Abstract
Background
The incidence of invasive pneumococcal disease (IPD) decreased worldwide in 2020 and the first quarter of 2021, concurrent with nonpharmaceutical interventions (NPIs) intended to stymie transmission of SARS-CoV-2. In 2021, the stringency of these NPI strategies has varied. We investigated age- and serotype-specific variations in IPD case counts in Germany in 2020–2021.
Methods
IPD cases through 30 November 2021 were stratified by age group, serotype, or geographic location. IPD surveillance data in 2020–2021 were compared with (1) IPD surveillance data from 2015–2019, (2) mobility data during 2020 and 2021, and (3) NPI stringency data in 2020 and 2021.
Results
IPD incidence began to return toward baseline among children 0–4 years old in April 2021 and exceeded baseline by June 2021 (a 9% increase over the average monthly values for 2015–2019). Children aged 5–14 years and adults aged 15–34 or ≥80 years showed increases in IPD cases that exceeded baseline values starting in July 2021, with increases also starting in spring 2021. The age distribution and proportion of vaccine-serotype IPD remained comparable to those in previous years, despite lower overall case counts in 2020 and 2021. The percentage change in IPD incidence compared with the previous 5 years was correlated with changes in mobility and with NPI stringency.
Conclusions
IPD levels began to return to and exceed seasonal levels in spring and summer 2021 in Germany, following sharp declines in 2020 that coincided with NPIs related to the coronavirus disease 2019 pandemic. Proportions of vaccine serotypes remained largely consistent throughout 2020–2021.
Keywords: Streptococcus pneumonia, invasive pneumococcal disease, Germany, SARS-CoV-2 pandemic
Invasive Pneumococcal Disease returned to or exceeded baseline levels in mid-2021 in Germany following substantial decreases in 2020, concurrent with the first wave of the SARS-CoV-2 pandemic. This reemergence was associated with increased mobility and decreased stringency to nonpharmaceutical interventions .
Streptococcus pneumoniae, or pneumococcus, causes disease ranging from routine (otitis media) to life-threatening (meningitis) and remains the most frequent cause of community-acquired pneumonia in adults as well as the cause of 300 000 annual deaths in children <5 years old worldwide [1, 2]. When pneumococci cause infection in normally sterile sites within the body, this is termed invasive pneumococcal disease (IPD).
The primary virulence factor of the pneumococcus is the polysaccharide capsule that surrounds the bacterium; approximately 100 capsular types, or serotypes, have been described [3]. There are highly effective vaccines against pneumococcal disease, which target a subset of the serotypes. New pneumococcal conjugate vaccines (PCVs), targeting up to 20 serotypes, have been approved for use in adults in the United States (Supplementary Table 1), but we have yet to see the effects of these next-generation vaccines deployed at the population level [4, 5].
Like many respiratory pathogens, worldwide reports of IPD declined sharply in 2020, concurrent with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic [6, 7]. While there are some reports of pneumococcal/SARS-CoV-2 coinfection [8, 9], they are not widespread. The unprecedented nonpharmaceutical interventions (NPIs) enacted to stymie SARS-CoV-2 transmission showed a temporal correlation with the declines in IPD and several other respiratory pathogens [10]. During the second quarter of 2021, other pathogens, such as respiratory syncytial virus (RSV), have reemerged and could influence the incidence of IPD [11].
Germany has strong surveillance for IPD that allows for tracking trends over time. The German National Reference Center for Streptococci (GNRCS) has been conducting active surveillance on IPD since 1992. Germany first instituted a recommendation for all infants to receive 4 PCV doses in July 2006 [12]. The recommendation does not specify which PCV formulation is to be used; caregivers and physicians can select either 10-valent PCV or 13-valent PCV (PCV13). The program has been updated over the years, and the current recommendation, implemented since 2015, is for infants to receive 2 primary doses and a booster dose [13]. At age 24 months (1 year after vaccination should be completed), children in Germany have only moderate PCV uptake, with a nationwide average of 69%, ranging from 58% to 75% by federal state [14]. There has also been a recommendation for adults aged ≥60 years to receive a dose of the 23-valent polysaccharide vaccine [15]. However, rates of pneumococcal vaccination in adults in Germany are very low: about 25% of adults with IPD have ever received a pneumococcal vaccination [16]. In this study, we evaluate changes in the frequency of IPD cases reported to the GNRCS before and during the coronavirus disease 2019 (COVID-19) pandemic and characterize the serotype distribution over time.
METHODS
Surveillance Methods
As part of the GNRCS, clinical microbiology laboratories throughout Germany are invited to send pneumococcal isolates and a case report form. A previous audit indicated that the GNRCS receives isolates from approximately 50% of IPD cases in Germany [17]. This stable IPD surveillance system [18], combined with publicly available data about SARS-CoV-2 infections, changes in mobility, and stringency of NPIs provide a wealth of information that can be used to describe and contextualize the epidemiology of an endemic respiratory pathogen during the SARS-CoV-2 pandemic.
Laboratory Methods
Pneumococcal isolates were identified by optochin sensitivity and bile solubility, with serotype determined by Neufeld capsular swelling (quellung) reaction with type and factor sera from Statens Serum Institute, Copenhagen, Denmark.
Analysis Methods
IPD cases were defined as those for which pneumococci were isolated from a normally sterile site within the body, most commonly blood or cerebrospinal fluid. Cases were divided into the following age groups: <5 , 5–14 , 15–34, 35–59, 60–79, and ≥80 years. Serotypes were grouped by their inclusion in current vaccine formulations (PCV13; serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) and future formulations (15-valent PCV [PCV15; PCV13 plus serotypes 22F and 33F] and 20-valent PCV [PCV20; PCV15 plus serotypes 8, 10A, 11A, 12F, and 15B]) and by invasiveness (high [serotypes 4, 7F, 9V, 14, and 18C], moderate [serotypes 8, 12F, 19A, and 22F], and low [serotypes 3, 6A, 6B, 9N,10A, 11A, 15B, 15C, 16F, 19F, 20, 21, 23A, 23F, 33F, 35F, and 38]) [19]. Cases were divided geographically by population-normalized region [20].
In addition to the GNRCS surveillance data, we used publicly available data on SARS-CoV-2 case counts, changes in mobility in 2020 and 2021, and stringency of NPIs. SARS-CoV-2 case counts were obtained from the Robert Koch Institute’s COVID-19 Datenhub (https://npgeo-corona-npgeo-de.hub.arcgis.com/). This database collects data on cases reported to the central health public health authority in Germany, based on SARS-CoV-2–positive polymerase chain reaction test results. Data on changes in mobility were obtained from Google’s Community Mobility Reports (https://www.google.com/covid19/mobility/). These reports use mobile phone location data signals to track changes in several categories of mobility, including retail-related, transit-related, and work-related mobility. These metrics are compared with baseline values for each category from January 2020. Finally, data on stringency of NPIs were obtained from the Oxford COVID-19 Government Response Tracker [21]. The tracker’s Stringency Index is calculated using a weighted composite of 23 metrics, grouped into 8 containment and control, 4 economic response, 8 health systems, and 3 vaccination program measures. These were used to determine average national adherence to NPIs in Germany.
We plotted monthly time series of IPD cases in Germany from 1 January 2015 to 30 November 2021, stratified by age group, serotype grouping (PCV13, PCV15, and PCV20 serotypes; high, moderate, and low invasiveness), or geographic region. We established baseline IPD case counts by averaging the number of cases in each calendar month for the years 2015–2019. We compared these values with IPD case counts during 2020 and 2021 and established a percentage change from baseline for each month of the year. We compared these changes with the percentage changes in several categories of mobility metrics and with NPI stringency during 2020 and 2021 using Spearman correlations. A Spearman ρ of 1 indicates a perfect positive association, 0 indicates no association, and −1 indicates a perfect negative association. Statistical analyses were performed using R software (version 4.0.3; R Foundation for Statistical Computing).
RESULTS
There were 15 704 cases of IPD reported to the GNRCS from 1 January 2015 to 31 December 2019 (the baseline prepandemic period) and 2532 from 1 March 2020 to 30 November 2021, during the SARS-CoV-2 pandemic. Age distributions of IPD cases remained consistent (Supplementary Figure 1), with the largest proportion of cases each occurring in the 60–79-year age group and the smallest proportion in 5–14-year-olds. Within age groups, there were also no differences in age distributions before and during the SARS-CoV-2 pandemic. (Supplementary Figure 2).
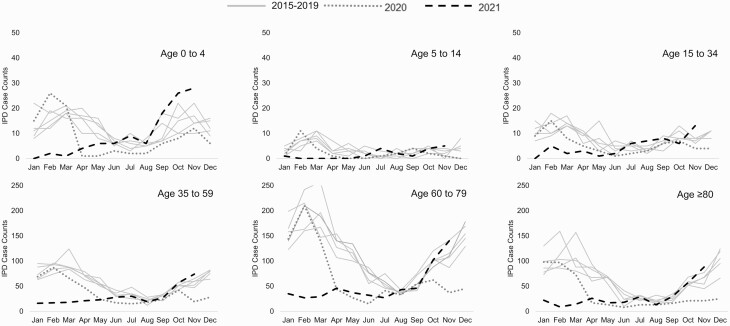
Cases of IPD exceeded prepandemic levels in June 2021, first in children aged 0–4 years, followed by children aged 5–14 years, adults aged 15–34 years, and adults aged ≥80 years in July 2021 (Figure 1). Although the IPD case counts first exceeded baseline values in the summer, the increasing trend began in spring 2021.
Figure 1.
Invasive pneumococcal disease (IPD) case count by age group, from January 2015 to November 2021. Gray lines represent cases counts in the years before 2020 (2015–2019); orange line, 2020; and green line, 2021.
The proportion of vaccine serotypes (for PCV13, PCV15, and PCV20) remained consistent in the population overall and by age group (Supplementary Figure 3. The proportions of serotypes with high , moderate, and low invasiveness were similarly consistent throughout the study period (Supplementary Figure 4, as were the proportions of respiratory versus nonrespiratory IPD cases (Supplementary Figure 5). Most individual serotypes also remained consistent throughout the study period (Supplementary Table 2). Serotype 3 remained the most common IPD serotype, comprising 16%–21% of all IPD cases, followed by serotypes 8 (7%–15%) and 22F (5%–8%).
IPD case counts decreased uniformly by geographic group in 2020 and have rebounded unevenly in 2021. The southernmost regional group, consisting of 2 large federal states, exceeded baseline IPD cases in July, as did 4 additional federal states. By the end of November, increases above baseline IPD had occurred in all 4 geographic regional groups (Supplementary Figures 6 and 7).
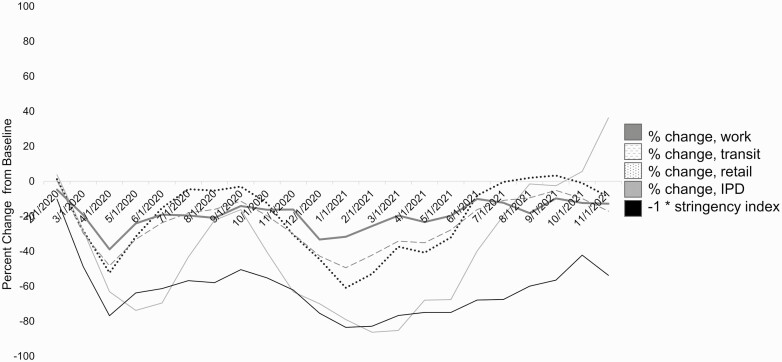
NPIs enacted in Germany included quarantine after exposure, gathering restrictions, mask ordinances, and school and business closures. Decreases in transit, work, and retail mobility were correlated with decreases in national-level rates of IPD (Spearman ρ, 0.85 [95% confidence interval, .67–.94], 0.75 [.49–.86], and 0.84 [.66–.93], respectively). Increases in the overall stringency of NPIs were also correlated with decreases in IPD (Spearman ρ, −0.80 [5% confidence interval, −.90 to −.60]) (Figure 2).
Figure 2.
Stringency Index for nonpharmaceutical interventions (NPIs), changes in work, transit, and retail mobility, and change in invasive pneumococcal disease (IPD), from March 2020 through July 2021 (dates given in month/date/year format). Mobility data were obtained from Google’s Community Mobility Reports, and Stringency Index data from the Oxford COVID-19 Government Response Tracker [21] (see Analysis Methods for details). Abbreviation: COVID-19, coronavirus disease 2019.
Decreases in mobility were associated overall with decreases in IPD, but the pattern varied by age group (Supplementary Figure 8): IPD case counts in children aged 0-4 or 5–14 years had few associations with mobility metrics, while the decreases in older groups tracked closely with the mobility metrics. The Stringency Index was associated with all age groups except children aged 0–4 years (Supplementary Table 3), though some of the confidence intervals were wide.
DISCUSSION
Here we describe the reemergence of IPD in Germany in 2021, after declines in 2020. Cases rebounded fully to or above baseline levels by the end of November 2021. The first age group to rebound to the prepandemic baseline was 0–4-year-olds, possibly reflecting this group’s role in population-wide transmission of pneumococci [22, 23]. Despite a sharp drop in the number of cases in 2020 and the first quarter of 2021, the proportion of vaccine serotypes remained consistent, both overall and stratified by age group and geographic regional group. There was some modest variation in the timing of the rebound between regions. There were associations between IPD counts and both stringency of NPIs and decreased mobility throughout 2020 and 2021.
Worldwide decreases in IPD incidence were widely reported in 2020 and early 2021 [6, 7, 24]. Unlike during pandemics and epidemics caused by other respiratory viruses [25, 26], we did not find a shift toward less invasive serotypes in 2020–2021, neither overall nor stratified by age or regional group.
The cause of the reemergence of IPD in Germany is likely multifactorial. Recent work indicates that despite global decreases in pneumococcal disease incidence during the SARS-CoV-2 pandemic, nasopharyngeal carriage of pneumococci, an important precursor to transmission and disease, remained constant. A decrease in disease while carriage remains constant may indicate the importance of a preceding or concomitant viral infection to invasive disease processes [24]. Mobility metrics showed a return to near-baseline levels of activity, which could allow the usual avenues of both pneumococcal and viral transmission to resume. A national survey of influenzalike illness indicated that the incidence of such illness decreased below baseline for the 2020–2021 winter season but returned to baseline in spring 2021, narrowly preceding the rise in IPD [27]. A further possibility is a particularly mutualistic relationship between IPD and RSV [28]. Unusually high levels of RSV hospitalizations were reported in children’s hospitals in the summer of 2021 [29], coinciding with the reemergence of IPD. There is also the possibility of post–SARS-CoV-2 vulnerability to pneumococcal infection [30], and when approximately 5% of the population has had a confirmed SARS-CoV-2 infection, this may compromise the baseline health of the population.
The possibility of an unusually high IPD season, either concurrent with or directly following outbreaks of SARS-CoV-2, would put additional strain on exhausted healthcare delivery systems and personnel. Increasing pneumococcal vaccine uptake across the population could reduce the burden of disease. Reports of disruptions to routine immunizations during the pandemic pose a further threat to ensuring adequate population-level protection against pneumococcal disease [31].
Limitations of the current study include the generalized, national level of the NPI stringency database, which does not capture the effect of geographic variations in NPIs, and the generalized, federal- and state-level mobility data, which do not capture age group–specific variations in mobility associated with the pandemic. Both of these data sets should be interpreted cautiously, because unknown geographic location (in the former) or age (in both data sets) may bias our results. In addition, it is possible that case counts were affected by reporting delays in the IPD surveillance system, but these were minimal (Supplementary Figure 9). The uptick in IPD cases in the spring and summer of 2021 persisted throughout the autumn of 2021, making it less likely to be a temporary artifact, and will likely continue to be affected by changes in mobility and NPI stringency: if lockdowns and NPIs are lax or nonexistent, IPD case counts may be particularly high in the winter of 2021–2022; conversely, if mobility is low and stringency of NPIs is high, there may be a return to the low IPD levels seen in 2020. The interplay between policy decisions, the baseline health of the population, respiratory viral transmission, and vaccine uptake all contribute to local levels of IPD and must be held in check to prevent severe illness and increased mortality rates.
In conclusion, IPD incidence decreased sharply in the second quarter of 2020 and rebounded to baseline levels in the beginning of the third quarter of 2021. Serotype distributions of IPD remained largely consistent throughout 2020–2021, despite varying NPI stringency. The potential for high case numbers this winter in undervaccinated populations is an unknown threat, which lends even greater importance to the arrival of new pneumococcal vaccines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the Robert Koch Institute, Google Mobility Reports, and the Oxford COVID-19 Government Response Tracker teams for providing high-quality, open-source data sets.
Financial support. This work was supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. We would also like to thank the Robert Koch Institute, Google Mobility Reports and the Oxford COVID-19 Government Response Tracker teams for providing high-quality, open-source datasets.
Contributor Information
Stephanie Perniciaro, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, Connecticut, USA; German National Reference Center for Streptococci, Department of Medical Microbiology, University Hospital RWTH Aachen, Aachen, Germany.
Mark van der Linden, German National Reference Center for Streptococci, Department of Medical Microbiology, University Hospital RWTH Aachen, Aachen, Germany.
Daniel M Weinberger, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, Connecticut, USA.
References
- 1. Pan American Health Organization/World Health Organization. About pneumococcal disease. Available at: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=1894:2009-about-pneumococcus-disease&Itemid=1630&lang=en. Accessed 28 September 2021.
- 2. Centers for Disease Control and Prevention. In: Hall E, Wodi AP, Hamborsky J, et al. eds. Epidemiology and prevention of vaccine-preventable diseases. 14th ed. Washington, DC: Public Health Foundation, 2021. [Google Scholar]
- 3. Ganaie F, Saad JS, McGee L, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio 2021; 11:e00937-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. U.S. FDA approves PREVNAR 20™, Pfizer’s pneumococcal 20-valent conjugate vaccine for adults ages 18 years or older. Available at: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-prevnar-20tm-pfizers-pneumococcal-20-valent#.YMDO5rFPVYg.twitter. Accessed 9 June 2021.
- 5. Merck announces U.S. FDA approval of VAXNEUVANCE™ (pneumococcal 15-valent conjugate vaccine) for the prevention of invasive pneumococcal disease in adults 18 years and older caused by 15 serotypes. Available at: https://www.merck.com/news/merck-announces-u-s-fda-approval-of-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-for-the-prevention-of-invasive-pneumococcal-disease-in-adults-18-years-and-older-caused-by-15-serot/. Accessed 28 September 2021.
- 6. Janapatla RP, Chen CL, Dudek A, et al. Serotype transmission dynamics and reduced incidence of invasive pneumococcal disease caused by different serotypes after implementation of non-pharmaceutical interventions during COVID-19 pandemic. Eur Respir J 2021; 58:2100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective national cohort study, England. Clin Infect Dis 2021; 72:e65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toombs J, Van den Abbeele K, Democratis J, Mandal AKJ, Missouris CG.. Pneumococcal coinfection in COV ID-19 patients. J Med Virol 2021; 93:177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cucchiari D, Pericàs JM, Riera J, Gumucio R, Md EC, Nicolás D.. Pneumococcal superinfection in COVID-19 patients: a series of 5 cases. Med Clin (Barc) 2020; 155:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health 2021; 3:e360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weinberger DM, Klugman KP, Steiner CA, Simonsen L, Viboud C.. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Med 2015; 12:e1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robert Koch Institute. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut: Begründungen zur allgemeinen Empfehlung der Impfungen gegen Pneumokokken und Meningokokken im Säuglings- und Kindesalter Epidemiologisches Bulletin 31/2006. Available at: 10.25646/4245. [DOI]
- 13. Robert Koch-Institute. Mitteilung der Ständigen Impfkommission (STIKO) am RKI Wissenschaftliche Begründung zur Änderung der Pneumokokken-Impfempfehlung für Säuglinge Epidemiologisches Bulletin 36 / 2015. doi: 10.17886/EpiBull-2015-005. [DOI]
- 14. Robert Koch Institute. Impfquoten von Kinderschutzimpfungen in Deutschland – aktuelle Ergebnisse aus der RKI-Impfsurveillance Epidemiologisches Bulletin 32-33 /2020. doi: 10.25646/7020 [DOI]
- 15. Robert Koch Institute. "Impfkalender (Standardimpfungen) für Säuglinge, Kinder, Jugendliche und Erwachsene; 2020/2021" 34/2020 DOI: 10.25646/7083.7. [DOI]
- 16. Perniciaro S, van der Linden M.. Pneumococcal vaccine uptake and vaccine effectiveness in older adults with invasive pneumococcal disease in Germany: a retrospective cohort study. Lancet Reg Health 2021; 7:100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reinert RR, Haupts S, Linden MVD, et al. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001–2003. Clin Microbiol Infect 2005; 11:985–91. [DOI] [PubMed] [Google Scholar]
- 18. van der Linden M, Imöhl M, Perniciaro S.. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PLoS One 2019; 14:e0220453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sleeman KL, Griffiths D, Shackley F, et al. Capsular serotype–specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis 2006; 194:682–8. [DOI] [PubMed] [Google Scholar]
- 20. Perniciaro S, Imöhl M, Fitzner C, van der Linden M.. Regional variations in serotype distribution and vaccination status in children under six years of age with invasive pneumococcal disease in Germany. PLoS One 2019; 14:e0210278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oxford COVID-19 Government Response Tracker. Available at: https://covidtracker.bsg.ox.ac.uk/. Accessed 18 January 2022 .
- 22. Weinberger DM, Pitzer VE, Regev-Yochay G, Givon-Lavi N, Dagan R.. Association between the decline in pneumococcal disease in unimmunized adults and vaccine-derived protection against colonization in toddlers and preschool-aged children. Am J Epidemiol 2019; 188:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flasche S, Lipsitch M, Ojal J, Pinsent A.. Estimating the contribution of different age strata to vaccine serotype pneumococcal transmission in the pre vaccine era: a modelling study. BMC Med 2020; 18:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danino D, Ben-Shimol S, van der Beek BA, et al. Decline in pneumococcal disease in young children during the COVID-19 pandemic associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. medRxiv [Preprint: not peer reviewed]. 1 August 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.07.29.21261308v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinberger DM, Harboe ZB, Viboud C, et al. Serotype-specific effect of influenza on adult invasive pneumococcal pneumonia. J Infect Dis 2013; 208:1274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernández S, Muñoz-Almagro C, Ciruela P, Soldevila N, et al. Invasive pneumococcal disease and influenza activity in a pediatric population: impact of PCV13 vaccination in pandemic and nonpandemic influenza periods. J Clin Microbiol 2019; 57:e00363-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robert Koch Institute. Arbeitsgemeinschaft influenza. Available at: https://influenza.rki.de/Diagrams.aspx?agiRegion=0. Accessed 3 January 2022.
- 28. Weinberger DM, Givon-Lavi N, Shemer-Avni Y, et al. Influence of pneumococcal vaccines and respiratory syncytial virus on alveolar pneumonia, Israel. Emerg Infect Dis J 2013; 19:1084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liese J, Adams O, Egbert H, et al. Zunahme an aufnahmen in kinderkliniken durch atemwegsinfektionen mit nachweis von respiratory syncytial virus (RSV). Stand 27.07.2021. DGPI: Deutsche Gesellschaft für Pädiatrische Infektiologie. Available at: https://dgpi.de/atemwegsinfektionen-nachweis-rsv-27-07-2021/. Accessed 8 September 2021.
- 30. Sender V, Hentrich K, Henriques-Normark B.. Virus-induced changes of the respiratory tract environment promote secondary infections with Streptococcus pneumoniae. Front Cell Infect Microbiol 2021; 11:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Causey K, Fullman N, Sorensen RJD, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet 2021; 398:522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.