Abstract
Background
Most studies of solid organ transplant (SOT) recipients with coronavirus disease 2019 (COVID-19) focus on outcomes within 1 month of illness onset. Delayed mortality in SOT recipients hospitalized for COVID-19 has not been fully examined.
Methods
We used data from a multicenter registry to calculate mortality by 90 days following initial acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection in SOT recipients hospitalized for COVID-19 and developed multivariable Cox proportional hazards models to compare risk factors for death by days 28 and 90.
Results
Vital status at day 90 was available for 936 of 1117 (84%) SOT recipients hospitalized for COVID-19; 190 of 936 (20%) died by 28 days, and an additional 56 of 246 deaths (23%) occurred between days 29 and 90. Factors associated with mortality by day 90 included age >65 years (adjusted hazard ratio [aHR], 1.8 [1.3–2.4]; P <.001), lung transplant (vs nonlung transplant; aHR, 1.5 [1.0–2.3]; P = .05), heart failure (aHR, 1.9 [1.2–2.9]; P = .006), chronic lung disease (aHR, 2.3 [1.5–3.6]; P < .001), and body mass index ≥30 kg/m2 (aHR, 1.5 [1.1–2.0]; P = .02). These associations were similar for mortality by day 28. Compared with diagnosis during early 2020 (1 March 2020–19 June 2020), diagnosis during late 2020 (20 June 2020–31 December 2020) was associated with lower mortality by day 28 (aHR, 0.7 [0.5–1.0]; P = .04) but not by day 90 (aHR, 0.9 [0.7–1.3]; P = .61).
Conclusions
In SOT recipients hospitalized for COVID-19, >20% of deaths occurred between 28 and 90 days following SARS-CoV-2 diagnosis. Future investigations should consider extending follow-up duration to 90 days for more complete mortality assessment.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been a global pandemic since March 2020. Many observational studies and randomized clinical trials to measure COVID-19 mortality have used ≤1 month follow-up periods to assess mortality [1–7]. However, a substantial number of patients remain alive but critically ill with continued risk for imminent death beyond 28 days after diagnosis, and some patients who appear to have recovered from COVID-19 experience worsening of symptoms later in their disease course [1]. In the general population, 15%–25% of all deaths in patients hospitalized for COVID-19 during the first 90 days of illness occur after day 30 [8–11]. Thus, mortality measured at 90 days, rather than 28 or 30 days, may provide a more comprehensive understanding of COVID-19–associated mortality.
Solid organ transplant (SOT) recipients are at risk for severe COVID-19 [4, 12, 13]. While short-term mortality is well characterized in SOT recipients with COVID-19, few investigations have focused on outcomes beyond 1 month. Capturing delayed mortality is particularly relevant in SOT recipients, as experience with the solid organ transplant process’ emphasis on survival may lead to greater pursuit of life-prolonging interventions during critical illness [14]. SOT recipients who receive chronic immunosuppressive therapies and/or certain COVID-19 therapies, such as high-dose corticosteroids, may be at higher risk for secondary opportunistic infections and associated late mortality [15]. We previously described outcomes in the first 28 days following SARS-CoV-2 diagnosis among SOT recipients enrolled in a multicenter registry [12, 16–18]. We used additional data from this registry to calculate mortality by 90 days among SOT recipients hospitalized for COVID-19 and to determine whether risk factors for death by 28 days are also predictive of death by 90 days.
METHODS
Study Design and Participants
We performed a multicenter, observational, cohort study of SOT recipients with laboratory-confirmed SARS-CoV-2 infection who were hospitalized for COVID-19 within 28 days of diagnosis and for whom vital status at day 28 was available, as previously described [12, 16, 17]. Patients were excluded if they were hospitalized for another indication prior to or concurrent with their first positive SARS-CoV-2 test. Patients who survived to day 28 were either followed through day 90 (last live encounter ≥90 days from diagnosis or documented day of death between days 29 and 90) or considered lost to follow-up. The University of Washington Institutional Review Board (IRB) approved this study with a waiver of informed consent. The University of Washington IRB issued a “no human subjects research” designation for local sites that contributed deidentified patient data. Individual sites sought local IRB approval as needed.
Data Collection
We invited transplant providers to submit deidentified data regarding SOT recipients with SARS-CoV-2 infections to REDCap (Research Electronic Data Capture), a secure, web-based data capture software program housed at the University of Washington [19]. Contributors were recruited from discussion boards of the American Society of Transplantation and International Society for Heart and Lung Transplantation and asked to complete 3 electronic surveys: an initial form at time of first positive SARS-CoV-2 test that included medical history and details regarding clinical presentation, a 28-day follow-up form with information on the clinical course during the first 28 days following first positive SARS-CoV-2 test, and a 90-day follow-up form with information on patients’ vital status at 90 days. On each follow-up form, the contributor was asked to report the number of days between first positive SARS-CoV-2 test (day 0) and most recent healthcare contact. For patients who died, contributors were asked to report the number of days between first positive SARS-CoV-2 test and death. The registry was open to new cases diagnosed between 1 March 2020 and 31 December 2020 and remained open to follow-up reports through 19 April 2021. Completed case report forms were reviewed by the study team, and inconsistencies were adjudicated via communication with the contributor whenever possible.
Statistical Analyses
Demographic and baseline characteristics were assessed as counts and percentages for categorical values and as a median (interquartile range) for continuous variables. We calculated the mortality by 90 days among all patients for whom vital status at day 90 was available. To account for the potential impact of patients who were lost to follow-up after day 28 and whose vital status at 90 days was unknown, we performed a sensitivity analysis to calculate the range of possible mortality values for the entire cohort of SOT recipients hospitalized for COVID-19. The minimum value of this range assumed that 0% of the patients lost to follow-up died before day 90, and the maximum value of this range assumed that 100% of the patients lost to follow-up died before day 90.
We used univariable and multivariable Cox proportional hazards models to assess associations between selected covariates and death by 28 and 90 days following first positive SARS-CoV-2 test. Patients were censored at last live encounter if lost to follow-up before 90 days. Specific covariates were selected based on hypotheses and prior publications indicating associations with worse outcomes in SOT recipients hospitalized for COVID-19 [12, 16, 17]. Covariates with a P value < .05 in univariable analysis for 28-day mortality were included in multivariable models. Time period of diagnosis was classified as a dichotomous variable, early 2020 (1 March 2020–19 June 2020) and late 2020 (20 June 2020–31 December 2020), because the time cutoff in June 2020 closely coincided with a paradigm shift in the care of patients with COVID-19 in response to evidence supporting use of corticosteroids for patients who required supplemental oxygen and the emergency use authorization of remdesivir in the United States [17, 20–22]. Most data were complete; multiple imputations by chained equations were used to account for missing covariates in multivariable analyses [23]. Stata version 16.1 (StataCorp, College Station, TX) and R version 4.0.2 were used to perform the statistical analyses. Figures were created using R version 4.0.2 and Excel (Microsoft, Redmond, WA).
RESULTS
Study Population
Of 1702 SOT recipients with SARS-CoV-2 infections, 1141 (67%) were hospitalized for COVID-19 within 28 days after first positive SARS-CoV-2 test; 1117 of 1141 (98%) were followed through death or day 28 and therefore included in the analyses (Supplementary Figure 1). There were 694 (62%) men, and the median age was 59 years (interquartile range, 49–67). A total of 368 (34%) had undergone transplantation within 2 years of SARS-CoV-2 infection; 563 (50%) had diabetes mellitus, 69 (6%) had heart failure, and 74 (7%) had chronic lung disease. There were 693 (62%) kidney recipients, 147 (14%) liver recipients, 137 (12%) heart recipients, and 127 (11%) lung recipients. First positive SARS-CoV-2 test occurred during early 2020 in 593 (53%) patients. Of patients with available laboratory and imaging results at presentation, lymphopenia was present in 325 (32%) and chest imaging was abnormal in 835 (82%), respectively. One hundred and ninety (17%) were deceased and 927 (83%) were alive at day 28. Among 927 who survived to day 28, 746 (80%) were followed through death or day 90, while 181 (20%) were lost to follow-up. Overall, characteristics of patients followed through death or day 90 were similar to those lost to follow-up (Supplementary Table 1, Supplementary Materials). Vital status at day 90 was available for 936 patients (190 who died within 28 days and 746 who survived beyond 28 days).
Calculated Mortality in the First 90 Days Following First Positive SARS-CoV-2 Test
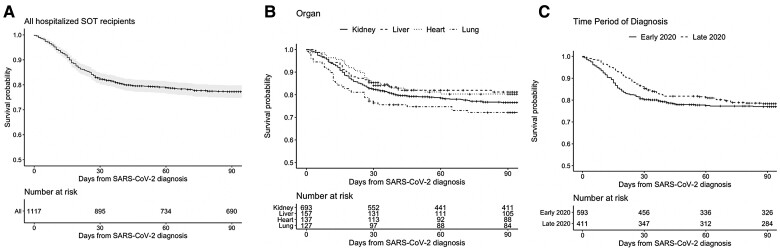
Among 936 hospitalized SOT recipients whose vital status at day 90 was confirmed, 246 (26.3%) died within the first 90 days following diagnosis. Fifty-six of 746 (7.5%) patients who survived to day 28 died by day 90. In a sensitivity analysis that included the entire cohort of 1117 SOT recipients, the minimum possible mortality by 90 days was 22% and the maximum possible mortality was 38% (Table 2). Figure 1 shows unadjusted survival curves during the first 90 days following first positive SARS-CoV-2 test in all 1117 hospitalized SOT recipients (Figure 1A) and stratified by organ (Figure 1B) and time period of diagnosis (Figure 1C).
Table 2.
Mortality by 90 Days Following First Positive Severe Acute Respiratory Syndrome Coronavirus 2 Test in Solid Organ Transplant Recipients Hospitalized for Coronavirus Disease 2019
| Patients With Known Status Vital Status at Day 90 (n = 936) | Entire Cohort (n = 1117) | |
|---|---|---|
| Day 0–28 | 190 (20.3%) | 190 (17.0%) |
| Day 29–90 | 56 (6.0%) | 56–237 (5.0%–21.2%)a |
| Overall mortality (day 0–90) | 246 (26.3%) | 246–427 (22.0%–38.0%)a |
Range includes the minimum and maximum possible value for mortality in the entire cohort. In sensitivity analysis, minimum mortality estimate assumes 0% mortality among patients lost to follow-up and the maximum mortality assumes 100% mortality among patients lost to follow-up.
Figure 1.
Unadjusted Kaplan-Meier survival curves in SOT recipients hospitalized for coronavirus disease 2019 (COVID-19). Unadjusted Kaplan-Meier survival curves for hospitalized SOT recipients (A) and stratified by organ (B) and time period of diagnosis: early 2020 (before 20 June 2020) and late 2020 (on or after 20 June 2020) (C). Gray shading represents 95% confidence intervals. Vertical marks represent patients censored at time of last live encounter. Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant.
Table 1.
Characteristics of Solid Organ Transplant Recipients Hospitalized for Coronavirus Disease 2019
| Characteristic, n (%) | Followed Through Day 28 (n = 1117) | Followed Through Day 90 (n = 936) |
|---|---|---|
| Men | 694 (62.2) | 581 (62.1) |
| Age >65 years | 343 (30.7) | 290 (31.0) |
| Median age (interquartile range), years | 59 (49–67) | 60 (49–67) |
| Black race | 370 (34.8) | 287 (32.0) |
| Hispanic or Latinx ethnicity | 338 (31.3) | 298 (33.0) |
| Transplant within 2 years | 368 (33.8) | 323 (35.4) |
| Organa | ||
| Kidney | 693 (62.0) | 567 (60.6) |
| Liver | 157 (14.1) | 134 (14.3) |
| Heart | 137 (12.3) | 114 (12.2) |
| Lung | 127 (11.4) | 119 (12.7) |
| Other | 3 (0.3) | 2 (0.21) |
| Comorbidities | ||
| Diabetes mellitus | 563 (50.4) | 475 (50.8) |
| Body mass index ≥30 kg/m2 | 382 (35.8) | 318 (35.7) |
| Chronic kidney disease or end-stage renal disease | 406 (36.4) | 325 (35.7) |
| Heart failure | 69 (6.2) | 57 (6.1) |
| Chronic lung disease | 74 (6.6) | 61 (6.5) |
| Baseline immunosuppression | ||
| Any CNI | 1024 (91.7) | 850 (90.8) |
| Any antimetabolite | 836 (74.8) | 709 (75.8) |
| Any corticosteroid | 818 (73.2) | 682 (72.9) |
| CNI, antimetabolite, steroids | 589 (52.7) | 498 (53.2) |
| Any mammalian target of rapamycin inhibitor | 65 (5.8) | 54 (5.8) |
| Recent intensive immunosuppressionb | 67 (6.0) | 63 (6.8) |
| Time period of diagnosis | ||
| Early 2020 (before 20 June 2020) | 593 (53.1) | 458 (48.9) |
| Late 2020 (on or after 20 June 2020) | 411 (36.8) | 371 (39.6) |
| Unknown | 113 (10.1) | 107 (11.4) |
| Surrogate markers of illness severity at presentation | ||
| Abnormal chest imagingc | 835 (82.3) | 689 (81.9) |
| Lymphopenia (absolute lymphocyte count <0.5 × 109/L) | 325 (31.5) | 275 (32.3) |
Among the 1117 patients followed through day 28: sex was missing for 1; race was missing for 54; ethnicity was missing for 38; year of most recent transplant was missing for 28; body mass index (BMI) was missing for 50; and chest imaging was not performed for 102. Presenting absolute lymphocyte count was missing for 85 patients. Among the 936 patients followed through day 90: sex was missing for 1; race was missing for 38; ethnicity was missing for 27; year of most recent transplant was missing for 22; BMI was missing for 46; time period of diagnosis was missing for 107; chest imaging was missing or not performed for 95; and absolute lymphocyte count at presentation was missing for 80. Patient race and ethnicity were collected as distinct variables and reported by the contributor based on observations or documentation in the electronic medical record. Race and ethnicity were collected as part of the registry to characterize the study population.
Abbreviation: CNI, calcineurin inhibitor.
Among the 1117 patients followed through day 28: kidney includes 19 kidney–pancreas recipients; liver includes 34 liver–kidney recipients, 1 liver–pancreas–small bowel recipient, and 1 liver–kidney–small bowel–stomach recipient; heart includes 15 heart–kidney recipients and 1 heart–kidney–small bowel recipient; lung includes 3 heart–lung, 1 lung–liver recipients, and 1 lung–kidney–small bowel recipient; other includes 2 small bowel and 1 vascular composite allograft recipients. Among the 926 patients followed through day 90: kidney includes 15 kidney–pancreas recipients; liver includes 23 liver–kidney recipients, 1 liver–pancreas–small bowel recipient, and 1 liver–pancreas–small bowel–stomach recipient; heart includes 14 heart–kidney recipients and 1 heart–kidney–small bowel recipient; lung includes 2 heart–lung recipients, 1 lung–liver recipient, and 1 lung–kidney–islet cell recipient.
Recent intensive immunosuppression refers to agents with significant T-cell, B-cell, or immunoglobulin depleting activity given within the 3 months prior to severe acute respiratory syndrome coronavirus 2 diagnosis. For the 1117 patients followed through day 28, agents included antithymocyte globulin (n = 37); alemtuzumab (n = 2); basiliximab (n = 17); pulse steroids, defined as equivalent of ≥500 mg methylprednisolone for ≥3 days (n = 24); rituximab (n = 4); plasmapheresis (n = 1); and bortezomib (n = 1). Among the 936 patients followed through day 90, agents included antithymocyte globulin (n = 34); alemtuzumab (n = 2); basiliximab (n = 17); pulse steroids, defined as equivalent of ≥500 mg methylprednisolone for ≥3 days (n = 21); rituximab (n = 4); plasmapheresis (n = 1); and bortezomib (n = 1).
Abnormal chest imaging findings on X-ray or computed tomography included lobar consolidation, multifocal or patchy opacities, including ground glass, interstitial abnormalities, or other findings deemed clinically significant by the contributing provider.
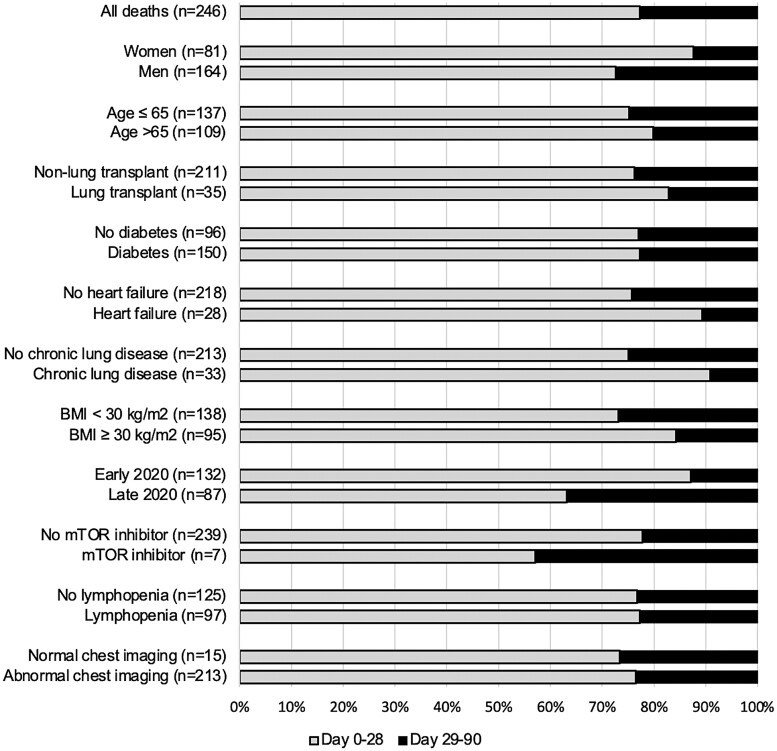
Of all 246 deaths within the first 90 days, 56 (23%) occurred beyond day 28 (Figure 2). Deaths after day 28 were more likely to occur in men (45 of 164, 27%) than in women (10 of 81, 12%) and more likely to occur in patients diagnosed in late 2020 (32 of 87, 37%) than in patients diagnosed in early 2020 (17 of 132, 13%; Supplementary Table 2, Supplementary Materials).
Figure 2.
Proportion of deaths before and after day 28 in solid organ transplant (SOT) recipients hospitalized for coronavirus disease 2019 (COVID-19). Covariates of interest (total number of deaths between days 0 and 90) and proportion of deaths that occurred between days 0 and 28 (gray) and days 29 and 90 (black) in SOT recipients hospitalized for COVID-19. Abbreviations: BMI, body mass index; mTOR, mammalian target of rapamycin.
Risk Factors for Mortality Within 90 Days of First Positive SARS-CoV-2 Test
Older age, comorbidities (heart failure, chronic lung disease, and body mass index ≥30 kg/m2), lung transplant (vs nonlung transplant), and factors associated with severe disease at presentation (lymphopenia and abnormal chest imaging) were associated with increased mortality by 28 days and 90 days in univariable (Table 3) and multivariable Cox proportional hazards models (Table 4). Compared with diagnosis in early 2020, diagnosis in late 2020 was associated with lower mortality at 28 days (adjusted hazard ratio [aHR], 0.7; 95% confidence interval [CI], .5–1.0; P = .04) but not at 90 days (aHR, 0.9; 95% CI, .7–1.3; P = .61). Similarly, mammalian target of rapamycin (mTOR) inhibitor use was associated with significantly lower mortality by 28 days (aHR, 0.3; 95% CI, .1–1.0; P = .05), but the association was weaker by 90 days (aHR, 0.5; 95% CI, .2–1.1; P = .07).
Table 3.
Risk Factors for Mortality by Day 28 and Day 90 in Solid Organ Transplant Recipients Hospitalized for Coronavirus Disease 2019, Univariable Cox Proportional Hazards (N = 1117)
| Covariate | Mortality by Day 28 | Mortality by Day 90 |
|---|---|---|
| Hazard Ratio (95% Confidence Interval), P Value | ||
| Men | 1.0 (.76–1.4), .91 | 1.2 (.9–1.6), .12 |
| Age >65 years | 2.1 (1.6–2.7), <.001 | 2.0 (1.6–2.6), <.001 |
| Transplant within 2 years | 0.76 (.55–1.0), .09 | 1.0 (.7–1.3), .81 |
| Lung transplant (vs nonlung) | 1.5 (1.0–2.2), .047 | 1.4 (1.0–1.9), .10 |
| Comorbidities | ||
| Hypertension | 1.3 (.9–1.9), .14 | 1.4 (1.0–1.9), .04 |
| Diabetes mellitus | 1.6 (1.2–2.2), .01 | 1.6 (1.3–2.1), <.001 |
| Body mass index ≥30 kg/m2 | 1.5 (1.1–2.0), .01 | 1.3 (1.0–1.7), .06 |
| Chronic kidney disease/end stage renal disease | 1.3 (.98–1.8), .06 | 1.2 (.9–1.5), .24 |
| Heart failure | 2.8 (1.8–4.2), <.001 | 2.5 (1.7–3.6), <.001 |
| Chronic lung disease | 3.4 (2.3–4.9), <.001 | 2.9 (2.0–4.2), <.001 |
| Baseline immunosuppression | ||
| Any CNI | 1.1 (.6–1.8), .76 | 1.0 (.65–1.6), .96 |
| Any antimetabolite | 0.87(.6–1.2), .40 | 0.9 (.7–1.2), .56 |
| Any corticosteroid | 1.1 (.8–1.5), .58 | 1.1 (.8–1.4), .64 |
| CNI, ant-metabolite, steroids | 0.98 (.7–1.3), .87 | 1.0 (.8–1.2), .75 |
| Any mammalian target of rapamycin inhibitor | 0.3 (.1–.9), .03 | 0.4 (.2–.9), .03 |
| Recent intensive immunosuppression | 0.5 (.2–1.1), .09 | 0.9 (.5–1.5), .65 |
| Time period of diagnosis | ||
| Late 2020 (on or after 20 June 2020) | 0.7 (.5–.9), .01 | 0.9 (.7–1.1), .29 |
| Markers of illness severity at presentation | ||
| Abnormal chest imaging | 3.5 (1.9–6.4), <.001 | 3.4 (2.0–5.8), <.001 |
| Lymphopenia (absolute lymphocyte count <0.5 × 109/L) | 1.8 (1.3–2.4), <.001 | 1.8 (1.4–2.4), <.001 |
Abbreviation: CNI, calcineurin inhibitor.
Statistically significant values (P ≤ .05) are shown in bold.
Table 4.
Risk Factors for Mortality by Day 28 and Day 90 in Solid Organ Transplant Recipients Hospitalized for Coronavirus Disease 2019, Multivariable Cox Proportional Hazards (N = 1117)
| Covariate | Mortality by 28 Days | Mortality by 90 Days |
|---|---|---|
| Adjusted Hazard Ratio (95% Confidence Interval), P Value | ||
| Age >65 years | 1.8 (1.3–2.6), <.001 | 1.8 (1.3–2.4), <.001 |
| Lung transplant (vs nonlung transplant) | 1.8 (1.1–2.8), .02 | 1.5 (1.0–2.3), .05 |
| Comorbidities a | ||
| Diabetes mellitus | 1.2 (.8–1.7), .34 | 1.3 (1.0–1.8), .09 |
| Heart failure | 1.9 (1.2–3.1), .01 | 1.9 (1.2–2.9), .006 |
| Chronic lung disease | 2.4 (1.5–3.7), <.001 | 2.3 (1.5–3.6), <.001 |
| Body mass index ≥30 kg/m2 | 1.6 (1.1–2.2), .01 | 1.5 (1.1–2.0), .02 |
| Baseline mammalian target of rapamycin inhibitor use | 0.3 (.1–1.0), .05 | 0.5 (.2–1.1), .07 |
| Late 2020 (on or after 20 June 2020) | 0.7 (.5–1.0), .04 | 0.9 (.7–1.3), .61 |
| Lymphopenia (absolute lymphocyte count <0.5 × 109/L) at presentation | 1.8 (1.3–2.6), <.001 | 1.8 (1.3–2.4), <.001 |
| Abnormal chest imaging at presentation | 2.9 (1.5–5.5), .002 | 2.7 (1.6–4.7), <.001 |
Statistically significant values (P ≤ .05) are shown in bold.
DISCUSSION
This large, multicenter study with standardized 90-day follow-up identified the frequency of and risk factors for delayed mortality in SOT recipients hospitalized for COVID-19. Approximately 8% of patients who survived to day 28 following initial positive SARS-CoV-2 test died between days 29 and 90, and more than 20% of deaths within the first 90 days occurred beyond day 28. These data emphasize the importance of extended follow-up in measurements of COVID-19–associated mortality.
Most large observational studies of SOT recipients with COVID-19 assessed mortality within 30 days of diagnosis, did not use a standardized follow-up period, or did not report median follow-up duration [4, 12, 13, 24]. Investigations with limited follow-up periods facilitated rapid reporting early in the pandemic, but mortality estimates based on short or variable follow-up periods are challenging to interpret. In the general population, 90-day mortality in patients hospitalized for COVID-19 ranges from 25% to 30%, with 15% to 25% of all deaths occurring after day 30 [8–11]. In a study of 1680 kidney transplant recipients (65% of whom were hospitalized), mortality at day 90 was 21%, and 22% of all deaths occurred more than 30 days after diagnosis [25]. Another investigation of 707 SOT recipients and 707 propensity-matched non-SOT controls hospitalized for COVID-19 followed patients for 60 days and found that 17% of all deaths occurred beyond day 30 in both SOT and non-SOT patients [26]. Likewise, in a randomized, controlled trial of baricitinib in the general population, 16% of all deaths occurred between 28 and 60 days after randomization [27]. Findings from our study, which focused on recipients of any solid organ who required hospitalization for COVID-19, highlight the significant proportion of patients who die beyond the initial month of observation.
The relatively high proportion of deaths after day 28 in both SOT and non-SOT populations may reflect unique aspects of COVID-19. Compared with patients hospitalized with influenza, patients hospitalized with COVID-19 have significantly longer lengths of stay and are more likely to develop complications of prolonged illness such as catheter-related infections, pressure ulcers, and venous thromboemboli [28, 29]. Thus, fatal and nonfatal sequelae of COVID-19 may manifest relatively late, and prolonged follow-up may be more important in COVID-19–focused studies compared with investigations of other infections.
Most comorbidities identified as risk factors for mortality during the first 28 days were also risk factors for death by 90 days. Diagnosis during late 2020 was associated with lower mortality by day 28 but not by day 90. Such temporal trends could suggest that advances in the care of COVID-19 patients only delay mortality by a matter of days to weeks, although changes in COVID-19 management cannot be causally linked to lower 28-day mortality. Notably, loss to follow-up after day 28 was more common during early 2020 than late 2020, and failure to capture late deaths in patients diagnosed during the early period may have led to the apparent elevation in mortality in the late period.
Critically ill SOT recipients may be less likely to receive recommendations for comfort-focused treatment as a result of the transplant process’ emphasis on recipient survival, potentially delaying mortality compared with non-SOT patients [14, 30–32]. Our findings, in conjunction with those from previous reports, suggest that the delayed mortality in patients hospitalized for COVID-19 is comparable in SOT and non-SOT populations [8–11, 25, 26]. The impact of transplant status on decisions regarding life-sustaining treatments is most pronounced early after transplant [31]. Most patients in this study underwent transplant more than 2 years before SARS-CoV-2 diagnosis, which may have limited detection of any associations between transplant status and time to death.
The reduced mortality seen in SOT recipients taking mTOR inhibitors prior to SARS-CoV-2 infection is intriguing. Multiple potential advantageous mechanisms of mTOR inhibition in COVID-19 have been proposed, including direct inhibition of viral replication and mitigation of cytokine release [33]. However, these mechanisms are speculative and not necessarily indicative of clinical benefits. Observational data from this study’s populations, among whom mTOR inhibitor use was sparse, need to be interpreted with caution.
This study’s size, inclusion of both kidney and nonkidney SOT recipients from multiple centers, and standardized follow-up duration are important strengths of this investigation. However, we acknowledge several potential limitations. Twenty percent of patients who survived to day 28 were lost to follow-up prior to day 90. Because loss to follow-up beyond day 28 may preclude capture of later deaths (or documentation of prolonged survival), any major differences in loss to follow-up by covariate category pose a potential threat to the accuracy of reported associations. Importantly, prevalence of older age and comorbidities, the strongest predictors of death in all subgroups at both 28 and 90 days, were similar between patients lost to follow-up and those followed through death or day 90. Thus, loss to follow-up was unlikely to have had major effects on the results of multivariable analyses. This study took place prior to the advent of SARS-CoV-2 vaccines, which dramatically reduce mortality from COVID-19 in SOT recipients and the general population [34–37]. Nonetheless, findings from this study remain relevant as serious infections despite vaccination may occur in SOT recipients, and emergence of SARS-CoV-2 variants that escape vaccine-induced immunity is an ongoing concern [38, 39]. We were unable to capture granular data on management strategies and indications for hospitalization among participating centers. Despite potential limitations of heterogeneity in COVID-19 management, the multicenter nature of the study provides a valuable summary of COVID-19 mortality in a broad range of clinical contexts. Additional restrictions to the study design, which was intended to rapidly collect data in a high-risk population during a worldwide pandemic, have been described elsewhere [12, 16, 17].
In this large multicenter study of SOT recipients hospitalized for COVID-19, a substantial proportion of deaths in the first 90 days following SARS-CoV-2 diagnosis occurred after the 28-day follow-up period that is frequently used when assessing mortality. Older age, comorbidities, and lung transplantation remained important predictors of both 28-day and 90-day mortality. Future investigations focusing on COVID-19 mortality should consider extending follow-up duration to 90 days for more accurate capture of mortality and associated risk factors.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Following are the members of the UW COVID-19 SOT Study Team, without whom this work would not have been possible: Behdad D. Besharatian, Maria Crespo, Rade Tomic, Sameep Sehgal, Dana Weisshaar, Reda Girgis, Cameron Lawrence, Joanna Nelson, William Bennett, Jennifer Leandro, Afrah Sait, Amy Rumore, Patricia West, Amy Jeng, Valida Bajrovic, Erin P. Bilgili, Tracy Anderson-Haag, Abigail Nastase, Abbas Badami, Jesus Alvarez-Garcia, Lyndsey Bowman-Anger, Lovelyn Julien, Carlos Ortiz-Bautista, Rachel Friedman-Morocco, Kiran Gajurel, Lizbeth Cahuayme-Zuniga, Mark Wakefield, Monica Fung, Nicole Theodoropoulos, Sally T. Chuang, Srividya Bhandaram, Massimiliano Veroux, Bhavna Chopra, Diana Florescu, Danielle Witteck, Daniela Diaz, Kathryn Ripley, Kapil Saharia, Sanjeev Akkina, Todd P. McCarty, Ally Webb, Akanksha Arya, Giridhar Vedula, Jose-Marie El-Amm, M. Katherine Dokus, Arun Narayanan, Priscila Cilene Leon Bueno de Camargo, Rosemary Ouseph, Andrew Breuckner, Alfred Luk, Avinash Aujayeb, Daniel Ganger, Douglas S. Keith, Federica Meloni, Ghady Haidar, Lori Zapernick, Megan Morales, Nitender Goyal, Tanvi Sharma, Uma Malhotra, Alexander Kuo, Ana P. Rossi, Angelina Edwards, Brian Keller, Christy Beneri, Darby Derringer, Edward Dominguez, Elise Carlson, Faris Hashim, Haris Murad, Heinrike Wilkens, Henry Neumann, Imran Gani, Joseph Kahwaji, Joyce Popoola, Marian Michaels, Niyati Jakharia, Oveimar De la Cruz, Alfredo Puing, Reza Motallebzadeh, Ravi Velagapudi, Rajan Kapoor, Sridhar Allam, Fernanda Silveira, Surabhi Vora, Ursala M. Kelly, Uttam Reddy, Vikas Dharnidharka, Hani Wadei, and Lominadze Zurabi.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID; T32AI118690 to M. R. H. and O. S. K.) and the National Heart, Lung, and Blood Institute (HL143050 to C. E. F.) at the NIH. REDCap (Research Electronic Data Capture) at the Institute of Translational Health Sciences at the University of Washington is supported by the National Center for Advancing Translational Sciences of the NIH (UL1 TR002319).
Data availability statement. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Contributor Information
Madeleine R Heldman, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
Olivia S Kates, Division of Infectious Diseases, Johns Hopkins University, Baltimore, Maryland, USA.
Kassem Safa, Massachusetts General Hospital, Boston, Massachusetts, USA.
Camille N Kotton, Massachusetts General Hospital, Boston, Massachusetts, USA.
Ashrit Multani, Department of Medicine, David Geffen School of Medicine at the University of California–Los Angeles, Los Angeles, California, USA.
Sarah J Georgia, Massachusetts General Hospital, Boston, Massachusetts, USA.
Julie M Steinbrink, Division of Infectious Diseases, Duke University, Durham, North Carolina, USA.
Barbara D Alexander, Division of Infectious Diseases, Duke University, Durham, North Carolina, USA.
Emily A Blumberg, Division of Infectious Diseases, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Brandy Haydel, Recanati/Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Vagish Hemmige, Division of Infectious Disease, Albert Einstein College of Medicine/Montefiore Medical Center, Bronx, New York, USA.
Marion Hemmersbach-Miller, Section of Infectious Diseases, Baylor College of Medicine, Houston, Texas, USA.
Ricardo M La Hoz, Division of Infectious Diseases and Geographic Medicine, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Lisset Moni, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Yesabeli Condor, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Sandra Flores, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Carlos G Munoz, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Juan Guitierrez, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Esther I Diaz, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Daniela Diaz, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Rodrigo Vianna, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Giselle Guerra, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Matthias Loebe, University of Miami/Jackson Memorial Hospital, Miami, Florida, USA.
Julie M Yabu, Department of Medicine, David Geffen School of Medicine at the University of California–Los Angeles, Los Angeles, California, USA.
Kailey Hughes Kramer, Transplant Infectious Diseases, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Sajal D Tanna, Division of Infectious Diseases, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Michael G Ison, Division of Infectious Diseases, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Robert M Rakita, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Maricar Malinis, Section of Infectious Diseases, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Marwan M Azar, Section of Infectious Diseases, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Margaret E McCort, Recanati/Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Pooja P Singh, Division of Nephrology, University of New Mexico, Albuquerque, New Mexico, USA.
Arzu Velioglu, Marmara University, School of Medicine, Department of Internal Medicine, Division of Nephrology, Istanbul, Turkey.
Sapna A Mehta, New York University Langone Transplant Institute, New York, New York, USA.
David van Duin, Division of Infectious Diseases, University of North Carolina, Chapel Hill, North Carolina, USA.
Jason D Goldman, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Swedish Medical Center, Seattle, Washington, USA.
Erika D Lease, Division of Pulmonology, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, Washington, USA.
Anna Wald, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA; Department of Epidemiology, University of Washington, Seattle, Washington, USA; Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Ajit P Limaye, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Cynthia E Fisher, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
UW Covid-19 SOT Study Team:
Behdad D Besharatian, Maria Crespo, Rade Tomic, Sameep Sehgal, Dana Weisshaar, Reda Girgis, Cameron Lawrence, Joanna Nelson, William Bennett, Jennifer Leandro, Afrah Sait, Amy Rumore, Patricia West, Amy Jeng, Valida Bajrovic, Erin P Bilgili, Tracy Anderson-Haag, Abigail Nastase, Abbas Badami, Jesus Alvarez-Garcia, Lyndsey Bowman-Anger, Lovelyn Julien, Carlos Ortiz-Bautista, Rachel Friedman-Morocco, Kiran Gajurel, Lizbeth Cahuayme-Zuniga, Mark Wakefield, Monica Fung, Nicole Theodoropoulos, Sally T Chuang, Srividya Bhandaram, Massimiliano Veroux, Bhavna Chopra, Diana Florescu, Danielle Witteck, Daniela Diaz, Kathryn Ripley, Kapil Saharia, Sanjeev Akkina, Todd P McCarty, Ally Webb, Akanksha Arya, Giridhar Vedula, Jose-Marie El-Amm, M Katherine Dokus, Arun Narayanan, Priscila Cilene Leon Bueno de Camargo, Rosemary Ouseph, Andrew Breuckner, Alfred Luk, Avinash Aujayeb, Daniel Ganger, Douglas S Keith, Federica Meloni, Ghady Haidar, Lori Zapernick, Megan Morales, Nitender Goyal, Tanvi Sharma, Uma Malhotra, Alexander Kuo, Ana P Rossi, Angelina Edwards, Brian Keller, Christy Beneri, Darby Derringer, Edward Dominguez, Elise Carlson, Faris Hashim, Haris Murad, Heinrike Wilkens, Henry Neumann, Imran Gani, Joseph Kahwaji, Joyce Popoola, Marian Michaels, Niyati Jakharia, Oveimar De la Cruz, Alfredo Puing, Reza Motallebzadeh, Ravi Velagapudi, Rajan Kapoor, Sridhar Allam, Fernanda Silveira, Surabhi Vora, Ursala M Kelly, Uttam Reddy, Vikas Dharnidharka, Hani Wadei, and Lominadze Zurabi
References
- 1. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180:1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 2020; 20:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buckner FS, McCulloch DJ, Atluri V, et al. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis 2020; 71:2167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Günster C, Busse R, Spoden M, et al. 6-month mortality and readmissions of hospitalized COVID-19 patients: a nationwide cohort study of 8679 patients in Germany. PLoS One 2021; 16:e0255427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 2021; 47:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zettersten E, Engerström L, Bell M, et al. Long-term outcome after intensive care for COVID-19: differences between men and women—a nationwide cohort study. Crit Care 2021; 25:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peñuelas O, Del Campo-Albendea L, de Aledo ALG, et al. Long-term survival of mechanically ventilated patients with severe COVID-19: an observational cohort study. Ann Intensive Care 2021; 11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis 2020. [Google Scholar]
- 13. Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butler CR, Reese PP, Perkins JD, et al. End-of-life care among US adults with ESKD who were waitlisted or received a kidney transplant, 2005-2014. J Am Soc Nephrol 2020; 31:2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fekkar A, Lampros A, Mayaux J, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med 2021; 203:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heldman MR, Kates OS, Safa K, et al. Covid-19 in hospitalized lung and non-lung solid organ transplant recipients: a comparative analysis from a multicenter study. Am J Transplant 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heldman MR, Kates OS, Safa K, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for Covid-19 during the course of the pandemic. Am J Transplant 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heldman MR, Kates OS, Haydel BM, et al. Healthcare resource use among solid organ transplant recipients hospitalized with COVID-19. Clin Transplant 2020:e14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) —a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finelli L, Gupta V, Petigara T, Yu K, Bauer KA, Puzniak LA. Mortality among US patients hospitalized with SARS-CoV-2 infection in 2020. JAMA Netw Open 2021; 4:e216556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open 2021; 4:e210417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matta A, Chaudhary S, Bryan Lo K, et al. Timing of intubation and its implications on outcomes in critically ill patients with coronavirus disease 2019 infection. Crit Care Explor 2020; 2:e0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Royston P. Multiple imputation of missing values. Stata Journal 2004; 4:227–41. [Google Scholar]
- 24. Quante M, Brake L, Tolios A, et al. SARS-CoV-2 in solid organ transplant recipients: a structured review of 2020. Transplant Proc 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Requião-Moura LR, Sandes-Freitas TV, Viana LA, et al. High mortality among kidney transplant recipients diagnosed with coronavirus disease 2019: results from the Brazilian multicenter cohort study. PLoS One 2021; 16:e0254822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hadi YB, Naqvi SF, Kupec JT, Sofka S, Sarwari A. Outcomes of coronavirus infectious disease-19 (COVID-19) in solid organ transplant recipients: a propensity matched analysis of a large research network. Transplantation 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cates J, Lucero-Obusan C, Dahl RM, et al. Risk for in-hospital complications associated with COVID-19 and influenza—Veterans Health Administration, United States, October 1, 2018-May 31, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shukla BS, Warde PR, Knott E, et al. Bloodstream infection risk, incidence, and deaths for hospitalized patients during coronavirus disease pandemic. Emerg Infect Dis 2021; 27:2588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Courtwright AM, Erler KS, Bandini JI, et al. Ethics consultation for adult solid organ transplantation candidates and recipients: a single centre experience. J Bioeth Inq 2021; 18:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Courtwright AM, Rubin E, Robinson EM, et al. An ethical framework for the care of patients with prolonged hospitalization following lung transplantation. HEC Forum 2019; 31:49–62. [DOI] [PubMed] [Google Scholar]
- 32. Maxwell BG, Levitt JE, Goldstein BA, et al. Impact of the lung allocation score on survival beyond 1 year. Am J Transplant 2014; 14:2288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng Y, Li R, Liu S. Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: a novel intervention strategy beyond vaccines and specific antiviral medicines. J Med Virol 2020; 92:1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anjan S, Natori Y, Fernandez Betances AA, et al. Breakthrough COVID-19 infections after mRNA vaccination in solid organ transplant recipients in Miami, Florida. Transplantation 2021; 105:e139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walensky RP, Walke HT, Fauci AS. SARS-CoV-2 variants of concern in the United States—challenges and opportunities. JAMA 2021; 325:1037–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.