Abstract
Objectives
Adolescents with juvenile-onset autoimmune inflammatory rheumatic diseases (AIIRDs) could be at risk for disease flare secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or to withholding anti-inflammatory therapy. While vaccination can protect against coronavirus disease 2019 (COVID-19), safety and immunogenicity data regarding anti-SARS-CoV-2 vaccines among adolescents with AIIRDs are limited. This international, prospective, multicentre study evaluated the safety and immunogenicity of the BNT162b2 anti-SARS-CoV-2 vaccine among adolescents and young adults with juvenile-onset AIIRDs, 80% of whom are on chronic immunomodulatory therapy.
Methods
Vaccine side effects, disease activity and short-term efficacy were evaluated after 3 months in 91 patients. Anti-spike S1/S2 IgG antibody levels were evaluated in 37 patients and 22 controls 2–9 weeks after the second dose.
Results
A total of 91 patients and 40 healthy controls were included. The safety profile was good, with 96.7% (n = 88) of patients reporting mild or no side effects and no change in disease activity. However, three patients had transient acute symptoms: two following the first vaccination (renal failure and pulmonary haemorrhage) and one following the second dose (mild lupus flare vs viral infection). The seropositivity rate was 97.3% in the AIIRD group compared with 100% among controls. However, anti-S1/S2 antibody titres were significantly lower in the AIIRD group compared with controls [242 (S.d. 136.4) vs 387.8 (57.3) BAU/ml, respectively; P < 0.0001]. No cases of COVID-19 were documented during the 3 month follow-up.
Conclusion
Vaccination of juvenile-onset AIIRD patients demonstrated good short-term safety and efficacy and a high seropositivity rate but lower anti-S1/S2 antibody titres compared with healthy controls. These results should encourage vaccination of adolescents with juvenile-onset AIIRDs, even while on immunomodulation.
Keywords: adolescent rheumatology, biologic therapies, DMARDs, JIA, paediatric/juvenile rheumatology, SLE
Rheumatology key messages.
An international study evaluated the safety and immunogenicity of mRNA COVID-19 vaccine among adolescents with juvenile-onset rheumatic diseases.
Vaccination demonstrated good safety and efficacy and a high seropositivity rate but lower anti-S1/S2 titres compared with controls.
Results should encourage vaccination of adolescents with juvenile-onset rheumatic diseases, even while on immunomodulatory therapy.
Introduction
Preventing the spread of coronavirus disease 2019 (COVID-19) infection through vaccinations is a worldwide goal. The BNT162b2 (Pfizer-BioNTech) vaccine is based on delivery of messenger RNA (mRNA) encoding the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike glycoprotein [1]. This vaccine was shown to have a favourable safety profile, immunogenicity and short-term efficacy in preventing COVID-19 in healthy individuals >16 years of age [2] and in healthy adolescents ages 12–15 years [3], leading to US Food and Drug Administration (FDA) and European Medicines Agency [4] approval for these age groups. Recently, expanded approval for use in children ages 5–11 years has been issued by the FDA [5] and the Centers for Disease Control [6].
Although children and adolescents generally have a milder COVID-19 disease course than adults, severe illness can occur in this population [7], especially among those with underlying comorbidities, such as obesity [8], cardiovascular disease, chronic lung disease, diabetes and asthma [9–11]. In addition, multisystem inflammatory syndrome in children (MIS-C) is a serious complication related to recent SARS-CoV-2 exposure [12, 13]. Long-term COVID-19 manifestations or post-COVID syndrome have been described in both children and adults [14].
With immunization of adults increasing, young adolescents and children account for an increased proportion of COVID-19 infections and may play an important role in SARS-CoV-2 transmission [15, 16].
Preliminary data from the EULAR COVID-19 Database regarding short-term outcomes of COVID-19 in children with pre-existing rheumatic diseases shows that COVID-19 presents mostly as a mild disease, with no need for hospitalization of most children with a rheumatic disease. However, severe, systemic rheumatic diseases, such as SLE and vasculitides, as well as obesity are risk factors for hospitalization [17].
Viruses are a well-recognized trigger for flare of rheumatic diseases and a recent publication reported that patients with JIA in remission can experience a flare following SARS-CoV-2 infection [18]. In addition, in the case of SARS-CoV-2 infection, it is usually advised to consider withholding some of the anti-inflammatory therapy, which also imposes a risk for disease flare. It is therefore crucial to try and prevent COVID-19 by vaccinating adolescents with autoimmune inflammatory rheumatic diseases (AIIRDs).
The ACR Vaccination Taskforce and the Paediatric Rheumatology European Society recommended prioritizing COVID-19 vaccination for patients with AIIRDs [19, 20]. Data from adults with AIIRDs who received the BNT162b2 vaccine demonstrated safety and immunogenicity in most, with up to 86% seropositivity and mostly mild and transient adverse events [21]. In addition, a large cohort of adults with RA showed no significant association between COVID-19 vaccines and arthritis flare [22]. However, antibody titres to the spike S1/S2 protein were significantly lower among patients with AIIRDs compared with healthy controls [21, 23].
While data on adult patients are accumulating, information on the safety and immunogenicity of COVID vaccines among adolescents with AIIRDs are limited. Therefore there is still hesitancy among patients and caregivers, with resultant suboptimal vaccination rates. The current study explored the safety and immunogenicity of the mRNA COVID-19 vaccine among adolescents with juvenile-onset AIIRDs treated with immunomodulatory medications and compared it with the results among healthy children.
Methods
This international, prospective, multicentre study was conducted between April and November 2021, at the Pediatric Rheumatology Clinics of Dana-Dwek Children’s Hospital (Tel Aviv), Meir Medical Center (Kfar Saba) and Rambam Medical Centers (Haifa), in Israel, and at the University of Ljubljana Children’s Hospital in Slovenia. The study was approved by the Institutional Review Board (IRB) of Tel Aviv Sourasky Medical Center (coordinating centre) and by the IRBs of the participating centres. Written informed consent was obtained from all participants.
Study endpoints
The primary endpoints of the study included evaluation of short-term side effects of the vaccination in adolescents with AIIRDs compared with controls, the impact of vaccination on clinical disease activity in adolescents with AIIRDs and immunogenicity of the BNT162b2 mRNA vaccine in adolescents with AIIRDs measured 2–9 weeks after the second vaccine dose compared with controls.
The secondary endpoint was evaluation of vaccination efficacy, defined as prevention of COVID-19 disease, confirmed by PCR testing.
Study population
The study population included adolescents (ages 12–18 years) and young adults (ages 18–21 years) with juvenile-onset of the following AIIRDs: JIA according to the EULAR 2001 classification criteria [24]; SLE according to the 1997 ACR or 2012 SLICC criteria [25]; systemic vasculitis, i.e. ANCA-associated vasculitis, including granulomatosis with polyangiitis (GPA), according to the Chapel Hill Consensus Conference definitions [26]; Behçet disease, idiopathic uveitis, IBD-related arthritis, systemic or localized scleroderma or juvenile dermatomyositis according to the EULAR/ACR classification criteria [27]; or autoinflammatory syndromes. Patients were instructed to continue all medications during the vaccination period.
The patient cohort included 91 patients evaluated for safety data. Among them, 37 patients who provided serum samples were checked for immunogenicity. A group of 40 healthy adolescents served as controls for comparison of vaccination side effects and COVID-19 rates, with immunogenicity data checked in 22 individuals who provided serum samples. Importantly, there was no difference in the demographic or clinical data between the cohorts who provided serum samples and those who did not. Patients were recruited from the four medical centres, with 64 (70%) from Israel and 27 (30%) from Slovenia. Healthy controls were recruited from Dana Dwek Children’s Hospital (coordinating centre). Exclusion criteria were previous COVID-19 infection and a history of an AIIRD or immunosuppressive treatment for controls.
All study participants were vaccinated with two doses of BNT162b2 mRNA vaccine (30 μg per dose, administered intramuscularly 3 weeks apart, as indicated by the national guidelines of Israel).
Safety and clinical assessment of the AIIRD
The participants were contacted by phone 2 weeks following the first vaccination and within 2–4 weeks following the second dose and completed a questionnaire regarding local and systemic side effects.
Medical history and the use of medications were recorded. Data regarding disease activity up to 3 months before vaccination were retrieved from the patients’ medical records. Post-vaccination disease activity was assessed by an in-person clinical examination ∼3 months after the first vaccine dose. The following disease activity indices were included: Juvenile Arthritis Disease Activity Score (JADAS) 10 for JIA [28], SLEDAI for SLE and patients’ and physicians’ global assessments (PGA, PhGA), using a visual analogue scale of 0–10 mm, for JIA, SLE, vasculitis, inflammatory myositis, scleroderma, uveitis and autoinflammatory syndromes.
Vaccine immunogenicity
Vaccine immunogenicity was evaluated for a subgroup of the patients and controls by measuring serum IgG antibody levels against SARS-CoV-2 trimeric spike S1/S2 glycoproteins 2–9 weeks after the second vaccine. We used the FDA authorized LIAISON (DiaSorin, Sallugia, Italy) quantitative assay. The assay provides an indication for the presence of neutralizing IgG antibodies against SARS-CoV-2 and has a clinical sensitivity and specificity >98% [29]. A value of >15 binding antibody units (BAU) was considered positive according to the manufacturer’s instructions.
Vaccine efficacy
Participants were queried for evidence of COVID-19 infection following each vaccine dose and electronic medical records were reviewed for evidence of infection 3 months following vaccination. As the Israeli electronic medical records system provides access to laboratory tests performed outside of the hospital setting, the results of SARS-CoV-2 PCR tests were available for review.
Statistical analysis
Differences between continuous variables were tested for significance using the independent-samples t-test. Differences between categorical variables were tested for significance using the chi-squared test or Fisher’s exact test. All tests applied were two-tailed and a P-value ≤0.05 was considered statistically significant. The data were analysed using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study sample
A total of 91 children with AIIRDs and 40 healthy controls vaccinated with two doses of BNT162b2 mRNA vaccine were enrolled (Table 1). The mean age at time of enrolment was 15.9 years (S.d. 2.1) and 14 years (S.d. 1.2) in the AIIRD and control groups, respectively. There was 47% and 48% females in the AIIRD and control groups, respectively. JIA was the most common disease, found in 42 (46%) patients, followed by 13 (14%) with SLE. The mean disease duration was 4.9 years (S.d. 4.4), with a median of 3.1 years (range 0.3–18).
Table 1.
Baseline characteristics of adolescents with AIIRDs and controls
| Characteristics | AIIRDs (n = 91) | Controls (n = 40) | P-value |
|---|---|---|---|
| Age, mean (S.d.), years | 15.9 (2.1) | 14.0 (1.2) | <0.0001 |
| Female, n (%) | 43 (47) | 19 (48) | 1 |
| Country, n (%) | |||
| Israel | 64 (70) | 40 (100) | 0.0003 |
| Slovenia | 27 (30) | 0 (0) | 0.0003 |
| Disease duration, mean (S.d.), years | 4.85 (4.4) | ||
| JIA, n (%) | 42 (46) | ||
| SLE, n (%) | 13 (14) | ||
| Autoinflammatory diseases, n (%) | 7 (8) | ||
| Scleroderma, n (%) | 5 (5) | ||
| Vasculitis, n (%) | 6 (7) | ||
| Uveitis, n (%) | 6 (7) | ||
| Myositis, n (%) | 4 (4) | ||
| Other, n (%) | 3 (3) |
A total of 73 (80.2%) adolescents with AIIRDs were treated with immunomodulatory medications (Table 2). Conventional synthetic DMARDs (csDMARDs) were used by 36 (39.7%) patients and as monotherapy by 18 (19.8%). Biologic DMARDs (bDMARDs) were used by 34 (37.4%) patients and as monotherapy by 23 (25.3%). Glucocorticoids [GCs at a mean prednisone dose of 10 mg/day (S.d. 9.8)] were used by 13 (14.3%). Janus kinase inhibitors (JAKis) were used as monotherapy or in combination with csDMARDs by 6 (6.6%) and 3 (3%), respectively, and 4 (4.4%) were treated with CD20-depleting (anti-CD20) therapies.
Table 2.
Treatments per diagnosis among adolescents with juvenile-onset AIIRDs
| AIIRD diagnosis | csDMARDs | bDMARDs | MTX | MMF | TNF inhibitor | IL-6 inhibitor | Anti-CD20 | Steroids | HCQ | JAKi |
|---|---|---|---|---|---|---|---|---|---|---|
| All AIIRDs (n = 91) | 36 (39.7) | 34 (37.4) | 19 (20.9) | 10 (10.9%) | 29 (31.87) | 1 (1.1) | 4 (4.4) | 13 (14.3) | 17 (18.7) | 6 (6.6) |
| JIA (n = 42) | 15 (35.71) | 17 (40.48) | 13 (30.95) | 1 (2.38) | 16 (38.1) | 1 (2.38) | 0 (0) | 1 (2.38) | 0 (0) | 4 (9.5) |
| SLE (n = 13) | 12 (92.31) | 1 (7.69) | 0 (0) | 6 (46.15) | 0 (0) | 0 (0) | 1 (7.69) | 7 (53.85) | 12 (92.3) | 0 (0) |
| Autoinflammatory diseases (n = 7) | 1 (14.29) | 2 (28.57) | 0 (0) | 0 (0) | 2 (28.57) | 0 (0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) |
| Scleroderma (n = 5) | 2 (40) | 1 (20) | 1 (20) | 3 (60) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 1 (2) | 0 (0) |
| Vasculitis (n = 6) | 0 (0) | 3 (50) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 2 (33.3) | 1 (16.7) | 0 (0) | 1 (16.7) |
| Uveitis (n = 6) | 3 (50) | 5 (83.3) | 3 (50) | 0 (0) | 5 (83.3) | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) |
| Myositis (n = 4) | 3 (75) | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (50) | 3 (75) | 1 (25) |
| IBD-related arthritis (n = 5) | 0 (0) | 5 (100) | 0 (0) | 0 (0) | 5 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other (n = 3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Values are presented as n (%).
IL-6 inhibitor: anti-IL-6 receptor monoclonal antibodies; anti-CD20: anti-CD20 monoclonal antibodies.
Safety of the BNT162b2 vaccine
The prevalence of mild adverse events was similar in patients with AIIRDs and controls. Local pain at the injection site was the most common side effect, with a prevalence of 79% in both cohorts following the first vaccine and 73% and 75%, respectively, following the second vaccine (Table 3). Some adverse events were more frequent in the adolescents with AIIRDs compared with controls, including fever following the first vaccine (6% vs 3%), fatigue following the second vaccine (33% vs 20%) and myalgia and arthralgia following the second vaccine (22% vs 10% and 11% vs 5%, respectively). However, none reached statistical significance.
Table 3.
Side effects of the BNT162b2 mRNA COVID-19 vaccines in adolescents with juvenile-onset AIIRDs and controls
| Side effects | Following first vaccine |
Following second vaccine |
||||
|---|---|---|---|---|---|---|
| AIIRDs (n = 90) | Controls (n = 39) | P-value | AIIRDs (n = 88) | Controls (n = 40) | P-value | |
| No symptoms, n (%) | 15 (17) | 7 (18) | 1 | 18 (20) | 5 (12) | 0.4019 |
| Local reactions, n (%) | ||||||
| Pain | 71 (79) | 31 (79) | 1 | 64 (73) | 30 (75) | 0.957 |
| Swelling | 11 (12) | 6 (15) | 0.8381 | 8 (9) | 3 (8) | 1 |
| Erythema | 3 (3) | 2 (5) | 1 | 2 (2) | 1 (2) | 1 |
| Itching | 4 (4) | 0 (0) | 0.4328 | 3 (3) | 0 (0) | 0.5813 |
| Pruritus | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 | |
| Systemic reactions, n (%) | ||||||
| Fever | 5 (6) | 1 (3) | 0.775 | 14 (16) | 7 (18) | 1 |
| Vomiting | 0 (0) | 2 (5) | 0.1647 | 2 (2) | 2 (5) | 0.7841 |
| Nausea | 4 (4) | 1 (3) | 0.9908 | 6 (7) | 3 (8) | 1 |
| Runny nose | 3 (3) | 0 (0) | 0.6047 | 4 (5) | 1 (2) | 0.9509 |
| 2 (2) | 0 (0) | 0.871 | 3 (3) | 1 (2) | 1 | |
| Muscle aches | 9 (10) | 3 (8) | 0.9327 | 19 (22) | 4 (10) | 0.1819 |
| Joint pain | 4 (4) | 1 (3) | 0.9908 | 10 (11) | 2 (5) | 0.4135 |
| Chills | 3 (3) | 2 (5) | 1 | 11 (12) | 3 (8) | 0.5929 |
| Feeling unwell | 10 (11) | 3 (8) | 0.7841 | 24 (27) | 8 (20) | 0.5089 |
| Headaches | 8 (9) | 5 (13) | 0.7167 | 22 (25) | 8 (21) | 0.7469 |
| Hospitalization within 2 weeks post-vaccine | 2 (2) | 0 (0) | 0.8727 | 1 (1) | 0 (0) | 1 |
| Allergic reaction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Weakness | 9 (10) | 4 (10) | 1 | 25 (29) | 10 (25) | 0.8229 |
| Tiredness | 15 (17) | 5 (13) | 0.7722 | 29 (33) | 8 (20) | 0.1977 |
| Exacerbationa | 5 (6) | 0 (0) | 0.315 | 1 (1) | 0 (0) | 1 |
Worsening of rheumatic disease symptoms.
Two AIIRD patients were hospitalized following the first vaccine and one patient presented to an emergency department (ED) following the second vaccine dose. The two patients hospitalized following the first vaccine both had systemic vasculitis (GPA); both were treated with rituximab and were 17 years old. The first patient was a female who was diagnosed at the age of 14 years. She was treated with azathioprine in addition to rituximab. She presented to the ED a few hours after the vaccine with acute symptoms of fever, cough and vomiting with dehydration. Laboratory results showing elevated CRP of 54 mg/dl (normal value <5) and acute renal failure (creatinine level increased from 1.19 mg/dl at baseline to 1.7 mg/dl). She was hospitalized and treated with i.v. fluids and i.v. antibiotics. She was discharged 2 days later with no change in her regular anti-inflammatory medications. The second patient was diagnosed with GPA at age 16.5 years after presenting with renal failure and pansinusitis. He was treated with rituximab and prednisone, with the first rituximab dose given 9 days after the second vaccine dose. Seven days after the first vaccine he presented with disease exacerbation and new-onset pulmonary haemorrhage. However, his symptoms began a few weeks prior to his first vaccine, when he presented with suspected otitis media, labyrinthitis and high inflammatory markers. The third patient, who presented to an ED following the second vaccine was a 14-year-old female diagnosed with SLE 6 months before receiving the first COVID-19 vaccine. She was treated with HCQ monotherapy due to low disease activity (SLEDAI score of 2), with SLE manifestations, including polyarthritis, pericarditis and leucopenia. She presented to the ED with fever, headache, vomiting and arthralgia 1 day after the second vaccine dose. She had normal inflammatory markers and was discharged on low-dose steroids, which were tapered over 2 weeks, with no change in her disease activity score.
BNT162b2 vaccine effect on disease activity in patients with AIIRDs
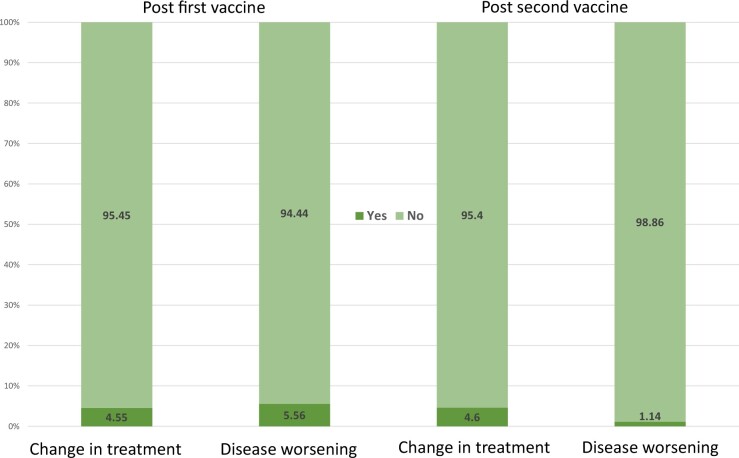
Post-vaccination disease activity remained stable in 85 (94.4%) of the adolescents with AIIRDs following the first vaccine and in 89 (98.8%) following the second dose (n = 90) (Fig. 1).
Fig. 1.
Disease activity and treatment change post-COVID-19 vaccines in percentages
During the study period, changes in immunomodulatory drugs were reported in 8 (9.2%) patients, 4 after the first dose and an additional 4 after the second dose (Fig. 1).
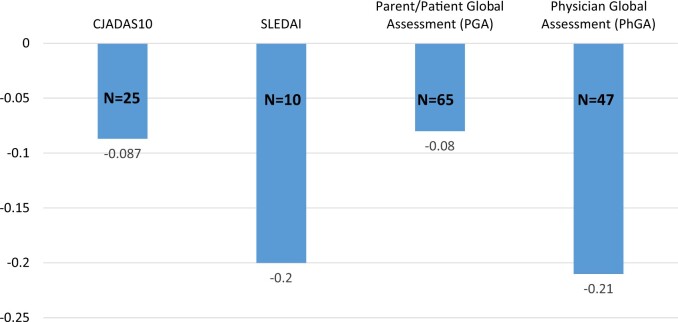
PGA and PhGA were available for 65 and 47 patients, respectively. The mean PGA remained stable or improved following the two-dose vaccine, at 0.89 and 0.77 pre- and post-vaccines, respectively, with a mean decrease of −0.08 (S.d. 0.97) (n = 65). Similarly, the mean PhGA scores pre- and post-vaccination were 0.67 and 0.6, respectively, with a mean decrease of −0.21 (S.d. 0.97) (n = 47) (Fig. 2). In patients with JIA and SLE, the post-vaccination indices of disease activity remained stable as well, with a mean clinical JADAS of 1.52 and 1.68 pre- and post-vaccine, a mean change of −0.09 (S.d. 1.53) for 23 JIA patients and a mean SLEDAI of 3.3 and 2.8 pre-and post-vaccine, with a mean change of −0.2 (S.d. 2.9) for 10 SLE patients (Fig. 2).
Fig. 2.
Change in disease activity scores before/after COVID-19 vaccines (N = 91)
cJADAS10: clinical Juvenile Arthritis Disease Activity Score 10.
Immunogenicity of the BNT162b2 vaccine
A total of 37 patients and 22 controls were evaluated for immunogenicity. The seropositivity rate was 97.3% (n = 36) in patients with AIIRDs compared with 100% (n = 22) in controls (P = 1). However, the anti-S1/S2 antibody levels were significantly lower in patients with AIIRDs compared with controls [mean 242 BAU/ml (S.d. 136.4)] vs 387.8 (57.3); P < 0.0001]. Analysis of the anti-S1/S2 antibody levels for the subgroups of 12–15 years and 16–21 years showed similar results, with mean levels of 241.8 BAU/ml (S.d. 133) and 242.5 (149.7), respectively.
Anti-S1/S2 antibody titres were highest in patients with JIA [mean 288 BAU/ml (S.d. 114), n = 6], followed by vasculitis [243 BAU/ml (S.d. 221), n = 2] and SLE [229.5 BAU/ml (S.d. 159), n = 10].
Analysis of immunogenicity according to the medication used showed that anti-S1/S2 antibody titres were highest among patients treated with HCQ or DMARDs as monotherapy [mean 300 BAU/ml (S.d. 172) and 305.5 (146.7), respectively] and lowest in patients treated with MMF as monotherapy [24 BAU/ml (S.d. 1.5)] or in combination with other treatments [142 BAU/ml (S.d. 139.6)] (Table 4). Patients on csDMARDs and bDMARDs developed anti-S1/S2 antibody titres similar to the general mean of the AIIRD group, with a level of 250 BAU/ml (S.d. 144.8) for any csDMARD and 238.4 (119.4) and 265.8 (98.5) for any bDMARD or bDMARD monotherapy, respectively (Table 4).
Table 4.
Seroconversion rate of BNT162b2 mRNA vaccine among adolescents with AIIRDs, categorized by immunomodulatory treatment
| Immunomodulatory treatment | Seropositivity rate, n (%) | Serum anti-S1/S2 antibody titre, mean (S.d.), BAU/ml |
|---|---|---|
| Any csDMARD | 16 (94) | 250.02 (144.8) |
| csDMARD monotherapy | 8 (100) | 305.5 (146.7) |
| Any bDMARD | 16 (100) | 238.38 (119.4) |
| bDMARD monotherapy | 9 (100) | 265.84 (98.5) |
| Glucocorticoids | 5 (100) | 187.14 (140.5) |
| HCQ | 9 (90) | 252.41 (150.5) |
| HCQ monotherapy | 3 (100) | 300.67 (172.1) |
| MTX | 7 (100) | 244.62 (153.1) |
| MTX monotherapy | 3 (100) | 400 (NA) |
| MMF | 7 (88) | 142.23 (139.6) |
| MMF monotherapy | 2 (100) | 23.95 (1.5) |
| TNF inhibitor | 13 (100) | 239.81 (119.6) |
| TNF inhibitor monotherapy | 9 (100) | 265.84 (98.5) |
| Anti-CD20 | 3 (100) | 232.67 (145.1) |
Anti-CD20: anti-CD20 monoclonal antibodies; NA: not available.
Nineteen (20.9%) patients were treated with MTX; 7 provided serum samples for immunogenicity. They had a 100% seropositivity rate and mean anti-S1/S2 level of 244.6 BAU/ml (S.d. 153). With respect to CD20-depleting therapies, 4 (4.4%) patients were treated with rituximab. Three provided serum samples, with a 100% seropositivity rate. The mean anti-S1/S2 level was 232.7 BAU/ml (S.d. 145; median 155; range 143–400). The interval between the last rituximab dose and the first COVID-19 vaccine dose was 6 months for two patients and 5 months for the third. The first patient, with a diagnosis of GPA, received rituximab every 6 months for 3 years prior to the COVID-19 vaccine, in addition to azathioprine, with a cumulative rituximab dose of 10 g. The second patient, with a diagnosis of SLE nephritis, started rituximab 6 months prior to the vaccine, in addition to MMF, with a cumulative dose of 2 g. This patient’s B cell count returned to normal prior to the COVID vaccine, with a total CD-19 count of 450/cm3 (normal range 116–613/cm3). Importantly, for these two patients, N antibodies were checked and found to be negative, i.e. there was no evidence of previous COVID-19 infection. The third patient, with a diagnosis of systemic scleroderma 5 years prior to the vaccine, had received rituximab every 6 months for 1 year, in addition to MMF, with a cumulative dose of 2.5 g.
Only one patient tested seronegative following the two vaccines. She was 17 years old with SSc diagnosed at the age of 6 years and is treated with MMF and HCQ.
Efficacy of the BNT162b2 vaccine
No COVID-19 cases were detected among AIIRD patients or controls during the 3 month post-vaccine follow-up.
Discussion
This prospective, multicentre, international study evaluated the safety and immunogenicity of mRNA-based anti-SARS-CoV-2 vaccine in a cohort of adolescents with juvenile-onset AIIRDs. Current COVID-19 vaccine recommendations do not specifically address children and adolescents with juvenile-onset AIIRDs and the guidelines rely mainly on data from adults with AIIRDs [19]. Therefore the current study supports the Paediatric Rheumatology European Society recommendations regarding COVID-19 vaccinations [20].
The safety profile of the vaccine was good, with minimal or no side effects in 88 (96.7%) individuals and severe transient adverse events in 3 (3.2%). The two vasculitis patients who were hospitalized had transient disease exacerbation following the first vaccine. Both recovered rapidly and received the second vaccine with no side effects. Furthermore, we did not observe a negative impact of the vaccine on disease activity, which remained stable following the second dose, with no changes in treatment, except in 9% of the patients.
Our results are compatible with a recently published article that demonstrates an acceptable side-effect profile in adolescents with AIIRDs [30].
In addition, we found that BNT162b2 mRNA vaccine was immunogenic and induced a humoral immune response in adolescent patients at comparable rates to healthy controls (seropositivity rate of 97.3% vs 100%, respectively). Except for one patient with systemic scleroderma treated with MMF, all patients had anti-S1/S2 antibody titres above the cut-off level defined as seropositive. The observed seropositivity rates in our study are higher than those previously demonstrated for adults with AIIRD [21, 23] and might be explained by younger age, which is associated with better immunologic response to COVID-19 vaccines [3], shorter disease duration and medication use. However, similar to studies in adults with AIIRDs, the mean anti-spike S1/S2 IgG antibody level was significantly lower in adolescents with AIIRDs compared with controls.
When considering vaccine immunogenicity according to the immunomodulatory treatments used, this study highlights several differences between adolescent and adult patients. Our results regarding lower anti-S1/S2 antibody titres in patients treated with MMF suggest a trend towards reduced humoral response and are consistent with the immunogenicity of the COVID-19 mRNA vaccine described in adults with AIIRDs [21, 23] and in the solid organ transplant population [31]. However, the seropositivity rates for patients receiving MTX (either as monotherapy or combination treatment) in our juvenile-onset cohort were 100%; significantly higher than the 84% in a large adult rheumatology cohort [21]. Similarly, while anti-CD20 treatment was shown to significantly impair vaccine immunogenicity in adults, with a seropositivity rate of 39% [21], all three patients in our juvenile-onset cohort treated with anti-CD20 tested seropositive. These differences can be potentially attributed to patient age, with a more robust immune response in younger patients, as well as disease and treatment durations.
Factors that were shown to have the potential to impair humoral response to COVID-19 in adults with AIIRDs treated with rituximab are the interval from the last medication dose to the vaccine administration [21], dose/duration of therapy and the degree of B cell recovery at the time of vaccination [32]. This was recently shown to be the most predictive marker of humoral response to the COVID-19 vaccine, regardless of the dose or duration of rituximab treatment [32]. As the juvenile cohort is smaller than the adult cohort, more data are needed to confirm these observations, and information on cellular response could potentially assist in interpretation.
Importantly, our study addresses ‘vaccine hesitancy’ and concerns of patients and families regarding the safety and efficacy of COVID-19 vaccine. These concerns pose a barrier to successful vaccination in many communities. Our results should provide reassurance to physicians, patients and families with AIIRDs regarding the short-term safety and immunogenicity of the mRNA COVID-19 vaccine, even while on immunomodulatory therapy. Encouraging this unique population to vaccinate will probably protect them against COVID-19 and from potentially harmful COVID-19 effects, including an increased risk for viral-induced disease flare.
The strength of this study is the inclusion of a heterogeneous international cohort exposed to widely diverse immunomodulatory treatments. Moreover, a quantitative, standardized FDA-authorized COVID-19 serology assay was used for measurement of the serum IgG antibody levels against SARS-CoV-2 spike S1/S2 glycoproteins. It has clinical sensitivity and specificity >98%. The patients’ treating paediatric rheumatologists, who were all acquainted with their disease course, evaluated AIIRD activity and adverse events.
There are some limitations to this study. Due to the diversity of rheumatic diseases and medications included in this juvenile-onset cohort, it was not possible to draw significant conclusions regarding the impact of immunomodulatory medications and type of disease on the anti-S1/S2 titres. In addition, the matching by age was not optimal, as the control group was younger than the patient group, with mean ages of 14.0 vs 15.9 years, respectively. However, this might actually strengthen our findings of high seropositivity rates, as younger control children could have more robust immune activity.
It is important to note that currently there are limited data demonstrating correlations between anti-spike antibody levels and vaccine efficacy [33], therefore caution is advised when instructing patients how to conduct themselves following serology testing or the significance of the results. The significantly lower antibody levels in our juvenile-onset patient cohort and the uncertainty regarding the meaning of the antibody titres support the rationale to continue following these patients clinically and serologically as planned and to assess whether antibody titres correlate with clinical outcomes (vaccine efficacy). Ongoing collection of longitudinal data can also assist in planning the appropriate timing for vaccine booster doses, which might be needed after a shorter time span in adolescents with juvenile-onset AIIRDs. Neither patients nor controls developed COVID-19 during our short-term, 3-month follow-up, but the small numbers can only be descriptive and not conclusive regarding efficacy.
In conclusion, immunization is the most efficient intervention to control the COVID-19 pandemic. The current study provides evidence of good short-term vaccine safety and adequate humoral immune response in the unique population of adolescents with juvenile-onset AIIRDs. Nevertheless, further studies are needed to determine the safety of the vaccine over time, the duration of the vaccine-induced immune response and its actual correlation with COVID-19 prevention in our patients. Ideally, additional studies could focus on assessing the impact of certain rheumatic diseases or medications on the humoral and cellular immune response and explore the effects of the mRNA COVID-19 vaccine on a younger cohort of children with AIIRDs.
Acknowledgements
We thank the families and adolescents who participated in the study. We thank Yishai Friedlander for performing the statistical analysis. We thank Faye Schreiber for editing the manuscript. M.H.B. and Y.U. are responsible for the study conception. M.H.B. directed and coordinated the study. M.H.B., Y.U. and O.E. designed the study. M.H.B., A.Z., N.T., D.K., Y.U., Y.A.B. and E.S. recruited participants into the study. G.S. and O.S. performed the serology tests. S.P. helped with sample handling and coordination. A.K. helped with blood collection. M.H.B. wrote the manuscript, which was critically reviewed by Y.U., O.E., N.T., D.H., A.Z., Y.A.B., E.S. and DK.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
All the data relevant to this study are included in the manuscript, tables and figures. Additional data will be provided upon request.
References
- 1. Fact sheet for healthcare providers administrating vaccine emergency use authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) for 12 years of age and older dilute before use for 12 years of age and old. www.cvdvaccine.com.
- 2. Polack FP, Thomas SJ, Kitchin N. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frenck RW, Klein NP, Kitchin N. et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. First COVID-19 vaccine approved for children aged 12 to 15 in EU. https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu (3 January 2022, date last accessed).
- 5. FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 Years of Age. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (1 January 2022, date last accessed).
- 6. Woodworth KR, Moulia D, Collins JP. et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years — United States, November 2021. MMWR Morb Mort Weekly Rep 2021;70:1579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liguoro I, Pilotto C, Bonanni M. et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 2020;179:1029–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsabouri S, Makis A, Kosmeri C, Siomou E.. Risk factors for severity in children with coronavirus disease 2019: a comprehensive literature review. Pediatr Clin North Am 2021;68:321–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belhadjer Z, Méot M, Bajolle F. et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020;142:429–36. [DOI] [PubMed] [Google Scholar]
- 10. Chao JY, Derespina KR, Herold BC. et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr 2020;223:14–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeBiasi RL, Song X, Delaney M. et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, Metropolitan Region. J Pediatr 2020;223:199–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Son MBF, Murray N, Friedman K. et al. Multisystem inflammatory syndrome in children – initial therapy and outcomes. N Eng J Med 2021;385:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feldstein LR, Tenforde MW, Friedman KG. et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021;325:1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buonsenso D, Munblit D, de Rose C. et al. Preliminary evidence on long COVID in children. Acta Paediatr 2021;110:2208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rumain B, Schneiderman M, Geliebter A.. Prevalence of COVID-19 in adolescents and youth compared with older adults in states experiencing surges. PLoS One 2021;16:e0242587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szablewski CM, Chang KT, Brown MM. et al. SARS-CoV-2 transmission and infection among attendees of an overnight camp — Georgia, June 2020. MMWR Morb Mort Weekly Rep 2020;69:1023–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kearsley-Fleet L, Lawson-Tovey S, Costello RE. et al. Outcomes of COVID-19 infection among children and young people with pre-existing rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:872–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hügle B, Krumrey-Langkammerer M, Haas JP.. Infection with SARS-CoV-2 causes flares in patients with juvenile idiopathic arthritis in remission or inactive disease on medication. Pediatr Rheumatol 2021;19:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curtis JR, Johnson SR, Anthony DD. et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021;73:e60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paediatric Rheumatology European Association. Guidelines and Recommendations. PRES update regarding COVID-19 vaccination in children with rheumatic diseases. https://www.pres.eu/clinical-affairs/guidelines.html (27 December 2021, date last accessed).
- 21. Furer V, Eviatar T, Zisman D. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 22. Li X, Tong X, Yeung WWY. et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis 2021;81:564–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braun-Moscovici Y, Kaplan M, Braun M. et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021;80:1317–21. [DOI] [PubMed] [Google Scholar]
- 24. Petty RE, Southwood TR, Manners P. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 25. Petri M, Orbai AM, Alarcõn GS. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jennette JC, Falk RJ, Andrassy K. et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994;37:187–92. [DOI] [PubMed] [Google Scholar]
- 27. Lundberg IE, Tjärnlund A, Bottai M. et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Consolaro A, Ruperto N, Bazso A. et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. [DOI] [PubMed] [Google Scholar]
- 29. Criscuolo E, Diotti RA, Strollo M. et al. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J Med Virol 2021;93:2160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haslak F, Gunalp A, Cebi MN. et al. Early experience of COVID-19 vaccine-related adverse events among adolescents and young adults with rheumatic diseases: a single-center study. Int J Rheum Dis 2022; doi: 10.1111/1756-185X.14279. [DOI] [PubMed] [Google Scholar]
- 31. Boyarsky BJ, Werbel WA, Avery RK. et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021;325:1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Floyd L, Elsayed ME, Seibt T. et al. SARS-CoV-2 vaccine response in patients with antineutrophil cytoplasmic autoantibody–associated vasculitis. Kidney Int Rep 2021;2022–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilbert PB, Montefiori DC, McDermott AB. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022;375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data relevant to this study are included in the manuscript, tables and figures. Additional data will be provided upon request.