maglia M, Benfatto F, Polosa R. Smoking behavior and psychological dynamics during COVID-19 social distancing and stay-at-home policies: a survey. Health Psychol Res. 2020;8(1):9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faro JM, Orvek EA, Blok AC, et al. . Dissemination and effectiveness of the peer marketing and messaging of a web-assisted tobacco intervention: protocol for a hybrid effectiveness trial. JMIR Res Protoc. 2019;8(7):e14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Scott KW, Lebo L, Hassan N, Lightner C, Pulley J. ResearchMatch: A national registry to recruit volunteers for clinical research. Acad Med. 2012;87(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patridge EF, Bardyn TP. Research electronic data capture (REDCap). J Med Libr Assoc. 2018;106(1):142. [Google Scholar]
- 23. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 24. Thoresen S, Tambs K, Hussain A, Heir T, Johansen VA, Bisson JI. Brief measure of posttraumatic stress reactions: impact of Event Scale-6. Soc Psychiatry Psychiatr Epidemiol. 2010;45(3):405–412. [DOI] [PubMed] [Google Scholar]
- 25. Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37. [PubMed] [Google Scholar]
- 26. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a U.S. national sample of patients with COVID-19. JAMA Netw. Open 2020;3(12):e2029058–e2029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ.. Tobacco product use among adults—United States, 2019. Morb Mortal Wkly Rep. 2020;69(46):1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. U.S. Department of Health and Human Services. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. [Google Scholar]
- 29. Rigotti NA, Chang Y, Regan S, et al. . Cigarette smoking and risk perceptions during the COVID-19 pandemic reported by recently hospitalized participants in a smoking cessation trial. J Gen Intern Med. 2021:36(12):3786–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ibuka Y, Chapman GB, Meyers LA, Li M, Galvani AP. The dynamics of risk perceptions and precautionary behavior in response to 2009 (H1N1) pandemic influenza. BMC Infect Dis. 2010;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grogan S, Walker L, McChesney G, Gee I, Gough B, Cordero MI. How has COVID-19 lockdown impacted smoking? A thematic analysis of written accounts from U.K. smokers. Psychol Health. 2020;37(1):1–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the project principal investigator, Dr. Sadasivam Rajani.